Abstract
Dysregulated calcium signaling has been increasingly implicated in tumor dissemination and progression. In a recent study we investigated the mechanism underlying calcium-mediated melanoma invasion and metastasis, and discovered that hyperactive Ca2+ oscillation in melanoma cells promoted invasion and metastasis through promoting invadopodium formation and extracellular matrix remodeling.
Gaining invasiveness and overcoming physical barrier imposed by the extracellular matrix is the first and arguably most critical steps of tumor metastasis (1). In addition to facilitating dissemination, remodeling of the extracellular matrix (ECM) is essential for tumor growth and the establishment of metastatic niches by malignant cells (2). Invadopodia in malignant cancer cells are actin-rich, ECM degrading membrane protrusions critical for tumor invasion and metastasis (1). In a recent study, we investigated the regulation of invadopodium formation and melanoma metastasis by Ca2+ signaling (3). We unexpectedly discovered that hyperactive Ca2+ signal in metastatic melanoma cells is organized in the form of oscillatory waves to orchestrate invadopodial assembly and melanoma invasion (3).
In recent years, dysregulated Ca2+ signaling has been increasingly implicated in cancer invasion and metastasis, and yet, the underlying mechanism was largely unclear (4, 5). To gain mechanistic insight underlying Ca2+-mediated invasion and metastasis, we examined the role of Ca2+ signaling in invadopodium formation in melanoma cells, and discovered that blocking store-operated calcium channel signaling significantly decreased invadopodium number and activity. Accompanying the assembly of invadopodia was oscillatory Ca2+ signal, mediated by SOC channel proteins STIM1 and ORAI1. Interestingly, disruption of the Ca2+ oscillation by either blocking store operated calcium entry (SOCE)(which decreased cytosolic Ca2+ concentration), or by inducing constitutive calcium entry with thapsigargin or ionophore A-23187 (which increased cytosolic Ca2+ concentration) similarly inhibited invadopodium assembly and melanoma invasion, signifying the importance of temporal Ca2+ signal coding during metastatic dissemination.
By screening a panel of protein kinases, we identified the non-receptor tyrosine kinase Src as a downstream effector of SOCE. The notion that SOCE regulates invadopodium assembly through Src is further supported by the rescue of invadopodium formation defect in STIM1 knockdown melanoma cells by constitutively active v-Src, and the abrogation of STIM1-mediated invadopodium assembly by Src inhibitor dasatinib. Since constitutive Ca2+ influx induced by thapsigargin and A-23187 was a robust activator of Src, this begs the question: why did melanoma cells use oscillatory Ca2+ signal instead of steady Ca2+ increase?
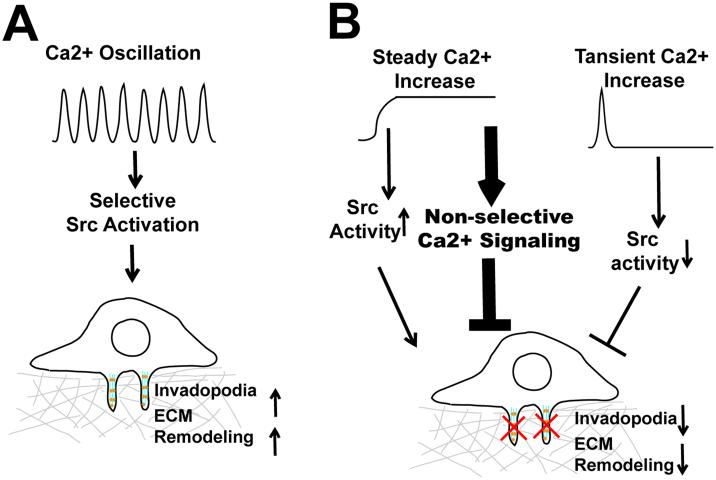
Ca2+ is a notoriously versatile second messenger. It is estimated that hundreds of genes in the human genome contain Ca2+ binding EF-hand or C2 domains (6). The specificity and versatility of Ca2+ signaling relies on the intricate spatial and temporal coding of cytosolic Ca2+ concentration (6). By compartmentalizing Ca2+ signals into spatial-temporal patterns, cells are able to activate selective downstream signaling events at defined time and subcellular location (6). It is believed that the frequency and amplitude of Ca2+ oscillations serve as digital signals that selectively activate threshold-dependent downstream events. The tight control of cytosolic Ca2+ is not only critical for signaling specificity, but also for cell survival, since prolonged and uncontrolled global increase in cytosolic Ca2+ is toxic to the cell and eventually leads to cell death (7). By organizing SOCE signal in the form of Ca2+ oscillation, melanoma cells are able to provide the Ca2+ signal necessary for invadopodium assembly and ECM remodeling over an extended period of time without causing cytotoxicity (Fig. 1A). In contrast, constitutive Ca2+ influx induced by thapsigargin and A23187, although robustly increased Src activity, might also indiscriminately activate hundreds of other Ca2+-dependent signaling pathways, which eventually reduced melanoma cell fitness and inhibited melanoma invasion (Fig. 1B) .
Figure 1.
Regulation of invadopodia by Ca2+ oscillation. A, Hyperactive store-operated calcium entry (SOCE) increase Ca2+ oscillation frequency and amplitude, which selectively activate Src to promote invadopodium assembly and extracellular matrix remodeling. B, Disruption of Ca2+ oscillation either by constitutive increase in cytosolic Ca2+, or by blockage of SOCE, inhibits invadopodium formation and melanoma invasion.
It is also possible that melanoma invasion and invadopodium assembly require coordinated cycle of Ca2+ peaks and valleys, as recently demonstrated in mast cell exocytosis by Wollman and Meyer (8). Ca2+ oscillation in antigen activated mast cells drive the cyclic assembly and disassembly of cortical actin. Newly assembled cortex actin serves as carrier to capture secretory vesicles, while disassembly of cortical actin allows the passage of vesicle to facilitate membrane fusion. Intriguingly, we discovered that the recycling of MT1-MMP (MMP14, commonly known as Membrane Type 1 Matrix Metalloprotease) to the plasma membrane required SOCE, and the blockage of which resulted in the entrapment of MT1-MMP in endosomes (3). It would be interesting to determine whether SOCE-mediated Ca2+ oscillation coordinated the recycling of endocytosed MT1-MMP to the plasma membrane.
Of note, tumor-promoting Ca2+ oscillation has also been recently observed in esophageal squamous cell carcinoma and hepatocellular carcinoma (HCC) (9, 10). Orai1 overexpresion is responsible for hyperactive Ca2+ oscillation in esophageal carcinoma cells, which promotes cancer cell motility and proliferation in vitro, and tumor growth in xenograft model (10). In HCC the voltage-gated calcium channel subunit CACNA2D1was found to be a marker for recurrent HCC cells. Recurrent HCC cells had higher expression of α2δ1 and hyperactive Ca2+ oscillation, which could be inhibited by a blocking antibody targeting CACNA2D1 (10). These observations, together with our recent finding, suggested that Ca2+ oscillation might be a signaling mechanism commonly hijacked by malignant cells to facilitate cancer progression. Future investigation into this area will likely significantly advance our understanding of how deregulated Ca2+ signaling promotes cancer malignancy.
Acknoledgement
This work is supported by the National Cancer Institute (R01175741).
Abbreviations
- SOCE
store-operated calcium entry
- ECM
extracellular matrix
- MT1-MMP
membrane-type 1 matrix metallopretease
- HCC
hepatocellular carcinoma\
References
- 1.Nurnberg A, Kitzing T, Grosse R. Nucleating actin for invasion. Nature reviews Cancer. 2011;11(3):177–87. doi: 10.1038/nrc3003. [DOI] [PubMed] [Google Scholar]
- 2.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114(1):33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 3.Sun J, Lu F, He H, Shen J, Messina J, Mathew R, Wang D, Sarnaik AA, Chang WC, Kim M, Cheng H, Yang S. STIM1- and Orai1-mediated Ca2+ oscillation orchestrates invadopodium formation and melanoma invasion. J Cell Biol. 2014;207(4):535–48. doi: 10.1083/jcb.201407082. PMCID: 4242838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nature reviews Cancer. 2011;11(8):609–18. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 5.Wang JY, Sun J, Huang MY, Wang YS, Hou MF, Sun Y, He H, Krishna N, Chiu SJ, Lin S, Yang S, Chang WC. STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression. Oncogene. 2014 doi: 10.1038/onc.2014.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4(7):552–65. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 8.Wollman R, Meyer T. Coordinated oscillations in cortical actin and Ca2+ correlate with cycles of vesicle secretion. Nat Cell Biol. 2012;14(12):1261–9. doi: 10.1038/ncb2614. PMCID: 3777337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu H, Zhang H, Jin F, Fang M, Huang M, Yang CS, Chen T, Fu L, Pan Z. Elevated Orai1 expression mediates tumor-promoting intracellular Ca2+ oscillations in human esophageal squamous cell carcinoma. Oncotarget. 2014;5(11):3455–71. doi: 10.18632/oncotarget.1903. PMCID: 4116495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao W, Wang L, Han H, Jin K, Lin N, Guo T, Chen Y, Cheng H, Lu F, Fang W, Wang Y, Xing B, Zhang Z. 1B50-1, a mAb raised against recurrent tumor cells, targets liver tumor-initiating cells by binding to the calcium channel alpha2delta1 subunit. Cancer Cell. 2013;23(4):541–56. doi: 10.1016/j.ccr.2013.02.025. [DOI] [PubMed] [Google Scholar]