Abstract
Objective
With no effective therapies to attenuate cartilage degeneration in osteoarthritis, the result is pain and disability. Activation of Hedgehog signaling causes osteoarthritic changes, with higher levels of Gli-mediated transcriptional activation associated with increased severity. To elucidate the mechanism through which this occurs, we identified genes regulated by Hedgehog signaling in human osteoarthritic chondrocytes.
Methods
Microarray analyses were performed to detect changes in gene expression when the Hh pathway was modulated in human osteoarthritic cartilage samples. Results were analyzed for differentially expressed genes, grouped into functional networks, and validated in independent samples. From this, cholesterol homeostatic genes were found to be modulated by Hh signaling. To investigate the effects of chondrocyte-specific sterol accumulation, we generated mice lacking Insig1 and Insig2, major negative regulators of cholesterol homeostasis, under Col2a1 regulatory elements.
Results
We show that Hedgehog signaling regulates genes that govern cholesterol homeostasis, and this alters cholesterol accumulation in chondrocytes; a higher level of Gli-mediated transcription results in accumulation of intracellular cholesterol. In genetically modified mice, chondrocyte-specific cholesterol accumulation causes an osteoarthritis phenotype. Reducing cholesterol accumulation attenuates the severity of osteoarthritis in mice in vivo and decreases expression of proteases in human cartilage in vitro.
Conclusion
Hedgehog signaling regulates cholesterol homeostasis in chondrocytes, and intracellular cholesterol accumulation contributes to osteoarthritis severity. Our findings have therapeutic implications since reduction of Hedgehog signaling reversed cholesterol accumulation and statin treatment attenuated cartilage degeneration.
Osteoarthritis is characterized by changes to the articular joint, including progressive degeneration of the articular cartilage. The pathogenesis of osteoarthritis is complex because it can result from multiple etiologies involving mechanical, genetic, traumatic, and metabolic factors1,2. Regardless of the cause, the changes that occur to the articular chondrocytes during osteoarthritis, such as chondrocyte hypertrophy, recapitulate some of the changes that occur in growth plate chondrocytes during elongation of the long bones3,4. Hedgehog (Hh) signaling is among the pathways that regulate chondrocyte differentiation in the growth plate of the long bones, including the change towards chondrocyte hypertrophy5,6. In vertebrates, Hh signaling is mediated by Gli transcription factors, with Gli2 acting as a transcriptional activator of the Hh target genes Gli1, Ptch1, and Hhip7. Previous studies demonstrate that genetically modified mice with higher levels of Gli-mediated transcription in chondrocytes develop more severe osteoarthritis, and that inhibition of Gli-mediated transcription reduces severity, but the mechanism through which this occurs remains unclear4,8.
To elucidate the role of Hh signaling in osteoarthritis severity, we identified targets of Gli-mediated transcription in human osteoarthritic cartilage. Using an unsupervised approach to analyze microarray results, we identified several pathways which potentially mediate the effect of Hh signaling in osteoarthritis pathogenesis. Among these, genes that are involved in cholesterol homeostasis were significantly represented, including the major negative regulator INSIG1, and the transcriptional regulator SREBF2. Because cholesterol is vital for cellular processes and has been previously implicated in osteoarthritis9-12, we focused on exploring the role of Hh signaling in regulating intracellular cholesterol biosynthesis in chondrocytes. We demonstrate that Hh signaling positively regulates cholesterol accumulation in chondrocytes, and that modulation of intracellular cholesterol level alters the severity of osteoarthritis.
Materials and Methods
Human osteoarthritic cartilage
Human cartilage samples were obtained from patients undergoing total knee replacement surgery for clinically diagnosed osteoarthritis. Articular cartilage was dissected from the subchondral bone of the lateral femoral condyle and incubated with pharmacologic Hh antagonist [10 μM N-[(3S,5S)-1-(2H-benzo[3,4-d]1,3-dioxolan-5-ylmethyl)-5-(piperazinylcarbonyl)pyrrolidin-3-yl]-N-(3-methoxyphenyl)methyl]-3,3-dimethylbutanamide (C31H42N4O5)], Hh ligand (5 μg/ml Shh-N, R&D Systems), 10 μM lovastatin hydroxy acid (Cayman Chemical Company), 10 μM purmorphamine (Sigma-Aldrich), or carrier for 48h. Explant processing and subsequent RNA extraction were conducted as previously described13. Because each patient sample was divided and used as its own control, extraneous variables were held constant and no additional patient history was required. All samples were obtained with informed consent under the approval of the Mount Sinai Hospital Research Ethnics Board (ON, Canada).
Gene expression analyses
Microarray was performed using the Affymetrix Human Gene 1.0 ST platform with human osteoarthritic articular cartilage samples. The three samples included two male and one female patient, with a mean age of 62.3 years. RNA was extracted from cartilage treated in vitro with 10 μM C31H42N4O5 (Hh antagonist) or control, using a method previously described13. Data can be accessed through the GEO database (GSE54749). Results were analyzed independently for paired samples from each of the three patients (Control1 vs. Hh antag1, Control2 vs. Hh antag2, Control3 vs. Hh antag3), and differentially expressed genes were filtered using Partek® Genomics Suite for those which were either upregulated or downregulated across all three samples. Ingenuity® Pathway analysis was used to identify functional gene networks represented in the microarray data. Real-time PCR experiments were conducted using TaqMan assays from Applied Biosystems. For this, human cartilage samples were obtained as described above and mouse samples were obtained from isolation of cartilage from knee joints as previously described14. Results were normalized to endogenous control genes (ASNS, BACTIN, GAPDH) and analyzed according to the comparative CT method (ΔΔCT). For promoter analyses, primary human chondrocytes or ATDC5 cells were transfected with an ADAMTS5 luciferase reporter construct or negative control vector (GeneCopoeia, Rockville, MD) using the Neon® Transfection System (Life Technologies, Burlington, ON) or Lipofectamine® (Life Technologies, Burlington, ON). Site-directed mutagenesis by inverse PCR was used to delete the SRE site, which was confirmed by sequencing.
Genetically modified mice
We crossed Col2a1-Cre mice15 with Insig1(fl/fl);Insig2(-/-) mice16 to excise Insig1 in Col2a1-expressing cells and generate mice with cartilage-specific cholesterol accumulation. Mice expressing Cre are referred to as InsigDKO [Insig1(-/-);Insig2(-/-);Col2a1-Cre] and are compared to their Cre-negative [Insig1(fl/fl);Insig2(-/-); “control”] littermates. Cre-mediated recombination and excision of Insig1 was confirmed by QPCR and Western blot analysis. To activate Hh signalling by increasing Gli-mediated transcription, InsigDKO mice were crossed with Col2a1-Gli2 mice17, the progeny for which were designated Col2a1-Gli2;InsigDKO. To inhibit Hh signalling by reducing Gli-mediated transcription, InsigDKO mice were crossed with Gli2zfd mice (Gli2+/-)18, the progeny for which were designated Gli2+/-;InsigDKO. To recapitulate osteoarthritis, we performed medial meniscectomy surgery as previously described4. Briefly, the medial meniscus was excised from the left knee of 8-week-old mice, and osteoarthritis developed predictably 8 weeks post-operatively. Sham surgeries were used as control, for which the entire surgical procedure was followed, except excision of the medial meniscus. To reduce cholesterol levels in vivo, mice were treated with 3mg/kg/day lovastatin hydroxy acid by surgical implantation of drug pellets with slow-release over 8 weeks. Within each genotype, mice were randomly selected for statin or placebo treatment. Effective statin treatment was confirmed by increased HMGCR in the chondrocytes, as assessed by immunohistochemistry. Knee joints were evaluated for the development of osteoarthritis by radiography, histology, and real-time PCR. Male and female mice and littermate comparisons were used for all experiments. All animal studies were approved by the Toronto Centre for Phenogenomics (ON, Canada).
Western blot analysis
Primary human chondrocytes were isolated from total knee arthroplasty samples as previously described13. Primary mouse chondrocytes were isolated from knee joints as previously described14. Protein lysates from chondrocytes were harvested using Reporter Lysis Buffer (Promega), according to the manufacturer's instructions. Antibodies against INSIG1 (1:100, sc-25124-R, Santa Cruz) and ACTIN (1:5000, A5441, Sigma) were used. The signals were detected and quantified using the ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA).
Radiography and histology
After sacrifice, mouse joints were harvested and fixed in 10% phosphate-buffered formalin. Radiographs of joints were taken using the Faxitron MX20 X-ray system. Samples were decalcified in 20% EDTA (pH 8.0), dehydrated, and embedded into paraffin for sectioning as previously described4. Histologic analysis was performed using antibodies against COL10A1 (X53, Quartett) and HMGCR (ab174830, abcam) as well as staining with Haematoxylin & Eosin and Safranin-O. Histomorphometry was performed to measure the osteophyte volume, and the bone volume relative to the total volume (BV/TV)19, as previously described4. Grading for osteoarthritis severity was performed in a blinded manner using the ICRS20 and OARSI21 scoring systems, as previously described4. For ICRS, images of knee joints were assessed for six features commonly associated with osteoarthritis, including changes to the subchondral bone, and categories were tallied into an overall score to represent disease severity (lower scores represent greater severity).
Sterol quantification
Primary mouse chondrocytes were isolated from knee joints and cultured as previously described14. To measure total sterol and lipid levels, these chondrocytes were fixed with 10% phosphate-buffered formalin for 10 minutes. Cells were stained with Oil-Red-O and extracted stain was quantified by spectrophotometry (OD 500), with readings normalized to crystal violet stain (OD 540). To measure cholesterol synthesis, chondrocytes were pooled and incubated with 50 μCi/mL 3H-acetic acid sodium salt overnight. Lipid extracted from the cells underwent thin layer chromatography for separation of components, including cholesterol. Incorporated 3H was measured in triplicate and reported as the relative change in counts per minute (cpm).
Chromatin Immunoprecipitation (ChIP)
ChIP was conducted using the SimpleChIP® Plus Enzymatic Chromatin IP Kit (Magnetic Beads; Cell Signaling, Cat No. 9005) according to the manufacturer's protocol with modifications. Cultured ATDC5 cells were treated with 10 μM lovastatin and fixed with 1% formaldehyde to maintain DNA-protein binding interactions. Following nuclei preparation and enzymatic chromatin digestion (manufacturer's protocol), sonication was performed to shear the chromatin and achieve a target size range of 200 to 700 base pairs. SREBF2 antibody (R&D Systems, Cat No. AF7119-SP) and negative control IgG antibody (Active Motif) were used to immunoprecipitate DNA-protein complexes. Quantification of DNA was performed by real-time PCR using custom primers for Adamts5, in the proximal promoter region and in a distal control region approximately 38 kilobases away.
Statistical analyses
Values are reported as the mean and error bars represent 95% confidence intervals unless stated otherwise. Power calculations were conducted based on the variation observed in our previous report4. Statistical significance for histologic grading was determined using a Mann-Whitney U test, and for average gene expression and spectrophotometric quantification using a t-test (P < 0.05).
Results
Hedgehog signaling regulates expression of cholesterol homeostatic genes
To identify Hh target genes, we performed microarray analysis of human osteoarthritic cartilage samples obtained from patients undergoing total knee replacement surgery. Articular cartilage explants were incubated overnight with an antagonist of Hh signaling [N-[(3S,5S)-1-(2H-benzo[3,4-d]1,3-dioxolan-5-ylmethyl)-5-(piperazinylcarbonyl)pyrrolidin-3-yl]-N-(3-methoxyphenyl)methyl]-3,3-dimethylbutanamide (C31H42N4O5)] or carrier as control22. RNA was extracted using an optimized protocol13 and modulation of Hh signaling was confirmed by downregulation of known Hh target genes in the treated groups (data not shown).
Microarray results from the Affymetrix Human Gene 1.0 ST array were analyzed independently for each of three biological replicates. Comparing treated to control, differentially expressed genes were filtered for those which changed in the same direction across all three datasets. This method of analysis was chosen in an effort to control for the heterogeneity that is inherent to human samples. Criteria surrounding arbitrary fold changes or statistical cutoffs were not applied so as to capture subtle changes across entire gene networks. This approach is biologically relevant for detecting small perturbations with effects that can accumulate over time to cause progressive diseases such as osteoarthritis.
Ingenuity® Pathway Analysis was used as an unbiased method for identifying signaling pathways, molecular networks, and biological processes that were represented in the microarray gene list. From an analysis set to filter all validated direct and indirect relationships according to the Ingenuity® Knowledge Base, the cholesterol biosynthetic pathway was found to be most significantly dysregulated (P = 3.91E-13; Table S1). Several genes known to be involved in cholesterol homeostasis23,24 were found to be upregulated with Hh inhibition (Fig. 1A). While systemic cholesterol dysregulation has been previously associated with osteoarthritis9-12, intracellular cholesterol homeostasis in osteoarthritic chondrocytes is not well understood. We sought to assess the role of Hh signaling in regulating the expression of cholesterol homeostatic genes in chondrocytes.
Figure 1. Hedgehog signaling regulates expression of cholesterol homeostatic genes.
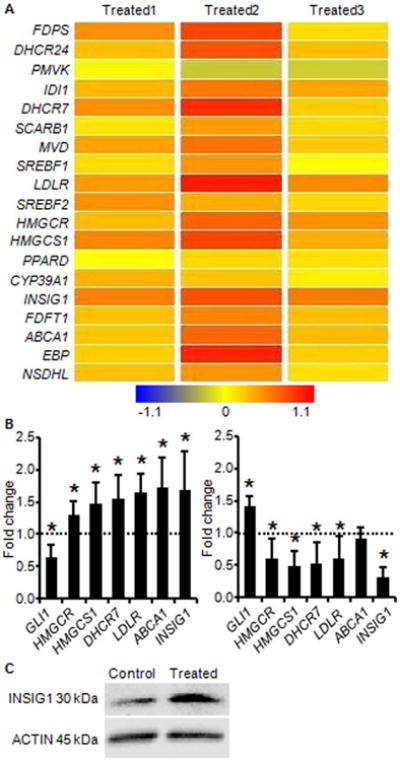
(A) Results from Affymetrix Human Gene 1.0 ST microarray of human osteoarthritic cartilage treated with Hh antagonist. Greater intensity of red represents increased gene expression with Hh inhibition. (B) Real-time PCR validation of Hh regulation of cholesterol homeostatic genes identified by microarray. Independent human osteoarthritic cartilage samples were treated identically to those used in microarray analyses. Expression in the control group was arbitrarily defined as ‘1’ (dashed line). Data from the groups treated with Hh antagonist (left) and Hh agonist (right) are given as the mean. Error bars are 95% confidence intervals (n = 3; *P < 0.05). (C) Western blot showing increased INSIG1 protein in extracts from human osteoarthritic cartilage explants treated with Hh antagonist (Treated). ACTIN is shown as a loading control.
Independent human osteoarthritic cartilage samples were subjected to Hh modulation and assayed for expression of genes that are known to be involved in cholesterol homeostasis23,24. Real-time PCR results validated microarray findings by showing that Hh inhibition, as evidenced by downregulation of GLI1, resulted in upregulation of cholesterol homeostatic genes HMGCR, HMGCS1, DHCR7, LDLR, ABCA1, and INSIG1. Consistent with this inverse relationship, Hh activation, as evidenced by upregulation of GLI1, resulted in a trend towards downregulation of the same genes (Fig. 1B). The changes in expression are small but occur across multiple genes in the pathway; this recapitulates microarray findings and previously reported trends for expression changes in cholesterol homeostatic genes16.
Transcription of cholesterol homeostatic genes is ultimately governed by SREBF2 in an end-product feedback manner that is dependent on regulators such as INSIG116,23. When intracellular cholesterol is high, the INSIG proteins prevent cholesterol biosynthesis, in part by tethering the SREBF proteins to the ER membrane and preventing transcription of target genes. When intracellular cholesterol is low, INSIG allows SREBF to be processed and to translocate to the nucleus, activating transcription and restoring cholesterol homeostasis16. In human osteoarthritic cartilage, INSIG1 expression is significantly downregulated (-2.38, P < 0.05) compared to normal cartilage25. Comparing more severely affected areas of human osteoarthritic cartilage to less severely affected areas, INSIG1 expression is downregulated -7.09 fold and INSIG2 expression is downregulated -3.89 fold. Taken together these data show that reduced INSIG expression correlates with increased OA severity. This suggests that cholesterol homeostasis is perturbed, possibly as a result of the Hh activation that is reported to accompany osteoarthritis4,8. When Hh signaling is inhibited in osteoarthritic cartilage, INSIG1 gene expression increases, and this translates to an increase in INSIG1 protein (Fig. 1C). As a critical negative regulator of cholesterol homeostasis, the inverse relationship between Hh signaling and INSIG1 expression suggests that inhibition of Hh signaling reduces cholesterol biosynthesis in chondrocytes. This is further supported by the overall increase in transcription of cholesterol homeostatic genes that is observed with Hh inhibition, an increase that typically occurs in response to reduced intracellular cholesterol levels.
Hedgehog signaling regulates cholesterol biosynthesis in chondrocytes
To investigate the net effect of Hh signaling on cholesterol homeostasis in chondrocytes, we used genetically modified mice in which Gli-mediated transcription was modulated. Previous studies demonstrated that Gli-mediated transcription is reduced in Gli2zfd mice (designated Gli2+/-) by disrupting one Gli2 allele18, and increased in Col2a1-Gli2 mice by overexpressing Gli217. Both Insig1 gene and INSIG1 protein expression increased in Gli2+/- cartilage and decreased in Col2a1-Gli2 cartilage (Fig. 2A and Fig. S1A). This confirmed an inverse relationship between Hh signaling and INSIG1 expression in chondrocytes.
Figure 2. Hedgehog signaling regulates cholesterol biosynthesis in chondrocytes.
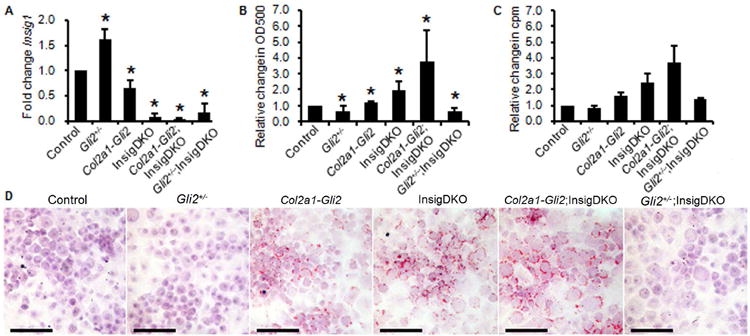
(A) Real-time PCR of Insig1 in murine cartilage with Gli transcriptional reduction (Gli2+/-), Gli transcriptional activation (Col2a1-Gli2), and/or sterol accumulation (InsigDKO). Expression in the Control group was arbitrarily defined as ‘1’ and data from other groups given as the mean. Error bars are 95% confidence intervals (n = 3; *P < 0.05). (B) Spectrophotometric quantification of alcohol-extracted Oil-Red-O stain from chondrocytes shown in D, normalized to crystal violet stain, to show sterol and lipid accumulation. Error bars are 95% confidence intervals (n = 3; *P < 0.05). (C) Cholesterol biosynthesis as measured by 3H- acetic acid sodium salt incorporation in pooled primary chondrocytes. Measured in triplicate and reported as the mean relative to Control in counts per minute (cpm). Error bars are SEM. (D) Representative images of primary chondrocytes stained with Oil-Red-O to show sterol and lipid accumulation according to genotype. Scale bar, 100 μm.
To assess the effects of modulated Hh signaling on total sterol and lipid, primary chondrocyte cultures were established using cartilage dissected from mouse knees as previously described14. Oil-Red-O staining for sterol and lipid accumulation showed lower levels with Gli transcriptional reduction (Gli2+/-) and higher levels with Gli transcriptional activation (Col2a1-Gli2; Fig. 2D). These differences were quantified by spectrophotometry and determined to be significant when compared to control chondrocytes (Fig. 2B). In primary human chondrocytes, stimulation of the Hh pathway with agonist purmorphamine resulted in a 3.70 fold increase in Oil-Red-O staining compared to carrier. This demonstrated that Hh signaling regulates total sterol and lipid content in chondrocytes.
To determine whether Insig1 was mediating the effect of Hh signaling on sterol homeostasis, we generated genetically modified mice in which Insig1 was removed from chondrocytes. There are two mammalian Insig genes, Insig1 and Insig2, which functionally compensate for each other in the regulation of sterol biosynthesis16,26. In their absence, mice accumulate cholesterol and triglycerides in a robust manner. To induce cholesterol accumulation in chondrocytes, we crossed Insig1(fl/fl);Insig2(-/-) mice16 to Col2a1-Cre transgenic mice15,27 (Fig. S1B). Analyses were performed by comparing double-knock-out Insig1(-/-);Insig2(-/-) mice (designated InsigDKO) with control littermates [Insig1(fl/fl);Insig2(-/-)]. To modulate Hh signaling, InsigDKO mice were crossed to Gli2+/- and Col2a1-Gli2 mice to generate Gli2+/-;InsigDKO and Col2a1-Gli2;InsigDKO mice (Fig. S2).
Using Oil-Red-O staining of primary chondrocyte cultures, we confirmed that InsigDKO chondrocytes had robust sterol and lipid accumulation. Despite the absence of Insig1, Gli-mediated transcription still modulated sterol and lipid accumulation in chondrocytes. Gli transcriptional activation (Col2a1-Gli2;InsigDKO) increased accumulation, and reduction (Gli2+/-;InsigDKO) decreased accumulation (Fig. 2B and 2D). To refine the potential sterol intermediates contributing to the accumulation, we took a candidate approach to determine changes to cholesterol specifically. Radiotracer studies were conducted to measure cholesterol production from 3H-acetic acid in primary chondrocyte cultures. These results were consistent with those of Oil-Red-O spectrophotometric quantification, showing that cholesterol was positively modulated by Hh signaling in chondrocytes (Fig. 2C). By examining changes to cholesterol level in both Insig1-expressing mice (Control, Gli2+/- and Col2a1-Gli2) and Insig1-knockout mice (InsigDKO, Col2a1-Gli2;InsigDKO and Gli2+/-;InsigDKO), we determined that Hh signaling could alter cholesterol level in the absence of Insig1, showing that additional regulators are involved in this process.
Intracellular cholesterol accumulation in chondrocytes exacerbates osteoarthritis
Since InsigDKO mice exhibit robust sterol accumulation in the chondrocytes, we evaluated the knees of 6-month-old mice for markers of osteoarthritis to determine whether intracellular sterol accumulation contributes to disease development. Compared to control littermates, InsigDKO mice showed characteristic signs of osteoarthritis. Histologic analyses revealed loss of proteoglycan and thinner articular cartilage (Fig. 3A). This was accompanied and potentially mediated by increased expression of proteases Mmp13 and Adamts5 in the cartilage (Fig. 3C). Expression of the hypertrophic marker Col10a1 was also increased and shown to be concentrated at the surface of the eroding cartilage in InsigDKO mice (Fig. 3D). Radiographic analyses revealed irregularity of the bone contour and increased sclerosis in the subchondral bone (Fig. 3B). Histomorphometry analyses indicated reduced bone volume in both the tibia and femur of InsigDKO mice (Fig. S3). Whether the subchondral bone changes preceded or resulted from changes to the articular cartilage remains unclear, but the contribution of subchondral bone to osteoarthritis progression is well recognized28,29. Following surgical induction of osteoarthritis, InsigDKO mice showed osteophyte-like protrusions that were 15% larger by volume than those in control littermates (Fig. S4).
Figure 3. Intracellular cholesterol accumulation in chondrocytes induces osteoarthritic changes.
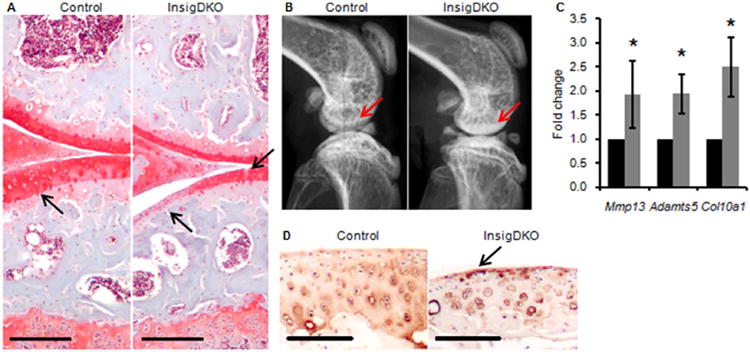
(A) Representative histological sections of mouse knees showing Safranin-O staining for proteoglycan at 6 months. Arrows point to loss of proteoglycan (red) and cartilage defects in InsigDKO. Scale bar, 100 μm. (B) Representative radiographic images of mouse knees showing a lateral view at 4 months. Arrows point to areas of subchondral sclerosis (whitening) in InsigDKO. (C) Real-time PCR of osteoarthritis markers in cartilage microdissected from mouse knees at 6 months. Expression of Control (black bars) was arbitrarily defined as ‘1’, and data for InsigDKO (grey bars) given as the mean. Error bars are 95% confidence intervals (n = 4, *P < 0.05). (D) Representative histological sections showing Col10a1 immunohistochemistry in the articular cartilage of the femur at 6 months. Arrow points to increased staining (brown) of hypertrophic chondrocytes in InsigDKO. Scale bar, 100 μm.
Blinded grading for osteoarthritis severity was conducted using the International Cartilage Repair Society (ICRS) Visual Histological Assessment Scale. This grading scale was chosen because it accounts for changes to the subchondral bone, which other scoring systems may minimize21,30. InsigDKO mice showed reduced overall ICRS scores, from 17.3 in control mice to 11.3 in InsigDKO mice (P < 0.05), where lower scores represent more severe osteoarthritis20 (Table S2). OARSI grading confirmed this finding; control mice score 1.4 and InsigDKO mice score 2.1 (P < 0.05), where higher scores represent more severe osteoarthritis21. There were no sex-dependent differences in overall ICRS scores (data not shown), but for the ‘Surface’ category, males showed a more severe phenotype (1.5 ± 1.0) than females (3.0 ± 0.0) at 6 months of age. This is consistent with previous reports of male mice developing a more severe osteoarthritis phenotype than female mice31. To determine whether the cholesterol accumulation in InsigDKO mice was exacerbating osteoarthritis by activating Hh signaling, we examined expression of Hh target genes in the cartilage. Because there was no change in expression of Gli1, Ptch1, and Hhip, this phenotype cannot be attributed to changes in Gli transcriptional activity in InsigDKO chondrocytes (Fig. S2). These results demonstrate that intracellular sterol accumulation in chondrocytes exacerbates the severity of osteoarthritis in mice.
Pharmacologic cholesterol inhibition in cartilage attenuates osteoarthritis
To assess whether inhibition of cholesterol production could mitigate osteoarthritis progression, mice were treated with 3mg/kg/day lovastatin. Drug pellets were placed adjacent to the synovial membrane of the knee in Col2a1-Gli2 mice in which Hh signaling was activated, InsigDKO mice in which cholesterol accumulation occurred, and surgical mice in which osteoarthritis was induced by medial meniscectomy4. Lovastatin treatment increased HMGCR expression by 25% in chondrocytes (n = 3; P < 0.05), the typical response to lowered intracellular cholesterol levels (Fig. S5). In all cases, lovastatin treatment reduced cartilage fibrillation and thinning (Fig. 4). This was reflected by improved overall ICRS scores20, for example in InsigDKO + Surgery mice, from 11.3 with placebo to 16.7 with lovastatin treatment (P < 0.05; Table S2). In Col2a1-Gli2, InsigDKO, and Col2a1-Gli2;InsigDKO mice, lovastatin treatment attenuated chondrocyte hypertrophy as indicated by Col10a1 staining (Fig. S6).
Figure 4. Pharmacologic cholesterol inhibition attenuates osteoarthritic changes in mice.
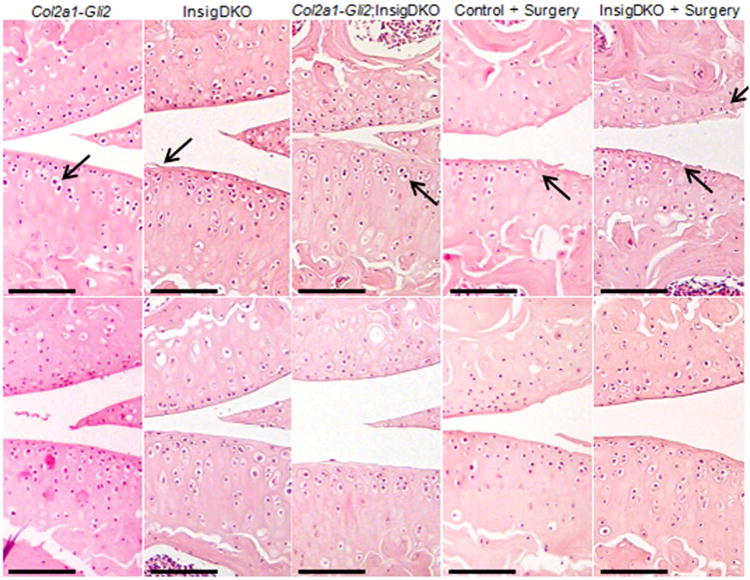
Representative histological sections showing haematoxylin and eosin staining of the knee joints in 4-month-old mice that were implanted with a slow release Placebo (top panels) or Statin pellet (bottom panels). Methods for inducing osteoarthritis include Hh activation (Col2a1-Gli2), cholesterol accumulation (InsigDKO), or both (Col2a1-Gli2;InsigDKO), as well as surgical excision of the medial meniscus (Surgery) in Control and InsigDKO mice. Arrows point to hypertrophic chondrocytes and cartilage fibrillation in Placebo-treated mice. Scale bar, 100 μm.
To investigate the role of cholesterol inhibition in human osteoarthritis, articular cartilage explants were treated with 10 μM lovastatin. Treatment induced increased HMGCR expression in chondrocytes, as expected in response to lowered intracellular cholesterol levels (Fig. S7). Lovastatin treatment significantly reduced expression of MMP1332,33 and ADAMTS5, and also decreased ADAMTS5 promoter activity in human OA chondrocytes (Fig. 5A and 5B). Chromatin immunoprecipitation showed binding of SREBF2 to the proximal promoter of Adamts5, a region that contains a conserved SRE binding site (Fig. 5C and 5D). Deletion of the SRE site diminishes ADAMTS5 promoter activity (Fig. S8), suggesting that Adamts5 is a target of the SREBF transcription factors. Since cholesterol levels regulate processing of the SREBF proteins34, these data suggest that cholesterol accumulation can impact osteoarthritis at least in part by modulating expression of ADAMTS5.
Figure 5. Pharmacologic cholesterol inhibition attenuates ADAMTS5 expression in human cartilage.
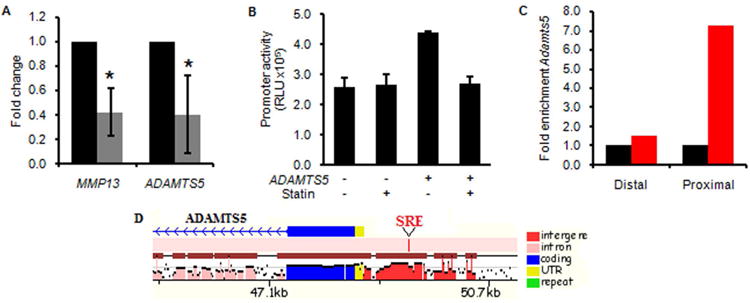
(A) Real-time PCR showing a reduction in osteoarthritis markers MMP13 and ADAMTS5 in human osteoarthritic cartilage explants treated with statin (grey bars). Expression in the control group (black bars) was arbitrarily defined as ‘1’ and data from the statin-treated group given as the mean. Error bars are 95% confidence intervals (n = 4; *P < 0.05). (B) Luciferase activity from the ADAMTS5 promoter construct transfected into primary human osteoarthritic chondrocytes, treated with control or statin. Measured in triplicate and reported as relative light units (RLU). Error bars are SEM. (C) Chromatin immunoprecipitation was performed in ATDC5 cells with IgG (black bars, arbitrarily defined as ‘1’) and anti-SREBF2 (red bars) antibodies. Real-time PCR of the Adamts5 gene with primers designed in the proximal promoter region (Proximal) and a control region (Distal). (D) Schematic of the ADAMTS5 promoter showing a SRE consensus binding site (red tick) that is conserved between mouse and human (from MULAN analysis, http://mulan.dcode.org).
Discussion
Here we show that Hh signaling regulates the cholesterol homeostatic pathway in human and mouse cartilage. In genetically modified mice, the level of Gli-mediated transcription positively correlates with the level of intracellular cholesterol accumulation and the severity of osteoarthritis. Furthermore, cholesterol blockade reduces the severity of osteoarthritis in Col2a1-Gli2 mice with Hh activation. This demonstrates that intracellular cholesterol biosynthesis at least partially mediates the effect of Hh signaling in chondrocytes in osteoarthritis4.
Sterols have been shown to modulate Hh signaling35-38, but this is the first study to demonstrate that Hh signaling modulates cholesterol homeostasis. Cholesterol modifies Hh signaling with the covalent addition of a cholesterol moiety to Hh ligands39. Key regulators of Hh signaling contain conserved sterol-sensing domains, suggesting that their behaviour may be affected by sterols40. Perturbations in either Hh signaling or cholesterol homeostasis produce similar central nervous system abnormalities, facial dysmorphisms, and skeletal defects41. These parallels point to a mutual regulatory relationship between Hh signaling and cholesterol homeostasis.
Engelking et al. noted the phenotypic similarity between total InsigDKO embryos and embryos with modulated Hh signaling42. They hypothesized that the sterol accumulation in InsigDKO mice caused Hh activation, but did not find differences in gene expression levels of Shh, Smo, Ptch1, or Gli1 in the palate tissues at 13.5 days post coitum26. Similarly, our InsigDKO mice did not show changes to Hh target gene expression in the chondrocytes. Thus, the phenotypic changes observed in response to cholesterol accumulation are not likely attributable to perturbations in Hh signaling in the cartilage. It is possible that Hh signaling is modulated in surrounding tissues due to altered ligand trafficking, or is modulated in cartilage by sterol intermediates other than cholesterol24. The potential mutual regulatory relationship between Hh signaling and sterol homeostasis merits further investigation.
Given that osteoarthritis is a degenerative disease, it conceivably results from the culmination of small changes over time. The magnitude of the changes we report here, to both gene expression and cholesterol accumulation, represent relatively small perturbations which accumulate over time, causing progressive cartilage degeneration and subchondral bone abnormalities. The resulting osteoarthritis phenotype is mild but reflective of disease presentation in humans. While the possibility exists that the phenotype was due in part to developmental alterations or structural changes to the joints, the rescue we observe with an 8-week statin treatment demonstrates at least a partial contribution of cholesterol dysregulation to the phenotype.
Studies examining the relationship between sterols and the occurrence of osteoarthritis have been largely epidemiological9,11,43,44. One limitation is the assumption that systemic sterol levels are representative of intra-articular sterol levels. Prete et al. measured total cholesterol levels of control and osteoarthritis patients and showed that plasma levels were comparable while synovial fluid levels were markedly increased, from 7-8 mg/dL in control patients to 4-169 mg/dL in osteoarthritis patients45. This suggests that any pharmacologic cholesterol inhibitor should target the synovial joint specifically46. Gierman et al. report rescue to the osteoarthritis phenotype with statin treatment but not ezetimibe treatment, suggesting that reductions in intracellular cholesterol production (by statin), and not reductions in serum cholesterol (by ezetimibe) are required to attenuate osteoarthritis progression47. In support of this, the osteoarthritic changes we observed resulted from chondrocyte-specific cholesterol accumulation. These changes were attenuated by statin treatment administered locally to the knee in vivo, which was effective in altering cholesterol homeostasis in the chondrocytes, and rescuing the osteoarthritis phenotype.
Statin treatment reduced expression of proteases in human osteoarthritic cartilage in vitro, including the promoter activity of ADAMTS5, the major protease responsible for cartilage degradation in osteoarthritis31. We show that activation of Hh signaling in the chondrocytes induces cholesterol accumulation, the levels of which govern processing of SREBF2 transcription factors. We also show that ADAMTS5 contains a conserved SRE binding site to which SREBF2 binds, making it a putative target of SREBF2 and subject to regulation by intracellular cholesterol levels. This is one mechanism through which cholesterol levels can regulate osteoarthritis progression. Alternate mechanisms include induction of chondrocyte hypertrophy by cholesterol-mediated activation of the lipid regulator Ror-alpha48, or cartilage degradation by proteases that are regulated by protein geranylgeranylation which requires intermediates from the cholesterol biosynthetic pathway32. Furthermore, statins have been shown to reduce inflammation49 and induce anabolic effects in cartilage33,46,50, so additional studies are required to elucidate the exact mechanism through which sterol levels impact cartilage biology. Identifying intracellular cholesterol as a mediator of osteoarthritis reinforces the importance of chondrocyte homeostasis in this disease, and puts forth a mechanism by which Hh signaling exacerbates osteoarthritis. Cholesterol biosynthesis is identified as a novel downstream pathway that is regulated by Hh signaling, a relationship which may be relevant in both physiologic and pathologic processes.
Supplementary Material
Figure S1. Hedgehog signaling modulates expression of Insig1, a major negative regulator of cholesterol biosynthesis. (A) Western blot analysis for INSIG1 in murine cartilage with Gli transcriptional reduction (Gli2+/-) or Gli transcriptional activation (Col2a1-Gli2). (B) Western blot analysis for INSIG1 in murine cartilage resulting from the cross between Insig1(fl/fl);Insig2(-/-) and Col2a1-Cre. Reduction of INSIG1 was observed in Insig1(-/-) cartilage [Insig1(-/-);Insig2(-/-), subsequently designated InsigDKO] but not Insig1(-/fl) cartilage [Insig1(-/fl);Insig2(-/-)], so all analyses focused on Insig1(-/-) and Insig1(fl/fl) cartilage [Insig1(fl/fl);Insig2(-/-), subsequently designated Control]. ACTIN is shown as a loading control.
Figure S2. Expression of Hedgehog target genes is unaltered in InsigDKO cartilage. Real-time PCR for Hh target genes Gli1, Ptch1, and Hhip in the articular cartilage of mice with cholesterol accumulation (InsigDKO), with reduced Gli-mediated transcription (Gli2+/-;InsigDKO), or with increased Gli-mediated transcription (Col2a1-Gli2;InsigDKO). Expression in Control mice was arbitrarily defined as ‘1’ (dashed line) and data for each genotype given as the mean. Error bars are 95% confidence intervals (n = 3; *P < 0.05).
Figure S3. InsigDKO mice show reduced bone volume compared to Control mice. Histomorphometry analysis for bone volume in the tibia and femur of InsigDKO (grey bars) and Control (black bars) mice at 6 months of age (*P < 0.05).
Figure S4. Osteophyte-like protrusions are more pronounced in InsigDKO mice compared to Control mice. Representative histological sections showing haematoxylin and eosin staining of the knee joints in 4-month-old mice following surgical induction of osteoarthritis (top panels). A magnified view of the joint space shows areas containing osteophyte-like protrusions at the periphery of the joint (arrows; bottom panels).
Figure S5. Effective statin treatment is shown by increased HMGCR in mouse cartilage. Representative histological sections showing HMGCR immunohistochemistry (brown) in the articular cartilage at 4 months for InsigDKO mice treated with placebo or statin. Scale bar, 50 μm.
Figure S6. Chondrocyte hypertrophy in the articular cartilage is altered by cholesterol accumulation. Representative histological sections showing Col10a1 immunohistochemistry in the articular cartilage of the femur at 4 months of age for each genotype/treatment group shown in Figure 4. Scale bar, 100 μm.
Figure S7. Effective statin treatment is shown by increased HMGCR in human cartilage. Real-time PCR of HMGCR in human osteoarthritic articular cartilage explants treated with statin. Expression in the control group was arbitrarily defined as ‘1′ and data from the statin-treated group given as the mean. Error bar is 95% confidence interval (n = 4; *P < 0.05).
Figure S8. Deletion of the SRE binding site in the ADAMTS5 promoter diminishes reporter activity. Luciferase activity from a negative control vector (Negative), the ADAMTS5 promoter construct (ADAMTS5), and the ADAMTS5 promoter construct with deleted SRE site (ADAMTS5-SRE), following transfection into ATDC5 cells. Measured in triplicate and reported as relative light units (RLU). Error bars are SEM.
Table S1. Summary of Ingenuity® Pathway Analysis. The gene list resulting from microarray analysis was subjected to Ingenuity® Pathway Analysis. The settings were adjusted to consider all molecules and/or relationships, direct and indirect, according to the Ingenuity® Knowledge Base reference set. This unsupervised analysis identified Cholesterol Biosynthesis to be the most significantly dysregulated pathway.
Table S2. ICRS scores for osteoarthritis. The International Cartilage Repair Society (ICRS) score was used to grade histologic sections in a blinded manner. As previously reported4, the mean and 95% confidence interval are given for each criterion, and a summary score is provided with Mann-Whitney U statistical analysis to determine significant differences. (A) Grading of knee joints from 6-month-old mice reveal more severe osteoarthritis in InsigDKO mice as compared to Control mice (P < 0.05). (B) Knee joints from 4-month-old mice were graded for genotypes (without surgery) shown in Figure 4. Statistical analyses compared placebo to statin treatment for each of Col2a1-Gli2, InsigDKO, and Col2a1-Gli2;InsigDKO. (C) Knee joints from 4-month-old mice were graded for genotypes (with surgery) shown in Figure 4. Statistical analyses compared placebo to statin treatment in mice that underwent excision of the medial meniscus. Statin treatment was found to have a statistically significant difference in all groups (P < 0.05).
Acknowledgments
We thank D. Backstein (Mount Sinai Hospital, Toronto) for assistance in obtaining human cartilage specimens. Scholarship support was provided to S.A.A. from the Ontario Government and the Toronto Musculoskeletal Centre, to M.A.J. from the Hospital for Sick Children, and to S.F. from Canadian Institutes of Health Research. This study was supported by the Canadian Institutes of Health Research (MOP-115092 to K.A., MOP-106587 to B.A.) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (R01-AR066765 to B.A.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions: S.A.A. and B.A. designed experiments, interpreted results, and wrote the manuscript. S.A.A. performed experiments with the assistance of M.A.J., who helped with protein and histologic analyses; H.M. and R.P. who performed ChIP and reporter construct experiments; H.W. who performed histologic experiments; and S.F., M.N., and K.A. who performed radiotracer experiments.
Competing financial interests: The authors declare no competing financial interests.
References
- 1.Hunter DJ, Eckstein F. Exercise and osteoarthritis. J Anat. 2009;214:197–207. doi: 10.1111/j.1469-7580.2008.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Issa RI, Griffin TM. Pathobiology of obesity and osteoarthritis: integrating biomechanics and inflammation. Pathobiol Aging Age Relat Dis. 2012;2 doi: 10.3402/pba.v2i0.17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aigner T, Soder S, Gebhard PM, McAlinden A, Haag J. Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis--structure, chaos and senescence. Nat Clin Pract Rheumatol. 2007;3:391–399. doi: 10.1038/ncprheum0534. [DOI] [PubMed] [Google Scholar]
- 4.Lin AC, et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. 2009;15:1421–1425. doi: 10.1038/nm.2055. [DOI] [PubMed] [Google Scholar]
- 5.Mak KK, Kronenberg HM, Chuang PT, Mackem S, Yang Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development. 2008;135:1947–1956. doi: 10.1242/dev.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vortkamp A, et al. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 7.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, et al. Disrupting the Indian hedgehog signaling pathway in vivo attenuates surgically induced osteoarthritis progression in Col2a1-CreERT2; Ihhfl/fl mice. Arthritis Res Ther. 2014;16:R11. doi: 10.1186/ar4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sturmer T, et al. Serum cholesterol and osteoarthritis. The baseline examination of the Ulm Osteoarthritis Study. J Rheumatol. 1998;25:1827–1832. [PubMed] [Google Scholar]
- 10.Kostopoulou F, et al. Central role of SREBP-2 in the pathogenesis of osteoarthritis. PLoS One. 2012;7:e35753. doi: 10.1371/journal.pone.0035753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Arfaj AS. Radiographic osteoarthritis and serum cholesterol. Saudi Med J. 2003;24:745–747. [PubMed] [Google Scholar]
- 12.Villalvilla A, Gomez R, Largo R, Herrero-Beaumont G. Lipid transport and metabolism in healthy and osteoarthritic cartilage. Int J Mol Sci. 2013;14:20793–20808. doi: 10.3390/ijms141020793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali SA, Alman B. RNA extraction from human articular cartilage by chondrocyte isolation. Anal Biochem. 2012;429:39–41. doi: 10.1016/j.ab.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3:1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 15.Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26:145–146. [PubMed] [Google Scholar]
- 16.Engelking LJ, et al. Schoenheimer effect explained--feedback regulation of cholesterol synthesis in mice mediated by Insig proteins. J Clin Invest. 2005;115:2489–2498. doi: 10.1172/JCI25614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopyan S, et al. A mutant PTH/PTHrP type I receptor in enchondromatosis. Nat Genet. 2002;30:306–310. doi: 10.1038/ng844. [DOI] [PubMed] [Google Scholar]
- 18.Mo R, et al. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- 19.Parfitt AM, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 20.Mainil-Varlet P, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS) J Bone Joint Surg Am. 2003;85-A Suppl 2:45–57. [PubMed] [Google Scholar]
- 21.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18 Suppl 3:S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 22.Williams JA, et al. Identification of a small molecule inhibitor of the hedgehog signaling pathway: effects on basal cell carcinoma-like lesions. Proc Natl Acad Sci U S A. 2003;100:4616–4621. doi: 10.1073/pnas.0732813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horton JD, et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci U S A. 2003;100:12027–12032. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill S, Chow R, Brown AJ. Sterol regulators of cholesterol homeostasis and beyond: the oxysterol hypothesis revisited and revised. Prog Lipid Res. 2008;47:391–404. doi: 10.1016/j.plipres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson C, et al. Genome-wide expression profiling reveals new candidate genes associated with osteoarthritis. Osteoarthritis Cartilage. 2010;18:581–592. doi: 10.1016/j.joca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Engelking LJ, et al. Severe facial clefting in Insig-deficient mouse embryos caused by sterol accumulation and reversed by lovastatin. J Clin Invest. 2006;116:2356–2365. doi: 10.1172/JCI28988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant TD, et al. Col2-GFP reporter marks chondrocyte lineage and chondrogenesis during mouse skeletal development. Dev Dyn. 2000;218:394–400. doi: 10.1002/(SICI)1097-0177(200006)218:2<394::AID-DVDY12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 28.Mansell JP, Bailey AJ. Abnormal cancellous bone collagen metabolism in osteoarthritis. J Clin Invest. 1998;101:1596–1603. doi: 10.1172/JCI867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang R, et al. Gene expression analyses of subchondral bone in early experimental osteoarthritis by microarray. PLoS One. 2012;7:e32356. doi: 10.1371/journal.pone.0032356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed] [Google Scholar]
- 31.Glasson SS, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 32.Barter MJ, et al. Lipophilic statins prevent matrix metalloproteinase-mediated cartilage collagen breakdown by inhibiting protein geranylgeranylation. Ann Rheum Dis. 2010;69:2189–2198. doi: 10.1136/ard.2010.129197. [DOI] [PubMed] [Google Scholar]
- 33.Simopoulou T, Malizos KN, Poultsides L, Tsezou A. Protective effect of atorvastatin in cultured osteoarthritic chondrocytes. J Orthop Res. 2010;28:110–115. doi: 10.1002/jor.20953. [DOI] [PubMed] [Google Scholar]
- 34.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 35.Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci U S A. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stottmann RW, et al. Cholesterol metabolism is required for intracellular hedgehog signal transduction in vivo. PLoS Genet. 2011;7:e1002224. doi: 10.1371/journal.pgen.1002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eaton S. Multiple roles for lipids in the Hedgehog signalling pathway. Nat Rev Mol Cell Biol. 2008;9:437–445. doi: 10.1038/nrm2414. [DOI] [PubMed] [Google Scholar]
- 38.Dwyer JR, et al. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem. 2007;282:8959–8968. doi: 10.1074/jbc.M611741200. [DOI] [PubMed] [Google Scholar]
- 39.Jeong J, McMahon AP. Cholesterol modification of Hedgehog family proteins. J Clin Invest. 2002;110:591–596. doi: 10.1172/JCI16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuwabara PE, Labouesse M. The sterol-sensing domain: multiple families, a unique role? Trends Genet. 2002;18:193–201. doi: 10.1016/s0168-9525(02)02640-9. [DOI] [PubMed] [Google Scholar]
- 41.Gofflot F, et al. Molecular mechanisms underlying limb anomalies associated with cholesterol deficiency during gestation: implications of Hedgehog signaling. Hum Mol Genet. 2003;12:1187–1198. doi: 10.1093/hmg/ddg129. [DOI] [PubMed] [Google Scholar]
- 42.Chiang C, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 43.Kadam UT, Blagojevic M, Belcher J. Statin use and clinical osteoarthritis in the general population: a longitudinal study. J Gen Intern Med. 2013;28:943–949. doi: 10.1007/s11606-013-2382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clockaerts S, et al. Statin use is associated with reduced incidence and progression of knee osteoarthritis in the Rotterdam study. Ann Rheum Dis. 2012;71:642–647. doi: 10.1136/annrheumdis-2011-200092. [DOI] [PubMed] [Google Scholar]
- 45.Prete PE, Gurakar-Osborne A, Kashyap ML. Synovial fluid lipoproteins: review of current concepts and new directions. Semin Arthritis Rheum. 1993;23:79–89. doi: 10.1016/s0049-0172(05)80014-9. [DOI] [PubMed] [Google Scholar]
- 46.Baker JF, Walsh P, Mulhall KJ. Statins: a potential role in the management of osteoarthritis? Joint Bone Spine. 2011;78:31–34. doi: 10.1016/j.jbspin.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 47.Gierman LM, et al. Osteoarthritis development is induced by increased dietary cholesterol and can be inhibited by atorvastatin in APOE*3Leiden.CETP mice--a translational model for atherosclerosis. Ann Rheum Dis. 2014;73:921–927. doi: 10.1136/annrheumdis-2013-203248. [DOI] [PubMed] [Google Scholar]
- 48.Woods A, James CG, Wang G, Dupuis H, Beier F. Control of chondrocyte gene expression by actin dynamics: a novel role of cholesterol/Ror-alpha signalling in endochondral bone growth. J Cell Mol Med. 2009;13:3497–3516. doi: 10.1111/j.1582-4934.2009.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leung BP, et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol. 2003;170:1524–1530. doi: 10.4049/jimmunol.170.3.1524. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita A, et al. Statin treatment rescues FGFR3 skeletal dysplasia phenotypes. Nature. 2014;513:507–511. doi: 10.1038/nature13775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Hedgehog signaling modulates expression of Insig1, a major negative regulator of cholesterol biosynthesis. (A) Western blot analysis for INSIG1 in murine cartilage with Gli transcriptional reduction (Gli2+/-) or Gli transcriptional activation (Col2a1-Gli2). (B) Western blot analysis for INSIG1 in murine cartilage resulting from the cross between Insig1(fl/fl);Insig2(-/-) and Col2a1-Cre. Reduction of INSIG1 was observed in Insig1(-/-) cartilage [Insig1(-/-);Insig2(-/-), subsequently designated InsigDKO] but not Insig1(-/fl) cartilage [Insig1(-/fl);Insig2(-/-)], so all analyses focused on Insig1(-/-) and Insig1(fl/fl) cartilage [Insig1(fl/fl);Insig2(-/-), subsequently designated Control]. ACTIN is shown as a loading control.
Figure S2. Expression of Hedgehog target genes is unaltered in InsigDKO cartilage. Real-time PCR for Hh target genes Gli1, Ptch1, and Hhip in the articular cartilage of mice with cholesterol accumulation (InsigDKO), with reduced Gli-mediated transcription (Gli2+/-;InsigDKO), or with increased Gli-mediated transcription (Col2a1-Gli2;InsigDKO). Expression in Control mice was arbitrarily defined as ‘1’ (dashed line) and data for each genotype given as the mean. Error bars are 95% confidence intervals (n = 3; *P < 0.05).
Figure S3. InsigDKO mice show reduced bone volume compared to Control mice. Histomorphometry analysis for bone volume in the tibia and femur of InsigDKO (grey bars) and Control (black bars) mice at 6 months of age (*P < 0.05).
Figure S4. Osteophyte-like protrusions are more pronounced in InsigDKO mice compared to Control mice. Representative histological sections showing haematoxylin and eosin staining of the knee joints in 4-month-old mice following surgical induction of osteoarthritis (top panels). A magnified view of the joint space shows areas containing osteophyte-like protrusions at the periphery of the joint (arrows; bottom panels).
Figure S5. Effective statin treatment is shown by increased HMGCR in mouse cartilage. Representative histological sections showing HMGCR immunohistochemistry (brown) in the articular cartilage at 4 months for InsigDKO mice treated with placebo or statin. Scale bar, 50 μm.
Figure S6. Chondrocyte hypertrophy in the articular cartilage is altered by cholesterol accumulation. Representative histological sections showing Col10a1 immunohistochemistry in the articular cartilage of the femur at 4 months of age for each genotype/treatment group shown in Figure 4. Scale bar, 100 μm.
Figure S7. Effective statin treatment is shown by increased HMGCR in human cartilage. Real-time PCR of HMGCR in human osteoarthritic articular cartilage explants treated with statin. Expression in the control group was arbitrarily defined as ‘1′ and data from the statin-treated group given as the mean. Error bar is 95% confidence interval (n = 4; *P < 0.05).
Figure S8. Deletion of the SRE binding site in the ADAMTS5 promoter diminishes reporter activity. Luciferase activity from a negative control vector (Negative), the ADAMTS5 promoter construct (ADAMTS5), and the ADAMTS5 promoter construct with deleted SRE site (ADAMTS5-SRE), following transfection into ATDC5 cells. Measured in triplicate and reported as relative light units (RLU). Error bars are SEM.
Table S1. Summary of Ingenuity® Pathway Analysis. The gene list resulting from microarray analysis was subjected to Ingenuity® Pathway Analysis. The settings were adjusted to consider all molecules and/or relationships, direct and indirect, according to the Ingenuity® Knowledge Base reference set. This unsupervised analysis identified Cholesterol Biosynthesis to be the most significantly dysregulated pathway.
Table S2. ICRS scores for osteoarthritis. The International Cartilage Repair Society (ICRS) score was used to grade histologic sections in a blinded manner. As previously reported4, the mean and 95% confidence interval are given for each criterion, and a summary score is provided with Mann-Whitney U statistical analysis to determine significant differences. (A) Grading of knee joints from 6-month-old mice reveal more severe osteoarthritis in InsigDKO mice as compared to Control mice (P < 0.05). (B) Knee joints from 4-month-old mice were graded for genotypes (without surgery) shown in Figure 4. Statistical analyses compared placebo to statin treatment for each of Col2a1-Gli2, InsigDKO, and Col2a1-Gli2;InsigDKO. (C) Knee joints from 4-month-old mice were graded for genotypes (with surgery) shown in Figure 4. Statistical analyses compared placebo to statin treatment in mice that underwent excision of the medial meniscus. Statin treatment was found to have a statistically significant difference in all groups (P < 0.05).


