Abstract
A major challenge in neuroscience is to reliably activate individual neurons, particularly those in deeper brain regions. Current optogenetic approaches require invasive surgical procedures to deliver light of specific wavelengths to target cells in order to activate or silence them. Here, we demonstrate the use of low-pressure ultrasound as a non-invasive trigger to activate specific ultrasonically-sensitized neurons in the nematode, Caenorhabditis elegans. We first show that wild-type animals are insensitive to low pressure ultrasound and require gas-filled microbubbles to transduce the ultrasound wave. We find that neuron-specific misexpression of TRP-4, the pore-forming subunit of a mechanotransduction channel, sensitizes neurons to ultrasound stimulus resulting in motor outputs. Furthermore, we use this approach to manipulate the function of sensory neurons and interneurons and identify a role for the PVD sensory neurons in modifying locomotory behaviors. We suggest this method can be broadly applied to manipulate cellular functions in vivo.
Introduction
Understanding how neural circuits generate specific behaviors requires identifying the participating neurons and subsequently recording and perturbing their activity. The best-understood motor circuit, the crab stomatogastric ganglion, has benefited from electrophysiological access to well-defined cell types as well as an ability to manipulate their activity1. A number of approaches have been developed for manipulating neuronal activity using light (optogenetics) or small molecules2,3. While these methods have revealed insights into circuit computations in a variety of model systems, they suffer from one drawback: difficulty in delivering stimulus to target neurons located in deeper brain regions4,5. To address this issue, we have developed a new method that genetically sensitizes targeted neurons to low-pressure ultrasound, a stimulus that can be delivered to small regions of deep tissue throughout an animal including its brain.
Ultrasound stimulation is non-invasive. This is particularly important for manipulating vertebrate neurons, as it eliminates the need for invasive surgery to insert fiber optics (required for some current optogenetic methods6). Furthermore, ultrasound is well-suited for stimulating neuron populations as it focuses easily through intact thin bone and deep tissue7 to volumes of a few cubic millimeters8,9. Previously, ultrasound has been used to directly stimulate clusters of neurons in vitro or within the brains of several model organisms10–15. Interestingly, activating neurons in these cases requires exposure to continuous or repeated pulses of ultrasound between 690 KHz-3 MHz16. Ultrasound has also been shown to safely manipulate deep nerve structures in human hands to reduce chronic pain17. Despite these observations and the development of theoretical models18, the mechanisms by which ultrasound stimulates these neurons remain poorly understood. Moreover, an additional challenge lies in developing a method to target individual neurons to ultrasound stimulation, as the minimum focal zone of the ultrasound is larger than an individual cell.
To overcome these challenges we developed a new method to stimulate neurons that we call ‘sonogenetics’, using the nematode, Caenorhabditis elegans. C. elegans with its small nervous system consisting of just 302 neurons connected by identified synapses19 has well-characterized robust behaviors20 and reliable methods to monitor neural activity21. We identified a pore-forming subunit of a mechanotransduction channel, TRP-4, that is sensitive to low-pressure ultrasound. Further, we show that individual neurons misexpressing TRP-4 show changes in neural activity upon low-pressure ultrasound stimulation. Finally, we correlate these neural activity changes with specific behaviors at the level of whole animals.
Results
Imaging setup delivers ultrasound waves to animals
To investigate the role of ultrasound on wild-type C. elegans neural activity and behavior, we developed a setup that aligns optical imaging with the ultrasound focal zone (Fig. 1a). Ultrasound with different peak negative pressures was generated from a transducer and focused onto an agar plate where animals were corralled into a small area using a copper solution (Fig. 1b). The transducer focused the ultrasound wave to a 1 mm diameter circular area at the agar surface (red circle in Fig. 1b). The entire setup was placed in a large tank filled with water to facilitate uniform transduction of the ultrasound wave (Fig. 1a). Depending on solution or tissue gas concentrations, high ultrasound peak negative pressures (> 2.5MPa) can create inertial cavitation with the resulting shockwaves compromising the integrity of cell membranes22,23. Consistently, we observed that animals exposed to multiple pulses of high ultrasound pressures were unable to maintain their normal body posture (Supplementary Fig 1). Therefore, we chose to use low-pressure ultrasound, which does not cause these damaging effects, to stimulate animal behavior.
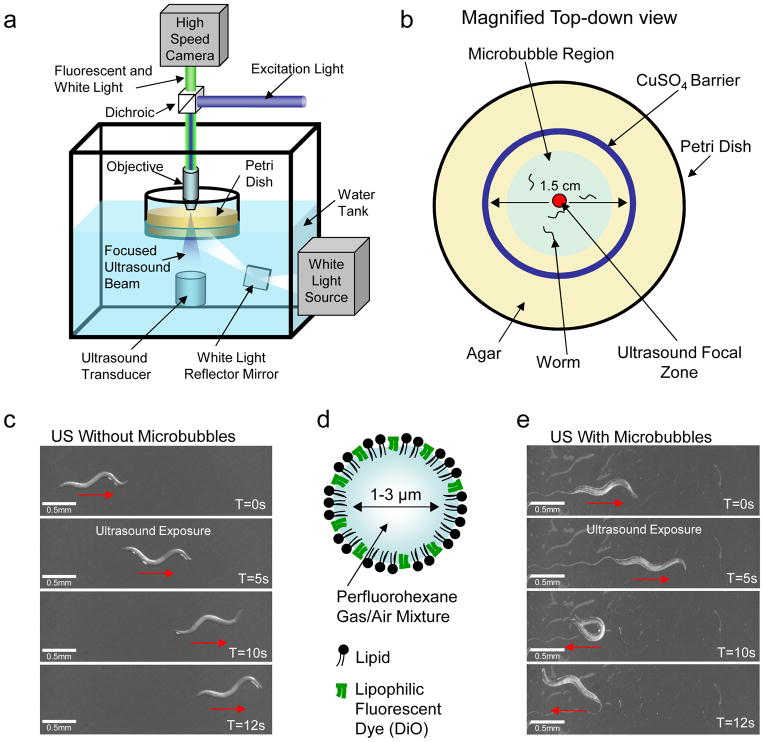
Figure 1. Amplifying ultrasound signals using microbubbles modifies animal behavior.
(a) Schematic of the computer-controlled imaging and ultrasound exposure system (frontal view) and (b) the agar plate with animals (top view) corralled into a small area by a copper barrier (1.5 cm in diameter). (c) Image sequence showing that animals do not respond to low-pressure ultrasound (US) alone. (d) Schematic of a stabilized microbubble. (e) Images showing that animals exhibit reversals and omega bends upon ultrasound (US) stimulus (single 10 ms pulse, 2.25 MHz with peak negative pressure of 0.9 MPa) in the presence of microbubbles.
We found that a single 10 ms duration ultrasound pulse of 2.25 MHz and peak negative pressures below 0.9 MPa had no effect on animal behavior (Fig. 1c). The mechanical disturbances24 of the fluid and tissue in the ultrasound focal zone take the form of compression and expansion deformations as well as bulk tissue distortions caused by acoustic radiation forces, but at low-pressures they were not large enough to influence C. elegans locomotion. Previous studies have shown that ultrasound waves can cause temperature changes25 in the focal zone. We first estimated the temperature increase as a result of ultrasound exposure. In a previous study, a continuous 1.1 MHz ultrasound pulse with a peak negative pressure of 2.6 MPa increased the temperature of the surrounding media at the rate of 35°C/sec25. Using these data, we estimated that the temperature increase around the worms on the agar surface to be 0.04°C for single ultrasound pulse at 0.9 MPa. Moreover, we directly measured the magnitude of temperature change on the agar surface using a miniature thermocouple (Supplementary Fig 2) and found that an ultrasound peak negative pressure of 0.7 MPa caused a temperature increase of less than 0.1°C (See Methods). This is a temperature stimulus that animals including C. elegans are unlikely to detect26,27. Together, these results show that C. elegans is unlikely to respond to the temperature and mechanical changes induced by the low-pressure ultrasound wave.
Microbubbles amplify the mechanical deformation of the ultrasound wave
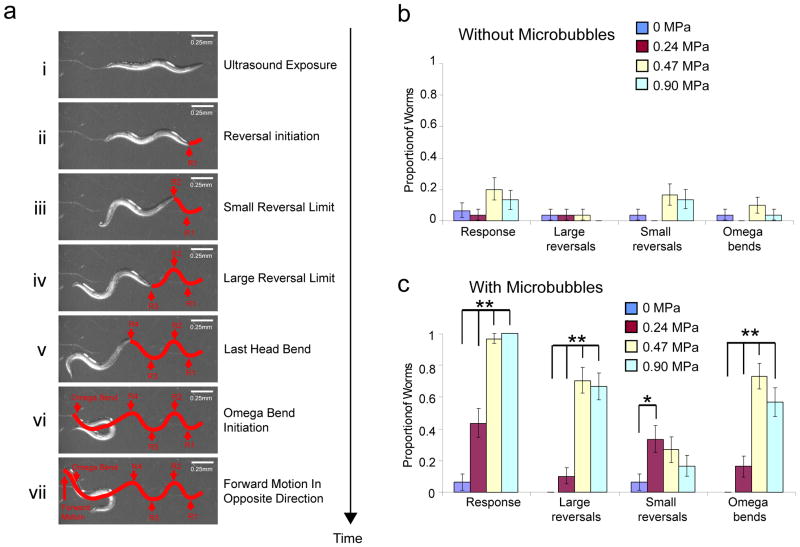
To test whether the tiny mechanical deformations created by the single low-pressure ultrasound pulse could be amplified we included gas-filled microbubbles in our assay (Fig. 1d,e). We found that animals showed a dramatic response to ultrasound when surrounded by microbubbles (Fig 1e). When the ultrasound wave was focused on the head of a worm surrounded by microbubbles, the animal immediately initiated a backward movement (termed “reversal”28) followed by a high-angled turn (identified as an “omega bend”28) (Fig 1e, 2a). These behaviors were scored as previously described (Methods, Fig 2a)28 and quantified as shown (Fig 2b,c). Reversals were separated into two categories: “Large” reversals involving three or more head bends and “small” reversals with two or less head bends (Fig 2a)28. All behavioral data were analyzed using a non-parametric Fisher’s exact test (2-sided) with error bars showing the standard error of the proportion. The animal’s behavioral responses were positively correlated with the pressure of the ultrasound wave in the presence of microbubbles (Fig. 2c). Importantly, using the thermocouple, we found that the presence of the microbubbles did not cause any measurable increase in the ultrasound-induced temperature change at the agar surface (See Methods). Collectively, these results showed that C. elegans responds behaviorally to a single pulse of low-pressure ultrasound in the presence of microbubbles.
Figure 2. C. elegans behavioral responses to ultrasound in the presence of microbubbles.
(ai–avii)) Panels show an animal reversing and generating a high-angled omega bend upon ultrasound stimulus. Reversals with greater than two head bends were scored as large, while those with fewer than two head bends were counted as small. Reversals and omega bends are shown with a red line overlaid on the animal tracks on the agar surface. Animal responses to ultrasound stimuli (single 10 ms pulse, 2.25 MHz) with varying peak negative pressures (b) without and (c) with microbubbles were quantified. n = 30 for each of the conditions. Proportion of animals responding and standard error of the proportion are shown. ** indicates p < 0.01 and * indicates p < 0.05 by Fisher’s exact test (2-sided).
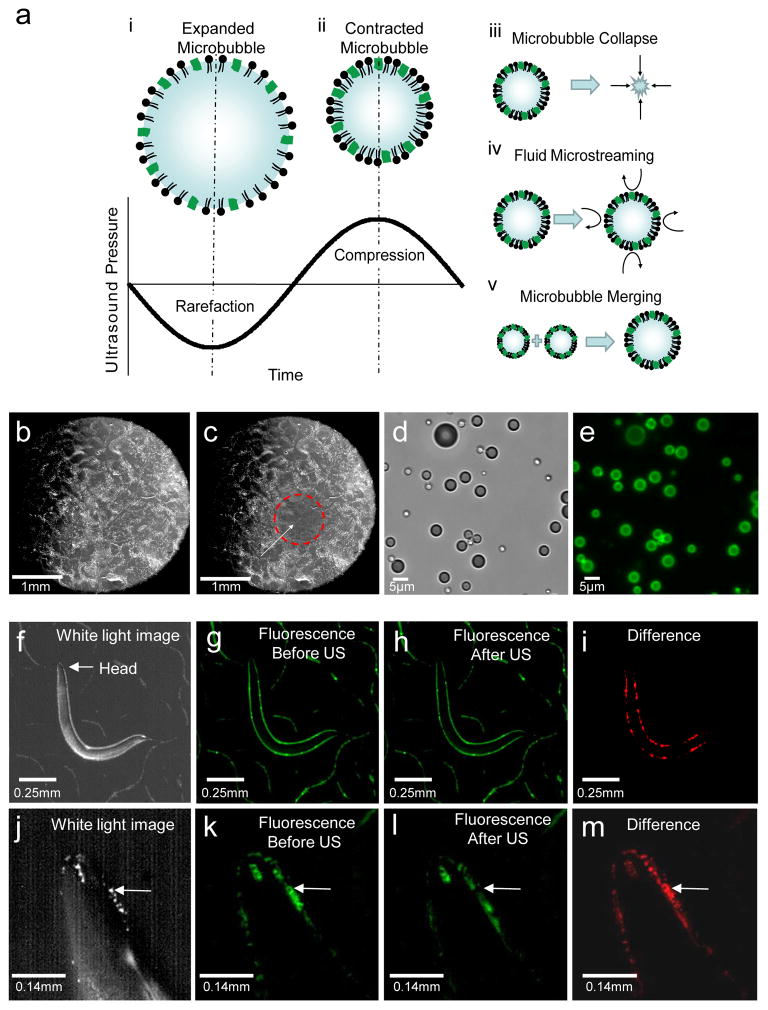
To probe how microbubbles transduce the ultrasound wave and modify animal behavior, we analyzed the behavior of microbubbles upon ultrasound stimulation. Previous studies have shown that the majority of the ultrasound energy propagates through water and soft tissue as a longitudinal wave with alternating compression and rarefaction phases. These two phases create pressures that are alternately higher and lower than the ambient pressure level respectively17. We designed our microbubbles to respond to the mechanical deformations induced by an ultrasound pulse. We filled our microbubbles with a stabilizing mixture of perfluorohexane and air that allows the compression and rarefaction phases of the ultrasound wave to shrink and expand the microbubbles from one half to four times their original diameters29 in a process known as stable cavitation. This occurs at the driving frequency of the underlying ultrasound pulse30,31 (Fig. 3a). These size oscillations create mechanical deformations of the surrounding fluid31 as well as create fluid microstreaming32 that can push against surfaces32,33. The microbubbles can also undergo lateral translations34, and complete collapse, creating large mechanical deformations that have been shown to propagate through water and tissue.31 We found that ultrasound exposure affected the distribution of microbubbles in the focal zone on agar plates confirming these mechanical deformations (Fig. 3b,c). Next, we hypothesized that these large mechanical deformation events created by the ultrasound-microbubble interaction likely propagate from the location of the microbubbles into the body of the worm, thereby affecting behavior. To test our hypothesis, we analyzed the behavior of microbubbles labeled with a fluorescent dye, DiO (Fig. 3d,e). We found that these microbubbles naturally distribute themselves around the animal, but are not attached to the animal’s body (Fig. 3f,3g,3j,3k). Upon ultrasound stimulation, some microbubbles collapse, some merge together, and others move (Fig. 3h,3l). These mechanical deformations occur all around the worm including the worm’s head and are specifically captured in the difference image (shown in red, Fig. 3i,3m). Taken together, these results suggest that the ultrasound-microbubble interaction creates mechanical disturbances around the worm that result in increased reversal behavior.
Figure 3. Ultrasound-microbubble interaction with C. elegans.
(a) The microbubble (i) expands and (ii) contracts in size with the rarefaction (low-pressure) and compression (high-pressure) portions of the ultrasound pressure wave. This oscillation behavior occurs at the frequency of the driving ultrasound resulting in a variety of behaviors including (iii) microbubble collapse, (iv) fluid microstreaming and (v) merging of microbubbles. These microbubble behaviors create mechanical distortions that can propagate through the agar and the body of the animal. (b) Microbubbles are uniformly distributed on an agar surface and appear white. (c) Ultrasound stimulus (10 pulses lasting 10 ms each with a duty cycle of 1Hz, 2.25MHz with peak negative pressure of 0.9 MPa) activates and destroys the microbubbles in the ultrasound focal zone of 1 mm diameter (white arrow). Microbubbles outside this focal zone (denoted by red circle) appear undisturbed. Microbubbles were labeled with DiO and (d) brightfield and (e) fluorescent images are shown. A whole animal view showing (f) brightfield, (g) fluorescence before and (h) after ultrasound stimulus and finally, (i) the difference in red showing the locations of microbubble induced mechanical deformations. A magnified view of the animal’s head in (j) brightfield, (k) before and (l) after ultrasound stimuli (US) and (m) the difference in red showing the locations of microbubble induced mechanical deformations. The white arrow points to a large microbubble that is destroyed upon ultrasound stimulation.
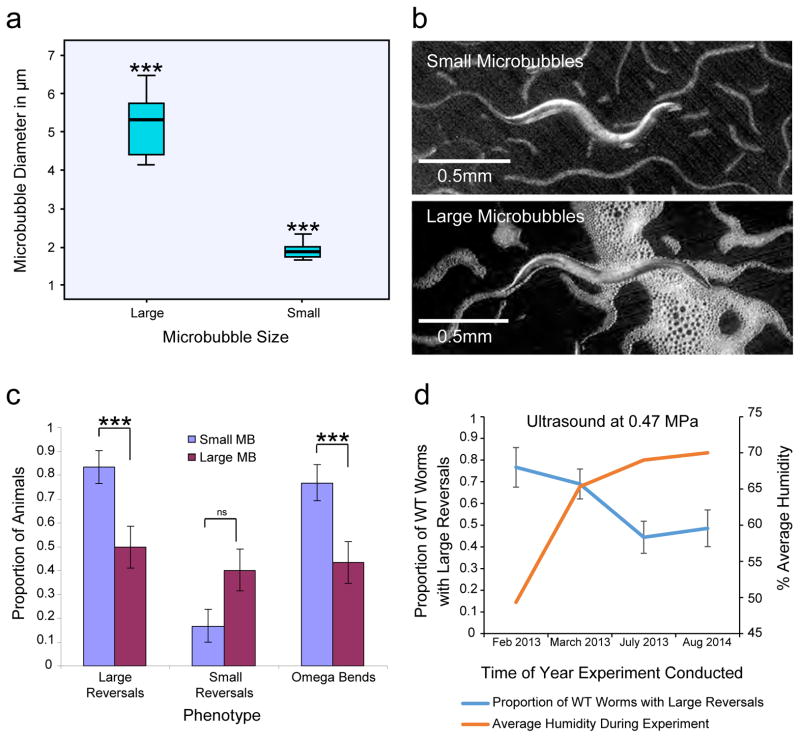
The microbubble synthesis process creates a size distribution of the microbubbles from 1.5 to 6.5 μm in diameter (see Methods). To better understand the effect of microbubbles from different size ranges we separated the mixed microbubbles into distinct large and small size categories based on settling time (see methods, Fig. 4a). Both small and large microbubbles surround the animals completely (Fig. 4b). Interestingly, we found that the smaller microbubbles stimulated a significantly larger proportion of the worms to generate large reversals when exposed to ultrasound, compared to larger microbubbles (Fig. 4c). In order to be consistent in the size distribution between different experiments we used well-mixed samples. This enabled us to ensure that worms were reproducibly exposed to the same microbubble size distributions for all experiments. We found that the proportion of wild-type animals that responded to the same ultrasound peak negative pressure (0.47 MPa) in the presence of microbubbles changed over several months. Recent work has shown that C. elegans detects humidity changes and modifies its locomotory behaviors35. We also tracked environmental conditions during the days that experiments were conducted and found that the wild-type animals’ reversal responses were related to the relative humidity level. At low humidities, more animals responded, while at higher humidities fewer animals executed reversal responses (Fig. 4d). We suggest that the observed variability in wild-type behavior is independent of the ultrasound stimulus and depends on environmental humidity. We controlled for this by testing wild-type animals along with the mutant strains on the same days. We also repeated our analysis over multiple days for each genotype to assure reproducibility.
Figure 4. Microbubbles transduce ultrasound stimuli.
(a) A bar and whisker plot showing the distribution of microbubbles fractionated based on their size. A one-way ANOVA test shows significance in the distribution (*** indicates p < 0.001 using the LSD post hoc test). A mixed size population was used for all experiments to maintain consistency. (b) Images showing animals incubated with small (1.5–2.5 μm; top) and large (4–6.5 μm; bottom) populations of microbubbles. (c) Behavioral responses of wild-type animals incubated with small and large microbubbles upon ultrasound stimulation with a single 10 ms pulse at 2.25 MHz with peak negative pressure of 0.9 MPa. Averages and standard error of the proportion are shown. *** indicates p < 0.001 using Fisher’s exact test. (d) A graph showing the effect of external humidity levels on animal reversal behavior. We observed that at different times of the year the animals had different reversal behavior in response to the same 0.47MPa ultrasound exposure. Under low humidity levels the animals would undergo more large reversals than under high humidity conditions. We accounted for this variable behavior by running a wild-type control for each of the genetically modified strains that was tested. These controls were run on the same day and under the same conditions as the tested strain. Error bars show standard error of the proportion.
TRP-4 stretch sensitive ion channels sensitize neurons to ultrasound
We hypothesized that mechanotransduction channels transduce the mechanical deformations of the ultrasound-microbubble interactions to stimulate individual neurons. Previous studies have identified TRP-4 as a stretch-sensitive, pore forming cation mechanotransduction channel in C. elegans36,37. This channel is specifically expressed in a few C. elegans neurons, the four CEPs (CEPDL, CEPDR, CEPVL and CEPVR) and the two ADE (ADEL and ADER) dopaminergic neurons and the DVA and DVC interneurons36,37. TRP-4 is both necessary and sufficient to generate mechanoreceptor currents in CEP neurons36. We tested whether TRP-4 was required to transduce the ultrasound-microbubble mechanical stimulus and modify animal behavior. To probe behaviors we examined the effects of three different ultrasound pressure levels and found the responses to be dose dependent. We consistently found a larger wild-type response to ultrasound (See wild-type data in Fig. 5a and Fig. 4d). We found that animals lacking TRP-4 have reduced large reversal responses to specific peak negative pressures (0.41 and 0.47 MPa) of ultrasound stimulation, suggesting that this channel increases the probability of large reversals (Fig. 5a). However, with an ultrasound pulse at 0.6 MPa peak negative pressure the trp-4 mutants have similar behavioral responses compared to wild-type (Fig. 5a), suggesting that there is an alternate pathway that detects ultrasound at these higher pressures. Moreover, these trp-4 mutants also show a significant increase in small reversal behaviors upon ultrasound stimulation at 0.41 and 0.46 MPa (Supplementary Fig. 3a), but no change in their omega bend behaviors (Supplementary Fig. 3b). Collectively, these results suggest that TRP-4 might be activated in response to ultrasound with peak negative pressure levels less than 0.5 MPa and modifies neurons involved in generating small and large reversals.
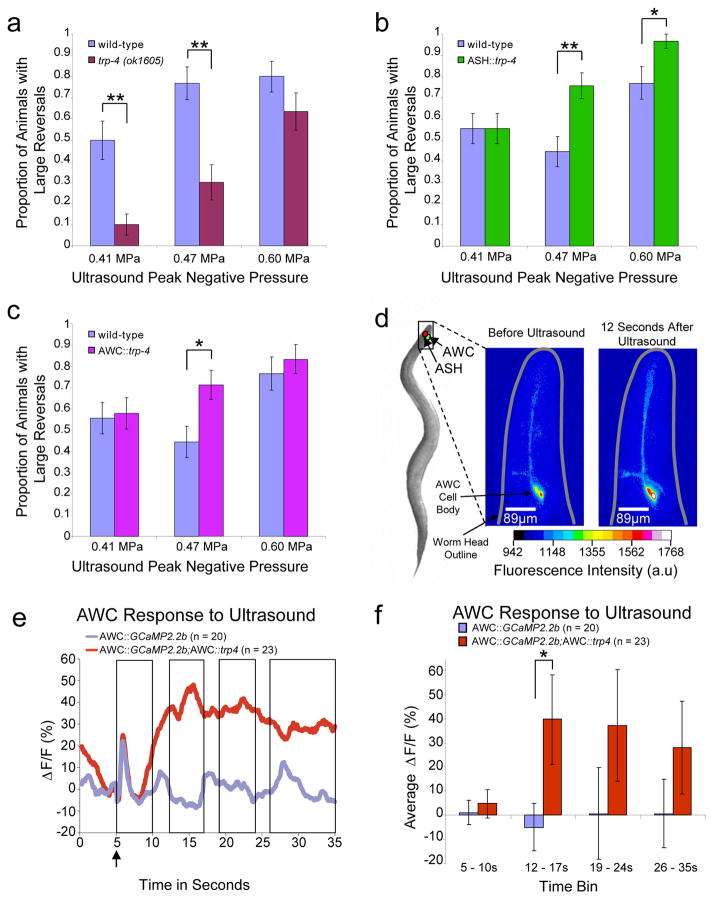
Figure 5. TRP-4 expression activates ASH and AWC neurons in the presence of microbubbles.
(a) Large reversal responses to low-pressure ultrasound require the pore-forming TRP-4 channel. n = 30 for each condition. Transgenic animals expressing TRP-4 in (b) ASH neurons and (c) AWC neurons execute more large reversals upon low-pressure ultrasound stimulation (single pulse, 2.25MHz, 10ms). n > 30 for each genotype and condition. Proportion and standard error of the proportion are shown in all data panels. ** indicates p < 0.01, while * indicates p < 0.05 by Fisher’s exact test (2-sided). (d) Schematic identifying chemosensory neurons ASH and AWC in C. elegans. False-colored images showing changes in GCaMP fluorescence in AWC neurons upon ultrasound stimulation. Warmer colors indicate increased calcium and neural activity. (e) Average AWC calcium responses upon ultrasound stimulation at t = 5 seconds (AWC::GCaMP2.2b n = 20, AWC::GCaMP2.2b;AWC::trp4 n = 23). (f) Average responses binned by distinct times for AWC::GCaMP2.2b and AWC::GCaMP2.2b;AWC::trp4 animals. Averages and s.e.m are shown. * indicates p < 0.05 by t-test (2-sided, equal variances not assumed, AWC::GCaMP2.2b n = 20, AWC::GCaMP2.2b;AWC::trp4 n = 23).
To test whether ultrasound sensitivity could be conferred to other neurons, we analyzed the behavior of transgenic animals misexpressing TRP-4 in specific chemosensory neurons. We tested for dose-dependent responses using three different ultrasound pressure levels. We initially misexpressed the TRP-4 channel in ASH, a well-studied polymodal nociceptive neuron38, whose activation leads to large reversals39. Consistently, we found that ASH expression of TRP-4 generated a significant increase in large reversals at ultrasound peak negative pressures of 0.47 and 0.6 MPa (Fig. 5b). Moreover, we found that these ASH::trp-4 transgenics show a decrease in small reversals (Supplementary Fig. 3c), but not omega bends (Supplementary Fig. 3d), indicating that activating ASH via ultrasound and TRP-4 specifically modifies the small and large reversal neural circuits. Next, we tested the effects of TRP-4 misexpression on the function of the AWC sensory neurons and AWC-driven behavior. Previous results have implied that AWC activation is correlated with an increase in the animal’s ability to generate large reversals21. We found that animals misexpressing TRP-4 in AWC neurons also initiated significantly more large reversals at the ultrasound peak negative pressure of 0.47 MPa (Fig. 5c), but we did not observe a change in small reversals or omega bends (Supplementary Fig. 3e, 3f). These results indicate that misexpressing the mechanotransduction channel, TRP-4, in ASH or AWC renders the neurons sensitive to low-pressure ultrasound, increasing the frequency of large reversals.
To test whether ultrasound could directly stimulate AWC neurons, we expressed the calcium indicator GCaMP340 in these neurons and recorded their activity levels upon ultrasound exposure (Fig. 5d). Consistent with our behavioral data, we found that ultrasound stimulation in the presence of microbubbles activated AWC neurons, as indicated by an increase in the fluorescence signal from the calcium sensor. The AWC calcium responses were greatly increased in transgenic animals expressing TRP-4 specifically in AWC neurons (Fig. 5d–f, Supplementary Fig. 4a–c). We also observed that AWC calcium responses were variable, with many transients lasting more than 30 seconds (the duration of our recording) and a few returning back to baseline (Supplementary Fig. 4c). Variability in AWC calcium responses has also been previously observed using odor stimuli21. To test whether AWC responses were related to artifacts in our imaging setup, we recorded AWC calcium in the absence of ultrasound stimulus and found no consistent responses (Supplementary Fig. 4d, 4e-g). Both wild-type AWC neurons and those misexpressing TRP-4 showed a response lasting about 2–3 seconds immediately upon exposure to a single ultrasound pulse in the presence of microbubbles. However, we also observed that AWC neurons misexpressing TRP-4 show a significant increase in their activity starting at 7 seconds after ultrasound exposure (t=12 seconds in Fig. 5f) and lasting for at least 5 seconds, which is not observed in wild-type neurons. This sustained increase in AWC calcium levels likely represents the activity of TRP-4, which could potentiate calcium entry into the neuron via other calcium channels. Interestingly, large reversals take approximately 10–20 seconds to complete, a time window where we also observe sustained AWC calcium activity in the AWC::trp-4 transgenics. We suggest that the sustained AWC calcium activity observed in these AWC::trp-4 transgenics is likely correlated with the increased frequency of large reversals generated by these animals after ultrasound stimulation. Taken together, these results show that TRP-4 channels are sensitive to low-pressure ultrasound, and ectopic expression of these channels in sensory neurons causes correlated changes in neuronal activity and behavior.
Newly identified roles for PVD sensory and AIY interneurons in generating behavior in the presence of microbubbles
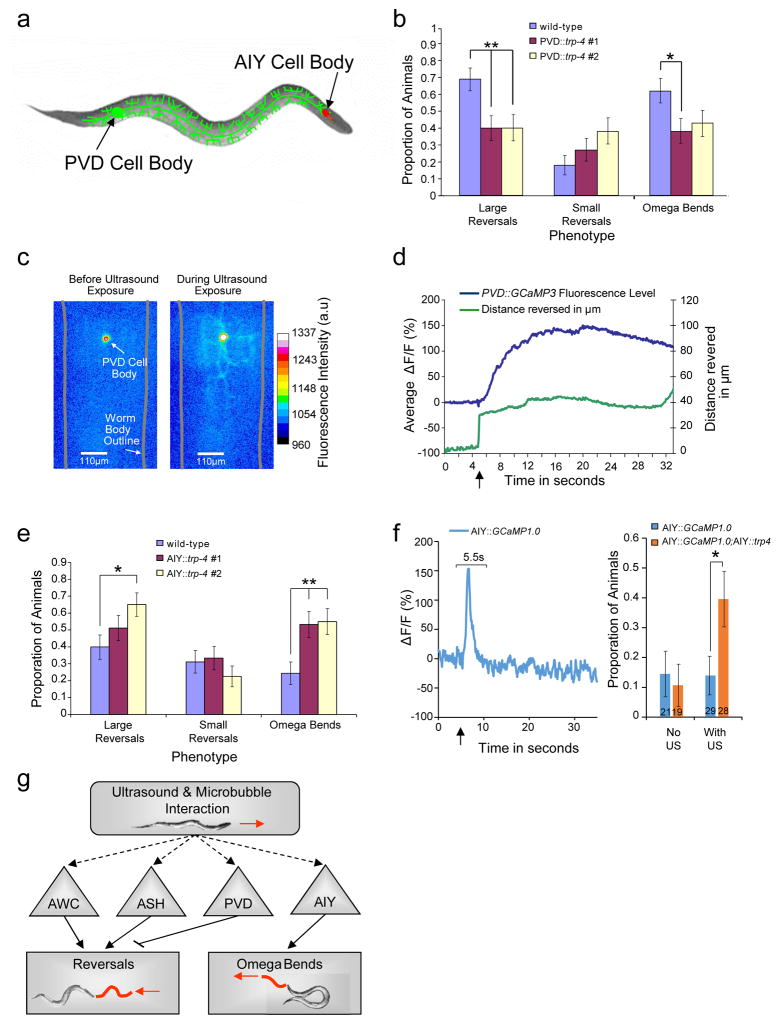
To test whether our method of ultrasound activation of neurons can reveal unidentified behavioral roles, we misexpressed TRP-4 in the poorly understood PVD neurons (Fig. 6a). PVD neurons have extensive, regularly spaced, and non-overlapping dendritic arbors that extend throughout most of the animal, excluding the head and the neck41. PVD shares promoters with the FLP, IL2 and PVC neurons42 and so the TRP-4 proteins were expressed in multiple neurons in the PVD::trp-4 transgenic animals. We find that expressing TRP-4 in PVD, FLP, IL2 and PVC neurons leads to a significant decrease in the animals’ reversal responses upon ultrasound stimulation (two independent transgenics, Fig. 6b). We did not observe changes in FLP or other neurons in response to the ultrasound stimulus (Supplementary Fig. 5a,b). We hypothesize that misexpressing TRP-4 channels activates PVD neurons upon ultrasound stimulation, which in turn suppresses reversals. To test our hypothesis we monitored PVD neuron activity in response to ultrasound stimulation. We observed is a sharp increase in PVD calcium over many seconds (Fig. 6c,d, Supplementary Fig. 6a). The initial decline is likely an artifact of the ultrasound stimulus as it was observed in both AWC and PVD recordings (Fig. 5e, 6d). Interestingly, we find a strong correlation between PVD activity and animal movement. In particular, we find that PVD neurons reach their maximum response when the animal has finished reversing (Fig. 6d). Moreover, we find that PVD neurons are more likely to be activated when the animal is moving backward rather than when moving forward in response to the ultrasound stimulus (Supplementary Fig. 6b–d). These results suggest that ultrasound stimulation activates TRP-4-expressing PVD neurons and causes premature suppression of backward movement, leading to fewer reversals.
Figure 6. PVD neurons inhibit reversals and AIY neurons stimulate omega bends in the presence of microbubbles.
(a) Schematic showing the PVD and AIY neurons in C. elegans. (b) Two independent transgenic strains expressing TRP-4 in PVD neurons show reduced reversals when stimulated with a single 10 ms 2.25 MHz 0.47 MPa peak negative pressure ultrasound pulse. n > 46 for each genotype. Proportion and standard error of the proportion are shown. ** indicates p < 0.01, while * indicates p < 0.05 by Fisher’s exact test (2-sided). (c) False-colored images showing changes in GCaMP3 fluorescence in PVD neurons upon ultrasound stimulation. Warmer colors indicate increased calcium and neural activity. (d) Average PVD calcium responses (n = 16) along with average distance moved by the animal shown as a function of time. Peak PVD response occurs when the animal has stopped moving. (e) Two independent transgenics expressing TRP-4 in AIY neurons show increased incidences of omega bends when stimulated with a single 10 ms 2.25 MHz 0.41 MPa peak negative pressure ultrasound pulse. n > 40 for each genotype. (f) AIY calcium responses to ultrasound stimuli. A representative trace showing the ratio of change in fluorescence to the baseline is shown. Ultrasound stimulus was given at t=5s and neurons that responded within a 5.5 second window after the stimulus were counted as responders. Bar graphs show % responders with and without ultrasound stimuli for AIY::GCaMP and AIY::GCaMP;AIY::trp-4. Numbers on the bars indicate the number of animals analyzed in each condition. * indicates p < 0.05 by fisher exact t-test. (g) Schematic showing the neural circuit that responds to ultrasound stimuli amplified by microbubbles. ASH and AWC neurons promote reversals, while PVD neurons inhibit reversals. AIY neurons promote omega bends.
We then tested whether our approach can manipulate the function of an interneuron, whose processes do not contact the external cuticle of the animal. We misexpressed TRP-4 in AIY interneurons, which are at least 25 μm from the cuticle19, and analyzed the behavior of these animals upon ultrasound stimulation. Optogenetic studies have previously shown that activating AIY interneurons reduces turns43. In contrast, we find that AIY::trp-4 transgenics are significantly more likely to initiate high-angled omega bends upon ultrasound stimulation (two independent transgenics, Fig. 6e). It is possible that expressing TRP-4 in AIY neurons has altered that neuron’s function, leading to increased turns. However, animals with genetically altered AIY function have been shown to have increased turns in a local search assay21,28. We found that these AIY::trp-4 transgenics did not show any defects in local search (Supplementary Fig. S7a–c), confirming that the AIY neurons were not altered in these animals. These data suggest that AIY can initiate different behaviors based on type of stimulation, ultrasound or light.
To confirm whether ultrasound stimulus is activating AIY interneurons, we used calcium imaging. AIY neural activity is typically measured from a bulb in the AIY neurite21. Consistent with previous observation, we found that AIY is a noisy neuron with a number of transients during our recordings (Supplementary Fig. 8). We collected from a number of AIY recordings from wild-type animals and defined the relevant transient. We counted all neurons that responded within a 5.5 second after the ultrasound pulse as responders. Using this criteria, AIY neurons in wild-type animals did not show a significant response to ultrasound stimulus (4/29) (Fig. 6f, Supplementary Fig. 8a,b). In contrast, we observed a significant number of AIY neurons in AIY::trp-4 transgenics (11/28 animals) had a positive response (Fig. 6f, Supplementary Fig. 8e,f). In contrast, we suggest that increased proportion of AIY responders in the AIY::trp-4 transgenics suggests that ultrasound stimulus activates AIY interneurons. These results show that mechanical deformations from the ultrasound-microbubble interaction can penetrate at least 25 μm into the worm and influence the function of AIY interneurons. Moreover, we find that misexpressing TRP-4 can influence both reversal and omega bend neural circuitry, suggesting that our sonogenetic approach is broadly applicable for manipulating circuit activity. Further, our results show that AIY interneurons are likely have at least three activity states with one suppressing turns21, one promoting forward turns (as revealed by optogenetic stimulation)43 and one increasing omega turns (as revealed by ultrasound stimulation). These studies validate our approach of using sonogenetics to reveal novel roles for both PVD and AIY neurons in modifying turn behavior.
Discussion
Our studies show that C. elegans neural circuits can be probed by combining low-pressure ultrasound stimulation with microbubbles that amplify the mechanical deformations. Specifically, we find that C. elegans are insensitive to low-pressure ultrasound but respond when surrounded by microbubbles. We find that animals missing the TRP-4 mechanosensitive ion channel have significantly reduced sensitivity to the ultrasound-microbubble stimulation, indicating that mechanosensitive ion channels play an important role in the mechanism of ultrasound stimulation. We also find that misexpressing the TRP-4 mechanosensitive ion channel in specific neurons modifies their neural activity upon ultrasound stimulation, resulting in altered animal behaviors. Specifically, misexpressing TRP-4 in ASH and AWC sensory neurons results in an increase in large reversals, while activating PVD neurons suppresses this behavior (Fig. 6g). We also define novel roles for PVD neurons in suppressing reversal behavior and AIY neurons in stimulating omega bend behavior (Fig. 6g).
Our novel method provides new insights into the neural activity patterns that drive whole-animal behavior. We note that persistent AWC neural activity might drive reversal behavior, providing a correlation between a distinct AWC neuronal activity pattern and whole-animal behavior. We suggest that ultrasound stimulation might activate neurons with different kinetics than what has been seen using optogenetics. For example, activating AIY interneurons using light leads to an increase in forward turns43, while using low-pressure ultrasound increases omega bend frequency. Our studies indicate an alternative role for AIY in promoting omega bends. The stimulation of AIY interneurons demonstrates that this ultrasound technique can also be applied to deep internal neurons that do not contact the skin of the worm. Taken together, these results and other studies44 show that TRP channels can be used to manipulate neuronal functions and thus provide insight into how neural circuits transform environmental changes into precise behaviors.
In order to target smaller groups of neurons, the resolution of the ultrasound focal zone can be made smaller than the 1 mm diameter. Frequencies above 2.25 MHz can produce sub-millimeter focal zone spot sizes45. Higher frequency ultrasound waves with their smaller focal zones are better suited to targets that are closer to the body surface as these waves do not penetrate tissues as well46. One of the advantages of ultrasound is that small focal zones can be maintained noninvasively even in deep brain tissue8. Outside the focal zone the peak negative pressures are significantly lower and are unlikely to result in neuron activation11,47. This was seen on the agar plates where only worms that were in the focal zone responded to the ultrasound and nearby worms that were outside the focal zone did not. Another advantage of ultrasound is that this focal zone can be moved arbitrarily within the tissue to simulate different regions without any invasive procedures8,47,48. With an electronically steerable ultrasound beam, multiple different targets can be noninvasively manipulated either simultaneously or in rapid succession48. Moreover, the genetic targeting of the stretch sensitive ion channels to individual neurons allows for targeting well below the resolution of the ultrasound focal zone.
We speculate that the use of ultrasound as a non-invasive neuronal activator can be broadly applied to decode neural circuits in larger vertebrate brains with opaque skin and intact skulls. Ultrasound waves with peak negative pressures of < 1 MPa have been shown to penetrate through skull and brain tissue with very little impedance or tissue damage49. Our results show that low-pressure ultrasound (with peak negative pressures 0.4 – 0.6 MPa) specifically activates neurons expressing the TRP-4 channel. Moreover, TRP-4 channels do not have mammalian homologs36,37, therefore, it is unlikely that expressing these channels in the mammalian brain would produce deleterious effects. We suggest that neurons in diverse model organisms misexpressing this channel can be activated by ultrasound stimulation, allowing us to probe their functions in influencing animal behavior. Additionally, other mechanosensitive channels can be explored that may be more sensitive to mechanic deformations than TRP-4. Of particular interest are the bacterial MscL and MscS channels that have different sensitivities to membrane stretch and are selective for different ions50. Moreover, TRP-4 and other channels may be mutated in and around the pore region in order to change their ion selectivity51 as well as their sensitivity to mechanical stretch to broaden the utility of this method.
Furthermore, if low-pressure ultrasound stimulation by itself does not activate TRP-4 expressing neurons, we demonstrate that the mechanical signals can be amplified by gas-filled microbubbles. Perfluorohexane microbubbles are well-established for use as ultrasound contrast agents in vivo52 and can be administered intravenously to circulate throughout the vertebrate body including the brain53–56. They can remain active for up to 60 minutes57 providing a time window where they could be used safely to amplify the ultrasound stimulus and manipulate neural activity. Microbubbles have been shown to undergo inertial cavitation when exposed to ultrasound with peak negative pressure of 0.58 MPa and higher58. Using ultrasound pressure levels lower than this will prevent damage to the brain from the microbubble-ultrasound interaction. Moreover, we used a third of the number of microbubbles that has been previously used to successfully image the mouse brain59 showing that the required microbubble dose would not be prohibitive for in vivo administration. Our experiments show that in the presence of microbubbles the low pressure ultrasound stimulated the deep AIY interneurons expressing TRP-4. This result enables us to estimate the distances at which the mechanical deformations from the ultrasound-microbubble interaction can effectively penetrate into brain tissue from the vasculature. The C. elegans cuticle is 0.5 μm thick60 and the AIY interneurons are 25 μm from the cuticle19, indicating that the mechanical deformations traveled at least 25.5 μm into the worm. In contrast, the mammalian blood-brain barrier is 0.2 μm thick61 and the average distance of a neuron from a capillary is less than 20 μm62. These distances are well within the range of our sonogenetic approach. With the data presented in this paper, we offer a novel, non-invasive approach to activate genetically targeted neurons using low-pressure ultrasound stimulation.
Methods
Ultrasound and Microscopy Setup
A schematic of the ultrasound and microscopy setup is shown in Fig. 1a and is previously described63. The single 10 ms, 2.25 MHz sine wave ultrasound pulse was generated with a submersible 2.25 MHz transducer (V305-Su, Panametrics, Waltham, MA) using a waterproof connector cable (BCU-58-6W, Panametrics, Waltham, MA). The resulting sound field was quantified using a needle hydrophone (HNP-0400, Onda Corporation, Sunnyvale, CA). An arbitrary waveform generator (PCI5412, National Instruments, Austin, TX) controlled by a custom designed program (LabVIEW 8.2, National Instruments, Austin, TX) was used to create the desired ultrasound pulse. The peak negative pressure of the ultrasound pulse was adjusted from 0 to 0.9 MPa using a 300 W amplifier (VTC2057574, Vox Technologies, Richardson, TX). Ultrasound attenuation though the plastic and agar was found to be minimal.
White light illumination was achieved by reflecting light from an external light source up at the petri dish using a mirror mounted at 45°. Behavior was captured using a high-speed camera (FASTCAM, Photron, San Diego, CA). Fluorescent images were collected using a Nikon 1-FL EPI-fluorescence attachment on the same setup as described. GCaMP imaging was performed using a 40× objective and the images were captured using a Quanti-EM 512C camera (Photometrics, Tucson, AZ).
The petri dish was held at the air-water interface with a three-prong clamp mounted to an XYZ micromanipulator stage, which allow the dish to be scanned in the XY plane while maintaining a constant Z distance between the objective and ultrasound transducer. This alignment positioned the agar surface in the focal zone of the ultrasound wave.
Microbubble Synthesis
Microbubbles were made using a probe sonication technique as described64. Briefly, the lipids were dissolved in chloroform and mixed together in the previously described molar ratios in a glass vial64. The chloroform was evaporated under an argon stream allowing the lipids to form a layer along the sides of the glass vial. The choice of lipids is essential for tuning the behavior of microbubbles since they can affect the stiffness of the monolayer that surrounds and protects the microbubbles. The formulation used here has a stabilizing lipid monolayer consisting of distearoyl phosphatidylcholine (DSPC, Avanti Polar Lipids Inc., Alabaster, AL), distearoyl phosphatidylethanolamine-methyl polyethylene glycol (mPEG-DSPE 5k, Layson Bio Inc., Arab, AL) and DiO (Biotium Inc., CA) in 85:13:2 molar ratio. The lipids were rehydrated in 500μl of phosphate buffered saline (PBS). The headspace of the vial was filled with the perfluorohexane and air gas mixture. The gas core of the microbubble also consisted of a perfluorohexane and air mixture (Sigma-Aldrich, St. Louis, MO) and was designed to attain stability under atmospheric pressure. A probe sonicator tip was then placed just below the surface of the PBS and run at maximum power for 3 seconds to cause the spontaneous formation of the lipid-coated microbubbles. Microbubbles were fractionated based on size by their settling time (Fig. 4a). We chose a mixed size of microbubbles from the manufacturing process to maintain uniformity across all the experiments. The microbubbles were shown to be stable on agar plates sealed with parafilm for up to 24 hours.
Behavioral Assays
A 15 μl solution of microbubbles at a density of 3.8×107/ml was added to an empty nematode growth media (NGM) 2% agar plate. The microbubbles were left on the plate for 20 min to ensure absorption of the liquid leaving the microbubbles on the surface. Well-fed young adult worms were placed on the plate and were corralled into the small microbubble area using a filter paper soaked in copper sulfate solution (200 mM). The worms were allowed to crawl around for 10 minutes before being stimulated by ultrasound. The agar plate was moved to localize the animal in the ultrasound focal zone where it was stimulated with one pulse. Resulting reversal and omega bend behavioral responses was recorded. Ultrasound pulse regime is described above. Reversals with fewer than two head bends were identified as small (Fig. 2aiii), while those with more than two were counted as large (Fig. 2aiv). High-angled turns that lead to a significant change in the direction of an animal’s movement were identified as omega bends (Fig. 2avi)28. Each animal was only exposed to a single ultrasound pulse. Since previous work has shown that C. elegans detects changes in humidity and modifies its locomotory behaviors35 we controlled for humidity effects by testing wild-type animals daily to ensure stable reversal behavior and repeating our analysis over multiple days for each genotype. Behavior data was collected on three separate days to ensure reproducibility. The data from all three days was combined for the final statistical analysis. Significance and the relevant comparisons are shown in each figure. Local search behavior28 was used to test whether AIY functionality was affected in the AIY::trp-4 transgenics. Briefly, day 1 adults were moved from food plates to a food-free plate and their behavior was scored as indicated. Reversals were scored as small (fewer than 2 head bends) or large (more than 2 head bends). 4 animals were scored for each genotype. Data was collected over 2 days and combined. AIY::trp-4 transgenics executed normal local search behavior (Supplementary Fig. 7).
Imaging
Transgenic animals expressing GCaMP in specific neurons were corralled into a small area by filter paper soaked in copper solution (as described above). The acetylcholine agonist and paralytic, tetramisole65, was used at a concentration of 1.3 mM to paralyze the animals to facilitate recording neural activity. Anesthetized animals were surrounded by a solution of microbubbles and stimulated using ultrasound peak negative pressures as described above. Fluorescence was recorded at 10 frames/second using an EMCCD camera (Photometrics, Quant-EM) and resulting movies were analyzed using Metamorph software (Molecular Devices)21. Briefly, a fluorescence baseline was calculated using a 2-second window from t=3 to t=5 seconds before ultrasound exposure. The ratio of change in fluorescence to baseline fluorescence was plotted in all graphs using custom MATLAB scripts21. For imaging PVD neurons, the concentration of the tetramisole was reduced to 1 mM, which allowed these animals greater movement. For imaging AIY interneurons, 2 doses of tetramisole at 3.9 mM was used to restrict animal movement. Here, we recorded fluorescence from the AIY process and the increased tetramisole enabled the animal to be paralyzed. Their motion and corresponding fluorescent intensity changes were captured and analyzed using Metamorph software. For all imaging experiments, one neuron was imaged in each animal and the animal was only used once. Imaging data was collected on three separate days to ensure reproducibility. The data from all three days was combined for the final statistical analysis.
Molecular Biology and Transgenic animals
All C. elegans strains were grown under standard conditions as described66. Cell-selective expression of TRP-4 was achieved by driving the full-length cDNA under odr-3 (AWC)67, sra-6 (ASH)68, des-2 (PVD and FLP)42 and ttx-3 (AIY)69 promoters. Germline transformations were obtained using the methods previously described70. Complete information for all strains is listed in Supplementary Table 1.
Temperature Measurements
Temperature was measured in the ultrasound focal zone on the surface of the agar using a High Precision Digital RTD/Thermocouple Thermometer/Data Logger (model number DP9602) along with a Fine Tip TJ Probe, both from Omega (Stamford, CT). The miniature probe was 2.4mm long by 0.5mm in diameter (Supplementary Fig. 1). These small dimensions are within the spot size of the ultrasound focal zone and close to the dimensions of the worms themselves. The small dimensions, and correspondingly small mass of the probe helped to reduce the effect of the probe itself removing heat from the ultrasound focal zone making our temperature measurements as accurate as possible. The tip was placed in the focal zone of the ultrasound on the agar surface both with and without the presence of microbubbles. The agar surface was ensonified with the same ultrasound pulse used in the experiments. We observed that both with and without the presence of microbubbles an ultrasound peak negative pressure of 0.7MPa caused a temperature increase of less than 0.1°C.
Statistical Analysis
Data was analyzed using SPSS software v22 (IBM, NY). The sample sizes used in the behavioral experiments were chosen to detect effect sizes of at least 15% with a power of 0.80. All behavior data were analyzed using a non-parametric Fisher’s exact test (2-sided). The sample sizes used in the imaging experiments were chosen to detect effect sizes of at least 20% with a power of 0.80. Animals were excluded from the study if they showed visible signs of injury or disorder and measures used to determine an animal’s health were established prior to the study. Animals were randomly chosen from large breeding populations of the different genotypes for each of the three replicates and analyzed on different days. The observer was not blind to the genotype of the group being tested. The populations used for imaging data (t-test) and microbubble size distribution (one way ANOVA) had adequate normal distribution to justify the use of those tests.
Supplementary Material
Acknowledgments
We thank S. Xu for providing the trp-4 cDNA, C. Bargmann, S. Xu and the CGC for strains and N. Chronis and the Chronis lab for insightful discussions. We also thank L. Hale, S. Leinwand, and C. Profaci and members of the Chalasani and Esener labs for helpful comments and suggestions on the manuscript. A Salk Institute Pioneer Fund Postdoctoral Fellowship (S.I.) and a Salk Institute Innovation Grant, The Rita Allen Foundation, The W.M. Keck Foundation and NIH R01MH096881-03 to S.H.C supported this work.
Footnotes
This manuscript contains Supplementary figures (1–8) and Supplementary Table 1.
Author Contributions
S.I. designed the microscope setup, performed experiments and co-wrote the paper. A.T. performed all the molecular biology and generated transgenic animals. C.S. designed and manufactured the microbubbles. S.E. provided mentorship and guidance for establishing the ultrasound and microscope setup. S.H.C. designed experiments and co-wrote the paper.
Competing financial interests
The authors declare no competing interests.
References
- 1.Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- 2.Aston-Jones G, Deisseroth K. Recent advances in optogenetics and pharmacogenetics. Brain Res. 2013;1511:1–5. doi: 10.1016/j.brainres.2013.01.026. S0006-8993(13)00100-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrell MS, Roth BL. Pharmacosynthetics: Reimagining the pharmacogenetic approach. Brain Res. 2013;1511:6–20. doi: 10.1016/j.brainres.2012.09.043. S0006-8993(12)01604-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Packer AM, Roska B, Hausser M. Targeting neurons and photons for optogenetics. Nat Neurosci. 2013;16:805–815. doi: 10.1038/nn.3427. nn.3427 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dugue GP, Akemann W, Knopfel T. A comprehensive concept of optogenetics. Prog Brain Res. 2012;196:1–28. doi: 10.1016/B978-0-444-59426-6.00001-X. B978-0-444-59426-6.00001-X [pii] [DOI] [PubMed] [Google Scholar]
- 6.Warden MR, Cardin JA, Deisseroth K. Optical neural interfaces. Annu Rev Biomed Eng. 2014;16:103–129. doi: 10.1146/annurev-bioeng-071813-104733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol. 1998;24:275–283. doi: 10.1016/s0301-5629(97)00269-x. S0301-5629(97)00269-X [pii] [DOI] [PubMed] [Google Scholar]
- 8.Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Annals of neurology. 2009;66:858–861. doi: 10.1002/ana.21801. [DOI] [PubMed] [Google Scholar]
- 9.Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol. 2002;47:1219–1236. doi: 10.1088/0031-9155/47/8/301. [DOI] [PubMed] [Google Scholar]
- 10.King RL, Brown JR, Newsome WT, Pauly KB. Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound in medicine & biology. 2013;39:312–331. doi: 10.1016/j.ultrasmedbio.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Tufail Y, et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Tufail Y, Yoshihiro A, Pati S, Li MM, Tyler WJ. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. nature protocols. 2011;6:1453–1470. doi: 10.1038/nprot.2011.371. [DOI] [PubMed] [Google Scholar]
- 13.Tyler WJ, et al. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS One. 2008;3:e3511. doi: 10.1371/journal.pone.0003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo SS, et al. Focused ultrasound modulates region-specific brain activity. Neuroimage. 2011;56:1267–1275. doi: 10.1016/j.neuroimage.2011.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menz MD, Oralkan O, Khuri-Yakub PT, Baccus SA. Precise neural stimulation in the retina using focused ultrasound. J Neurosci. 2013;33:4550–4560. doi: 10.1523/JNEUROSCI.3521-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bystritsky A, et al. A review of low-intensity focused ultrasound pulsation. Brain stimulation. 2011;4:125–136. doi: 10.1016/j.brs.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien WD., Jr Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol. 2007;93:212–255. doi: 10.1016/j.pbiomolbio.2006.07.010. S0079-6107(06)00091-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krasovitski B, Frenkel V, Shoham S, Kimmel E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proceedings of the National Academy of Sciences. 2011;108:3258–3263. doi: 10.1073/pnas.1015771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Transact R Soc Lond B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 20.de Bono M, Maricq AV. Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci. 2005;28:451–501. doi: 10.1146/annurev.neuro.27.070203.144259. [DOI] [PubMed] [Google Scholar]
- 21.Chalasani SH, et al. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature. 2007;450:63–70. doi: 10.1038/nature06292. nature06292 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J Acoust Soc Am. 1990;88:2059–2069. doi: 10.1121/1.400102. [DOI] [PubMed] [Google Scholar]
- 23.Bao S, Thrall BD, Miller DL. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med Biol. 1997;23:953–959. doi: 10.1016/s0301-5629(97)00025-2. S0301562997000252 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Brysev AP, et al. Spectroscopy of spontaneous Raman scattering of a liquid-water local structure in the field of an intense ultrasound pulse. Opt Spectrosc. 2002;93:282–285. doi: 10.1134/1.1503760. [DOI] [Google Scholar]
- 25.Farny CH, Holt RG, Roy RA. The Correlation Between Bubble-Enhanced HIFU Heating and Cavitation Power. Biomedical Engineering, IEEE Transactions on. 2010;57:175–184. doi: 10.1109/tbme.2009.2028133. [DOI] [PubMed] [Google Scholar]
- 26.Mori I, Sasakura H, Kuhara A. Worm thermotaxis: a model system for analyzing thermosensation and neural plasticity. Curr Opin Neurobiol. 2007;17:712–719. doi: 10.1016/j.conb.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Clark DA, Gabel CV, Gabel H, Samuel AD. Temporal activity patterns in thermosensory neurons of freely moving Caenorhabditis elegans encode spatial thermal gradients. J Neurosci. 2007;27:6083–6090. doi: 10.1523/JNEUROSCI.1032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2005;102:3184–3191. doi: 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Postema M, van Wamel A, Lancee CT, de Jong N. Ultrasound-induced encapsulated microbubble phenomena. Ultrasound Med Biol. 2004;30:827–840. doi: 10.1016/j.ultrasmedbio.2004.02.010. S0301562904000687 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Schutt C, Ibsen S, Benchimol M, Hsu M, Esener S. Manipulating Nanoscale Features on the Surface of Dye-Loaded Microbubbles to Increase their Ultrasound-Modulated Fluorescence Output. Small. 2014;10:3316–3324. doi: 10.1002/smll.201302786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauterborn W, Kurz T. Physics of bubble oscillations. Reports on Progress in Physics. 2010;73:106501. [Google Scholar]
- 32.Ferrara RPK, Borden M. Ultrasound Microbubble Contrast Agents: Fundamentals and Application to Gene and Drug Delivery. Annu Rev Biomed Eng. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Lewis TN, Prausnitz MR. Non-Invasive Assessment and Control of Ultrasound-Mediated Membrane Permeabilization. Pharmaceutical Research. 1998;15:918–924. doi: 10.1023/a:1011984817567. [DOI] [PubMed] [Google Scholar]
- 34.Dayton PA, Allen JS, Ferrara KW. The magnitude of radiation force on ultrasound contrast agents. J Acoust Soc Am. 2002;112:2183–2192. doi: 10.1121/1.1509428. [DOI] [PubMed] [Google Scholar]
- 35.Russell J, Vidal-Gadea AG, Makay A, Lanam C, Pierce-Shimomura JT. Humidity sensation requires both mechanosensory and thermosensory pathways in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2014;111:8269–8274. doi: 10.1073/pnas.1322512111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang L, Gao J, Schafer WR, Xie Z, Xu XZC. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. S0896-6273(10)00516-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W, Feng Z, Sternberg PW, Xu XZ. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;440:684–687. doi: 10.1038/nature04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hilliard MA, Bergamasco C, Arbucci S, Plasterk RH, Bazzicalupo P. Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. Embo J. 2004;23:1101–1111. doi: 10.1038/sj.emboj.7600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo ZV, Hart AC, Ramanathan S. Optical interrogation of neural circuits in Caenorhabditis elegans. Nat Methods. 2009;6:891–896. doi: 10.1038/nmeth.1397. nmeth.1397 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albeg A, et al. C. elegans multi-dendritic sensory neurons: morphology and function. Mol Cell Neurosci. 2011;46:308–317. doi: 10.1016/j.mcn.2010.10.001. S1044-7431(10)00246-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Treinin M, Gillo B, Liebman L, Chalfie M. Two functionally dependent acetylcholine subunits are encoded in a single Caenorhabditis elegans operon. Proc Natl Acad Sci USA. 1998;95:15492–15495. doi: 10.1073/pnas.95.26.15492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kocabas A, Shen CH, Guo ZV, Ramanathan S. Controlling interneuron activity in Caenorhabditis elegans to evoke chemotactic behaviour. Nature. 2012;490:273–277. doi: 10.1038/nature11431. nature11431 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tobin D, et al. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. S0896627302007572 [pii] [DOI] [PubMed] [Google Scholar]
- 45.Foster FS, et al. A new 15–50 MHz array-based micro-ultrasound scanner for preclinical imaging. Ultrasound in medicine & biology. 2009;35:1700–1708. doi: 10.1016/j.ultrasmedbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 46.d’Astous F, Foster F. Frequency dependence of ultrasound attenuation and backscatter in breast tissue. Ultrasound in medicine & biology. 1986;12:795–808. doi: 10.1016/0301-5629(86)90077-3. [DOI] [PubMed] [Google Scholar]
- 47.Chen L, et al. Histological changes in rat liver tumours treated with high-intensity focused ultrasound. Ultrasound in medicine & biology. 1993;19:67–74. doi: 10.1016/0301-5629(93)90019-k. [DOI] [PubMed] [Google Scholar]
- 48.Hand J, et al. A random phased array device for delivery of high intensity focused ultrasound. Physics in medicine and biology. 2009;54:5675. doi: 10.1088/0031-9155/54/19/002. [DOI] [PubMed] [Google Scholar]
- 49.Tyler WJ. Noninvasive neuromodulation with ultrasound? A continuum mechanics hypothesis. Neuroscientist. 2011;17:25–36. doi: 10.1177/1073858409348066. 1073858409348066 [pii] [DOI] [PubMed] [Google Scholar]
- 50.Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Annual review of microbiology. 2010;64:313–329. doi: 10.1146/annurev.micro.112408.134106. [DOI] [PubMed] [Google Scholar]
- 51.Kang L, Gao J, Schafer WR, Xie Z, Xu XS. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Jong N, Bouakaz A, Frinking P. Basic acoustic properties of microbubbles. Echocardiography. 2002;19:229–240. doi: 10.1046/j.1540-8175.2002.00229.x. [DOI] [PubMed] [Google Scholar]
- 53.Fan CH, et al. SPIO-conjugated, doxorubicin-loaded microbubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery. Biomaterials. 2013;34:3706–3715. doi: 10.1016/j.biomaterials.2013.01.099. S0142-9612(13)00167-1 [pii] [DOI] [PubMed] [Google Scholar]
- 54.Haggag KJ, Russell D, Walday P, Skiphamn A, Torvik A. Air-filled ultrasound contrast agents do not damage the cerebral microvasculature or brain tissue in rats. Investigative radiology. 1998;33:129–135. doi: 10.1097/00004424-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Martina AD, Meyer-Wiethe K, Allémann E, Seidel G. Ultrasound contrast agents for brain perfusion imaging and ischemic stroke therapy. Journal of Neuroimaging. 2005;15:217–232. doi: 10.1177/1051228405277342. [DOI] [PubMed] [Google Scholar]
- 56.Bloch SH, Dayton PA, Ferrara KW. Targeted imaging using ultrasound contrast agents. Engineering in Medicine and Biology Magazine, IEEE. 2004;23:18–29. doi: 10.1109/memb.2004.1360405. [DOI] [PubMed] [Google Scholar]
- 57.Simon RH, Ho SY, D’ARRIGO J, WAKEFIELD A, HAMILTON SG. Lipid-coated ultrastable microbubbles as a contrast agent in neurosonography. Investigative radiology. 1990;25:1300–1304. doi: 10.1097/00004424-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Miller DL, Thomas RM. Ultrasound Contrast Agents Nucleate Inertial Cavitation In Vitro. Ultrasound in Med & Biol. 1995;21:1059–1065. doi: 10.1016/0301-5629(95)93252-u. [DOI] [PubMed] [Google Scholar]
- 59.Ellegala DB, et al. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation. 2003;108:336–341. doi: 10.1161/01.CIR.0000080326.15367.0C. [DOI] [PubMed] [Google Scholar]
- 60.Cox GN, Kusch M, Edgar RS. Cuticle of Caenorhabditis elegans: its isolation and partial characterization. The Journal of cell biology. 1981;90:7–17. doi: 10.1083/jcb.90.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlageter KE, Molnar P, Lapin GD, Groothuis DR. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvascular research. 1999;58:312–328. doi: 10.1006/mvre.1999.2188. [DOI] [PubMed] [Google Scholar]
- 63.Ibsen S, Benchimol M, Esener S. Fluorescent microscope system to monitor real-time interactions between focused ultrasound, echogenic drug delivery vehicles, and live cell membranes. Ultrasonics. 2013;53:178–184. doi: 10.1016/j.ultras.2012.05.006. S0041-624X(12)00103-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schutt CE, Ibsen SD, Benchimol MJ, Hsu MJ, Esener SC. Manipulating Nanoscale Features on the Surface of Dye-Loaded Microbubbles to Increase Their Ultrasound-Modulated Fluorescence Output. Small. 2014 doi: 10.1002/smll.201302786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewis JA, Wu CH, Levine JH, Berg H. Levamisole-resistant mutants of the nematode Caenorhabditis elegans appear to lack pharmacological acetylcholine receptors. Neuroscience. 1980;5:967–989. doi: 10.1016/0306-4522(80)90180-3. [DOI] [PubMed] [Google Scholar]
- 66.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roayaie K, Crump JG, Sagasti A, Bargmann CI. The G alpha protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron. 1998;20:55–67. doi: 10.1016/s0896-6273(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 68.Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell. 1995;83:207–218. doi: 10.1016/0092-8674(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 69.Hobert O, et al. Regulation of interneuron function in the C. elegans thermoregulatory pathway by the ttx-3 LIM homeobox gene. Neuron. 1997;19:345–357. doi: 10.1016/s0896-6273(00)80944-7. [DOI] [PubMed] [Google Scholar]
- 70.Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. Embo J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.