Abstract
Background
Remnants are partially-hydrolyzed, triglyceride-rich lipoproteins that, like other apolipoprotein B-containing lipoproteins, are atherogenic. Prior observational studies suggest paradoxically better outcomes in hypercholesterolemic patients who sustain an acute myocardial infarction (AMI), one of several known recurrent risk paradoxes. To date, the association of directly-measured remnant lipoprotein cholesterol (RLP-C) with survival has not been examined after an AMI.
Methods
We examined 2,465 AMI survivors in TRIUMPH, a prospective, 24-center US study of AMI outcomes. Lipoprotein cholesterol subfractions were directly measured by ultracentrifugation. RLP-C was defined as IDL-C+VLDL3-C. Given a linear relationship between RLP-C and mortality, we examined RLP-C by tertiles and continuously. Cox regression hazard ratios were adjusted for the GRACE score and 23 other co-variates.
Results
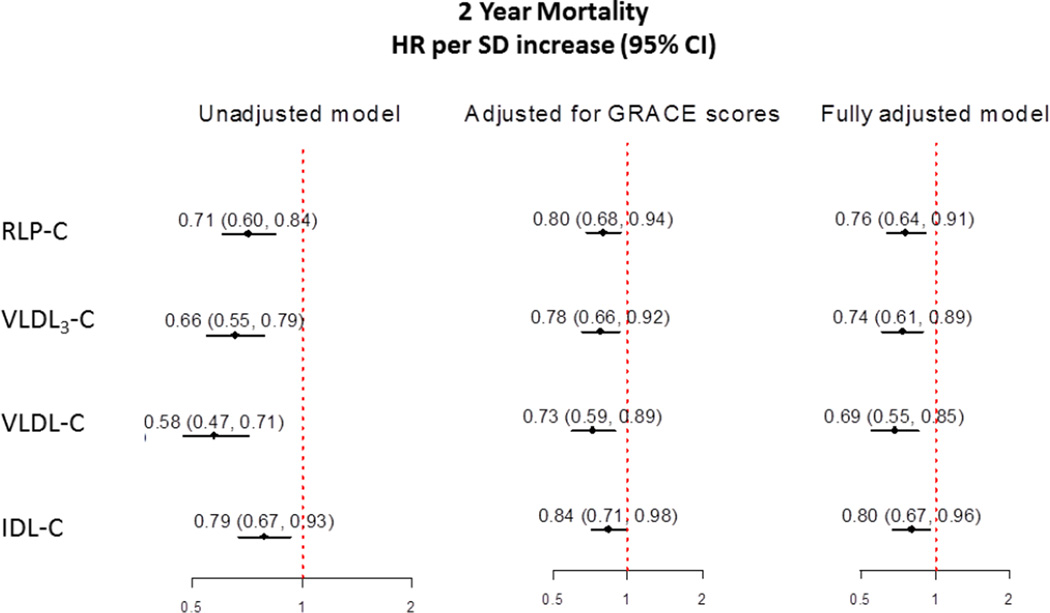
Participants were 58±12 years old (mean±SD) and 68% were men. After 2 years of follow-up, 226 (9%) participants died. The mortality proportion was 12.4% in the lowest tertile of RLP-C (0–15 mg/dL), 8.5% in the middle tertile (16–23 mg/dL), and 6.8% in the highest tertile (24–120 mg/dL) (p<0.001). A one SD increase in RLP-C (11 mg/dL) predicted a 24% lower adjusted risk of 2-year mortality (HR, 0.76; 95% CI 0.64–0.91). Similar results were found for a one SD increase in IDL-C (HR per 8 mg/dL, 0.80; 0.67–0.96), VLDL3-C (HR per 4 mg/dL, 0.74; 0.61–0.89), and VLDL-C (HR per 8 mg/dL, 0.69; 0.55–0.85).
Conclusion
In conclusion, higher RLP-C levels were associated with lower mortality 2 years after AMI despite rigorous adjustment for known confounders. Unknown protective factors, or a lead-time bias, likely explain the paradox.
Keywords: acute myocardial infarction, coronary heart disease, mortality, secondary prevention, lipids, triglycerides, remnant lipoprotein cholesterol
INTRODUCTION
Coronary heart disease (CHD) is the leading cause of death in the US1 and worldwide,2 contributing to over 7 million deaths per year globally. Hypercholesterolemia is a well-established causal factor for CHD, and low-density lipoprotein cholesterol (LDL-C) lowering reduces cardiovascular (CV) risk in both the primary and secondary prevention settings.3 Remnants are partially-hydrolyzed, triglyceride-rich lipoproteins that, like other apolipoprotein B-containing lipoproteins, are atherogenic. Remnant particles consist of intermediate-density lipoproteins (IDL) and dense forms of very-low density lipoproteins (VLDL). Remnants are associated with development of CHD, independent of LDL-C and HDL-C.4–9 Mendelian randomization analysis mapped to Friedewald-estimated VLDL-C levels also suggests a causal role of remnants in CHD.10
Paradoxically, epidemiologic studies of patients with acute coronary syndromes (ACS) have linked hypercholesterolemia, based on LDL-C, to lower future mortality following the initial event.11–15 To some extent, this paradox could be related to death from non-cardiovascular causes. However, epidemiologic bias may also be operative. The hypercholesterolemia ACS paradox is one of a number of described paradoxes in recurrent risk research, which may be subject to bias introduced by selecting on an event – index event bias.16–19 If the primary exposure of interest is unfavorable at the time of the event, other risk factors may tend to be more favorable, making the exposure appear protective in relation to follow-up for events unless other factors are fully accounted for. Selecting on an event could also introduce lead-time bias. If an unfavorable exposure leads to ACS presentation earlier in the atherosclerosis disease process, then this could lead to apparently greater longevity in post-ACS follow-up.
To date, the association of directly measured RLP-C with survival has not been examined after an AMI. It is especially timely to study this association because the obesity and diabetes epidemics have led to a much greater prevalence of hypertriglyceridemia and elevations in RLP-C in modern AMI patients. Individuals with AMI, particularly those with diabetes, are one of the highest atherosclerotic cardiovascular risk groups seen in clinical practice, and are often the target of the most aggressive pharmaceutical interventions. Although RLP-C is not routinely measured in clinical practice, it is felt to be the causal mediator of atherosclerosis, rather than triglycerides per se. As such, we sought to investigate the association of RLP-C levels and mortality in patients following an acute myocardial infarction (AMI) using the Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH) registry. Based on prior studies of LDL-C, we hypothesized that higher RLP-C levels may be paradoxically associated with lower mortality, but explained by confounders, such that the association would become null or positive between RLP-C and post-AMI survival. The uniquely rich set of measurements obtained in TRIUMPH allowed for adjustment beyond what was possible in prior studies in this field.
METHODS
TRIUMPH Study
The TRIUMPH prospective cohort study enrolled AMI patients 18 years of age or older from April 11, 2005 to December 31, 2008 at 24 centers in the US, and has been previously described in detail.20 AMI was defined as clinical features of ischemia (e.g., prolonged ischemic signs/symptoms, ST-segment changes in ≥2 contiguous leads on electrocardiogram) combined with cardiac biomarker elevation (troponin per local laboratory cutpoints) outside the setting of elective coronary revascularization. Among those eligible for TRIUMPH, 74% of patients enrolled. All patients in this study provided informed consent and each enrolling site obtained Institutional Review Board approval.
Lipid Measurements
Serial laboratory testing was a voluntary substudy performed within a median of 1 day of discharge (25th–75th: 0 to 2 days). Blood samples were processed, serum separated, refrigerated, and sent by overnight mail in freezer packs to the core laboratory (Clinical Reference Laboratories, Lenexa, KS). Serum specimens were aliquoted, stored frozen at −70°C, and then sent to Atherotech (Birmingham, AL) by overnight mail on dry ice.
Cholesterol concentrations of major lipoprotein fractions and subfractions were measured by Atherotech’s Vertical Auto Profile (VAP) method.20,21 RLP-C was defined as the sum of IDL-C and the denser subfraction 3 of VLDL-C (VLDL3-C).4 The VAP method separates lipoproteins based on their density using single vertical-spin density gradient ultracentrifugation, then quantifies cholesterol content using an enzymatic reaction and spectrophotometric absorbance. Results from VAP testing were available for research purposes, but not to the treating providers.
Risk Factor and Outcome Measurements
Research staff conducted detailed chart abstractions to capture baseline characteristics, including the sociodemographic and clinical parameters presented in Table 1, as previously described.19 All-cause mortality was adjudicated using the Social Security Death Index. We did not specifically assess cardiovascular mortality.
Table 1.
Baseline Characteristics of TRIUMPH Participants by Remnant Lipoprotein Cholesterol Tertiles
| Remnant Lipoprotein Cholesterol | Total | P-Value | |||
|---|---|---|---|---|---|
| Tertile 1 (0 to <16 mg/dL) n = 772 |
Tertile 2 (16 to <24 mg/dL) n = 846 |
Tertile 3 (24 to 120 mg/dL) n = 847 |
n = 2465 | ||
| Age | 60.4 ± 12.6 | 58.0 ± 12.2 | 56.5 ± 11.5 | 58.2 ± 12.2 | <0.001 |
| Sex | |||||
| Male | 550 (71.2%) | 581 (68.7%) | 545 (64.3%) | 1676 (68.0%) | 0.011 |
| Female | 222 (28.8%) | 265 (31.3%) | 302 (35.7%) | 789 (32.0%) | |
| Race | |||||
| White/Caucasian | 498 (64.7%) | 601 (71.0%) | 567 (67.1%) | 1666 (67.7%) | 0.024 |
| Black/African-American | 222 (28.8%) | 197 (23.3%) | 210 (24.9%) | 629 (25.6%) | |
| Other/unknown | 52 (6.5%) | 48 (5.7%) | 70 (8.0%) | 170 (6.7%) | |
| High school education | 607/769 (78.9%) | 677/840 (80.6%) | 667/845 (78.9%) | 1951/2454 (79.5%) | 0.626 |
| Insured | 597/754 (79.2%) | 643/828 (77.7%) | 625/830 (75.3%) | 1865/2412 (77.3%) | 0.177 |
| Family history of coronary disease | 568/763 (74.4%) | 621/835 (74.4%) | 629/837 (75.1%) | 1818/2435 (74.7%) | 0.922 |
| History of dyslipidemia | 369 (47.8%) | 384 (45.4%) | 417 (49.2%) | 1170 (47.5%) | 0.279 |
| History of diabetes | 247 (32.0%) | 251 (29.7%) | 284 (33.5%) | 782 (31.7%) | 0.229 |
| History of hypertension | 510 (66.1%) | 540 (63.8%) | 580 (68.5%) | 1630 (66.1%) | 0.130 |
| Systolic blood pressure, mm Hg | 139 ± 29 | 143 ± 30 | 146 ± 30 | 143 ± 30 | <0.001 |
| Diastolic blood pressure, mm Hg | 80 ± 18 | 83 ± 20 | 86 ± 18 | 83 ± 19 | <0.001 |
| Chronic kidney disease | 50 (6.5%) | 62 (7.3%) | 64 (7.6%) | 176 (7.1%) | 0.677 |
| Chronic heart failure | 78 (10.1%) | 82 (9.7%) | 54 (6.4%) | 214 (8.7%) | 0.013 |
| Prior myocardial infarction | 168 (21.8%) | 172 (20.3%) | 159 (18.8%) | 499 (20.2%) | 0.326 |
| Prior PCI | 185 (24.0%) | 140 (16.5%) | 148 (17.5%) | 473 (19.2%) | <0.001 |
| Prior CABG | 109 (14.1%) | 87 (10.3%) | 80 (9.4%) | 276 (11.2%) | 0.007 |
| Prior stroke | 44 (5.7%) | 37 (4.4%) | 37 (4.4%) | 118 (4.8%) | 0.358 |
| Smoking Status | |||||
| Current | 297/767 (38.7%) | 367/840 (43.7%) | 358/842 (42.5%) | 1022/2449 (41.7%) | 0.008 |
| Former | 268/767 (34.9%) | 259/840 (30.8%) | 230/842 (27.3%) | 757/2449 (30.9%) | |
| Never | 202/767 (26.3%) | 214/840 (25.5%) | 254/842 (30.2%) | 670/2449 (27.4%) | |
| Body mass index, kg/m2 | 28.6 ± 6.4 | 30.0 ± 6.3 | 30.7 ± 6.5 | 29.8 ± 6.4 | <0.001 |
| Waist circumference, inches | 38.0 ± 5.8 | 39.0 ± 5.7 | 39.2 ± 5.5 | 38.8 ± 5.7 | 0.003 |
| Activity during leisure time | |||||
| Mainly sedentary | 329/766 (43.0%) | 350/841 (41.6%) | 412/844 (48.8%) | 1091/2451 (44.5%) | |
| Mild exercise | 242/766 (31.6%) | 263/841 (31.3%) | 241/844 (28.6%) | 746/2451 (30.4%) | 0.023 |
| Moderate exercise | 163/766 (21.3%) | 185/841 (22.0%) | 169/844 (20.0%) | 517/2451 (21.1%) | |
| Strenuous exercise | 32/766 (4.2%) | 43/841 (5.1%) | 22/844 (2.6%) | 97/2451 (4.0%) | |
| Alcohol use in past year | |||||
| Never | 321/770 (41.7%) | 347/842 (41.2%) | 372/843 (44.1%) | 1040/2455 (42.4%) | 0.005 |
| Monthly or less | 186/770 (24.2%) | 250/842 (29.7%) | 233/843 (27.6%) | 669/2455 (27.3%) | |
| 2–4 times a month | 129/770 (16.8%) | 125/842 (14.8%) | 141/843 (16.7%) | 395/2455 (16.1%) | |
| 4–5 times a week | 66/770 (8.6%) | 75/842 (8.9%) | 57/843 (6.8%) | 198/2455 (8.1%) | |
| 6 or more times a week | 68/770 (8.8%) | 45/842 (5.3%) | 40/843 (4.7%) | 153/2455 (6.2%) | |
| Type of myocardial infarction | |||||
| STEMI | 310 (40.2%) | 377 (44.6%) | 364 (43.0%) | 1051 (42.6%) | 0.195 |
| NSTEMI | 462 (59.8%) | 469 (55.4%) | 483 (57.0%) | 1414 (57.4%) | |
| Multi-vessel disease* | |||||
| No | 366 (47.4%) | 391 (46.4%) | 393 (46.5%) | 1150 (46.7%) | 0.635 |
| Yes | 343 (44.4%) | 398 (47.2%) | 388 (45.9%) | 1129 (45.9%) | |
| No cath/missing | 63 (8.2%) | 57 (6.4%) | 66 (7.6%) | 186 (7.4%) | |
| GRACE score (6 month mortality) | 104.1 ± 29.6 | 98.4 ± 30.1 | 94.8 ± 28.3 | 98.9 ± 29.6 | <0.001 |
| Lipid medications at discharge | |||||
| Statin | 685 (88.7%) | 743 (87.8%) | 728 (86.0%) | 2156 (87.5%) | 0.223 |
| Ezetimibe | 73 (9.5%) | 73 (8.6%) | 81 (9.6%) | 227 (9.2%) | 0.770 |
| Niacin | 23 (3.0%) | 11 (1.3%) | 18 (2.1%) | 52 (2.1%) | 0.064 |
| Fibrate | 13 (1.7%) | 32 (3.8%) | 37 (4.4%) | 82 (3.3%) | 0.007 |
| Fish oil | 25 (3.2%) | 19 (2.2%) | 27 (3.2%) | 71 (2.9%) | 0.395 |
Cells represent mean (SD) or N (%) with the denominators at the top of the table, unless otherwise specified within cells
Single-, double-, or triple-vessel disease defined by ≥70% stenosis in each major epicardial vessel, with left main stenosis ≥50% counting as two vessels
GRACE = Global Registry of Acute Coronary Events; STEMI = ST-segment Elevation Myocardial Infarction; NSTEMI = non- ST-segment Elevation Myocardial Infarction; PCI = Percutaneous Coronary Intervention; CABG = Coronary Artery Bypass Grafting
Statistical Analysis
Descriptive statistics were used to characterize the populations. Continuous variables approximating a normal distribution were reported as mean (standard deviation) and their differences were assessed by independent t tests. Continuous variables deviating from a normal distribution were reported as medians (25th–75th percentile) with differences compared by the Wilcoxon rank-sum test. Categorical variables were reported as proportions and differences were compared using the Chi-square method and Fisher’s exact test.
To examine the association of RLP-C, IDL-C, VLDL3-C, and VLDL-C with outcomes, we used unadjusted Kaplan-Meier estimates by tertile groups, fully-adjusted restricted cubic spline curves, and sequentially-adjusted Cox proportional hazards regression at 2 years. Model 1 was unadjusted. Model 2 was adjusted for the Global Registry of Acute Coronary Events (GRACE) 1.0 score, a composite score of the following components: age, heart rate, systolic blood pressure, creatinine, congestive heart failure, in-hospital percutaneous coronary intervention, in-hospital coronary artery bypass surgery, prior MI, ST-segment depression on electrocardiogram, and elevated cardiac biomarkers.22,23 Model 3 was adjusted for GRACE 1.0 score plus the following potential confounders as individual variables: site, age, sex, race, insurance, education, tobacco use, diabetes mellitus, hypertension, body mass index, alcohol use, physical activity, kidney disease, heart failure, prior MI, extent of coronary disease, “real” LDL-C, HDL-C, statin, ezetimibe, niacin, fibrate, and fish oil. “Real” LDL-C is the biologic form of LDL-C in contrast to the traditional Friedewald definition of LDL-C, which is inclusive of IDL-C. Medications use was based on discharge (similar results with adjustment for use prior to admission).
Linear assumptions were satisfied and, therefore, hazard ratios were calculated per one standard deviation increases in RLP-C variables. Statistical analyses were conducted with SAS version 9.2 (SAS Institute, Cary, NC) and R version 2.7.2 (R Foundation for Statistical Computing, Vienna, Austria). Two-tailed p-values <0.05 were considered statistically significant.
RESULTS
Baseline Characteristics
The baseline sociodemographic and clinical characteristics are shown in Table 1, by RLP-C tertiles and overall. The highest RLP-C tertile group was youngest in age, most likely to be female, least likely to have chronic heart failure, and had the lowest GRACE score. However, other risk factors, in particular blood pressure, were less favorable in those in the highest RLP-C compared to other groups. Overall, the population had a mean age of 58±12 years and 68% were men. Nearly a third of patients had diabetes mellitus and <10% had a history of heart failure. A statin was prescribed at discharge to 88% of patients, while much smaller proportions received prescriptions for other lipid-modifying agents such as ezetimibe, fibrates, niacin, or fish oil (omega 3). Those in the highest RLP-C tertile were most likely to receive a fibrate whereas there was no significant difference in the prescription of fish oil (omega-3).
Baseline Lipids
Baseline data on the standard lipid profile and remnant subfractions are presented in Table 2. Overall, atherogenic cholesterol concentrations were relatively controlled, but not optimal. The median (25th–75th) baseline RLP-C, IDL-C, VLDL3-C, and VLDL-C levels were 20 (14–27), 8 (5–13), 11 (9–13), and 19 (16–23) mg/dL, respectively.
Table 2.
Baseline Lipid Parameters in TRIUMPH
| TRIUMPH (N=2,465) |
|
|---|---|
| Standard lipid profile | |
| Total cholesterol, mg/dL | 156.2 (38.5) |
| HDL-C, mg/dL | 40.0 (10.6) |
| Triglycerides, mg/dL | 132.0 (100.0–179.0) |
| LDL-C (direct), mg/dL | 95.4 (32.3) |
| Non-HDL-C, mg/dL | 116.2 (36.4) |
| TC/HDL-C | 4.1 (1.2) |
| TG/HDL-C | 3.5 (2.4–5.0) |
| Remnants | |
| RLP-C, mg/dL | 20 (14–27) |
| IDL-C, mg/dL | 8 (5–13) |
| VLDL3-C, mg/dL | 11 (9–13) |
| VLDL-C, mg/dL | 19 (16–23) |
Mean (SD) or median (25th–75th percentile)
Unadjusted Outcomes
Follow-up was 100% complete at 2 years with mortality occurring in 226 (9%) patients. Table 3 shows unadjusted mortality across tertile groups of VLDL-C and remnant variables. There was an inverse relationship between mortality and RLP-C. The mortality rate was highest in the lowest tertile of RLP-C (12.4%) and progressively lower in the middle tertile (8.5%) to upper tertile (6.8%), a difference that was highly statistically significant (p<0.001). A similar inverse pattern was observed across tertile groups of IDL-C, VLDL3-C, and VLDL-C.
Table 3.
Unadjusted Kaplan-Meier Estimates of Mortality by RLP-C, IDL-C, VLDL3-C, and VLDL-C Tertiles
| Tertile 1 (mg/dL) |
Tertile 2 (mg/dL) |
Tertile 3 (mg/dL) |
p for trend | |
|---|---|---|---|---|
| (n = 2,465) | ||||
| RLP-C | (<16) | (16–23) | (24–120) | |
| 2 years | 12.4% | 8.5% | 6.8% | <0.001 |
| IDL-C | (<6) | (6–11) | (12–69) | |
| 2 years | 11.9% | 8.0% | 7.4% | 0.005 |
| VLDL3-C | (<10) | (10–12) | (13–69) | |
| 2 years | 12.8% | 9.2% | 6.2% | <0.0001 |
| VLDL-C | (<17) | (17–21) | (22–142) | |
| 2 years | 13.2% | 9.5% | 5.7% | <0.0001 |
Adjusted Outcomes
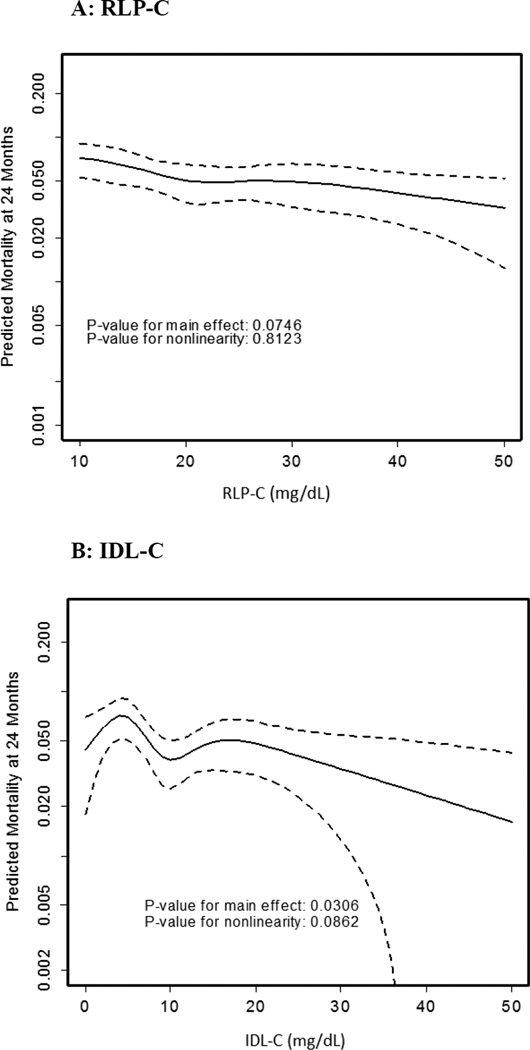
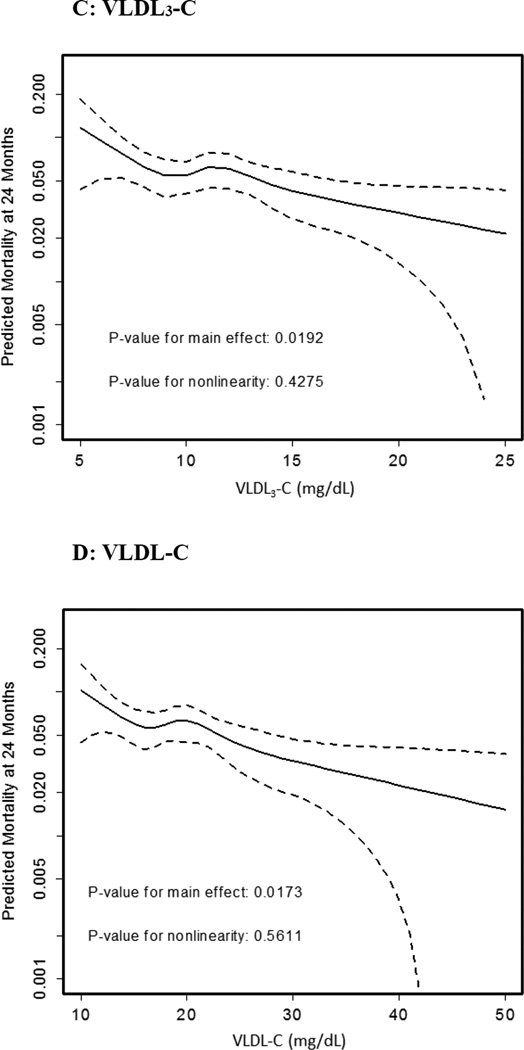
Using fully-adjusted models, Figure 1 shows restricted cubic spline curves examining the association of RLP-C, IDL-C, VLDL3-C, and VLDL-C with mortality. For RLP-C, we observed a strong trend towards a linear inverse association with mortality (p=0.07). The relationships were even stronger and reached formal statistical significance for each of the components of RLP-C, IDL-C and VLDL3-C, as well as for VLDL-C.
Figure 1. Adjusted Spline Curves of Remnant Lipoprotein Cholesterol Parameters in Association with Predicted Mortality.
Restricted cubic spline curves are presented. The x-axis represents observed values for remnant lipoprotein cholesterol parameters and the y-axis represents predicted mortality (log scale) at 2 years in TRIUMPH after adjusting for all covariates in the fully-adjusted model 3 (covariates listed in methods). Dotted lines indicate the 95% confidence interval. Panel A shows RLP-C, B) IDL-C, C) VLDL3-C, and D) VLDL-C. Results were consistent across model 1, 2, and 3; therefore, results from model 3 only are highlighted.
Figure 2 presents forest plots of point estimates and 95% confidence intervals for the relationship of RLP-C, IDL-C, VLDL3-C, and VLDL-C with mortality in fully-adjusted models. A one SD increase in RLP-C (11 mg/dl) predicted a 24% lower risk of 2-year mortality (HR, 0.77; 95% CI, 0.65 to 0.90). Similar results were found for a one SD increase in VLDL3-C (HR per 4 mg/dl, 0.74; 0.61–0.89), IDL-C (HR per 8 mg/dl, 0.80; 0.67–0.96), and VLDL-C (HR per 8 mg/dl, 0.69; 0.55–0.85).
Figure 2. Hazard Ratios for Mortality in Association with Remnant Lipoprotein Cholesterol Parameters.
Models were adjusted for all covariates in the fully-adjusted model 3.
DISCUSSION
While there is a previously described paradoxical relationship between LDL-C and mortality after AMI, this is the first study, of which we are aware, to document such an association between RLP-C and mortality. After stratifying patients by directly measured levels of RLP-C collected during hospitalization for AMI, we found that the mortality rate of the highest RLP-C tertile group (6.8%) was almost half that seen in the lowest tertile group (12.4%).
A hypercholesterolemia paradox has also been observed in prior studies on the basis of a clinical diagnosis of hypercholesterolemia or LDL-C levels. In an analysis of over 84,000 patients with NSTEMI from CRUSADE, a history of hypercholesterolemia was associated with lower in-hospital mortality after adjusting for baseline characteristics, including prior statin use. Among patients without a history of hypercholesterolemia, however, a new in-hospital diagnosis was not associated with lower mortality compared to those without prior hypercholesterolemia and LDL-C <100 mg/dL.11 An established diagnosis of hypercholesterolemia may correlate with increased access to medical care and improved utilization of evidence-based medications. Similarly, a history of hypercholesterolemia has been associated with lower 30-day mortality in patients with NSTEMI in the PURSUIT trial and lower in-hospital mortality in patients with ACS in the GRACE study.12,13 In AMI patients who underwent percutaneous coronary intervention in Korea, in-hospital mortality and 1-year mortality were significantly lower in patients with higher LDL-C levels.21 Most recently, in 115,492 patients hospitalized for AMI, Reddy et al reported adjusted odds ratios for in-hospital mortality of 0.79, 0.80, and 0.85 for the 2nd–4th LDL-C quartiles compared with the lowest quartile.15 Our work extends these prior observational studies by examining RLP-C, as opposed to just LDL-C, and by adjusting for a broader range of potential confounding patient characteristics.
As an epidemiologic study of a secondary prevention population, index event bias likely influenced these findings.16–19 Index event bias results from selection of a group of patients based on a disease episode – in this case AMI. Those suffering an AMI in the context of higher RLP-C presumably had a larger contribution of RLP-C to the event and generally carry a more favorable profile of other causal AMI factors than their counterparts with better controlled RLP-C. Also from selecting on an event, lead-time bias may be operative in relation to mortality. For example, if 2 otherwise identical patients were both to live exactly 80 years, and one had a risk factor that led to an AMI at age 60 and the other had an AMI at age 70 because they did not have that risk factor, it would paradoxically appear as if the patient with the risk factor lived 10 years longer than the second patient, even though they both lived exactly 80 years. In such an example, the risk factor might appear to be paradoxically associated with better post event survival.
In addition, we can speculate that those presenting with an AMI and higher RLP-C may have cardiovascular mortality risk that is more modifiable with lipid-lowering therapy than those who present with lower RLP-C. We do not believe that it is reasonable to attribute the results to inherent protective properties of remnants, as our study cannot establish causality, and because such properties are not biologically plausible or consistent with the totality of evidence. It is notable that lead-time bias, unlike other biases, is difficult to account for in an observational study design and cannot, therefore, be proven. Our data serve as a reminder to beware of conflating observational studies with randomized controlled trials, as doing so has great potential to mislead.
Compared with LDL-C, the atherogenic potential of remnant lipoproteins is less widely appreciated. Like LDL, triglyceride-rich remnant-like lipoprotein particles contain apolipoprotein B on their surface, the necessary mediator for intimal retention. Remnants are formed by modification of triglycerides contained in chylomicrons and VLDLs by lipoprotein lipase, hepatic lipase and cholesterol ester transfer protein, resulting in smaller cholesterol-rich particles. These particles have been documented to induce smooth muscle cell proliferation and foam cell formation, modulating monocyte-endothelial interactions, inhibiting vasodilatation, inducing endothelial dysfunction, and preventing the normal development of endothelial progenitor cells.7–9 A Mendelian randomization study in which VLDL-C was estimated from triglyceride levels suggests a causal role of lipoprotein remnants in CHD.10
Clinically, RLP-C levels tend to increase in patients with type II diabetes, renal disease, metabolic syndrome, and familial hypercholesterolemia, all of which were adjusted for in the current analysis.7 RLP-C is associated with cardiovascular disease independent of LDL-C and HDL-C.5 Elevated RLP-C levels have also been associated with a greater burden of atherosclerosis in patients with CHD and a higher risk of recurrent coronary events in patients with CHD and diabetes.6,22
Our findings should be interpreted in the context of several potential limitations. First, our observational study design precludes establishing causal relationships between measured variables and outcomes. Though we controlled for many clinical characteristics in our analyses, there are likely confounding factors that we did not account for and may have affected our results. For example, lower RLP-C levels may reflect malnutrition and we did not have access to albumin or protein levels or other measures of nutritional status to address that possibility. Additionally, the causal risk of RLP-C would likely be better reflected in a CHD or cardiovascular mortality endpoint, whereas our analyses were limited to all-cause mortality. Nevertheless, all-cause mortality is an objective and clinically-relevant endpoint and cardiovascular causes of death are most common in patients who have suffered an AMI. Lastly, it is possible that higher RLP-C levels may be associated with increased mortality at intervals >2 years following an AMI, when the long-term atherogenic effects RLP-C are more likely to result in cardiovascular events. Finally, while direct measurement of RLP-C in this research study allowed for a unique opportunity for scientific inquiry, our study was not intended to determine whether or not RLP-C should be routinely measured in clinical practice.
Conclusion
In conclusion, we observed a paradoxically lower risk of two-year mortality associated with higher RLP-C in contemporary AMI patients from the US. This observation survived rigorous adjustment, and therefore other unknown protective factors, or a lead-time bias, likely explain the paradoxically better outcomes.
Acknowledgement
None
Funding/support: This work was supported by the National Heart, Lung and Blood Institute [P50 HL 077113]. SSM and PHJ were supported by the Pollin Cardiovascular Prevention Fellowship and by NIH training grants (T32HL07024 and T32HL007227, respectively). SSM was also supported by the Marie-Josée and Henry R. Kravis endowed fellowship.
SRJ: Medical advisory board for Atherotech. KRK: Atherotech Research Director; receives royalty from the University of Alabama in Birmingham. PPT: consultancy for Amgen, AstraZeneca, Atherotech, Kowa, Liposcience, Lilly, Merck, and Novartis; speakers bureau for Amarin, AstraZeneca, Genzyme, Kowa, and Merck. JAS: Research grants from Amgen, Bristol-Myers Squibb/Sanofi-Aventis, Eli Lilly, Medtronic; consultancy for Amgen, Novartis, St. Jude Medical, United Healthcare.
Footnotes
Conflicts of interest: None for KF, AAK, MJB, PHJ, TMM, EPH, FT. SSM and SRJ: listed as co-inventors on a pending patent filed by Johns Hopkins University for a method of low-density lipoprotein cholesterol estimation.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global status report on noncommunicable diseases 2010. Geneva: WHO; 2011. [Google Scholar]
- 3.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 4.Jones SR, Martin SS, Brinton EA. Letter by Jones et al regarding article, "Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation". Circulation. 2014;129:e655. doi: 10.1161/CIRCULATIONAHA.113.005954. [DOI] [PubMed] [Google Scholar]
- 5.Twickler TB, Dallinga-Thie GM, Cohn JS, Chapman MJ. Elevated remnant-like particle cholesterol concentration: a characteristic feature of the atherogenic lipoprotein phenotype. Circulation. 2004;109:1918–1925. doi: 10.1161/01.CIR.0000125278.58527.F3. [DOI] [PubMed] [Google Scholar]
- 6.Lamon-Fava S, Herrington DM, Reboussin DM, et al. Plasma levels of HDL subpopulations and remnant lipoproteins predict the extent of angiographically-defined coronary artery disease in postmenopausal women. Arterioscler Thromb Vasc Biol. 2008;28:575–579. doi: 10.1161/ATVBAHA.107.157123. [DOI] [PubMed] [Google Scholar]
- 7.Twickler T, Dallinga-Thie GM, Chapman MJ, Cohn JS. Remnant lipoproteins and atherosclerosis. Curr Atheroscler Rep. 2005;7:140–147. doi: 10.1007/s11883-005-0037-x. [DOI] [PubMed] [Google Scholar]
- 8.Kawakami A, Yoshida M. Remnant lipoproteins and atherogenesis. J Atheroscler Thromb. 2005;12:73–76. doi: 10.5551/jat.12.73. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Wen T, Zheng XY, et al. Remnant-like particles accelerate endothelial progenitor cells senescence and induce cellular dysfunction via an oxidative mechanism. Atherosclerosis. 2009;202:405–414. doi: 10.1016/j.atherosclerosis.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Varbo A, Benn M, Tybjaerg-Hansen A, Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 11.Wang TY, Newby LK, Chen AY, et al. Hypercholesterolemia paradox in relation to mortality in acute coronary syndrome. Clin Cardiol. 2009;32:E22–E28. doi: 10.1002/clc.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boersma E, Pieper KS, Steyerberg EW, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation. 2000;101:2557–2567. doi: 10.1161/01.cir.101.22.2557. [DOI] [PubMed] [Google Scholar]
- 13.Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345–2353. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 14.Spencer FA, Allegrone J, Goldberg RJ, et al. Association of statin therapy with outcomes of acute coronary syndromes: the GRACE study. Ann Intern Med. 2004;140:857–866. doi: 10.7326/0003-4819-140-11-200406010-00006. [DOI] [PubMed] [Google Scholar]
- 15.Reddy VS, Bui QT, Jacobs JR, et al. Relationship Between Serum Low-Density Lipoprotein Cholesterol and In-hospital Mortality Following Acute Myocardial Infarction (The Lipid Paradox) Am J Cardiol. 2015;115:557–562. doi: 10.1016/j.amjcard.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Flanders WD, Eldridge RC, McClellan W. A nearly unavoidable mechanism for collider bias with index-event studies. Epidemiology. 2014;25:762–764. doi: 10.1097/EDE.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 17.Dahabreh IJ, Kent DM. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA. 2011;305:822–823. doi: 10.1001/jama.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smulders YM. Index event bias: why causal factors appear not to apply to disease recurrence. Ned Tijdschr Geneeskd. 2011;155:A3458. [PubMed] [Google Scholar]
- 19.Smits LJ, van Kuijk SM, Leffers P, Peeters LL, Prins MH, Sep SJ. Index event bias-a numerical example. J Clin Epidemiol. 2013;66:192–196. doi: 10.1016/j.jclinepi.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Arnold SV, Chan PS, Jones PG, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho KH, Jeong MH, Ahn Y, et al. Low-density lipoprotein cholesterol level in patients with acute myocardial infarction having percutaneous coronary intervention (the cholesterol paradox) Am J Cardiol. 2010;106:1061–1068. doi: 10.1016/j.amjcard.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Fukushima H, Sugiyama S, Honda O, et al. Prognostic value of remnant-like lipoprotein particle levels in patients with coronary artery disease and type II diabetes mellitus. J Am Coll Cardiol. 2004;43:2219–2224. doi: 10.1016/j.jacc.2003.09.074. [DOI] [PubMed] [Google Scholar]