Abstract
Neurotransmitter effects on calcium currents activated by sensory neuron action potentials have been previously studied in embryonic or neonatal dorsal root ganglion (DRG) cells in culture. In the present study we examined the effects of serotonin (5-HT) on the shape of action potentials recorded from fully differentiated primary afferent neurons in isolated DRG of adult bullfrogs. Intracellular recordings were obtained from cell bodies of type A and C neurons. Concentrations of 5-HT that had no effect on membrane potential or input resistance had little or no effect on action potential shape, Treatment with 5–20 mm tetraethylammonium ion (TEA) led to the appearance of a plateau phase on the falling limb of the spike. This plateau phase appears to result from calcium influx, as it was dramatically reduced in amplitude and duration by solutions containing low concentrations of calcium or the calcium channel blocker, manganese. In preparations treated with 7.5 mm TEA, low concentrations of 5-HT (10 nm–1 µm) produced a dose-dependent narrowing of the calcium-dependent plateau phase of the mixed sodium/calcium spike. A decrease in spike afterhyperpolarization was also noted. The decrease in spike duration was recorded from 74% of type A neurons and 57% of type C neurons, and was not secondary to a change in resting potential or input resistance. The 5-HT receptor antagonists methysergide and metergoline did not block the response to 5-HT. Instead, they exhibited weak agonist-like actions. Serotonin also reduced the rate of rise and peak amplitude of calcium spikes recorded in the presence of tetrodotoxin and TEA. Since these effects on spike shape could occur in the absence of any 5-HT-induced depolarization or resistance change, the involvement of a 5-HT receptor system different from that which mediates the depolarizing actions of much higher concentrations of 5-HT (Holz et al., 1985) is indicated.
Dunlap and Fischbach (1978) reported that serotonin (5-HT) decreases the duration of action potentials recorded from the somata of embryonic chick dorsal root ganglion cells (DRG cells) grown in primary dissociated cell culture. Specifically, 5-HT produced a narrowing of the calcium-dependent plateau phase on the falling limb of the spike. On the basis of voltage clamp analysis, it was suggested that this decrease in spike duration results from an inhibition of calcium currents activated by the depolarizing phase of the action potential (Dunlap and Fischbach, 1981). Such an inhibitory action of 5-HT may explain its ability to reduce the calcium-dependent release of substance P from primary afferent neurons (Fischbach et al., 1981; Mudge et al., 1979). Although the above conclusions are supported by studies of embryonic DRG cells in dissociated cell culture, studies comparing adult and cultured fetal DRG cells from rodents have led to the suggestion that Ca channels are lost, or cease to function, as these cells differentiate (Matsuda et al., 1978; Yoshida et al., 1978). If this is the case, it is uncertain whether transmitter modulation of Ca influx occurs in fully differentiated sensory neurons. Therefore, the present study was undertaken to determine what effects 5-HT has on action potentials recorded from DRG cells of the adult frog. It was found that 5-HT had little or no effect on the duration of spikes recorded from DRG cells in normal Ringer’s solution. In contrast, the application of tetraethylammonium ion (TEA) resulted in the appearance of a prominent plateau phase on the falling limb of the spike, which was reduced by low concentrations of 5-HT. A preliminary report of these observations has appeared (Holz et al., 1984).
Materials and Methods
Dorsal root ganglion (DRG) cells L9–L10 of Rana catesbiana were isolated, desheathed, and pinned to the floor of a superfusion chamber as previously described (Holz et al., 1985). The ganglion was superfused with Ringer’s solution of the following composition: NaCl, 100.0 mm; KCl, 2.4 mm; NaHCO3, 9.5 mm; Tris, 10.0 mm; CaCl2, 1.9 mm; and dextrose, 5.6 mm, and was saturated with 95/5% O2/CO2, pH 7.4. Experiments were performed at room temperature (21–23°C). The flow rate was 6.5 ml/min, and the equilibration time for complete exchange of chamber contents was 20 sec. Drugs were dissolved in Ringer’s solution and administered in the bath by means of a valve system to switch between control and drug-containing solutions. Serotonin creatinine sulfate, d,l-arterenol HCl (NE), gamma-aminobutyric acid (GABA). and tetrodotoxin (TTX) were obtained from Calbiochem-Behring. Methysergide maleate was obtained from Sandoz, metergoline from Farmitalia, yohimbine HC1 from Sigma, TEA and 4-aminopyri-dine (4-AP) from Eastman Organic Chemical Co. Sodium-free solutions were prepared by equiosmolar substitution of NaCl with 133 mm Tris (pH 7.4). Low-calcium solutions were prepared by omitting CaCl2 from the Ringer’s. Solutions containing elevated levels of calcium, manganese, cobalt, or barium were prepared by adding CaCl2, MnCl2, CoCl2, or BaCl2 to Ringer’s containing 1.9 mm calcium.
Intracellular recordings from DRG somata were obtained with glass microelectrodes (20–80 MΩ) filled with 2 m KCl. Potentials were recorded by an amplifier with an active bridge circuit allowing current injection through the recording electrode. Current and voltage traces were displayed on a pen recorder and digitizing storage oscilloscope. Input resistance was measured by passing hyperpolarizing constant current pulses of sufficient duration (200–400 msec) to fully charge the membrane capacitance and reach a steady-state voltage deflection. Action potentials recorded intracellularly in DRG somata were evoked either by direct injection of depolarizing current through the recording electrode (direct action potential) or indirectly by stimulation of the dorsal root (antidromic action potential) or spinal nerve (orthodromic action potential). The dorsal root and spinal nerve were led out through Vaseline-coated slits into oil pools adjacent to the central superfusion chamber and were mounted on wire electrodes for stimulation with square-wave pulses (0.1–0.2 msec, 0.5–40 V). The distance between the cathode and the DRG was 1 cm.
Results
DRG cell action potentials
The dorsal root or spinal nerve was stimulated and the latency of antidromically or orthodromically evoked somatic action potentials measured. The conduction velocity (CV) of each cell’s axon was determined from the latency measurement, and the cells were classified as type A (CV 4–20 m/set) or type C (CV 0.2–0.7 m/set) neurons as previously described (Holz et al., 1985). The fastest conducting type A neurons (CV 13–20 m/set) generated somatic action potentials (Fig. 1A, trace 1) of relatively short duration (0.5–1.0 msec, measured at half-peak amplitude), and these spikes were followed by brief afterhyperpolarizations lasting 1–20 msec (measured at return to resting membrane potential). Action potentials recorded from these cells showed no inflection on the spike’s falling limb. In contrast, a brief plateau phase (hump) was noted on the falling limb of action potentials recorded from most slowly conducting type A neurons (CV 8–11 m/set) and all type C neurons (see trace 1 on Fig. 1, B and C). These spikes were of longer duration (2.0–6.0 msec) and were followed by prolonged afterhyperpolarizations lasting up to 620 msec. Parameters describing the shape of action potentials recorded from type A and C DRG cells are summarized in Table 1. Note that action potentials recorded from type A and C neurons did not differ in spike amplitude but did show significant differences in spike duration, afterhyperpolarization amplitude and duration, and rates of rise and fall.
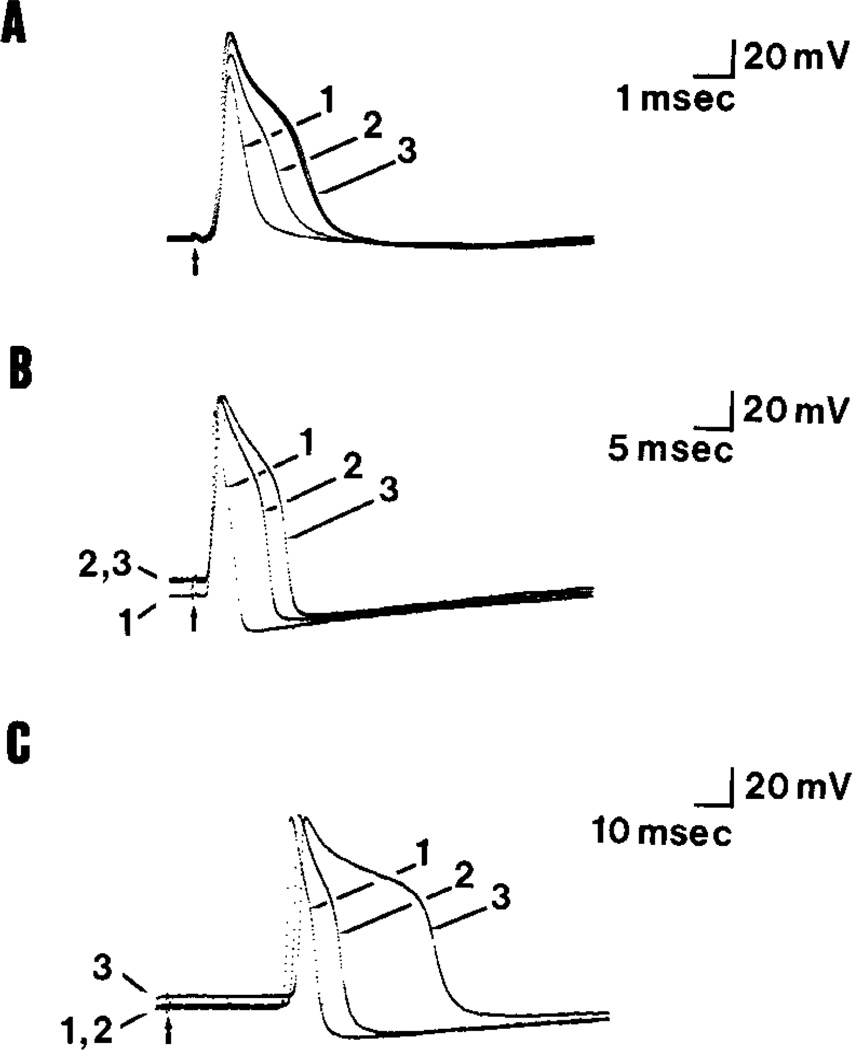
Figure 1.
Prolongation of action potentials by TEA in three types of DRG cells. Action potentials were recorded in the soma following stimulation of the dorsal root or spinal nerve; times at which stimuli were applied are indicated by the arrows. The position of the DC baseline on the oscilloscope traces indicates the resting potential and was not readjusted when superimposing these traces (here, and in subsequent figures). The effects of 7.5, 10, and 20 mm TEA are shown in parts A, B, and C, respectively. The traces marked 1 show action potentials recorded in normal Ringer’s solution; traces marked 2 were recorded during development of the TEA effect; and traces marked 3 show the maximum (steady-state) response to TEA. Note the different time scales in parts A, B, and C. A, Action potentials recorded from a type A neuron with a CV of 20 m/set. The control spike (trace 1) was of short duration (0.6 msec) and was followed by a brief afterhypernolarization. TEA (7.5 mm) increased the spike duration by 325% (trace 3) Action potentials recorded from a different type A neuron (CV 10 m/set), which under the control condition (trace 1) was of longer duration (1.8 msec) and was followed by a prolonged afterhyperpolarization. TEA (10 mm) increased the spike duration by 425% (trace 3) and depolarized the cell by about 10 mV (traces 2 and 3). C, Action potentials recorded from this type C neuron (CV 0.4 m/set) were of longer duration (4.7 msec) followed by a prolonged afterhyperpolarization (trace 1). TEA (20 mm) increased the spike duration by 520% and depolarized the resting potential by 6 mV (trace 3).
Table 1.
Parameters describing the shape of action potentials recorded from type A and C neurons in the frog dorsal root ganglion
| Cell type | Spike amplitude (mV) |
Spike duration (msec) |
AHP amplitude (mV) |
AHP duration (msec) |
Rate of rise (V/sec) |
Rate of fall (V/sec) |
|---|---|---|---|---|---|---|
| A | ||||||
| Mean | 73 | 1.3 | 11 | 41 | 161 | 79 |
| SEM | 2.5 | 0.1 | 0.8 | 11.8 | 21.5 | 8.2 |
| n | 43 | 37 | 36 | 16 | 32 | 32 |
| C | ||||||
| Mean | 74 | 3.2 | 14 | 248 | 68 | 32 |
| SEM | 3.3 | 0.2 | 0.8 | 52.0 | 7.3 | 3.5 |
| n | 24 | 23 | 26 | 13 | 18 | 18 |
| n.s.a | sig.a | sig.a | sig.b | sig.b | sig.b | |
| p > 0.5 | p < 0.001 | p < 0.01 | p < 0.002 | p < 0.05 | p < 0.002 |
AHP = afterhyperpolarization.
Values for A and C were compared by Student’s t test.
Sample variances were heterogeneous as tested by the Fmax test; therefore, a Mann-Whitney U test was used to compare values for A and C.
Actions of 5-HT recorded in standard Ringer’s solution
When DRG from adult bullfrogs were superfused with Ringer’s solution containing 5-HT (10–100 µm), the effect of 5-HT on spike shape was determined for those cells that showed no change in membrane potential or input resistance. In nine type A and C neurons, 5-HT had no effect on spike shape. A small decrease in spike duration was recorded from one type A neuron. In contrast, Dunlap and Fischbach (1978) reported that similar concentrations of 5-HT decreased the duration of action potentials recorded from a majority of embryonic chick DRG cells in culture. It is important to note that action potentials recorded from chick sensory neurons exhibit a prominent plateau phase on their falling limb (Dichter and Fischbach, 1977) whereas bullfrog DRG spikes have either no plateau phase (trace 1, Fig. 1A) or an abbreviated plateau phase (trace 1, Fig. 1, B and C). For this reason, it seemed possible that a response to 5-HT might be unmasked in the frog DRG once the spike duration was prolonged by treatment with TEA.
Prolongation of the action potential by TEA
TEA is a well-known blocker of the voltage-dependent potassium channels that contribute to the repolarizing phase of the action potential (Stanfield, 1983). Addition of 7.5–20 mm TEA to Ringer’s solution produced a dose-dependent increase in the duration of action potentials recorded from both type A (Fig. 1, A and B) and type C (Fig. 1C) bullfrog DRG cells. In 94% of the neurons tested (n = 49) the prolongation was associated with the appearance of a long-duration (2–30 msec) plateau phase on the falling limb of the spike (trace 3, Fig. 1, A–C). TEA (7.5 mm) submaximally increased the spike duration 2- to 4-fold. In addition, this concentration of TEA increased the spike amplitude (Fig. 1A), depolarized the resting potential by 4–8 mV, and increased the input resistance. As described below, frog DRG preparations were treated with 7.5 mm TEA in order to further examine the effects of 5-HT on spike shape.
Serotonin decreases the duration of “TEA-broadened” spikes in frog DRG cells
The effect of 5-HT was tested on action potentials recorded from 46 type A and seven type C cells during superfusion with Ringer’s solution containing 7.5 mm TEA. Serotonin (10 nm–10 µm) decreased the duration of TEA-broadened action potentials in 74% of type A neurons (Fig. 2, A and D) and 57% of type C neurons (Fig. 2C). The decrease in action potential duration was dose-dependent between 10 nm and 1 µm and resulted from a narrowing of the TEA-induced plateau phase on the falling limb of the spike. This effect of 5-HT was not accompanied by a change in the rate of rise or peak amplitude of the spike, but a clear decrease in the amplitude of the spike afterhyperpolarization was frequently noted (Fig. 2, A and B). The average percentage decrease in spike duration (at half-amplitude) during exposure to 10 µm 5-HT was 32 ± 4% in type A neurons (n = 16) and 33 ± 6% for type C neurons (n = 4). The effect of 5-HT was rapid in onset (within 10–20 set) and offset (within 60 set), and no tachyphylaxis to repeated or prolonged (1–2 min) applications of 5-HT was noted.
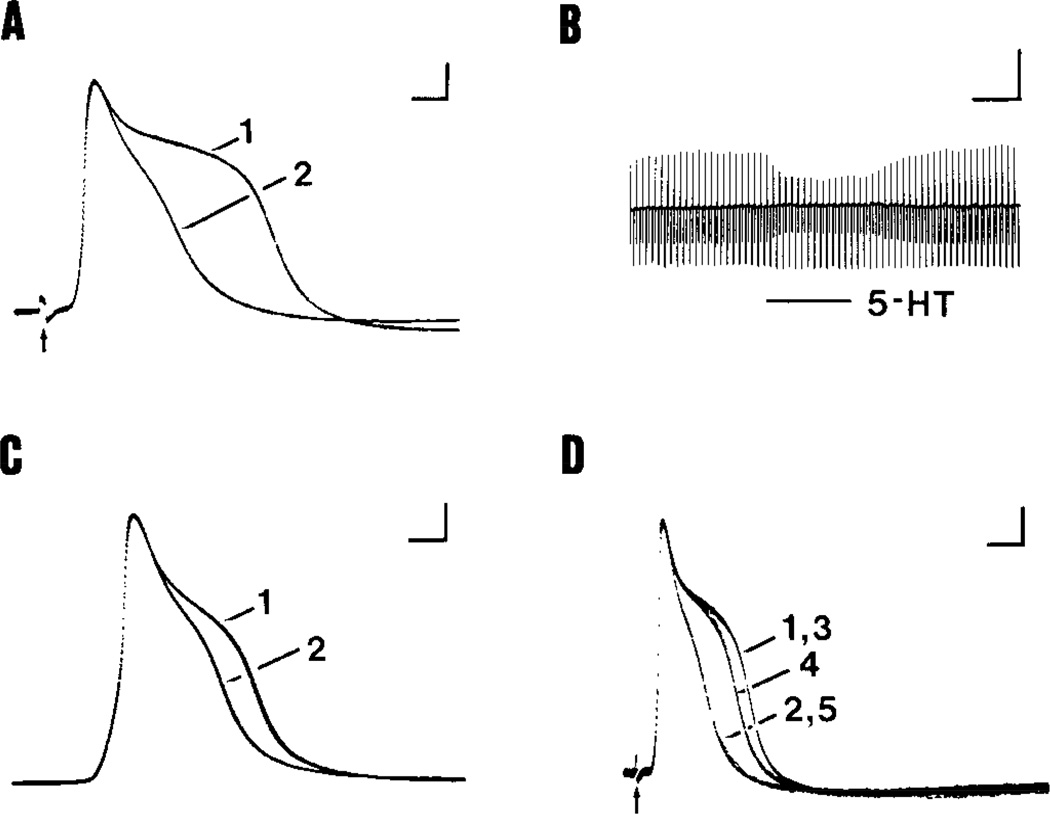
Figure 2.
Effects of 5-HT and methysergide on the shape of action potentials in type A and C neurons. The Ringer’s solution contained 7.5 mm TEA in all cases. A, Action potentials evoked in a type A neuron (CV 14 m/set) before (trace 1) and during (trace 2) application of 1 µm 5-HT. Serotonin decreased the spike duration by 56%. The duration of the spike returned to control value during washout of 5-HT (not shown). Note the reduction in afterhyperpolarization amplitude. B, DC voltage record of the same response to 5-HT as shown in A, on a slower time base. Injection of constant-current hyperpolarizing pulses (0.4 nA) between action potentials produced the longer downward voltage deflections that were used to monitor input resistance. Action potentials (upward deflections) and associated afterhyperpolarizations (small downward deflections) were evoked by stimulation of the spinal nerve. The frequency response of the pen recorder caused attenuation of the spike amplitude and the height of the upward deflection was determined by the duration of the spike. During exposure to 1 µm 5-HT (dark bar), the resting potential and input resistance remained constant, while the amplitude of the upward deflection decreased, indicating a reduction in spike duration. As in Fig. 3A, the amplitude of the spike afterhyperpolarization (shorter downward deflections) was reduced. C, Recordings from a type C neuron (CV 0.4 m/set) show the action potential before (trace 1) and during (trace 2) exposure to 10 µm 5-HT. 5-HT decreased the duration of the TEA-broadened mike by 26%. D. Trace 1 shows action potentials recorded from a type A neuron (CV 10 m/set). A 50% decrease in spike duration was recorded on exposure to 10 µm 5-HT (trace 2). Following washout of 5-HT. the spike returned to its original duration (trace 3). Next, the superfusion medium was switched to TEA-Ringer’s containing 100 µm methysergide, and a 19% decrease in spike duration recorded (trace 4). Addition of 10 µm 5-HT to the superfusate containing TEA and 100 µm methysergide caused a decrease in spike duration (truce 5) identical to that recorded in the presence of 10 µm 5-HT without methysergide (trace 2). Calibration bars, 20 mV in A–D; 1 msec in A, 60 set in B, 5 msec in C, 2 msec in D.
Recordings from type A neurons showed an additional increase in spike duration when 7.5 mm 4-AP or 1 mm barium was added to the 7.5 mm TEA-Ringer’s. When 10 mm 5-HT was tested, a 45 ± 10% (n = 3) decrease in spike duration was recorded in the 4-AP/TEA solutions, and a 40 ± 12% (n = 3) decrease in the barium/TEA solution. Although these 5-HT-induced decreases in spike duration were larger than those observed in preparations treated only with TEA, the spike duration at the peak of the 5-HT effect was similar in each of these three solutions. Attempts to analyze the effects of 5-HT on neurons impaled with 2 m CsCl-filled electrodes proved inconclusive because cesium-loaded neurons generated action potentials of such varied duration that even qualitative observations could not be made.
In concentrations of 1 µm or less, 5-HT decreased the duration of TEA-broadened spikes while having no effect on resting membrane potential or input resistance (see Fig. 2B). With higher concentrations of 5-HT (10–100 µm), changes in membrane potential and input resistance were sometimes observed. Of 12 type A neurons showing a decrease in spike duration following 10 µm 5-HT, 58% exhibited no change in input resistance or resting potential, while 42% responded with a slow depolarization (average amplitude 6 mV). This slow depolarizing response to 5-HT was accompanied by an underlying increase in input resistance and has been described in detail in a previous report (Holz et al., 1985).
Methysergide and metergoline, two drugs known to be 5-HT antagonists in a number of systems (Peroutka et al., 1981), were tested for their ability to block the effect of 5-HT on spike shape. In concentrations of 1–100 µm (n = 10), neither substance blocked the decrease in TEA spike duration caused by 10 µm 5-HT. Instead, their action resembled that of 5-HT, with both agents producing small (10–20%) decreases in spike duration (Fig. 2D, n = 4). The 5-HT-induced decrease in TEA spike duration measured in six type A neurons was also insensitive to the α2-adrenoreceptor antagonist yohimbine (1–10 µm). Yohimbine had no effect of its own on the TEA spike duration. When tested on TEA-treated type A neurons that showed clear 5-HT-induced decreases in spike duration, 10–50 µm of GABA (n = 5) or norepinephrine (n = 6) had no effect on spike shape.
Analysis of the components of the TEA spike
An action potential recorded from a type A neuron superfused with Ringer’s solution containing 7.5 mm TEA is shown in Figure 3A (trace 1). Omission of calcium from the TEA-Ringer’s reduced the amplitude and duration of the spike (trace 2), suggesting that the plateau phase results from calcium influx triggered by depolarization. When the extracellular calcium concentration was raised from 1.9 to 9.5 mm, the amplitude of the spike increased, but instead of a prolongation, the plateau phase was reduced (compare traces 1 and 2, Fig. 3B). This rather paradoxical effect of high-calcium solutions was previously reported by Koketsu et al. (1963). Such a decrease in spike duration was not the consequence of a depolarizing shift in membrane potential seen in high-calcium solutions (Fig. 3B, trace 2), since an increase in spike duration was always observed when the resting potential was depolarized by direct current injection.
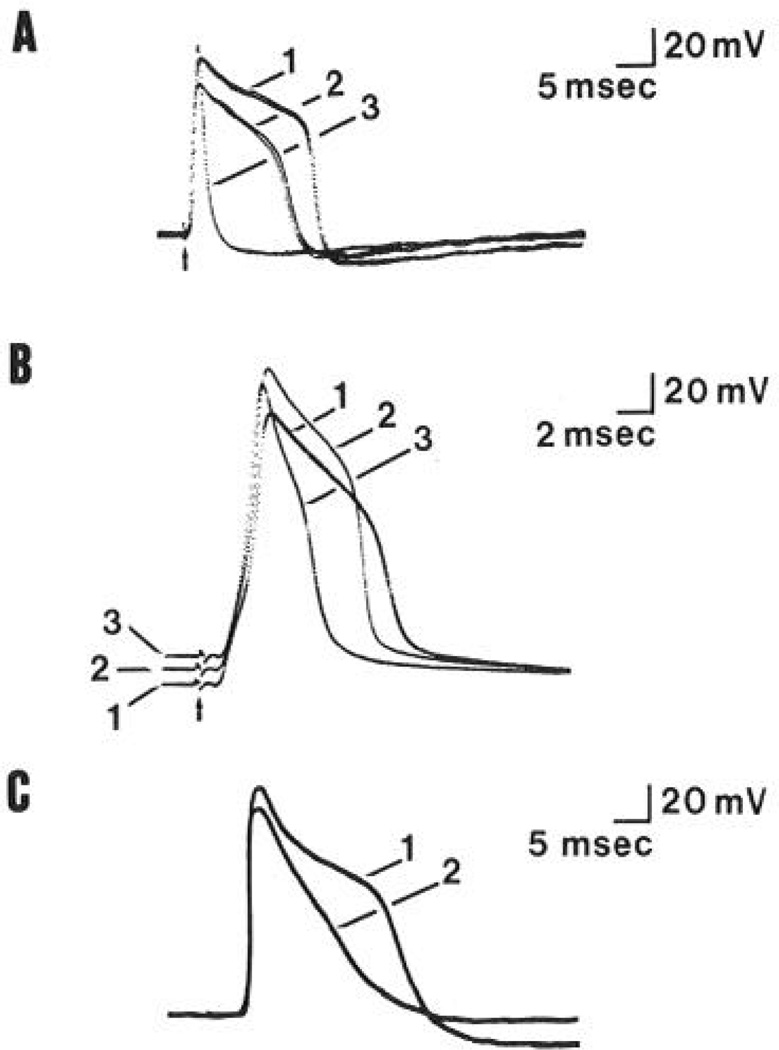
Figure 3.
Effects of calcium and manganese on the shape of action potentials recorded from TEA-treated type A and C neurons. A, Action potentials recorded from a type A neuron (CV 18 m/set) superfused with Ringer’s solutions containing 7.5 mm TEA and either the normal amount (1.9 mm) of calcium (trace 1), no added calcium (trace 2), or 1.9 mm calcium and 4.0 mm manganese (trace 3). B, Action potentials recorded from a type A neuron (CV 8 m/set) superfused with Ringer’s solutions containing 7.5 mm TEA and either normal (1.9 mm) calcium (trace 1), high (9.5 mm) calcium (truce 2), or 1.9 mm calcium and 4.0 mm manganese (truce 3). Note that the cell was depolarized by solutions containing high calcium or manganese. C, Action potentials recorded horn a type C neuron (CV 0.4 m/set) superfused with Ringer’s solutions containing 5.0 mm TEA and either 1.9 mm calcium (trace 1) or 1.9 mm calcium and 4.0 mm manganese (truce 2).
Manganese ion is a relatively ineffective carrier of inward current through voltage-dependent calcium channels in frog (Ishizuka et al., 1984) and in rodent DRG cells (Kostyuk et al., 1981b). Addition of 2–4 mm manganese to the TEA-Ringer’s reduced the amplitude and duration of the plateau phase of spikes recorded from type A (trace 3, Fig. 3, A and B) and type C (trace 2, Fig. 3C) neurons. Manganese-induced reductions in duration at half-amplitude ranged from 37 to 93% (n = 6) and were accompanied by a depolarization of the membrane potential in some (Fig. 3B, trace 3), but not all, cases (Fig. 3, A and C). In addition, high-manganese or low-calcium solutions reduced the amplitude of the spike afterhyperpolarization (see Fig. 3, A and C).
When the medium superfusing the ganglia was switched from normal Ringer’s solution to a sodium-free solution containing 7.5 mm TEA, action potentials recorded from type A and C neurons began to fail and were eventually blocked (Fig. 4A, traces 1 and 2). Increasing the depolarizing stimulus by 1.5- to 2-fold resulted in the appearance of a new type of higher-threshold regenerative response (Fig. 4A, trace 3). This new type of action potential appeared to result from calcium influx, since it was not blocked by 1 µm TTX, but was blocked by addition of 2 mm manganese or cobalt to the Ringer’s solution (see Fig. 4B).
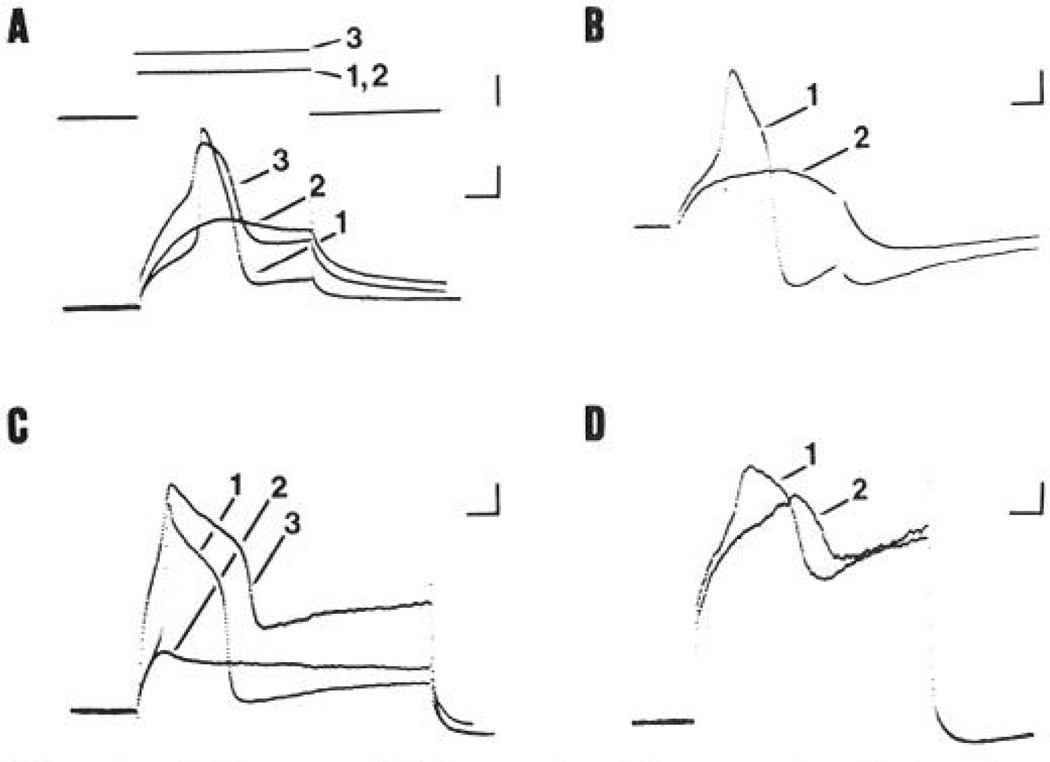
Figure 4.
Action potentials in type A and C neurons in which calcium is the main carrier of inward current can be demonstrated and are reduced in duration by 5-HT. Action potentials were evoked by injection of depolarizing current through the recording electrode. A, Upper traces show the current injected, and lower truces show voltage recording of action potentials from a type C neuron (CV 0.7 m/sec). Current trace 1 shows the threshold current needed to evoke a spike during superfusion of the ganglion in Ringer’s solution. The superfusate was then switched to a sodium-free solution containing 133 mm Tris, 1.9 mm calcium, and 7.5 mm TEA, whereupon the spike failed (trace 2). Note that TEA treatment increased the input resistance of the cell, as indicated by the larger size of the electrotonic potential. When the stimulus current was increased to 1.5× the original threshold level (trace 3), a new type of action potential (calcium action potential) was recorded. B, Calcium action potential recorded from a type A neuron superfused in sodium-free solution containing 7.5 mm TEA (trace 1). The spike was blocked by application of a solution of the same composition with the addition of 2 mm cobalt (trace 2). The spike returned following washout of cobalt (not shown). C, Effect of TTX on action potentials recorded from a TEA-treated type A neuron. Action potential evoked by injection of just suprathreshold current during superfusion of the ganglion with Ringer’s containing 7.5 mm TEA (trace 1). Addition of 1 µm TTX blocked this spike (trace 2). A TTX-resistant action potential (“calcium” action potential) was recorded when the current strength was increased 2-fold (trace 3). D, Effect of 5-HT on the shape of TTX-resistant “calcium” action potentials in the same type A neuron as shown in C. Trace 1, Action potential obtained in the presence of 7.5 mm TEA and 1 µm TTX, trace 2, bath application of 10 µm 5-HT reduced the rate of rise and peak amplitude of this spike. The afterhyperpolarization following the spike also appears to be reduced. These responses were fully reversible and repeatable. Calibration bars, time and voltage bars in all frames are 5 msec and 20 mV, upper current trace in A, 0.2 nA.
TTX-resistant action potentials in type A and C frog DRG cells
Addition of 1 µm TTX to Ringer’s solution (no TEA added) did not block action potentials recorded from type C neurons. These TTX-resistant spikes persisted during exposure to solutions containing 2 mm cobalt in addition to TTX (n = 10) but failed in sodium-free Ringer’s solution (n = 7). In type C neurons, therefore, a significant portion of the regenerative inward current appears to enter through TTX-resistant sodium channels. Type A neurons differed from type C neurons in that regenerative potentials could be evoked in the presence of TTX only when TEA was included in the Ringer’s solution. The effect of TTX on a TEA-treated type A neuron is shown in Fig. 4C. The TEA spike evoked by a just suprathreshold current pulse (trace 1) failed when TTX was added to the superfusate (trace 2). When the stimulus current was increased to twice threshold, a TTX-resistant spike was recorded (trace 3). Such high-threshold, TTX-resistant spikes in TEA-treated type A neurons were completely blocked by solutions containing 2–4 mm manganese or cobalt (not shown) and, therefore, appear to be pure calcium action potentials. Serotonin (10 µm) reduced the rate of rise and peak amplitude of these “calcium” spikes (Fig. 4D).
Discussion
Effects of 5-HT on DRG cell action potentials
In the present study, we found that 5-HT (10–l00 µm) had little, if any, effect on the duration of action potentials recorded from fully differentiated bullfrog DRG cells when tested in Tris-Ringer’s solutions containing 1.9 mm Ca2+. This observation differs from the findings of a previous report (Dunlap and Fischbach, 1978) indicating that 5-HT clearly decreased the duration of action potentials recorded from cultured embryonic chick DRG cells bathed in HEPES-buffered saline containing 5.4 mm Ca2+. The different actions of 5-HT seen in these two preparations may be explained by the fact that embryonic chick DRG cells generate action potentials with a prominent calcium-dependent plateau phase on their falling limb (Dichter and Fischbach, 1977), whereas fully differentiated frog DRG cells do not. When frog DRG cells were treated with TEA, however, spike duration increased 2- to 4-fold and a plateau phase appeared. Only then was a response to 5-HT unmasked. In concentrations as low as 10 nm, 5-HT produced a clear narrowing of the TEA-induced plateau phase. The effect of 5-HT on spikes recorded from frog DRG cells treated with TEA resembles the action of 5-HT on cultured chick sensory neurons in several ways. The concentration range over which 5-HT reduces spike duration is the same in the two preparations, and the response is not secondary to changes in resting membrane potential or input resistance. In addition, the response to 5-HT is rapid in onset and offset and shows no tachyphylaxis. One pharmacological difference between the two preparations is that yohimbine was reported to block this action of 5-HT on chick sensory neurons (Canfield and Dunlap, 1984) while no such antagonism was observed in the frog DRG. Thus, the receptor mediating this response in adult frog DRG does not appear to be a nonspecific amine receptor, as postulated for embryonic chick sensory neurons (Canfield and Dunlap, 1984). Furthermore, NE and GABA were previously reported to decrease the duration of action potentials recorded from chick DRG cells (Dunlap and Fischbach, 1978), but these transmitters had no effect on the duration of action potentials recorded from TEA-treated type A frog DRG cells (see also Minota and Koketsu, 1977). Whether NE or GABA has an effect on the shape of action potentials recorded from type C neurons remains to be determined.
Effects of TEA on frog DRG action potentials
The TEA-induced increase in spike duration can be understood in terms of its well-known ability to block voltage-dependent potassium channels; these channels are responsible for the rapid repolarization of the membrane and thereby control the duration of the action potential (Stanfield, 1983). The plateau phase observed during treatment with TEA appears to result from depolarization-induced calcium influx, as it was reduced in amplitude and duration by low-calcium solutions or manganese-containing solutions. In fact, voltage-dependent calcium currents were previously demonstrated in voltage-clamp studies of internally perfused frog DRG cells (Ishizuka et al., 1984; Veselovskii et al., 1977). One seemingly paradoxical finding of our study was that during treatment with TEA, an increase in extracellular calcium concentration decreased the duration of the spike. Koketsu et al. (1963) first reported this unexpected narrowing of the TEA spike produced by high extracellular calcium solutions and suggested that this might be explained by a membrane-stabilizing effect of calcium. Indeed, elevated extracellular calcium has been shown to shift the voltage dependence of calcium channel activation to more depolarized levels, thereby increasing the threshold for activation of the calcium current (Hagiwara and Byerly, 1981). Increased extracellular calcium might also decrease spike duration by enhancing calcium-dependent potassium conductance, as previously described in studies of cardiac Purkinje fiber spikes (Kass and Tsien, 1977). For these reasons, the decrease in TEA spike duration seen with high extracellular calcium is not incompatible with the proposal that the plateau phase results from calcium influx.
Frog DRG cells can generate action potentials in sodium-free media if TEA is present, as noted in the present study, and previously by Koketsu et al. (1959a). These regenerative responses also appear to result from calcium influx since they were blocked by the calcium channel blockers, manganese or cobalt (as demonstrated in the present study), or by superfusion in calcium-free solution, as previously reported by Koketsu et al. (1959b).
TTX-resistant action potentials
Previous studies of rodent DRG cells (Kostyuk et al., 1981a; Yoshida et al., 1978) have demonstrated spikes resistant to TTX in most type C and a few type A neurons. In the present study, TTX-resistant spikes were observed in all type C neurons tested. These TTX-resistant spikes were recorded even when TEA was not included in the Ringer’s solution. TTX-resistant spikes recorded from type C neurons were blocked in sodium-free media, but not by cobalt, and therefore appear to depend primarily on sodium entry through TTX-resistant sodium channels. In contrast, action potentials recorded from type A neurons failed during superfusion with Ringer’s solutions containing TTX. Type A neurons, therefore, do not appear to generate sufficient inward current through TTX-resistant sodium channels to support regenerative activity. TTX-resistant spikes could be evoked in type A neurons only when TEA was present, and were completely blocked by cobalt or manganese. These data suggest that the TTX-resistant action potentials in TEA-treated type A neurons were pure calcium spikes. Serotonin (10 µm) reduced the rate of rise and peak amplitude of these “calcium” action potentials.
Possible mechanisms by which 5-HT decreases spike duration
The present findings indicate that 5-HT reduces voltage-dependent calcium influx in some, but not all, fully differentiated primary afferent neurons. This conclusion is supported by the observation that, in TEA-treated neurons, 5-HT reduced the duration of the calcium-dependent plateau phase of the spike. Also, in TEA/TTX-treated neurons, 5-HT reduced the amplitude of the calcium action potential. The decrease in afterhyperpolarization amplitude that accompanies the response to 5-HT may be secondary to a decrease in calcium influx, since the spike afterhyperpolarization may depend, in part, on the activation of a calcium-dependent gK. The simplest explanation for these effects is that 5-HT directly inhibits calcium conductance (gCa). Evidence for a direct inhibitory action of 5-HT on gCa was previously demonstrated in a voltage-clamp study of cultured chick DRG cells in which 5-HT was found to reduce calcium tail currents recorded at the potassium equilibrium potential (Dunlap and Fischbach, 1981). It is also possible that 5-HT may shorten the spike duration by enhancing an outward potassium current. The fact that the addition of 4-AP to the medium did not alter 5-HT’s ability to shorten the action potential suggests that the action of 5-HT is not dependent on the early potassium current (IA). Also, the lack of effect of barium on the action of 5-HT suggests that 5-HT’s action is not dependent on calcium-dependent gK. Further resolution of the site of action of 5-HT will probably require single-channel recording techniques.
Multiple actions of 5-HT on primary afferent neurons
On the basis of sucrose gap recordings from isolated frog spinal cord, we reported that 5-HT directly depolarized primary afferent nerve terminals (Holz and Anderson, 1984). Intracellular recordings from adult frog DRG cells reveal at least three distinct membrane actions of 5-HT on primary afferent neurons (Holz et al., 1984, 1985). Low concentrations of 5-HT (10 nm–1 µm) reduce calcium influx without altering membrane potential or input resistance. Higher concentrations (100 µm–-1 mm) produce a slow membrane depolarization with increased membrane resistance and/or a faster, transient membrane depolarization with decreased resistance (Holz et al., 1985). These three membrane responses appear to result from the activation of three distinct 5-HT receptor systems, which may or may not coexist on the same neurons. Specifically, which 5-HT receptor subtypes are involved remains to be determined. Methysergide, metergoline, and cinanserin mimicked, rather than blocked, the ability of 5-HT to reduce calcium influx, or to induce slow depolarization of DRG cells (Holz et al., 1985). These agents have a significant binding affinity for both 5-HT1 and 5-HT2 receptors. However, the fact that the 5-HT antagonistic actions of these compounds appear to be mediated by 5-HT2, and not 5-HT1, receptors (Peroutka et al., 1981) suggests that the actions of these compounds on the DRG are not mediated by 5-HT2 receptors. Our observation that methysergide showed agonist activity similar to that of 5-HT may explain the observation that methysergide, like 5-HT, directly inhibits the response of dorsal horn interneurons to peripheral nerve stimulation (Griersmith et al., 1981), an action that may reflect presynaptic inhibition of primary afferent transmission.
The effects of 5-HT on primary afferent neurons may modify sensory transmission in several ways. For example, if action potential current invading sensory nerve terminals activates large numbers of 5-HT sensitive calcium channels, then 5-HT might markedly inhibit calcium-dependent transmitter release (Shapiro et al., 1980). The previously reported (Holz et al., 1985) fast depolarizing action of 5-HT, which is accompanied by a decreased input resistance, would be likely to inhibit transmission in a manner similar to GABAergic presynaptic inhibition. These effects could explain the ability of 5-HT to inhibit sensory afferent activation of dorsal horn interneurons (Belcher et al., 1978; Headley et al., 1978). In contrast, the slow depolarizing action of 5-HT with increased input resistance would be expected to increase the action potential current invading primary afferent terminals, thereby facilitating sensory transmission. Thus, multiple mechanisms by which 5-HT may modulate primary afferent transmission appear to be available.
References
- Belcher G, Ryall RW, Schaffner R. The differential effects of 5-hydroxytryptamine, noradrenaline and raphe stimulation on nociceptive and non-nociceptive dorsal horn intemeurones in the cat. Brain Res. 1978;151:307–321. doi: 10.1016/0006-8993(78)90887-9. [DOI] [PubMed] [Google Scholar]
- Canfield DR, Dunlap K. Pharmacological characterization of amine receptors on embryonic chick sensory neurones. Br. J. Pharmacol. 1984;82:557–563. doi: 10.1111/j.1476-5381.1984.tb10794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter MA, Fischbach GD. The action potential of chick dorsal root ganglion neurones maintained in cell culture. J. Physiol. (Lond.) 1977;267:281–298. doi: 10.1113/jphysiol.1977.sp011813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium component of sensory neurone action potentials. Nature. 1978;276:837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J. Physiol. (Lond.) 1981;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach GD, Dunlap K, Mudge A, Leeman S. Peptide and amine transmitter effect on embryonic chick sensory neurons in vitro. In: Martin JB, Reichlin S, Bick KL, editors. Neurosecretion and Brain Peptides. New York: Raven; 1981. pp. 175–188. [PubMed] [Google Scholar]
- Griersmith BT, Dugan AW, North RA. Methysergide and supraspinal inhibition of the spinal transmission of nociceptive information in the anaesthetized cat. Brain Res. 1981;204:147–158. doi: 10.1016/0006-8993(81)90658-2. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Byerly L. Calcium channel. Annu. Rev. Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Headley PM, Duggan AW, Griersmith BT. Selective reduction by noradrenaline and 5-hydroxytryptamine of nociceptive responses of cat dorsal horn neurones. Brain Res. 1978;145:185–189. doi: 10.1016/0006-8993(78)90809-0. [DOI] [PubMed] [Google Scholar]
- Holz GG, IV, Anderson EG. The actions of serotonin of frog primary afferent terminals and cell bodies. Comp. Biochem. Physiol. C. 1984;77:13–21. doi: 10.1016/0742-8413(84)90124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, IV, Shefner SA, Anderson EG. Serotonin decreases the calcium-dependent component of action potentials recorded from frog dorsal root ganglion cells. Soc. Neurosci. Abstr. 1984;10:992. doi: 10.1523/JNEUROSCI.06-03-00620.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, IV, Shefner SA, Anderson EG. Serotonin depolarizes type A and C primary afferents: An intracellular study in bullfrog dorsal root ganglion. Brain Res. 1985;327:71–79. doi: 10.1016/0006-8993(85)91500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka S, Hattori K, Akaike N. Separation of ionic currents in the somatic membrane of frog sensory neurons. J. Membr. Biol. 1984;78:19–28. doi: 10.1007/BF01872528. [DOI] [PubMed] [Google Scholar]
- Kass RS, Tsien RW. Control of action potential duration by calcium ions in cardiac Purkinje fibers. J. Gen. Physiol. 1977;67:599–617. doi: 10.1085/jgp.67.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu K, Cerf JA, Nishi S. Effect of quaternary ammonium ions on electrical activity of spinal ganglion cells in frogs. J. Neurophysiol. 1959a;22:177–194. doi: 10.1152/jn.1959.22.2.177. [DOI] [PubMed] [Google Scholar]
- Koketsu K, Cerf JA, Nishi S. Further observations on electrical activity of frog spinal ganglion cells in sodium-free solutions. J. Neurophysiol. 1959b;22:693–703. doi: 10.1152/jn.1959.22.6.693. [DOI] [PubMed] [Google Scholar]
- Koketsu K, Nishi S, Soeda H. Effects of calcium ions on prolonged action potentials and hyperpolarizing responses. Nature [New Biol.] 1963;200:786–787. doi: 10.1038/200786a0. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Veselovsky NS, Tsydrenko AY. Ionic currents in the somatic membrane of rat dorsal root ganglion neurones. I. Sodium currents. Neuroscience. 1981a;6:2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Veselovsky NS, Fedulova SA. Ionic currents in the somatic membrane of rat dorsal root ganglion neurones. II. Calcium currents. Neuroscience. 1981b;6:2431–2437. doi: 10.1016/0306-4522(81)90089-0. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Yoshida S, Yonezawa T. Tetrodotoxin sensitivity and Ca component of action potentials of mouse dorsal root ganglion cells cultured in vitro. Brain Res. 1978;154:69–82. doi: 10.1016/0006-8993(78)91052-1. [DOI] [PubMed] [Google Scholar]
- Minota S, Koketsu K. Effects of adrenaline on the action potential of sympathetic ganglion cells in bullfrogs. Jpn. J. Physiol. 1977;27:353–366. doi: 10.2170/jjphysiol.27.353. [DOI] [PubMed] [Google Scholar]
- Mudge AW, Leeman SE, Fischbach GD. Enkephalin inhibits release of substance P from sensory neurons in culture and decreases action potential duration. Proc. Natl. Acad. Sci. USA. 1979;76:526–530. doi: 10.1073/pnas.76.1.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peroutka SJ, Lebovitz RM, Snyder S. Two distinct serotonin receptors and different physiological functions. Science. 1981;212:827–829. doi: 10.1126/science.7221567. [DOI] [PubMed] [Google Scholar]
- Shapiro E, Castellucci VF, Kandel ER. Presynaptic inhibition in Aplysia involves a decrease in the Ca2+ current of the presynaptic neuron. Proc. Natl. Acad. Sci. USA. 1980;77:1185–1189. doi: 10.1073/pnas.77.2.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield PR. Tetraethylammonium ions and the potassium permeability of excitable cells. Rev. Physiol. Biochem. Pharmacol. 1983;97:1–67. doi: 10.1007/BFb0035345. [DOI] [PubMed] [Google Scholar]
- Veselovskii NS, Kostyuk PG, Kryshtal OA, Pidoplichkos VI. Separation of ionic currents responsible for action potential generation in isolated neurons of frog spinal ganglia. Neirofiziologiya. 1977;9:638–640. [PubMed] [Google Scholar]
- Yoshida S, Matsuda Y, Smaejima A. Tetrodotoxin-resistant sodium and calcium components of action potentials in dorsal root ganglion cells of the adult mouse. J. Neurophysiol. 1978;41:1096–1106. doi: 10.1152/jn.1978.41.5.1096. [DOI] [PubMed] [Google Scholar]