Traumatic axonal injury resulting in disruption of functional networks in the brain is thought to be a major contributor to cognitive dysfunction in survivors of traumatic brain injury (TBI).1 However, the true significance of this disruption is not known as current clinical imaging modalities do not directly assess axonal injury or functional network connectivity. Modern MRI techniques such as resting-state fMRI correlation analysis may overcome this limitation.2,3
A 21-year-old right-handed woman presented to the outpatient TBI clinic because she was “having trouble in school and remembering things.” At 15, she was a passenger in a head-on motor vehicle collision. She had a severe TBI, clavicle fracture, and pulmonary contusions. Her initial Glasgow Coma Score was 4 (range: 3, deeply comatose, to 15, normal). Her initial head CT revealed a large left subdural hematoma with 1 cm left-to-right shift, bifrontal hemorrhagic contusions, subarachnoid hemorrhage, intraventricular hemorrhage, and punctate hemorrhages in the subcortical white matter, pons, and splenium of the corpus callosum. A left craniotomy was performed and the subdural hematoma was evacuated. She made a progressive neurologic recovery over 2 months. At discharge she was able to speak complete sentences and had no cranial nerve, motor, or sensory deficits. However, she has experienced persistent difficulties with memory and concentration affecting school performance and everyday life. Neuropsychological testing at age 21 revealed a moderately severe verbal memory deficit for auditory information but otherwise generally intact cognition (see supplementary data on the Neurology® Web site at www.neurology.org).
Conventional MRI scans of the brain revealed mild atrophy and bilateral superior frontal abnormalities with high T2 and FLAIR signal surrounding small regions of encephalomalacia (figure, A). These lesions were not thought to account for her memory loss. Brain regions associated with memory—specifically hippocampi, medial temporal lobes, thalami, and related white matter structures—were normal on conventional structural images.
Figure. Conventional MRI and resting-state fMRI correlation analysis in a 21-year-old with verbal memory deficits following traumatic brain injury.
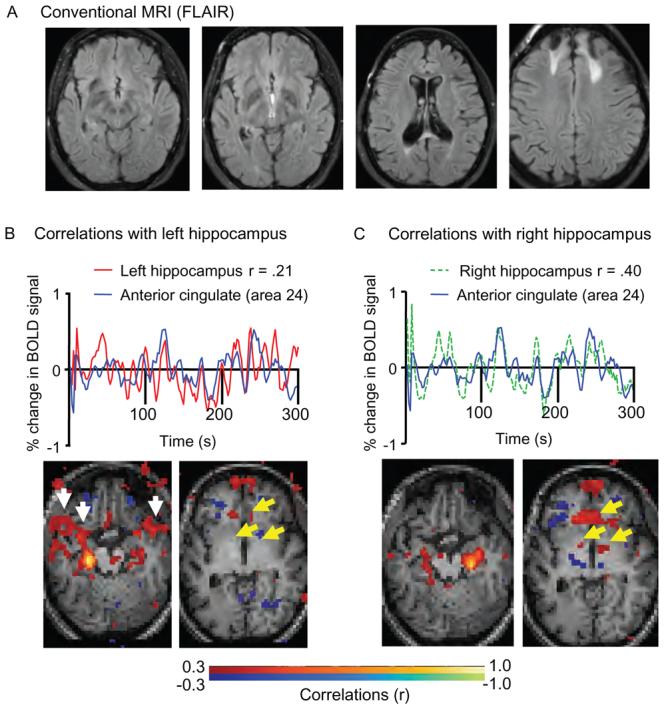
(A) Conventional MRI (FLAIR) revealed bilateral superior frontal lesions but no abnormalities that would explain the patient's verbal memory deficit (left to right: transverse slices at the level of hippocampus, thalamus, fornix, cingulum). (B) Resting-state fMRI correlation analysis revealed abnormal left hippocampal functional connectivity. Top panel: resting BOLD signal time course in the left hippocampus (red line) and anterior cingulate (blue line) were not well correlated (r = 0.21). Bottom panel: spatial map of resting BOLD correlations with the left hippocampus. Yellow arrows indicate absence of significant correlations between the left hippocampus and anterior cingulate or between left hippocampus and anterior thalamus. White arrows point to areas of abnormally increased correlation with the left hippocampus, of unknown importance. (C) Normal right hippocampal functional connectivity. Top panel: BOLD signal time course in the right hippocampus (green line) and anterior cingulate (blue line) were normally correlated (r = 0.40). Bottom panel: spatial map of resting BOLD correlations with right hippocampus. Significant correlations were observed between the right hippocampus and anterior cingulate as well as anterior thalamus (yellow arrows). Images displayed in anatomic space; patient's left side on the left side of the images.
Quantitative hippocampal volumetry4 revealed that the left hippocampus was 18% smaller than the right (3,538 mm3 vs 4,185 mm3). Although each side individually was within the range of 10 normal controls (3,516 to 4,303 mm3), this degree of hippocampal asymmetry was abnormal; absolute right to left difference in controls was 1–10%.
Diffusion tensor imaging revealed subtle abnormalities in the left and right cingulum bundles as well as left and right fornices. This technique measures the diffusion of water in many directions. It is sensitive to axonal injury as it detects microstructural abnormalities which reduce directional anisotropy of water diffusion.5 Relative anisotropy in the cingulum and fornix were lower than the mean of 10 control subjects matched in age, gender, and handedness. However, relative anisotropy was not more than 2 standard deviations below the mean in any of the regions, and so cannot be considered definitively abnormal (see supplementary data).
Thus, it was initially not clear whether the lower left hippocampal volume and borderline low relative anisotropy in the cingulum and fornix were functionally significant. Resting state fMRI correlation analysis, however, clearly demonstrated disruption of the normal connectivity of the left hippocampal network (figure, B). Specifically, this analysis revealed that the BOLD fluctuations in the left hippocampus correlated poorly with those in several other structures implicated in memory including anterior thalamus and the ventral anterior cingulate cortex (vACC). In contrast, the fluctuations in the right hippocampus were normally correlated with those in these structures (figure, C). No such left-right asymmetry in the hippocampal-anterior thalamic and hippocampal-vACC correlations was seen in 10 age-matched controls (not shown).
From this, we conclude that the patient had a functional disruption of the left hippocampal network. This is likely responsible for her verbal memory deficit.6 We hypothesize that the combined effects of her three relatively mild lesions (to the left hippocampus, fornix, and cingulum bundle) caused the disruption. The cingulum bundle injury may have been due to subfalcine herniation, as this region is not commonly affected by traumatic axonal injury. Traumatic axonal injury in the fornix, instead, has been frequently reported,4,7 as has post-traumatic hippocampal volume loss.4
Resting-state fMRI correlation analysis appears to be a remarkably powerful tool for assessing the sequelae of TBI. This technique may be especially helpful in clarifying the functional significance of subtle anatomic abnormalities uncovered using other imaging methods such as DTI and quantitative volumetry. The general utility of this combined advanced MRI approach for diagnosis, prognosis, rehabilitative planning, and therapeutic trial stratification will be the subject of future investigations.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Maurizio Corbetta for insightful comments and advice regarding this line of investigation.
Supported by Burroughs Wellcome Career Award in the Biomedical Sciences, NIH K08 NS049237, and the Washington University Neuroimaging Lab.
Footnotes
Disclosure: The authors report no disclosures.
References
- 1.Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. J Head Trauma Rehabil. 2003;18:307–316. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 3.Arfanakis K, et al. Diffusion tensor MR imaging in diffuse axonal injury. AJNRAm J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- 4.Tomaiuolo F, Carlesimo GA, et al. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1 weighted MRI study. J Neurol Neurosurg Psychiatry. 2004;75:1314–1322. doi: 10.1136/jnnp.2003.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Milner B. Internemispheric differences in the localization of psychological processes in man. Br Med Bull. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- 7.Strich SJ. Shearing of nerve fibers as a cause of brain damage to head injury. Lancet. 1961;2:443–448. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


