Abstract
Objective
Neighborhood characteristics may be important for promoting walking, but little research has focused on older adults, especially those with cognitive impairment. We evaluated the role of neighborhood characteristics on cognitive function and decline over a 2-year period adjusting for measures of walking.
Method
In a study of 64 older adults with and without mild Alzheimer's disease (AD), we evaluated neighborhood integration and connectivity using geographical information systems data and space syntax analysis. In multiple regression analyses, we used these characteristics to predict 2-year declines in factor analytically derived cognitive scores (attention, verbal memory, mental status) adjusting for age, sex, education, and self-reported walking.
Results
Neighborhood integration and connectivity predicted cognitive performance at baseline, and changes in cognitive performance over 2 years. The relationships between neighborhood characteristics and cognitive performance were not fully explained by self-reported walking.
Discussion
Clearer definitions of specific neighborhood characteristics associated with walkability are needed to better understand the mechanisms by which neighborhoods may impact cognitive outcomes. These results have implications for measuring neighborhood characteristics, design and maintenance of living spaces, and interventions to increase walking among older adults. We offer suggestions for future research measuring neighborhood characteristics and cognitive function.
Keywords: neighborhood, cognitive decline, Alzheimer's disease, walking
Introduction
Older adults are among the most sedentary groups in the United States, spending 60% of waking time in sedentary activities (Caspersen, Pereira, & Curran, 2000; Matthews et al., 2008). They rarely engage in structured exercise or high intensity physical activity, such as sports or running. In our recent study of older adults with and without Alzheimer's disease (AD), the majority participated only in unstructured and low intensity physical activities, including walking and housework (Watts, Vidoni, Loskutova, Johnson, & Burns, 2013). Several studies are beginning to suggest that even light intensity activity can have health benefits in older adults (Buman et al., 2010; Woodcock, Franco, Orsini, & Roberts, 2011). Walking is the most common physical activity of older adults with and without dementia (Sun, Norman, & While, 2013; Taraldsen, Chastin, Riphagen, Vereijken, & Helbostad, 2012). Walking is a relatively safe form of activity even for older adults with impaired cognitive and physical function (Lautenschlager et al., 2008; Murtagh, Murphy, & Boone-Heinonen, 2010), and it requires no special equipment or training. Thus, walking is a practical target for interventions to increase physical activity among older adults.
Walking is associated with a number of positive health and cognitive outcomes in older adults (Abe et al., 2010; Howe, Rochester, Neil, Skelton, & Ballinger, 2011; Weuve et al., 2004). Walking interventions in community-dwelling older adults have been associated with improved memory among individuals with good adherence to the intervention (van Uffelen, Chinapaw, van Mechelen, & Hopman-Rock, 2008). Prohaska et al. (2009) studied patterns of walking among older adults and found that the community setting in which people walked and intensity of neighborhood walking were associated with cognitive scores. A meta-analysis of exercise intervention studies indicated there are benefits of exercise to physical and cognitive function even among individuals with existing cognitive impairment and dementia (Heyn, Abreu, & Ottenbacher, 2004).
Walking most frequently occurs in one's own neighborhood (Eyler, Brownson, Bacak, & Housemann, 2003) and is highly influenced by characteristics of the physical environment (Van Cauwenberg et al., 2011). Walking for exercise and transportation purposes is associated with a number of neighborhood characteristics including availability of sidewalks, accessibility of desirable destinations, aesthetic attributes, and perceptions of safety from traffic or crime (Owen, Humpel, Leslie, Bauman, & Sallis, 2004). Though recent research points to the importance of neighborhood walkability on physical activity (Frank, Engelke, & Schmid, 2003; Van Dyck et al., 2012), little of this work has focused on older adults with or without AD.
The ecology theory of aging (Lawton, 1986) suggests that personal competence, including physical and cognitive functioning, interacts with the characteristics of the physical environment, including neighborhood characteristics, to determine an individual's optimal level of functioning. The more disability an individual faces, the greater the impact of the environment on that individual. Therefore, it is imperative to build our evidence base on the relationship between the environmental determinants and neighborhood characteristics to increase activity in older adults and understand the pathways leading to poor health outcomes.
Although a number of studies have considered the relationship of “neighborhood walkability” to health outcomes (Brown et al., 2009; Marshall, Brauer, & Frank, 2009; Owen et al., 2011), there is currently no single, universal definition of how this concept should be measured or of what individual components it is comprised (Glicksman, Ring, Kleban, & Hoffman, 2013). It can be measured by subjective reports, expert evaluation, or objective measures such as geographical mapping data. How it is measured may be important for understanding the mechanisms by which it impacts health outcomes. In the present study, we focus on two particular characteristics that may be related to neighborhood walking behavior, connectivity and integration.
Connectivity is a measure of the number of paths, streets, homes, or businesses directly linked to an individual's home within a defined distance. For example, a farm house on a rural road with no neighbors would have a low connectivity score, while a neighborhood with many homes, streets, walking paths, or businesses would have a high connectivity score. We would expect higher connectivity to be associated with more walking, and thus better health outcomes, because there are a greater number of destinations (paths, streets, homes, businesses) within walking distance. It is limited in that it does not directly measure the number of each type of destination or the desirability of the destinations available to particular individuals.
Integration is a measure of how many turns, or choice points, a person must experience to access all locations in the delimited system. For example, a neighborhood with a grid-like pattern of streets allows fairly direct access from one point to another, and an error at one point may be easily corrected at the next intersection. This would be considered highly integrated. Conversely, a neighborhood with winding roads, dead-ends, and cul-de-sacs requires more convoluted pathways to reach destinations and an error may lead to significant backtracking as there is no readily available means of correction. Integration is a particularly good predictor of movement that has been correlated with movement patterns in several studies (Choi, 2012; Lawton, 1986). We chose integration as it is the feature most commonly associated with cognitive complexity and thus likely to be a unique influence on cognitively impaired individuals. Such indicators of cognitive complexity are not often reported in other papers of neighborhood walkability. The expected effect of integration on walking and health and cognitive outcomes is less clear. Integration theoretically represents the cognitive complexity of reaching a destination within a given neighborhood (Long, Baran, & Moore, 2007; Wang, Zhu, & Mao, 2007). Higher levels of integration might make walking more likely because it is cognitively simpler (e.g., fewer choice points to sequence correctly) and requires the least amount of turns to reach a desired destination, especially among individuals with reduced cognitive capacity. Or, it may have a heavier initial cognitive burden in that there are multiple routes by which to achieve a locomotive goal. However, higher integration might also indicate shorter distances walked, which would be less beneficial for health outcomes associated with walking.
The present study had two aims: (a) to determine how objective measures of two different neighborhood characteristics, connectivity and integration, are related to cognitive function and decline among older adults with and without dementia, and (b) to evaluate whether these characteristics influence older adults without dementia and with dementia in different ways.
Method
Study Participants
Data used in the present analyses were collected from participants enrolled in the Brain Aging Project at the University of Kansas Medical Center with data for tests of cognitive performance at baseline and 2-year follow-up, neighborhood characteristics, and self-reported physical activity. These data were available for 64 participants. In all, 25 participants had early stage AD (Clinical Dementia Rating [CDR] = 0.5 or 1; Morris, 1993), and 39 were healthy older adult controls (CDR = 0). See Table 1 for a summary of participant characteristics. Participants were recruited by self-referral in response to media coverage in the Kansas City metro and word of mouth. Study exclusions include diabetes mellitus (clinical diagnosis, use of an anti-diabetic agent, or 2-hr post-load serum glucose) and unstable ischemic heart disease within the last 2 years as previously described (Burns et al., 2008). Participants were also excluded if they had mobility impairments that would interfere with exercise testing.
Table 1.
Descriptions of Participant and Neighborhood Characteristics (N= 64).
| Healthy controls (n= 39) | Mild AD (n= 25) | |
|---|---|---|
|
| ||
| M (SD) | M (SD) | |
| Participant characteristics | ||
| Age (years) | 74.41 (6.83) | 75.64 (5.69) |
| Education (years) | 16.38 (2.84) | 15.28 (2.01) |
|
| ||
| n(%) | n(%) | |
|
| ||
| Female | 24 (61.5) | 16 (64) |
| Caucaian | 39 (100.0) | 22 (88.0) |
|
| ||
| M (SD) | M (SD) | |
|
| ||
| Neighborhood characteristics | ||
| Integration | 1.13 (0.18) | 1.11 (0.19) |
| Connectivity | 10.56 (13.19) | 12.52 (11.80) |
Note. No statistically significant differences were found between groups on these variables. AD = Alzheimer's disease; SD = standard deviation.
Detailed clinical assessment procedures have been described previously (Burns et al., 2008). Briefly, dementia status of the participant was based on an in-depth clinical evaluation and interview with the participant and a study partner with whom they had regular contact. Participants were classified as having AD if they met established diagnostic criteria: gradual and progressive impairment in memory and at least one other cognitive or functional domain (McKhann et al., 1984). All study procedures were conducted in accordance with the Helsinki Declaration of 1975 and approved in compliance of the ethical standards of the University of Kansas Medical Center Institutional Review Board. Informed consent was obtained for all participants prior to enrollment into the study.
Measures
Neighborhood characteristics and space syntax
We selected this subset of addresses in the Kansas City metro from a larger sample based on observed diversified spatial configurations of the road networks to maximize the variability in the types of neighborhood characteristics. Recognizing that few public health empirical studies have incorporated objective neighborhood environmental measures (Moudon et al., 2006), the study had the aim of limiting itself to publicly available, secondary objective data so as to enhance its transferability to other situations. Using participant addresses, ArcGIS allowed access to map data including roads, sidewalks, and topography and the data were translated into a vector format applicable for space syntax analysis. Space syntax is a set of descriptive techniques for representing, quantifying, and modeling spatial configuration in buildings and settlements. It is a way of researching urban configuration and cities to understand how social and economic processes shape space over time. The methodological innovation of space syntax for analyzing patterns of space (or spatial configuration) in the built environment could uncover spatial structures in cities and therefore relate them to the way people move, stop, and interact (Hillier, 1996; Hillier & Hanson, 1984). It has several numerical measures for describing the configurational attributes of a spatial layout by projecting the mid- and long-term effects of design and planning decisions and therefore allows designers and planners to work with social and economic processes (Frank et al., 2003; Van Dyck et al., 2012). We used a 0.5-mile radius of each address (Qureshi, & Siong, 2012; Vargo, Stone, & Glanz, 2012) and digitally mapped the area to make the objective neighborhood environment compatible with space syntax analysis. We used DepthMap software (Turner, 2007) to generate the axial map (Bafna, 2003) and other syntactic measures.
The fundamental association of spatial configuration is movement. The relationship between the configuration of urban grid and walking or movement densities along the grid can be described by the theory of “natural movement” (Hillier, Penn, Hanson, Grajewski, & Xu, 1993). According to Hillier (1996), movement is largely determined by spatial configuration and dictates the spatial pattern and form of the city. According to Hillier, in any urban system, configuration or urban street network is the primary generator of pedestrian movement patterns and attractors, which work as multipliers on the urban grid. In this context, attractor means shops, residential, commercial, educational, and recreational facilities and even neighborhood public space that generate activities in any urban system. In urban areas, attractors tend to be clustered in specific locations, and they can function both positively or negatively (Hillier et al., 1993). These multiplier effects of attractors influence the natural movement or walkability along the road or grid.
Connectivity
Connectivity is defined as the number of paths, streets, or nodes directly linked to each individual street or node in the road network. It is the number of spatial units directly connected to it or the number of axial lines that connect to or intersect with another axial line in the system (Bafna, 2003; Figure 1). Weighted with a metric of distance, this numerical measure provides an objective measure of accessibility from one point to another (Jiang, Claramunt, & Batty, 1999).
Figure 1. A visual display of neighborhood connectivity calculated using space syntax analysis.
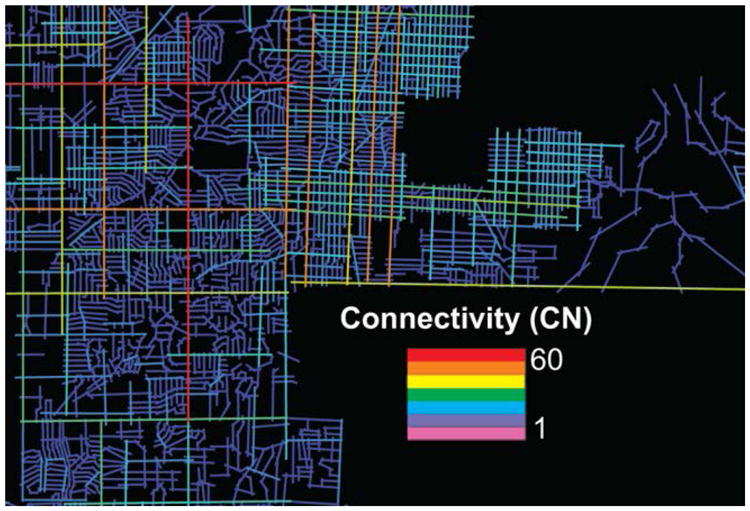
Integration
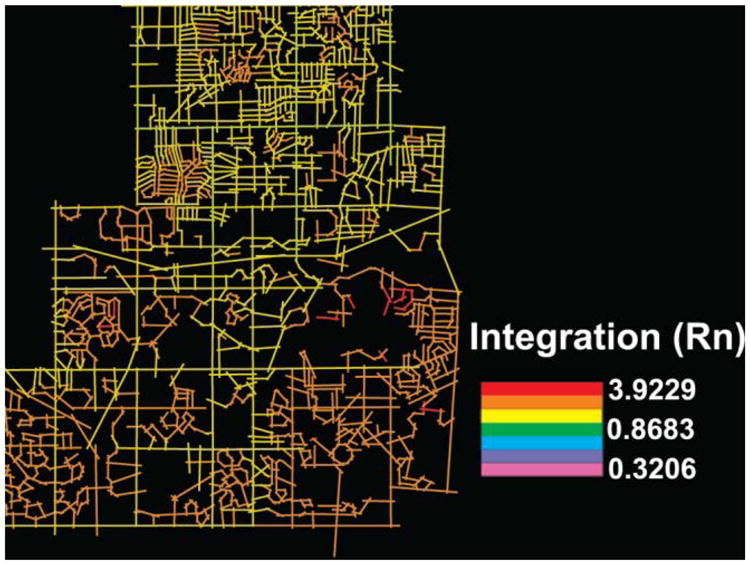
In space syntax, an axial map of a layout is comprised of the fewest number of axial lines needed to get to every space. Integration measures how many turns must be made from a street segment to reach all other street segments in the network, using the shortest paths. The first intersecting segment requires only one turn, the second two turns, and so on. The street segments that require the least amount of turns to reach all other streets are most integrated and are visually represented with vibrant colors. (See Figure 2.) In this way, axes with highest integration values of spatial configuration will be most accessible (Talavera, 2012). The spaces of a system can be ranked from the most integrated to the most segregated. Integration may theoretically represent the cognitive complexity of reaching a street (Long et al., 2007; Wang et al., 2007).
Figure 2. A visual display of neighborhood integration calculated using space syntax analysis.
Self-reported walking
Self-reported physical activity was measured by the Physical Activity Scale for the Elderly (PASE; Washburn, Smith, Jette, & Janney, 1993). The PASE is a commonly used geriatric self-report scale designed to assess weekly physical activity of older individuals. It assesses the frequency and duration of leisure (e.g., walking, sports) and household activities (e.g., housework, gardening) and paid or volunteer employment. As cognitive impairment can compromise information provided by individuals with AD (Wadley, Harrell, & Marson, 2003), each participant enrolled with a study partner knowledgeable about the participant's daily activities. Self-reports were used for the participants without AD and study partner reports for those with AD. We analyzed response for walking frequency and duration.
Cognitive performance and decline
A trained psychometrician administered a standard psychometric battery that included the Mini-Mental State Exam (MMSE), Wechsler Memory Scale (WMS)–Revised Logical Memory I and II, Free and Cued Selective Reminding Task, Wechsler Adult Intelligence Scale (WAIS) letter–number sequencing, digit symbol, and Stroop Color–Word Test (color reading). Higher scores indicate better performance. Cognitive scores were summarized using factor scores established using confirmatory factor analysis (Watts, Loskutova, Burns, & Johnson, 2013) using six cognitive indices. The scores fit a two-factor structure measuring attention and verbal memory (χ2/df = 7/7, comparative fit index [CFI] = .99, root mean square error of approximation [RMSEA] = .03). Attention was indicated by digit symbol, letter–number sequencing, and Stroop interference. Verbal memory was indicated by logical memory, delayed logical memory, and the selective reminding task. Factorial invariance was established across the healthy control and early AD groups and at both baseline and the 2-year follow-up visit (Watts, Loskutova, et al., 2013).
Decline in cognitive performance was estimated by using the follow-up score as the dependent variable, while adjusting for the baseline score. This method is less problematic than subtracting one time point from the other (Wright, 2006).
Statistical analyses
We conducted multiple regression analysis in Mplus version 6 (Muthén & Muthén, 2010) to evaluate our hypotheses including age, sex, and years of education as covariates. We included self-reported walking scores in the model to estimate the effect of walking on cognitive performance. Using Mplus allowed us to estimate measurement error and account for missing data using the full information maximum likelihood algorithm.
Results
Table 1 provides descriptions of participant and neighborhood characteristics. All regression analyses are adjusted for age, sex, and years of education.
Predictors of Self-Reported Walking
In healthy older adult controls, none of the variables included (age, sex, education, integration, connectivity) predicted self-reported walking. These variables together had an R2 = .131 and p = .238 for predicting self-reported walking.
For individuals with mild AD, higher neighborhood integration (β = –.667, p = .022) was associated with lower rates of self-reported walking, though the equation including age, sex, education, and neighborhood connectivity did not account for a significant proportion of R2 = .218, p = .152.
Predictors of Baseline Cognitive Performance
In healthy older adults, higher neighborhood integration and lower neighborhood connectivity predicted poorer baseline MMSE (Table 2). Older age predicted poorer baseline cognitive performance on attention and verbal memory. Self-reported walking did not predict baseline cognitive performance in healthy older adult controls.
Table 2.
Standardized Regression Coefficients for Neighborhood Characteristics Predicting Baseline Cognitive Performance.
| Healthy controls | Mild AD | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Attention (β, p) | Verbal memory (β, p) | MMSE (β, p) | Attention (β, p) | Verbal memory (β, p) | MMSE (β, p) | |
| Neighborhood integration | .178, .344 | .143, .431 | −.449, .019* | .559, .059 | −.280, .413 | .443, .160 |
| Neighborhood connectivity | −.061, .740 | −.005, .979 | .539, .003** | −.231, .358 | .199, .487 | .024, .930 |
| Age (years) | −.509, <.001*** | −.528, <.001*** | −.226, .129 | −.300, .159 | .098, .689 | −.150, .513 |
| Sex (female = 0, male = 1) | −.061, .712 | −.168, .288 | −.231, .176 | −.058, .817 | −.018, .950 | .158, .550 |
| Education (years) | −.039, .804 | .050, .740 | .185, .254 | .495, .002** | .356, .054 | .217, .228 |
| Walking | .150, .321 | .135, .356 | .065, .681 | .297, .101 | −.098, .638 | −.158, .419 |
| R2 | .354, .009** | .405, .002** | .293, .030* | .393, .013* | .210, .165 | .306, .056 |
Note. AD = Alzheimer's disease; MMSE = Mini-Mental State Exam.
p< .05.
p< .01.
p< .001.
In participants with mild AD, neighborhood integration and connectivity were not associated with cognitive performance (Table 2). Higher levels of education were associated with better baseline attention. Self-reported walking was not associated with baseline cognitive performance.
Predictors of 2-Year Cognitive Decline
In healthy older adults, higher levels of neighborhood integration predicted greater declines in attention and verbal memory over the 2-year follow-up period. Higher neighborhood connectivity predicted fewer declines in attention (Table 3). Neighborhood characteristics were not associated with change in MMSE. Self-reported walking was associated with changes in MMSE over 2 years in healthy older adult controls. Higher baseline cognitive performance predicted fewer declines over the 2-year follow-up period in all three cognitive domains.
Table 3.
Standardized Regression Coefficients for Neighborhood Walkability Indicators Predicting 2-Year Cognitive Change.
| Healthy controls | Mild AD | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Change in attention (β, p) | Change in verbal memory (β, p) | Change in MM SE (β, p) | Change in attention (β, p) | Change in verbal memory (β, p) | Change in MM SE (β, p) | |
| Neighborhood integration | −.390, .011* | −.414, .006** | .019 .908 | −.521, .030* | −.183, .241 | −.189, .583 |
| Neighborhood connectivity | .324, .025* | .239, .089 | −.105, .506 | .207, .271 | .246, .073 | .161, .585 |
| Age (years) | −.141, .285 | −.131, .318 | −.113, .329 | .418, .012* | −.066, .532 | −.092, .698 |
| Sex (female = 0, male = 1) | −.093, .475 | .011, .933 | −.359, .007** | .071, .657 | .118, .295 | .036, .887 |
| Education (years) | .039, .753 | −.019, .878 | .220, .077 | −.420, .005** | −.004, .964 | .120, .532 |
| Base line cognitive score | .621, <.001*** | .664, <.001*** | .645, <.001*** | 1.000, <.001*** | .839, < .001*** | .590, .002** |
| Walking | .162, .185 | .125, .293 | −.237, .048* | −.251, .059 | −.051, .539 | .197, .308 |
| R2 | .622, <.001*** | .635, <.001*** | .623, <.001*** | .754, <.001*** | .882, < .001*** | .375, .026* |
Note. AD = Alzheimer's disease; MMSE = Mini-Mental State Exam.
p< .05.
p< .01.
p< .001.
In participants with mild AD, higher neighborhood integration predicted greater declines in attention over the 2-year follow-up (Table 3). Self-reported walking was not significantly associated with changes in cognitive performance in participants with AD.
Discussion
We evaluated the role of objective measures of neighborhood characteristics in cognitive performance and decline among older adults with and without mild AD. Our results suggest that neighborhood integration and neighborhood connectivity are independently and differentially associated with cognitive performance and decline and that this pattern of results differs between individuals with and without AD.
Neighborhood Characteristics and Walking
Neighborhood characteristics were not consistent predictors of self-reported walking. One potential explanation for this is that self-reports of walking, and physical activity generally, have greater degrees of measurement error and recall bias compared with more objective measures (Tudor-Locke & Myers, 2001). Thus, in the prospective extension of our study, we are currently collecting accelerometry data in older adults with and without AD to gather more precise estimates of walking behavior in this population.
Another possible explanation is that the purpose of the walking, for transportation versus for leisure, have been found to relate differently to neighborhood characteristics (Kerr, Rosenberg, & Frank, 2012), and the present retrospective study did not distinguish between different purposes for walking. Neighborhood connectivity may be more essential to walking for transportation (i.e., to a specific destination) than for leisure walking (Kerr et al., 2012). In our prospective extension study, we are collecting data regarding different types of walking and subjective impressions of neighborhood quality and aesthetics, which may be stronger influences in walking for leisure.
It is also possible that the relationship between neighborhood characteristics and cognitive decline is explained by another mechanism, besides walking, that was not measured here. For example, the cognitive complexity of navigating a neighborhood may affect cognitive function via mental visuospatial representations required for driving (Carr, Shead, & Storandt, 2005; Uc, Rizzo, Anderson, Shi, & Dawson, 2004). Connectivity might be associated with a greater number of opportunities to socialize, which could also be important for cognitive function. The finding that neighborhood characteristics predicted cognitive function after adjusting for walking also lends support to the idea that other mechanisms may indeed be at work.
Neighborhood Integration and Connectivity
Neighborhood integration is a measure of the number of turns required to travel between two points. The more direct a path is between two points (e.g., the fewer choice points), the less cognitive complexity required to navigate the route (Long et al., 2007; Wang et al., 2007). However, greater integration may create a greater menu of initial choices that may inhibit self-initiation of a walking route. Among the elderly, walking is a cognitively complex task and gait is correlated with cognitive function (Hausdorff, Yogev, Springer, Simon, & Giladi, 2005). Our results suggest that neighborhoods with higher levels of integration were associated with poorer cognitive function and greater declines in attention and verbal memory over a 2-year period in cognitively intact individuals. In older adults with mild AD, higher integration was associated with greater declines in attention over the 2-year follow-up. We could speculate that greater cognitive complexity required to navigate a neighborhood might discourage people with cognitive impairment from venturing out and walking. For example, topographical disorientation is common in community-dwelling individuals with AD and appears to occur more frequently in individuals who have relocated their place of residence (Pai & Jacobs, 2004). Individuals with disorientation may also require an escort to ensure their safety during outdoor walking activities.
Neighborhoods low in integration have often been designed to reduce through traffic, and therefore in comparison, highly integrated neighborhoods may have greater automobile traffic flow, making walking potentially more risky for those with impairments. One study found that individuals with poorer cognitive functioning were more likely to use indoor venues for walking activities (Prohaska et al., 2009). Thus, neighborhood characteristics such as perceptions of safety or climate may be more important aspects to measure in individuals with AD. Our ongoing prospective study includes these more subjective aspects of neighborhood walkability.
Connectivity is associated with the availability of potential walkable destinations. High intersection densities and connectivity provide more potential routes for walking and greater accessibility. In healthy older adult controls, connectivity was associated with better cognitive function. We did not find evidence that this was because of self-reported walking. Another potential explanation is that greater physical connectivity could indicate more opportunities for social engagement or activity participation (e.g., nearby neighbors, services, business).
To summarize, neighborhoods with greater connectivity, that is, number of paths, streets, homes, and businesses were associated with maintained cognitive function among older adults without cognitive impairment. Neighborhoods with fewer turns required to reach a destination were associated with greater declines in cognitive function among older adults without cognitive impairment. A potential explanation for this is that less direct routes require more cognitive complexity to navigate, perhaps allowing individuals to practice and strengthen cognitive abilities. It may also be that neighborhoods with more potential routes of travel challenge initiation and that this may be the more significant cognitive difficulty compared with sequencing. Cognitive and motivational factors may interact to influence out of home activities especially given increasing cognitive resources required with aging (Wahl et al., 2012).
Limitations
Some limitations of our study include our inability to evaluate the role of retirement communities, often selected for leisure amenities, which may play an important role in the characteristics of both the neighborhoods and the participants involved in the study. From our data, we were unable to illustrate the role of neighborhood characteristics such as poverty, crime, safety, and food insecurity (Chung & Docherty, 2011) on walking and cognitive outcomes. As a secondary data analysis, we were unable to include measures of land mix use or the types of destinations to which residents might walk. Although our sample is well characterized clinically, it is a relatively healthy, educated, and high functioning group of older adults free of diabetes mellitus and ischemic heart disease that may not be generalizable to other groups of older adults. The sample size is small and although we attempted to maximize variability in the neighborhood characteristics studied, we are unlikely to have captured the full range of neighborhood features that may impact walking and cognitive function. Our ongoing prospective study will attempt to replicate these findings in a larger sample. As our data were retrospective, we were unable to measure other potential explanations for the relationships between neighborhood characteristics and cognitive function such as social participation (Richard et al., 2013). Although we cannot infer causality between neighborhood characteristics and cognitive outcomes, the association is present over a period of 2 years even after accounting for potential confounders such as education.
Unique Contributions
Unique contributions of this study include the use of innovative and objective measures of the immediate neighborhood environment using GIS mapping and space syntax to quantify integration and connectivity. Few studies of neighborhood walkability have focused on older adult communities, cognitive outcomes, and individuals with AD. The associations linking objective measures of the physical neighborhood environment with cognitive outcomes suggest that other studies could be similarly enriched by inclusion of physical environment characteristics, which are widely publicly available.
Conclusion and Future Directions
Neighborhood characteristics may be an important determinant of cognitive function and decline in older adults. An important contribution of the present study is the conceptualization of particular neighborhood characteristics, integration and connectivity, that are differentially related to cognitive performance. A recent study suggested that further evaluation of the term “walkability” is needed to better understand its individual components to enable its effective use for research in gerontology (Glicksman et al., 2013). The mechanisms that drive the relationship between neighborhoods and health outcomes may differ by these components as suggested by the differing relationships between connectivity and integration in our study of older adults with and without AD. Neighborhood characteristics may influence health by other mechanisms than walking behaviors, such as social behaviors, driving behaviors, and cognitive complexity of navigating the environment. Our results also demonstrate that these mechanisms may function differently in individuals with cognitive impairment. Our findings have implications for design and maintenance of living spaces for older adults with and without cognitive impairment and may be helpful in eliminating barriers to physical activity in sedentary older adults.
Acknowledgments
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors wish to acknowledge research support from National Institute on Aging as a pilot award from the University of Kansas Alzheimer's Disease Center (NIA 5P30AG035982-3) and from Frontiers: The Heartland Institute for Clinical and Translational Research, University of Kansas Medical Center's CTSA (NCRR #UL1RR033179, now at NCATS #UL1TR000001). The authors acknowledge additional support from the National Institute on Aging (P30AG035982-01A1; R01AG033673-01; R01AG034614-02; R03 AG026374; R21 AG029615), National Institute on Neurological Disorders and Stroke (K23NS058252), and the University of Kansas GCRC/CTSU (M01RR023940). Additional support was received from the University of Kansas Strategic Initiative Award.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abe T, Sakamaki M, Fujita S, Ozaki H, Sugaya M, Sato Y, Nakajima T. Effects of low-intensity walk training with restricted leg blood flow on muscle strength and aerobic capacity in older adults. Journal of Geriatric Physical Therapy. 2010;33:34–40. doi: 10.1152/japplphysiol.01267.2005. [DOI] [PubMed] [Google Scholar]
- Bafna S. Space syntax: A brief introduction to its logic and analytical techniques. Environment & Behavior. 2003;35:17–29. doi: 10.1177/0013916502238863. [DOI] [Google Scholar]
- Brown BB, Yamada I, Smith KR, Zick CD, Kowaleski-Jones L, Fan JX. Mixed land use and walkability: Variations in land use measures and relationships with BMI, overweight, and obesity. Health & Place. 2009;15:1130–1141. doi: 10.1016/j.healthplace.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buman MP, Hekler EB, Haskell WL, Pruitt L, Conway TL, Cain KL, et al. King AC. Objective light-intensity physical activity associations with rated health in older adults. American Journal of Epidemiology. 2010;172:1155–1165. doi: 10.1093/aje/kwq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, et al. Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Shead V, Storandt M. Driving cessation in older adults with dementia of the Alzheimer's type. The Gerontologist. 2005;45:824–827. doi: 10.1093/geront/45.6.824. [DOI] [PubMed] [Google Scholar]
- Caspersen CJ, Pereira MA, Curran KM. Changes in physical activity patterns in the United States, by sex and cross-sectional age. Medicine & Science in Sports & Exercise. 2000;32:1601–1609. doi: 10.1097/00005768-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Choi E. Urban diversity and pedestrian behavior: Refining the concept of land-use mix for walkability; Eighth international space syntax symposium, Pontifical Catholic University of Chile; Santiago, Chile. 2012. [Google Scholar]
- Chung HL, Docherty M. The protective function of neighborhood social ties on psychological health. American Journal of Health Behavior. 2011;35:785–796. doi: 10.5993/ajhb.35.6.14. [DOI] [PubMed] [Google Scholar]
- Eyler AA, Brownson RC, Bacak SJ, Housemann RA. The epidemiology of walking for physical activity in the United States. Medicine & Science in Sports & Exercise. 2003;35:1529–1536. doi: 10.1249/01.mss.0000084622.39122.0c. [DOI] [PubMed] [Google Scholar]
- Frank L, Engelke P, Schmid T. Health and community design: The impact of the built environment on physical activity. Washington, DC: Island Press; 2003. [Google Scholar]
- Giles-Corti B, Donovan RJ. Socioeconomic status differences in recreational physical activity levels and real and perceived access to a supportive physical environment. Preventive Medicine. 2002;35:601–611. doi: 10.1006/pmed.2002.1115. [DOI] [PubMed] [Google Scholar]
- Glicksman A, Ring L, Kleban M, Hoffman C. Is “walkability” a useful concept for gerontology? Journal of Housing for the Elderly. 2013;27:241–254. doi: 10.1080/02763893.2012.754825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: Gait in the elderly as a complex cognitive task. Experimental Brain Research. 2005;164:541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Archives of Physical Medicine and Rehabilitation. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Hillier B. Space is the machine: A configurational theory of architecture. Cambridge, UK: Cambridge University Press; 1996. [Google Scholar]
- Hillier B, Hanson J. The social logic of space. Cambridge, UK: Cambridge University Press; 1984. [Google Scholar]
- Hillier B, Penn A, Hanson J, Grajewski T, Xu J. Natural movement: Or, configuration and attraction in urban pedestrian movement. Environment and Planning B: Planning and Design. 1993;20:29–66. [Google Scholar]
- Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people (review) Cochrane Database of Systematic Reviews. 2011;9(11) doi: 10.1002/14651858.cd004963.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Claramunt C, Batty M. Geometric accessibility and geographic information: Extending desktop GIS to space syntax. Computers, Environment and Urban Systems. 1999;23:127–146. doi: 10.1016/s0198-9715(99)000174. [DOI] [Google Scholar]
- Kerr J, Rosenberg D, Frank L. The role of the built environment in healthy aging community design, physical activity, and health among older adults. Journal of Planning Literature. 2012;27:43–60. doi: 10.1177/0885412211415283. [DOI] [Google Scholar]
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, et al. Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. The Journal of the American Medical Association. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- Lawton MP. Environment and aging. Los Angeles, CA: Brooks Cole; 1986. [Google Scholar]
- Long Y, Baran PK, Moore R. The role of space syntax in spatial cognition: Evidence from urban China. 6th International Space Syntax Symposium; Istanbul, Turkey. 2007. [Google Scholar]
- Marshall JD, Brauer M, Frank LD. Healthy neighborhoods: Walkability and air pollution. Environmental Health Perspectives. 2009;117:1752–1759. doi: 10.1289/ehp.0900595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003–2004. American Journal of Epidemiology. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease Report of the NINCDS‐ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–939. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Moudon AV, Lee C, Cheadle AD, Garvin C, Johnson D, Schmid TL, et al. Lin L. Operational definitions of walkable neighborhood: Theoretical and empirical insights. Journal of Physical Activity & Health. 2006;3:S99. doi: 10.1123/jpah.3.s1.s99. [DOI] [PubMed] [Google Scholar]
- Murtagh EM, Murphy MH, Boone-Heinonen J. Walking: The first steps in cardiovascular disease prevention. Current Opinion in Cardiology. 2010;25:490–496. doi: 10.1097/hco.0b013e32833ce972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén BO, Muthén LK. Mplus (Version 6) Los Angeles, CA: 2010. Author. [Google Scholar]
- Owen N, Humpel N, Leslie E, Bauman A, Sallis JF. Understanding environmental influences on walking: Review and research agenda. American Journal of Preventive Medicine. 2004;27:67–76. doi: 10.1016/j.amepre.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Owen N, Sugiyama T, Eakin EE, Gardiner PA, Tremblay MS, Sallis JF. Adults' sedentary behavior: Determinants and interventions. American Journal of Preventive Medicine. 2011;41:189–196. doi: 10.1016/j.amepre.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Pai MC, Jacobs WJ. Topographical disorientation in community-residing patients with Alzheimer's disease. International Journal of Geriatric Psychiatry. 2004;19:250–255. doi: 10.1002/gps.1081. [DOI] [PubMed] [Google Scholar]
- Prohaska TR, Eisenstein AR, Satariano WA, Hunter R, Bayles CM, Kurtovich E, et al. Ivey SL. Walking and the preservation of cognitive function in older populations. The Gerontologist. 2009;49(S1):S86–S93. doi: 10.1093/geront/gnp079. [DOI] [PubMed] [Google Scholar]
- Qureshi S, Siong HC. Redefining walking distance of operational planned walkable neighborhood in Malaysian cities the case of Putrajaya City. Paper presented at the AESOP 26th Annual Congress, Middle East Technical University (METU); Ankara, Turkey. 2012. [Google Scholar]
- Richard L, Gauvin L, Kestens Y, Shatenstein B, Payette H, Daniel M, et al. Mercille G. Neighborhood resources and social participation among older adults results from the VoisiNuage Study. Journal of Aging and Health. 2013;25:296–318. doi: 10.1177/0898264312468487. [DOI] [PubMed] [Google Scholar]
- Sun F, Norman IJ, While AE. Physical activity in older people: A systematic review. BMC Public Health. 2013;13:449. doi: 10.1186/1471-2458-13-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera R. Improving pedestrian accessibility to public space through space syntax analysis. Paper presented at the Eighth International Space Syntax Symposium, Pontifical Catholic University of Chile; Santiago, Chile. 2012. [Google Scholar]
- Taraldsen K, Chastin SF, Riphagen II, Vereijken B, Helbostad JL. Physical activity monitoring by use of accelerometer-based body-worn sensors in older adults: A systematic literature review of current knowledge and applications. Maturitas. 2012;71:13–19. doi: 10.1016/j.maturitas.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke CE, Myers AM. Challenges and opportunities for measuring physical activity in sedentary adults. Sports Medicine. 2001;31:91–100. doi: 10.2165/00007256-200131020-00002. [DOI] [PubMed] [Google Scholar]
- Turner A. UCL Depthmap 7.12. 2007 Retrieved from http://www.vr.ucl.ac.uk/depthmap/
- Uc EY, Rizzo M, Anderson SW, Shi Q, Dawson JD. Driver route-following and safety errors in early Alzheimer disease. Neurology. 2004;63:832–837. doi: 10.1212/01.wnl.0000139301.01177.35. [DOI] [PubMed] [Google Scholar]
- Van Cauwenberg J, De Bourdeaudhuij I, De Meester F, Van Dyck D, Salmon J, Clarys P, Deforche B. Relationship between the physical environment and physical activity in older adults: A systematic review. Health & Place. 2011;17:458–469. doi: 10.1016/j.healthplace.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Van Dyck D, Cerin E, Conway TL, De Bourdeaudhuij I, Owen N, Kerr J, et al. Sallis JF. Associations between perceived neighborhood environmental attributes and adults' sedentary behavior: Findings from the USA, Australia and Belgium. Social Science & Medicine. 2012;74:1375–1384. doi: 10.1016/j.socscimed.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomized controlled trial. British Journal of Sports Medicine. 2008;42:344–351. doi: 10.1136/bjsm.2007.044735. [DOI] [PubMed] [Google Scholar]
- Vargo J, Stone B, Glanz K. Google walkability: A new tool for local planning and public health research? Journal of Physical Activity & Health. 2012;9:689–697. doi: 10.1123/jpah.9.5.689. [DOI] [PubMed] [Google Scholar]
- Wadley VG, Harrell LE, Marson DC. Self- and informant report of financial abilities in patients with Alzheimer's disease: Reliable and valid? Journal of the American Geriatric Society. 2003;51:1621–1626. doi: 10.1046/j.1532-5415.2003.51514. [DOI] [PubMed] [Google Scholar]
- Wahl HW, Wettstein M, Shoval N, Oswald F, Kaspar R, Issacson M, Heinik J, et al. Interplay of cognitive and motivational resources for out-of-home behavior in a sample of cognitively heterogeneous older adults: Findings of the SenTra project. The Journals of Gerontology, Series B: Psychological Sciences & Social Sciences. 2012;68:691–702. doi: 10.1093/geronb/gbs106. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhu Q, Mao Q. Integration may theoretically represent the cognitive complexity. Paper presented at the 6th International Space Syntax Symposium; Istanbul, Turkey. 2007. [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. Journal of Clinical Epidemiology. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Watts AS, Loskutova N, Burns JM, Johnson DK. Metabolic syndrome and cognitive decline in early Alzheimer's disease and healthy older adults. Journal of Alzheimer's Disease. 2013;35:253–265. doi: 10.1016/j.jalz.2012.05.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AS, Vidoni ED, Loskutova N, Johnson DK, Burns JM. Measuring physical activity in older adults with and without early stage Alzheimer's disease. Clinical Gerontologist. 2013;36:356–374. doi: 10.1080/07317115.2013.788116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. Journal of the American Medical Association. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Woodcock J, Franco OH, Orsini N, Roberts I. Non-vigorous physical activity and all-cause mortality: Systematic review and meta-analysis of cohort studies. International Journal of Epidemiology. 2011;40:121–138. doi: 10.1093/ije/dyq104. [DOI] [PubMed] [Google Scholar]
- Wright DB. Comparing groups in a before–after design: When t test and ANCOVA produce different results. British Journal of Educational Psychology. 2006;76:663–675. doi: 10.1348/000709905x52210. [DOI] [PubMed] [Google Scholar]


