Abstract
Tuberculosis (TB) is a global pandemic, partially due to the failure of vaccination approaches. Novel anti-TB vaccines are therefore urgently required. Here we show that aerosol immunization of macaques with the Mtb mutant in SigH (MtbΔsigH) results in significant recruitment of inducible bronchus-associated lymphoid tissue as well as CD4+ and CD8+ T cells expressing activation and proliferation markers to the lungs. Further, the findings indicate that pulmonary vaccination with MtbΔsigH elicited strong central memory CD4+ and CD8+ T cell responses in the lung. Vaccination with MtbΔsigH results in significant protection against a lethal TB challenge, as evidenced by a ~three log reduction in bacterial burdens, significantly diminished clinical manifestations and granulomatous pathology and characterized by the presence of profound iBALT. This highly protective response is virtually absent in unvaccinated and BCG-vaccinated animals after challenge. These results suggest that future TB vaccine candidates can be developed based on MtbΔsigH.
Despite widespread use of the BCG vaccine, Mtb infection and the resulting incidence of TB remains a major global concern. The BCG vaccine, to a large extent and with some exceptions, mitigates only the most severe aspects of infection and exhibits a highly variable efficacy, especially in high-burden areas.1 The majority of Mtb-infected individuals, including BCG-vaccinated ones, develop persistent but asymptomatic TB infection following Mtb exposure, rather than sterilizing immunity.2 These individuals retain a finite risk of reactivation, due to comorbidities such as HIV or diabetes. Developing new and efficacious TB vaccines is clearly the most effective intervention for containing the TB pandemic.3, 4, 5 To this end, a number of novel candidates are currently being evaluated either as potential replacements for BCG or to boost BCG-generated responses using a variety of approaches6. However, a frontline candidate that attempted to boost existing BCG responses failed to protect a target population against TB in a high-burden setting.7 These results provide further impetus to the objective of replacing BCG with a new live attenuated vaccine.
The failure to generate an effective TB vaccine is attributed to a lack of specific immune correlates of protection from Mtb infection and TB disease. Of interest are adaptive responses, particularly pathogen-specific memory CD4+ and CD8+ T cell mediated responses essential for successful bacterial control during LTBI. BCG is generally considered to be inefficient in generating central memory CD4+ and CD8+ T cell responses,8 although long-term immunity following BCG administration is possible in some settings.9 This contributes to the widely perceived inability of the vaccine to confer long-term protection against TB.10 Thus, understanding related immune parameters and how perturbation by comorbidities leads to TB may be useful for effective TB vaccine design.
Aerosol-delivery of BCG to the lung enhances protective efficacy,11 including in macaques.12 Further, aerosol TB vaccination might allow co-delivery of vaccine and adjuvants.13 Aerosol-BCG resulted in significantly improved protection in guinea pigs against Mtb challenge compared to conventional vaccination.11 As well, an adenoviral vector expressing Mtb antigens elicited robust responses following aerosol delivery.14
Attenuated mycobacteria have evoked interest as potential BCG-replacement vaccines15. The Mtb antigen-repertoire counter-intuitively evokes strong immunity, suggesting that such responses work in the pathogen’s favor.16 Immune-evasive pathways of Mtb are therefore key to understanding mechanisms of Mtb persistence.17 SigH orchestrates a key stress-response pathway in Mtb that mitigates oxidative stress through induction of anti-oxidant production18. The Mtb mutant in sigE, which is part of the SigH-regulon, elicited protection from Mtb infection.19 Further, the M. avium paratuberculosis sigH mutant is being evaluated as a candidate vaccine for protecting cattle [US20140271719A1 (Pending US Patent, Adel Talaat)]. As well, aerosol immunization of macaques with the MtbΔsigH mutant failed to cause disease.20 This was in direct contrast to the phenotype of this mutant in C57Bl/6 mice, where the sigH-null strain replicated to levels comparable to those of parental Mtb.18 While the MtbΔsigH mutant in H37Rv did not have a phenotype in monocytes,21 the mutant in the CDC1551 strain exhibited growth restriction in BMDMs.22 These results suggest that MtbΔsigH I) fails to neutralize host-generated oxidants in vivo and II) is controlled in an elite fashion by the primate innate immune system. Macaques accurately model several aspects of the human TB syndrome including partial protection from BCG-vaccination,23, 24 different outcomes25 and the full-spectrum of pathology,26 27 and have the unique capacity to model Mtb/HIV co-infection.28, 29 A World Health Organization (WHO) working group strongly recommended validation of candidate live TB vaccines in this model.30
In the current study, aerosol vaccination with MtbΔsigH elicited strong CD4+ and CD8+ central memory T cell responses as well as a robust Th1 response correlating with significantly greater protection from lethal Mtb challenge in rhesus macaques. Furthermore, protection from lethal TB in MtbΔsigH-vaccinated animals was characterized by the presence of highly organized iBALT associated with granulomas, following Mtb challenge.
Results
MtbΔsigH infection and increased iBALT
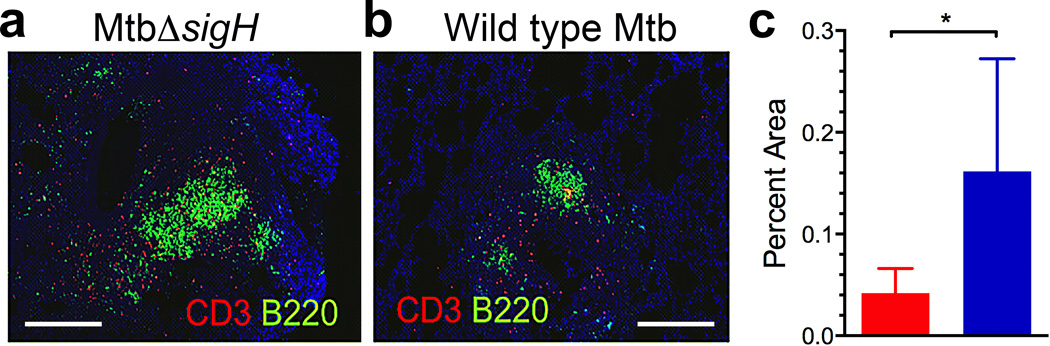
We have previously established a correlation between the levels of ectopic lymphoid structures known as iBALT and the control of Mtb infection in a latent state. Experimentally infected macaques had significantly greater levels of iBALT in their lungs during LTBI, and this response was markedly reduced during TB disease.31 iBALT was characterized by the presence of CD20+ B and CXCR5+ T cells. The expression of CXCR5 on T cells within iBALT follicles governed correct orientation and localization of T cells and macrophage activation.31 Since aerosol infection of macaque lungs with MtbΔsigH resulted in the virtually complete clearance of infection, we used samples from this previous study20 to assess if the lungs of MtbΔsigH-infected macaques harbored increased iBALT. Lung sections from six macaques each exposed to high-doses of Mtb CDC1551 strain or the isogenic MtbΔsigH mutant were co-stained for expression of CD3 and CD45R (B220).31 Immunofluorescence revealed that lung lesions from animals infected with the mutant contained significantly greater iBALT signal (Fig 1a), relative to those infected with Mtb (Fig 1b). The percentage of area occupied by iBALT was significantly greater in samples from MtbΔsigH-infected than Mtb-infected animals (P<0.05) (Fig 1c). In addition, the total lung area involved in iBALT follicles (µm2) (P<0.01) and the average size of these iBALT follicles (µm2) (P<0.0001, student’s t-test) was also significantly greater in sections derived from MtbΔsigH-infected, relative to Mtb-infected animals (Supplementary Fig 1a–b). These results further support our previous observations that protection from Mtb-infection directly correlates with the presence of granuloma-associated iBALT and suggested that the lungs of MtbΔsigH-infected macaques could exemplified an environment conducive to protection from TB.
Figure 1. iBALT formation induced by MtbΔsigHvaccination.
Co-staining with CD3 and B220 revealed that lung granulomas following infection with MtbΔsigH (a) exhibited a significantly increased iBALT response relative to infection with Mtb (b). The white bar represents 500 µm. Data from multiple lesions from six different animals were used in the analyses. (c) The percentage of area occupied by iBALT follicles relative to total lung area was analyzed in animals challenged with Mtb ( ) and MtbΔsigH (
) and MtbΔsigH ( ). *P<0.05 (student’s t-test). Data are means ± S.D. Samples from 4–5 animals in each group were used for analysis.
). *P<0.05 (student’s t-test). Data are means ± S.D. Samples from 4–5 animals in each group were used for analysis.
Analysis of aerosol-MtbΔsigH as an anti-TB vaccine candidate
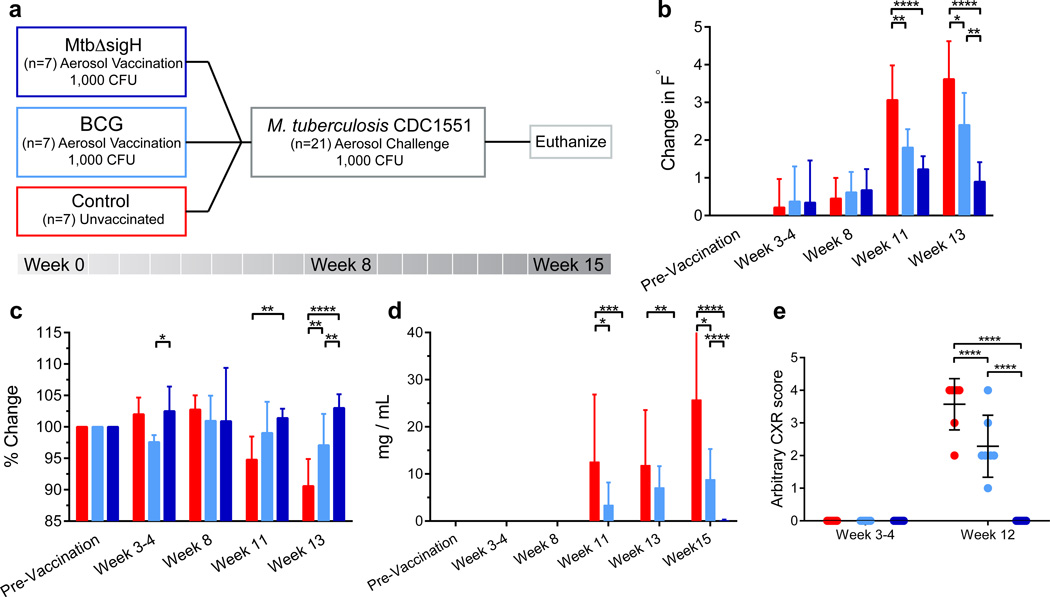
Encouraged by the magnitude of iBALT induction in macaques infected with MtbΔsigH, we conceived a vaccine study to assess both the immunogenicity as well as the efficacy of this mutant as a potential vaccine against pulmonary TB. The design of the macaque study is outlined in Fig 2a. Since enhanced iBALT responses were observed in the lungs of animals infected with the mutant strain via the aerosol route, we postulated that vaccination via the same route would have a greater chance of eliciting protection. Furthermore, BCG vaccination is more effective via the pulmonary route, indicating that local responses, elicited by matching the route of the vaccination to that of infection, may be critical to protect against TB.11, 12 Prior to and following single aerosol vaccination with MtbΔsigH at a dose that elicited strong iBALT response,20 both peripheral blood and lung compartments were repeatedly sampled to obtain cells for immune studies and transcriptomics. Eight weeks post-vaccination, animals were challenged via aerosol, with a highly lethal dose of Mtb. Unvaccinated and BCG (aerosol)-vaccinated groups were included as appropriate controls (Fig 2a). All animals that received aerosol BCG- or MtbΔsigH-vaccination became TST positive (Supplementary table 1).
Figure 2. Study outline and clinical correlates of vaccination and infection.
(a) Three groups of seven macaques were used: unvaccinated ( ); vaccinated with BCG (
); vaccinated with BCG ( ) and vaccinated with MtbΔsigH (
) and vaccinated with MtbΔsigH ( ). (b) Changes (Δ-°F) in body temperature; (c) changes in percentage of body weight; (d) changes in serum CRP (mg/mL) levels; and (e) changes in relative thoracic radiograph (CXR) scores, over the course of the vaccination and infection phases. CXRs were scored in a blinded fashion by categorizing between 0–4 based on increasing involvement in granulomatous pathology. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 using two-way ANOVA with Tukey’s correction for multiple comparisons. Data are means ± S.D. Samples from all seven animals in each group were used for analysis at each time point.
). (b) Changes (Δ-°F) in body temperature; (c) changes in percentage of body weight; (d) changes in serum CRP (mg/mL) levels; and (e) changes in relative thoracic radiograph (CXR) scores, over the course of the vaccination and infection phases. CXRs were scored in a blinded fashion by categorizing between 0–4 based on increasing involvement in granulomatous pathology. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 using two-way ANOVA with Tukey’s correction for multiple comparisons. Data are means ± S.D. Samples from all seven animals in each group were used for analysis at each time point.
Absence of disease upon vaccination with MtbΔsigH or BCG
Aerosolization of broth-cultured, log-phase BCG and MtbΔsigH was performed as described in the methods section.20, 25, 29, 32, 33 Microbial efficiency of BCG and MtbΔsigH was highly equivalent during aerosolization. Aerosol vaccination of macaques with MtbΔsigH and BCG deposited ~1000 CFU bacilli into deep lung. Aerosol-vaccination with either strain resulted in a positive TST (Supplementary table 1) but did not induce dyspnea, anorexia, or significant changes in body temperatures relative to pre-infection values (Fig 2b). The body weights of all vaccinated animals also remained relatively normal during the post-vaccination/pre-challenge phase with the exception of a slight decline (<3%) in the body weights of BCG-vaccinated animals 3 to 4 weeks post-vaccination (Fig 2c). None of the vaccinated animals exhibited an increase in serum CRP levels relative to unvaccinated animals at any time post-vaccination (Fig 2d). Further, thoracic radiographs acquired post-vaccination on all fourteen vaccinated animals were normal (Fig 2e).
Differential persistence of MtbΔsigH and BCG in BAL
The persistence of live mycobacteria was evaluated in BAL from both MtbΔsigH- and BCG-vaccinated macaques. Three weeks after vaccination, greater levels of MtbΔsigH were recovered from BAL compared to BCG (Fig 3a). At week 5 post-vaccination, BCG could not be recovered from BAL, while detectable levels of MtbΔsigH were still recovered (Fig 3a), indicating this strain might persist longer than BCG in human lungs. Eight weeks after vaccination, CFU for neither strain were recovered from the BAL of vaccinated macaques. Furthermore, macaque BMDM were able to kill BCG at a faster rate than both Mtb and MtbΔsigH in vitro (Fig. 3b). BMDM’s infected with MtbΔsigH expressed greater levels of TNF-α and IL-1β but not IL-6 transcripts relative to BCG (Fig 3c). Additionally BMDMs infected with MtbΔsigH also secreted significantly lower levels of inflammatory chemokines CXCL9 (Fig 3d) and CXCL10 (Fig 3e) in supernatants, relative to the BCG-vaccinated or the unvaccinated groups. Both the expression and anti-Mtb activity of TNF-α is strongly enhanced by IL-1β via direct augmentation of caspase-dependent apoptosis.34 Infection of BMDMs with MtbΔsigH results in both higher TNF-α expression and greater apoptosis relative to Mtb.22 The activity of IL-1β is itself regulated by Type I interferons, whose expression is positively controlled by IRF1 and negatively by IRF2. Elicitation of Type I interferon response inhibits IL-1β and TNF-α activity, and promotes the progression of active TB, as is the case during infection with hypervirulent Mtb strains35. Abrogation of Type I responses reverses this trend and is being considered as a host-directed therapy for TB36. Accordingly, the expression of Type I genes was significantly higher in BAL samples obtained three weeks after vaccination from animals that received BCG, and significantly lower in animals that received MtbΔsigH (Fig 3f). The IRF2 gene-expression level was lower in the BAL of BCG-vaccinated and >2-fold higher in the BAL of MtbΔsigH vaccinated animals (Fig 3f). The expression of prototypical Type I molecule IFN-γ was also higher in BMDMs infected with BCG and Mtb, relative to those infected with MtbΔsigH (Fig 3c).
Figure 3. Comparative measures of bacterial burden in BAL following aerosol vaccination and restriction during intra-phagosomal culturing in vitro.
(a) BCG ( ) and MtbΔsigH (
) and MtbΔsigH ( ) CFU levels in total BAL samples at week 3, 5, and 8 after vaccination. (b) Rhesus macaque bone marrow derived macrophages (BMDM) in vitro killing assay in CFU/mL with Mtb (
) CFU levels in total BAL samples at week 3, 5, and 8 after vaccination. (b) Rhesus macaque bone marrow derived macrophages (BMDM) in vitro killing assay in CFU/mL with Mtb ( ), BCG, and MtbΔsigH. **P < 0.01 using two-way ANOVA with Tukey’s correction for multiple comparisons. Data are means ± S.D. (c) Relative expression (2ΔΔCt) of TNF-α, IL-1β, IL-6 and IFN1A in BMDMs infected with Mtb, BCG and MtbΔsigH by real-time RT-PCR. (d, e) Absolute expression of CXCL9 (d) and CXCL10 (e) (pg/mL) in supernatants derived from BMDMs infected with Mtb, BCG and MtbΔsigH by cytokine analysis assay. (f) Microarray-derived fold changes of gene expression of 12 Type I interferon genes in the BAL of BCG- and MtbΔsigH-vaccinated animals. For analysis involving BAL, samples from all seven animals in each group were used for analysis at each time point (a). For CFU analysis in vitro, the experiment was performed twice, with four biological replicates in each instance (b). Transcript and cytokine analysis were performed on biological replicates (c–f).
), BCG, and MtbΔsigH. **P < 0.01 using two-way ANOVA with Tukey’s correction for multiple comparisons. Data are means ± S.D. (c) Relative expression (2ΔΔCt) of TNF-α, IL-1β, IL-6 and IFN1A in BMDMs infected with Mtb, BCG and MtbΔsigH by real-time RT-PCR. (d, e) Absolute expression of CXCL9 (d) and CXCL10 (e) (pg/mL) in supernatants derived from BMDMs infected with Mtb, BCG and MtbΔsigH by cytokine analysis assay. (f) Microarray-derived fold changes of gene expression of 12 Type I interferon genes in the BAL of BCG- and MtbΔsigH-vaccinated animals. For analysis involving BAL, samples from all seven animals in each group were used for analysis at each time point (a). For CFU analysis in vitro, the experiment was performed twice, with four biological replicates in each instance (b). Transcript and cytokine analysis were performed on biological replicates (c–f).
MtbΔsigH induces protective immune signatures in the lung
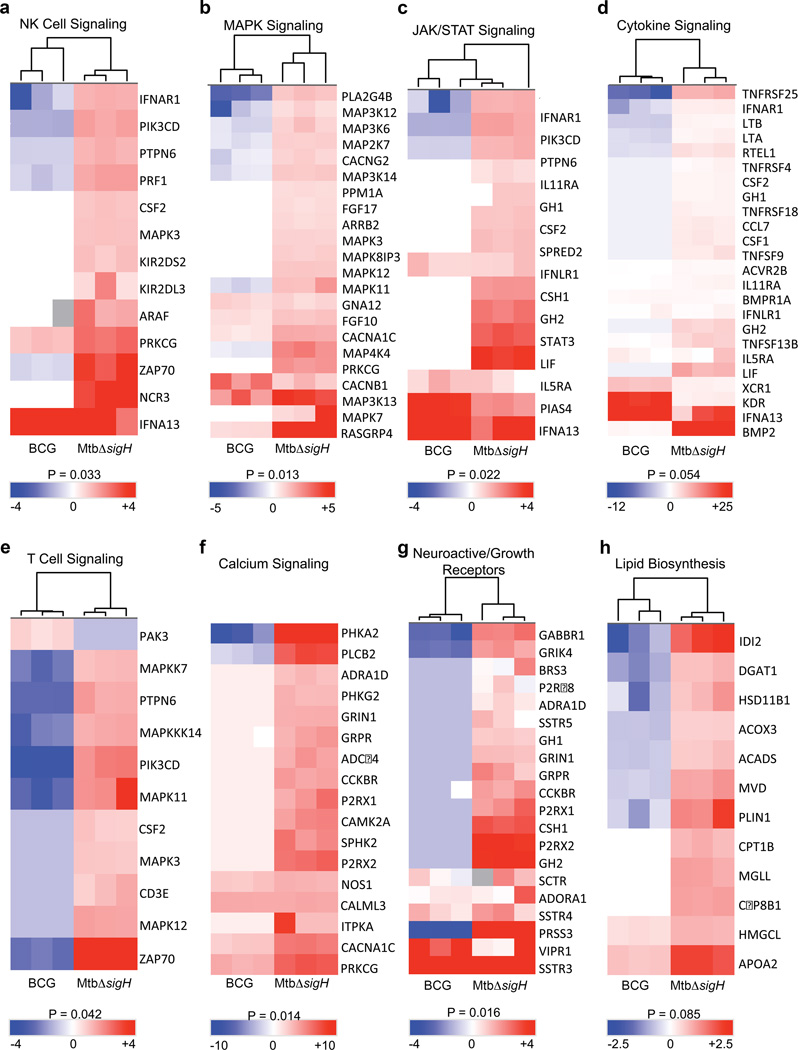
Global immune responses to vaccination with MtbΔsigH or BCG were analyzed by transcriptome profiling of BAL samples collected prior to vaccination and 3 weeks post-vaccination. The genes significantly induced in BAL after BCG vaccination were involved in cellular transport, DNA binding by regulatory protein, RNA processing, or were part of the lumen, non-membrane bound organelle, or macromolecular complex assembly (Fig. 4). The only major category of genes induced following BCG vaccination was that categorized as pertaining to immune system development (Fig. 4). Conversely, the expression of genes involved in NK cell signaling, MAPK signaling, and JAK/STAT signaling, cytokine signaling, T cell signaling, calcium signaling, neuroactive/growth receptors, and lipid biosynthesis were induced to higher levels in the BAL from MtbΔsigH-vaccinated macaques (Fig. 4). Further, a large majority of these genes were either not expressed, or were expressed to significantly lower levels in the BAL of animals vaccinated with BCG (Fig. 4). Thus, vaccination with MtbΔsigH appeared to result in the induction of a markedly stronger innate immune response, as indicated by the differential induction of NK cell, MAPK, and JAK/STAT signaling pathways as well as genes from the cytokine, T cell receptor, and calcium signaling pathways (Fig. 4), although the differential persistence of the two mycobacterial strains could have played a role in this differential response. In addition, enhanced immune cell differentiation, proliferation, activation, and processing, as well as macromolecular synthesis, which are associated with heightened cytokine and T cell responses, were evidenced by the increased expression levels of neuroactive/growth factor receptor signaling, potentiation, and lipid biosynthesis pathways in BAL samples derived from animals vaccinated with MtbΔsigH (Fig. 4).
Figure 4. Transcriptomics from BAL three weeks after immunization.
Total RNA isolated from BAL samples of three animals vaccinated with BCG and another three with MtbΔsigH, obtained three weeks after vaccination, was subjected to amplification and macaque-specific DNA microarray analysis. The expression of genes belonging to natural killer cells (a), MAP Kinase (b), JAK/STAT (c), cytokine (d), T cells (e), calcium signaling (f), neuroactive/growth receptors (g), and lipid biosynthesis (h) pathways were induced to significantly higher levels in the BAL of animals vaccinated with MtbΔsigH, relative to those vaccinated with BCG. P-values shown are derived from the analysis of significant terms in DAVID with Bonferroni correction for multiple comparisons. BAL samples from three animals in each group were used for microarray experiments.
Local increases in T cells due to MtbΔsigH-vaccination
Local and systemic immune responses were compared following vaccination by analysis of BAL and blood, respectively. Marked increases in CD4+ and CD8+ T cell numbers were found in BAL immediately post-vaccination (P<0.001; Two-way ANOVA with Tukey’s correction) (Fig. 5a–b), but no differences in the frequency of CD4+ and CD8+ T cells were observed in the periphery post-vaccination (Supplementary Fig. 2). In addition, no differences in the chemokine receptors CXCR3, CXCR4, CCR7, or activation marker CD69 were detected systemically, which indicated no significant variation in the functional phenotype of T cells in the blood (Supplementary Fig. 3). However, a significant increase in the number of circulating CD4+CCR5+ T cells was discovered in the MtbΔsigH group between weeks 2 and 3 (P<0.05; Two-way ANOVA with Tukey’s correction) (Supplementary Fig. 3). No differences were observed in the memory status of circulating T cells, including central (TCM-CD28+CD95+) and effector memory (TEM-CD28−CD95+) cells, or naïve T cells (CD28+CD95−) (Supplementary Fig. 2).37
Figure 5. T cell response to immunization in BAL.
Vaccination with MtbΔsigH ( ) induced a significantly higher central memory immune response relative to BCG vaccination (
) induced a significantly higher central memory immune response relative to BCG vaccination ( ), or no vaccination (
), or no vaccination ( ). Quantification of CD4+ (a) and CD8+ (b) T cells migrating to the lung after vaccination. Representative plots of central memory (CD28+CD95+), effector memory (CD28−CD95+), and naïve (CD28+CD95−) CD4+ (c, e) and CD8+ (d, f) T cells in BAL. Quantification of CD4+ (e) central memory (TCM) and effector memory (TEM) cells, CD8+ TCM, and TEM cells (f) in BAL, and the proliferative capability of these cells (g, h). **P < 0.01; ***P < 0.001; ****P < 0.0001 using two-way ANOVA with Tukey’s correction for multiple comparisons. Data are means ± S.D. BAL samples from all 21 animals (n=7, three groups) were included in the flow cytometry experiments and analyses. Samples from all seven animals in each group were used for analysis at each time point.
). Quantification of CD4+ (a) and CD8+ (b) T cells migrating to the lung after vaccination. Representative plots of central memory (CD28+CD95+), effector memory (CD28−CD95+), and naïve (CD28+CD95−) CD4+ (c, e) and CD8+ (d, f) T cells in BAL. Quantification of CD4+ (e) central memory (TCM) and effector memory (TEM) cells, CD8+ TCM, and TEM cells (f) in BAL, and the proliferative capability of these cells (g, h). **P < 0.01; ***P < 0.001; ****P < 0.0001 using two-way ANOVA with Tukey’s correction for multiple comparisons. Data are means ± S.D. BAL samples from all 21 animals (n=7, three groups) were included in the flow cytometry experiments and analyses. Samples from all seven animals in each group were used for analysis at each time point.
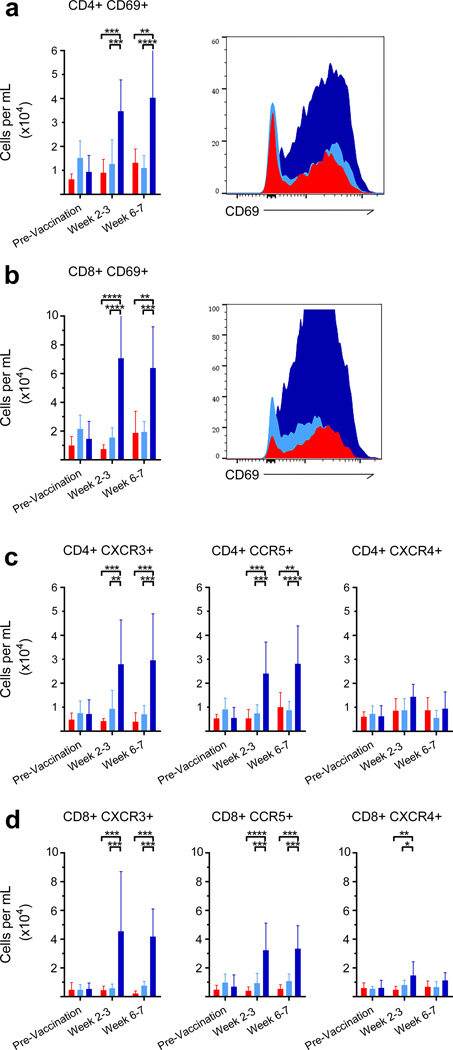
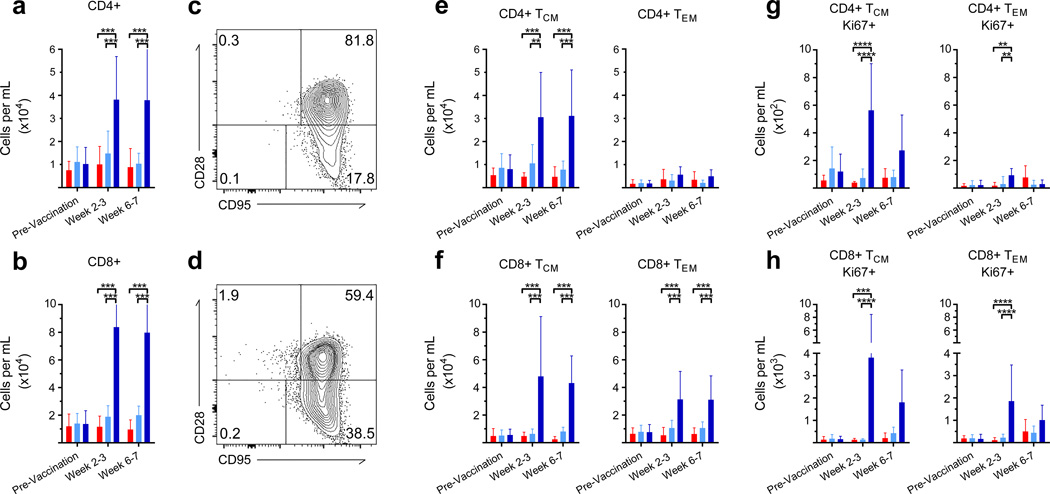
While, no significant changes were discovered in T cell frequencies in the peripheral blood, a strong lung-specific central and effector memory response was initiated post-vaccination with MtbΔsigH. This response was apparent immediately after vaccination by significant increases in the number of CD4+ TCM (P<0.01–0.001; Two-way ANOVA with Tukey’s correction) as well as CD8+ TCM and TEM (P<0.001) in the BAL (Fig. 5c–f), with a marked increase in proliferation as measured by Ki67 positivity (P< 0.01–0.0001) (Fig. 5g–h). A significant increase in the number of CD69+ T cells (P<0.001 for MtbΔsigH vs. BCG and P<0.01–0.001 for MtbΔsigH vs. unvaccinated groups) (Fig. 6a–b) indicated that T cells in BAL were antigen-stimulated via the T cell receptor. The polarity of the T cell response was examined using the markers CCR5 and CXCR3, which are preferentially expressed by T helper 1 (Th1) cells, as well as CXCR4, which is preferentially expressed by T-helper 2 (Th2) cells. The results revealed that T cells in BAL immediately post-MtbΔsigH vaccination preferentially expressed high levels of CCR5 and CXCR3 compared to T cells recruited following vaccination with BCG (P<0.01–0.001) (Fig. 6c–d). Thus, aerosol vaccination with MtbΔsigH induced transcriptomic and cellular signatures indicative of a strong Th1 response that resulted in accumulation of memory T cells.
Figure 6. Local T cell phenotype to immunization in BAL.
Vaccination with MtbΔsigH ( ) induced a significantly stronger TH1 cell response relative to BCG vaccination (
) induced a significantly stronger TH1 cell response relative to BCG vaccination ( ), or no vaccination (
), or no vaccination ( ). Quantification of and representative histograms of CD4+CD69+ (a) and CD8+CD69+ (b) T cells migrating to the lung after vaccination. Absolute cell counts of phenotypic markers CXCR3, CCR5, and CXCR4 in CD4+ (c) and CD8+ (d) T cells in BAL at different stages of infection. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 using two-way ANOVA with Tukey’s correction for multiple comparisons. Data are means ± S.D. BAL samples from all 21 animals (n=7, three groups) were included in the flow cytometry experiments and analyses. Samples from all seven animals in each group were used for analysis at each time point.
). Quantification of and representative histograms of CD4+CD69+ (a) and CD8+CD69+ (b) T cells migrating to the lung after vaccination. Absolute cell counts of phenotypic markers CXCR3, CCR5, and CXCR4 in CD4+ (c) and CD8+ (d) T cells in BAL at different stages of infection. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 using two-way ANOVA with Tukey’s correction for multiple comparisons. Data are means ± S.D. BAL samples from all 21 animals (n=7, three groups) were included in the flow cytometry experiments and analyses. Samples from all seven animals in each group were used for analysis at each time point.
Vaccination with MtbΔsigH protects from lethal Mtb challenge
To evaluate the potential of the attenuated mycobacterium to serve as a TB vaccine, all animals were challenged with a high dose of Mtb CDC1551, which has historically produced lethal TB in rhesus macaques within 10 weeks.20, 32 Unvaccinated animals rapidly developed pulmonary granulomatous pathology with rapid increases in body temperatures (P<0.01 for MtbΔsigH relative to BCG and P<0.0001 for MtbΔsigH vs. unvaccinated groups; Two-way ANOVA with Tukey’s correction) and serum CRP levels (P<0.0001 for MtbΔsigH vs. both other groups) (Fig. 2b, d), as well as a swift declines in body weights P<0.01 for MtbΔsigH relative to BCG and P<0.0001 for MtbΔsigH vs. unvaccinated groups) (Fig. 2c). Control animals also exhibited high levels of pulmonary granulomatous involvement by radiology (Fig. 2e). The same clinical measure also increased, albeit to a lesser degree, in animals that were aerosol vaccinated with BCG, while animals vaccinated with MtbΔsigH, exhibited virtually no evidence of disease (P<0.0001 for MtbΔsigH vs. unvaccinated as well as vs. BCG) (Fig. 2b–e). Three weeks post-Mtb challenge (week 11), the increase in body temperatures in unvaccinated animals was significant relative to BCG-vaccinated (P<0.01) and highly significant relative to MtbΔsigH-vaccinated animals (P<0.0001) (Two-way ANOVA with Tukey’s correction) (Fig. 2b). Five weeks post-challenge (week 13), the differences in body temperature between the unvaccinated and BCG-vaccinated groups were significant (P<0.05), the differences between the BCG and MtbΔsigH-vaccinated groups were very significant (P<0.01), and the differences between the unvaccinated and MtbΔsigH-vaccinated groups were highly significant (P<0.0001) (Fig. 2b). The differences in body weight between the unvaccinated and MtbΔsigH-vaccinated groups and the BCG and MtbΔsigH-vaccinated groups were very significant at week 11 (P<0.01) (Fig. 2c) and while by week 13, these differences had become highly significant (P<0.0001) (Two-way ANOVA with Tukey’s correction). Profoundly lower serum CRP levels were detected in the macaques vaccinated with MtbΔsigH relative to macaques that were not vaccinated at weeks 11 and 13 and at euthanasia (P<0.0001). At both week 11 (P<0.05) and euthanasia (P<0.0001) (Two-way ANOVA with Tukey’s correction), the serum CRP levels detected in the macaques vaccinated with MtbΔsigH were also significantly lower than levels measured in the BCG-vaccinated group (Fig. 2d). Similarly, animals vaccinated with MtbΔsigH exhibited significantly lower CXR scores, consistent with lack of disease following lethal challenge, in this group, relative to either BCG-vaccinated, or unvaccinated macaques (P<0.0001 in both cases). While extensive pathology was not discernable in the lungs of macaques vaccinated with the mutant, a majority of those vaccinated with BCG exhibited moderate CXR scores, again significantly lower than those in the unvaccinated group, where pathology consistent with military TB could be observed in a majority of animals.
Vaccination with MtbΔsigH reduces in vivo bacterial burdens
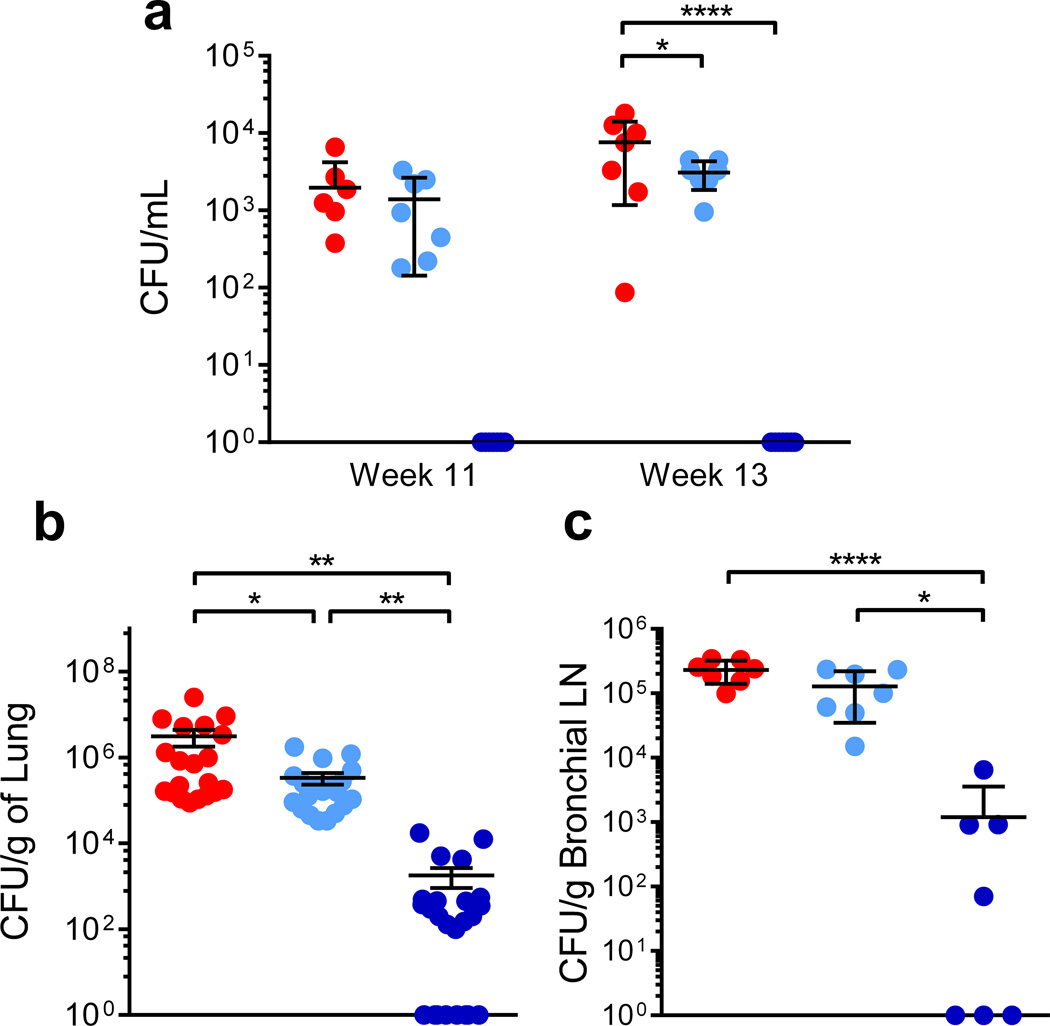
The protection conferred by MtbΔsigH was also evident following evaluation of bacterial burdens. Significantly lower Mtb was recovered from BAL from MtbΔsigH-vaccinated animals relative to those vaccinated with BCG at 3 and 5 weeks after challenge (weeks 11 and 13; Fig. 7a). At euthanasia, the total lung burden in animals vaccinated with MtbΔsigH was three-logs lower than burdens in unvaccinated (P<0.01) and two-logs lower than burdens in BCG-vaccinated (P<0.01) animals, while bacterial loads in animals vaccinated with BCG were only 0.5–1 logs lower than in the control group (P<0.05) (One-way ANOVA with Tukey’s correction) (Fig. 7b). We were unable to culture Mtb from >42% of all lung sections obtained from macaques vaccinated with MtbΔsigH. In contrast, every section obtained from either unvaccinated animals or those vaccinated with BCG- was positive for Mtb. Thus, the Mtb burden was significantly lower in the lungs of MtbΔsigH-vaccinated animals following lethal challenge compared to those vaccinated with BCG. Similar results were obtained when bronchial lymph node bacterial burdens were analyzed at necropsy (Fig. 7c). Using a combination of hygromicin resistance/sensitivity and PCR, we verified that the obtained CFU were Mtb and not residual MtbΔsigH or BCG.
Figure 7. Bacterial burden following lethal Mtb challenge.
(a) Mtb CFU levels in total BAL samples at week 11 (3 weeks after challenge) and week 13 (5 weeks after challenge) (b). Mtb levels per gram of lung tissue at necropsy (c). Mtb burdens per gram of bronchial lymph node tissue at *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, (a) two-way ANOVA and (b, c) one-way ANOVA with Tukey’s multiple testing correction. Data are means ± S.D. Samples from all seven animals in each group were used for analysis at each time point. For analysis of lung burdens, four pooled samples (two each from left and right lung) from each macaque, each representing five distinct lung sections were used (b). For analysis of bronchial lymph node burdens, one section from each animal was analyzed.
Vaccination with MtbΔsigH leads to reduced lung pathology
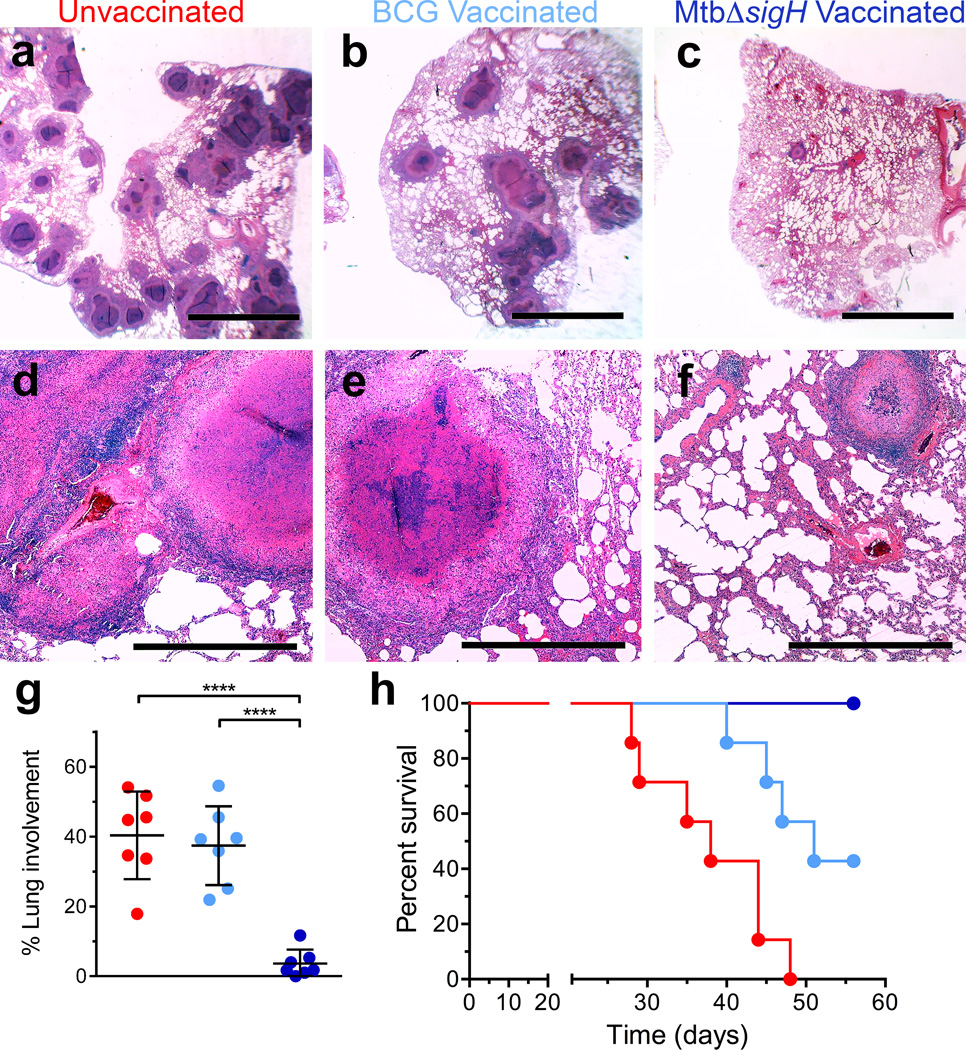
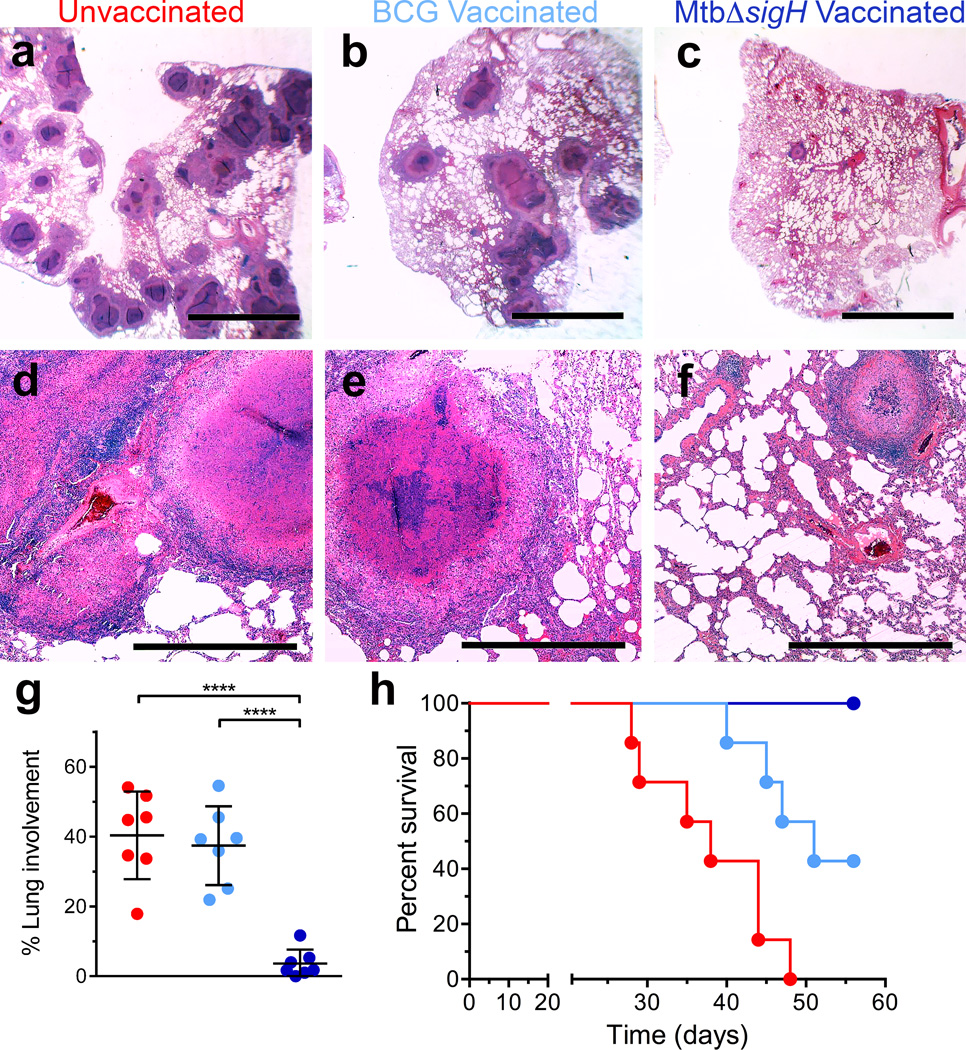
The results of the pulmonary pathology analyses mirrored those obtained following analysis of bacterial burdens. Animals vaccinated with MtbΔsigH exhibited significantly fewer pulmonary lesions upon challenge, as determined by both gross and histopathological examination (Fig. 8a–f, Supplementary Fig 4) and morphometric quantitation (Fig. 8g). Thus, the animals vaccinated with MtbΔsigH had fewer granulomas (the extent of lung affected by TB lesions following Mtb infection encompassed an average of 4%), and less TB-related pathology (e.g. edema, pneumonia and generalized foci of inflammation) than animals in the other two groups (where ~40% of the lung was affected) (P<0.0001 in both cases; One-way ANOVA with Tukey’s correction) (Fig 8g). The clinical and microbiological differences also correlated with significant differences in overall survival rates following challenge (Fig. 8h). All seven unvaccinated animals succumbed to massive pulmonary TB following a lethal aerosol Mtb challenge within a median of 36 days. Five out of seven BCG-vaccinated animals also succumbed to TB, within a median of 48 days. None of the MtbΔsigH-vaccinated animals exhibited any overt signs of disease or required euthanasia (Fig. 8h).
Figure 8. Histopathological and survival analysis of lungs of Mtb infected animals.
Hematoxylin and eosin (H&E) staining from representative animals in each of the three groups. (a, d) Vaccine-naïve group with miliary, white 2–4 mm granulomas and scattered lobular multicolored areas of consolidation, (b, e) BCG-vaccinated with localized dark red lobar pneumonia, and (c, f) MtbΔsigH-vaccinated with no apparent gross lesions. The black bars in (a–c) represent 5mm and (d–f) represent 500µm. (g) Morphometric measures of pulmonary pathology in the different groups of unvaccinated ( ), BCG-vaccinated (
), BCG-vaccinated ( ) and MtbΔsigH-vaccinated (
) and MtbΔsigH-vaccinated ( ) animals. ****P < 0.0001 using (g) One-way ANOVA using Tukey’s multiple testing correction. Data are means ± S.D. (h) Survival proportion Kaplan-Meier curves for the three groups of animals, using Mantel-Cox (logrank) survival analysis. At least three systematic random microscopic fields from each lung, representing most lung lobes, from each of the animals in every group were used for morphometric analysis (g). Data from each of the seven animals per group was used for survival proportions analysis (h).
) animals. ****P < 0.0001 using (g) One-way ANOVA using Tukey’s multiple testing correction. Data are means ± S.D. (h) Survival proportion Kaplan-Meier curves for the three groups of animals, using Mantel-Cox (logrank) survival analysis. At least three systematic random microscopic fields from each lung, representing most lung lobes, from each of the animals in every group were used for morphometric analysis (g). Data from each of the seven animals per group was used for survival proportions analysis (h).
Differential T cell responses in animals post-challenge
To assess an immunologic basis for protection induced by MtbΔsigH, the functional phenotype of circulating CD4+ and CD8+ T cells was analyzed by employing the same markers used for evaluating pulmonary responses following vaccination. No significant differences in the percentages of CD4+ and CD8+ central or effector memory cells were observed in peripheral blood immediately following challenge (Supplementary Fig. 2). However, a significant increase in CD4+ CCR5+ T cells was identified 7 weeks after Mtb challenge, as well as a significant decrease in CD8+ CXCR3+ T cells in peripheral blood at weeks 3 and 7 following challenge in unvaccinated animals (Supplementary Fig. 3).
Consistent with overall lung involvement and chest radiograph scores, a significantly greater number of CD4+ and CD8+ T cells including TCM and TEM were present in BAL in the unvaccinated animals compared to both BCG and MtbΔsigH vaccinated animals (Fig. 9a–d). The increased numbers of T cells in BAL in unvaccinated animals was also evident from representative histograms of CD69+ T cells (Supplementary Fig. 5a–b). While no significant differences were observed in CXCR3, CCR5, and CXCR4 expression by CD4+ or CD8+ T cells between the two vaccinated groups (Supplementary Fig. 5c–d), a significant decrease in the number of CD8+CXCR4+ was noted at week 11 and 15 in the MtbΔsigH vaccinated group compared to the other two groups (Supplementary Fig. 5d).
Figure 9. Immune responses in the lung post Mtb challenge.
(a, b) Quantification of cells per mL of BAL of (a) CD4+ and (b) CD8+ T cells and of (c) CD4+ TCM and TEM cells and of (d) CD8+ TCM and TEM cells migrating to the lung after challenge in groups of unvaccinated ( ), BCG-vaccinated (
), BCG-vaccinated ( ) and MtbΔsigH-vaccinated (
) and MtbΔsigH-vaccinated ( ) animals. (e, f) Percentage of CD4 T cells from PBMCs prepared from whole blood, producing gamma interferon, interleukin 2, and tumor necrosis factor alpha in response to (e) Mtb cell wall and to (f) Mtb cell filtrate protein. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 using two-way ANOVA with Tukey’s correction for multiple comparisons. Data are means ± S.D. BAL samples from all 21 animals (n=7, three groups) were included in the flow cytometry experiments and analyses (a–d). For antigen-specific studies samples from four animals per group were employed (e–d).
) animals. (e, f) Percentage of CD4 T cells from PBMCs prepared from whole blood, producing gamma interferon, interleukin 2, and tumor necrosis factor alpha in response to (e) Mtb cell wall and to (f) Mtb cell filtrate protein. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 using two-way ANOVA with Tukey’s correction for multiple comparisons. Data are means ± S.D. BAL samples from all 21 animals (n=7, three groups) were included in the flow cytometry experiments and analyses (a–d). For antigen-specific studies samples from four animals per group were employed (e–d).
Comparison of polyfunctional Mtb-specific T cell responses
To examine antigen specific responses to Mtb, isolated PBMCs collected 5 weeks post-challenge were stimulated with whole Mtb cell wall and cell filtrate protein, and responses were analyzed using intracellular cytokine staining for IFN-γ, IL-2, and TNF-α. The results indicated that vaccination with MtbΔsigH and BCG correlated with significant increases in the percentage of polyfunctional, IFN-γ+, IL-2+, TNF-α+ CD4+ T cells whereas unvaccinated animals displayed a significantly greater number percentage of monofunctional CD4+ T cells (Fig. 9e–f). Unfortunately, samples from other time points were not available for analysis.
Profound iBALT post-challenge in MtbΔsigH-vaccinated animals
Lung samples collected after lethal Mtb challenge from unvaccinated (Fig. 10a, d), BCG-vaccinated (Fig. 10b, e) and MtbΔsigH vaccinated (Fig. 10c, f) macaques at the time of necropsy were assayed for iBALT by histopathology and immunofluorescence with CD3 and CD20 followed by confocal microscopy and image analysis. We observed that protection in each of these groups of macaques was strongly associated with levels of iBALT. Thus, the few, small granulomas in the lungs of animals vaccinated with MtbΔsigH and challenged with Mtb were characterized by the presence of multiple well organized, iBALT per lesion (Fig. 10c). The presence of the follicles was also apparent in the cognate H&E stains (Fig. 10 c, f). These follicles were, in general associated with granulomas and appeared to be an outgrowth of the outer lymphocyte-rich layer (Fig. 10 c, f). While B cells constituted the majority in these follicles, CD3+ cells were also present (Fig. 10c). In contrast to the multiple well-developed iBALT associated with granulomas in MtbΔsigH immunized animal, animals from the other groups (Fig 10b, e), (Fig 10a, d), had fewer iBALT that were less well organized. The total area of iBALT in MtbΔsigH-vaccinated macaques was significantly greater after Mtb challenge (Fig. 10g) than in either of the other groups, despite the fact that the area occupied by granulomas in the MtbΔsigH-vaccinated group was significantly less compared to the other two groups, after Mtb challenge (Fig. 10h). This further emphasizes the difference in BALT induction and the correlation between iBALT and latent control of Mtb infection.
Figure 10. Induction of BALT after lethal Mtb challenge correlates with protection from pulmonary granulomatous TB.
(a, b, c) Co-staining of lung sections with CD3 (green) and CD20 (red) and staining of corresponding sections (d, e, f) with Hematoxylin and eosin (H&E) revealed that (a, d) vaccine-naïve animals and (b, e) BCG-vaccinated animals had significantly reduced iBALT follicle formation in comparison to (c, f) MtbΔsigH-vaccinated animals, at necropsy, after lethal Mtb challenge. The white and black bars represent 500 µm. Quantification of multiple lesions in six different animals were used the analysis. (g) Total area (mm2) of iBALT follicles and (h) granulomatous pathology in unvaccinated ( ), BCG-vaccinated (
), BCG-vaccinated ( ) and MtbΔsigH-vaccinated (
) and MtbΔsigH-vaccinated ( ) animals. ****P < 0.0001 using students t-test. Data are means ± S.D. At least ten sections from each slide derived from a lung block from multiple animals in each group were used for statistical analysis (g–h).
) animals. ****P < 0.0001 using students t-test. Data are means ± S.D. At least ten sections from each slide derived from a lung block from multiple animals in each group were used for statistical analysis (g–h).
Discussion
Previously, we showed that aerosolization of a high-dose of MtbΔsigH into the lungs of rhesus macaques resulted in nonpathogenic infection.20 Here, we demonstrate that pulmonary vaccination with this mutant protected against lethal challenge in macaques via the induction of a potent memory T cell response. Further, we establish a strong correlation between the latent control of infection and iBALT, suggesting that these lymphoid structures facilitate control of TB by maintaining LTBI or sterilizing infection. Induction of BALT in response to respiratory infections is known to not only result in protection, but such responses are also less pathologic38. Secondary TFH cells, which constitute the great majority of lymphoid follicle (including iBALT) T cells, expand from memory T cells in a B cell-aided manner.39 Our results therefore suggest that the interplay between B and T cells is critical for the proper development of granulomatous protective responses to TB. This study substantiates previous work using the macaque model of TB, which showed that B cells produced antibody and were important for control of TB.40 Our results suggest that future studies should unravel the function of B cells recruited to the lung in order to understand immunity from TB.
While the correlates of vaccine-induced protective immunity against TB are not completely understood, T lymphocytes expressing markers of central memory response (dual positivity for CD28 and CD95),37 are critical for protection. A model of protective immunity that is currently gaining credence suggests that initial vaccination(s) resulting in a spike in effector response can result in the establishment of a central memory response, which is likely to result in long-term protection.41 TCM are extremely proliferative and can rapidly evolve into large numbers of effector cells expressing high levels of pro-inflammatory cytokines (e.g. IFN–γ). Consequently, the finding that pulmonary vaccination with MtbΔsigH resulted in potent TCM (as well as TEM, particularly CD8+) responses during the post-vaccination phase that corresponded to a strong TEM response in the challenge phase merits further evaluation. The role of chemokine receptors, including CXCR3, is critical to the recruitment of CD8+ T cells, and CXCR3 expression on TCM is strongly correlated with effective anti-pathogen memory responses.42 BAL transcriptome profiling results indicated that animals vaccinated with MtbΔsigH developed a highly productive pulmonary immune response, both in terms of magnitude and breadth. Prior studies have established that CD4+ T cells with specificity for mycobacterial antigens are critical for the control of Mtb infection,43 and that CD8+ T cells play an increasingly important role in this process.41, 44 SigH induces pathogen antioxidant responses during stress.45, 46 MtbΔsigH is unable to induce expression of the transcriptionally linked thioredoxins and thiol peroxidases45 that act as antioxidant buffers against the host oxidative burst.47 Consequently, the antioxidant response of the MtbΔsigH mutant was strongly crippled,47 and the resulting production of oxidants likely enhanced antigen-specific CD8+ T cell responses through the cross-priming of antigen presentation.48 Additionally, the ability of MtbΔsigH to persist longer than BCG allowed for increased stimulation and antigen presentation, inducing a stronger, more robust, T cell repertoire.49 Further characterization of this TCM response is however necessary in order to substantiate that these cells are not long-lived TEM cells, since MtbΔsigH antigens persist longer than BCG.
The ability of aerosol MtbΔsigH-vaccination to elicit highly protective responses in macaques could have roots in the fundamental processes that govern Mtb-macrophage interactions. In vitro, macrophages infected with this mutant elicited greater expression of Th1 pro-inflammatory cytokines TNF-α and IL-1β, and lower Type I interferon, relative to those infected with Mtb and BCG (this study and22). Together, these results indicate that the interaction of MtbΔsigH with host phagocytes is critically altered by the loss of the bacillus’s ability to induce SigH-mediated antioxidant functions, relative to BCG. TNF can restrict macrophage-contained Mtb by several mechanisms, including by potently inducing early inflammatory responses,50 transducing apoptotic signals through activation of caspase 8,51 activating CD8 cells,52 and potentiating IFN-γ induced killing of Mtb.53, 54 On the other hand, BCG induces strong expression of the SigH regulon (including antioxidants) and is therefore unable to alter these interactions. In fact most BCG strains exhibit duplication of sigH, likely rendering it even less susceptible to phagocyte oxidative mechanisms.55 In vitro results thus hint at the mechanism by which pulmonary vaccination with MtbΔsigH could generate protective responses. Unable to counter the host oxidative burst, the mutant elicited greater IL-1β and TNF-α expression leading to significantly greater antimicrobial response, apoptosis and antigen-presentation. That Mtb uses Type I-signaling to modulate IL-1β expression is well established.56
CD4+ T cells play a key role in the control of Mtb infection, and polyfunctional cytokine production of antigen-specific Th1 cells is associated with this control.57 Animals vaccinated with MtbΔsigH displayed higher frequencies of Mtb cell wall (CW) and culture filtrate protein (CFP) responsive polyfunctional CD4+ T cells compared to unvaccinated animals. Recent data has shown that markers of T cell immune activation in human TB are associated with smear and culture conversion during anti-TB treatment.58 Nonetheless, the current study had some limitations, e.g., protection was only demonstrated in a homologous strain. Thus, future experiments should test the efficacy of the current and related mutants against high-virulence heterologous strains of the same clade as CDC1551, as well as of a different lineage. Secondly, a lethal challenge at 8 weeks post-vaccination may not sufficiently model protection arising from long-term antigen-specific memory T cell responses. A prior report suggested that BCG failed to induce long-term central memory responses to Mtb infection, which may account for its failure to protect adults against TB.10 Successful vaccination against TB will require elicitation of both a stable and broad repertoire of memory responses to ensure effective protection against the pathogen during a subsequent infection.41 While the current results did not establish long-term immunity based on effective elicitation of TCM responses, the findings did demonstrate that prior pulmonary vaccination with MtbΔsigH resulted in a markedly superior elicitation of both CD4+ and CD8+ TCM responses relative to BCG vaccination. An abundance of data has indicated that TCM lymphocytes retain a potent proliferative capacity and can differentiate into the TEM phenotype when re-exposed to the cognate antigen.59 The current data demonstrated that MtbΔsigH elicited a more efficient post-vaccination TCM response, a greater TEM response following challenge and conferred stronger protection. In this study, vaccines were delivered via aerosol, although BCG is given to recipients intradermally. It is impossible to directly compare the results from the aerosol-BCG group to the effectiveness of intradermal BCG vaccination. There have been recent attempts however, to deliver vaccines, directly to the lung,11 driven by the pioneering work of Barclay et al, who showed that macaques could be protected against TB by aerosolized BCG.12 While a direct comparison of the effectiveness of aerosol-to intradermal-administered BCG was beyond the scope of this study, preliminary data and prior reports of intradermal BCG vaccination14, 24, 60 suggest that aerosol BCG outperformed intradermal vaccination in macaques.
Although BCG induces potent cellular responses in infants and protects them, neither the responses nor the efficacy are generally considered to be long-lasting,61 although some reports strongly suggest that this is possible.9 Furthermore, BCG does not completely protect against pulmonary TB including reactivation and reinfection.61 New vaccines against TB are therefore urgently needed. It has therefore been hypothesized that rationally attenuated strains of Mtb are likely better at serving as vaccine platforms against TB, since these strains are human adapted, in contrast to bovine-adapted BCG.61 There are other issues, which limit the effectiveness of BCG. It is only able to induce cytokines associated with long-lasting control of Mtb infection several months post-vaccination, meanwhile enabling a window of dissemination.62 Furthermore, Mtb, unlike BCG, retains known immunodominant epitopes for humans. Finally, it has been argued that Mtb expresses genes important for the evasion of host immune responses. SigH is one such gene, allowing the pathogen to significantly induce the expression of thioredoxins, which attenuate phagocyte oxidative burst.20, 22, 47 Thus, MtbΔsigH, a human-adapted strain with a significant immune-evasion impairment is exceedingly safe even after direct high-dose delivery into primate lungs.
In summary, our results provide a roadmap for identifying correlates of protection from TB in a highly human-like model. While it remains to be seen if such responses can also be elicited following intradermal vaccination, there is considerable renewal of interest in matching the route of vaccination with that of infection in generating protection against TB.13
Online methods
Nonhuman primates
All animal procedures were approved by the Institutional Animal Care and Use Committee and were performed in strict accordance with NIH guidelines. Twenty-one specific-pathogen free, retrovirus-free, mycobacteria-naive, adult rhesus macaques, bred and housed at the TNPRC, between the ages of 3–12 years were assigned to three groups of seven based on power calculations which suggested that statistical significance could be detected with sufficient power in these group sizes if addressing a reduction in the average lung bacterial burden by 1 log. One group of macaques (n=7) remained unvaccinated, a second group (n=7) was vaccinated with a target dose of 1,000 CFU of M. bovis BCG (Danish) while the third group (n=7) was vaccinated with an equivalent dose of the MtbΔsigH isogenic mutant in the CDC1551 background. Aerosol vaccination was conducted using the same equipment and procedural configuration as the Mtb challenge component of the study (see below). The average ages of the animals within each group were 7.42+/− 4.26 years for the unvaccinated group, 7.38+/− 4.50 years for the BCG-vaccinated group and 6.75+/− 4.02 years for the MtbΔsigH group, and these were not statistically significant differences (Supplementary Fig. 6).
Aerosol procedures for both vaccination and Mtb-challenge
Animals were both aerosol-vaccinated and challenged with Mtb using the same methodology and equipment configuration. A custom head-only dynamic inhalation system housed within a class III biological safety cabinet was used for this purpose.63 The use of this inhalation system for aerosol delivery to NHP63 has been described for numerous studies involving Mtb,20, 25, 29, 32 as well as other agents.64 Initially, the respective microbial efficiencies of MtbΔsigH gene mutant and the parental Mtb CDC1551 strains were determined through a series of aerosol-only studies. The microbial efficiencies were used to estimate achievable aerosol doses that could be delivered to each animal using mathematical formula catered to this purpose65 for both the aerosol vaccination and subsequent challenge experiments. The animal vaccinations (and challenge exposures) were performed singly, and a ‘target’ dose is reported for the group based on prevailing experimental conditions, including individual animal respiratory rate, during each exposure event. Actual individual dose is based upon post hoc analysis of active aerosol sampling of the inhalation chamber during the time of each vaccination and bacterial challenge. All aerosol infections were performed in a single day.
Clinical procedures including sampling and euthanasia
Vaccines were delivered directly to the deep lung via the aerosol route in a manner similar to how the Mtb challenge is administered. Eight weeks post-vaccination, each of the three groups was infected via the inhalation route with a target dose of 1,000 CFU of Mtb CDC1551. Samples were collected prior to vaccination, post-vaccination, and post-infection. For the sake of clarity, results from vaccinated and infected NHPs are reported in two phases: post-vaccination, which means after aerosol vaccination with either BCG or MtbΔsigH, put prior to challenge with Mtb and ii) post-infection (or post-challenge) i.e. after challenge with Mtb.
A tuberculin skin test was performed prior to vaccination (−2 weeks), post-vaccination (3 weeks), and post-infection (11 weeks) as previously described, by administration of mammalian tuberculin into the right eyelid.20, 24, 25, 29, 32, 33 Thoracic radiographs (CXRs) were acquired 2 weeks prior to vaccination and at 3, 7, 11, and 14 weeks post-vaccination as previously described.20, 24, 25, 29, 32, 66 Briefly, the CXRs were scored by veterinary clinicians in a blinded-fashion on a subjective scale of 0–4, with a score of 0 denoting normal lung and a score of 4 denoting severe tuberculous pneumonia and pathology. Prior to vaccination/infection, all 21 animals received a score of 0, as their lungs were perfectly normal at this time. The following subjective scoring system was used: 0 (no pathological involvement); 1 (mild pathological involvement); 2 (moderate pathological involvement); 3 (extensive pathological involvement) and 4 (severe pathological involvement).
Blood was drawn prior to vaccination (−2 weeks) and then weekly thereafter for the performance of complete blood count (CBC) and serum chemistry.20, 24, 25, 29, 32 Blood collected in EDTA tubes (Sarstedt AG & co.) was used for whole blood flow cytometry using panels described previously67. Blood collected in Cell Preparation tubes (CPT) (Sarstedt AG & co.) was used to isolate peripheral blood mononuclear cell (PBMC) for antigen-specific assays. BAL samples were obtained as previously described, using two washes of 40 mL sterile saline, 2 weeks prior to vaccination and at 3, 7, 11, and 14 weeks20, 24, 25, 29, 32 and analyzed for colony forming units (CFU).
Necropsy to collect tissues was performed at euthanasia. There were two different reasons for euthanasia. In all 100% (7/7) unvaccinated control animals, as well as ~57% (4/7) of BCG-vaccinated animals, disease progressed to an extent that humane euthanasia was deemed necessary by clinical veterinarians on this team (Fig. 8h; Supplementary Table 1). These humane endpoints were pre-defined in the animal use protocol and applied as a measure of reduction of discomfort. The TNPRC Institutional Animal Care and Use Committee approved all animal-related procedures and activities. 43% (3/7) BCG-vaccinated and 100% of MtbΔsigH-vaccinated animals, were either disease-free or had not progressed to an extent that required human euthanasia till day 60. Such animals were euthanized for tissue collection via necropsy at that point (between days 60–62). At necropsy, lung, spleen, and liver tissue were collected and processed as previously described, and CFU were determined per gram of tissue, using four pooled lung samples per animal, each of which were comprised of five sections each, thus representing every lung lobe with at least one sample.20, 24, 25, 29, 32 A subset of colonies obtained after post-challenge necropsy were assayed for resistance to hygromicin, to classify them as residual MtbΔsigH, and subjected to PCR for sigH and esat6, to classify them as residual MtbΔsigH and BCG respectively. No evidence of residual MtbΔsigH or BCG was found. Endpoint criteria for euthanasia were previously described. Briefly, animals were euthanized if they exhibited four or more of the following: I) a 2°F increase in body temperature relative to pre-infection values that persisted for three or more consecutive weeks; II) a 15% or greater loss in body weight; III) serum C-reactive protein (CRP) values greater than 10 mg/mL for two or more consecutive readings; IV) Chest X-Rays (CXR) values higher than 2; V) respiratory discomfort resulting in vocalization; VI) significant-to-complete loss of appetite; and VII) detectable bacilli in bronchoalveolar lavage (BAL) samples. CRP values were included as criteria for euthanasia because CRP is a marker for systemic inflammation that exhibits a high degree of correlation with active TB in macaques.20, 24, 25, 29, 32, 33 Lung pathology at necropsy was determined as described earlier, 21, 26, 29, 30, 38 using stereological principles described by Sharpe and colleagues.27 Briefly, lung involvement was quantified by point counting using an overlaid grid with 18.5-mm point spacing. Towards this end, digital images of three systematic random microscopic fields with an original magnification of 2.5× per slide were employed. At least one sample from each of the 4 lobes of each lung was used. Intersections representing normal lung included interstitium and air space, while lesions comprised of intersections with massive areas of inflammatory cells, hemorrhage, edema, necrosis or individual to multifocal to lobar granulomas. Differences between completely normal and somewhat inflamed airspace containing localized, small zones of subacute inflammation sans fibrous or cellular encapsulation were not differentiated.
Transcriptomics
BAL samples were obtained from animals prior to vaccination (−2 weeks), post-vaccination (weeks 3 and 7) and post-infection (weeks 11 and 14) as previously described.20, 24, 25, 29, 32, 33 68 For stabilization, 8 mL of 100% fetal bovine serum (Invitrogen, Life Sciences) was immediately added to 80 mL of the BAL sample. The sample was centrifuged at 400 rpm for 10 min at 4°C in an Allegra benchtop centrifuge. The pelleted cells were washed with cold RPMI media (Invitrogen, Life Sciences) and stored at −80°C until analysis. For transcriptomics, total macaque RNA was isolated from total BAL obtained at the pre-vaccination and the 3 week post-vaccination time points as previously described30 using an RNAEasy kit (Qiagen), followed by RNA amplification (Ambion MessageAmp). The cDNA derived from the amplified RNA samples from pre-vaccination time points were labeled with Cy3, and samples from the 3-week post-vaccination time point were labeled with Cy5 (Agilent Technologies). Microarray analyses were performed as previously described by assessing the relative expression of transcripts in the Cy5 (experimental)-labeled samples relative to the Cy3(control)-labeled samples, using Agilent 4×44k Rhesus Monkey microarrays.20, 24, 25, 68 Global impact of expression profiles was analyzed using Database for Annotation, Visualization and Integrated Discovery (DAVID). 30
Inducible bronchus-associated lymphoid tissue (iBALT)
Immunofluorescence confocal microscopy was used to measure iBALT as previously described, using formalin-fixed, paraffin-embedded tissue. 31, 69
Flow cytometry
Flow cytometry was performed on whole blood and BAL samples obtained from all 21 animals as previously described.25, 33 For T cell phenotyping the following antibodies were used: CD3 V500 (1:50, clone SP34-2), CD4 PerCP-Cy5.5 (1:10, clone L200), CD8 PE-TxRed (1:30, clone RPA-T8), CD28 APC (1:5, clone CD28.2), CD69 APC-Cy7 (1:20, clone FN50), CD95 PE-Cy5 (1:5, clone DX2), CD183 AL488 (1:10, clone 1C6/CXCR3), CD184 PE-Cy5 (1:5, clone 12G5), CD195 APC (1:5, clone 3A9), CD197 PE-Cy7 (1:20, clone 3D12), HLA-DR APC-Cy7 (1:75, clone L243), and Ki67 PE-Cy7 (1:50, clone B56) all purchased from BD Biosciences (San Jose, CA, USA). Flow cytometry analyses were conducted by gating first on lymphocytes followed by the elimination of B cells by gating for CD20. The remaining cells were gated for the selection of T cells using CD3, followed by gating into CD3+CD4+ and CD3+CD8+ subpopulations. The frequencies of CD4+ and CD8+ T cells expressing activation and homing markers were compared using Ki67, CXCR3, CCR5, and CCR7.70 The levels of Foxp3+ were determined as a measure of the Treg response. Finally, the extent of CD4+ and CD8+ cells belonging to either central memory (TCM) or effector memory (TEM) relative to the naïve T cell population were measured using a combination of CD28 and CD95 markers.37
Antigen-specific immune response
PBMCs isolated from whole blood collected from unvaccinated, vaccinated, and infected NHPs were stimulated with Mtb cell-wall extract (BEI Resources) for the performance of intracellular cytokine staining as previously described, using Mtb cell filtrate protein and and cell-wall extract for stimulation.57
In vitro infection of rhesus macaque BMDMs
Rh-BMDMs were generated and infected with Mtb CDC1551, the isogenic MtbΔsigH mutant in this strain, and BCG at an MOI of 1:10 as previously described, for four hours.22, 46 CFUs were measured as described at different time points including 4, 24, 48, 72, 96, and 120 hrs. CXCL13 expression was measured using DNA microarray25 and quantitative RT-PCR.22 The expression of pro-inflammatory mediators TNF-α, IL-1β and IL-6 was measured by real-time RT-PCR as described earlier.22 The expression of specific chemokines e.g. CXCL9 and CXCL10 was measured using Cytokine Monkey Magnetic 29-Plex Panel kit from Life-Tech, essentially as described earlier.22
Statistical analyses
Statistical comparisons were performed using one-way or two-way analysis of variance (ANOVA) in GraphPad Prism with Sidak’s correction for multiple hypotheses. Analysis of transcriptome data was performed using Spotfire DecisionSite24, 25, 68 LOWESS scripts, Ingenuity Pathways Analysis (IPA) and Database for Annotation, Visualization and Integrated Discovery (DAVID) as previously described.25 Specifically, genes with two-fold or greater induction in triplicate samples from either vaccination were uploaded to DAVID and statistically significant accumulation of terms calculated.
Supplementary Material
Acknowledgements
This research was supported by startup funds and a Center for Biomedical Research Excellence (COBRE) award to SM (P30GM110760), awards to DK (AI089323, HL106790, HL106790-S1, AI111943, RR026006), SAK & DK (AI111914) and the TNPRC (OD011104, AI058609), including its pilot program, the Emory Center for AIDS Research (CFAR; AI050409), the Louisiana Board of Regents and the Tulane Office of the Vice-President for Research.
Abbreviations
- BCG
bacille Calmette-Guérin
- TB
tuberculosis
- Mtb
Mycobacterium tuberculosis
- iBALT
inducible bronchus associated lymphoid tissue
- TST
tuberculin skin test
- BAL
bronchoalveolar lavage
- BMDMC
bone marrow derived macrophages
- CRP
C-Reactive Protein
- CXR
Chest X Ray
- NK
Natural Killer
- MAPK
Mitogen-activated protein kinase
- (JAK/STAT)
Janus Kinase/ Signal Transducer and Activator of Transcription
- PBMC
Peripheral blood mononuclear cell
Footnotes
Author contributions. D.K. provided funding, advised on the experimental design, carried out the experiments, analyzed the results, and participated in the manuscript preparation; T.W.F. carried out the experiments, analyzed the results, and participated in the manuscript preparation; U.S.G. carried out the experiments and analyzed results; X.A. carried out the experiments and analyzed results; T.A. carried out the experiments and analyzed results; J.R-M. carried out the experiments; N.A.G. carried out the experiments; A-M.F.J. carried out the experiments; B.L.P. carried out the experiments; M.H.A. carried out the experiments; K.R-L provided veterinary assistance; L.D. provided veterinary assistance; C.J.R. carried out experiments; P.J.D performed veterinary pathology; J.L.B provided veterinary assistance; J.R. analyzed the results; A.A.L performed veterinary pathology and assisted in data interpretation and the manuscript preparation; S.A.K. provided funding and assisted in data interpretation and manuscript preparation; S.M. provided funding, advised on the experimental design, analyzed the results, participated in the manuscript preparation. All authors reviewed the manuscript before submission.
Accession codes
DNA microarray data have been deposited in the Gene Expression Omnibus under the Accession number GPL10183.
Competing financial interests
There are no competing financial interests for any of the authors.
REFERENCES
- 1.Organization WH. Global tuberculosis report. 2014. [Google Scholar]
- 2.Kaufmann SH, Lange C, Rao M, Balaji KN, Lotze M, Schito M, et al. Progress in tuberculosis vaccine development and host-directed therapies--a state of the art review. The lancet Respiratory medicine. 2014;2(4):301–320. doi: 10.1016/S2213-2600(14)70033-5. [DOI] [PubMed] [Google Scholar]
- 3.Orme IM. Prospects for new vaccines against tuberculosis. Trends in microbiology. 1995;3(10):401–404. doi: 10.1016/s0966-842x(00)88987-8. [DOI] [PubMed] [Google Scholar]
- 4.Hingley-Wilson SM, Sambandamurthy VK, Jacobs WR., Jr Survival perspectives from the world’s most successful pathogen, Mycobacterium tuberculosis. Nat Immunol. 2003;4(10):949–955. doi: 10.1038/ni981. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann SH, Lange C, Rao M, Balaji KN, Lotze M, Schito M, et al. Progress in tuberculosis vaccine development and host-directed therapies-a state of the art review. The lancet Respiratory medicine. 2014;2(4):301–320. doi: 10.1016/S2213-2600(14)70033-5. [DOI] [PubMed] [Google Scholar]
- 6.Wilkie ME, McShane H. TB vaccine development: where are we and why is it so difficult? Thorax. 2015;70(3):299–301. doi: 10.1136/thoraxjnl-2014-205202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381(9871):1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McShane H, Jacobs WR, Fine PE, Reed SG, McMurray DN, Behr M, et al. BCG: myths, realities, and the need for alternative vaccine strategies. Tuberculosis (Edinb) 2012;92(3):283–288. doi: 10.1016/j.tube.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aronson NE, Santosham M, Comstock GW, Howard RS, Moulton LH, Rhoades ER, et al. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: A 60-year follow-up study. JAMA. 2004;291(17):2086–2091. doi: 10.1001/jama.291.17.2086. [DOI] [PubMed] [Google Scholar]
- 10.Henao-Tamayo M, Ordway DJ, Orme IM. Memory T cell subsets in tuberculosis: what should we be targeting? Tuberculosis (Edinb) 2014;94(5):455–461. doi: 10.1016/j.tube.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Contreras L, Wong YL, Muttil P, Padilla D, Sadoff J, Derousse J, et al. Immunization by a bacterial aerosol. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(12):4656–4660. doi: 10.1073/pnas.0800043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barclay WR, Busey WM, Dalgard DW, Good RC, Janicki BW, Kasik JE, et al. Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guerin. Am Rev Respir Dis. 1973;107(3):351–358. doi: 10.1164/arrd.1973.107.3.351. [DOI] [PubMed] [Google Scholar]
- 13.Manjaly Thomas ZR, McShane H. Aerosol immunisation for TB: matching route of vaccination to route of infection. Trans R Soc Trop Med Hyg. 2015;109(3):175–181. doi: 10.1093/trstmh/tru206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darrah PA, Bolton DL, Lackner AA, Kaushal D, Aye PP, Mehra S, et al. Aerosol vaccination with AERAS-402 elicits robust cellular immune responses in the lungs of rhesus macaques but fails to protect against high-dose Mycobacterium tuberculosis challenge. J Immunol. 2014;193(4):1799–1811. doi: 10.4049/jimmunol.1400676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen MH, Biermann K, Chen B, Hsu T, Sambandamurthy VK, Lackner AA, et al. Efficacy and safety of live attenuated persistent and rapidly cleared Mycobacterium tuberculosis vaccine candidates in non-human primates. Vaccine. 2009;27(34):4709–4717. doi: 10.1016/j.vaccine.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell DG. The evolutionary pressures that have molded Mycobacterium tuberculosis into an infectious adjuvant. Current opinion in microbiology. 2013;16(1):78–84. doi: 10.1016/j.mib.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hmama Z, Pena-Diaz S, Joseph S, Av-Gay Y. Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis. Immunol Rev. 2015;264(1):220–232. doi: 10.1111/imr.12268. [DOI] [PubMed] [Google Scholar]
- 18.Kaushal D, Schroeder BG, Tyagi S, Yoshimatsu T, Scott C, Ko C, et al. Reduced immunopathology and mortality despite tissue persistence in a Mycobacterium tuberculosis mutant lacking alternative sigma factor, SigH. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(12):8330–8335. doi: 10.1073/pnas.102055799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez Pando R, Aguilar LD, Smith I, Manganelli R. Immunogenicity and protection induced by a Mycobacterium tuberculosis sigE mutant in a BALB/c mouse model of progressive pulmonary tuberculosis. Infection and immunity. 2010;78(7):3168–3176. doi: 10.1128/IAI.00023-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehra S, Golden NA, Stuckey K, Didier PJ, Doyle LA, Russell-Lodrigue KE, et al. The Mycobacterium tuberculosis stress response factor SigH is required for bacterial burden as well as immunopathology in primate lungs. The Journal of infectious diseases. 2012;205(8):1203–1213. doi: 10.1093/infdis/jis102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manganelli R, Voskuil MI, Schoolnik GK, Dubnau E, Gomez M, Smith I. Role of the extracytoplasmic-function sigma factor sigma(H) in Mycobacterium tuberculosis global gene expression. Molecular microbiology. 2002;45(2):365–374. doi: 10.1046/j.1365-2958.2002.03005.x. [DOI] [PubMed] [Google Scholar]
- 22.Dutta NK, Mehra S, Martinez AN, Alvarez X, Renner NA, Morici LA, et al. The stress-response factor SigH modulates the interaction between Mycobacterium tuberculosis and host phagocytes. PloS one. 2012;7(1):e28958. doi: 10.1371/journal.pone.0028958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barclay WR, Anacker RL, Brehmer W, Leif W, Ribi E. Aerosol-Induced Tuberculosis in Subhuman Primates and the Course of the Disease After Intravenous BCG Vaccination. Infection and immunity. 1970;2(5):574–582. doi: 10.1128/iai.2.5.574-582.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehra S, Alvarez X, Didier PJ, Doyle LA, Blanchard JL, Lackner AA, et al. Granuloma correlates of protection against tuberculosis and mechanisms of immune modulation by Mycobacterium tuberculosis. The Journal of infectious diseases. 2013;207(7):1115–1127. doi: 10.1093/infdis/jis778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehra S, Foreman TW, Didier PJ, Ahsan MH, Hudock TA, Kissee R, et al. The DosR Regulon Modulates Adaptive Immunity and is Essential for M. tuberculosis Persistence. American journal of respiratory and critical care medicine. 2015 doi: 10.1164/rccm.201408-1502OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaushal D, Mehra S, Didier PJ, Lackner AA. The non-human primate model of tuberculosis. Journal of medical primatology. 2012;41(3):191–201. doi: 10.1111/j.1600-0684.2012.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharpe SA, Eschelbach E, Basaraba RJ, Gleeson F, Hall GA, McIntyre A, et al. Determination of lesion volume by MRI and stereology in a macaque model of tuberculosis. Tuberculosis (Edinb) 2009;89(6):405–416. doi: 10.1016/j.tube.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Diedrich CR, Mattila JT, Klein E, Janssen C, Phuah J, Sturgeon TJ, et al. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PloS one. 2010;5(3):e9611. doi: 10.1371/journal.pone.0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehra S, Golden NA, Dutta NK, Midkiff CC, Alvarez X, Doyle LA, et al. Reactivation of latent tuberculosis in rhesus macaques by coinfection with simian immunodeficiency virus. Journal of medical primatology. 2011;40(4):233–243. doi: 10.1111/j.1600-0684.2011.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamath AT, Fruth U, Brennan MJ, Dobbelaer R, Hubrechts P, Ho MM, et al. New live mycobacterial vaccines: the Geneva consensus on essential steps towards clinical development. Vaccine. 2005;23(29):3753–3761. doi: 10.1016/j.vaccine.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, et al. CXCR5(+) T helper cells mediate protective immunity against tuberculosis. The Journal of clinical investigation. 2013;123(2):712–726. doi: 10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dutta NK, Mehra S, Didier PJ, Roy CJ, Doyle LA, Alvarez X, et al. Genetic requirements for the survival of tubercle bacilli in primates. The Journal of infectious diseases. 2010;201(11):1743–1752. doi: 10.1086/652497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips BL, Mehra S, Ahsan MH, Selman M, Khader SA, Kaushal D. LAG3 expression in active Mycobacterium tuberculosis infections. The American journal of pathology. 2015;185(3):820–833. doi: 10.1016/j.ajpath.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jayaraman P, Sada-Ovalle I, Nishimura T, Anderson AC, Kuchroo VK, Remold HG, et al. IL-1beta promotes antimicrobial immunity in macrophages by regulating TNFR signaling and caspase-3 activation. J Immunol. 2013;190(8):4196–4204. doi: 10.4049/jimmunol.1202688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manca C, Tsenova L, Freeman S, Barczak AK, Tovey M, Murray PJ, Hypervirulent M, et al. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res. 2005;25(11):694–701. doi: 10.1089/jir.2005.25.694. [DOI] [PubMed] [Google Scholar]
- 36.Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzales J, et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511(7507):99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168(1):29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 38.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nature medicine. 2004;10(9):927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 39.Fairfax KC, Everts B, Amiel E, Smith AM, Schramm G, Haas H, et al. IL-4-secreting secondary T follicular helper (Tfh) cells arise from memory T cells, not persisting Tfh cells, through a B cell-dependent mechanism. J Immunol. 2015;194(7):2999–3010. doi: 10.4049/jimmunol.1401225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phuah JY, Mattila JT, Lin PL, Flynn JL. Activated B cells in the granulomas of nonhuman primates infected with Mycobacterium tuberculosis. The American journal of pathology. 2012;181(2):508–514. doi: 10.1016/j.ajpath.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nunes-Alves C, Booty MG, Carpenter SM, Jayaraman P, Rothchild AC, Behar SM. In search of a new paradigm for protective immunity to TB. Nature reviews Microbiology. 2014;12(4):289–299. doi: 10.1038/nrmicro3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kohlmeier JE, Reiley WW, Perona-Wright G, Freeman ML, Yager EJ, Connor LM, et al. Inflammatory chemokine receptors regulate CD8(+) T cell contraction and memory generation following infection. The Journal of experimental medicine. 2011;208(8):1621–1634. doi: 10.1084/jem.20102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162(9):5407–5416. [PubMed] [Google Scholar]
- 44.Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS pathogens. 2009;5(4):e1000392. doi: 10.1371/journal.ppat.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehra S, Kaushal D. Functional genomics reveals extended roles of the Mycobacterium tuberculosis stress response factor sigmaH. Journal of bacteriology. 2009;191(12):3965–3980. doi: 10.1128/JB.00064-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehra S, Dutta NK, Mollenkopf HJ, Kaushal D. Mycobacterium tuberculosis MT2816 encodes a key stress-response regulator. The Journal of infectious diseases. 2010;202(6):943–953. doi: 10.1086/654820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kernodle DS. SigH, antioxidants, and the pathogenesis of pulmonary tuberculosis. The Journal of infectious diseases. 2012;205(8):1186–1188. doi: 10.1093/infdis/jis108. [DOI] [PubMed] [Google Scholar]
- 48.Winau F, Hegasy G, Kaufmann SH, Schaible UE. No life without death--apoptosis as prerequisite for T cell activation. Apoptosis : an international journal on programmed cell death. 2005;10(4):707–715. doi: 10.1007/s10495-005-2940-6. [DOI] [PubMed] [Google Scholar]
- 49.Obst R, van Santen HM, Melamed R, Kamphorst AO, Benoist C, Mathis D. Sustained antigen presentation can promote an immunogenic T cell response, like dendritic cell activation. Proc Natl Acad Sci U S A. 2007;104(39):15460–15465. doi: 10.1073/pnas.0707331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris J, Keane J. How tumour necrosis factor blockers interfere with tuberculosis immunity. Clinical and experimental immunology. 2010;161(1):1–9. doi: 10.1111/j.1365-2249.2010.04146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10(1):45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 52.Bruns H, Meinken C, Schauenberg P, Harter G, Kern P, Modlin RL, et al. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. The Journal of clinical investigation. 2009;119(5):1167–1177. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris J, Hope JC, Keane J. Tumor necrosis factor blockers influence macrophage responses to Mycobacterium tuberculosis. The Journal of infectious diseases. 2008;198(12):1842–1850. doi: 10.1086/593174. [DOI] [PubMed] [Google Scholar]
- 54.Schaible UE, Sturgill-Koszycki S, Schlesinger PH, Russell DG. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol. 1998;160(3):1290–1296. [PubMed] [Google Scholar]
- 55.Kernodle DS. Decrease in the effectiveness of Bacille Calmette-Guerin vaccine against pulmonary tuberculosis: a consequence of increased immune suppression by microbial antioxidants, not overattenuation. Clin Infect Dis. 2010;51(2):177–184. doi: 10.1086/653533. [DOI] [PubMed] [Google Scholar]
- 56.Novikov A, Cardone M, Thompson R, Shenderov K, Kirschman KD, Mayer-Barber KD, et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1beta production in human macrophages. J Immunol. 2011;187(5):2540–2547. doi: 10.4049/jimmunol.1100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adekambi T, Ibegbu CC, Kalokhe AS, Yu T, Ray SM, Rengarajan J. Distinct effector memory CD4+ T cell signatures in latent Mycobacterium tuberculosis infection, BCG vaccination and clinically resolved tuberculosis. PloS one. 2012;7(4):e36046. doi: 10.1371/journal.pone.0036046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riou C, Gray CM, Lugongolo M, Gwala T, Kiravu A, Deniso P, et al. A subset of circulating blood mycobacteria-specific CD4 T cells can predict the time to Mycobacterium tuberculosis sputum culture conversion. PLoS One. 2014;9(7):e102178. doi: 10.1371/journal.pone.0102178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamilton SE, Jameson SC. CD8 T cell memory: it takes all kinds. Frontiers in immunology. 2012;3:353. doi: 10.3389/fimmu.2012.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, Bigbee C, et al. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. The Journal of clinical investigation. 2012;122(1):303–314. doi: 10.1172/JCI46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: where are we and where do we need to go? PLoS pathogens. 2012;8(5):e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cruz A, Torrado E, Carmona J, Fraga AG, Costa P, Rodrigues F, et al. BCG vaccination-induced long-lasting control of Mycobacterium tuberculosis correlates with the accumulation of a novel population of CD4(+)IL-17(+)TNF(+)IL-2(+) T cells. Vaccine. 2015;33(1):85–91. doi: 10.1016/j.vaccine.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 63.Hartings JM, Roy CJ. The automated bioaerosol exposure system: preclinical platform development and a respiratory dosimetry application with nonhuman primates. J Pharmacol Toxicol Methods. 2004;49(1):39–55. doi: 10.1016/j.vascn.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Roy CJ, Brey RN, Mantis NJ, Mapes K, Pop IV, Pop LM, et al. Thermostable ricin vaccine protects rhesus macaques against aerosolized ricin: Epitope-specific neutralizing antibodies correlate with protection. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(12):3782–3787. doi: 10.1073/pnas.1502585112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swearengen JR. Biodefense : research methodology and animal models. 2nd edn. Boca Raton: Taylor & Francis; 2012. [Google Scholar]
- 66.Darrah PA, Bolton DL, Lackner AA, Kaushal D, Aye PP, Mehra S, et al. Aerosol Vaccination with AERAS-402 Elicits Robust Cellular Immune Responses in the Lungs of Rhesus Macaques but Fails To Protect against High-Dose Mycobacterium tuberculosis Challenge. J Immunol. 2014 doi: 10.4049/jimmunol.1400676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dutta NK, McLachlan J, Mehra S, Kaushal D. Humoral and lung immune responses to Mycobacterium tuberculosis infection in a primate model of protection. Trials in vaccinology. 2014;3::47–51. doi: 10.1016/j.trivac.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mehra S, Pahar B, Dutta NK, Conerly CN, Philippi-Falkenstein K, Alvarez X, et al. Transcriptional reprogramming in nonhuman primate (rhesus macaque) tuberculosis granulomas. PloS one. 2010;5(8):e12266. doi: 10.1371/journal.pone.0012266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gopal R, Monin L, Torres D, Slight S, Mehra S, McKenna K, et al. S100A8/A9 Proteins Mediate Neutrophilic Inflammation and Lung Pathology during Tuberculosis. American journal of respiratory and critical care medicine. 2013 doi: 10.1164/rccm.201304-0803OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Veazey RS, Mansfield KG, Tham IC, Carville AC, Shvetz DE, Forand AE, et al. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. Journal of virology. 2000;74(23):11001–11007. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.