Abstract
Objective
This systematic review was performed to elucidate dosing practices, dosing conversions, and related outcomes from randomized controlled trials that directly compared onabotulinumtoxinA (ONA) and abobotulinumtoxinA (ABO) at various dose conversion ratios for therapeutic use in movement disorders.
Methods
A systematic review of 3 medical literature databases (PubMed, the Cochrane Library, and EMBASE) was performed to identify relevant comparative clinical studies, systematic reviews, and meta-analyses published in the English language between January 1991 and January 2015. Studies that met predefined inclusion criteria were selected for formal data extraction and quality assessment.
Results
A total of 182 manuscripts were identified, of which 4 were included for analysis. Targeted clinical applications included neurological disorders. The studies compared ONA to ABO dose conversion ratios of 1:2.5 (n=1), 1:3 (n=2), and 1:4 (n=2). One study compared both 1:3 and 1:4 ratios. An ONA:ABO conversion factor of 1:2.5 was associated with similar efficacy and side effects. An ONA:ABO ratio of 1:3 provided similar or higher efficacy but an increased rate of adverse effects, and an ONA:ABO ratio of 1:4 was associated with higher efficacy but with an excessive rate of intolerable side effects.
Conclusion
A dose conversion ratio of ONA to ABO between 1:2.5 and 1:3.0 provides comparable safety and efficacy for therapeutic movement disorders chemodenervation procedures.
Keywords: botulinum toxin, abobotulinumtoxinA, onabotulinumtoxinA, dose conversion ratio, cervical dystonia, blepharospasm
Introduction
Seven different serotypes of botulinum neurotoxins (BoNTs) have been identified, but only serotypes A and B are commercially available for use in clinical practice.1–5 Currently, 3 different BoNTs of serotype A (BoNTs-A) have been approved in the United States for various clinical applications in aesthetics and dermatological, neurological, and urological indications.1,6 These preparations are Botox® (onabotulinumtoxinA [ONA]; Allergan, Inc., Irvine, CA),2 Dysport® (abobotulinumtoxinA [ABO]; Ipsen Biopharmaceuticals, Inc., Basking Ridge, NJ),3 and Xeomin® (incobotulinumtoxinA [INCO]; Merz Pharmaceuticals, LLC; Greensboro, NC).5
BoNTs-A have been used therapeutically for more than 20 years throughout the world.1,2 In the United States, ONA is available in 50, 100, and 200 unit (U) vials, 2,7 and ABO is available in 300 and 500 U vials.3 Each unit of BoNT corresponds to the calculated intraperitoneal median lethal dose (LD50) of reconstituted product in a biologic mouse model.8 Many factors affect the LD50 bioassay, including type of assay used,8 mouse strain, gender and age, route and volume of injection, time of examination post-injection,9 and delivery vehicle or reconstitution buffer.10 Because the commercial BoNTs are produced by different pharmaceutical laboratories using different proprietary methodologies and assay methods, the potency of a unit of one product is not equivalent to a unit of another, and the unit potencies of different BoNT formulations cannot be easily compared.8–14
Although the unit doses of BoNT products are not interchangeable, clinicians are required to estimate the approximate dose equivalence between BoNTs-A when changes are warranted due to secondary nonresponse, development of side effects, or changes in insurance coverage.
Multiple studies have been performed to establish the comparative potency of ONA and ABO, but the issue remains unresolved and controversial.9–14 Investigators have compared ONA:ABO at conversion ratios ranging from 1:1 to 1:13.3.15,16 Currently among experts, dose conversions range from 1:1.5 to 1:4, although without definitive support. The few head-to-head studies have had different methodologies and outcomes, diminishing their individual value for wider clinical application.
Because ONA and ABO share the same BoNT serotype, and because no strong evidence exists to suggest differences in local or distant spreading or in efficacy between products,17 we sought to establish the collective safest and most effective conversion dose between ONA and ABO across all available head-to-head randomized, controlled trials in movement disorders.
Methods
We conducted a systematic literature review of clinical trials comparing ONA to ABO for therapeutic uses in any of the medical fields in which these toxins have been examined. The primary objective was to examine dosing practices, dosing conversions, and related outcomes from selected studies in order to produce the tightest estimate of the conversion ratio between these two BoNTs-A, which can be universally applicable.
Literature Search Strategy
Based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), a literature search strategy and methods for systematic review were specified in advance of data collection. The authors agreed upon a PRISMA search strategy that they felt would identify the highest quality data available in the medical literature to recommend a dose conversion ratio between ONA and ABO. The systematic literature review focused on identifying comparative clinical studies, systematic reviews, and meta-analyses across countries. The search was intended to capture all ONA vs ABO clinical trials within the following conditions of interest: movement disorders (head/neck), movement disorders (face/eye), movement disorders (jaw/tongue/mouth), spasticity (all limbs), focal limb/other dystonia, sweating, pain, and aesthetics (glabellar lines or crow’s feet). Only studies with movement disorder applications were included for this systematic literature review.
The authors agreed on a strategy that used a combination of Medical Subject Heading (MeSH) terms and key words. The initial terms for inclusion were the indications as well as the terms onabotulinumtoxinA (with alternative spellings onabotulinumtoxin A and onabotulinum toxin A), Botox, abobotulinumtoxinA (with alternative spellings abobotulinumtoxin A and abobotulinum toxin A), Dysport, and clinical trial. Studies that were published in languages other than English or outside the date range (January 1991 to January 2015) were excluded. The search was performed in 3 electronic medical literature databases: PubMed, the Cochrane Library, and EMBASE.
Study Selection
At level 1 screening, titles and abstracts identified from the electronic databases were reviewed by 2 researchers independently (K.D. and J.C.) to evaluate potential study relevance. All publications reporting preclinical, phase 1, prognostic/biomarker, genetic retrospective, registry, case report, and/or noncomparative studies were excluded, as were letters, consensus reports, editorials, and nonsystematic reviews. At level 2 screening, full-text studies selected at level 1 were reviewed with the same inclusion and exclusion criteria. In addition, bibliographic references of systematic reviews and meta-analyses were searched to find relevant studies that may not have been identified during the primary search. At both screening levels, the authors met to reach consensus about any cases where there was initial disagreement or uncertainty about study inclusion. The overall study-selection process is illustrated in Figure 1.
Figure 1.
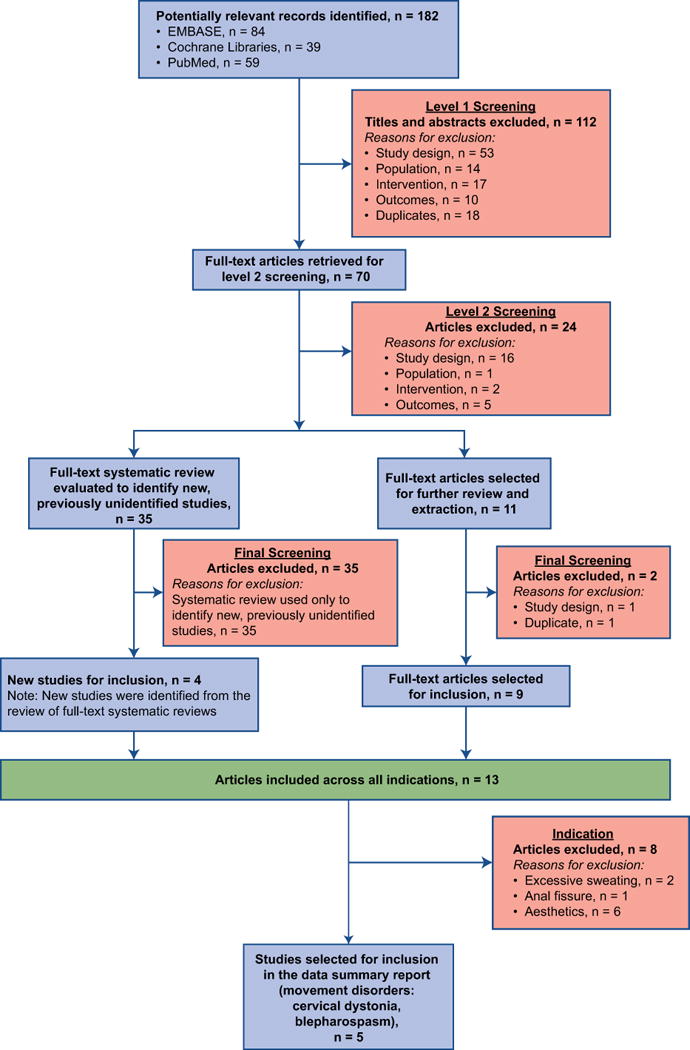
PRISMA flow diagram reporting the results of the systematic literature search.
Level 1 screening = review of titles and abstracts; Level 2 screening = review of full-text articles; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Data Extraction and Bias Assessment
After screening, randomized, controlled clinical trials that met the predefined inclusion criteria were selected for formal data extraction and quality assessment. Data were extracted from the full-text publications under the following predefined headings: Study Title, Sponsor, Methodology, Patients, Treatment, Efficacy Results (primary and secondary endpoints), Safety Results, Conclusion. Each study underwent quality assessment of risk for bias based on Cochrane metrics, which addressed 6 types of potential bias: selection, performance, detection, attrition, reporting, and other sources of bias not covered by other domains.18
Determination of Appropriate Dose Conversion
For the purposes of this review, we defined a comparable dose conversion as one in which the efficacy and safety were equivalent (i.e., no statistically significant difference) between ONA and ABO. If differences in efficacy and/or safety were observed between ONA and ABO in the study, we concluded that the dose conversion ratio was not comparable.
Results
A total of 182 manuscripts were identified during the level 1 screening. Of these, 112 records were excluded based on titles and abstracts, and 70 full-text articles were retrieved. After level 2 screening, 14 primary publications were included for data extraction and analysis. Upon data extraction, it became clear that 2 of these were duplicates of the same study,19,20 leaving 13 studies for the analysis. Of these 13 studies, only the 4 movement disorder studies were included for the current systematic review analyses due to the fact that the dosage, efficacy, and profile of adverse effects can be different from the non-movement disorders indications of botulinum toxins.
In the quality assessment of risk for bias for the studies that compared ONA and ABO for the treatment of movement disorders, the studies fulfilled the Cochrane criteria for low-risk (n=2) or unclear risk (n=2) of bias.
Of the 4 studies meeting criteria for final analysis, 1 compared ONA and ABO at a dose conversion ratio of 1:2.523 and 2 compared the toxins at a 1:3 dose conversion.21,22 One of the 1:3 studies also had a 1:4 arm.22 The fourth study included in the final analysis also compared the toxins using a 1:4 dose conversion ratio.30
Clinical Trials for Cervical Dystonia
Three randomized clinical trials compared ONA and ABO for cervical dystonia (Supplemental Table 1). Odergren and colleagues evaluated the dose equivalence of ONA and ABO at a conversion ratio of 1:3 in a double-blind, randomized, parallel group study of 73 patients treated for predominantly rotational cervical dystonia. The toxins were administered to 1 or more affected muscles, at 1 or more sites per muscle.21 Patients in both groups experienced substantial improvement in Tsui scores by Week 2, with a peak effect at Week 4. The mean post-treatment Tsui scores for the 2 groups were not statistically different (P = 0.66), and there were no significant differences in the incidence rates of adverse events (P = 0.35). Overall, there was no significant difference in the number of treatment successes between groups based on a global assessment of efficacy and safety (76% ABO; 66% ONA; P = 0.32). The authors concluded that patients with predominantly rotational cervical dystonia had similar improvements and experienced similar safety profiles when treated with ONA or ABO at a 1:3 conversion ratio.21
Ranoux and colleagues evaluated patients with cervical dystonia in a double-blind, randomized cross-over study designed to compare the efficacy and safety of ONA with ABO at 2 conversion ratios.22 In a random order, patients received ONA at the usually effective dose for the patient, ABO at a conversion ratio of 1:3, and ABO at a conversion ratio of 1:4. In the study, patients treated with ABO had significantly better improvement in Tsui score at both conversion ratios compared with patients in the ONA group. The mean change from baseline to 1 month post-injection was 3.25 for the ONA group, it was 4.27 for the ABO 1:3 conversion, and it was 4.92 for the ABO 1:4 conversion (P = 0.02 and 0.01 for the 1:3 and 1:4 conversions, respectively). The mean duration of action was not significantly different between ONA and ABO at the 1:3 conversion ratio (89.3 vs 96.9 days, P = 0.58), but at the 1:4 conversion, ABO had a significantly longer duration (89.3 vs 114 days, P = 0.02). The number of adverse events was higher with both ABO treatments. The most frequent event was dysphagia, which occurred in 3%, 15.6%, and 17.3% of patients in the ONA, ABO 1:3, and ABO 1:4 groups, respectively. The authors concluded that ABO is more effective than ONA for cervical dystonia but with a higher incidence of adverse effects.22
Yun and colleagues compared ONA with ABO at a dose conversion ratio of 1:2.5 in 103 patients with cervical dystonia in a randomized, double-blind, noninferiority cross-over trial (Supplemental Table 1).23 Patients were assessed at baseline and follow-up using the Tsui scale and the Toronto western spasmodic torticollis rating scale (TWSTRS). In the study, no significant differences between ONA and ABO treatment were observed from baseline to assessment at 1 month in in Tsui scales scores (−4.77 vs −3.98, P = 0.091) or TWSTRS (−.78 vs −9.76, P = 0.429). In addition, no significant differences in clinical global impression or patient global impression were identified. Muscle weakness and dysphagia were the most common adverse events with both treatments, and no significant differences were observed in the incidence of adverse events between treatments.23
Clinical Trial for Blepharospasm
One randomized clinical trial compared ONA and ABO for essential blepharospasm (Supplemental Table 1). Nussgens and Roggenkamper found that a dose conversion ratio of 1:4 was associated with similar duration of effect for both drugs (extent of improvement in blepharospasm was not reported), but patients in the ABO group had higher rates of adverse events.30 Thus, the ONA to ABO ratio of 1:4 appeared noncomparable, with data in this study limited to a tradeoff between duration and adverse events.
Summary Results of Dose Conversion Ratios
The outcomes of the clinical trials by dose conversion ratio and indication are shown in Figure 2. The 2 randomized clinical trials (total n = 264) that compared ONA and ABO at a dose conversion ratio of 1:4 were both associated with greater occurrence of adverse effects with ABO.22,30 In 1 of the trials (n=73) that used a 1:3 dose conversion, efficacy and safety did not differ between ABO and ONA.21 In the other trial (n=51) that used a 1:3 dose conversion, treatment with ABO resulted in higher efficacy and higher rates of adverse events.22 In the trial (n=103) that used a 1:2.5 dose conversion, ABO was associated with similar efficacy and adverse events.23
Figure 2.
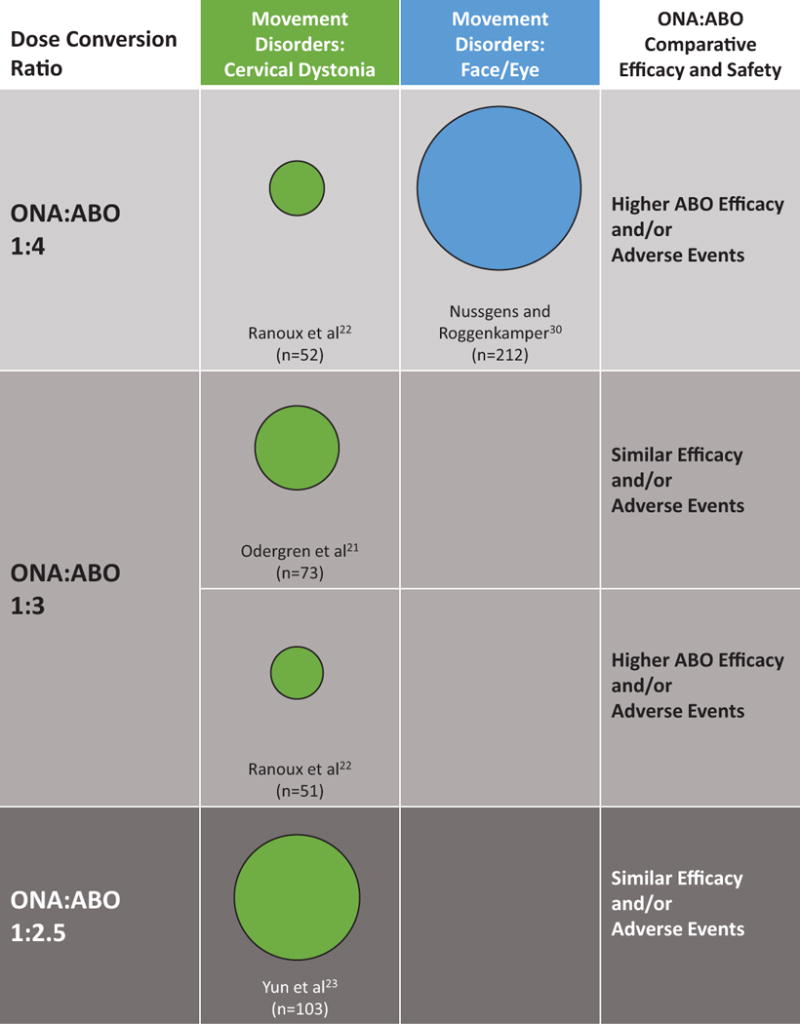
Distribution of studies by indication, dose conversion ratio, and outcomes. Note: the sizes of the circles reflect number of subjects in each study.
Discussion
The converging evidence from BoNT comparative clinical trials suggests that a dose conversion ratio of ONA to ABO between 1:2.5 and 1:3 provides comparable safety and efficacy for therapeutic movement disorders chemodenervation procedures. A conversion ratio of 1:4 appears to yield an undesirable tradeoff between greater efficacy/longer duration and poorly tolerable side effects.
While the unit doses of different BoNTs are not interchangeable due to differences in proprietary manufacturing methodologies and assays, chemodenervation in a variety of clinical settings requires making informed inter-BoNT conversion decisions. We performed this systematic review to develop a detailed summary of the dosing practices, dosing conversions, and related outcomes from randomized controlled trials that compared use of ABO to ONA for movement disorders. The data reviewed herein serve to establish an appropriate conversion dose ratio for ABO and ONA based on the best available evidence.
For the purposes of this review, we defined a comparable conversion dose as one in which the safety and efficacy results did not differ for patients treated with ABO or ONA. A noncomparable conversion may result from disproportionate rates of adverse events, most notably dysphagia.22
In addition to conversion ratios based on efficacy and tolerability, dose conversions may need to factor in cost-effectiveness differences between toxins, which cannot be adequately extracted from the controlled clinical trials selected here. Because BoNTs are widely used around the world for multiple indications, optimizing the cost per injection may have a substantial impact on costs for the individual patient as well as the overall healthcare system.
Conclusion
In this systematic review, we found that ONA:ABO dose conversion ratios above 1:3 significantly increased rates of adverse events in patients treated with ABO and should be discouraged. Overall, a dose conversion of 1:2.5 resulted in equivalent or lower rates of adverse events, with equivalent or, in some cases, lower efficacy associated with ABO. In the 2 studies using a dose conversion of 1:3, ABO was as effective as or more effective than ONA, and associated with equivalent (1 study) or higher (1 study) occurrence of adverse effects.
In summary, data extracted from randomized clinical studies suggest that a dose conversion of 2.5 to no more than 3 U of ABO for each unit of ONA provides an appropriate balance of safety and efficacy and can be used as a guidance range. However, the most appropriate dose ratio when switching from one BoNT to another depends on multiple factors, including the severity of patients’ symptoms, any comorbid conditions, and previous response to BoNT treatment.
Supplementary Material
Supplemental Table 1. Trials for Movement Disorders.
Acknowledgments
The authors thank Catherine Masaquel (RTI Health Solutions, funded by Ipsen) for assistance with conducting the literature search and Brian Atkinson and Brian Kearney (funded by Ipsen), for editorial support, including referencing.
The study was partially funded by Ipsen Biopharmaceuticals, Inc., for data collection and editorial support. Dr. Dashtipour developed the protocol, and data collection was coordinated and designated by RTI Health Solutions. Aside from procuring the data collection and editorial support, Ipsen Biopharmaceuticals, Inc., did not contribute to the study conduct or reporting of results.
Financial Disclosures (for the preceding 12 months)
Khashayar Dashtipour has received compensation/honoraria for services as a consultant/scientific advisory board member or speaker from Allergan, Inc., Ipsen Biopharmaceuticals, Inc., Lundbeck Inc., Merz Pharmaceuticals, Teva Pharmaceutical Industries Ltd., UCB Inc., Impax Pharmaceutical and US World Meds.
Jack J. Chen has received grant support from Lundbeck Inc. He also received compensation/honoraria for services as a consultant or an advisory committee member from Ipsen Biopharmaceuticals, Inc. He serves on the editorial board for the Journal of Toxins.
Dr. Espay is supported by the K23 career development award (NIMH, 1K23MH092735); has received grant support from CleveMed/Great Lakes Neurotechnologies and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for AbbVie, Chelsea Therapeutics, TEVA, Impax, Merz, Pfizer, Acadia, Cynapsus, Solstice Neurosciences, Eli Lilly, Lundbeck, and USWorldMeds; royalties from Lippincott Williams & Wilkins and Cambridge University Press; and honoraria from UCB, TEVA, the American Academy of Neurology, and the Movement Disorders Society. He serves as Associate Editor of Movement Disorders, Frontiers in Movement Disorders, and Journal of Clinical Movement Disorders, and on the editorial boards of Parkinsonism and Related Disorders and The European Neurological Journal.
Dr. Mari received grant support in the past 12 months from the NIH, MJFF, NPF, AbbVie, AVID, Great Lakes Neurotechnology, Inc., and P-CORI. He also received compensation/honoraria as consultant and/or advisory board member from Ipsen, Impax, Navidea, and Medtronic.
Dr. Ondo received consultant fees from Auspex, ACADIA, UCB Pharma, InSightec, Jazz, Xenoport and compensation/honoraria as the speaker from TEVA, USWorldMeds, Lundbeck, Avanir, Merz, and Xenoport.
Footnotes
Author’s Roles
All authors had full access to all data, contributed to manuscript revisions, and had final approval for submission. Dr. Dashtipour wrote the initial draft and had final responsibility for the decision to submit the paper for publication.
Drs. Chen, Espay, Mari, and Ondo critically reviewed the manuscript and each is responsible for parts of the writing and interpretation of data.
References
- 1.Setler PE. Therapeutic use of botulinum toxins: background and history. Clinical J Pain. 2002;18(6 suppl):S119–S124. doi: 10.1097/00002508-200211001-00002. [DOI] [PubMed] [Google Scholar]
- 2.Allergen. Botox (onabotulinumtoxinA) [prescribing information] Irvine, CA: Allergan, Inc; Feb, 2014. [Google Scholar]
- 3.Ipsen Biopharmaceuticals. Dysport® for injection (abobotulinumtoxinA) [prescribing information] Basking Ridge, NJ: Ipsen Biopharmaceuticals, Inc; Sep, 2013. [Google Scholar]
- 4.Solstice Neurosciences. Myobloc (rimabotulinumtoxinB) injection [prescribing information] South San Francisco, CA: Solstice Neurosciences, Inc; May, 2010. [Google Scholar]
- 5.Merz Pharmaceuticals. Xeomin (incobotulinumtoxinA) [prescribing information] Greensboro, NC: Merz Pharmaceuticals, LLC; Apr, 2014. [Google Scholar]
- 6.Chen JJ, Dashtipour K. Abo-, inco-, ona-, and rima-botulinum toxins in clinical therapy: a primer. Pharmacotherapy. 2013;33:304–318. doi: 10.1002/phar.1196. [DOI] [PubMed] [Google Scholar]
- 7.Allergan Pharmaceuticals. BOTOX COSMETIC (onabotulinumtoxinA) for injection, for intramuscular use [prescribing information] Irvine, CA: Allergan, Inc; Sep, 2013. [Google Scholar]
- 8.Dressler D, Mander G, Fink K. Measuring the potency labelling of onabotulinumtoxinA (Botox®) and incobotulinumtoxinA (Xeomin®) in an LD50 assay. J Neural Transm. 2012;119:13–15. doi: 10.1007/s00702-011-0719-1. [DOI] [PubMed] [Google Scholar]
- 9.Mclellan K, Das RE, Ekong TA, Sesardic D. Therapeutic botulinum type A toxin: factors affecting potency. Toxicon. 1996;34:975–985. doi: 10.1016/0041-0101(96)00070-0. [DOI] [PubMed] [Google Scholar]
- 10.Hambleton P, Pickett AM. Potency equivalence of botulinum toxin preparations. J R Soc Med. 1994;87:719. [PMC free article] [PubMed] [Google Scholar]
- 11.Pickett A, O’Keeffe R, Panjwani N. The protein load of therapeutic botulinum toxins. Eur J Neurol. 2007;14:e11. doi: 10.1111/j.1468-1331.2007.01683.x. [DOI] [PubMed] [Google Scholar]
- 12.Frevert J. Content of botulinum neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/Bocouture®. Drugs R D. 2010;10:67–73. doi: 10.2165/11584780-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickett A. Consistent biochemical data are essential for comparability of botulinum toxin type A products. Drugs R D. 2011;11:97–98. doi: 10.2165/11590750-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenzel R, Jones D, Borrego JA. Comparing two botulinum toxin type A formulations using manufacturers’ product summaries. Journal of Clinical Pharmacy and Therapeutics. 2007;32:387–402. doi: 10.1111/j.1365-2710.2007.00835.x. [DOI] [PubMed] [Google Scholar]
- 15.Rystedt A, Nyholm D, Naver H. Clinical experience of dose conversion ratios between 2 botulinum toxin products in the treatment of cervical dystonia. Clin Neuropharmacol. 2012;35:278–282. doi: 10.1097/WNF.0b013e3182711fc0. [DOI] [PubMed] [Google Scholar]
- 16.Bentivoglio AR, Ialongo T, Bove F, De NF, Fasano A. Retrospective evaluation of the dose equivalence of Botox((R)) and Dysport ((R)) in the management of blepharospasm and hemifacial spasm: a novel paradigm for a never ending story. Neurol Sci. 2012;33:261–267. doi: 10.1007/s10072-011-0672-7. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez-Castaneda J, Jankovic J, Comella C, Dashtipour K, Fernandez HH, Mari Z. Diffusion, spread, and migration of botulinum toxin. Mov Disord. 2013;28:1775–1783. doi: 10.1002/mds.25582. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. [updated March 2011] The Cochrane Collaboration. 2011 [Google Scholar]
- 19.Nestor MS, Ablon GR. Duration of action of abobotulinumtoxina and onabotulinumtoxina: a randomized, double-blind study using a contralateral frontalis model. Journal of Clinical and Aesthetic Dermatology. 2011;4:43–49. [PMC free article] [PubMed] [Google Scholar]
- 20.Nestor MS, Ablon GR. Comparing the clinical attributes of abobotulinumtoxinA and onabotulinumtoxinA utilizing a novel contralateral Frontalis model and the Frontalis Activity Measurement Standard. Journal of Drugs in Dermatology. 2011;10:1148–1157. [PubMed] [Google Scholar]
- 21.Odergren T, Hjaltason H, Kaakkola S, et al. A double blind, randomised, parallel group study to investigate the dose equivalence of Dysport® and Botox® in the treatment of cervical dystonia. J Neurol Neurosurg Psychiatry. 1998;64:6–12. doi: 10.1136/jnnp.64.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranoux D, Gury C, Fondarai J, Mas JL, Zuber M. Respective potencies of Botox and Dysport: a double blind, randomised, crossover study in cervical dystonia. J Neurol Neurosurg Psychiatry. 2002;72:459–462. doi: 10.1136/jnnp.72.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yun JY, Kim JW, Kim HT, et al. Dysport and Botox at a ratio of 2.5:1 units in cervical dystonia: a double-blind, randomized study. Mov Disord. 2015 Feb;30(2):206–13. doi: 10.1002/mds.26085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talarico-Filho S, Mendonca do Nascimento M, Sperandeo de Macedo F, de Sanctis Pecora C. A double-blind, randomized, comparative study of two type A botulinum toxins in the treatment of primary axillary hyperhidrosis. Dermatol Surg. 2007;33:S44–S50. doi: 10.1111/j.1524-4725.2006.32331.x. [DOI] [PubMed] [Google Scholar]
- 25.Brisinda G, Albanese A, Cadeddu F, et al. Botulinum neurotoxin to treat chronic anal fissure: results of a randomized “Botox vs Dysport” controlled trial. Aliment Pharmacol Ther. 2004;19:695–701. doi: 10.1111/j.1365-2036.2004.01895.x. [DOI] [PubMed] [Google Scholar]
- 26.Firoz B, Pilato T, Zhou W. A randomized, double blind, split-face comparison of Botox verses dysport for glabellar and forehead lines [abstract] J Am Acad Dermatol. 2012;66(4 suppl 1):AB21. [Google Scholar]
- 27.Karsai S, Adrian R, Hammes S, Thimm J, Raulin C. A randomized double-blind study of the effect of Botox and Dysport/Reloxin on forehead wrinkles and electromyographic activity. Arch Dermatol. 2007;143:1447–1449. doi: 10.1001/archderm.143.11.1447-b. [DOI] [PubMed] [Google Scholar]
- 28.Lowe PL, Patnaik R, Lowe NJ. A comparison of two botulinum type a toxin preparations for the treatment of glabellar lines: double-blind, randomized, pilot study. Dermatological Surgery. 2005;31:1651–1654. doi: 10.2310/6350.2005.31303. [DOI] [PubMed] [Google Scholar]
- 29.Lowe P, Patnaik R, Lowe N. Comparison of two formulations of botulinum toxin type A for the treatment of glabellar lines: a double-blind, randomized study. J Am Acad Dermatol. 2006;55:975–980. doi: 10.1016/j.jaad.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Nussgens Z, Roggenkamper P. Comparison of two botulinum-toxin preparations in the treatment of essential blepharospasm. Graefe’s Archive for Clinical and Experimental Ophthalmology. 1997;235:197–199. doi: 10.1007/BF00941758. [DOI] [PubMed] [Google Scholar]
- 31.Simonetta Moreau M, Cauhepe C, Magues JP, Senard JM. A double-blind, randomized, comparative study of Dysport vs Botox in primary palmar hyperhidrosis. Br J Dermatol. 2003;149:1041–1045. doi: 10.1111/j.1365-2133.2003.05620.x. [DOI] [PubMed] [Google Scholar]
- 32.Lew H, Yun YS, Lee SY, Kim SJ. Effect of botulinum toxin A on facial wrinkle lines in Koreans. Ophthalmologica. 2002;216:50–54. doi: 10.1159/000048297. [DOI] [PubMed] [Google Scholar]
- 33.Aoki KR, Guyer B. Botulinum toxin type A and other botulinum toxin serotypes: a comparative review of biochemical and pharmacological actions. Eur J Neurol. 2001;8(suppl 5):21–29. doi: 10.1046/j.1468-1331.2001.00035.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Trials for Movement Disorders.


