Abstract
WT1126 (RMFPNAPYL) is a human leukocyte antigen-A2 (HLA-A2) restricted peptide derived from Wilms tumor protein (WT1), which is widely expressed in a broad spectrum of leukemias, lymphomas and solid tumors. A novel T-cell-receptor (TCR)-like single chain variable fragment (scFv) antibody specific for the T cell epitope consisting of the WT1/HLA-A2 complex was isolated from a human scFv phage library. This scFv was affinity-matured by mutagenesis combined with yeast display, and structurally analyzed using a homology model. This monovalent scFv showed a 100-fold affinity improvement (dissociation constant [KD]= 3nM) and exquisite specificity towards its targeted epitope or HLA-A2+/WT1+ tumor cells. Bivalent scFv-huIgG1-Fc fusion protein demonstrated an even higher avidity (KD = 2pM) binding to the T cell epitope and to tumor targets, and was capable of mediating antibody-dependent cell-mediated cytotoxicity or tumor lysis by chimeric antigen receptor (CAR)-expressing human T or NK-92-MI transfected cells. This antibody demonstrated specific and potent cytotoxicity in vivo towards WT1-positive leukemia xenograft that was HLA-A2 restricted. In summary, T cell epitopes can provide novel targets for antibody-based therapeutics. By combining phage and yeast displays and scFv-Fc fusion platforms, a strategy for developing high affinity TCR-like antibodies could be rapidly explored for potential clinical development.
Keywords: TCR-like antibodies, HLA-A2, WT1, chimeric antigen receptors, yeast display
Introduction
Major histocompatibility complex (MHC) class I molecules play a central role in the surveillance of aberrant or foreign proteins within cells. Peptides derived from endogenous proteins fill the MHC class I pockets and are recognized by T cell receptors (TCRs) on CD8(+) T lymphocytes.1, 2 These MHC class I complexes are constitutively expressed by all nucleated cells. In cancer, virus-specific or tumor-specific peptide/MHC complexes represent a unique class of cell surface targets for immunotherapy, with promise in vaccine research,3 adoptive cell therapy,4 and more recently, TCR-like antibodies.5, 6
Although soluble TCRs have been successfully developed to target T cell epitope on tumors, their inherent low affinity has limited their potential as therapeutics.7 Even more importantly, the low density of MHC molecules and of the individual displayed peptides put further constraints on low-affinity TCRs.8 Attempts to affinity mature TCRs were sometimes hampered by cross-reactivity.9, 10 Monoclonal antibodies are now an accepted modality in cancer treatment. Yet, most of these antibodies have targeted surface antigens whose repertoire on solid tumors is limited. TCR-like antibodies, with high affinity and controlled specificity, could be ideal therapeutics.5, 11 To this end, several TCR-like antibodies targeting peptides derived from viral or tumor antigens in the context of human leukocyte antigen(HLA)-A1 or HLA-A2 have been reported.12–18 In addition to their therapeutic values, they are desirable for characterizing specific peptide presentation on malignant and infected cells.
One of the most studied tumor associated antigens has been the product of the Wilms tumor gene 1, which encodes a zinc-finger transcription factor (Wilms tumor protein 1; WT1) important in cell growth and differentiation.19 In a tissue-specific manner, it is expressed mainly in the urogenital system of the developing embryo as well as the central nervous and hematopoietic systems in adults.20 In its aberrant state, WT1 expression is found in leukemias, lymphomas and solid tumors including astrocytic tumors, sarcomas, breast, lung and colorectal cancer, and neuroblastoma,20, 21 with the characteristics of an oncogene.22 Several peptides derived from endogenous WT1 protein are presented in the context of MHC class I molecules and are immunogenic.1, 23 The 9-mer WT1-derived peptide 126–134, RMFPNAPYL (WT1126), is the most extensively studied.24, 25 As vaccines, the WT1126 peptide induced a durable WT1-specific cytotoxic T cell (CTL) response in patients with acute myeloid leukemia (AML).3, 26
To develop a reagent with diagnostic or even therapeutic potential, we first isolated a novel TCR-like antibody against WT1126/HLA-A2 from a human scFv phage-displayed library. This anti-WT1126/HLA-A2 scFv was affinity matured by yeast display selection. By using the scFv-Fc fusion protein and the scFv-chimeric antigen receptor (CAR) platforms, the therapeutic potential of this TCR-like antibody was tested in vitro and in xenograft models.
Materials and Methods
Human lymphocytes and tumor cell lines
Human peripheral blood mononuclear cells (PBMC) were isolated from whole blood by ficoll Hypaque density gradient separation. T cells were purified by negative magnetic separation using magnetic beads containing antibodies against CD19, CD20, CD14, CD56 (Pan T-cell isolation kit, Miltenyi Biotech). LAN-1 tumor cells were obtained from Children’s Hospital Los Angeles. JN-DSRCT tumor cells were obtained from Fukuoka University, Japan. Tap-deficient HLA-A2+ T2 cells, NK-92-MI and all other cell lines used were purchased from ATCC or developed at Memorial Sloan Kettering Cancer Center. Cells were cultured in RPMI 1640 with 2 mM L-glutamine and 10% fetal bovine serum (FBS). All cell lines have been tested and authenticated by short tandem repeat profiling using PowerPlex 10 System (Promega) and tested for mycoplasma contamination. NK-92-MI cells and genetically CAR modified NK-92-MI cells were propagated in Alpha Minimum Essential medium with 2 mM L-glutamine, 12.5% horse serum and 12.5% fetal bovine serum.
MHC-peptide complexes
Using peptides synthesized by Genscript, biotinylated soluble MHC class I-peptide complexes were generated by the Tetramer facility at Memorial Sloan Kettering Cancer Center (MSKCC), and the phycoerythrin (PE) conjugated MHC/peptide tetramers were obtained from the National Institutes of Health Tetramer Core Facility (Emory University, Atlanta, GA). The specific WT1 peptide used was RMFPNAPYL (WT1126); control peptides included: (1) NLVPMVATV derived from pp65 of human cytomegalovirus, (2) RIITSTILV derived from HUD (alias ELAVL4, embryonic lethal, abnormal vision, drosophila-like 4), (3) LLEEMFLTV from cerebellar degeneration-related protein 2 (CDR2), (4) SLGEQQYSV and (5) CMTWNQMNL derived from WT1, (6) LMLGEFLKL derived from Survivin, and (7) FLTPKKLQCV derived from prostate specific antigen PSA.
Phage display selection
The Tomlinson I + J human scFv phage display libraries,27 containing approximately 2.85 × 108 independent scFv clones, were used for selection according to previously published methods with modifications17. Phages were first preincubated with streptavidin paramagnetic Dynabeads (Invitrogen) and unbiotinylated HLA-A2-NLVPMVATV (irrelevant complex). The supernatant (phage and irrelevant complex mixture) was reacted with biotinylated HLA-A2-RMFPNAPYL (WT1126) before capture on fresh Dynabeads (preincubated with 2% milk and washed with PBS). After the final round of panning, the eluted phages were used to infect both TG1 and HB2151 E. coli. TG1 cells were cultured overnight while the HB2151 cells were spread on 2YT plus Ampicillin (100 µg/ml) agar plates.
Mutagenesis by error-prone PCR
Error-prone PCR of the entire scFv gene was performed using Stratagene GeneMorph® II Random Mutagenesis Kit according to the instructions of the manufacturer.
Yeast display selection
Construction of yeast libraries and the protocol for generating and isolating high affinity mutants28 were followed with minor modifications. Briefly, induced yeast library (2 × 109cells) was negatively selected with 10 µg-HLA-A2/ELMLGEFLKL-conjugated magnetic beads for 1 h at RT in PBSA buffer (0.1% BSA in PBS), followed by magnetic separation. The subtracted yeast cells were subsequently incubated with 10 µg-HLA-A2/RMFPNAPYL-conjugated magnetic beads for 3 h at RT in PBSA buffer. The magnetically isolated yeast cells were washed 3 times with PBSA buffer, and added into 10 ml of SDCAA yeast media for amplification overnight in a 30°C shaker with 250 rpm. The amplified yeast cells were induced in SG/RCAA media for 18 h at 20°C shaker with 250 rpm. With 3consecutive fluorescence-activated cell sorting (FACS) selections, yeast cells were sorted at100, 33 and 10 µg/ml biotinylatedHLA-A2/RMFPNAPYL, respectively, each time with sorting gates set for yeasts cells with high binding signals.
Expression and purification of soluble scFv and scFv-Fc
The soluble scFv was expressed and purified as previously described29, 30.The scFv-Fc variant genes were synthesized for CHO cells (Genscript), transfected into CHO-S cells and selected with G418 (Invitrogen).31 High expression clones were selected for culture in Opticho serum free medium (Invitrogen),17 and scFv-Fc protein purified using MabSelect affinity chromatography (GE Healthcare). After concentration with a 50,000 MWCO Vivaspin centrifuge tube (Sartorius Stedim), the scFv-Fc was tested for binding by ELISA or by flow cytometry on FACS Calibur (BD Biosciences) using peptide-loaded T2 cells.
ELISA
The specificity of individual phage clones, soluble scFv and scFv-Fc antibodies was assessed by ELISA at RT with indirectly coated HLA-A2/peptide complexes, where the bound scFv or scFv-Fc was detected using a HRP-conjugated anti-Flag tag antibody or HRP-conjugated anti-human Fc antibody, respectively.17
Surface plasmon resonance
Kinetics and affinities of various antibodies and WT1126/HLA-A2 were analyzed by surface plasmon resonance using Biacore T100 (GE healthcare). Biotinylated WT1126/HLA-A2 was captured by streptavidin-fusion protein on a sensor chip (CM5). A control reference surface was prepared for nonspecific binding and refractive index changes. For analysis of the kinetics of interactions, varying concentrations of antibodies were injected at a flow rate of 30 µl/min using a running buffer containing 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.05% Surfactant P-20 (pH 7.4). The association and dissociation phase data were fitted simultaneously to a 1:1 model by using BIAevaluation 3.2. All the experiments were done at 25°C.
Flow cytometric analysis
T2 cells were harvested and incubated in serum-free IMDM containing 20–25 µg/ml β2-microglobulin (β2M) in the presence of 40 µM or less of WT1126 peptide or control peptides at 37°C for 5 h, washed and reacted with purified scFv-Fc (human Fc) for 30 min on ice, and after further washing, incubated with PE-conjugated goat anti-Fc specific antibody (Jackson ImmunoResearch Laboratories). For epitope mapping, T2 cells were incubated with either the wild type or alanine substituted WT1126 peptides at 37°C overnight before reaction with the APC-conjugated goat anti-Fc antibody (Jackson ImmunoResearch Laboratories). The same method was used to determine the binding of the antibodies to tumor cell lines: first scFv-Fc, then PE-conjugated goat anti-Fc secondary antibody. For CAR modified cells, fluorescent antibodies for surface staining were purchased from BD Biosciences. CAR expression on CD4(+) and CD8(+) T cells was analyzed using MHC/peptide tetramer, while for NK-92-MI, PG-13 and K562 cells, both MHC/peptide tetramer and the reporter GFP were used.
Generation of HLA-A2/WT1126-specific CAR construct
CAR expression vector was obtained from Dr. Dario Campana at St. Jude Children’s Research Hospital.32 The scFv sequences were fused in-frame to the scFv-4-1BB-CD3ζ DNA (Genscript). In the construct, the CAR gene was under a CMV promoter, followed by IRES-GFP. The CAR gene was inserted into the expression vector for transformation into E.coli, plated on LB plus Ampicillin (100 µg/ml). Once the sequences were validated, the DNA was packaged into retrovirus and used to infect human T cells, K562 or NK-92-MI cells.
Retroviral production and transduction
For T-cell or K562 transduction, vector DNA was transfected into H29 packaging cells in the presence of CaCl2. Viral supernatant was collected for two consecutive days to be stored, or to transfect the packaging cell line PG-13. PG-13 cells expressing the transduced vector DNA were sorted using GFP as the selection marker, cloned and expanded, and culture supernatants collected for T-cell transduction. Purified T-cells were first stimulated with CD3/CD28 beads for 24 hours. PG-13 viral supernatant was added to retronectin coated plates, followed by T-cells or K562 cells. The plates were spun down and incubated for 48 h. Cells expressing the transduced vector were detected using GFP and the WT1126/HLA-A2-PE-labeled tetramer by FACS.
For transduction of NK-92-MI cells, the following procedure was employed which used a 293T-based retroviral production cell line (GP2) as previously described.17
Cytotoxicity Assay
Antibody-dependent cell-mediated cytotoxicity (ADCC) assays were performed using NK-92-MI cells stably transfected with the human CD16 Fc receptor as previously described.31 The cytolytic capacity of T-cells or NK-92-MIcells was tested against HLA-A2/WT1+ tumor cell lines as well as autologous EBV-BLCL loaded with the WT1126 peptide using the standard 51Cr release assay.17 Alloreactivity was assessed using HLA mismatched EBV-BLCL, and NK like activity was evaluated against the erythroleukemia cell line K562 lacking the expression of HLA but with high expression of WT1.
Molecular Modeling
Molecular modeling, energy calculations, docking simulations, and image renderings were done using Discovery Studio 4.0 (Accelrys, San Diego, CA) or Pymol (Schrodinger LLC, New York, NY). A homology model of the anti-WT1-HLA-A2 scFv antibody was built using pdb structure of the anti-SARS scFv antibody from pdb 2GHW as a template (68% sequence identity). Each CDR loop was then refined using additional homologous templates shown in parentheses: L1 (2BX5, 1RZI, 2UZI), L2 (2VH5, 2UZI, 2BX5), L3 (2BX5, 3NCJ, 2FGW), H1 (2QQN, 1H3P, 3QOS), H2 (2QQN, 3SKJ, 3SOB), and H3 (1MRD, 1MRE, 1MRC). The final model underwent 2 nanoseconds of molecular dynamics simulation to reach a low energy conformation for use in docking simulations. Docking simulations were run using the energy minimized homology model of ZDOCK with anti-WT1-HLA-A2 scFv and the crystal structure of HLA-A2-WT1-RMF (pdb 3HPJ).
Therapy of human leukemia xenograft models
All animal procedures were performed in compliance with Institutional Animal Care and Use Committee (IACUC) guidelines. Two million BV173 human leukemia cells were injected intravenously into Rag2(−/−)gammaC(−/−) double knockout (DKO) male mice (7–10 weeks in age). On day 6, tumor engraftment was confirmed by luciferase imaging in all mice that were to be treated. Mice were then assigned to treatment and control groups by simple randomization (n = 5). Sample size was sufficient to detect significant differences between groups using log-rank Mantel-Cox test. Antibodies (50 µg/mouse) were administered intravenously twice a week for a total of 4 doses, in a non-blinded manner. In animals that also received human effector cells with or without antibodies, PBMC from healthy donors were injected intravenously into mice (10 millions cells per mouse) on day 7 and 14, as well as cytokine IL15/IL15Rα complex (10 µg per subcutaneous injection). Tumor growth was assessed by bioluminescence imaging at least once a week.
Results
Selecting for human scFvs specific for HLA-A2/WT1126 using phage display
With the assumption that TCR-like antibodies are under-represented in a mature B-cell library,5 we chose the recombinant “Tomlinson I + J” human scFv library for phage display. After negative selection against streptavidin beads and the HLA-A2/pp65 control peptide, and removing those clones which were cross-reactive with HLA-A2or irrelevant recombinant HLA-A2/peptide complexes, 48 clones were isolated of which three individual scFv clones were specific for the HLA-A2/WT1126 complex. All three clones shared identical DNA sequence, designated as Clone45 (Supplementary Table S1). By ELISA Clone45 scFv was specific for HLA-A2/WT1126, and by flow cytometry only reactive with T2 cells loaded with the WT1126 peptide (Supplementary Figure S1).
Affinity maturation of scFv using yeast display
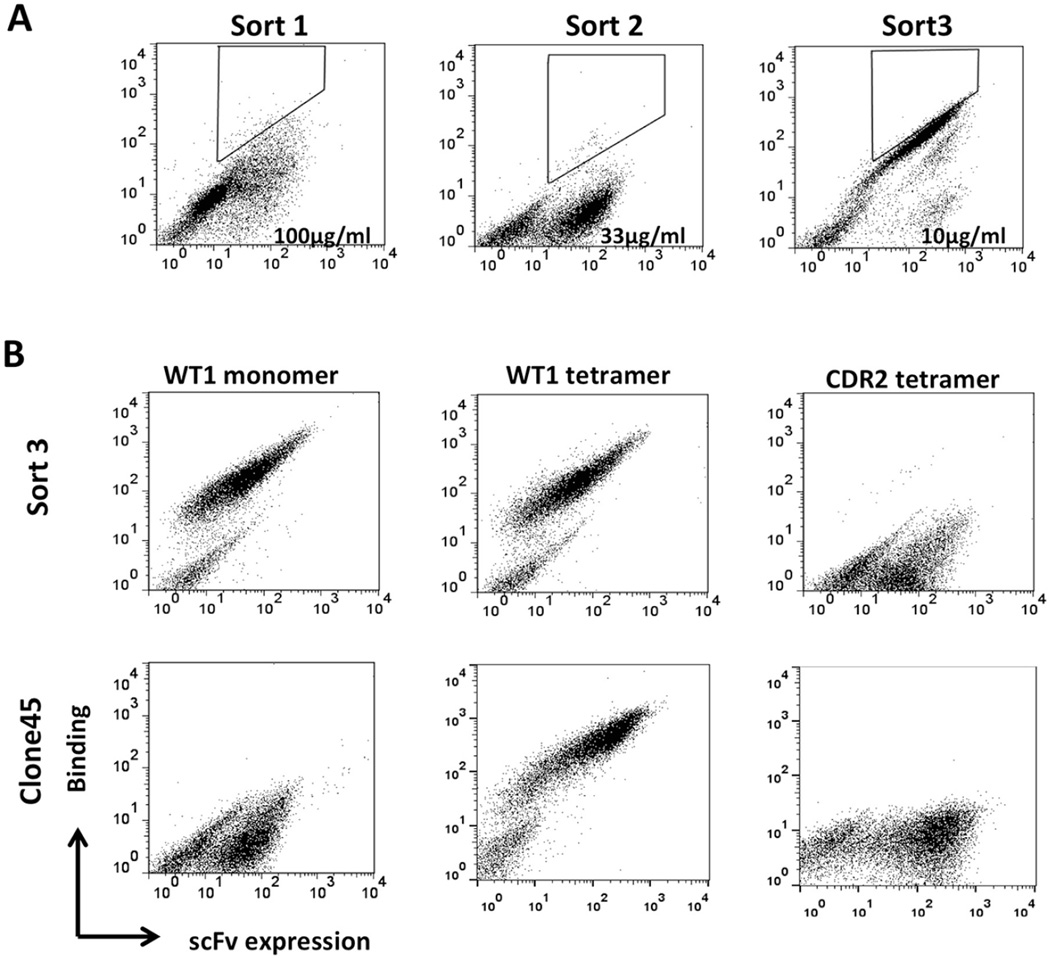
By Biacore, the binding affinity of scFv Clone45 (KD=300 nM) was low (Table 1).33, 34 To affinity mature Clone45, we created a randomly diversified libraries, comprised of scFv mutants with low (<5/1000 bp), moderate (5–9/1000 bp), and high (>9/1000 bp) mutation rates, displayed on yeast cells by homologous recombination using a vector containing a C-terminal Aga2 protein and c-myc tag.35 The final library (5 × 108 independent clones) was first enriched using HLA-A2/WT1126 conjugated magnetic beads, followed by sequential FACS sorting based on stringent mean fluorescence intensity (MFI) for specific binding to HLA-A2/WT1126 (Figure 1a) but not to irrelevant HLA-A2/CDR2-derived peptide (LLEEMFLTV) (Figure 1b).
Table 1.
Binding kinetics and affinities of scFvs or scFv-Fc by Biacore
| Antibodies | kon (M−1s−1) | koff (s−1) | KD(nM) |
|---|---|---|---|
| Clone45 scFv | 2.73E+05 | 7.18E−02 | 263 |
| S3.1 scFv | 1.48E+05 | 1.91E−03 | 12.9 |
| S3.3 scFv | 2.20E+04 | 5.35E−05 | 2.43 |
| S3.6 scFv | 1.26E+05 | 1.77E−03 | 14.1 |
| Q1L scFv | 9.43E+04 | 5.50E−03 | 58.3 |
| Q2L scFv | 1.15E+05 | 3.55E−04 | 3.08 |
| Q2L scFv-Fc | 4.84E+05 | 1.15E−06 | 0.002 |
Figure 1. FACS for yeast display selection.
(a) Sorting of yeast mutant library. Yeast library was labeled with mouse anti-c-myc antibody followed by fluorescent goat anti-mouse antibody, as well as biotinylated HLA-A2-WT1 monomer followed by fluorescent streptavidin (SA). During the three FACS selections, yeast cells were stained with decreasing concentrations of biotinylated HLA-A2-WT1 monomer at 100µg/ml (sort1), 33µg/ml (sort2) and 10µg/ml (sort3), respectively. Each time, the brightest 0.1–0.3% cells were selected. (b) The binding and specificity of selected scFv-displayed yeast cell. Yeast cells from the 3rd round selection (the first row) or parental Clone45 (the second row) were stained with biotinylated HLA-A2-WT1 monomer (1st column) followed by fluorescent Streptavidin, PE conjugated HLA-A2-WT1 tetramer (2nd column), or the negative control (HLA-A2-CDR2) tetramer (3rd column). The x axe represents scFv expression. The y axe represents the monomer or tetramer binding.
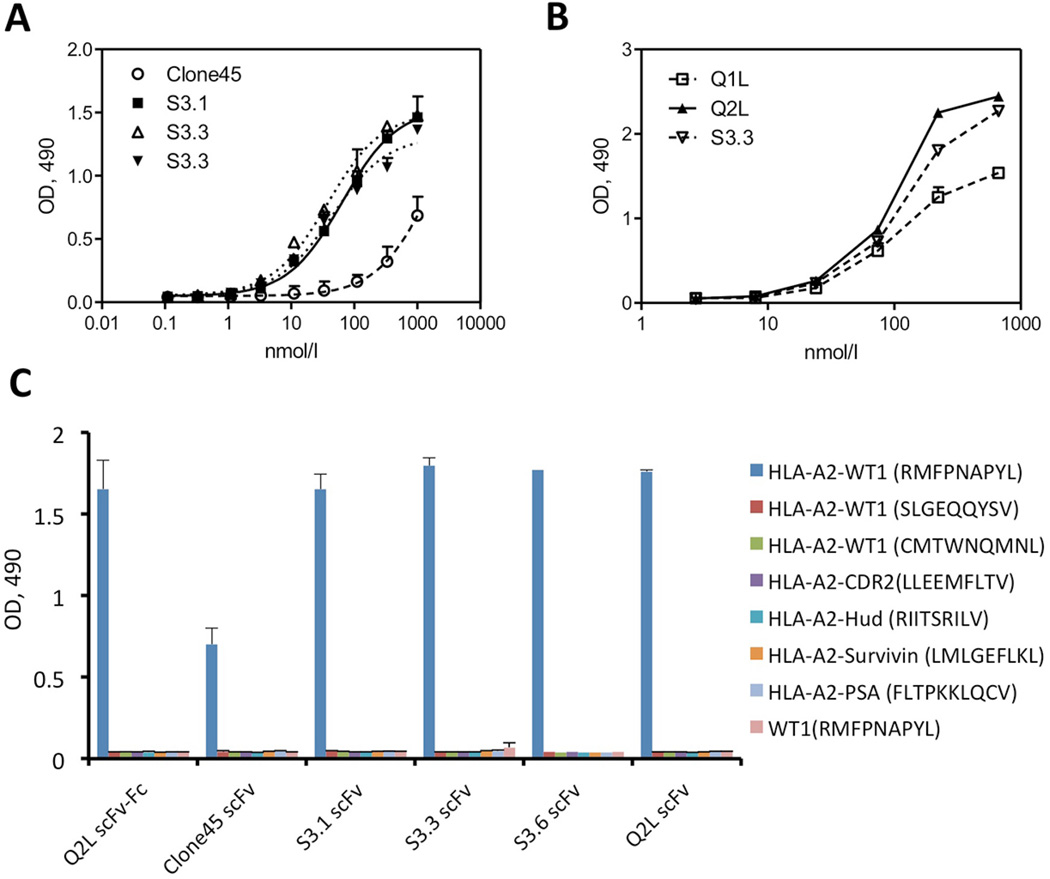
When highest affinity clones from the final round of sorting were sequenced, 4 recurrent scFv sequences (S3.1, S3.3, S3.6 and S3.22) were identified. Compared with parental clone Clone45, the most dominant mutations contained 9 amino acid substitutions in the variable regions of the heavy chain and light chains (Supplementary Table S2). All three scFv (S3.1, S3.3 and S3.6) exhibited a stronger binding signal than parental scFv Clone45 at all concentrations by ELISA on HLA-A2/WT1126 complex (Figure 2a), while maintaining specificity (Figure 2c). As shown in Table 1, the three scFvs (S3.1, S3.3 and S3.6) bound HLA-A2/WT1126 monomer with dissociation constants (KD) of 13 nM, 2.4 nM and 14 nM, respectively, compared to KD = 263 nM of parental Clone45. With a KD of 2.4 nM, scFv S3.3 exhibited the highest improvement in binding affinity of nearly 100-fold, with a significantly prolonged dissociation time (Supplementary Figure S2).
Figure 2. ELISA of scFv variants and Q2L scFv-Fc against HLA-A2-peptide monomers.
(a) Three scFvs (S3.1, S3.3 and S3.6) from the FACS sorting and parental Clone45 scFv were serially diluted and tested for binding to wells coated with HLA-A2-WT1 (RMFPNAPYL) monomer. (b) Q1L (single mutation), Q2L (double mutations) and S3.3 scFvs were serially diluted and added to wells coated with HLA-A2-WT1 (RMFPNAPYL) monomer. (c) The S3.1, S3.3, S3.6, Q2L, parental scFvs and Q2L scFv-Fc were serially diluted and added to wells coated with WT1 peptide (RMFPNAPYL), three types of HLA-A2-WT1 monomers and four irrelevant HLA-A2 monomers. Bound scFv or scFv-Fc were detected with an HRP-conjugated anti-Flag tag antibody or HRP-conjugated anti-human Fc antibody and the optical densities (O.D.) at 490 nm measured by Dynex MRX Microplate reader. For panels (a), (b) and (c), all samples were prepared in duplicate, and experiments were repeated 1–2 times with similar results. Values are shown as mean ± SD.
Identifying crucial amino acid positions for affinity maturation of TCR-like antibodies
For affinity maturation the identification of key residues as the interaction of antibody and its antigen was crucial.9, 36 The crystal structure of WT1126 bound to HLA-A2 at 2 Å resolution has revealed the usual architecture of class I MHC/peptide complexes.25 TCR-like antibodies are known to recognize MHC-bound peptides either by contacting the peptide directly, as a TCR usually does, or by recognizing a unique conformation of the MHC protein bound to a particular peptide.37 TCR generally recognizes the extended conformation characterized by a bulge at proline (P) and asparagine (N) at residues 4 and 5, respectively of the WT1126 peptide.25 The structure of the scFv Clone45 was generated using homology modeling. The CHARMm force field was then used to perform energy minimizations and molecular dynamic simulations of the structure. The alignment of 4 scFv mutants (Supplementary Table S2) suggests Q50L in the heavy chain as the first critical position for affinity maturation. Q53L in the light chain of the best mutant (S3.3) was the second critical position. Based on homology modeling, these two glutamine residues located in CDR2 regions of heavy and light chains, respectively, were involved in antigen recognition.
Binding properties and specificity of TCR-like antibodies
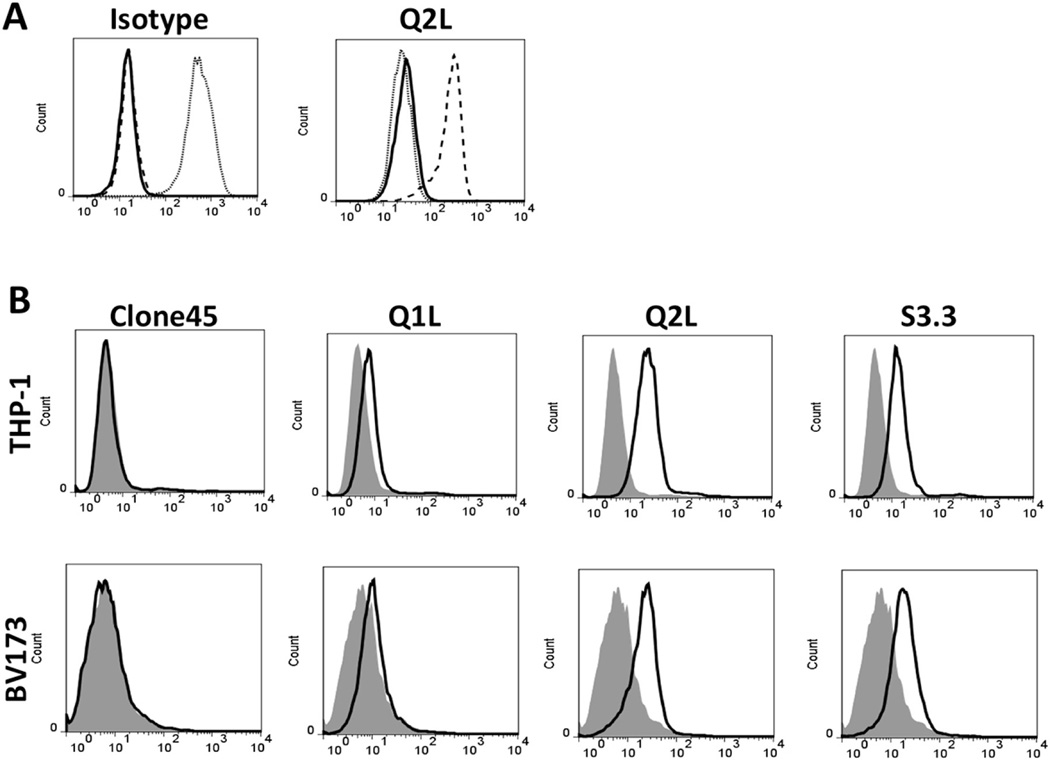
To confirm our predicted “hotpots”, Q1L with VH-Q50L mutation and Q2L with VH-Q50L/VL-Q53L mutations were created. Q2L exhibited an equivalent binding by ELISA to S3.3 (scFv mutant with the highest affinity), whereas scFv Q1L was weaker (Figure 2b). By Biacore, Q2L showed comparable affinity (KD =3 nM) to S3.3 (KD=2.4 nM) while Q1L was inferior (KD = 58 nM) (Table 1). When reshaped into a scFv-Fc fusion protein, Q2L showed an even higher apparent affinity (2 pM). By ELISA (Figure 2c), Q2L as scFv or scFv-Fc maintained its specificity, showing no cross-reactivity with WT1187-195 (SLGEQQYSV), 235–243(CMTWNQMNL),or with the WT1 126–134 (RMFPNAPYL) peptide itself. Specificity was further confirmed by flow cytometry of peptide loaded T2 cells stained with Q2L (Figure 3a).
Figure 3. Binding of TCR-like antibodies to WT1/HLA-A0201 complexes on live cells measured by flow cytometry.
(a) Binding of Q2L scFv-Fc (right) and isotype matched TCR-like scFv-Fc (left) to T2 cells pulsed with WT1 peptide (dashed line), without peptide (solid line), or with irrelevant peptide(dotted line). T2 cells were then stained with TCR-like antibodies at 1µg/ml, followed by fluorescent secondary antibody. (b) Recognition of the naturally presented WT1/HLA-A2 complex on tumor cells by scFv variants. The human leukemia cells, THP-1 and BV173, were stained with scFvs at 10µg/ml, followed by fluorescent secondary antibody. Experiments from panel (a) and (b) were repeated 1–2 times with similar results.
Q2L showed staining of human tumor cell lines positive for both HLA-A2 and WT1, but not to cell lines that were either HLA-A2(−) or WT1(−) (Supplementary Table S3).The intensity of staining correlated with the expression level of HLA-A2 molecule. Cell lines that were genotypically positive for HLA-A2 with little HLA-A2 expression were also negative for binding to Q2L. When the staining with Clone 45 (low affinity), Q1L (modest affinity), Q2L or S3.3 (high affinity) was compared against WT1/HLA-A2 positive leukemia cell lines, MFI correlated with antibody affinities (Figure 3b).
To check for potential cross-reactivity of the affinity matured Q2L versus clone 45 for normal cells, we stained both HLA-A2[+] and HLA-A2[−] PBMC (Supplementary Table S4). No increase in staining of the Q2L was observed with HLA-A2[−] cells. However there was a modest elevation in Q2L staining of HLA-A2[+] PBMC relative to clone 45.
Epitope Mapping
To confirm the precise molecular epitope of the Q2L scFv, we used both in silico docking simulations and experimental binding with alanine-substituted WT1126 peptides (Supplementary Figure S3). For in silico modeling, a homology model of Q2L scFv was docked onto the known crystal structure of HLA- HLA-A2/WT1126. The top docked pose (Supplementary Figure S3a) revealed that the binding epitope involved the interaction of the heavy chain CDR2 of the Q2L scFv with Tyr 8 of WT1126. The mutation VH-Q50L enhanced this interaction at this site. The model showed that the second mutation VL-Q53L enhanced the interaction of Q2L with the helical peptide-binding cleft of the HLA molecule. We verified the predicted epitope with binding experiments using WT1126 peptides substituted with alanine at positions 1, 3, 4, 5, 7 and 8 (Supplementary Figure S3b). T2 cells were pulsed with these peptides and Q2L binding was measured by flow cytometry. Reduced binding was only observed when Tyr8 was mutated to Ala, confirming the epitope.
Antibody-dependent Cell-mediated Cytotoxicity (ADCC)
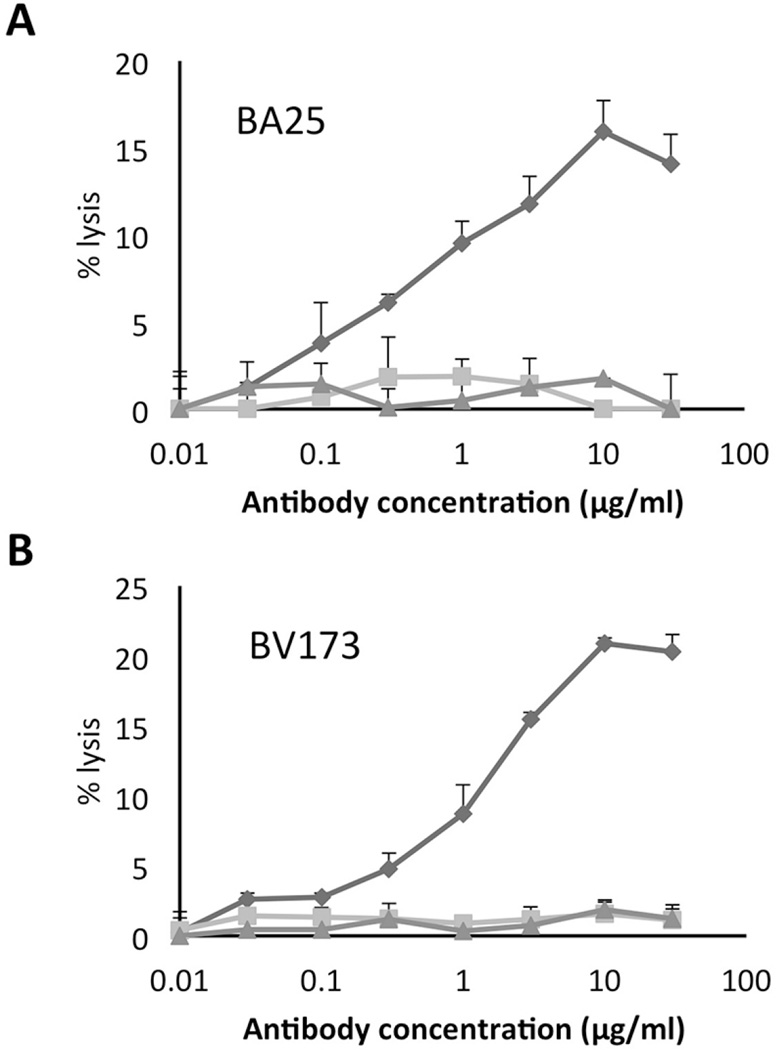
We next tested if Q2L scFv-Fc could induce mediate ADCC of leukemia targets carrying the HLA-A2/WT1126 complex. For ADCC, we used NK-92-MI cells transfected with human CD16.31 Q2L mediated dose-dependent ADCC against the WT1126 epitope naturally presented by HLA-A2 molecules on BV173 and BA25 leukemia targets (Figure 4). The low-affinity parental Clone45 and the irrelevant isotype matched TCR-like scFv-Fc antibody (HLA-A2/HUD) did not kill these tumor cells. Complement-mediated cytotoxicity (CMC) was ineffective (data not shown).
Figure 4. Antibody-dependent cell mediated cytotoxicity of TCR-like antibodies against leukemia cells BA25.
(a) and BV173 (b). Cytotoxicity of Q2L (blue), Clone45 (green) and isotype TCR-like scFv-Fc (red) was measured by chromium release assay. Samples were prepared in triplicate. Values are shown as mean ± SD.
Arming NK cells and T cells with chimeric antigen receptor (CAR)
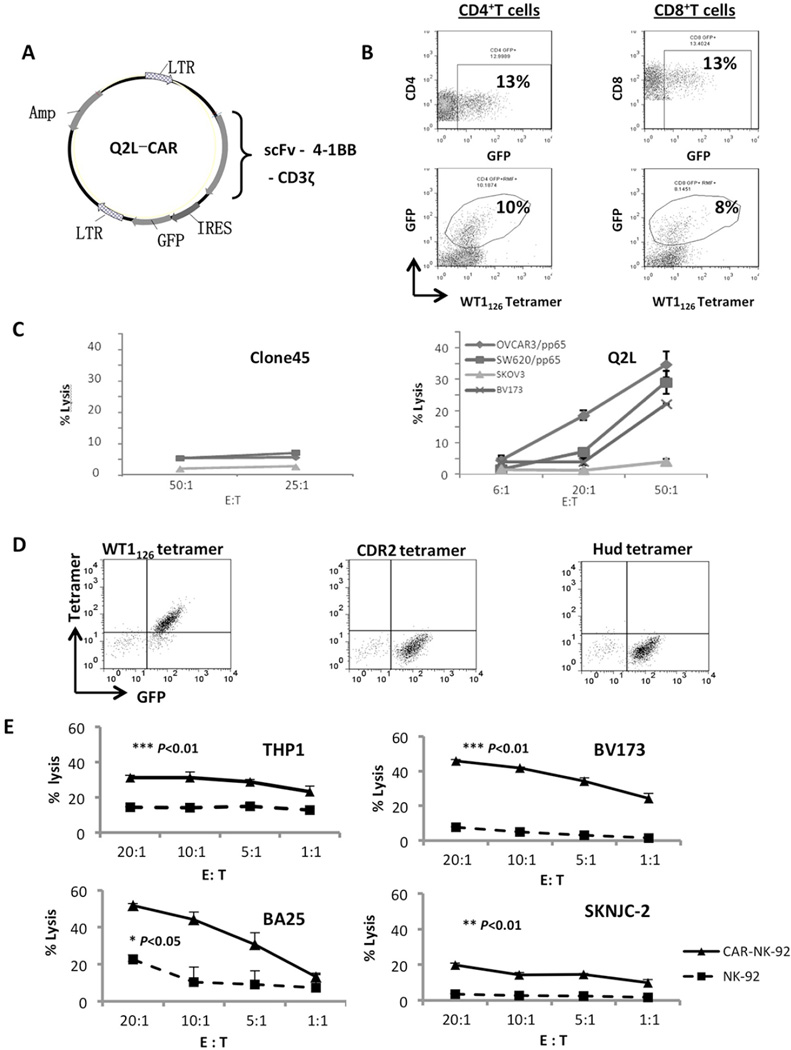
CAR was constructed using the Q2L scFv linked to the intracellular signaling domains of 4-1BB and CD3ζ (Figure 5a). NK-92-MI cells were genetically modified to express Q2L CAR using retroviral MSCV vector carrying an IRES-GFP sequence downstream used for FACS sorting, in order to produce a fairly pure population (~90%) of stable NK-92-MI cells carrying anti-HLA-A2/WT1126 CAR on their cell surface (Figure 5d). Their antigen specificity was confirmed by specific tetramer staining. When tested againstHLA-A2(+) and WT1(+)leukemia cell lines (THP-1, BV173 and BA25) or neuroblastoma cell line (SKNJC2),specific lysis was observed only with NK-92-MI-scFv(Q2L), but not with unmodified NK-92-MI cells (Figure 5e).
Figure 5. Chimeric antigen receptor expressing human lymphocytes specific for HLA-A2-WT1126.
(a) Schematic diagram of the CAR construct. The scFv sequence was cloned into the CAR gene and further cloned into a murine stem cell virus-based vector, which contained an internal ribosome entry site (IRES)-green fluorescence protein (GFP) sequence along with ampicillin-resistance. The resulting CAR was composed of the leader sequence, scFv and hinge region on the extracellular surface, a CD8α transmembrane domain, along with 4-1BB and the CD3ζ chain. (b) Transduced T cells derived from a single healthy donor. Both CD4 and CD8 T cells were genetically modified. CAR modified T cells were stained with HLA-A2-WT1126 tetramer, anti-CD4, or anti-CD8 and analyzed by flow cytometry. (c) Specific cytotoxicity of Clone45-CAR (left) or Q2L-CAR (right) T cells against the tumor cell lines by chromium release assay. Samples were prepared in triplicate and values are shown as mean ± SE. Experiment was repeated twice with similar results. (d) CAR NK-92 cells were stained with PE conjugated HLA-A2/WT1126 tetramer (right) and two isotype controls: HLA-A2/Hud tetramer and HLA-A2/CDR2 tetramer. (e) Specific cytotoxicity of Q2L-CAR NK-92 (solid line) and mock (dashed line) cells against the tumor cell lines by chromium release assay. Samples were prepared in triplicate, and values are shown as mean ± SD. Experiment was repeated twice with similar results. The P values of the difference between CAR and mock groups were analyzed by log-rank Mantel-Cox test, with treatment groups showing similar variance.
We next modified CD3(+) T cells isolated from the peripheral blood of healthy donors, using retroviral transduction in vitro with either the Q2L-CARor the Clone45-CAR. Transduction efficacy varied between 20% and 40%, and correct functional assembly of immune receptors was confirmed by HLA-A2/WT1126 tetramer staining (Figure 5b and Supplementary Figure S4). Low affinity Clone45-CAR did not stain well with the tetramer and the CAR-modified T cells were not cytotoxic for WT1(+) HLA-A2(+) tumor targets (data not shown). In contrast, the high affinity Q2L-CAR bound strongly to the tetramer and mediated efficient tumor lysis in a dose-dependent manner (Figure 5c). Q2L-CAR grafted T cells specifically recognized and killed HLA-A2(+)/WT1(+)targets (e.g. BV173, SW620/pp65, OVCAR3/pp65in a dose-dependent manner, but not HLA-A2(+)/WT1(−) cells (SKOV3).
Therapy of human leukemia cells by Q2L in vivo
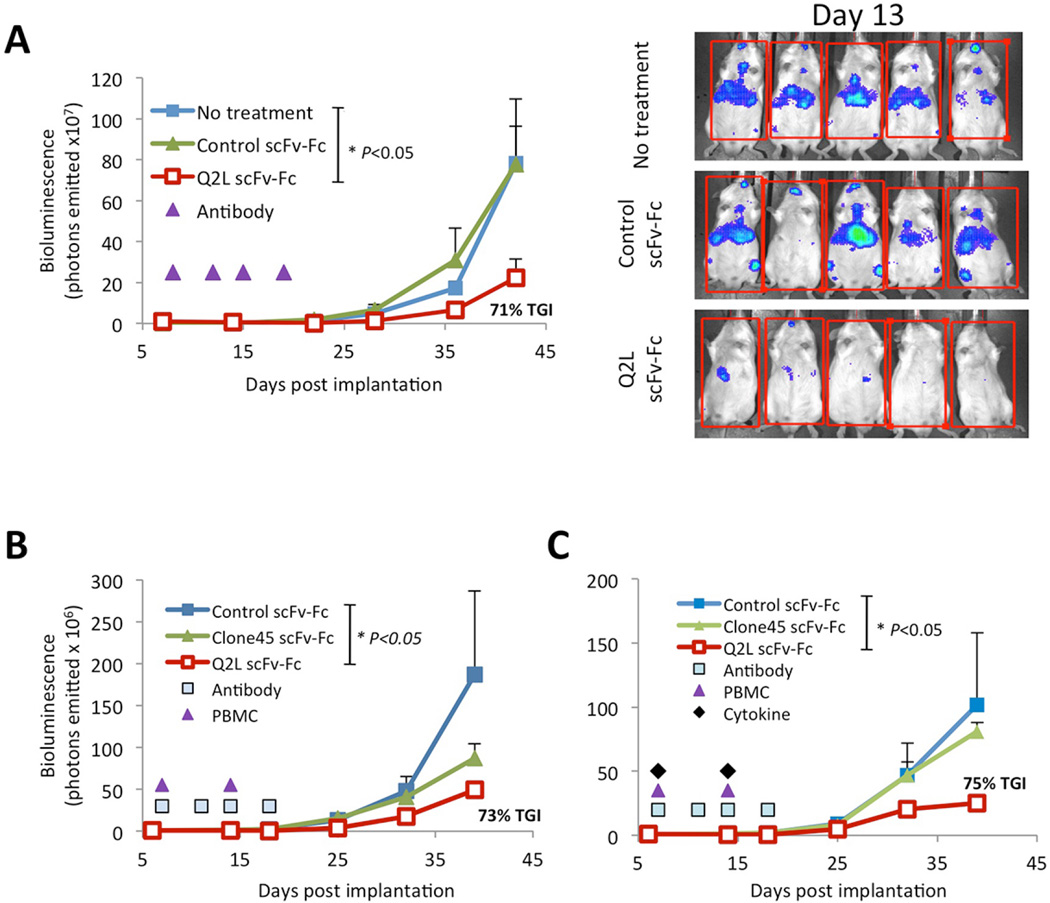
Q2L scFv-Fc was next tested for their anti-tumor effect in vivo in DKO mice xenografted intravenously 7 days prior with BV173 acute lymphoblastic leukemia cells. In the first tumor model, four IV injections of Q2L suppressed subcutaneous tumor growth, but not when control scFv-Fc was used; anti-tumor effect was observed even without the infusion of human PBMC (Figure 6a). In the second tumor model, injection of human PBMC along with four doses (100 µg per dose) of Q2L nearly eliminated the leukemia in comparison to treatments with effector alone (Figure 6b). When PBMC and cytokine IL15/IL15α were added to enhance lymphocyte survival, leukemia cells rapidly disseminated in the body with no activity by Clone45 in comparison to Q2L-treated mice (Figure 6c). These results suggest that the higher affinity of Q2L translated into a significantly enhanced anti-tumor effect.
Figure 6. Therapeutic effect of Q2L in vivo against human leukemia xenografts.
Tumor burden was calculated by the luminescence signal of each mouse, and averaged (n = 5 per group). scFv-Fc fusion antibody (Q2L, Clone45 or anti-HLA-A2/Hud [isotope control]) was administered (100 µg per dose) intravenously twice a week for a total of 4 doses. (a) Q2L alone without human effectors significantly reduced tumor burden (p <0.05). (b and c) Human PBMC(10 million per iv injection, and cytokine IL15/IL15α (10 µg each sc injection) were given on day 7 and 14. Q2L was more effective in tumor suppression when compared to parental Clone45, in the absence (b) or presence (c) of IL15-IL15Rα. In contrast, the group treated with isotype control had rapid tumor growth. Percent tumor growth inhibition in Q2L treatment group was calculated as Tumor Growth Inhibition (TGI). Values are shown as mean ± SE. The P values of the difference between Q2L treatment and control groups were analyzed by log-rank Mantel-Cox test, with treatment groups showing similar variance.
However, tumor growth suppression Q2L alone treatment was transient, compared with Q2L with PBMC effectors (data not shown). It confirmed that Q2L-mediated human ADCC likely plays an important role in eliminating tumor cells long term.
Discussion
Therapeutic antibodies are now an established modality for cancer therapy. Peptides originating from intracellular proteins are presented on the surface of all nucleated cells, including tumor cells, by their MHC-I molecules. If specific antibodies can be made against these peptide-HLA complexes, a huge repertoire of targets is theoretically possible.5 In contrast to the TCR where low affinity is the rule, TCR-like antibodies can be made to have high affinity while retaining specificity.38 A number of TCR-like antibodies have been described directed against a large variety of MHC-class-I–peptide complexes derived from tumors as well as from pathogens.5, 11, 39 However, few of these antibodies have yet been tested in the clinic, and the optimal specificity and affinity of this class of therapeutic antibodies need to be defined.
In this study, we described an algorithm for the discovery of TCR-like antibodies directed toward an endogenous tumor–associated antigen, WT1, which is overexpressed by human malignant cells. We showed that these antibodies bind to a conformational epitope of HLA-A2-restricted WT1126 peptide, contacting the 126–134 residues of the WT1 protein, an epitope previously validated as a tumor target for CD8+ T cells in patients with AML and CML.23 The WT1 protein has also been found to be characteristic of CML stem cells40, and utilizing this TCR-like antibody approach may be a useful strategy to target cancer initiating cells in other tumor types. However, whether the particular WT1126 epitope was presented on HLA-A2 in all tumor cells has yet to be proven in the clinical setting.
The evidence for naturally occurring high affinity TCR-like antibodies in human is scant. TCR-like antibodies were previously generated using hybridoma approaches13 or phage-display libraries.41 We started this investigation using a synthetic antibody gene library where the genes were recombinant. The affinity of TCR-like antibodies isolated from human antibody phage-display library was relatively low (50–250 nM) and not always sufficient for therapeutic purposes.13 As tumors evade T cell immunity by downregulating HLA antigens,42–44 affinity of TCR-like antibody is critical. In this study, we adopted an efficient yeast display system for affinity maturation. From the initial anti-WT1126/HLA-A2 scFv Clone45 with low affinity (KD=263 nM), to the final affinity matured variant (KD=2.4nM) representing a 100-fold improvement. Functionally the parental Clone45 as either monovalent scFv or bivalent scFv-Fc did not recognize the naturally expressed WT1126/HLA-A2 epitope on tumor cells, even though it was able to recognize WT1126/HLA-A2 complexes loaded on the surface of T2 cells. Most importantly, the parental Clone45 scFv was not recognizable by tetramer in FACS analysis and did not mediate cytotoxicity either as scFv-Fc in ADCC or as CAR in transduced NK-92-MI cells or human T cells.
The affinity maturation of Clone45 was carried out using complementary technologies: yeast display and in silico computation. The yeast display library was initially generated based on scFv Clone45 where the CDR residues were randomized and clones selected for enhanced binding to WT1126/HLA-A2 but not to irrelevant complexes. Using a minimal 20-fold to a maximal100-fold affinity improvement boundary, 3 clones were selected. Using homology modeling, the simulated structure of scFv recognizing the HLA-A2-WT1126 complex was used to identify the two key residues responsible for interaction with the peptide motif, while residues facing the MHC helices were left unchanged. The final mutant, Q2L with two crucial leucine mutations at glutamine residues of CDR2 regions of the heavy and light chains, achieved a 100-fold improvement in affinity. It was noteworthy that the picomolar KD (2 pM) of Q2L as bivalent scFv-Fc was the highest among reported TCR-like antibodies (9.9 to 294 nM).13 Two other groups have developed anti-WT1126/HLA-A2 antibodies with lower affinity, including the IgG1 ESK1 (100pM KD)16 and the Fab fragments F2 (400nM KD) and F3 (30nM KD)18. We show that the high affinity Q2L, with its long retention time (slow koff=3.55 × 10−4 S−1) was not just better at binding compared to the parental Clone45 (koff=7.18×10−2 S−1), it was also more effective in ADCC in vitro, and anti-tumor effect in vivo. Our studies showed that in DKO mice, the addition of human effectors and cytokine could enhance the antibody effects and extend survival, most likely through Fc-receptor dependent ADCC mechanisms in the presence of human natural killer (NK) cells and myeloid cells.
In order to exploit cytotoxic T cells, genetic modifications using CARs seem to hold great promise45. The affinity matured Q2L enabled CAR-modified T cells to display potent cytolytic capacity in vitro against AML and breast cancer cell lines. Additionally, we demonstrated that Q2L-CAR T cells recognized tumor cells in a WT1-dependent fashion. In contrast, no lysis was observed for low affinity parental Clone45 in the same format. Oren et al.18 also generated T cell CARs with scFvs based on their low affinity F2 (400nM Kd) and their moderate affinity F3 (30nM kD), and postulated that F3-CAR likely cross-reacted and led to poor viability, implying an affinity barrier to developing effective anti-peptide/HLA CAR T cells. Here we show that Q2L CAR can retain specificity despite having high affinity (3nM KD for the scFv).
NK cells are also a vital component of the innate immune system and the body’s first line of defense against viral infection and malignance.46 Unlike T cells expressing the TCR, NK cells are devoid of receptors for common tumor antigens.47 In addition, unlike transformed cells of hematopoietic origin which express NK activation ligands, solid tumors are relatively resistant to NK killing.47 In fact, most neuroblastoma cells were resistant to NK cells48 and to NK-92-MI cells (data not shown). However, they were effectively lysed by Q2L-CAR modified NK-92-MI even when their HLA expression was low. Whether CAR-modified NK cells could overcome resistance mechanisms of neuroblastoma will require testing of patient NK cells and their tumor samples. Since the NK-92-MI cell line was safe in adoptive cancer immunotherapy,46 Q2L-CAR modified NK-92-MI cell could have therapeutic potential for WT1-expressing tumors. As adoptive NK cell therapy becomes clinically established, Q2L-CAR modified NK cells may be another therapeutic possibility.49
While we did observe a higher level of binding of Q2L to normal HLA-A2[+] PBMC relative to clone 45, we have previously observed that WT1 is present on bone marrow cells (data not shown). It has also been shown that an anti-WT1/HLA-A2 antibody can bind some CD19[+] cells16. A thorough analysis on HLA-typed normal tissue arrays will be needed to ensure specificity for clinical development. Our work, nevertheless, shows that targeting peptide-MHC complexes with Fc-mediated and NK cell CAR-mediated modalities are powerful methods to target intra-cellular proteins.
In conclusion, we described a readily applicable strategy for generating TCR-like antibodies with picomolar affinity and high specificity. Such antibodies are critical diagnostic tools to study individual peptide expression in fresh tumors and as a biomarker of peptide vaccine immunotherapy. When the peptide is derived from tumor-associated antigens (e.g.WT1) or viral antigens,17 they could provide a sensitive and specific probe for detecting or isolating circulating tumor cells in patients. As therapeutics either in the form of IgG antibodies, bispecific constructs or CAR-modified lymphocytes, these TCR-mimics directed against critical tumor-specific or tumor-associated internal antigens among common human cancers50 should open possibilities in the emerging field of cancer immunotherapy.
Supplementary Material
Acknowledgments
This study was supported in part by Katie Find a Cure Fund, Cycle for Survival, and Robert Steel Foundation. Technical services provided by the MSKCC Small-Animal Imaging Core Facility were supported in part by NIH Cancer Center Support Grant No 2 P30 CA008748-48. We want to thank Dr. Mamoru Ito of Central Institute for Experimental Animals, Kawasaki, Japan, for kindly providing the DKO mice for our studies. We are grateful to Dr. Gloria Koo, Hospital for Special Surgery, New York, NY, for her expertise and advice in handling these DKO mice. We thank Dr. Dario Campana and St. Jude Children’s Research Hospital for sharing with us the vector for chimeric antigen receptor. We also want to thank Dr. Jian Hu for technical assistance.
Footnotes
Disclosure:
QZ, MA, DVT, RJO and NKC were named as inventors in patents related to WT1 filed by Memorial Sloan Kettering Cancer Center for which a license has been obtained. The other authors declare they have no competing interests or other interests that might be perceived to influence the results and discussion reported in this paper.
References
- 1.Doubrovina E, Carpenter T, Pankov D, Selvakumar A, Hasan A, O'Reilly RJ. Mapping of novel peptides of WT-1 and presenting HLA alleles that induce epitope-specific HLA-restricted T cells with cytotoxic activity against WT-1(+) leukemias. Blood. 2012 Aug 23;120(8):1633–1646. doi: 10.1182/blood-2011-11-394619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santomasso BD, Roberts WK, Thomas A, Williams T, Blachere NE, Dudley ME, et al. A T-cell receptor associated with naturally occurring human tumor immunity. Proc Natl Acad Sci U S A. 2007 Nov 27;104(48):19073–19078. doi: 10.1073/pnas.0704336104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keilholz U, Letsch A, Busse A, Asemissen AM, Bauer S, Blau IW, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009 Jun 25;113(26):6541–6548. doi: 10.1182/blood-2009-02-202598. [DOI] [PubMed] [Google Scholar]
- 4.Yee C. Adoptive T cell therapy: Addressing challenges in cancer immunotherapy. J Transl Med. 2005 Apr 28;3(1):17. doi: 10.1186/1479-5876-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahan R, Reiter Y. T-cell-receptor-like antibodies - generation, function and applications. Expert Rev Mol Med. 2012;14:e6. doi: 10.1017/erm.2012.2. [DOI] [PubMed] [Google Scholar]
- 6.Cohen CJ, Denkberg G, Lev A, Epel M, Reiter Y. Recombinant antibodies with MHC-restricted, peptide-specific, T-cell receptor-like specificity: new tools to study antigen presentation and TCR-peptide-MHC interactions. J Mol Recognit. 2003 Sep-Oct;16(5):324–332. doi: 10.1002/jmr.640. [DOI] [PubMed] [Google Scholar]
- 7.Chames P, Hufton SE, Coulie PG, Uchanska-Ziegler B, Hoogenboom HR. Direct selection of a human antibody fragment directed against the tumor T-cell epitope HLA-A1-MAGE-A1 from a nonimmunized phage-Fab library. Proc Natl Acad Sci U S A. 2000 Jul 5;97(14):7969–7974. doi: 10.1073/pnas.97.14.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lev A, Noy R, Oved K, Novak H, Segal D, Walden P, et al. Tumor-specific Ab-mediated targeting of MHC-peptide complexes induces regression of human tumor xenografts in vivo. Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):9051–9056. doi: 10.1073/pnas.0403222101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart-Jones G, Wadle A, Hombach A, Shenderov E, Held G, Fischer E, et al. Rational development of high-affinity T-cell receptor-like antibodies. Proc Natl Acad Sci U S A. 2009 Apr 7;106(14):5784–5788. doi: 10.1073/pnas.0901425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holler PD, Chlewicki LK, Kranz DM. TCRs with high affinity for foreign pMHC show self-reactivity. Nat Immunol. 2003 Jan;4(1):55–62. doi: 10.1038/ni863. [DOI] [PubMed] [Google Scholar]
- 11.Denkberg G, Reiter Y. Recombinant antibodies with T-cell receptor-like specificity: novel tools to study MHC class I presentation. Autoimmun Rev. 2006 Apr;5(4):252–257. doi: 10.1016/j.autrev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Sastry KS, Too CT, Kaur K, Gehring AJ, Low L, Javiad A, et al. Targeting hepatitis B virus-infected cells with a T-cell receptor-like antibody. J Virol. 2011 Mar;85(5):1935–1942. doi: 10.1128/JVI.01990-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sergeeva A, Alatrash G, He H, Ruisaard K, Lu S, Wygant J, et al. An anti-PR1/HLA-A2 T-cell receptor-like antibody mediates complement-dependent cytotoxicity against acute myeloid leukemia progenitor cells. Blood. 2011 Apr 21;117(16):4262–4272. doi: 10.1182/blood-2010-07-299248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma B, Neethling FA, Caseltine S, Fabrizio G, Largo S, Duty JA, et al. TCR mimic monoclonal antibody targets a specific peptide/HLA class I complex and significantly impedes tumor growth in vivo using breast cancer models. J Immunol. 2010 Feb 15;184(4):2156–2165. doi: 10.4049/jimmunol.0902414. [DOI] [PubMed] [Google Scholar]
- 15.Willemsen RA, Debets R, Hart E, Hoogenboom HR, Bolhuis RL, Chames P. A phage display selected fab fragment with MHC class I-restricted specificity for MAGE-A1 allows for retargeting of primary human T lymphocytes. Gene therapy. 2001 Nov;8(21):1601–1608. doi: 10.1038/sj.gt.3301570. [DOI] [PubMed] [Google Scholar]
- 16.Dao T, Yan S, Veomett N, Pankov D, Zhou L, Korontsvit T, et al. Targeting the Intracellular WT1 Oncogene Product with a Therapeutic Human Antibody. Science translational medicine. 2013 Mar 13;5(176):176ra133. doi: 10.1126/scitranslmed.3005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tassev DV, Cheng M, Cheung NK. Retargeting NK92 cells using an HLA-A2-restricted, EBNA3C-specific chimeric antigen receptor. Cancer Gene Ther. 2012 Feb;19(2):84–100. doi: 10.1038/cgt.2011.66. [DOI] [PubMed] [Google Scholar]
- 18.Oren R, Hod-Marco M, Haus-Cohen M, Thomas S, Blat D, Duvshani N, et al. Functional Comparison of Engineered T Cells Carrying a Native TCR versus TCR-like Antibody-Based Chimeric Antigen Receptors Indicates Affinity/Avidity Thresholds. J Immunol. 2014 Oct 31; doi: 10.4049/jimmunol.1301769. [DOI] [PubMed] [Google Scholar]
- 19.Renshaw J, Orr RM, Walton MI, Te Poele R, Williams RD, Wancewicz EV, et al. Disruption of WT1 gene expression and exon 5 splicing following cytotoxic drug treatment: antisense down-regulation of exon 5 alters target gene expression and inhibits cell survival. Mol Cancer Ther. 2004 Nov;3(11):1467–1484. [PubMed] [Google Scholar]
- 20.Yang L, Han Y, Suarez Saiz F, Minden MD. A tumor suppressor and oncogene: the WT1 story. Leukemia. 2007 May;21(5):868–876. doi: 10.1038/sj.leu.2404624. [DOI] [PubMed] [Google Scholar]
- 21.Sugiyama H. WT1 (Wilms' tumor gene 1): biology and cancer immunotherapy. Jpn J Clin Oncol. 2010 May;40(5):377–387. doi: 10.1093/jjco/hyp194. [DOI] [PubMed] [Google Scholar]
- 22.O'Reilly RJ, Dao T, Koehne G, Scheinberg D, Doubrovina E. Adoptive transfer of unselected or leukemia-reactive T-cells in the treatment of relapse following allogeneic hematopoietic cell transplantation. Semin Immunol. 2010 Jun;22(3):162–172. doi: 10.1016/j.smim.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezvani K, Brenchley JM, Price DA, Kilical Y, Gostick E, Sewell AK, et al. T-cell responses directed against multiple HLA-A*0201-restricted epitopes derived from Wilms' tumor 1 protein in patients with leukemia and healthy donors: identification, quantification, and characterization. Clin Cancer Res. 2005 Dec 15;11(24 Pt 1):8799–8807. doi: 10.1158/1078-0432.CCR-05-1314. [DOI] [PubMed] [Google Scholar]
- 24.Kohrt HE, Muller A, Baker J, Goldstein MJ, Newell E, Dutt S, et al. Donor immunization with WT1 peptide augments antileukemic activity after MHC-matched bone marrow transplantation. Blood. 2011 Nov 10;118(19):5319–5329. doi: 10.1182/blood-2011-05-356238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borbulevych OY, Do P, Baker BM. Structures of native and affinity-enhanced WT1 epitopes bound to HLA-A*0201: implications for WT1-based cancer therapeutics. Mol Immunol. 2010 Sep;47(15):2519–2524. doi: 10.1016/j.molimm.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mailander V, Scheibenbogen C, Thiel E, Letsch A, Blau IW, Keilholz U. Complete remission in a patient with recurrent acute myeloid leukemia induced by vaccination with WT1 peptide in the absence of hematological or renal toxicity. Leukemia. 2004 Jan;18(1):165–166. doi: 10.1038/sj.leu.2403186. [DOI] [PubMed] [Google Scholar]
- 27.de Wildt RM, Mundy CR, Gorick BD, Tomlinson IM. Antibody arrays for high-throughput screening of antibody-antigen interactions. Nat Biotechnol. 2000 Sep;18(9):989–994. doi: 10.1038/79494. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Q, Zhu Z, Dimitrov DS. Yeast display of engineered antibody domains. Methods Mol Biol. 2012;899:73–84. doi: 10.1007/978-1-61779-921-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Q, Chan YW, Lee SS, Cheung WT. One-step expression and purification of single-chain variable antibody fragment using an improved hexahistidine tag phagemid vector. Protein Expr Purif. 2009 Dec;68(2):190–195. doi: 10.1016/j.pep.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Feng Y, Zhao Q, Zhu Z, Dimitrov DS. Human monoclonal antibodies targeting nonoverlapping epitopes on insulin-like growth factor II as a novel type of candidate cancer therapeutics. Mol Cancer Ther. 2012 Jul;11(7):1400–1410. doi: 10.1158/1535-7163.MCT-12-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung NK, Guo H, Hu J, Tassev DV, Cheung IY. Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. Oncoimmunology. 2012 Jul 1;1(4):477–486. doi: 10.4161/onci.19864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004 Apr;18(4):676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 33.Wittrup KD, Thurber GM, Schmidt MM, Rhoden JJ. Practical theoretic guidance for the design of tumor-targeting agents. Methods in enzymology. 2012;503:255–268. doi: 10.1016/B978-0-12-396962-0.00010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimitrov DS, Marks JD. Therapeutic antibodies: current state and future trends--is a paradigm change coming soon? Methods Mol Biol. 2009;525:1–27. xiii. doi: 10.1007/978-1-59745-554-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Q, Feng Y, Zhu Z, Dimitrov DS. Human monoclonal antibody fragments binding to insulin-like growth factors I and II with picomolar affinity. Mol Cancer Ther. 2011 Sep;10(9):1677–1685. doi: 10.1158/1535-7163.MCT-11-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Li H, Yang F, Smith-Gill SJ, Mariuzza RA. X-ray snapshots of the maturation of an antibody response to a protein antigen. Nat Struct Biol. 2003 Jun;10(6):482–488. doi: 10.1038/nsb930. [DOI] [PubMed] [Google Scholar]
- 37.Mareeva T, Martinez-Hackert E, Sykulev Y. How a T cell receptor-like antibody recognizes major histocompatibility complex-bound peptide. J Biol Chem. 2008 Oct 24;283(43):29053–29059. doi: 10.1074/jbc.M804996200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epel M, Carmi I, Soueid-Baumgarten S, Oh SK, Bera T, Pastan I, et al. Targeting TARP, a novel breast and prostate tumor-associated antigen, with T cell receptor-like human recombinant antibodies. European journal of immunology. 2008 Jun;38(6):1706–1720. doi: 10.1002/eji.200737524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noy R, Eppel M, Haus-Cohen M, Klechevsky E, Mekler O, Michaeli Y, et al. T-cell receptor-like antibodies: novel reagents for clinical cancer immunology and immunotherapy. Expert Rev Anticancer Ther. 2005 Jun;5(3):523–536. doi: 10.1586/14737140.5.3.523. [DOI] [PubMed] [Google Scholar]
- 40.Gerber JM, Qin L, Kowalski J, Smith BD, Griffin CA, Vala MS, et al. Characterization of chronic myeloid leukemia stem cells. American journal of hematology. 2011 Jan;86(1):31–37. doi: 10.1002/ajh.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engberg J, Yenidunya AF, Clausen R, Jensen LB, Sorensen P, Kops P, et al. Human recombinant Fab antibodies with T-cell receptor-like specificities generated from phage display libraries. Methods Mol Biol. 2003;207:161–177. doi: 10.1385/1-59259-334-8:161. [DOI] [PubMed] [Google Scholar]
- 42.Gilham DE, Debets R, Pule M, Hawkins RE, Abken H. CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol Med. 2012 Jul;18(7):377–384. doi: 10.1016/j.molmed.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Ramos CA, Dotti G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin Biol Ther. 2011 Jul;11(7):855–873. doi: 10.1517/14712598.2011.573476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mardiros A, Dos Santos C, McDonald T, Brown CE, Wang X, Budde LE, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and anti-tumor effects against human acute myeloid leukemia. Blood. 2013 Sep 12; doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheung NK, Dyer MA. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer. 2013 Jun;13(6):397–411. doi: 10.1038/nrc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esser R, Muller T, Stefes D, Kloess S, Seidel D, Gillies SD, et al. NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. Journal of cellular and molecular medicine. 2012 Mar;16(3):569–581. doi: 10.1111/j.1582-4934.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kruschinski A, Moosmann A, Poschke I, Norell H, Chmielewski M, Seliger B, et al. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proc Natl Acad Sci U S A. 2008 Nov 11;105(45):17481–17486. doi: 10.1073/pnas.0804788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho D, Shook DR, Shimasaki N, Chang YH, Fujisaki H, Campana D. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin Cancer Res. 2010 Aug 1;16(15):3901–3909. doi: 10.1158/1078-0432.CCR-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shook DR, Campana D. Natural killer cell engineering for cellular therapy of cancer. Tissue Antigens. 2011 Dec;78(6):409–415. doi: 10.1111/j.1399-0039.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009 Sep 1;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.