Abstract
A distributed network of neurons regulates wake, non-rapid eye movement sleep (NREM), and rapid eye movement sleep. However, there are also glia in the brain, and there is growing evidence that neurons and astroglia communicate intimately to regulate behavior. To identify the effect of optogenetic stimulation of astrocytes on sleep, the promoter for the astrocyte specific cytoskeletal protein, glial fibrillary acidic protein (GFAP) was employed to direct the expression of channelrhodopsin-2 (ChR2) and the linked reporter gene, enhanced yellow fluorescent protein (EYFP), in astrocytes. rAAV-GFAP-ChR2 (H134R) -EYFP or rAAV-GFAP-EYFP were microinjected (750nl) into the posterior hypothalamus (bilateral) of mice. Three weeks later baseline sleep was recorded (0 Hz) and 24h later optogenetic stimulation applied during the first 6h of the lights-off period. Mice with ChR2 were given 5, 10 or 30Hz stimulation for 6h (10 msec pulses; 1mW; one minute on- 4 minutes off). At least 36h elapsed between the stimulation periods (5, 10, 30Hz) and although 0Hz was always first, the order of the other three stimulation rates were randomized. In mice with ChR2 (n=7), 10 Hz, but not 5 or 30Hz stimulation increased both NREM and REM sleep during the 6hr period of stimulation. Delta power did not increase. In control mice (no ChR2; n=5) 10Hz stimulation had no effect. This study demonstrates that direct stimulation of astrocytes powerfully induces sleep during the active phase of the sleep-wake cycle and underscores the inclusion of astrocytes in network models of sleep-wake regulation.
Keywords: Channelrhodopsin-2, REM sleep, orexin, melanin concentrating hormone, recombinant adenoassociated virus
Introduction
It is now firmly established that astrocytes actively control neuronal activity and synaptic transmission (Araque et al., 2001; Panatier et al., 2011). A single astrocyte contacts hundreds of dendrites, and tens of thousands of synapses (Bushong et al., 2002; Halassa et al., 2007). The concept of a tripartite synapse has emerged whereby neuronal transmission increases Ca2+ in astrocytes (Araque et al., 1999). Astrocytes do not generate an action potential, but they respond to stimulation by intracellular [Ca2+]i waves, also known as ‘Ca2+ excitability’ that can be recorded in vivo (Hirase et al., 2004; Wang et al., 2006; Dombeck et al., 2007; Bekar et al., 2008; Schummers et al., 2008; Shigetomi et al., 2010), in vitro (Cornell-Bell et al., 1990) and also in human brain slices (Oberheim et al., 2009). The Ca2+ wave spreads within a single mature astrocyte causing the release of its chemical transmitter, dubbed gliotransmitter, that modulates neuronal transmission (Araque et al., 1999). Astrocytes release glutamate that locally excites neurons (Fellin et al., 2006). Astrocytes also release ATP a major source of extracellular adenosine in the brain (Zhang et al., 2003; Pascual et al., 2005; Serrano et al., 2006). Optogenetic activation of astrocytes in the medulla oblongata releases ATP and increases breathing (Gourine et al., 2010). Adenosine inhibits neurons via the adenosine A1 receptor (Deng et al., 2011) and increased levels of adenosine stimulate sleep (Porkka-Heiskanen et al., 1997). Conversely, sleep is decreased when adenosine released by astrocytes is decreased (Halassa et al., 2009). Astrocytes regulate food intake by modulating adenosine levels and inhibiting agouti-related peptide (AGRP) neurons via adenosine A1 receptor (Yang et al., 2015).
Glia outnumber neurons and since they are considered to be partners with neurons in regulating sleep (Frank, 2013) it is important to directly manipulate them in local circuits to better understand their impact on a specific behavior such as sleep. This is now feasible because of the development of optogenetics (Boyden et al., 2005). Recently, we used optogenetics to selectively stimulate sleep-active neurons in the posterior hypothalamus and found that it robustly increased sleep (Konadhode et al., 2013). However, the effects of optogenetic stimulation of astrocytes on sleep are not known. Therefore, the present study tests the hypothesis that optogenetic stimulation of astrocytes increases sleep. In wildtype C57BL/6J mice, we found that sleep was increased during the active phase in response to optogenetic stimulation of astrocytes.
Materials and Methods
All manipulations done to the mice adhered to the ackn
Guide for the humane care and use of laboratory animals and were approved by the Medical University of South Carolina (protocol #3294) and the Ralph H. Johnson VA (protocol #537) Institutional Animal Care and Use Committee.
rAAV-GFAP-ChR2 (H134R)-EYFP plasmid construction
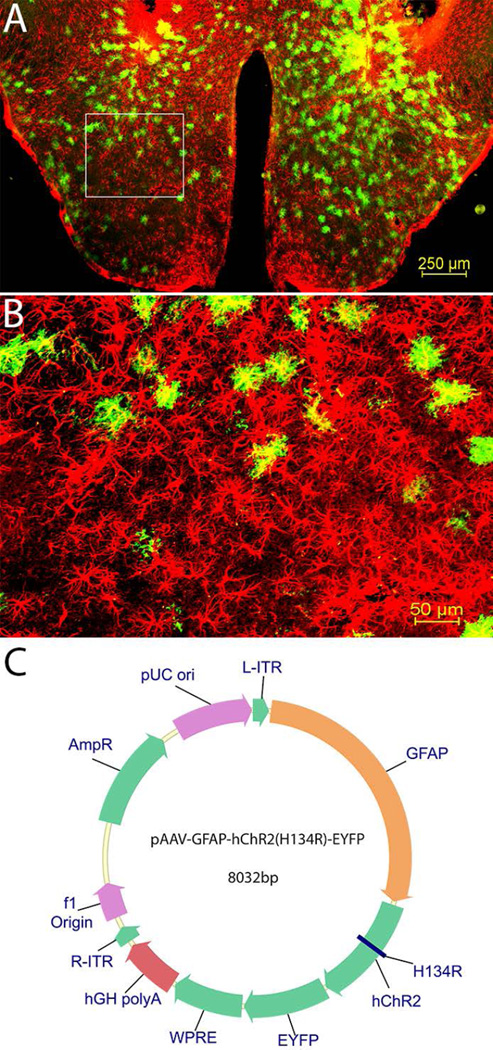
The entire plasmid containing GFAP-ChR2 (H134R)-EYFP was graciously supplied by Karl Deisseroth (Fig. 1C). We then submitted it for packaging in rAAV serotype 5 virus (University of North Carolina, Chapel Hill, NC) and titers of up to 1.5 ×1012 virus molecules/ml were injected bilaterally into the posterior hypothalamus. A separate plasmid that did not contain channelrhodopsin-2 was used as control (rAAV-GFAP-EYFP) and was similarly packaged.
Figure 1.
Distribution of astrocytes in the posterior hypothalamus. The photomicrographs illustrate the astrocytes containing GFAP (red) and EYFP (green) in a representative mouse (panels A and B). A low magnification view of the area of disbursement of the ChR2-EYFP in the posterior hypothalamus is depicted in panel A. Panel B is a high magnification view of the boxed area in panel A. Map of ChR2 linked GFAP reporter plasmid that was packaged into recombinant adenoassociated virus (rAAV; Panel C).
Implantation of sleep electrodes and delivery of virus
Twenty wildtype (9 males and 11 females) C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) that were approximately 6 months old and weighing 20–35 g were used. Using a stereotaxic apparatus (under 2% isoflurane anesthesia) the rAAV-GFAP-ChR2 (H134R)-EYFP or rAAV-GFAP-EYFP virus was delivered in 750 nl per injection with an injector cannula (33 gauge, cat#C3151-SPC, Plastics1) coupled to a 10ul Hamilton syringe. In each mouse, one microinjection per each side targeted the posterior hypothalamus. The virus was injected slowly over 10 minutes and we waited further 10 minutes before withdrawing the injector cannula. Two guide cannulae (26 gauge with 1.4 mm spacing and 4mm below pedestal height) were implanted and targeted the posterior hypothalamus. The optogenetic fiber optic probes (200 microns; cat # FT200EMT, Thor labs) were inserted into the guide cannulae.
At this time the electrodes to record the electroencephalogram (EEG) and electromyogram (EMG) were implanted as described previously (Shiromani et al., 2004). Briefly, four screw electrodes (cat # E363-96-1.6-SPC, Plastics1, Roanoke, VA) were placed in the skull to record cortical EEG, one on the frontal cortex and one on the contralateral parietal cortex of each side. Two flexible wire electrodes (cat # E363-76-SPC, Plastics1) were inserted into the nuchal muscles to record muscle activity (EMG). All the electrodes were inserted into a six-pin acrylic socket and secured to the skull with dental cement. The EEG was recorded from two contralateral screws (frontal- occipital). The electromyography (EMG) signal was recorded from nuchal muscles. The EEG and EMG signals were amplified via a Grass polygraph and recorded onto the hard disk using an analog– digital board (Kissei Comtec Co, Nagano, Japan).
After surgery the animals were housed singly in Plexiglas cages with bedding; food and water were available ad libitum. The temperature in the sleep recording room was 25°C and a 12 h light/dark cycle (6:00 A.M. – 6:00 P.M. lights on; 100 lux) was maintained.
Optogenetic stimulation paradigm
Ten days after surgery the mice were tethered to lightweight recording cables mounted to swivels and adapted for 10 days. The swivels allowed the mice to engage in unimpeded freedom of movement. Three weeks after surgery and viral delivery, sleep was recorded for 6h during the first half of the lights-off period (the wake-active phase in nocturnal rodents). During this first sleep recording session there was no optogenetic stimulation and this 6h session represents 0 Hz. Twenty-four hours later, the animals with ChR2 were given either 5, 10 or 30 Hz stimulation for 6h (1 minute stimulation followed by 4 minutes no stimulation, repeated every 5 minutes for 6h). The blue light (473 nm; 1mW power at tip of probe) pulses (10msec duration) were delivered through a programmable stimulator (Master 8, AMPI, Jerusalem, Israel). Sleep and behavior were recorded for the 6h stimulation period. At least 36h elapsed between the stimulation periods (5, 10, 30Hz) and although 0Hz was always first, the order of the other three stimulation rates were randomized.
In the group of mice administered the control virus, rAAV-GFAP-EYFP, sleep was recorded after 0 and 10 Hz stimulation using the above protocol. These animals determined the effect of light stimulation in astrocytes without ChR2.
Immunohistochemistry
At the end of the experimental paradigm the animals were deeply anesthetized (4% isoflurane) and the brains perfused (25ml saline followed by 100ml 10% formaldehyde). After equilibration in 30% sucrose the brains were cut on a cryostat (40 µm coronal sections; 1 in 4 series). The free-floating sections were incubated overnight in a primary antibody mouse anti-GFAP (1:500; Cat# MAb-3402 EMD Millipore) followed by 2h incubation in the secondary antibody (donkey anti-mouse-Alexa Fluor 568; 1:500; Invitrogen). For staining of neurons the sections were incubated overnight in mouse anti-NeuN (1:500, Cat # MAB377, EMD Millipore) primary antibody, followed by 2h incubation in donkey anti mouse-Alexa Fluor 568 (1:500; Invitrogen) secondary antibody. For staining of microglia the sections were incubated overnight in rabbit anti-Iba1 (1:500, Cat # 019–19741, Wako Chemicals) primary antibody, followed by 2h incubation in donkey anti rabbit-Alexa Fluor 568 (1:500; Invitrogen) secondary antibody. For staining of oligodendrocytes the sections were incubated overnight in mouse anti-oligodendrocyte (1:500, Cat # MAB328, EMD Millipore) primary antibody, followed by 2h incubation in donkey anti mouse-Alexa Fluor 568 (1:500; Invitrogen) secondary antibody. For double staining of astrocytes with GFAP and orexin or melanin concentrating hormone (MCH) neurons, the sections were incubated overnight in primary antibodies i.e., mouse anti-GFAP (1:500; Cat# MAb-3402 EMD Millipore), goat anti-Orexin (1:500; Cat# SC-8070, Santa Cruz biotechnology) or rabbit anti-MCH (1:500; Cat# 070-47, Phoenix Pharmaceuticals) respectively, followed by 2h incubation in secondary antibodies (donkey anti mouse-Alexa Fluor 568, donkey anti goat-Alexa Fluor 647 or donkey anti rabbit –Alexa Fluor 647 respectively; 1:500; Invitrogen). For double staining of astrocytes for GFAP and histamine neurons for histidine decarboxylase (HDC), the sections were incubated overnight in primary antibodies, mouse anti-GFAP (1:500) and rabbit anti-HDC (1:200; Cat# 03–16045, American Research Products). Next day, the sections were incubated for 2hr in secondary antibodies (donkey anti mouse-Alexa Fluor 568 and donkey anti rabbit-Alexa Fluor 647 respectively; 1:500; Invitrogen). The sections were washed, mounted onto gelatin-coated slides, and cover slipped. The fluorescent images were visualized with a Nikon A1RSi confocal microscope.
Identification of sleep–wake states
The sleep records were scored by blinding the scorer (DP) to the type of rAAV administered to the mice. After the EEG data were scored and the brain tissue examined for presence of EYFP+ astrocytes, the code was broken to identify the type of rAAV administered to the mouse. The 6hr EEG, EMG, and video recordings were scored manually on a computer (Sleep Sign Software) in 12 s epochs for wake, NREM sleep, and REM sleep (Liu et al., 2011). Wakefulness was identified by the presence of desynchronized EEG, and high EMG activity. NREM sleep consisted of high amplitude slow waves together with a low EMG tone relative to waking. REM sleep was identified by the presence of regular EEG theta activity coupled with low EMG relative to slow-wave sleep. The behavior of the mice was recorded on video and this aided in identifying sleep-wake states.
Analysis of delta power
A Fast Fourier transform (FFT) of the EEG during wake, NREM, and REM sleep was automatically calculated by the software (Sleep Sign) in 0.5 Hz bins. Because of the variability in the amplitude of the EEG across mice, FFT values were normalized by dividing the EEG power during NREM sleep (0.5-4Hz) by the EEG power during wake (0.5–4 Hz).
Counting of astrocytes
The total number of astrocytes and the infected astrocytes were counted for mice with ChR2 by drawing a 4 × 3 grid (250um x 330um each) in each hemisphere on the fluorescence microscopy images. The number of EYFP+ astrocytes, and single GFAP+ astrocytes were counted inside the grids. The percentage of infected astrocytes, i.e., EYFP+, as a function of total number of astrocytes (GFAP+ + EYFP+) was determined.
Statistical analysis
The sleep data in the experimental group was analyzed with a one way repeated measures ANOVA (Holm-Sidak post hoc test against 0Hz) and a-priori paired t-tests. Pearson correlation determined the relationship between percentage of infected astrocytes and REM, NREM and total sleep. p<0.05 was used to determine level of significance.
Results
Distribution of rAAV expressing astrocytes
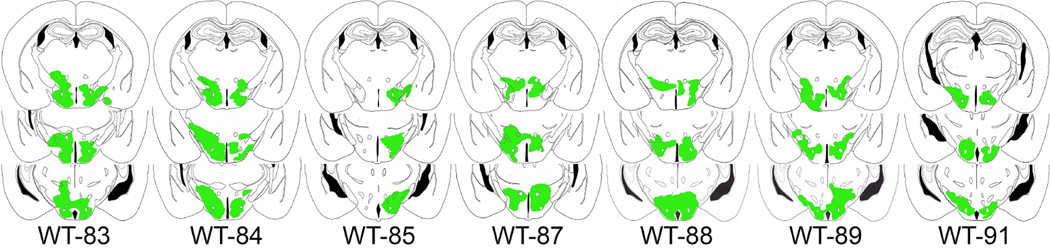
The promoter for glial fibrillary acidic protein (GFAP) directed the expression of the ChR2-EYFP fusion protein (Fig. 1). The rAAV-GFAP-ChR2 (H134R)-EYFP was present in GFAP+ astrocytes only in the posterior hypothalamus (Fig. 1A; the boxed area is shown in Fig. 1B). Astrocytes outside the injection zone did not contain the reporter gene indicating that the infected cells did not migrate outside the intended target site. In mice given ChR2-EYFP, the percentage of astrocytes containing the reporter gene (proxy for ChR2) relative to GFAP+ astrocytes was 22.21% (+/− 2.23). Figure 2 shows the distribution of the infection zone in mice with ChR2.
Figure 2.
The drawings represent coronal sections of the mouse brain with rAAV-GFAP-ChR2 (H134R)-EYFP infected astrocytes in the posterior hypothalamus. The transfected regions where the EYFP signal was present were traced and are shown in green color. The numbers below the sections indicate the mouse ID. The distance of the sections range from 1.22mm to 2.46mm posterior to bregma (Franklin & Paxinos, 1997).
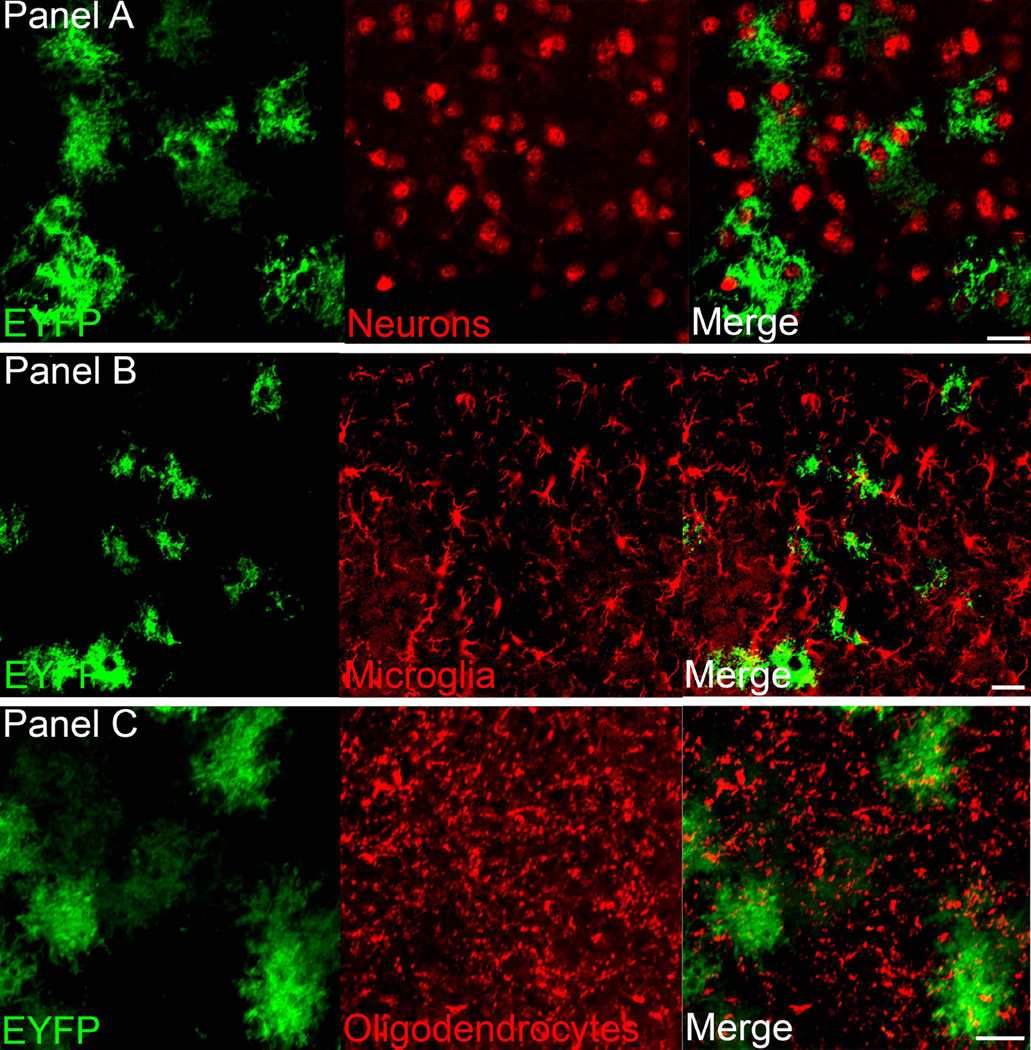
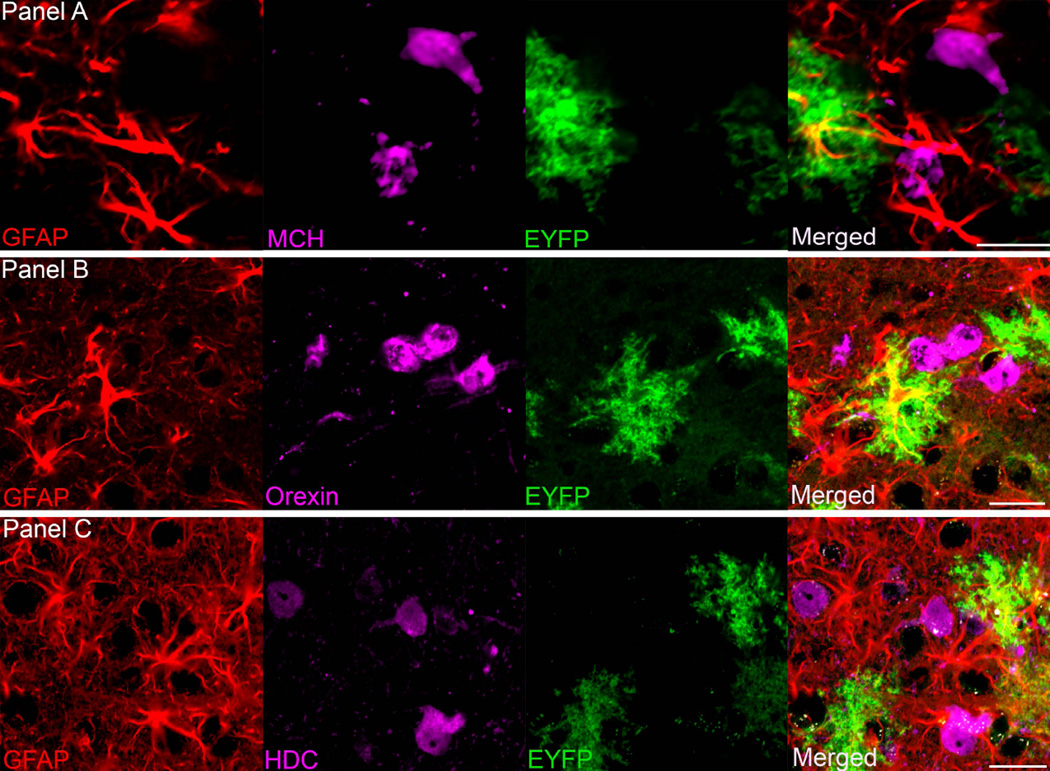
Figure 3 shows the relationship between surrounding neurons (panel 3A), microglia (panel 3B) and oligodendrocytes (panel 3C) with ChR2-EYFP-positive astrocytes in the infected region. This shows that there is no ChR2-EYFP infection in neurons, microglial cells or oligodendrocytes at the target site respectively. Figure 4 indicates the close proximity of the ChR2-EYFP-positive astrocytes with the sleep-active neurons containing melanin concentrating hormone (panel 4A) or the arousal neurons orexin (panel 4B) and histidine decarboxylase (histamine synthesizing enzyme) neurons (panel 4C)
Figure 3.
ChR2-EYFP is expressed in astrocytes and not in other cell types. The photomicrographs shows that the GFAP promoter drives ChR2-EYFP (green) into astrocytes and not neurons (panel A; anti-NeuN), microglia (panel B; anti-Iba-1) or oligodendrocytes (panel C; anti- oligodendrocytes). Scale bar is 25um.
Figure 4.
The close relationship between astrocytes that contain ChR2-EYFP (green), and sleep regulating MCH (melanin concentrating hormone) neurons (panel A), wake active orexin neurons (panel B) and neurons containing the histamine synthesizing enzyme, histidine decarboxylase (HDC) (panel C). Scale bar is 25um.
Effect of optogenetic stimulation of astrocytes on sleep in mice
Twelve mice were administered rAAV-GFAP-ChR2 (H134R)-EYFP and implanted with sleep recording electrodes. In two mice the sleep data could not be analyzed because of noise artifact in the EEG/EMG signal. In three mice, post-mortem analysis revealed no EFYP+ cells in the brain, perhaps because the microinjection cannula was clogged during injection of the virus. The sleep data presented in figures 5 and 6 summarizes the effects of optogenetic stimulation of astrocytes on wake, NREM and REM sleep during the first 6h of the lights-off period in n=7 mice (5 males; 2 females) given ChR2.
Figure 5.
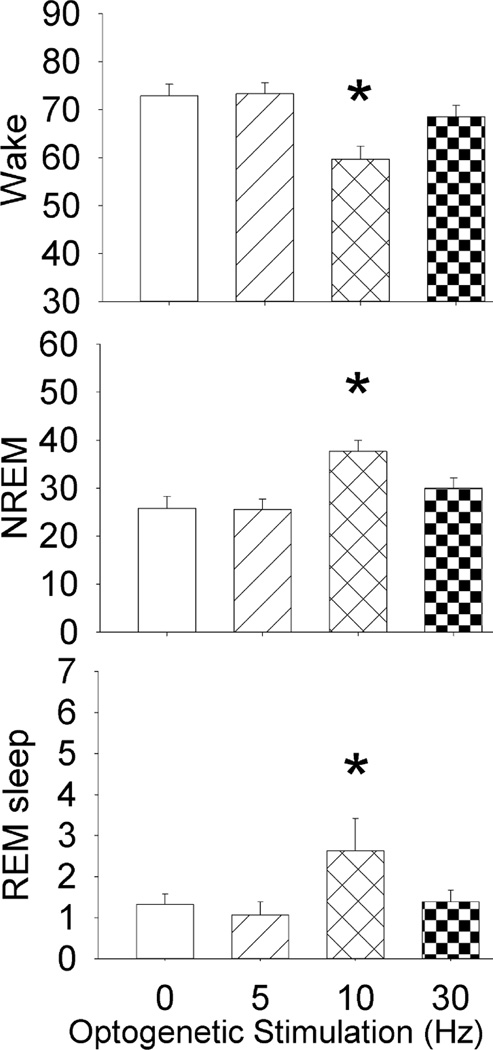
Effects of optogenetic stimulation of astrocytes on percent wake, NREM and REM sleep during the first 6h of the lights-off period. Optogenetic stimulation was applied in the first 6h of the lights-off period. Blue (473nm) light pulses (10msec duration) were applied at 0, 5, 10 and 30Hz rates for one minute every five minutes for 6h (1 minute with stimulation followed by 4 minutes without stimulation). Sleep was continuously recorded and the behavior was videotaped. *p<0.05 versus 0 Hz.
Figure 6.
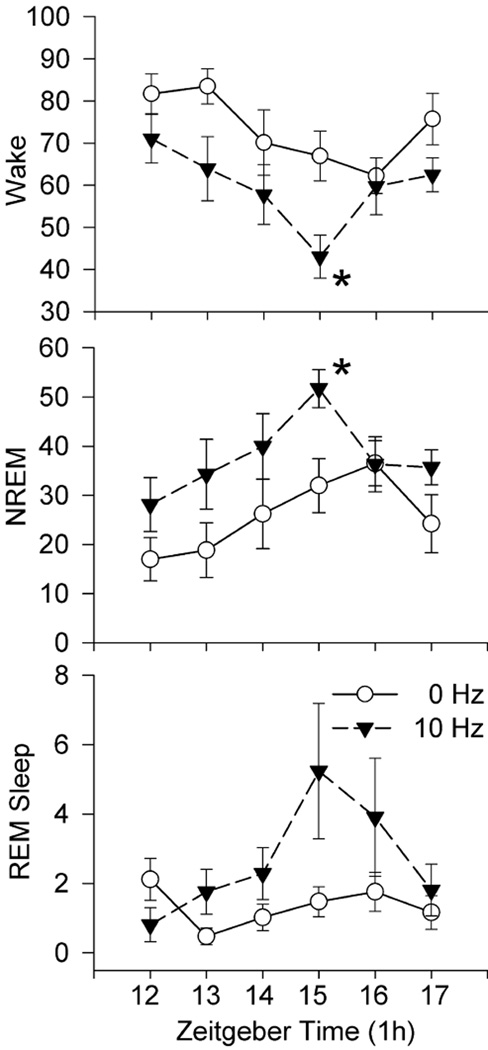
Time course of effects of optogenetic stimulation of astrocytes in the posterior hypothalamus on percent wake, NREM and REM sleep. 10Hz stimulation began at the onset of the lights-off period (Zeitgeber time 12) and continued for 6h (1minute on, 4 minutes off). A repeated measures ANOVA determined significant effect of stimulation (0 versus 10Hz) for wake, NREM and REM sleep. There was also a significant effect of the time (ZT) for wake and NREM, but not for REM sleep. There was no interaction (time x treatment) effect for wake, NREM or REM sleep. Asterisk denotes significant difference with the 0Hz for that hour (Holm-Sidak post-hoc comparison; p<0.05). There was no effect of 5 and 30Hz stimulation on sleep (figure 5 and supplementary figure 1). 10Hz stimulation produced a progressive decrease in waking and increased NREM with a peak after 4h. REM sleep lagged but also peaked at 4h. The waxing and waning of sleep suggests a physiological response mediated by adenosine (see profile of adenosine in response to 6h of sleep deprivation in Blanco et al., 2006).
The sleep data were initially averaged over the 6h of the experiment (Fig. 5) to identify the stimulation rate that produced the changes in sleep. Optogenetic stimulation at 10 Hz compared to 0 Hz significantly increased NREM sleep in mice with ChR2 (+68%; p<0.003), and decreased wake (p<0.003). 10Hz stimulation significantly increased REM sleep (p<0.05). Five or 30Hz stimulation rates did not significantly change percent wake, NREM or REM sleep.
Figure 6 summarizes the time course of changes in sleep induced by the 10Hz stimulation. The data were analyzed using a nested repeated measures ANOVA design (time factor (6 one hour blocks) nested within the treatment factor (two blocks; 0, 10 Hz) (Kirk, 1968). There was a significant effect of the treatment factor (0 versus 10Hz stimulation) for wake (F (1, 66) =13.23; p<0.01), NREM (F (1, 66) =11.31; p<01), and REM sleep (F (1, 66) =6.74; p<0.05). There was also a significant effect of the time factor for wake (F (5, 66) =3.06; p<0.05) and NREM (F (5, 66) =2.56; p<0.05), but not for REM sleep (F (5, 66) =2.12; n.s). There was no interaction (time x treatment) effect for wake, NREM or REM sleep. The impact of the optogenetic stimulation was evident on wake and NREM by the end of the first hour and it progressively increased so that peak effects were significant at the end of the fourth hour (figure 6). 5 and 30Hz stimulation did not produce such a progressive change over the 6h of stimulation (supplementary figure 1).
10 Hz stimulation did not increase delta power during NREM sleep (0Hz = 2.63+0.23; 10Hz = 2.22+0.18). There was no significant relationship between the number of transfected astrocytes and REM sleep (r=0.55), NREM sleep (r=0.23) or total sleep (REM+NREM; r= 0.35) in the mice with ChR2.
With 10Hz stimulation there was a significant increase in the number of NREM sleep bouts (+79.8%; Table 1) and a decrease in length of NREM bouts (−22.76%; Table 1). The stimulation increased the number of wake bouts (+74.9%; Table 1) but the length of these bouts was significantly decreased (−59.69%). The increase in number of NREM bouts coupled with a decreased length of wake bouts may have contributed to the increase in percent NREM sleep and a decrease in waking.
Table 1.
Effects of optogenetic stimulation (0 versus 10Hz) of astrocytes on number and length (seconds) of bouts of wake, NREM and REM sleep in wildtype mice (n=7). The data represents average (+ SEM) number and length of bouts during the 6h stimulation period (first half of lights-off period).
| Number of Bouts | Length of Bouts (Seconds) | |||
|---|---|---|---|---|
| 0 Hz | 10 Hz | 0 Hz | 10 Hz | |
| Wake | 19.36 (3.72) |
33.86* (2.29) |
989.96 (196.2) |
399* (45.5) |
| NREM | 19.07 (3.16) |
34.29* (2.8) |
315.89 (27.8) |
244* (20.37) |
| REM | 4.14 (0.59) |
8.43 (2.17) |
69.77 (7.43) |
66.29 (7.18) |
(Standard error is in parentheses)
Paired t-test versus 0 Hz; p < 0.05
The effects of light stimulation were examined in a control group of mice that received the virus without ChR2 (n=8; rAAV-GFAP-EYFP). However, three mice in this group were deleted because of excessive noise artifact in the EEG/EMG signal. 10Hz stimulation in mice without ChR2 (n=5 female) produced no significant change in percent wake (0Hz=87.89+ 3.73; 10Hz =86.4+ 4.26), NREM (0Hz =11.21+ 3.45; 10Hz=11.97+ 3.54) or REM sleep (0Hz =0.82+ 0.35; 10Hz=1.44+ 0.76).
Discussion
The utility of astrocyte activation as a therapeutic target in the brain has been proposed (Halassa et al., 2007). However, the approach has not been tried in sleep disorders because it is not readily obvious where in the brain to stimulate astrocytes. Here we have provided the first evidence that optogenetic activation of astrocytes in the posterior hypothalamus increases sleep in C57BL/6J wildtype mice. The posterior hypothalamus was targeted as it contains neurons regulating both sleep and wake (Konadhode et al., 2014). Orexin neurons drive arousal (Carter et al., 2010) and there is considerable evidence that MCH neurons induce sleep (Hassani et al., 2009; Konadhode et al., 2013). The astrocytes containing ChR2-EYFP were found distributed among neurons containing melanin concentrating hormone (panel 4A), orexin (panel 4B) and histidine decarboxylase (histamine synthesizing enzyme) neurons (panel 4C).
In the present study the parameters for optogenetic stimulation of astrocytes were the same as those used to optogenetically stimulate MCH neurons (Konadhode et al., 2013), which permits comparison between effects of astrocyte versus neuron stimulation. In both studies, C57BL/6J wildtype mice were used, which required creating specific viral vectors. The advantage of this approach is that the viral vectors can also be used in rats, and other mammals that sleep, thereby strengthening the generalizability of the effect. Recently, we have confirmed in rats that optogenetic stimulation of MCH neurons increases sleep (Blanco-Centurion et al., 2014). In both studies, the ChR2 was inserted into the posterior hypothalamus, a region implicated in sleep. The stimulation occurred at night, when nocturnal rodents are mostly awake, and it increased both NREM and REM sleep. This attests to the significance of the posterior hypothalamus in sleep-wake regulation. However, other brain areas need to be similarly manipulated to fully identify the role of astrocytes and neurons in regulating states of consciousness.
Astrocytes release D-serine, glutamate, ATP, and cytokines, such as tumor necrosis factor-α (TNF-α), among other molecules (Hines & Haydon, 2014). Glutamate is the primary excitatory neurotransmitter in the brain and at least in the hippocampus half of the excitatory synapses are closely coupled to an astrocytic process (Ventura & Harris, 1999). D-serine and glutamate released from astrocytes enhance excitation of neurons (Panatier et al., 2006). In the primary visual cortex, optogenetic stimulation of astrocytes enhances both excitatory and inhibitory synaptic transmission (Perea et al., 2014).
ATP released from astrocytes and converted to extracellular adenosine inhibits synaptic transmission (Pascual et al., 2005). There is considerable evidence that accumulation of adenosine in the brain increases sleep (Porkka-Heiskanen et al., 1997; Frank, 2013). Astrocytes are a major source of ATP and extracellular adenosine, which acts on pre-synaptic adenosine 1-receptors. Adenosine A1R agonists potently stimulate sleep whereas the antagonists decrease sleep (Blanco-Centurion et al., 2006). Genetically engineered mice with reduced adenosine levels (expression of dominant negative SNARE selectively within astrocytes) have normal amounts of sleep and wake in the baseline period but do not have a sleep rebound after sleep deprivation (Halassa et al., 2009). Microinjection of adenosine in the rat posterior hypothalamus, the site of optogenetic stimulation of astrocytes in the present paper, increases sleep (Alam et al., 2009) and this has been linked to reduction in activity of the arousal orexin neurons (Alam et al., 2005). In the present study, we found that the impact of the stimulation was progressive (Figure 6) and this is consistent with the accumulation of adenosine. A future study could use the dnSNARE mice and we hypothesize that optogenetic stimulation of the astrocytes should release less adenosine and consequently there should be a blunted sleep response.
Besides adenosine, cytokines released from astrocytes may also have contributed to the increase in sleep. Astrocytes are a source of cytokines in the brain (Benveniste et al., 1990), and there is considerable evidence linking cytokines and sleep (Imeri & Opp, 2009). In particular, interleukin-1β (IL-1β) and TNF-α increase sleep in response to immune challenge (Krueger et al., 2011). Both are produced by neurons and glia, and their receptors are also present on neurons and glia (Krueger et al., 2011). Previously, infection of mice with murine gammaherpesvirus-68 has been shown to increase sleep (Olivadoti et al., 2011). In the present study, rAAV was used to insert the ChR2 and EYFP genes into astrocytes. However, it is unlikely that an inflammatory response to the rAAV was responsible for the increase in sleep because sleep was not increased in the 5Hz or 30Hz animals. It is more likely that optogenetic stimulation of astrocytes released cytokines, which then affected the orexin and MCH neurons regulating sleep –a possibility we would like to explore further.
Will optogenetic activation of astrocytes in other brain regions also increase sleep? It is important to answer this question because although astrocytes are pervasive throughout the brain there are specific neurons localized in the basal forebrain, hypothalamus and brainstem that are identified as regulating wake, NREM and REM sleep (Pelluru et al., 2013). The basal forebrain contains both wake-active and sleep-active neurons (Jones, 2011). Moreover, only in this region there is an increase in adenosine in response to waking (Porkka-Heiskanen et al., 1997). Thus, activation of astrocytes in the basal forebrain should increase sleep. Similarly, we postulate that in the pons where the sleep-wake neurons are also located, astrocytic activation should increase sleep.
A potential concern regarding the use of optogenetics to stimulate astrocytes is that the astrocytes are not excitable cells like neurons, and that the presence of ChR2 might be considered abnormal. However, specific behaviors have been manipulated by optogenetic stimulation of astrocytes (Gourine et al., 2010; Perea et al., 2014). In the present study, the stimulation was applied for one minute every five minutes. Thus, the light stimulation was intermittent and lasted only 20% of the time. Moreover, each animal was stimulated at three different rates (5, 10 and 30Hz, randomized) with a 36h break between each rate. The effects on sleep were evident with 10Hz stimulation but not 5 or 30Hz (Figure 5, 6 and supplementary figure 1). The impact of 10Hz stimulation was that it progressively increased sleep, with a peak at 4h (Figure 6). Such a time course was not seen with 5 or 30Hz stimulation (supplementary figure 1), perhaps because 5Hz did not provide sufficient stimulation whereas 30Hz was excessive. The progressive increase in sleep in response to 10Hz astrocyte stimulation suggests that there was a slow cumulative load from the gradual accumulation of gliotransmitters, such as adenosine. However, the effects subsided after 4h even though light continued to be delivered, suggesting activation of endogenous factors regulating levels of gliotransmitters, such as adenosine. This is consistent with the time course of adenosine which increases in response to four hours of sleep deprivation, but then declines in the subsequent two hours (Blanco-Centurion et al., 2006).
One limitation of the optogenetic approach is that light may not reach all of the astrocytes containing ChR2. In the present study sleep was increased when 22% of the total astrocytes in the target region were infected, indicating the potency of the glial effect. Alternatively, a pharmacogenetic approach is likely to reach widely distributed phenotype of cells. This approach uses a drug, clozapine-N-oxide, to exclusively activate genetically engineered receptors (designer receptors exclusively activated by designer drugs (DREADD) (Armbruster et al., 2007). Recently, this approach was used to link astrocytes to feeding behavior. In the study, the engineered muscarinic receptors, hM3Dq (excited by drug) or hM4Di (inhibited by drug) were inserted into astrocytes (GFAP promoter driven) in the medial basal hypothalamus and feeding behavior was monitored after injection of the drug, clozapine-N-oxide (CNO). Drug-induced stimulation of the hM3Dq receptors on the astrocytes inhibited feeding behavior. The effect of astrocyte stimulation was linked to release of adenosine from the astrocytes which then inhibited neurons via the adenosine A1 receptor (Yang et al., 2015). We anticipate that the DREADD method will be used to manipulate astrocytes in specific brain regions and the effects on sleep will be determined.
In the brain, astrocytes may function as wakefulness integrators (Frank, 2013). The brain is most active during waking and astrocytes show changes in intracellular calcium concentrations. These changes in calcium may dampen the waking drive and promote sleep (Frank, 2013). In the present study, optogenetic stimulation of astrocytes may have produced similar changes in calcium, and coupled with secretion of glutamate, ATP and cytokines, imparted a cumulative load on the posterior hypothalamic neurons to promote sleep.
Optogenetics is increasingly used as a powerful tool to mechanistically test the roles of specific CNS cell types in behavior. Using this tool, this is the first study to demonstrate that stimulation of hypothalamic astrocytes increases sleep. The effects of astrocytic stimulation are likely to be more localized compared to neuronal stimulation because neurons project to multiple targets. Future studies will focus on the nature of astrocyte released factors (known and novel) potentially involved in sleep induction.
Supplementary Material
Acknowledgments
We thank Dr. Kumar Sambamurti, Dr. Carlos Blanco-Centurion and Dr. Meng Liu for helpful comments during the writing of the manuscript. We thank Dr. Amanda LaRue for use of the confocal microscope. Supported by The Medical Research Service of the Department of Veterans Affairs and NIH grants NS084477 and NS079940.
Abbreviations
- AGRP
agouti-related peptide
- ChR2
channelrhodopsin-2
- EEG
electroencephalogram
- EMG
electromyogram
- EYFP
enhanced yellow fluorescent protein
- GFAP
glial fibrillary acidic protein
- HDC
histidine decarboxylase
- MCH
Melanin Concentrating Hormone
- NREM
non rapid eye movement
- rAAV
recombinant Adeno associated virus
- REM
rapid eye movement
- TNFα
Tumor necrosis factor-α
- ZT
Zeitgeber Time
Footnotes
Conflict of Interests: No conflicts of interest, financial or otherwise are declared by the author(s).
References
- Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. The Journal of physiology. 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, Kumar S, Rai S, Methippara M, Szymusiak R, McGinty D. Role of adenosine A(1) receptor in the perifornical-lateral hypothalamic area in sleep-wake regulation in rats. Brain research. 2009;1304:96–104. doi: 10.1016/j.brainres.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annual review of physiology. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends in neurosciences. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekar LK, He W, Nedergaard M. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cerebral cortex. 2008;18:2789–2795. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste EN, Sparacio SM, Norris JG, Grenett HE, Fuller GM. Induction and regulation of interleukin-6 gene expression in rat astrocytes. Journal of neuroimmunology. 1990;30:201–212. doi: 10.1016/0165-5728(90)90104-u. [DOI] [PubMed] [Google Scholar]
- Blanco-Centurion C, Xu M, Murillo-Rodriguez E, Gerashchenko D, Shiromani AM, Salin-Pascual RJ, Hof PR, Shiromani PJ. Adenosine and sleep homeostasis in the Basal forebrain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:8092–8100. doi: 10.1523/JNEUROSCI.2181-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Centurion CA, Pelluru D, Konadhode R, Liu MA, van den Pol AN, Shiromani P. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2014. Optogenetic activation of melanin-concentrating hormone (MCH) containing neurons powerfully increases sleep in rats. Program No. 257.26/OO3. 2014. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature neuroscience. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nature neuroscience. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Deng Q, Terunuma M, Fellin T, Moss SJ, Haydon PG. Astrocytic activation of A1 receptors regulates the surface expression of NMDA receptors through a Src kinase dependent pathway. Glia. 2011;59:1084–1093. doi: 10.1002/glia.21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pozzan T, Carmignoto G. Purinergic receptors mediate two distinct glutamate release pathways in hippocampal astrocytes. The Journal of biological chemistry. 2006;281:4274–4284. doi: 10.1074/jbc.M510679200. [DOI] [PubMed] [Google Scholar]
- Frank MG. Astroglial regulation of sleep homeostasis. Current opinion in neurobiology. 2013;23:812–818. doi: 10.1016/j.conb.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. ed 2. San Diego, CA USA: Academic Press Inc; 1997. [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines DJ, Haydon PG. Astrocytic adenosine: from synapses to psychiatric disorders. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2014;369:20130594. doi: 10.1098/rstb.2013.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase H, Qian L, Bartho P, Buzsaki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS biology. 2004;2:E96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nature reviews. Neuroscience. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. Neurobiology of waking and sleeping. Handbook of clinical neurology. 2011;98:131–149. doi: 10.1016/B978-0-444-52006-7.00009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. Belmont, CA: Brooks/Cole; 1968. [Google Scholar]
- Konadhode RR, Pelluru D, Blanco-Centurion C, Zayachkivsky A, Liu M, Uhde T, Glen WB, Jr, van den Pol AN, Mulholland PJ, Shiromani PJ. Optogenetic stimulation of MCH neurons increases sleep. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:10257–10263. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konadhode RR, Pelluru D, Shiromani PJ. Neurons containing orexin or melanin concentrating hormone reciprocally regulate wake and sleep. Frontiers in systems neuroscience. 2014;8:244. doi: 10.3389/fnsys.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Clinton JM, Winters BD, Zielinski MR, Taishi P, Jewett KA, Davis CJ. Involvement of cytokines in slow wave sleep. Progress in brain research. 2011;193:39–47. doi: 10.1016/B978-0-444-53839-0.00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Blanco-Centurion C, Konadhode R, Begum S, Pelluru D, Gerashchenko D, Sakurai T, Yanagisawa M, van den Pol AN, Shiromani PJ. Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6028–6040. doi: 10.1523/JNEUROSCI.6069-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivadoti MD, Weinberg JB, Toth LA, Opp MR. Sleep and fatigue in mice infected with murine gammaherpesvirus 68. Brain, behavior, and immunity. 2011;25:696–705. doi: 10.1016/j.bbi.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, Robitaille R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 2011;146:785–798. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pelluru D, Konadhode R, Shiromani PJ. MCH neurons are the primary sleep-promoting group. Sleep. 2013;36:1779–1781. doi: 10.5665/sleep.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Yang A, Boyden ES, Sur M. Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nature communications. 2014;5:3262. doi: 10.1038/ncomms4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:5370–5382. doi: 10.1523/JNEUROSCI.5255-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Kracun S, Sofroniew MV, Khakh BS. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nature neuroscience. 2010;13:759–766. doi: 10.1038/nn.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiromani PJ, Xu M, Winston EM, Shiromani SN, Gerashchenko D, Weaver DR. Sleep rhythmicity and homeostasis in mice with targeted disruption of mPeriod genes. Am J Physiol Regul Integr Comp Physiol. 2004;287:R47–R57. doi: 10.1152/ajpregu.00138.2004. [DOI] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nature neuroscience. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- Yang L, Qi Y, Yang Y. Astrocytes Control Food Intake by Inhibiting AGRP Neuron Activity via Adenosine A1 Receptors. Cell Rep. 2015;11:798–807. doi: 10.1016/j.celrep.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.