Abstract
Background and Purpose
Contrast agent (CA) extravasation has been shown to confound brain tumor perfusion measurements using DSC-MRI, necessitating the use of correction techniques (e.g. Weisskoff, Bjornerud). Model parameters (K2 and Ka) postulated to reflect vessel permeability can be extracted from these correction methods, however the biophysical interpretation of these parameters and their relationship to commonly used MR measures of vascular permeability (e.g. Ktrans) remains unclear. Given that vascular density, as assessed by blood volume, and vascular permeability, as reflected by Ktrans (and potentially K2 or Ka), report on unique and clinically informative vascular characteristics, there is a compelling interest to simultaneously assess these features.
Materials and Methods
Multi-echo DSC-MRI data was acquired, allowing the simultaneous computation and voxel-wise comparison of single- and dual-echo derived measures of K2, Ka and Ktrans in glioma patients. This acquisition enabled the investigation of competing T1 and T2* leakage effects and echo time dependency on these parameters.
Results
K2 and Ka displayed non-significant (p = 0.150 and p = 0.060, respectively) voxel-wise linear correlations with Ktrans, while a significant (p < 0.0001) inverse relationship was observed between K2 and Ka [r2 = 0.466–0.984]. Significantly different (p < 0.005) mean estimates were found between voxels exhibiting predominately T1 or T2* leakage effects for K2 and Ka. Ktrans, however, was observed to be similar between these voxels (0.109 min−1 vs 0.092 min−1). Significant differences (p < 0.0005) in ve (0.285 vs 0.167) were also observed between cohorts. Additionally, K2 and Ka were found to have a significant quadratic relationship (p = 0.031 and p = 0.005, respectively) with ve.
Conclusion
Estimates of vascular permeability in brain tumors may be simultaneously acquired from multiple-echo DSC-MRI via Ktrans, however caution should be used in assuming a similar relationship for K2 and Ka.
Keywords: permeability, perfusion, leakage, DSC-MRI, dynamic contrast enhanced, multi-echo, tumor
Introduction
Brain tumors are characterized by abnormal, poorly constructed vasculature that is often permeable1, making them identifiable on contrast-enhanced MR images. With dynamic contrast enhanced (DCE)-MRI methods, contrast agent (CA) wash-in and extravasation alters the tissue T1 relaxation time and kinetic analysis of the associated signal change permits the computation of the volume transfer constant, Ktrans, which reflects vascular permeability and perfusion. In dynamic susceptibility contrast (DSC)-MRI studies, CA flowing through blood vessels decreases tissue T2* and the acquired signal changes can be used to estimate tumor blood volume. However, CA extravasation has been shown to confound measurements of tissue perfusion (e.g. underestimation of blood volume), particularly in high-grade brain tumors2–4. When corrected for CA leakage effects, DSC-MRI measures of blood volume correlate with brain tumor grade and may be useful for monitoring treatment response2, 5.
CA extravasation leads to simultaneous and competing T1 and T2* effects that can substantially alter the temporal dynamics of DSC-MRI signals2, 6, necessitating the use of correction techniques. One such technique, developed by Weisskoff et al.7 and Boxerman et al.2, incorporates knowledge of the average signal time-course across the brain in non-enhancing voxels to model and correct time-courses in tumor voxels. As a result, a parameter termed ‘K2’ can be extracted that reflects the degree of CA extravasation. Though initially developed to correct T1 leakage effects, the Weisskoff method has been adapted to also account for T2* leakage effects8. A known limitation of this method, however, is that it assumes the mean transit times (MTT) of both healthy and diseased tissue to be equal, which has been observed to not be true in gliomas9. To address this issue, Bjornerud et al. recently developed a MTT insensitive approach for correcting both T1 and T2* leakage effects on DSC-MRI signals10, 11. In this method, the tissue residue function, which describes the CA passage through a voxel, is separated into an intravascular and an extravascular component, from which a parameter ‘Ka’ (similar to K2) can be estimated. A third technique aims to remove T1-based CA leakage effects, through the use of multiple gradient-echo acquisitions3, 12–14. A feature of this approach is that dynamic T1-weighted information can be separated and quantified15–17. Traditional pharmacokinetic modeling18, 19 can then be applied to this data to extract a measure of Ktrans, in a manner similar to DCE-MRI. This approach has been validated in animal brain tumor models and has been recently applied in high-grade glioma patients16, 17, 20. To collect both DCE-MRI and DSC-MRI datasets, an alternative strategy is to acquire traditional DCE-MRI data during a pre-load injection of contrast agent, which is a technique also commonly used to reduce T1 leakage effects in single-echo based DSC-MRI data3.
In the case of brain tumors, Ktrans is largely considered to reflect vascular permeability19 and has demonstrated promise in tumor grading21, 22 and identifying disease progression and treatment response23–26. It has been postulated that measures of K2 and Ka may also directly report on vascular permeability, however their relationship with imaging biomarkers such as Ktrans is not entirely clear and may be dependent on CA kinetics, tissue microstructure, and imaging parameters. Preliminary studies have also investigated the use of K2 and Ka for assessing tumor type27, grade28, 29, and treatment response11.
Inherent to the aforementioned DSC-MRI correction techniques, estimates of K2 and Ka may assume positive or negative values depending on whether T1 (+K2, −Ka) or T2* (−K2, +Ka) leakage effects are the dominating source of signal error. Unlike K2 and Ka, estimates of Ktrans assume the use of a ‘purely’ T1-weighted signal and, therefore, presume insensitivity to competing T1 and T2* leakage effects. In this regard, a previous simulation study reported a non-linear relationship between Ka and Ktrans when large flip angles (>70) were used10. In a follow up in vivo study11, a positive quadratic relationship between Ka and Ktrans was observed. A more recent study found a positive linear correlation between K2 and Ktrans when comparing maximum whole tumor values across patients30. These studies, however, were limited to ROI-based estimates and measures of Ktrans acquired from separate DCE-MRI acquisitions, and did not take into consideration the dominating CA leakage effect.
As suggested by previous works, the presence of simultaneous T1 and T2* leakage effects within a tumor may influence the magnitude and interpretation of K2 and Ka. The overarching goal of this study, therefore, was to investigate the contribution of both T1 and T2* effects on K2 and Ka, while evaluating these parameters as imaging biomarkers of vascular permeability in brain tumors. This was achieved through voxel-wise comparisons of DSC-MRI derived measures of K2, Ka, and Ktrans using the previously described methods. The multi-echo nature of this study allowed simultaneous measurement of these parameters from the same data set, permitting a more accurate comparison free of registration errors and/or sequence specific differences. In addition, the multi-echo data allowed further exploration of potential echo time dependencies of both Weisskoff and Bjornerud correction techniques.
Methods
MRI data were acquired in high-grade glioma patients (n = 7, See Table 1) under Vanderbilt University Institutional Review Board guidelines at 3T (Achieva, Philips Healthcare, Best, The Netherlands) using a 32-channel head coil. Multiple flip angle (MFA) data (TR = 7.6ms, TE = 4.6ms, FA = 2°-20° in 2° increments) were acquired to compute pre-contrast R1 (R10) maps. Dual-echo (DE) DSC-MRI data were then acquired using either a dual gradient-echo (DGE) EPI or SAGE EPI protocol17, 31 with: TR = 1.5s (DGE) or 1.8s (SAGE), TE1/TE2 = 7.0/31.0ms (DGE) or 8.3/25ms (SAGE), SENSE = 2, FOV = 240 × 240mm2, Reconstructed Voxel Size = 2.5 × 2.5 × 5.0mm3, and slices = 15. For SAGE data, only the first two echoes were used in the analysis. Measurements were made before, during, and after administration of Gd-DTPA (0.1 mmol/kg, 4ml/s infusion rate followed by a 20ml saline flush). The scan duration was 7.5 minutes, including 80s of pre-bolus baseline data. A high-resolution T1-weighted data set was collected following the DSC-MRI experiment. Dynamic estimates of ΔR2* were computed for each echo ( and ) and for the dual-echo data () as previously described12, 13.
Table 1.
Patient Demographics
| Patient | Age (yr) | Sex | Prior Resection | Pathology | OS (mo) |
|---|---|---|---|---|---|
| 1 | 61 | Female | Yes | Grade IV Glioblastoma | 17.9 |
| 2 | 66 | Male | Yes | Grade IV Glioblastoma | 18.2 |
| 3 | 65 | Male | Yes | Grade III Anaplastic Astrocytoma | N/A |
| 4 | 51 | Male | Yes | Grade IV Glioblastoma | 4.3 |
| 5 | 55 | Male | No | Grade III Oligodendroglioma | 13.1 |
| 6 | 40 | Male | Yes | Grade IV Glioblastoma | 11.0 |
| 7 | 42 | Female | Yes | Grade IV Glioblastoma | N/A |
OS = overall survival after radiologically confirmed tumor recurrence/progression
K2 Computation
The method proposed by Weisskoff et al.7 allows the extraction of K2 from Eq 1,
| (1) |
where is the average ΔR2* from a mask of non-enhancing brain voxels and is the leakage affected estimate of ΔR2*. A voxel-wise least squares fit to Eq. 1 was performed to extract K2 using 80s of pre-bolus baseline data and 70s of post-bolus data (2.5 min total) consistent with previous reports2, 3, 29.
Ka Computation
In the presence of CA extravasation, the tissue concentration time-course, Ct(t), can be represented as
| (2) |
where f is proportional to tissue blood flow, R(t) is defined as the tissue-specific residue function, Tc is the capillary transit time of the CA, ve is the extracellular-extravascular volume fraction, and Cp is the tracer [CA] in plasma (computed from an AIF extracted from the dual-echo data using an automated selection process32, 33). In DSC-MRI, Ct(t) is estimated in relative terms through measurements of ΔR2,t*(t)10, where ΔR2,t*(t) ∝ r2*·Ct(t) and r2* is the effective transverse relaxivity. Circular deconvolution of Eq. 2 with the AIF34 (over the same time-course used in the Weisskoff correction), results in a composite residue function H(t) described by an early vascular phase (0 ≤ t < Tc) and an extravasation phase (t ≥ Tc)10:
| (3) |
In the context of a single-echo DSC-MRI acquisition, H(t) ≈ Ka for t >> Tc. In this study, Ka was estimated as the mean value of H(t) following a time Tc, equivalent to 1.5× the mean transit time (MTT), up to H(t=60s).
Ktrans Computation
To compute an estimate of Ktrans from multi-echo DSC-MRI data, a T1-weighted signal time-course (ST1w(t)) was first extracted from dual-echo data via Eq. 415, 16, 35.
| (4) |
A pre-contrast R1 (R10) map was combined with the ST1w(t) data to produce dynamic R1 time-courses (R1t(t)) for each voxel36, 37. Ktrans and ve were estimated by fitting R1t(t) and Cp(t) (AIF) with the standard Tofts model18, 19.
Voxel Selection
Voxels selected for this analysis were obtained from enhancing regions on the post-Gd T1-weighted images, determined using a 50% signal threshold (based on the maximum signal intensity in tumor-containing slices) over a manually drawn tumor ROI. These voxels were further categorized by the predominate leakage effect (T1 or T2*) exhibited in their dynamic ΔR2* time-course. In this study, ‘T2* voxels’ were defined by a positive mean ΔR2* over the last 20s of the time-course used for computation of Ka and K2. ‘T1 voxels’ were defined as those in which this estimate was negative.
Statistical Analysis
Voxel-wise measures of K2 and Ka were compared with Ktrans and ve to examine the relationship between these parameters. Associations between the aforementioned parameters were first analyzed on an individual basis using simple linear regression and reported using the r-squared (r2) statistic. Unless otherwise noted, group voxel-wise comparisons were conducted using analysis of covariance in a generalized linear model for repeated measures. Generalized estimating equations (GEE) were used with an exchangeable covariance structure to model the correlation among voxels across patients.
Results
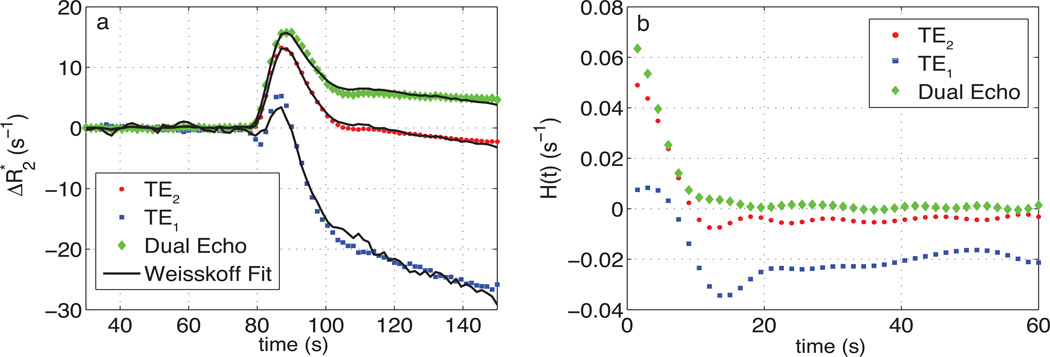
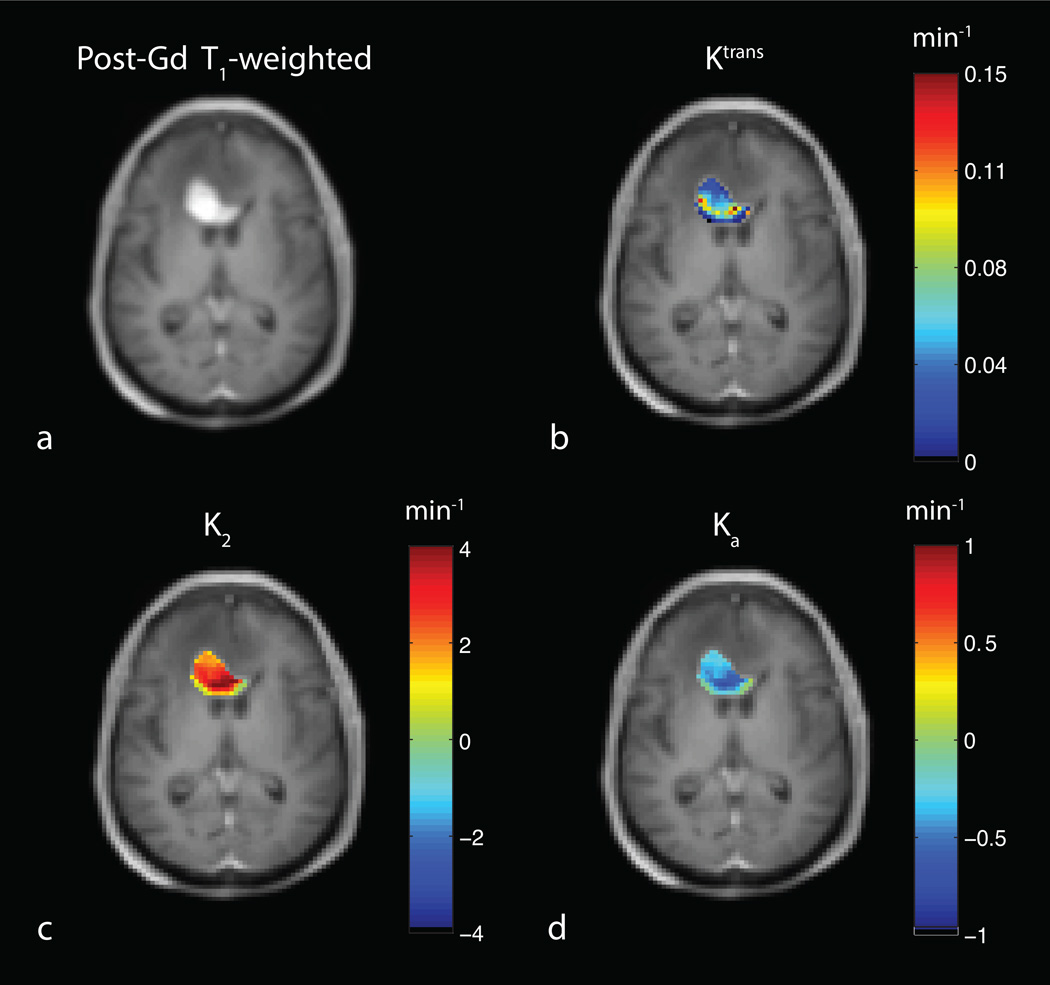
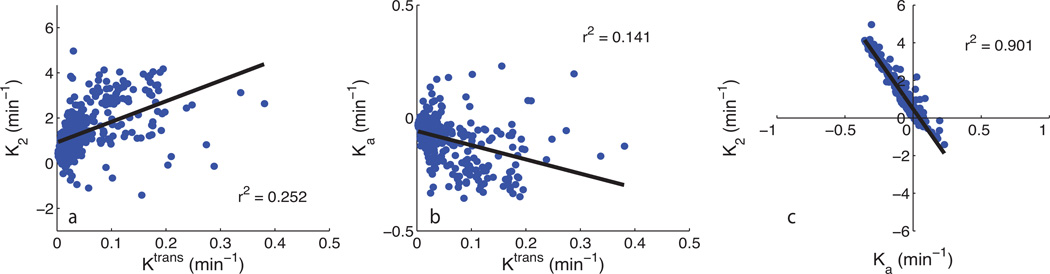
Fig. 1a shows a representative uncorrected tumor ΔR2* time-courses for each echo time and the dual-echo signal, along with the associated Weisskoff model fit. Fig. 1b shows the corresponding tissue residue functions used to compute Ka from the same patient. The computed Ktrans, K2, and Ka maps (overlaid on post-Gd T1-weighted images) for this patient (at TE2) can be seen in Fig. 2b–d, respectively, along with the corresponding post-Gd T1-weighted image (Fig. 2a). Fig. 3a and 3b show an example voxel-wise comparison of K2 and Ka (computed at TE2) with the parameter Ktrans. The range of correlations at TE2 were r2 = [0.014 – 0.430] for K2 and r2 = [0.0001 – 0.403] for Ka. Across patients, both K2 and Ka were found to have non-significant (p = 0.150 and p = 0.060, respectively) linear correlations with Ktrans. A significant (p < 0.0001) inverse relationship was observed (Fig. 3c), however, between K2 and Ka [r2 = 0.466–0.984]. To help elucidate these observed relationships, further analysis was performed.
Fig. 1.
a) Representative uncorrected tumor ΔR2* time-course and the associated Weisskoff model fit (solid) used to compute K2 at TE1 (square), TE2 (dot), and DE (diamond). b) Corresponding tissue residue function used to compute Ka at TE1, TE2, and DE.
Fig. 2.
a) T1-weighted post-Gd anatomical image showing a high-grade brain tumor. Example computed permeability maps (units in min−1) for b) Ktrans, c) K2 and d) Ka.
Fig. 3.
a) Example voxel-wise comparison between K2 at TE2 and Ktrans. b) Example voxel-wise comparison between Ka at TE2 and Ktrans. c) Voxel-wise comparison between K2 (y-axis) and Ka (x-axis). Linear regression line shown in black.
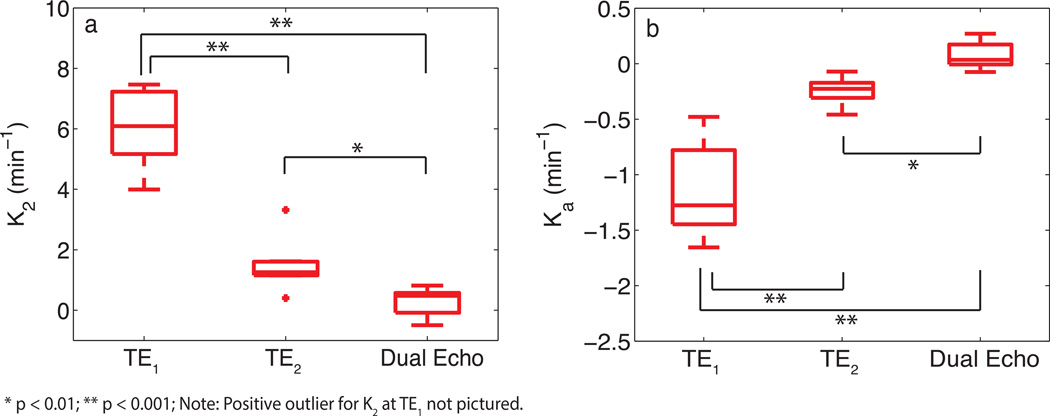
With the availability of multi-echo data, the effect of echo time on K2 and Ka was investigated. Fig. 4a and 4b show box plots using the median values of K2 and Ka, across all patients. A statistically significant difference (Mann-Whitney U test) was observed between K2 at TE1 and TE2 (p<0.001), K2 at TE1 and DE (p<0.001), and K2 at TE2 and DE (p<0.01) acquisitions. Similar differences were observed for Ka. For TE2, voxel-wise estimates of K2 were observed to be predominately positive for high-grade gliomas whereas Ka was predominately negative. A decrease in echo time (TE1) resulted in a broader voxel-wise distribution of values across patients with estimates of K2 becoming increasingly positive and Ka becoming increasingly negative. The computation of K2 using the time-course resulted in a negative shift in the distribution of values, with an increase in the number of voxels near K2 = 0. A similar shift in the distribution towards positive values was observed for Ka.
Fig. 4.
Box plots of median parameter estimates (from all patients) calculated at various echo times for K2 (a) and Ka (b). Box plots display the median, 25th and 75th percentiles (edges of box), and extreme data points (whiskers). Outliers are plotted individually (+). Significance determined by Mann-Whitney U test.
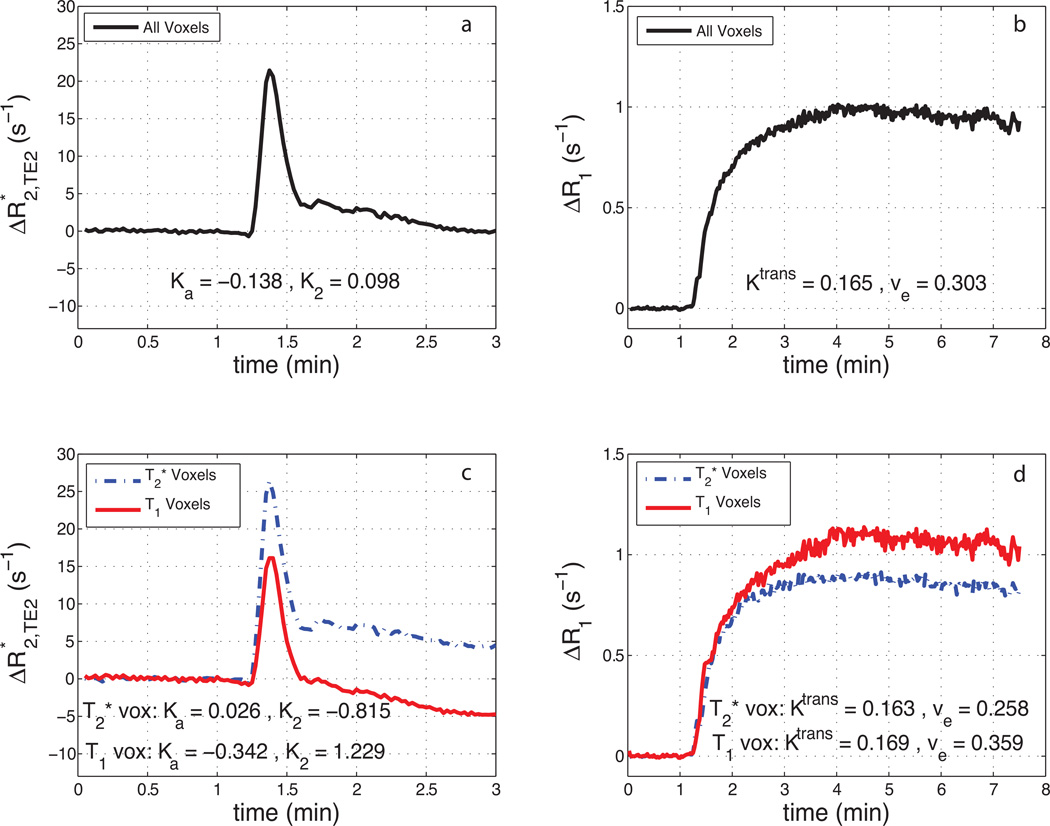
Fig. 5 shows the contribution of both T1 and T2* leakage effects on the relaxation rate time-courses. Fig. 5a shows the mean ΔR2* time course (TE2) for a tumor ROI from patient 2. The resulting ΔR1 time-course from the same tumor can be seen in Fig. 5b. Though the ΔR2* time-course appears to show no appreciable signs of CA leakage, the ΔR1 time-course exhibits large changes in R1 with bolus passage. This indicates CA extravasation and results in a moderate estimate of Ktrans. Similarly, focusing on the smallest 10% of all voxels (based on the magnitude of Ka) in a given patient, Ka = −0.043 ± 0.050 min−1, K2 = 0.113 ± 0.553 min−1, and Ktrans = 0.060 ± 0.099 min−1 (weighted mean (meanw) ± pooled std (stdp)). Fig. 5c and 5d show mean ΔR2* and ΔR1 time-courses from the same tumor with voxels separated by predominate T1 or T2* leakage effects. Note that, in Fig. 5c and 5d, voxels from the same tumor exhibited positive and negative values of K2 and Ka, while Ktrans was observed to be almost identical between the two cohorts.
Fig. 5.
a) Example mean ΔR2* time-course (TE = 31ms) for a tumor ROI and b) the resulting ΔR1 time-course. c) Mean ΔR2* and d) ΔR1 time-courses from the same tumor with voxels separated by whether they predominately exhibit T2* leakage effects (‘T2* voxels’) or T1 leakage effects (‘T1 voxels’).
Table 2 displays the mean estimates of K2, Ka, and Ktrans (separated by T1 and T2* voxels) across all patients. On average, 63% of voxels in the high-grade gliomas were found to predominately exhibit T1 leakage effects. In addition, a significant difference (p < 0.005, paired t-test) was observed, across patients, between mean estimates from T1 and T2* voxel cohorts for both K2 and Ka. While the difference between T1 and T2* cohorts for Ktrans trended toward significance (p ≈ 0.05), the weighted mean for each cohort across patients were similar (0.109 min−1 vs 0.092 min−1). In all voxels across patients, we observed ve = 0.241 ± 0.207. When separated by leakage effect, a significant difference (p < 0.0005, paired t-test) in mean estimates of ve was also observed. Additionally, both K2 and Ka were found to have a significant quadratic relationship (p = 0.031 and p = 0.005, respectively) with ve.
Table 2.
Patient specific estimates of DSC-MRI and DCE-MRI parameters separated by the predominant leakage effect
| # Voxels (%) | K2 (min−1) | Ka (min−1) | Ktrans (min−1) | ve | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | T1 | T2* | T1 | T2* | T1 | T2* | T1 | T2* | T1 | T2* |
| 1 | 44 (79%) | 12 (21%) | 1.807 | 1.205 | −0.373 | −0.250 | 0.223 | 0.066 | 0.221 | 0.072 |
| 2 | 214 (45%) | 265 (55%) | 1.229 | −0.815 | −0.342 | 0.026 | 0.169 | 0.163 | 0.359 | 0.258 |
| 3 | 126 (61%) | 79 (39%) | 2.374 | 0.822 | −0.372 | −0.117 | 0.089 | 0.038 | 0.328 | 0.150 |
| 4 | 368 (47%) | 417 (53%) | 1.767 | 0.700 | −0.536 | −0.469 | 0.104 | 0.078 | 0.228 | 0.140 |
| 5 | 187 (56%) | 147 (44%) | 1.975 | 0.787 | −0.149 | −0.025 | 0.069 | 0.044 | 0.284 | 0.107 |
| 6 | 734 (93%) | 52 (7%) | 3.726 | 0.240 | −0.256 | 0.004 | 0.099 | 0.050 | 0.290 | 0.138 |
| 7 | 16 (64%) | 9 (36%) | 2.591 | 0.025 | −0.418 | 0.024 | 0.200 | 0.179 | 0.203 | 0.107 |
| meanw | 2.627 | 0.289 | −0.329 | −0.208 | 0.109 | 0.092 | 0.285 | 0.167 | ||
Discussion
DCE-MRI estimates of vascular permeability, often reported via Ktrans, have been shown to be helpful in deciphering brain tumor grade21 and in predicting disease prognosis25, 38. Unlike DCE-MRI, DSC-MRI acquisitions can actually be confounded by the increased vascular permeability present in brain tumors, requiring strategies for leakage correction of the MR signal time-courses. Rate constants (K2 and Ka) computed from these correction techniques have been suggested to reflect vessel permeability7, 28. To evaluate this relationship, a simultaneous comparison between Ktrans and the parameters K2 and Ka was performed using multi-echo DSC-MRI. In general, the range of K2 and Ka estimates in this study were observed to be larger than that of Ktrans, though they were consistent with previous measures in brain tumors8, 10, 28. Voxel-wise linear relationships between K2 and Ka and the parameter Ktrans were found to be non-significant when computed from the same data set. Though a non-linear relationship between Ka and Ktrans was previously presented in simulations10, this work provides additional in vivo confirmation. The individual correlations observed here between K2 and Ktrans in gliomas were similar to that observed by Bonekamp et al. using max Ktrans and K2 values from whole tumor ROIs30. Though the lack of a strong linear correlation with Ktrans suggests potential limitations with extracting permeability estimates from DSC-MRI correction methods themselves, it should not, however, be interpreted as a failure of these techniques to reliably correct CBV measures for CA leakage.
The effect of echo time on K2 and Ka was also studied. From Fig. 4 we observed a significant increase (decrease) in estimates of K2 (Ka) with a shorter echo time. This is due, in part, to the decrease in T2* weighting with decreasing echo time and subsequent dominance of T1 leakage effects. Liu et al. previously explored the effect of echo time on K2 in numerical simulations8 and noted that changes in the actual vascular permeability should not affect the polarity of K2, though changes in imaging parameters (e.g. echo time) could. Prior to the current study, a similar analysis with Ka had not yet been performed.
In addition to echo time, the intrinsic presence of competing and simultaneous T1 and T2* leakage effects, within a given voxel, were integral in determining the value of K2 and Ka. As shown in Fig. 5, competing T1 and T2* leakage effects can produce a ΔR2* time-course that paradoxically appears to be free of CA extravasation effects. This is misleading, as the dynamic ΔR1 information reveals appreciable CA leakage, resulting in moderate estimates of Ktrans. As noted by Bjornerud et al., the presence of both T1 and T2* relaxation effects in the extracellular-extravascular space may drive Ka (and K2) towards 0, resulting in artifactually low estimates. As an example, in the smallest 10% of all voxels (based on the magnitude of Ka), the mean Ktrans was observed to be 50% larger than |Ka|. Conversely, the magnitude of the mean Ka was ≈3× larger than Ktrans when computed using all voxels. Additionally, the mean value of K2 and Ka, computed from the aforementioned subset of voxels (smallest 10%), were almost an order of magnitude smaller than the respective mean K2 and Ka computed using all voxels. These findings clearly have implications on the reliability of these parameters as measures of vascular permeability.
In general, the relationship of K2 and Ka with Ktrans may indicate an inaccurate assumption that these parameters solely reflect vessel permeability in brain tumors. When separated into T1 and T2* voxel cohorts, the mean values of K2 and Ka across patients were found to be significantly different from one another (Table 2). The same was true for ve. Similar to the previous observation between Ka and Ktrans in vivo11, a significant quadratic relationship was observed between K2 and Ka and ve across all patients. To this end, a recent theoretical study by Liu et al. demonstrated a potential relationship between ve and the ratio of the parameters K1 and K2 from the Weisskoff correction method39. These results indicate that K2 and Ka may also be influenced by the extravasation space of the CA.
The data in Table 2 also revealed that ‘T1 voxels’ demonstrated larger ve values than those found in ‘T2* voxels’. This likely originates from the underlying biophysical basis of T1 and T2* leakage effects. As in DCE-MRI, T1 leakage effects result from the direct interaction of CA with the extracellular-extravascular water. Accordingly, the physiological factors that drive the tissue [CA] (compartmental volume fractions, perfusion and vascular permeability) as well as physical properties (CA T1 relaxivity, pre-contrast T1) and pulse sequence parameters (TR, flip angle) all influence the shape and magnitude of T1 leakage effects on DSC-MRI signals. In addition to physiological factors and imaging parameters, T2* leakage effects are influenced by intravoxel susceptibility differences created by the spatial distribution of the CA within a voxel. Recently, Semmineh et al. demonstrated that these effects are predominantly influenced by cellular properties including density, size, distribution and shape40. Consistent with the results presented herein, stronger T2* leakage effects were observed for tissues with higher cell density (or lower ve). In general, the dependency of T2* leakage effects on tumor cellularity manifests as changes in the CA’s effective T2* relaxivity. So unlike T1 leakage effects, where the CA’s T1 relaxivity is essentially constant within and across tumors, the T2* relaxivity may vary from voxel to voxel as the cellular properties change41.
The variable CA T2* relaxivity also has important implications on the interpretation of the extracted K2 and Ka parameters. Though voxels were designated as predominantly exhibiting either T1 or T2* leakage effects, each voxel’s signal is the summation of these competing effects, as previously discussed. In the limiting case where T2* leakage effects are absent and the signals only reflect T1 leakage effects, the K2 and Ka parameters are primarily driven by the underlying CA kinetics and the assumptions built into the correction models and can be understood accordingly. However, when there are competing T1 and T2* effects K2 and Ka represent a complex balance between the CA kinetics and the tissue microstructure. Practically, this implies that a positive and negative estimate of K2 or Ka of the same absolute value may not reflect the same combination of vascular permeability, tissue compartment size, or microstructural geometry. Similarly, K2 and Ka values that are equivalent within or across tumors may not reflect the same underlying physiological environment since they could originate from unique combinations of competing T1 and T2* effects. This observation may help further explain the discrepancies in using K2 and Ka to evaluate tumor grade and to assess treatment response11, 28, 29. Computational studies that account for the underlying biophysical basis of the DSC-MRI signal could be used to systematically investigate and provide insight into the complex interaction between T1 and T2* leakage effects and the derived K2 and Ka values.
The use of multi-echo DSC-MRI in this study enabled measures of DCE-MRI signals and, subsequently, computation of the associated Ktrans maps. As mentioned above, an alternative approach to collect both datasets in the same exam is to acquire DCE-MRI data during a pre-load of CA. This enables the use of traditional DCE-MRI pulse sequences, ones that typically have higher spatial (and lower temporal) resolution. For the purpose of the study, this approach would have enabled the comparison of more conventionally derived Ktrans values to K2 and Ka. It is interesting to note, however, that the addition of a pre-load to this study would have reduced T1 leakage effects and increased T2* leakage effects. It is unclear how this would influence the correlation between Ktrans, K2 and Ka. Another limitation of this study is the small sample size. While the findings are likely to hold in a larger population of glioma patients, it would be valuable to expand the tumor types considered (e.g. primary central nervous system lymphoma and brain metastasis) since different histologic subtypes have been shown to express varying degrees of T1 and T2* leakage effects.
Conclusion
This study investigated the use of DSC-MRI for estimating vascular permeability in brain tumors. Implementation of common DSC-MRI leakage corrections techniques afforded the computation of rate constants (K2 and Ka) postulated to report on vessel permeability. Additionally, the acquisition of multi-echo data allowed the computation of the DCE-MRI pharmacokinetic parameter Ktrans. A voxel-wise comparison between the parameters K2, Ka and Ktrans revealed non-significant linear correlations that may be attributed, in part, to competing T1 and T2* leakage effects and the effect of echo time on K2 and Ka. Further investigation also revealed a significant quadratic relationship between K2 and Ka and the DCE-MRI parameter ve. Based on these findings, caution should be used in assuming a direct relationship between K2 and Ka and vascular permeability in brain tumors. Furthermore, the acquisition of Ktrans from multi-echo DSC-MRI data may provide a convenient method for simultaneously measuring vascular permeability and perfusion in brain tumors.
Acknowledgments
Grant Sponsors: NIH R01CA158079, NCI 2R25CA092043, VICC Young Ambassadors Grant (CCQ)
Abbreviations
- CA
contrast agent
- DSC
dynamic susceptibility contrast
- DCE
dynamic contrast enhanced
- Ktrans
CA volume transfer constant
- ve
extracellular-extravascular volume fraction
- CBV
cerebral blood volume
- CBF
cerebral blood flow
- MTT
mean transit time
- MFA
multiple flip angle
- AIF
arterial input function
References
- 1.Shubik P. Vascularization of tumors: a review. Journal of cancer research and clinical oncology. 1982;103:211–226. doi: 10.1007/BF00409698. [DOI] [PubMed] [Google Scholar]
- 2.Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006;27:859–867. [PMC free article] [PubMed] [Google Scholar]
- 3.Paulson ES, Schmainda KM. Comparison of dynamic susceptibility-weighted contrast-enhanced MR methods: recommendations for measuring relative cerebral blood volume in brain tumors. Radiology. 2008;249:601–613. doi: 10.1148/radiol.2492071659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quarles CC, Gochberg DF, Gore JC, et al. A theoretical framework to model DSC-MRI data acquired in the presence of contrast agent extravasation. Phys Med Biol. 2009;54:5749–5766. doi: 10.1088/0031-9155/54/19/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmainda KM, Prah M, Connelly J, et al. Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro-oncology. 2014;16:880–888. doi: 10.1093/neuonc/not216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulson E, Prah DE, Schmainda KM. Compensation of Confounding T1 and T2 Dipolar and residual Susceptibility Effects in DSC-MRI using Dual-Echo SPIRAL. Proc Int Soc of Magn Reson Med. 2007:2811. [Google Scholar]
- 7.Weisskoff RM, Boxerman JL, Sorensen AG. Proc Soc Magn Reson Med. San Francisco, California: 1994. Simultaneous blood volume and permeability mapping using a single Gd-based contrast agent; p. 279. [Google Scholar]
- 8.Liu HL, Wu YY, Yang WS, et al. Is Weisskoff model valid for the correction of contrast agent extravasation with combined T1 and T2* effects in dynamic susceptibility contrast MRI? Med Phys. 2011;38:802–809. doi: 10.1118/1.3534197. [DOI] [PubMed] [Google Scholar]
- 9.Quarles CC, Ward BD, Schmainda KM. Improving the reliability of obtaining tumor hemodynamic parameters in the presence of contrast agent extravasation. Magn Reson Med. 2005;53:1307–1316. doi: 10.1002/mrm.20497. [DOI] [PubMed] [Google Scholar]
- 10.Bjornerud A, Sorensen AG, Mouridsen K, et al. T1- and T2*-dominant extravasation correction in DSC-MRI: part I--theoretical considerations and implications for assessment of tumor hemodynamic properties. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:2041–2053. doi: 10.1038/jcbfm.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emblem KE, Bjornerud A, Mouridsen K, et al. T(1)- and T(2)(*)-dominant extravasation correction in DSC-MRI: part II-predicting patient outcome after a single dose of cediranib in recurrent glioblastoma patients. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:2054–2064. doi: 10.1038/jcbfm.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vonken EJ, van Osch MJ, Bakker CJ, et al. Measurement of cerebral perfusion with dual-echo multi-slice quantitative dynamic susceptibility contrast MRI. J Magn Reson Imaging. 1999;10:109–117. doi: 10.1002/(sici)1522-2586(199908)10:2<109::aid-jmri1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Miyati T, Banno T, Mase M, et al. Dual dynamic contrast-enhanced MR imaging. J Magn Reson Imaging. 1997;7:230–235. doi: 10.1002/jmri.1880070136. [DOI] [PubMed] [Google Scholar]
- 14.Uematsu H, Maeda M, Sadato N, et al. Blood volume of gliomas determined by double-echo dynamic perfusion-weighted MR imaging: a preliminary study. AJNR Am J Neuroradiol. 2001;22:1915–1919. [PMC free article] [PubMed] [Google Scholar]
- 15.Vonken EP, van Osch MJ, Bakker CJ, et al. Simultaneous quantitative cerebral perfusion and Gd-DTPA extravasation measurement with dual-echo dynamic susceptibility contrast MRI. Magn Reson Med. 2000;43:820–827. doi: 10.1002/1522-2594(200006)43:6<820::aid-mrm7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 16.Quarles CC, Gore JC, Xu L, et al. Comparison of dual-echo DSC-MRI- and DCE-MRI-derived contrast agent kinetic parameters. Magn Reson Imaging. 2012;30:944–953. doi: 10.1016/j.mri.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skinner JT, Robison RK, Elder CP, et al. Evaluation of a multiple spin- and gradient-echo (SAGE) EPI acquisition with SENSE acceleration: Applications for perfusion imaging in and outside the brain. Magn Reson Imaging. 2014 doi: 10.1016/j.mri.2014.08.032. Early View. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997;7:91–101. doi: 10.1002/jmri.1880070113. [DOI] [PubMed] [Google Scholar]
- 19.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Schmiedeskamp H, Andre JB, Straka M, et al. Simultaneous perfusion and permeability measurements using combined spin- and gradient-echo MRI. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:732–743. doi: 10.1038/jcbfm.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang N, Zhang L, Qiu B, et al. Correlation of volume transfer coefficient Ktrans with histopathologic grades of gliomas. J Magn Reson Imaging. 2012;36:355–363. doi: 10.1002/jmri.23675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cha S, Yang L, Johnson G, et al. Comparison of microvascular permeability measurements, K(trans), determined with conventional steady-state T1-weighted and first-pass T2*-weighted MR imaging methods in gliomas and meningiomas. AJNR Am J Neuroradiol. 2006;27:409–417. [PMC free article] [PubMed] [Google Scholar]
- 23.Ah-See ML, Makris A, Taylor NJ, et al. Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2008;14:6580–6589. doi: 10.1158/1078-0432.CCR-07-4310. [DOI] [PubMed] [Google Scholar]
- 24.George ML, Dzik-Jurasz AS, Padhani AR, et al. Non-invasive methods of assessing angiogenesis and their value in predicting response to treatment in colorectal cancer. The British journal of surgery. 2001;88:1628–1636. doi: 10.1046/j.0007-1323.2001.01947.x. [DOI] [PubMed] [Google Scholar]
- 25.Armitage PA, Schwindack C, Bastin ME, et al. Quantitative assessment of intracranial tumor response to dexamethasone using diffusion, perfusion and permeability magnetic resonance imaging. Magn Reson Imaging. 2007;25:303–310. doi: 10.1016/j.mri.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toh CH, Wei KC, Chang CN, et al. Differentiation of primary central nervous system lymphomas and glioblastomas: comparisons of diagnostic performance of dynamic susceptibility contrast-enhanced perfusion MR imaging without and with contrast-leakage correction. AJNR Am J Neuroradiol. 2013;34:1145–1149. doi: 10.3174/ajnr.A3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Provenzale JM, Wang GR, Brenner T, et al. Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. AJR American journal of roentgenology. 2002;178:711–716. doi: 10.2214/ajr.178.3.1780711. [DOI] [PubMed] [Google Scholar]
- 29.Donahue KM, Krouwer HG, Rand SD, et al. Utility of simultaneously acquired gradient-echo and spin-echo cerebral blood volume and morphology maps in brain tumor patients. Magn Reson Med. 2000;43:845–853. doi: 10.1002/1522-2594(200006)43:6<845::aid-mrm10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 30.Bonekamp D, Deike K, Wiestler B, et al. Association of overall survival in patients with newly diagnosed glioblastoma with contrast-enhanced perfusion MRI: Comparison of intraindividually matched T - and T -based bolus techniques. J Magn Reson Imaging. 2014 doi: 10.1002/jmri.24756. [DOI] [PubMed] [Google Scholar]
- 31.Schmiedeskamp H, Straka M, Newbould RD, et al. Combined spin- and gradient-echo perfusion-weighted imaging. Magn Reson Med. 2012;68:30–40. doi: 10.1002/mrm.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carroll TJ, Rowley HA, Haughton VM. Automatic calculation of the arterial input function for cerebral perfusion imaging with MR imaging. Radiology. 2003;227:593–600. doi: 10.1148/radiol.2272020092. [DOI] [PubMed] [Google Scholar]
- 33.Newton AT, Skinner JT, Quarles CC. Proc Int Soc Magn Reson Med. Salt Lake City, Utah: 2013. Automatic AIF Estimation in Multi-Echo DSC-MRI of Pediatric Patieints: Avoiding the Noise Floor. [Google Scholar]
- 34.Liu HL, Pu Y, Liu Y, et al. Cerebral blood flow measurement by dynamic contrast MRI using singular value decomposition with an adaptive threshold. Magn Reson Med. 1999;42:167–172. doi: 10.1002/(sici)1522-2594(199907)42:1<167::aid-mrm22>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 35.Kuperman VY, Karczmar GS, Blomley MJ, et al. Differentiating between T1 and T2* changes caused by gadopentetate dimeglumine in the kidney by using a double-echo dynamic MR imaging sequence. J Magn Reson Imaging. 1996;6:764–768. doi: 10.1002/jmri.1880060509. [DOI] [PubMed] [Google Scholar]
- 36.Landis CS, Li X, Telang FW, et al. Determination of the MRI contrast agent concentration time course in vivo following bolus injection: effect of equilibrium transcytolemmal water exchange. Magn Reson Med. 2000;44:563–574. doi: 10.1002/1522-2594(200010)44:4<563::aid-mrm10>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Skinner JT, Yankeelov TE, Peterson TE, et al. Comparison of dynamic contrast-enhanced MRI and quantitative SPECT in a rat glioma model. Contrast media & molecular imaging. 2012;7:494–500. doi: 10.1002/cmmi.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mills SJ, Patankar TA, Haroon HA, et al. Do cerebral blood volume and contrast transfer coefficient predict prognosis in human glioma? AJNR Am J Neuroradiol. 2006;27:853–858. [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Ding W, Bensheng Q. Proc Int Soc Magn Reson Med. Milan, Italy: 2014. Extravascular extracellular space fraction measurement by DSC-MRI: a theoretical study. [Google Scholar]
- 40.Semmineh NB, Xu J, Boxerman JL, et al. An efficient computational approach to characterize DSC-MRI signals arising from three-dimensional heterogeneous tissue structures. PloS one. 2014;9:e84764. doi: 10.1371/journal.pone.0084764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semmineh NB, Xu J, Skinner JT, et al. Assessing tumor cytoarchitecture using multiecho DSC-MRI derived measures of the transverse relaxivity at tracer equilibrium (TRATE) Magn Reson Med. 2014 doi: 10.1002/mrm.25435. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]