Synopsis
Premature infants suffer significant respiratory morbidity during infancy with long-term negative consequences on health, quality of life, and health care costs. Enhanced susceptibility to a variety of infections and inflammation play a large role in early and prolonged lung disease following premature birth, though the mechanisms of susceptibility and immune dysregulation are active areas of research. This chapter will review aspects of host-pathogen interactions and immune responses that are altered by preterm birth and that impact chronic respiratory morbidity in these children.
Keywords: prematurity, neonatal immunology, neonatal infection, virus, lymphocytes, bronchopulmonary dysplasia, chronic lung disease of prematurity, preterm
Introduction
Each year, approximately 1 in 9 infants in the United States, more than 440,000 babies yearly, are born prematurely (<37 weeks gestation) [1]. These infants suffer from complications of exposure to a diverse environment at a time in development when the respiratory tract and immune system are intended to be protected and maintained in a relatively naïve intrauterine state. During infancy and early childhood, premature infants suffer significant inflammatory and infectious respiratory morbidities with extended negative consequences for health, quality of life, and health care costs. As compared to approximately 8% of full term newborns, 17% of late-preterm (LPT, born at 34 0/7 – 36 6/7 weeks) and 30–40% of early preterm infants (EPT, born at < 32 weeks) are re-hospitalized within the first year of life, most commonly for viral respiratory infections [2–4]. Respiratory infections that are less severe, not requiring hospitalization, are even more common, recurrent and, in total, costly in the very young [5]. The incidence and severity of respiratory tract infections in babies less than one year of age is attributed at least in part to immune immaturity, a problem magnified by preterm birth and influenced by genetic traits and environmental exposures. Differences in gastrointestinal tract colonization patterns, the development and balance of the intestinal microbiome, have been shown to influence immunologic development in full term infants, and have begun to be evaluated in the premature [6–8]. Viral infections, either subclinical or severe, may also alter immunologic development both directly and by altering the bacterial microflora. Preterm babies are exposed to maternal and hospital-based flora, frequently with additional pressures of antibiotics, in-dwelling catheters and tubes, that alter the establishment of diverse, health promoting microbiota on the skin and respiratory mucosa, as well as in the gastrointestinal tract, and increase the risk of invasive disease with predominant organisms.
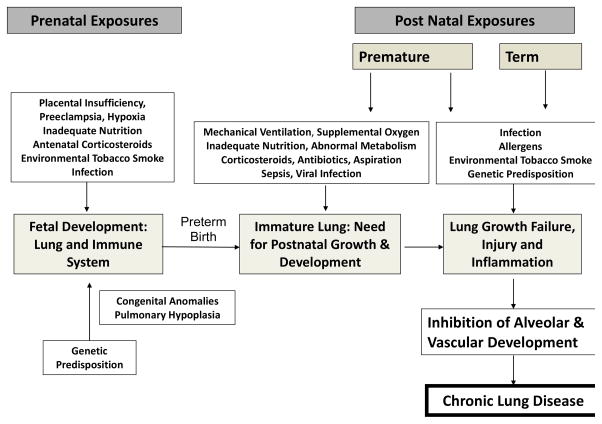
Recurrence of respiratory symptoms in the first year of life correlates inversely with gestational age at birth, directly with in utero exposure to inflammation (chorioamnionitis) and with non-white race. The pathogenesis of chronic lung disease of prematurity, bronchopulmonary dysplasia, has been recently reviewed and is closely correlated with in utero inflammation, oxygen toxicity, ventilator-induced trauma and pre-alveolar lung development at birth (Figure 1). [10–12] Premature birth induces a slowing or arrest of lung development that underlies BPD and likely occurs in a spectrum of severity in all prematurely born infants. Perinatal therapeutic and environmental exposures, most notably oxygen exposure and environmental tobacco smoke, have been reproducibly related to chronic respiratory morbidity, independent of mechanical ventilation and the diagnosis of BPD. A recent study of very low birth weight (VLBW) infants without BPD demonstrated significant relationships between an integrated estimate of oxygen exposure in the first 3–14 days of life and symptomatic respiratory disease (SRD) over the first year of life. [9] Lower gestational age, non-white race, greater oxygen exposure and chorioamnionitis significantly increased the odds ratio of infants having SRD. A recent murine model demonstrated that early neonatal exposure to hyperoxia dramatically increases, in a dose-responsive manner, the severity of influenza infection when induced in adulthood, with markedly enhanced inflammation and fibrotic repair. [10, 11] These observations, and an increasing understanding of the preterm infant immune system, as well as their exposures, colonization and infections with microorganisms, suggest that interventions to modify the immunologic response may significantly improve respiratory and general outcome for these children. This chapter will review prenatal and postnatal exposures that induce lung inflammation in preterm infants in the context of unique susceptibility factors that occur because of premature delivery.
Figure 1.
Inflammation as a Mechanism for Respiratory Morbidity
Several lines of evidence suggest that the inflammatory response of the fetal or premature lung to injury or infection, if not causative of disease, exacerbates the severity of chronic lung disease in infants at risk. [12, 13] Recent reviews highlight the current understanding of the role of inflammatory mediators and the immunobiology of BPD. [14–16] Increased levels of pro-inflammatory mediators in amniotic fluid [17, 18], early tracheal effluents [19–24], lung tissue [23] and serum [25, 26] of at-risk premature infants support a role for both intrauterine and extra-uterine inflammation in the development and severity of BPD. Airway and bronchoalveolar lavage samples demonstrate increased inflammatory cells and multiple pro-inflammatory mediators in ventilated, oxygen-exposed infants progressing toward BPD [19–23]. Genome-wide expression profiling of BPD lungs, as compared to gestational age matched controls, identified 159 differentially expressed genes. [27] Pathway analysis identified cell cycle, immunodeficiency signaling and B cell development pathways associated with BPD. In addition, of the top 25 differentially expressed gene sets, 9 were related to chymase expressing mast cells, the presence of which was confirmed by PCR and immunohistochemistry. Consistent with active inflammation, the transcription factor, NF-κB, a prototypical regulator of inflammation and cell survival, was elevated in neutrophils and macrophages in preterm infant airways, correlating with the presence of U Urealyticum and need for prolonged mechanical ventilation. [28] Interestingly, NF-κB activation in fetal lung and fetal lung macrophages has been shown to inhibit airway morphogenesis and activity of fibroblast growth factor 10, a critical factor in lung development, linking inflammation to the growth arrest of the preterm lung. [29, 30] Several animal models demonstrate that mediators of inflammation, including endotoxins, tumor necrosis factor α (TNF-α) and transforming growth factor α, enhance lung maturation but also impair alveolar septation and vascular remodeling, and thus contribute to the development of BPD even without frank tissue destruction. [31–33]
Pro-inflammatory stimuli come from multiple sources in the premature infant both prenatally and after birth. The most common causes are considered next.
Prenatal Induction of Inflammation and Respiratory Morbidity
Chorioamnionitis
Once thought to be sterile, modern molecular techniques independent of culture demonstrated that amniotic fluid and placental tissues frequently contain microbes. [34–40] Maternal-fetal inflammation is clinically identified as chorioamnionitis by maternal fever with one or more of maternal/fetal tachycardia, maternal leukocytosis, uterine tenderness and/or foul amniotic fluid. A recent study, utilizing amniocentesis to sample amniotic fluid of 46 mothers with signs and symptoms of clinical chorioamnionitis, detected microorganisms by culture and/or polymerase chain reaction/mass spectrometry, frequently more than one microbe, in 61%. [41] Fifteen percent had neither inflammation or infection and 24% had amniotic fluid evidence of inflammation without detectable microorganisms, suggesting other non-infectious causes of clinical symptoms. Of those with clinical chorioamnionitis, 51–62% also have histological evidence of placental inflammation. [41, 42] Severity of acute histological chorioamnionitis has been correlated with amniotic fluid matrix-metalloproteinase-8 and IL-6 levels supporting the presence of active inflammation [43, 44]. It is not uncommon, however, to have evidence of acute histological chorioamnionitis without detectable microorganisms, ranging from 30% to more than 50%. The cause of “sterile inflammation” of the fetal-placental tissues may be non-infectious disease or lack of sensitivity for microbial detection. Inflammatory placental lesions of a more chronic form, characterized by lymphocytes, plasma cells and macrophages, sometimes eosinophils, also occur in association with preterm birth and recurrent placental failure. Most frequently these lesions are of unknown etiology. [45]
Chorioamnionitis has been associated with chronic lung disease of prematurity in multiple small series [46] and in focused studies of specific organisms such as Ureaplasma [47]. In experimental models, chorioamnionitis caused by intra-amniotic injections of endotoxin or Ureaplasma initially cause fetal lung inflammation followed by persistent low-grade inflammation and evidence of enhanced lung maturation. [48–50] A more aggressive inflammatory response to oxygen or mechanical ventilation in newborns with a history of chorioamnionitis has been suggested in animal models [51] and some clinical reports [52]. The severity of the fetal inflammatory response to infection, as indicated by amniotic fluid IL-6, is inversely related to gestational age, suggesting that more premature infants are at greater risk of inflammatory injury. [44]
The most common organisms isolated from infected amniotic fluid and placentas are U. parvum and U. urealyticum. Likewise, it is relatively common to identify these organisms in the bodily fluids of preterm infants. Compelling evidence for an association between pulmonary Ureaplasma colonization and bronchopulmonary dysplasia in preterm infants has been recently reviewed. [53, 54] Further details and discussion of clinical trials for treatment of Ureaplasma found in respiratory secretions of preterm infants are reviewed in Chapter XX of this Journal.
However, the role of chorioamnionitis as a risk factor for BPD remains controversial and recently debated. [55, 56] Several large studies question the relationship of in utero infection to chronic lung disease. As part of the ELGAN Study, exhaustive placental bacterial cultures were done from deliveries at 23–27 weeks of gestation [57]. There was no correlation between placental culture results and the phenotypes of the infants assessed by oxygen need at day of life 14 or the development of BPD. The Canadian Neonatal Network, also reported that 3,094 infants born at <33wks gestation exposed to clinical chorioamnionitis had no increase in the incidence of BPD [58]. Further, Lahra, et al. [59] reported, using a 13 year experience from Sydney, that a fetal inflammatory response was protective for BPD.
These and other similar studies demonstrate that clinical or culture proven chorioamnionitis are not good predictors of BPD. Chorioamnionitis/infection has a major association with preterm premature rupture of membranes and preterm labor at early gestations [60, 61]. Also, chorioamnionitis is associated with inflammation in lungs of preterm infants soon after birth [62] and causes lung inflammation and altered immune modulation in animal models where the type and duration of fetal exposures can be controlled [63]. Clinically, variation in detection and virulence of causative organisms, as well as in the duration of infection and the maternal-fetal inflammatory response complicates the determination of effect on outcomes. The assessment of influence on preterm infant chronic lung disease is further confounded by the imprecise diagnosis of BPD. [64]
Other Prenatal Pro-inflammatory Exposures
As outlined in Figure 1, there are a number of other maternal-fetal-placental abnormalities that alter lung growth and/or induce fetal inflammation. The association of maternal preeclampsia, placental insufficiency and associated intrauterine growth restriction with BPD however remains controversial with some studies suggesting increased and others decreased or no effect. [65–71] Antenatal corticosteroids enhance fetal lung maturation and likely reduce inflammation but, although one study suggested that corticosteroids reduced BPD in those with histologic chorioamnionitis, over all they have had little effect on rates of BPD. [72]
Postnatal Induction of Inflammation and Respiratory Morbidity
Many exposures in the post-natal period promote inflammation [52].
Oxygen and Mechanical Ventilation
Both oxygen and mechanical ventilation, together and independently, induce inflammation via direct cellular injury, induction of cytokines and chemokines, recruitment of neutrophils and macrophages, and oxidation of DNA, lipids and proteins. Oxygen toxicity and barotrauma or volutrauma are important hazards of mechanical ventilation that are associated with the release of inflammatory cytokines and chemokines that cause pulmonary injury. [73] Higher levels of cytokines correlate with more prolonged duration of ventilation. [73] Supplemental oxygen also contributes to inflammation through biochemical pathways of oxidant stress. [74–76]
Bacterial Infection and Sepsis
Sepsis beyond the first days of life is frequent in ELBW infants at risk of BPD and often presents with respiratory instability [59]. Both early and late microbial presence in neonatal lung fluid samples was significantly associated with the development of chronic lung disease suggesting that both ante- and post-natal infection play a role in the development of disease. [24] Numerous studies associate postnatal sepsis, both early- and late-onset and typically with common infectious agents such as coagulase negative staphylococcus and gram-negative bacteria, with BPD suggesting that sepsis-induced inflammation compromises lung development and healing. [52, 77–81] Administration of intravenous immunoglobulin however, although associated with a small reduction in sepsis, was not shown by meta-analysis of randomized controlled trials to reduce the incidence of BPD. [82]
Viral Infections
Broad respiratory virus surveillance in the NICU is a relatively new approach augmented by more readily available culture-independent methods of detection. Previous NICU viral studies targeted patients with threshold symptoms. With this approach, small pandemics of viral infection, such as with adenovirus or respiratory syncytial virus (RSV) were detected but the overall infection rate in NICUs appeared relatively low. [83] As example, using a symptom based testing strategy, viral infection was confirmed in 51 of 5396 infants (1%) admitted to the NICU; of these 20 (39%) had an enterovirus / parechovirus infection, 15 (29%) RSV, 5 (10%) rotavirus, 3 (6%) cytomegalovirus (CMV), 2 (4%) adenovirus, 2 (4%) parainfluenza virus, 2 (4%) herpes simplex virus, 1 (2%) rhinovirus and 1 (2%) rubella virus. [84]
Recent data, including that from our collection of expedited autopsy human neonatal distal lung tissue, suggest a relatively high prevalence of lung viral infections in those who succumb to respiratory failure in the NICU; 21 out of 63 samples tested were virus positive. ([85] and data not shown) Coronavirus, rhinovirus, parainfluenza, and CMV were detected by RT-PCR. Interestingly, in this small post-mortem sample, RSV, influenza A & B, parainfluenza type 1 and metapneumovirus were not detected.
Surveillance studies utilizing PCR and genomic sequencing for detection have begun to report a closer to true incidence of nosocomial viral respiratory infections (NVRI) in neonates and children hospitalized in pediatric and neonatal intensive care units. In a NICU surveillance study, nasal brush samples were taken weekly from all neonates (age < or = 28 days) and children (age>28 days) hospitalized through a winter viral season. Out of a total of 120 patients enrolled (64 neonates and 56 children), 20 patients were virus positive by PCR (incidence 16.7%). Seven positive samples for human coronaviruses were detected (incidence 11%). Risk factors for NVRI in the neonates were duration of hospitalization, antibiotic treatment and duration of parenteral nutrition (P<0.01). [86]
A one-year NICU surveillance study of infants born at < 33 weeks gestation, using PCR detection of 17 viral subtypes, identified at least one positive respiratory virus during the hospitalization in 26 of 50 subjects, most asymptomatic. Testing positive was associated with longer length of stay and length of mechanical ventilation, as well as diagnosis of BPD. Similar ongoing studies should determine if viral infection is such a common occurrence in the NICU as to warrant more frequent surveillance and development of interventions to reduce exposure and illness.
Neonatal Cytomegalovirus
Human cytomegalovirus (CMV), a betaherpesvirinae virus, latent in leukocytes, is highly prevalent in the human population; approximately 50% of adults are CMV seropositive and 60% of mothers of preterm infants. Congenital, in utero, infection of the fetus occurs in 0.1–2% of all pregnancies and may arise through primary infection of the mother, reactivation during pregnancy of a latent infection or re-infection with a different strain of CMV. Postnatal, the virus is spread even more efficiently from mother to the newborn via breastmilk. Since it reactivates in 95% or more of CMV seropositive women in the postpartum period and can be detected in breastmilk as early as 3.5 days after delivery, CMV is a relatively common viral infection of the newborn period. [87] Transmission to full term newborns is reported in approximately 40%, while in preterm infants it varies from 6% to 55%, potentially due to differing strains, use of fresh/frozen milk and maternal factors affecting viral shedding. [87] A surveillance study of 175 NICU neonates, testing serum CMV-titers and CMV-DNA, demonstrated an overall prevalence of CMV of 12.6%. Ten (5.71%) of the infants had congenital infection, while 12 cases (6.86%) had perinatal infection. [88] Post-natal infection in the newborn can be detected by molecular diagnostics as early as 12 days of life. Infection remains clinically silent in the majority but 9 – 12% of post-nataly infected low birth weight preterm infants have been reported to demonstrate severe, sepsis-like infection. [89] Although lower gestational age infants are at increased risk of developing symptoms with post-natal infection and are also at greatest risk of bronchopulmonary dysplasia there remains relatively little evidence of cause and effect. Prosch et al [90] found approximately 29% of VLBW infants with BPD to be CMV positive but 12% of those without BPD. This study and others have found postnatal infection symptoms in preterms to be transient and to have no effect on neonatal outcome including BPD or necrotizing enterocolitis. [91, 92] A review of PubMed papers describing CMV pneumonitis, however, concludes that CMV infection can be protracted with diffuse interstitial pneumonitis associated with fibrosis and BPD. [93] It would appear that more surveillance and outcome studies are needed to determine if a causative relationship exists and if anti-CMV therapy or methods to reduce transmission of CMV to the fetus and neonates could effectively reduce disease.
Interestingly, CMV has a notable influence on the human immune system inducing a substantial cytolytic CD8+ T cell population. [94] CMV infection in infants induces the differentiation of not only phenotypically mature cells, but also functionally active cells that produce interferon gamma (IFN-γ) upon re-stimulation. [95] Serum cytokine concentrations measured in CMV congenitally infected infants show evidence of a strong Th1 bias with a predominance of IFN-γ, Il-2, IL-12 and IL-8 production and diminished IL-4. [96] Since, the generation of IFN-γ secreting T cells and CD8+ effector cells is associated with successful recovery from viral infections in general and RSV in particular such data suggest that CMV infection in infancy could be beneficial. There is, however, concern that CMV-induced immuno-ageing of lymphocytes may ultimately result in immunosuppression suggested by poor vaccine response in the elderly. [97]
Respiratory Syncytial Virus and Other Common Viruses
Recurrent wheezing in later childhood has been associated with infections with respiratory syncytial virus (RSV), metapneumovirus (hMPV), parainfluenza (PIV), rhinovirus, and human coronavirus NL63. [98–102] RSV infections have best demonstrated that effects of viral respiratory tract infections in infancy may be long-lived. In premature infants born at less than 32 weeks gestation, with and without BPD, those with a history of RSV LRTI were found to have more days of cough and wheeze at one year of age than those without RSV LRTI.[103] Additionally, those with RSV LRTI and hMPV LRTI were found to have increased airway resistance at one year of age on pulmonary function testing.[101] In some infants, airway function has been shown to deteriorate during the first years of life. [104] When the group with BPD was followed up at school entry, those who had been hospitalized with RSV LRTI or another respiratory illness within the first two years of life had a greater cumulative number of outpatient visits and costs of care compared to former premature infants with BPD without a respiratory hospitalization.[105] A subset of these children with pulmonary function testing at 8–10 years of age demonstrated significantly reduced lung function (lower forced expiratory volume in 0.75 s (FEV0.75), FEV0.75/forced vital capacity, and flows at 50% and 75% of vital capacity) in those with an RSV LRTI compared to children without. Whether viral LRTI cause subsequent airway disease or are merely markers for pre-existing abnormal lung function has not been definitively determined.[100] The role of atopy, predisposition to asthma and post-infection airway remodeling in relationship to LRTI and subsequent wheezing in childhood is also not clear.[100, 106] A combination of viral factors and innate and adaptive immune responses, in the setting of a susceptible genetic background and a young or elderly host appear to drive clinical outcome. [107, 108] An ongoing study of premature infants and viral LRTI, including baseline pulmonary function testing, seeks to determine if the viral respiratory infection is causal of the increased long-term morbidity or merely a marker for children with more severe pre-existing lung disease, as has been suggested for term infants (ClinicalTrials.gov NCT01789268). This question is important when evaluating selective vaccine strategies to prevent severe LRTI and chronic disease.
Susceptibility Factors for Enhanced Inflammation
The immune system is a double-edged sword – too little response and microbes invade and injure, too robust a host response may result in bystander injury and disease. In addition to exposure to infectious agents, there appear to be certain intrinsic factors that result in enhanced inflammatory responses in some individuals as adult, child or preterm infant, when compared to others. There is evidence to suggest that preterm infants may be affected by both relative immunosuppression and more robust immune responses than full term infants. We conclude this chapter with a review of susceptibility factors identified or suggested to enhance inflammation in the prematurely born.
Genetics
Genetics that predispose to shortened pregnancy, especially if related to increased inflammation, naturally increase the risk of inflammatory lung disease of prematurity. For example, elevated mid-trimester vaginal IL-1β is associated with increased risk for spontaneous preterm birth. Homozygous carriers of IL1RN*1, a single nucleotide polymorphism in the IL-1 receptor antagonist (IL-1ra) gene, a genotype associated with elevated IL-1β, are at increased risk for preterm birth and an example of genetic polymorphisms that affect the innate immune system and risks of prematurity. [109] In women who had a preterm birth, the combination of clinical chorioamnionitis and IL-10 (−1082)*G allele was associated with an increased risk for delivery before 29 weeks’ gestation, suggesting a gene-environment interaction. [110]
In infants, twin studies suggest significant genetic susceptibility to BPD. [111] Relative to inflammation, genotype analysis, after multiple comparisons correction, revealed two significant single-nucleotide polymorphism (SNPs), rs3771150 (IL-18RAP) and rs3771171 (IL-18R1), in African Americans (AAs) with BPD (vs. AAs without BPD; q < 0.05). No associations with Caucasian (CA) BPD, AA or CA respiratory distress syndrome (RDS), or prematurity in either AAs or CAs were identified with these SNPs. [112] Functional polymorphisms in the promoter of NFKBIA that encodes IκBα, a negative regulator of NFκB, is associated with differential susceptibility to severe bronchopulmonary dysplasia, as well as other common inflammatory diseases of infant lung. [113] A number of additional studies, evaluating exome sequencing in extremes of disease, epigenomic regulation, transcriptome responses to exposures such as hyperoxia, and pathway analyses are ongoing to identify gene and gene regulatory susceptibility factors involved in pathogenesis of BPD [114–124].
Alterations in Immune Responses Due to Developmental Window of Preterm Delivery
Recent developments in miniaturization of technologies including assays based on polychromatic flow cytometry, multiplexed protein assays and low-input transcriptional analyses have begun to advance the field of neonatal immunology. Dowling and Levy provide a recent review of both in vivo and in vitro approaches to studying early-life immuno-development, as well as a summary of unique characteristics of the preterm and term innate and adaptive immune systems. [125]
The innate immune responses of full term infants, including the function and recruitment of granulocytes, natural killer (NK) cells and antigen presenting cells are characterized as immature and functionally suppressed. [125] Innate immune responses in human preterm infants have been less well characterized. [126, 127] Fetal cells, including NK cells, have enhanced sensitivity to the immuno-suppressive effects of TGF beta. [128] Early life antigen presenting cells tend to produce more IL-6, IL-10 and IL-27, predominantly immunosuppressive cytokines. Intriguingly, a recent study has in addition suggested that CD71+ nucleated red blood cells (erythroid precursor cells) that are typically increased in fetal blood, especially in pregnancies complicated by placental insufficiency, appear to suppress phagocyte and antigen presenting cell stimulus-induced TNF alpha production suggesting an immunosuppressive function. [126]
Lymphocytes and the adaptive immune system provide a critical defense against intracellular, including viral, infections. Reduced CD4+ T cells result in impaired immune response to pathogens. CD8+ T cells and NK cells provide protection from viral infection but also contribute to immunopathology by contact-dependent effector functions (ex. perforin and FasL). IFN-γ and particularly TNF-α are thought to be primary perpetrators of T-cell-mediated lung injury yet are also important for antimicrobial defense.[129]
The fetal and neonatal period are unique immune developmental stages in which adaptive responses are highly plastic and dependent on gestational age. [130, 131] Although relatively little literature refers to detailed phenotyping of lymphocytic maturation in the prematurely born infant, investigators have numerically evaluated classes of lymphocytes in the human fetus and young child. The total circulating white cell counts increase through the latter half of gestation until term delivery and then decreases slightly to adult levels. The percentage of lymphocytes decreases from approximately 80% at 18–36wks (median 26wks) to 40% at term delivery to 21% in the adult human, based on cord blood sampling at delivery or cordocentesis [132, 133]. The proportion of CD3+ cells however increases with gestational age and in the presence of an infection; In normal pregnancies, circulating CD4+ T cells numbers are inversely related to gestational age and the fetal percentage of CD8+ T cells was reduced, increasing before term (9.5 – 15.7%) such that CD4+/CD8+ ratios also vary inversely with gestational age, higher in very-low-birth-weight (VLBW) infants than full-term. [134, 135] Maternal disease may alter fetal lymphocytes. Preeclampsia had a significant effect on T cell distribution associating with fewer CD4+ cells and CD4+CD8+ double-positive cells, decreased CD4+/CD8+ ratios, reduced Th2 and regulatory T cell subsets in cord blood, while maternal betamethasone therapy also associates with higher CD3+ cell proportion and a lower proportion of NK cells. [136, 137]
Evidence for Lymphocytic Abnormalities in Premature Infants with Lung Disease
Several lines of investigation suggest a role for dysregulation of CD4+ responses in BPD. In animal models, T cells accumulate in the lungs of preterm lambs exposed to LPS in utero [138] and preterm baboons that develop BPD were found to have abundant CD4+ T cells in the lung parenchyma [139]. Significant infiltrates of T cells were noted in distal lung of infants who died with BPD as compared to gestational age matched without lung disease. [140] In serial blood samples from premature infants with RDS born <1200g and <30wks gestation, Ballabh et al demonstrated a reduction in absolute lymphocyte count, as well as the percentage and the absolute number of CD4+ T cells, in those who progressed to BPD (p<0.03), significant even on day one of life [141]. More activated T cells in those who go on to develop BPD may reflect sequestration and activation of cells within the lung. [141, 142] CD4+ T cell percentage continued to decrease with postnatal age. Berrington et al measured lymphocyte subclasses in premature infants just prior to first immunizations [143]. At 7–8 weeks of age, prematurely born infants had lower absolute lymphocyte, T cell, B cell, and T helper cell counts, and lower CD4+/CD8+ T cell ratio, than term infants, as well as increased proportion of Treg (CD4+CD25+) cells and decreased CD45RA+ naïve cells. By 6 months, the B cell population had numerically normalized but T cell abnormalities persisted.
Recent studies have challenged the concept that CD3+ T cell responses are uniformly impaired in neonates, especially in preterm infants. Although the majority of the newborn CD4+ T cells are naïve, activation markers like CD25, CD69 and CD45RO+ are enhanced on CD4+ cells of prematurely born infants [144]. Likewise, the proportion of cord blood CD8+ T cells that are CD45RO+, suggesting activation, is also higher at lower gestational age. [145] Several reports now demonstrate a correlation between T cell activation, as measured by CD45RO expression, and premature infants’ adverse outcomes, such as BPD, necrotizing enterocolitis and periventricular leukomalacia. [141, 146, 147] CD4+ and CD8+ T cells at lower gestational age are also shown to have enhanced cytokine production with in vitro stimulation suggesting that enhanced CD45RO expression in preterms is accompanied by inducible effector functions that may contribute to the severity of lung disease. [145, 148] A report that regulatory CD4+ T cells (CD4+CD25hiFoxP3+CD127Dim) were significantly reduced in cord blood of preterm infants who developed BPD further raises the potential for enhanced inflammation due to reduced inhibitory control. [148] It has been suggested that the relatively activated CD4+ and CD8+ T cell phenotype at early gestational ages is reminiscent of recovery from bone marrow ablation in adults and represents rapid homeostatic expansion in a lymphopenic host. [147] Further, intra-amniotic administration of IL-1beta to rhesus monkeys at 80% gestation resulted in reduction in frequency of Treg cells in lymphoid organs while Th17, IL-17A producing, cells were increased potentially linking in utero innate immune activity to inflammatory lymphocyte-mediated injury. [149]
Some insight into potential T cell immunopathology in BPD may be gained from animal and adult models of inflammatory lung disease. In a baboon model of BPD, thymic involution, increased peripheral T cells carrying markers of maturation, robust non-specific cytokine secretion and increased autoreactive CD4+ T cells in the lung interstitium, were associated with an increase in bombesin-like peptides (BLP) [139, 150]. Treatment with a neutralizing antibody to BLP corrected the thymic and lung pathology seen in preterm baboons treated with 100% oxygen [139]. BLP is also elevated in preterm human infants with BPD [151] suggesting a mechanism linking lymphocyte dysregulation and BPD.
Age at First Infection
The degree of lung and immune system maturation at the time of infection influences cytopathogenic responses to virus and perhaps bacteria but also appears to set a trajectory of immune response to subsequent challenge. Newborn mice infected with RSV have, compared to mice infected at a slightly later age, increased BAL fluid numbers of Th2 type CD4+IL-4+ cells and fewer CD4+IFNγ + cells when re-infected in adulthood.[152] Likewise, mice infected with influenza A within one week of birth showed enhanced airway hyper-reactivity, chronic pulmonary inflammation, and diffuse emphysematous-type lesions as adults. An adaptive immune insufficiency was most apparent in the neonatal CD8+ T cells. Newborn infection was associated with reduced and delayed IFN-γ responses as compared to infection in older animals. RSV infected neonatal mice recruited CD8+ T cells defective in IFN-γ production in association with mild symptoms. Re-infection as adults, however, resulted in limited viral replication but enhanced inflammation and T cell recruitment, including Th2 cells and eosinophils [153, 154]. Depletion of CD8+ T cells (but not CD4) cells during the primary neonatal infection was protective against the adult challenge. Recall responses from neonatal-primed and adult-primed mice were associated with IFN-γ secretion, indicative of a Th1 response. However, interleukin IL-4 and IL-5 secretion were enhanced only in neonatal-primed mice. Re-challenge of these mice, primed as newborns, was also associated with increased concentrations of monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α) and RANTES in the lung. It is suggested then that neonatal T cells, in particular IFN-γ-deficient CD8+ T cells, play a crucial role in regulation of immune responses after neonatal infection. In these neonatal animal models, adoptive transfer of naive CD8+ cells, from wild-type but not from IFN-γ deficient donors, significantly lowered pulmonary viral titers and greatly improved pulmonary function as adults, supporting the importance of IFN-γ secreting CD8+ T cells in determining disease outcome [155]
A strong argument has now been advanced that childhood wheezing and atopy is related to reduced cord blood IFN-γ. In a study of infants predisposed to asthma and atopy, less robust mitogen- or specific antigen-induced IFN-γ and IL-13 responses from cord blood cells were associated with more wheezing episodes in the first year of life in children infected with RSV and rhinovirus.[156] In the Childhood Origins of Asthma (COAST) Project, cytokine-response profiles of cord blood and 1-year mononuclear cells stimulated in vitro identified that cord blood IFN-γ responses were inversely related to the frequency of viral respiratory infections and wheezing in infancy while enhanced IFN-γ responses at one year correlated positively with the frequency of preceding viral infections. [157] Severity of asthma has been associated with excessive IFN-γ production, particularly by CD8+ T cells, potentially reflecting the cytotoxic effect of the cytokine. These data suggest that neonatal IFN-γ responses influence subsequent antiviral activity. Conversely, the frequency of viral infections in infancy can influence IFN-γ responses.
Neutralizing antibodies provide important antiviral protection in infants. Higher RSV neutralizing antibody titers in both premature and term infants are associated with protection from infection and LRTI, an effect also supported by the success of palvizumab in preventing severe RSV disease in premature infants.[158] Transplacental transfer of maternal antibodies is inversely related to length of gestation such that the more preterm infants have relatively less humoral protection contributing to disease risk. Since viral loads of RSV, hMPV, PIV and rhinovirus correlate with the severity of clinical disease, [159–162] it is suggested that infants with a greater ability to control viral replication upon first infection, via the presence of neutralizing antibody and a more robust IFN-γ response, are successful in limiting excessive antigen presentation, generating protective immune responses associated with viral clearance, and avoiding immuno-pathogenesis. To date, no study has evaluated antibody and cellular immune phenotype together with viral load measurements in infants with respiratory infections. Additionally, the association between these factors and disease severity has not been explored in premature infants.
Overall, alterations in lymphocyte-related immunity occur and are dependent on gestational age, maternal influences, postnatal oxidant stress and viral diseases. There is burgeoning data in this area in premature infants though as yet minimal knowledge of specific mechanisms by which lymphocytes participate in respiratory outcomes in premature infants.
Altered Establishment of Colonizing Microbiota
A developing body of research suggests that both the acquisition and maintenance of bacterial populations in the gut soon after birth are important drivers of the development of both systemic and mucosal immunity.[7] Recent advances in high-throughput sequencing technology have provided insight into the gut microbiome and are beginning to describe the diversity and dynamics of the microbial populations in both health and disease. Although the exact factors that control the interactions between the gut epithelial cells, the gut associated lymphoid tissue (GALT), and the gut microbiome are not yet clear, all three components appear to play a significant role in the induction of immune tolerance to luminal bacterial antigens and the maintenance of homeostasis. Proposed mechanisms identified in animal models include the blocking of innate signaling via TLR-4 receptors, the development or expansion of Fox P3+ T regulatory cells, and enhanced IL-10 production in the gut induced by commensal bacteria.[163, 164] The presence of specific species of bacteria may also be crucial to the development of gut tolerance as suggested by the relatively decreased amounts of Bacteroides, Bifidobacter, and Lactobacillus species in patients with inflammatory bowel disease.[7] Additionally, the timing of acquisition of gut bacteria may be critical for the positive effects on health. Neonatal IL-10 deficient mice exposed to bacterial antigens had delayed development of colitis at 18 weeks of age compared to those not exposed. Decreased IFN-γ and IL-17 production in explanted intestinal tissue and spleen cells following stimulation with gut bacteria also suggests that exposure of the neonatal immune system to antigens of the microbiome is associated with both mucosal and systemic immune tolerance.[165] These findings may be especially relevant to the pre-term infant in view of recent data showing an inverse relationship between antibiotic therapy and parenteral nutrition with fecal diversity in the infants born at < 29 weeks gestation.[166] A recent study in elderly adults, also suggests improved protection from influenza infection with oral provision of a Bifidobacterium longum species. [167]
Attention has recently turned to determining the microbiome of the respiratory tract in premature infants. The conventional theory that the lower airways are sterile has been challenged by identification of organisms in the deep lung of adults and now infants and children, initially, not surprisingly associated with diseases such as cystic fibrosis, chronic obstructive pulmonary disease and asthma, but also now as a “normal microbiome” in healthy patients. [168–170] In preterm infants, Lohmann, et.al. described non-sterile tracheal aspirates with a predominance of Acinetobacter in samples taken at intubation in the delivery room, and a persistent decrease in diversity of organisms over the first month of life in those who went on to develop BPD. [171] Early sustained airway bacterial colonization in infants < 1250 grams at birth and intubated for at least 3 weeks was detected within 7 days of life, dominated by Staphylococcus and Ureaplasma.[172] Ongoing studies promise further longitudinal intestinal and respiratory microbiome and viral infection data and correlations to respiratory outcomes in preterm and full term infants (Clinicaltrials.gov: NCT01607216 and NCT01789268, funding U01HL101813 and HHSN272201200005C, respectively)
That the microbes, the bacterial, virus, fungi and others, that flourish on human skin and mucosa affect the metabolism, immune system, health and disease of their host is becoming more clear. Just what those effects are in the premature infant and how they affect susceptibility to infections and alter respiratory outcomes is an important area of current research.
Key Points.
In the first year of life, preterm infants are re-hospitalized 2–5 fold more frequently than infants born at term, primarily for respiratory symptoms.
Mediators of inflammation tend to enhance lung maturation but impair alveolar septation and developmental vascular remodeling.
The developmental age of the immune system at birth, and at early age infections, may significantly alter the acute response, and the sequelae, to inflammatory stimuli.
Pre- and post-natal infection and immune responses contribute to the severity of chronic lung disease of prematurity.
Acknowledgments
Funding Source: Department of Pediatrics, University of Rochester Medical Center
NIH/NHLBI/NICHD 1U01 HL101813
NIH/NHLBI 1U01 HL 122628
NIH/NIAID HHSN272201200005C
Abbreviations
- (G/N/B)ALT
Gut/Nasal/Bronchial Associated Lymphoid Tissue
- BLP
Bombesin Like Peptide
- BPD
Bronchopulmonary Dysplasia
- COAST
Childhood Origins of Asthma Study
- CMV
Cytomegalovirus
- DC
Dendritic Cell
- ELBW
Extremely Low Birth Weight
- EPT
Early Preterm
- FEV
Forced Expiratory Volume
- hMPV
Human Metapneumovirus
- IFN-γ
Interferon gamma
- IL
Interleukin
- LPT
Late Preterm
- LRTI
Lower Respiratory Tract Infection
- NFκB
Nuclear Factor kappa beta
- NK
Natural Killer Cell
- NICU
Neonatal Intensive Care Unit
- NVRI
Nosocomial Viral Respiratory Infections
- rtPCR
Real Time Polymerase Chain Reaction
- RSV
Respiratory Syncytial Virus
- SRD
symptomatic respiratory dysfunction
- TGF-β
Transforming Growth Factor beta
- TNF-α
Tumor Necrosis Factor alpha
- Treg
CD4+ T Regulatory Cell
- VLBW
very low birth weight
Footnotes
Disclosures: The authors have no conflicts of interest or relevant financial interests to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65. [PubMed] [Google Scholar]
- 2.McLaurin KK, Hall CB, Jackson EA, Owens OV, Mahadevia PJ. Persistence of morbidity and cost differences between late-preterm and term infants during the first year of life. Pediatrics. 2009;123(2):653–659. doi: 10.1542/peds.2008-1439. [DOI] [PubMed] [Google Scholar]
- 3.Gunville CF, Sontag MK, Stratton KA, Ranade DJ, Abman SH, Mourani PM. Scope and impact of early and late preterm infants admitted to the PICU with respiratory illness. J Pediatr. 2010;157(2):209–214. e201. doi: 10.1016/j.jpeds.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Underwood MA, Danielsen B, Gilbert WM. Cost, causes and rates of rehospitalization of preterm infants. J Perinatol. 2007;27(10):614–619. doi: 10.1038/sj.jp.7211801. [DOI] [PubMed] [Google Scholar]
- 5.Wade KC, Lorch SA, Bakewell-Sachs S, Medoff-Cooper B, Silber JH, Escobar GJ. Pediatric care for preterm infants after NICU discharge: high number of office visits and prescription medications. J Perinatol. 2008;28(10):696–701. doi: 10.1038/jp.2008.74. [DOI] [PubMed] [Google Scholar]
- 6.Dimmitt RA, Staley EM, Chuang G, Tanner SM, Soltau TD, Lorenz RG. Role of postnatal acquisition of the intestinal microbiome in the early development of immune function. J Pediatr Gastroenterol Nutr. 2010;51(3):262–273. doi: 10.1097/MPG.0b013e3181e1a114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conroy ME, Shi HN, Walker WA. The long-term health effects of neonatal microbial flora. Curr Opin Allergy Clin Immunol. 2009;9(3):197–201. doi: 10.1097/ACI.0b013e32832b3f1d. [DOI] [PubMed] [Google Scholar]
- 8.Sydora BC, McFarlane SM, Doyle JS, Fedorak RN. Neonatal exposure to fecal antigens reduces intestinal inflammation. Inflamm Bowel Dis. 2010 doi: 10.1002/ibd.21453. [DOI] [PubMed] [Google Scholar]
- 9.Panthagani ID, Stevens TP, Lynch K, Conn KM, Halterman JS. Recurrent wheezing in VLBW infants without bronchopulmonary dysplasia (BPD) E-PAS2006. Abstract# 751028. [Google Scholar]
- 10.O’Reilly MA, Marr SH, Yee M, McGrath-Morrow SA, Lawrence BP. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med. 2008;177(10):1103–1110. doi: 10.1164/rccm.200712-1839OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maduekwe ET, Buczynski BW, Yee M, Rangasamy T, Stevens TP, Lawrence BP, O’Reilly MA. Cumulative neonatal oxygen exposure predicts response of adult mice infected with influenza A virus. Pediatr Pulmonol. 2014 doi: 10.1002/ppul.23063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speer CP. New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate. 2001;79(3–4):205–209. doi: 10.1159/000047092. [DOI] [PubMed] [Google Scholar]
- 13.Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev. 1998;53(1):81–94. doi: 10.1016/s0378-3782(98)00045-0. [DOI] [PubMed] [Google Scholar]
- 14.Viscardi RM. Perinatal inflammation and lung injury. Semin Fetal Neonatal Med. 2012;17(1):30–35. doi: 10.1016/j.siny.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhandari V. Postnatal inflammation in the pathogenesis of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol. 2014;100(3):189–201. doi: 10.1002/bdra.23220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan RM, Ahmed Q, Lakshminrusimha S. Inflammatory mediators in the immunobiology of bronchopulmonary dysplasia. Clin Rev Allergy Immunol. 2008;34(2):174–190. doi: 10.1007/s12016-007-8031-4. [DOI] [PubMed] [Google Scholar]
- 17.Baud O, Emilie D, Pelletier E, Lacaze-Masmonteil T, Zupan V, Fernandez H, Dehan M, Frydman R, Ville Y. Amniotic fluid concentrations of interleukin-1beta, interleukin-6 and TNF-alpha in chorioamnionitis before 32 weeks of gestation: histological associations and neonatal outcome. Br J Obstet Gynaecol. 1999;106(1):72–77. doi: 10.1111/j.1471-0528.1999.tb08088.x. [DOI] [PubMed] [Google Scholar]
- 18.Viscardi RM, Muhumuza CK, Rodriguez A, Fairchild KD, Sun CC, Gross GW, Campbell AB, Wilson PD, Hester L, Hasday JD. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55(6):1009–1017. doi: 10.1203/01.pdr.0000127015.60185.8a. [DOI] [PubMed] [Google Scholar]
- 19.Kotecha S, Wilson L, Wangoo A, Silverman M, Shaw RJ. Increase in interleukin (IL)-1 beta and IL-6 in bronchoalveolar lavage fluid obtained from infants with chronic lung disease of prematurity. Pediatr Res. 1996;40(2):250–256. doi: 10.1203/00006450-199608000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Kotecha S, Mildner RJ, Prince LR, Vyas JR, Currie AE, Lawson RA, Whyte MK. The role of neutrophil apoptosis in the resolution of acute lung injury in newborn infants. Thorax. 2003;58(11):961–967. doi: 10.1136/thorax.58.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baier RJ, Majid A, Parupia H, Loggins J, Kruger TE. CC chemokine concentrations increase in respiratory distress syndrome and correlate with development of bronchopulmonary dysplasia. Pediatr Pulmonol. 2004;37(2):137–148. doi: 10.1002/ppul.10417. [DOI] [PubMed] [Google Scholar]
- 22.Munshi UK, Niu JO, Siddiq MM, Parton LA. Elevation of interleukin-8 and interleukin-6 precedes the influx of neutrophils in tracheal aspirates from preterm infants who develop bronchopulmonary dysplasia. Pediatr Pulmonol. 1997;24(5):331–336. doi: 10.1002/(sici)1099-0496(199711)24:5<331::aid-ppul5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Bose CL, Dammann CE, Laughon MM. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal Ed. 2008;93(6):F455–461. doi: 10.1136/adc.2007.121327. [DOI] [PubMed] [Google Scholar]
- 24.Beeton ML, Maxwell NC, Davies PL, Nuttall D, McGreal E, Chakraborty M, Spiller OB, Kotecha S. Role of pulmonary infection in the development of chronic lung disease of prematurity. Eur Respir J. 2011;37(6):1424–1430. doi: 10.1183/09031936.00037810. [DOI] [PubMed] [Google Scholar]
- 25.Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, Thorsen P, Higgins RD Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123(4):1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bose C, Laughon M, Allred EN, Van Marter LJ, O’Shea TM, Ehrenkranz RA, Fichorova R, Leviton A Elgan Study I. Blood protein concentrations in the first two postnatal weeks that predict bronchopulmonary dysplasia among infants born before the 28th week of gestation. Pediatr Res. 2011;69(4):347–353. doi: 10.1203/PDR.0b013e31820a58f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharya S, Go D, Krenitsky DL, Huyck HL, Solleti SK, Lunger VA, Metlay L, Srisuma S, Wert SE, Mariani TJ, et al. Genome-wide transcriptional profiling reveals connective tissue mast cell accumulation in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2012;186(4):349–358. doi: 10.1164/rccm.201203-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheah FC, Winterbourn CC, Darlow BA, Mocatta TJ, Vissers MC. Nuclear factor kappaB activation in pulmonary leukocytes from infants with hyaline membrane disease: associations with chorioamnionitis and Ureaplasma urealyticum colonization. Pediatr Res. 2005;57(5 Pt 1):616–623. doi: 10.1203/01.PDR.0000156209.37627.82. [DOI] [PubMed] [Google Scholar]
- 29.Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC, Yull FE, Prince LS. NF-kappaB signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol. 2011;187(5):2740–2747. doi: 10.4049/jimmunol.1101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamin JT, Carver BJ, Plosa EJ, Yamamoto Y, Miller JD, Liu JH, van der Meer R, Blackwell TS, Prince LS. NF-kappaB activation limits airway branching through inhibition of Sp1-mediated fibroblast growth factor-10 expression. J Immunol. 2010;185(8):4896–4903. doi: 10.4049/jimmunol.1001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jobe AH, Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res. 2001;2(1):27–32. doi: 10.1186/rr35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Cras TD, Hardie WD, Deutsch GH, Albertine KH, Ikegami M, Whitsett JA, Korfhagen TR. Transient induction of TGF-alpha disrupts lung morphogenesis, causing pulmonary disease in adulthood. Am J Physiol Lung Cell Mol Physiol. 2004;287(4):L718–729. doi: 10.1152/ajplung.00084.2004. [DOI] [PubMed] [Google Scholar]
- 33.Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1178–1185. doi: 10.1152/ajplung.00049.2004. [DOI] [PubMed] [Google Scholar]
- 34.Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, Rael J, Grove T, Morgan TK, Clewell W, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e121–125 e115. doi: 10.1016/j.ajog.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Buhimschi CS, Temoin S, Bhandari V, Han YW, Buhimschi IA. Comparative microbial analysis of paired amniotic fluid and cord blood from pregnancies complicated by preterm birth and early-onset neonatal sepsis. PLoS One. 2013;8(2):e56131. doi: 10.1371/journal.pone.0056131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ardissone AN, de la Cruz DM, Davis-Richardson AG, Rechcigl KT, Li N, Drew JC, Murgas-Torrazza R, Sharma R, Hudak ML, Triplett EW, et al. Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS One. 2014;9(3):e90784. doi: 10.1371/journal.pone.0090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wassenaar TM, Panigrahi P. Is a foetus developing in a sterile environment? Lett Appl Microbiol. 2014;59(6):572–579. doi: 10.1111/lam.12334. [DOI] [PubMed] [Google Scholar]
- 38.Payne MS, Bayatibojakhi S. Exploring preterm birth as a polymicrobial disease: an overview of the uterine microbiome. Front Immunol. 2014;5:595. doi: 10.3389/fimmu.2014.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antony KM, Ma J, Mitchell KB, Racusin DA, Versalovic J, Aagaard K. The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am J Obstet Gynecol. 2015;212(5):653.e651–616. doi: 10.1016/j.ajog.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra265. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, Gotsch F, Dong Z, Ahmed AI, Shaman M, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43(1):19–36. doi: 10.1515/jpm-2014-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smulian JC, Shen-Schwarz S, Vintzileos AM, Lake MF, Ananth CV. Clinical chorioamnionitis and histologic placental inflammation. Obstet Gynecol. 1999;94(6):1000–1005. doi: 10.1016/s0029-7844(99)00416-0. [DOI] [PubMed] [Google Scholar]
- 43.Kim SM, Romero R, Park JW, Oh KJ, Jun JK, Yoon BH. The relationship between the intensity of intra-amniotic inflammation and the presence and severity of acute histologic chorioamnionitis in preterm gestation. J Matern Fetal Neonatal Med. 2014:1–10. doi: 10.3109/14767058.2014.961009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2014:1–16. doi: 10.3109/14767058.2014.958463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katzman PJ. Chronic inflammatory lesions of the placenta. Semin Perinatol. 2015;39(1):20–26. doi: 10.1053/j.semperi.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- 47.Viscardi RM, Hasday JD. Role of Ureaplasma species in Neonatal Chronic Lung Disease: Epidemiologic and Experimental Evidence. Pediatr Res. 2009 doi: 10.1203/PDR.0b013e31819dc2f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kallapur SG, Moss JTM, Newnham JP, Ikegami M, Jobe AH. Recruited inflammatory cells mediate endotoxin-induced lung maturation in preterm fetal lambs. Am J Respir Crit Care Med. 2005;172:1315–1321. doi: 10.1164/rccm.200506-1007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moss TJM, Knox CL, Kallapur SG, Nitsos I, Theodoropoulos C, Newnham JP, Ikegami M, Jobe AH. Experimental amniotic fluid infection in sheep; effects of Ureaplasma parvum. Am J Obstet Gynecol. 2007 doi: 10.1016/j.ajog.2007.06.065. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willet KE, Jobe AH, Ikegami M, Brennan S, Newnham J, Sly PD. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr Res. 2000;48:782–788. doi: 10.1203/00006450-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Ikegami M, Jobe A. Postnatal lung inflammation increased by ventilation of preterm lambs exposed antenatally to E. coli endotoxin. Pediatr Res. 2002;52:356–362. doi: 10.1203/00006450-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, Martin C. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140(2):171–176. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- 53.Lowe J, Watkins WJ, Edwards MO, Spiller OB, Jacqz-Aigrain E, Kotecha SJ, Kotecha S. Association between pulmonary ureaplasma colonization and bronchopulmonary dysplasia in preterm infants: updated systematic review and meta-analysis. Pediatr Infect Dis J. 2014;33(7):697–702. doi: 10.1097/INF.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 54.Kallapur SG, Kramer BW, Jobe AH. Ureaplasma and BPD. Semin Perinatol. 2013;37(2):94–101. doi: 10.1053/j.semperi.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lacaze-Masmonteil T. That chorioamnionitis is a risk factor for bronchopulmonary dysplasia--the case against. Paediatr Respir Rev. 2014;15(1):53–55. doi: 10.1016/j.prrv.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Thomas W, Speer CP. Chorioamnionitis is essential in the evolution of bronchopulmonary dysplasia--the case in favour. Paediatr Respir Rev. 2014;15(1):49–52. doi: 10.1016/j.prrv.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 57.Laughon M, Allred EN, Bose C, O’Shea TM, Van Marter LJ, Ehrenkranz RA, Leviton A. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics. 2009;123(4):1124–1131. doi: 10.1542/peds.2008-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soraisham AS, Singhal N, McMillan DD, Sauve RS, Lee SK. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol. 2009;200(4):372 e371–376. doi: 10.1016/j.ajog.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 59.Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics. 2009;123(5):1314–1319. doi: 10.1542/peds.2008-0656. [DOI] [PubMed] [Google Scholar]
- 60.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 61.Stimac M, Juretic E, Vukelic V, Matasic NP, Kos M, Babic D. Effect of chorioamnionitis on mortality, early onset neonatal sepsis and bronchopulmonary dysplasia in preterm neonates with birth weight of < 1,500 grams. Coll Antropol. 2014;38(1):167–171. [PubMed] [Google Scholar]
- 62.Watterberg KL, Scott SM, Naeye RL. Chorioamnionitis, cortisol, and acute lung disease in very low birth weight infants. Pediatrics. 1997;99:E6. doi: 10.1542/peds.99.2.e6. [DOI] [PubMed] [Google Scholar]
- 63.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced Chorioamnionitis Modulates Innate Immunity of Monocytes in Preterm Sheep. Am J Respir Crit Care Med. 2005;171(1):73–77. doi: 10.1164/rccm.200406-745OC. [DOI] [PubMed] [Google Scholar]
- 64.Maitre NL, Ballard RA, Ellenberg JH, Davis SD, Greenberg JM, Hamvas A, Pryhuber GS Prematurity Respiratory Outcomes P. Respiratory consequences of prematurity: evolution of a diagnosis and development of a comprehensive approach. J Perinatol. 2015;35(5):313–321. doi: 10.1038/jp.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vinnars MT, Nasiell J, Holmstrom G, Norman M, Westgren M, Papadogiannakis N. Association between placental pathology and neonatal outcome in preeclampsia: a large cohort study. Hypertens Pregnancy. 2014;33(2):145–158. doi: 10.3109/10641955.2013.842584. [DOI] [PubMed] [Google Scholar]
- 66.Yen TA, Yang HI, Hsieh WS, Chou HC, Chen CY, Tsou KI, Tsao PN Taiwan Premature Infant Developmental Collaborative Study G. Preeclampsia and the risk of bronchopulmonary dysplasia in VLBW infants: a population based study. PLoS One. 2013;8(9):e75168. doi: 10.1371/journal.pone.0075168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lees C, Marlow N, Arabin B, Bilardo CM, Brezinka C, Derks JB, Duvekot J, Frusca T, Diemert A, Ferrazzi E, et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE) Ultrasound Obstet Gynecol. 2013;42(4):400–408. doi: 10.1002/uog.13190. [DOI] [PubMed] [Google Scholar]
- 68.Eriksson L, Haglund B, Odlind V, Altman M, Kieler H. Prenatal inflammatory risk factors for development of bronchopulmonary dysplasia. Pediatr Pulmonol. 2014;49(7):665–672. doi: 10.1002/ppul.22881. [DOI] [PubMed] [Google Scholar]
- 69.Ozkan H, Cetinkaya M, Koksal N. Increased incidence of bronchopulmonary dysplasia in preterm infants exposed to preeclampsia. J Matern Fetal Neonatal Med. 2012;25(12):2681–2685. doi: 10.3109/14767058.2012.708371. [DOI] [PubMed] [Google Scholar]
- 70.O’Shea JE, Davis PG, Doyle LW Victorian Infant Collaborative Study G. Maternal preeclampsia and risk of bronchopulmonary dysplasia in preterm infants. Pediatr Res. 2012;71(2):210–214. doi: 10.1038/pr.2011.27. [DOI] [PubMed] [Google Scholar]
- 71.Mestan KK, Check J, Minturn L, Yallapragada S, Farrow KN, Liu X, Su E, Porta N, Gotteiner N, Ernst LM. Placental pathologic changes of maternal vascular underperfusion in bronchopulmonary dysplasia and pulmonary hypertension. Placenta. 2014;35(8):570–574. doi: 10.1016/j.placenta.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahn HM, Park EA, Cho SJ, Kim YJ, Park HS. The association of histological chorioamnionitis and antenatal steroids on neonatal outcome in preterm infants born at less than thirty-four weeks’ gestation. Neonatology. 2012;102(4):259–264. doi: 10.1159/000339577. [DOI] [PubMed] [Google Scholar]
- 73.Jonsson B, Tullus K, Brauner A, Lu Y, Noack G. Early increase of TNF alpha and IL-6 in tracheobronchial aspirate fluid indicator of subsequent chronic lung disease in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1997;77(3):F198–201. doi: 10.1136/fn.77.3.f198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lorch SA, Banks BA, Christie J, Merrill JD, Althaus J, Schmidt K, Ballard PL, Ischiropoulos H, Ballard RA. Plasma 3-nitrotyrosine and outcome in neonates with severe bronchopulmonary dysplasia after inhaled nitric oxide. Free Radic Biol Med. 2003;34(9):1146–1152. doi: 10.1016/s0891-5849(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 75.Varsila E, Pesonen E, Andersson S. Early protein oxidation in the neonatal lung is related to development of chronic lung disease. Acta Paediatr. 1995;84(11):1296–1299. doi: 10.1111/j.1651-2227.1995.tb13552.x. [DOI] [PubMed] [Google Scholar]
- 76.Schlenzig JS, Bervoets K, von Loewenich V, Bohles H. Urinary malondialdehyde concentration in preterm neonates: is there a relationship to disease entities of neonatal intensive care? Acta Paediatr. 1993;82(2):202–205. doi: 10.1111/j.1651-2227.1993.tb12639.x. [DOI] [PubMed] [Google Scholar]
- 77.Shah J, Jefferies AL, Yoon EW, Lee SK, Shah PS Canadian Neonatal N. Risk Factors and Outcomes of Late-Onset Bacterial Sepsis in Preterm Neonates Born at < 32 Weeks’ Gestation. Am J Perinatol. 2015;32(7):675–682. doi: 10.1055/s-0034-1393936. [DOI] [PubMed] [Google Scholar]
- 78.Landry JS, Menzies D. Occurrence and severity of bronchopulmonary dysplasia and respiratory distress syndrome after a preterm birth. Paediatr Child Health. 2011;16(7):399–403. doi: 10.1093/pch/16.7.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klinger G, Levy I, Sirota L, Boyko V, Lerner-Geva L, Reichman B Israel Neonatal N. Outcome of early-onset sepsis in a national cohort of very low birth weight infants. Pediatrics. 2010;125(4):e736–740. doi: 10.1542/peds.2009-2017. [DOI] [PubMed] [Google Scholar]
- 80.Lardon-Fernandez M, Uberos J, Molina-Oya M, Narbona-Lopez E. Epidemiological factors involved in the development of bronchopulmonary dysplasia in very low birth-weight preterm infants. Minerva Pediatr. 2015 doi: 10.23736/S0026-4946.16.04215-8. [DOI] [PubMed] [Google Scholar]
- 81.Ivarsson M, Schollin J, Bjorkqvist M. Staphylococcus epidermidis and Staphylococcus aureus trigger different interleukin-8 and intercellular adhesion molecule-1 in lung cells: implications for inflammatory complications following neonatal sepsis. Acta Paediatr. 2013;102(10):1010–1016. doi: 10.1111/apa.12350. [DOI] [PubMed] [Google Scholar]
- 82.Ohlsson A, Lacy JB. Intravenous immunoglobulin for preventing infection in preterm and/or low-birth-weight infants. Cochrane Database Syst Rev. 2004;(1):CD000361. doi: 10.1002/14651858.CD000361.pub2. [DOI] [PubMed] [Google Scholar]
- 83.Faden H, Wynn RJ, Campagna L, Ryan RM. Outbreak of adenovirus type 30 in a neonatal intensive care unit. J Pediatr. 2005;146(4):523–527. doi: 10.1016/j.jpeds.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 84.Verboon-Maciolek MA, Krediet TG, Gerards LJ, Fleer A, van Loon TM. Clinical and epidemiologic characteristics of viral infections in a neonatal intensive care unit during a 12-year period. Pediatr Infect Dis J. 2005;24(10):901–904. doi: 10.1097/01.inf.0000180471.03702.7f. [DOI] [PubMed] [Google Scholar]
- 85.Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L811–823. doi: 10.1152/ajplung.00325.2001. [DOI] [PubMed] [Google Scholar]
- 86.Gagneur A, Sizun J, Vallet S, Legr MC, Picard B, Talbot PJ. Coronavirus-related nosocomial viral respiratory infections in a neonatal and paediatric intensive care unit: a prospective study. J Hosp Infect. 2002;51(1):59–64. doi: 10.1053/jhin.2002.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meier J, Lienicke U, Tschirch E, Kruger DH, Wauer RR, Prosch S. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J Clin Microbiol. 2005;43(3):1318–1324. doi: 10.1128/JCM.43.3.1318-1324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morgan MA, el-Ghany el SM, Khalifa NA, Sherif A, Rasslan LR. Prevalence of cytomegalovirus (CMV) infection among neonatal intensive care unit (NICU) and healthcare workers. Egypt J Immunol. 2003;10(2):1–8. [PubMed] [Google Scholar]
- 89.Maschmann J, Hamprecht K, Dietz K, Jahn G, Speer CP. Cytomegalovirus infection of extremely low-birth weight infants via breast milk. Clin Infect Dis. 2001;33(12):1998–2003. doi: 10.1086/324345. [DOI] [PubMed] [Google Scholar]
- 90.Prosch S, Lienicke U, Priemer C, Flunker G, Seidel WF, Kruger DH, Wauer RR. Human adenovirus and human cytomegalovirus infections in preterm newborns: no association with bronchopulmonary dysplasia. Pediatr Res. 2002;52(2):219–224. doi: 10.1203/00006450-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 91.Neuberger P, Hamprecht K, Vochem M, Maschmann J, Speer CP, Jahn G, Poets CF, Goelz R. Case-control study of symptoms and neonatal outcome of human milk-transmitted cytomegalovirus infection in premature infants. J Pediatr. 2006;148(3):326–331. doi: 10.1016/j.jpeds.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 92.Capretti MG, Lanari M, Lazzarotto T, Gabrielli L, Pignatelli S, Corvaglia L, Tridapalli E, Faldella G. Very low birth weight infants born to cytomegalovirus-seropositive mothers fed with their mother’s milk: a prospective study. J Pediatr. 2009;154(6):842–848. doi: 10.1016/j.jpeds.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 93.Coclite E, Di Natale C, Nigro G. Congenital and perinatal cytomegalovirus lung infection. J Matern Fetal Neonatal Med. 2013;26(17):1671–1675. doi: 10.3109/14767058.2013.794207. [DOI] [PubMed] [Google Scholar]
- 94.Kuijpers TW, Vossen MT, Gent MR, Davin JC, Roos MT, Wertheim-van Dillen PM, Weel JF, Baars PA, van Lier RA. Frequencies of circulating cytolytic, CD45RA+CD27-, CD8+ T lymphocytes depend on infection with CMV. J Immunol. 2003;170(8):4342–4348. doi: 10.4049/jimmunol.170.8.4342. [DOI] [PubMed] [Google Scholar]
- 95.Miles DJ, van der Sande M, Jeffries D, Kaye S, Ismaili J, Ojuola O, Sanneh M, Touray ES, Waight P, Rowland-Jones S, et al. Cytomegalovirus infection in Gambian infants leads to profound CD8 T-cell differentiation. J Virol. 2007;81(11):5766–5776. doi: 10.1128/JVI.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hassan J, Dooley S, Hall W. Immunological response to cytomegalovirus in congenitally infected neonates. Clin Exp Immunol. 2007;147(3):465–471. doi: 10.1111/j.1365-2249.2007.03302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, Parson W, Bock G, Schonitzer D, Trannoy E, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168(11):5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 98.Lemanske RF, Jr, Dick EC, Swenson CA, Vrtis RF, Busse WW. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. The Journal of clinical investigation. 1989;83(1):1–10. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simoes EA, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick L, Groothuis JR. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J Allergy Clin Immunol. 2010;126(2):256–262. doi: 10.1016/j.jaci.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stein RT, Martinez FD. Respiratory syncytial virus and asthma: still no final answer. Thorax. 2010;65(12):1033–1034. doi: 10.1136/thx.2009.133967. [DOI] [PubMed] [Google Scholar]
- 101.Broughton S, Thomas MR, Marston L, Calvert SA, Marlow N, Peacock JL, Rafferty GF, Greenough A. Very prematurely born infants wheezing at follow-up: lung function and risk factors. Arch Dis Child. 2007;92(9):776–780. doi: 10.1136/adc.2006.112623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee KK, Hegele RG, Manfreda J, Wooldrage K, Becker AB, Ferguson AC, Dimich-Ward H, Watson WT, Chan-Yeung M. Relationship of early childhood viral exposures to respiratory symptoms, onset of possible asthma and atopy in high risk children: the Canadian Asthma Primary Prevention Study. Pediatr Pulmonol. 2007;42(3):290–297. doi: 10.1002/ppul.20578. [DOI] [PubMed] [Google Scholar]
- 103.Broughton S, Roberts A, Fox G, Pollina E, Zuckerman M, Chaudhry S, Greenough A. Prospective study of healthcare utilisation and respiratory morbidity due to RSV infection in prematurely born infants. Thorax. 2005;60(12):1039–1044. doi: 10.1136/thx.2004.037853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jacob SV, Coates AL, Lands LC, MacNeish CF, Riley SP, Hornby L, Outerbridge EW, Davis GM, Williams RL. Long-term pulmonary sequelae of severe bronchopulmonary dysplasia. J Pediatr. 1998;133(2):193–200. doi: 10.1016/s0022-3476(98)70220-3. [DOI] [PubMed] [Google Scholar]
- 105.Greenough A, Alexander J, Boit P, Boorman J, Burgess S, Burke A, Chetcuti PA, Cliff I, Lenney W, Lytle T, et al. School age outcome of hospitalisation with respiratory syncytial virus infection of prematurely born infants. Thorax. 2009;64(6):490–495. doi: 10.1136/thx.2008.095547. [DOI] [PubMed] [Google Scholar]
- 106.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, Gustafsson PM. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 107.Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82(5):2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Graham BS. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev. 2011;239(1):149–166. doi: 10.1111/j.1600-065X.2010.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Genc MR, Onderdonk A. Endogenous bacterial flora in pregnant women and the influence of maternal genetic variation. BJOG. 2011;118(2):154–163. doi: 10.1111/j.1471-0528.2010.02772.x. [DOI] [PubMed] [Google Scholar]
- 110.Kerk J, Dordelmann M, Bartels DB, Brinkhaus MJ, Dammann CE, Dork T, Dammann O. Multiplex measurement of cytokine/receptor gene polymorphisms and interaction between interleukin-10 (−1082) genotype and chorioamnionitis in extreme preterm delivery. J Soc Gynecol Investig. 2006;13(5):350–356. doi: 10.1016/j.jsgi.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 111.Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, Ment LR, Gruen JR Neonatal Genetics Study G. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006;117(6):1901–1906. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 112.Floros J, Londono D, Gordon D, Silveyra P, Diangelo SL, Viscardi RM, Worthen GS, Shenberger J, Wang G, Lin Z, et al. IL-18R1 and IL-18RAP SNPs may be associated with bronchopulmonary dysplasia in African-American infants. Pediatr Res. 2012;71(1):107–114. doi: 10.1038/pr.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ali S, Hirschfeld AF, Mayer ML, Fortuno ES, 3rd, Corbett N, Kaplan M, Wang S, Schneiderman J, Fjell CD, Yan J, et al. Functional genetic variation in NFKBIA and susceptibility to childhood asthma, bronchiolitis, and bronchopulmonary dysplasia. J Immunol. 2013;190(8):3949–3958. doi: 10.4049/jimmunol.1201015. [DOI] [PubMed] [Google Scholar]
- 114.Wang H, St Julien KR, Stevenson DK, Hoffmann TJ, Witte JS, Lazzeroni LC, Krasnow MA, Quaintance CC, Oehlert JW, Jelliffe-Pawlowski LL, et al. A genome-wide association study (GWAS) for bronchopulmonary dysplasia. Pediatrics. 2013;132(2):290–297. doi: 10.1542/peds.2013-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hagood JS. Beyond the genome: epigenetic mechanisms in lung remodeling. Physiology (Bethesda) 2014;29(3):177–185. doi: 10.1152/physiol.00048.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Park J, Wick HC, Kee DE, Noto K, Maron JL, Slonim DK. Finding novel molecular connections between developmental processes and disease. PLoS Comput Biol. 2014;10(5):e1003578. doi: 10.1371/journal.pcbi.1003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoffmann TJ, Shaw GM, Stevenson DK, Wang H, Quaintance CC, Oehlert J, Jelliffe-Pawlowski LL, Gould JB, Witte JS, O’Brodovich HM. Copy number variation in bronchopulmonary dysplasia. Am J Med Genet A. 2014;164A(10):2672–2675. doi: 10.1002/ajmg.a.36659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stouch AN, Zaynagetdinov R, Barham WJ, Stinnett AM, Slaughter JC, Yull FE, Hoffman HM, Blackwell TS, Prince LS. IkappaB kinase activity drives fetal lung macrophage maturation along a non-M1/M2 paradigm. J Immunol. 2014;193(3):1184–1193. doi: 10.4049/jimmunol.1302516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lingappan K, Srinivasan C, Jiang W, Wang L, Couroucli XI, Moorthy B. Analysis of the transcriptome in hyperoxic lung injury and sex-specific alterations in gene expression. PLoS One. 2014;9(7):e101581. doi: 10.1371/journal.pone.0101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sorensen GL, Dahl M, Tan Q, Bendixen C, Holmskov U, Husby S. Surfactant protein-D-encoding gene variant polymorphisms are linked to respiratory outcome in premature infants. J Pediatr. 2014;165(4):683–689. doi: 10.1016/j.jpeds.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 121.Bhattacharya S, Zhou Z, Yee M, Chu CY, Lopez AM, Lunger VA, Solleti SK, Resseguie E, Buczynski B, Mariani TJ, et al. The genome-wide transcriptional response to neonatal hyperoxia identifies Ahr as a key regulator. Am J Physiol Lung Cell Mol Physiol. 2014;307(7):L516–523. doi: 10.1152/ajplung.00200.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ambalavanan N, Cotten CM, Page GP, Carlo WA, Murray JC, Bhattacharya S, Mariani TJ, Cuna AC, Faye-Petersen OM, Kelly D, et al. Integrated genomic analyses in bronchopulmonary dysplasia. J Pediatr. 2015;166(3):531–537. e513. doi: 10.1016/j.jpeds.2014.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carrera P, Di Resta C, Volonteri C, Castiglioni E, Bonfiglio S, Lazarevic D, Cittaro D, Stupka E, Ferrari M, Somaschini M, et al. Exome sequencing and pathway analysis for identification of genetic variability relevant for bronchopulmonary dysplasia (BPD) in preterm newborns: A pilot study. Clin Chim Acta. 2015 doi: 10.1016/j.cca.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 124.Li J, Yu KH, Oehlert J, Jeliffe-Pawlowski LL, Gould JB, Stevenson DK, Snyder M, Shaw GM, O’Brodovich HM. Exome Sequencing of Neonatal Blood Spots Identifies Genes Implicated in Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201501-0168OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol. 2014;35(7):299–310. doi: 10.1016/j.it.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 127.Hillman NH, Moss T, JM, Nitsos I, Kramer BW, Bachurski C, Ikegami M, Jobe AH, Kallapur SG. Toll-like receptors and agonist responses in the developing fetal sheep lung. Pediatr Res. 2008;63(4):388–393. doi: 10.1203/PDR.0b013e3181647b3a. [DOI] [PubMed] [Google Scholar]
- 128.Ivarsson MA, Loh L, Marquardt N, Kekalainen E, Berglin L, Bjorkstrom NK, Westgren M, Nixon DF, Michaelsson J. Differentiation and functional regulation of human fetal NK cells. J Clin Invest. 2013;123(9):3889–3901. doi: 10.1172/JCI68989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bruder D, Srikiatkhachorn A, Enelow RI. Cellular immunity and lung injury in respiratory virus infection. Viral Immunol. 2006;19(2):147–155. doi: 10.1089/vim.2006.19.147. [DOI] [PubMed] [Google Scholar]
- 130.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 131.Marchant A, Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clin Exp Immunol. 2005;141(1):10–18. doi: 10.1111/j.1365-2249.2005.02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao Y, Dai ZP, Lv P, Gao XM. Phenotypic and functional analysis of human T lymphocytes in early second- and third-trimester fetuses. Clin Exp Immunol. 2002;129(2):302–308. doi: 10.1046/j.1365-2249.2002.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schultz C, Reiss I, Bucsky P, Gopel W, Gembruch U, Ziesenitz S, Gortner L. Maturational changes of lymphocyte surface antigens in human blood: comparison between fetuses, neonates and adults. Biol Neonate. 2000;78(2):77–82. doi: 10.1159/000014253. [DOI] [PubMed] [Google Scholar]
- 134.Ballow M, Cates KL, Rowe JC, Goetz C, Pantschenko AG. Peripheral blood T-cell subpopulations in the very low birth weight (less than 1,500-g) infant. Am J Hematol. 1987;24(1):85–92. doi: 10.1002/ajh.2830240111. [DOI] [PubMed] [Google Scholar]
- 135.Series IM, Pichette J, Carrier C, Masson M, Bedard PM, Beaudoin J, Hebert J. Quantitative analysis of T and B cell subsets in healthy and sick premature infants. Early Hum Dev. 1991;26(2):143–154. doi: 10.1016/0378-3782(91)90018-x. [DOI] [PubMed] [Google Scholar]
- 136.Kotiranta-Ainamo A, Apajasalo M, Pohjavuori M, Rautonen N, Rautonen J. Mononuclear cell subpopulations in preterm and full-term neonates: independent effects of gestational age, neonatal infection, maternal pre-eclampsia, maternal betamethason therapy, and mode of delivery. Clin Exp Immunol. 1999;115(2):309–314. doi: 10.1046/j.1365-2249.1999.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Suursalmi P, Kopeli T, Korhonen P, Lehtimaki L, Nieminen R, Luukkaala T, Moilanen E, Korppi M, Paassilta M, Tammela O. Very low birthweight bronchopulmonary dysplasia survivors show no substantial association between lung function and current inflammatory markers. Acta Paediatr. 2015;104(3):264–268. doi: 10.1111/apa.12837. [DOI] [PubMed] [Google Scholar]
- 138.Kuypers E, Collins JJ, Kramer BW, Ofman G, Nitsos I, Pillow JJ, Polglase GR, Kemp MW, Newnham JP, Gavilanes AW, et al. Intra-amniotic LPS and antenatal betamethasone: inflammation and maturation in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol. 2012;302(4):L380–389. doi: 10.1152/ajplung.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rosen D, Lee JH, Cuttitta F, Rafiqi F, Degan S, Sunday ME. Accelerated thymic maturation and autoreactive T cells in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2006;174(1):75–83. doi: 10.1164/rccm.200511-1784OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ryan RM, Ahmed Q, D’Angelis CA, Kumar VH, Lakshminrusimha S, Metlay L, Wang H, Pryhuber GS. CD8+ T-Lymphocytes in Infants with Bronchopulmonary Dysplasia (BPD) Pediatric Academic Society. 2009:3858.137. E-PAS2009. [Google Scholar]
- 141.Ballabh P, Simm M, Kumari J, Krauss AN, Jain A, Auld PA, Cunningham-Rundles S. Lymphocyte subpopulations in bronchopulmonary dysplasia. Am J Perinatol. 2003;20(8):465–475. doi: 10.1055/s-2003-45387. [DOI] [PubMed] [Google Scholar]
- 142.Turunen R, Vaarala O, Nupponen I, Kajantie E, Siitonen S, Lano A, Repo H, Andersson S. Activation of T Cells in Preterm Infants with Respiratory Distress Syndrome. Neonatology. 2009;96(4):248–258. doi: 10.1159/000220764. [DOI] [PubMed] [Google Scholar]
- 143.Berrington JE, Barge D, Fenton AC, Cant AJ, Spickett GP. Lymphocyte subsets in term and significantly preterm UK infants in the first year of life analysed by single platform flow cytometry. Clin Exp Immunol. 2005;140(2):289–292. doi: 10.1111/j.1365-2249.2005.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Luciano AA, Yu H, Jackson LW, Wolfe LA, Bernstein HB. Preterm labor and chorioamnionitis are associated with neonatal T cell activation. PLoS One. 2011;6(2):e16698. doi: 10.1371/journal.pone.0016698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Emo J, Yang H, Holden-Wiltse JAS, Huyck H, Misra SK, Topham D, Ryan RM, Reynolds AM, Mariani TJ, et al. Developmentally determined reduction in CD31 during gestation is associated with CD8+ T cell effector differentiation in preterm infants. Clin Immunol. 2015 doi: 10.1016/j.clim.2015.07.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, Thorsen P, Higgins RD. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123(4):1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Duggan PJ, Maalouf EF, Watts TL, Sullivan MH, Counsell SJ, Allsop J, Al-Nakib L, Rutherford MA, Battin M, Roberts I, et al. Intrauterine T-cell activation and increased proinflammatory cytokine concentrations in preterm infants with cerebral lesions. Lancet. 2001;358(9294):1699–1700. doi: 10.1016/s0140-6736(01)06723-x. [DOI] [PubMed] [Google Scholar]
- 148.Misra RS, Shah S, Fowell DJ, Wang H, Scheible K, Misra SK, Huyck H, Wyman CP, Ryan RM, Reynolds AM, et al. Preterm cord blood CD4(+) T cells exhibit increased IL-6 production in chorioamnionitis and decreased CD4(+) T cells in bronchopulmonary dysplasia. Hum Immunol. 2015;76(5):329–338. doi: 10.1016/j.humimm.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kallapur SG, Presicce P, Senthamaraikannan P, Alvarez M, Tarantal AF, Miller LM, Jobe AH, Chougnet CA. Intra-amniotic IL-1beta induces fetal inflammation in rhesus monkeys and alters the regulatory T cell/IL-17 balance. J Immunol. 2013;191(3):1102–1109. doi: 10.4049/jimmunol.1300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sunday ME, Yoder BA, Cuttitta F, Haley KJ, Emanuel RL. Bombesin-like peptide mediates lung injury in a baboon model of bronchopulmonary dysplasia. J Clin Invest. 1998;102(3):584–594. doi: 10.1172/JCI2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Scher H, Miller YE, Aguayo SM, Johnson KJ, Miller JE, McCray PB., Jr Urinary bombesin-like peptide levels in infants and children with bronchopulmonary dysplasia and cystic fibrosis. Pediatr Pulmonol. 1998;26(5):326–331. doi: 10.1002/(sici)1099-0496(199811)26:5<326::aid-ppul4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 152.Culley FJ, Pollott J, Openshaw PJ. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med. 2002;196(10):1381–1386. doi: 10.1084/jem.20020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tregoning JS, Yamaguchi Y, Harker J, Wang B, Openshaw PJ. The role of T cells in the enhancement of respiratory syncytial virus infection severity during adult reinfection of neonatally sensitized mice. J Virol. 2008;82(8):4115–4124. doi: 10.1128/JVI.02313-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tasker L, Lindsay RW, Clarke BT, Cochrane DW, Hou S. Infection of mice with respiratory syncytial virus during neonatal life primes for enhanced antibody and T cell responses on secondary challenge. Clin Exp Immunol. 2008;153(2):277–288. doi: 10.1111/j.1365-2249.2008.03591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.You D, Ripple M, Balakrishna S, Troxclair D, Sandquist D, Ding L, Ahlert TA, Cormier SA. Inchoate CD8+ T cell responses in neonatal mice permit influenza-induced persistent pulmonary dysfunction. J Immunol. 2008;181(5):3486–3494. doi: 10.4049/jimmunol.181.5.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gern JE, Brooks GD, Meyer P, Chang A, Shen K, Evans MD, Tisler C, Dasilva D, Roberg KA, Mikus LD, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117(1):72–78. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 157.Friedlander SL, Jackson DJ, Gangnon RE, Evans MD, Li Z, Roberg KA, Anderson EL, Carlson-Dakes KT, Adler KJ, Gilbertson-White S, et al. Viral infections, cytokine dysregulation and the origins of childhood asthma and allergic diseases. Pediatr Infect Dis J. 2005;24(11 Suppl):S170–176. doi: 10.1097/01.inf.0000187273.47390.01. discussion S174–175. [DOI] [PubMed] [Google Scholar]
- 158.Wang EE, Law BJ, Robinson JL, Dobson S, al Jumaah S, Stephens D, Boucher FD, McDonald J, Mitchell I, MacDonald NE. PICNIC (Pediatric Investigators Collaborative Network on Infections in Canada) study of the role of age and respiratory syncytial virus neutralizing antibody on respiratory syncytial virus illness in patients with underlying heart or lung disease. Pediatrics. 1997;99(3):E9. doi: 10.1542/peds.99.3.e9. [DOI] [PubMed] [Google Scholar]
- 159.DeVincenzo JP, Wilkinson T, Vaishnaw A, Cehelsky J, Meyers R, Nochur S, Harrison L, Meeking P, Mann A, Moane E, et al. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2010;182(10):1305–1314. doi: 10.1164/rccm.201002-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Houben ML, Coenjaerts FE, Rossen JW, Belderbos ME, Hofland RW, Kimpen JL, Bont L. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol. 2010;82(7):1266–1271. doi: 10.1002/jmv.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bosis S, Esposito S, Osterhaus AD, Tremolati E, Begliatti E, Tagliabue C, Corti F, Principi N, Niesters HG. Association between high nasopharyngeal viral load and disease severity in children with human metapneumovirus infection. J Clin Virol. 2008;42(3):286–290. doi: 10.1016/j.jcv.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Utokaparch S, Marchant D, Gosselink JV, McDonough JE, Thomas EE, Hogg JC, Hegele RG. The relationship between respiratory viral loads and diagnosis in children presenting to a pediatric hospital emergency department. Pediatr Infect Dis J. 2011;30(2):e18–23. doi: 10.1097/INF.0b013e3181ff2fac. [DOI] [PubMed] [Google Scholar]
- 163.Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31(3):401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 164.Biswas A, Wilmanski J, Forsman H, Hrncir T, Hao L, Tlaskalova-Hogenova H, Kobayashi KS. Negative regulation of Toll-like receptor signaling plays an essential role in homeostasis of the intestine. Eur J Immunol. 2011;41(1):182–194. doi: 10.1002/eji.201040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sydora BC, McFarlane SM, Doyle JS, Fedorak RN. Neonatal exposure to fecal antigens reduces intestinal inflammation. Inflamm Bowel Dis. 2010 doi: 10.1002/ibd.21453. [DOI] [PubMed] [Google Scholar]
- 166.Jacquot A, Neveu D, Aujoulat F, Mercier G, Marchandin H, Jumas-Bilak E, Picaud JC. Dynamics and clinical evolution of bacterial gut microflora in extremely premature patients. J Pediatr. 2011;158(3):390–396. doi: 10.1016/j.jpeds.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 167.Namba K, Hatano M, Yaeshima T, Takase M, Suzuki K. Effects of Bifidobacterium longum BB536 administration on influenza infection, influenza vaccine antibody titer, and cell-mediated immunity in the elderly. Biosci Biotechnol Biochem. 2010;74(5):939–945. doi: 10.1271/bbb.90749. [DOI] [PubMed] [Google Scholar]
- 168.Warner BB, Hamvas A. Lungs, microbes and the developing neonate. Neonatology. 2015;107(4):337–343. doi: 10.1159/000381124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tracy M, Cogen J, Hoffman LR. The pediatric microbiome and the lung. Curr Opin Pediatr. 2015;27(3):348–355. doi: 10.1097/MOP.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bisgaard H, Hermansen MN, Bonnelykke K, Stokholm J, Baty F, Skytt NL, Aniscenko J, Kebadze T, Johnston SL. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lohmann P, Luna RA, Hollister EB, Devaraj S, Mistretta TA, Welty SE, Versalovic J. The airway microbiome of intubated premature infants: characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr Res. 2014;76(3):294–301. doi: 10.1038/pr.2014.85. [DOI] [PubMed] [Google Scholar]
- 172.Mourani PM, Harris JK, Sontag MK, Robertson CE, Abman SH. Molecular identification of bacteria in tracheal aspirate fluid from mechanically ventilated preterm infants. PLoS One. 2011;6(10):e25959. doi: 10.1371/journal.pone.0025959. [DOI] [PMC free article] [PubMed] [Google Scholar]