Abstract
Sarcoidosis is a granulomatous disease of unknown etiology, characterized by a Th1 immunophenotype, most commonly involving the lung, skin, lymph node and eyes. Molecular and immunologic studies continue to strengthen the association of sarcoidosis with infectious antigens, particularly those derived from Propionibacterium and Mycobacterium species. Independent studies report the presence of microbial nucleic acids and proteins within sarcoidosis specimens. Complementary immunologic studies also support the role of infectious agents in sarcoidosis pathogenesis. Th-1 immune responses directed against mycobacterial virulence factors have been detected within sarcoidosis diagnostic bronchoalveolar lavage (BAL). Th1 and Th17 immune responses against propionibacteria have also been reported. More recently, case reports and clinical trials from Japanese, European and American investigators have emerged regarding the efficacy of antimicrobials against Propionibacterium and Mycobacterium species on pulmonary and cutaneous sarcoidosis. While these clinical investigations are not conclusive, they support increasing efforts to identify novel therapeutics, such as antimicrobials, that will impact the observed increase in sarcoidosis morbidity and mortality.
Sarcoidosis epidemiology suggest exposure to microbial bioaerosols
Sarcoidosis is a granulomatous disease of unknown etiology, most commonly involving the lung, skin, lymph node and eyes 1. Granulomatous inflammation can be initiated by infectious agents, such as fungi or Mycobacterium tuberculosis (MTB), or by non-infectious agents such as beryllium (chronic beryllium disease [CBD]). Analysis of sarcoidosis epidemiology suggests that infectious agents have a role in sarcoidosis pathogenesis. Investigators in A Case Control Etiologic Study of Sarcoidosis (ACCESS) observed positive associations between sarcoidosis risk and certain occupations, such as agricultural employment, exposure to insecticides and moldy environments2. Another study noted that the hospitalization admissions for African Americans with sarcoidosis in South Carolina increased with proximity to the Atlantic Ocean3. A unifying factor in environmental and geographic reports is the possibility of exposure to microbial bioaerosols. Natural waters, water distribution systems, biofilm in pipes, peat and potting soil, water droplets, equipment such as bronchoscopes and catheters, and moldy buildings are natural habitats for environmental opportunistic mycobacteria4. Aerosolization of environmental opportunistic mycobacteria has been associated with the development of other granulomatous diseases of mycobacterial origin, such as hypersensitivity pneumonitis5.
Molecular and immunologic investigations reveal microbial proteins and DNA
The inability to identify microorganisms by histologic staining or to culture microorganisms from pathologic tissues continues to be one of the strongest arguments against a potential role for infectious agents in sarcoidosis pathogenesis. As molecular analysis continues to grow in sensitivity and specificity, we now realize that current culture and staining methods identify less than two percent of current microbial communities present within the human biological specimens6, 7. Advanced molecular techniques such as Deep Sequencing technologies also have demonstrated successful identification of novel microorganisms in pathologic tissues not easily identified by traditional methods8, 9. Molecular analysis of pathologic tissue for microbial nucleic acids and proteins serves as an alternative means of identifying a putative infectious agent. PCR was used to identify the etiologic agents of Whipple’s disease (Tropheryma whippelii)10, as well as the novel coronavirus as the agent of Severe Acute Respiratory Syndrome (SARS)11.
A growing scientific interest involves defining the microbial community within distinct diseases, ie microbiome analysis. Microbiome analysis was performed on the upper and lower airway of subjects with interstitial lung diseases, including idiopathic interstitial pneumonia (IIP), non-IIP and sarcoidosis, as well as Pneumocystis jiroveci pneumonia and healthy controls. The microbiota in lower airways of the majority of patients (30; 90%) primarily consisted of Prevotellaceae, Streptococcaceae and Acidaminococcaceae; α and β diversity measurements revealed no significant differences in airway microbiota composition between the five different groups of patients. It was concluded that IIP, non-IIP and sarcoidosis are not associated with disordered airway microbiota and a pathogenic role of commensals in the disease process is therefore unlikely12. A more targeted molecular approach for microbial pathogens in sarcoidosis granulomas most strongly supports that propionibacteria and/or mycobacteria have a role in sarcoidosis pathogenesis. Japanese researchers report molecular evidence of Propionibacterium acnes (P. acnes) DNA in sarcoidosis specimens, although the DNA could also be isolated from control specimens13. The distinction lies in the quantitative differences in P. acnes DNA between sarcoidosis and controls. The number of genomes of P. acnes in bronchoalveolar lavage (BAL) cells was correlated with the serum angiotensin-converting enzyme (ACE) level and the percentage of macrophages in BAL fluid from patients with sarcoidosis. No significant difference was detected between P. granulosum and controls14. A murine model of sarcoidosis pathogenesis was successfully developed using heat-killed propionibacteria by intratracheal challenge. This model demonstrated the contribution of toll-like receptor-1 (TLR1), -2 (TLR2) and -9 (TLR9) to the development of the polarized, Th-1 immune response15. Another study further confirmed the role of TLR2 in P. acnes-specific sarcoidosis immune responses by demonstrating that P. acnes-induced granulomatous pulmonary inflammation was markedly attenuated in TLR-2(−/−) mice compared to wild-type C57BL/6 animals16. A recent meta-analysis involving nine case-control studies of P. acnes associated with sarcoidosis revealed that a significantly elevated sarcoidosis risk (OR = 19.58, 95% CI = 13.06 – 29.36)17.
Investigations from independent laboratories worldwide have also reported molecular evidence supporting a significant association between mycobacteria and sarcoidosis. One study reported evidence of mycobacterial 16S rRNA or RNA polymerase B in 60% of the sarcoidosis granulomas and in none of the controls (p<0.00002, chi square)18. Sequence analysis of the 16S rRNA and rpoB amplicons revealed the presence of a novel Mycobacterium, genetically most similar to MTB complex (99% positional identity). Using matrix-assisted laser desorption/ionization time of flight mass spectrometry, Song et al found MTB katG peptides in 75% of sarcoidosis specimens compared to 14% of control specimens (p=0.0006); in situ hybridization localized MTB katG and 16S rRNA DNA to the inside of sarcoidosis granulomas19. Analysis of Polish sarcoidosis lymph nodes revealed MTB complex heat shock protein (hsp) 70, hsp65, and hsp16 20. Molecular analysis of American sarcoidosis granulomas also revealed the presence of nucleic acids of the mycobacterial virulence factor, superoxide dismutase A (sodA) in 70% of the sarcoidosis specimens, compared to 12% of controls (p=0.001). Sequence analysis of the amplicons demonstrated close positional identity with MTB complex, yet genetically distinct21. DNA of mycobacterial heat shock proteins has been detected in cutaneous lesions of Chinese sarcoidosis patients, but absent from control specimens. Sequence analysis was consistent with MTB, M. chelonae, and M. gordonae22. Another study reported the ability of real-time PCR analysis to quantitatively differentiate sarcoidosis from tuberculosis using Receiver-operating characteristic (ROC) curves23. Real-time PCR analysis from these independent laboratories demonstrates that if viable mycobacteria are present within the sarcoidosis granulomas, they are present below the sensitivity of the acid-fast bacilli (AFB) histologic stain21, 23. Future molecular efforts should delineate if the identified nucleic acids or proteins reflect actively replicating organisms or persistent proteins.
Immune responses against mycobacterial virulence factors are present in systemic and active sarcoidosis involvement
An equally important modality to delineate if infectious agents have a role in idiopathic disease is to assess for immune responses against microbial proteins. The presence of humoral and cellular responses against microbial antigens is an insightful method for assessing exposure to infectious agents. Increased lymphocyte proliferation induced by P. acnes has been reported in patients with active sarcoidosis; however, these responses did not correlate with clinical, roentgenographic, physiologic, and bronchoalveolar lavage findings in regards to disease severity24, 25. Sarcoidosis Th1 and Th17 immune responses against viable P. acnes that were significantly different from healthy controls was recently reported26.
Immune responses against mycobacteria have also been reported. Along with the detection of peptide fragments consistent with katG protein within sarcoidosis granulomas, the existence of humoral immune responses against mycobacterial katG proteins was demonstrated in sarcoidosis patients. Song et al noted IgG antibodies to recombinant MTB katG in sera from 48% of sarcoidosis patients compared to 0% in sera from PPD negative controls (p=0.0059)16. Sarcoidosis is characterized by polarized CD4+ T cells with a Th-1 immunophenotype. The identification of Th-1 CD4+ cellular immune responses against mycobacterial ESAT-6 and katG peptides in sarcoidosis peripheral blood mononuclear cells (PBMC) suggested that the sarcoidosis immune response may be against mycobacterial virulence factors27. Distinctions in cellular recognition patterns against virulence factors such as Antigen 85A (Ag85A) can differentiate mycobacterial species. For example, patients infected with MTB recognize distinct Ag85A peptides than those infected with M. leprae28. Further investigation of the sarcoidosis immune response pattern against Ag85A confirmed that the pattern detected was distinct from those in patients infected with MTB or M. leprae29. Another report demonstrated systemic CD4+ Th-1 immune responses against multiple mycobacterial virulence factors in sarcoidosis patients. These responses were not only against multiple secreted proteins, but also against multiple epitopes within a given protein30. These findings are more analogous with what is observed in patients with active bacterial infection.
A dual molecular and immunologic analysis of sarcoidosis specimens for the mycobacterial virulence factor, superoxide dismutase A, demonstrated nucleic acids sequences closest to MTB, yet distinct. Translation of those sequences into peptides to stimulate sarcoidosis PBMC resulted in reproduction of the sarcoidosis Th-1 immunophenotype21. Mycobacterial proteins such as sodA are virulence factors that confer pathogenicity to Mycobacterium species31. It has been demonstrated that the protein secretion system SecA2 is required for the optimal secretion of sodA and katG. Both of these proteins are synthesized without Sec signal sequences and function to detoxify reactive oxygen intermediates (ROI) generated by the host macrophage. SecA2 is part of a specialized secretion system that contributes to the virulence of pathogenic mycobacteria by countering the oxidative attack of the host, and confers their ability to survive within the host macrophage 32, 33.
In addition, CD4+ and CD8+ T cell immune responses against MTB katG have been detected in sarcoidosis BAL. Comparison of immune responses to mycobacterial katG whole protein between American and Swedish sarcoidosis subjects revealed no differences despite distinctions in patient phenotypic, genetic, and prognostic characteristics. It was also demonstrated that while Th-1 immune responses were present systemically, katG-reactive CD4(+) Th1 cells preferentially accumulated in the lung, indicating a compartmentalized response34. Patients with or without Löfgren’s syndrome had similar frequencies of katG specific IFNγ-expressing peripheral T cells. This study also demonstrated that circulating katG-reactive T cells were found in chronic active sarcoidosis but not in patients with inactive disease34. The loss of immune responses to mycobacterial virulence factors after resolution of tuberculosis has also been observed35. Another report demonstrated that immune responses against these mycobacterial virulence factors are present in sarcoidosis diagnostic BAL, and that induction of innate immunity by Toll-like receptor 2 contributes to the polarized Th1 immune response. Recognition was significantly absent from BAL fluid cells of patients with other lung diseases, including infectious granulomatous diseases36. The detection of immune responses against ESAT-6, katG, and sodA confirms exposure of sarcoidosis patients to a pathogenic mycobacterial species. These proteins are typically secreted during the stage of active mycobacterial replication, compared to expression of other proteins that are expressed when mycobacteria are in the latent state37, 38. The immunologic analysis performed to date provides a mechanism for more indepth analysis of sarcoidosis pathogenesis. These proteins can be used to delineate immunologic pathways that contribute to sarcoidosis resolution or disease progression.
Noninfectious etiologies of sarcoidosis
It has been reported that the amyloid precursor protein serum amyloid A (SAA) is strikingly abundant in sarcoidosis tissues, predominantly in a non-fibrillar form, and localized to epithelioid granulomas. SAA has been detected in numerous pulmonary infections, such as tuberculosis, nontuberculous mycobacteria (NTM) infection and leprosy39–42. By comparison, quantitative immunohistochemistry showed that the extent and distribution of SAA in sarcoidosis is significantly lower in other diseases of granulomatous inflammation43. Chen et al elaborate a concept of chronic stimulation of the innate immune system by disaggregated host protein serum amyloid A within granulomas following a microbial infection that induces a hyperimmune Th1 immune response to microbial antigens in the absence of ongoing infection SAA levels are reduced in pulmonary tuberculosis subjects following the initiation on antimicrobial therapy40. In addition, antibodies against autoantigens, such as zinc finger protein 688 and mitochondrial ribosomal protein L43, have been identified in sarcoidosis BAL and serum. High interindividual heterogeneity was noted45. Using pulmonary CD4+ T cells from 16 HLA-DRB1*0301+ patients, HLA-DR molecules were affinity purified and bound peptides acid eluted. The peptidies were separated by reversed-phase high performance liquid chromatography and analyzed by liquid chromatography-mass spectrometry, resulting in the identification of autoantigens such as vimentin and ATP synthase46, These data support that immune responses against self antigens are present in local and systemic sites of sarcoidosis subjects.
Microbial induction of sarcoidosis CD4+ T cell dysfunction
Investigation of sarcoidosis immune function upon T cell receptor stimulation reveals significant distinctions from healthy controls. The presence of chronic immune stimulation due to persistent microbial antigens has been reported to reduce T cell function. Sarcoidosis T lymphocytes have also been characterized by reduced cytokine expression and proliferative capacity, as well as upregulation of the inhibitory receptor, Programmed Death-1 (PD-1), all immunologic phenomena associated with elevated antigenic burdens.
As defined by Wherry and colleagues, T cell exhaustion occurs as a result of chronic antigen stimulation that, over the duration of antigen exposure, results in a gradual reduction in the cell’s ability to optimally respond to TCR stimulation. As such, while healthy T cells produce high levels of cytokine and exhibit high levels of proliferation and low levels of apoptosis in response to antigen, exhausted T cells gradually lose these normal functions until they can no longer respond to antigen and instead undergo apoptosis upon TCR activation. PD-1 upregulation on T cells plays a significant role in acquisition of the exhaustion phenotype. As an inhibitory coreceptor, signaling between PD-1 and its ligands, PDL1 and PDL2, functions to modulate tolerance to self antigens and limit the robustness of the adaptive immune response to foreign antigens. Exhausted T cells express high levels of PD-1 that correlates well with the systematic loss of cellular function. Recent findings that PD-1 is upregulated on dysfunctional sarcoidosis T cells, as well as the T cells of other granulomatous diseases characterized by microbial antigens, such as MTB47–49 and schistosomiasis50, suggesting that this phenotype could result from persistent antigen exposure.
Upregulation of the Programmed Death-1 (PD-1) receptor and reduced proliferative capacity in sarcoidosis bronchoalveolar lavage (BAL) and peripheral CD4+ T cells was recently reported51. Restoration of sarcoidosis CD4+ T cell proliferative capacity to healthy control levels was apparent after PD-1 pathway blockade51. Various mechanisms by which PD-1 interferes with T cell proliferation have been well described. PD-1 has been reported to inhibit CD4+ T cell proliferation by blocking cell cycle progression through the suppression of Skp2 transcription52, 53. Skp2 is the substrate recognition component of the ubiquitin ligase complex SCFSkp2 that binds to and degrades p27kip1, a cdk inhibitor, thereby allowing continuation of the cell cycle. PD-1 cell cycle impediment, and therefore proliferation hindrance, has been shown to be the result of PI3K/Akt and ERK pathway inactivation52, 53. PD-1 inhibition of T cell proliferation has been correlated with increased p27 availability and repression of Cdc25A, a cdk-activating phosphatase52, 53. PD-1 engagement has also been demonstrated to attenuate T cell receptor (TCR) signaling by preventing ZAP70 and PKCθ activation54.
The reported upregulation of PD-1 is particularly important as it has been associated with the emergence of human lymphotropic viruses, such as Epstein-Barr virus (EBV) and cytomegalovirus (CMV)55–57. These same viruses have been associated with sarcoidosis pathogenesis58, 59.
Clinical trials of antimicrobial therapy in sarcoidosis
Following the publication of molecular and immunologic support for a role of microorganisms in sarcoidosis pathogenesis, such as fungi, propionibacteria and mycobacteria, there has been an increasing number of case reports and clinical trials regarding efficacy with antimicrobial therapy. Numerous prior reports of the tetracyclines, particularly doxycycline and minocycline, have been published in subjects with cutaneous sarcoidosis60–62. Although minocycline has antimicrobial effects against Propionibacterium acnes, its mechanism of action is felt to be immunomodulatory63. A recent report of the efficacy of clarithromycin, which has efficacy against propionibacteria and mycobacteria, was reported in a Japanese female with systemic sarcoidosis64. Conclusive delineation of the mechanism of action is pending.
Fungal antigens are also reported to contribute to sarcoidosis pathogenesis65. Reports of clinical and radiographic improvement following administration of antifungal therapy such as posaconazole 300mg/d or ketoconazole 200mg/d with or without corticosteroids have been reported in Slovenic sarcoidosis patients. The authors conducted an open-labelled, patient-preference trial of steroids (methylprednisolone 0.4mg/kg, antifungal agents (posaconazole 300mg/d or ketoconazole 200mg/d) or steroids/antifungal agents. The most significant clinical radiographic improvement was detected in the antifungal group; they also reported a reduction in disease recurrence among those on antifungal therapy. Study limitations include the lack of randomization, as well as not being conducted in a double blind fashion.
Two clinical trials regarding the efficacy of antimycobacterial therapy in sarcoidosis pathogenesis have been reported. A double blind, placebo-controlled investigation of an antimycobacterial regimen consisting of concomitant Levaquin, Ethambutol, Azithromycin and Rifampin (CLEAR) compared to placebo was conducted in subjects with cutaneous sarcoidosis. In the intention-to-treat analysis, the CLEAR-treated group had a mean (SD) decrease in lesion diameter of −8.4 (14.0) mm compared with an increase of 0.07 (3.2) mm in the placebo-treated group (P = .05). The CLEAR group had a significant reduction in granuloma burden and experienced a mean (SD) decline of −2.9 (2.5) mm in lesion severity compared with a decline of −0.6 (2.1) mm in the placebo group (P = .02). The observed clinical reductions were present at the 180-day follow-up period. Transcriptome analysis of sarcoidosis CD4+ T cells revealed reversal of pathways associated with disease severity and enhanced T-cell function following T-cell receptor stimulation66.
In addition, an open-label investigation of this same regimen was conducted in pulmonary sarcoidosis subjects. Fifteen chronic, pulmonary sarcoidosis patients with forced vital capacities (FVC) between 45–80% of predicted were enrolled. The primary efficacy endpoint was change in absolute FVC from baseline to completion of therapy. Secondary endpoints were change in functional capacity measured by Six Minute Walk Distance (6MWD) and quality of life assessment measured by St. George’s Respiratory Questionnaire (SGRQ). Of 15 patients enrolled, 11 completed 4 weeks of therapy, and 8 completed 8 weeks of therapy. The CLEAR regimen was associated with an FVC increase of 0.23 liters at 4 weeks and 0.42 liters at 8 weeks (P=0.0098 and 0.016, respectively). The 6MWD increased by 87 meters from baseline to 8 weeks (p=0.0078). The mean score of the validated SGRQ was improved at 8 weeks over baseline (p=0.023)67.
These early trials are promising. Future investigation of the mechanisms by which the antimicrobials work—as antimicrobials, immune modulators or both—is warranted.
Summary
Recent molecular, genetic, and immunologic studies from independent laboratories support an association with sarcoidosis and microbial antigens, particularly mycobacteria or propionibacteria. The findings among American sarcoidosis subjects are most strongly associated with mycobacteria, and among Japanese sarcoidosis subjects, propionibacteria. Because epidemiologic studies indicate that both sarcoidosis morbidity and mortality is increasing68, the impetus on current sarcoidosis researchers is to translate their strong basic research investigations into innovative therapeutics that will impact sarcoidosis pathogenesis and hopefully lead to a cure. The progress to date strongly supports advances toward this goal.
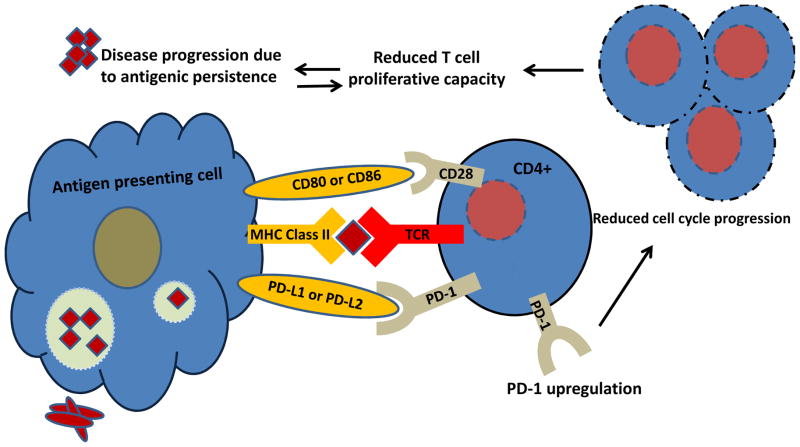
Figure 1. PD-1 inhibits sarcoidosis cellular proliferation.
High antigenic loads induce PD-1 upregulation, which alters cell cycle progression. Cell cycle progression is necessary for normal CD4+ T cell proliferation to clear microbial or autoantigens, thus leading to clinical resolution. Persistent antigen further PD-1 upregulation and loss of cellular function.
Table 1.
Evidence for Etiologic Agents in Sarcoidosis pathogenesis
| Etiology | Evidence |
|---|---|
| Mycobacteria | M, I, E 18–22,27,29,30 |
| Propionibacteria | M, I13–15, 24–26 |
| Fungal Antigens | M 65 |
| Autoantigens | M, I 45,46 |
M=Molecular; I=Immu no lo gic; E=Epidemiologic
Key Points.
There is a growing body of literature supporting the role of infectious antigens, particularly mycobacteria and propionibacteria, in sarcoidosis pathogenesis.
Immunologic studies reveal that mycobacterial virulence factors are the targets of the immune response in sarcoidosis diagnostic bronchoalveolar lavage.
Recently, case reports and clinical trials have emerged reporting the efficacy of antimicrobial therapy on cutaneous and pulmonary sarcoidosis. While the studies are not conclusive, they demonstrate efficacy on endpoints associated with sarcoidosis morbidity and mortality, such as forced vital capacity.
Acknowledgments
This work was supported by National Institutes of Health grants (T32 HL069765 to L.J.C.; T32 HL094296 to C.H.; R01 HL117074, U01 112694 to W.P.D).
Footnotes
Drs. Celada and Hawkins have no conflicts of interest to disclose. Dr. Drake serves as a scientific advisor for Celgene.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Baughman RP. Sarcoidosis. Clin Dermatol. 2007;25(3):231. doi: 10.1016/j.clindermatol.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170(12):1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 3.Kajdasz DK, Judson MA, Mohr LC, Jr, Lackland DT. Geographic variation in sarcoidosis in South Carolina: its relation to socioeconomic status and health care indicators. Am J Epidemiol. 1999;150(3):271–278. doi: 10.1093/oxfordjournals.aje.a009998. [DOI] [PubMed] [Google Scholar]
- 4.Falkinham JO., III Nontuberculous mycobacteria in the environment. Clin Chest Med. 2002;23(3):529–551. doi: 10.1016/s0272-5231(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 5.Hanak V, Kalra S, Aksamit TR, Hartman TE, Tazelaar HD, Ryu JH. Hot tub lung: presenting features and clinical course of 21 patients. Respir Med. 2006;100(4):610–615. doi: 10.1016/j.rmed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 6.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3(8):e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bik EM, Eckburg PB, Gill SR, et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A. 2006;103(3):732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitley R. The new age of molecular diagnostics for microbial agents. N Engl J Med. 2008;358(10):988–989. doi: 10.1056/NEJMp0708085. [DOI] [PubMed] [Google Scholar]
- 9.Palacios G, Druce J, Du L, et al. A new arenavirus in a cluster of fatal transplant-associated diseases. N Engl J Med. 2008;358(10):991–998. doi: 10.1056/NEJMoa073785. [DOI] [PubMed] [Google Scholar]
- 10.Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med. 1992;327(5):293–301. doi: 10.1056/NEJM199207303270501. [DOI] [PubMed] [Google Scholar]
- 11.Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 12.Garzoni C, Brugger SD, Qi W, et al. Microbial communities in the respiratory tract of patients with interstitial lung disease. Thorax. 2013;68(12):1150–1156. doi: 10.1136/thoraxjnl-2012-202917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishige I, Eishi Y, Takemura T, et al. Propionibacterium acnes is the most common bacterium commensal in peripheral lung tissue and mediastinal lymph nodes from subjects without sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22(1):33–42. [PubMed] [Google Scholar]
- 14.Ichikawa H, Kataoka M, Hiramatsu J, et al. Quantitative analysis of propionibacterial DNA in bronchoalveolar lavage cells from patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2008;25(1):15–20. [PubMed] [Google Scholar]
- 15.McCaskill JG, Chason KD, Hua X, et al. Pulmonary immune responses to Propionibacterium acnes in C57BL/6 and BALB/c mice. Am J Respir Cell Mol Biol. 2006;35(3):347–356. doi: 10.1165/rcmb.2005-0285OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabrilovich MI, Walrath J, van LJ, et al. Disordered Toll-like receptor 2 responses in the pathogenesis of pulmonary sarcoidosis. Clin Exp Immunol. 2013;173(3):512–522. doi: 10.1111/cei.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Hu Y, Li H. Role of Propionibacterium Acnes in Sarcoidosis: A Meta-analysis. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30(4):262–267. [PubMed] [Google Scholar]
- 18.Drake WP, Pei Z, Pride DT, Collins RD, Cover TL, Blaser MJ. Molecular analysis of sarcoidosis tissues for mycobacterium species DNA. Emerg Infect Dis. 2002;8(11):1334–1341. doi: 10.3201/eid0811.020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song Z, Marzilli L, Greenlee BM, et al. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med. 2005;201(5):755–767. doi: 10.1084/jem.20040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubaniewicz A, Dubaniewicz-Wybieralska M, Sternau A, et al. Mycobacterium tuberculosis complex and mycobacterial heat shock proteins in lymph node tissue from patients with pulmonary sarcoidosis. J Clin Microbiol. 2006;44(9):3448–3451. doi: 10.1128/JCM.01433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen SS, Evans W, Carlisle J, et al. Superoxide dismutase A antigens derived from molecular analysis of sarcoidosis granulomas elicit systemic Th-1 immune responses. Respir Res. 2008;9:36. doi: 10.1186/1465-9921-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding XL, Cai L, Zhang JZ. Detection and identification of mycobacterial gene in skin lesions and lymph nodes in patients with sarcoidosis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2009;31(1):20–23. [PubMed] [Google Scholar]
- 23.Zhou Y, Li HP, Li QH, et al. Differentiation of sarcoidosis from tuberculosis using real-time PCR assay for the detection and quantification of Mycobacterium tuberculosis. Sarcoidosis Vasc Diffuse Lung Dis. 2008;25(2):93–99. [PubMed] [Google Scholar]
- 24.Nakata Y, Ejiri T, Kishi T, et al. Alveolar lymphocyte proliferation induced by Propionibacterium acnes in sarcoidosis patients. Acta Med Okayama. 1986;40(5):257–264. doi: 10.18926/AMO/31929. [DOI] [PubMed] [Google Scholar]
- 25.Nakata Y, Ejiri T, Kishi T, et al. Alveolar lymphocyte proliferation in sarcoidosis patients induced by Propionibacterium acnes. Nihon Kyobu Shikkan Gakkai Zasshi. 1985;23(4):413–419. [PubMed] [Google Scholar]
- 26.Furusawa H, Suzuki Y, Miyazaki Y, Inase N, Eishi Y. Th1 and Th17 immune responses to viable Propionibacterium acnes in patients with sarcoidosis. Respir Investig. 2012;50(3):104–109. doi: 10.1016/j.resinv.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Drake WP, Dhason MS, Nadaf M, et al. Cellular recognition of Mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis. Infect Immun. 2007;75(1):527–530. doi: 10.1128/IAI.00732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Launois P, DeLeys R, Niang MN, et al. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect Immun. 1994;62(9):3679–3687. doi: 10.1128/iai.62.9.3679-3687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajizadeh R, Sato H, Carlisle J, et al. Mycobacterium tuberculosis Antigen 85A induces Th-1 immune responses in systemic sarcoidosis. J Clin Immunol. 2007;27(4):445–454. doi: 10.1007/s10875-007-9080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlisle J, Evans W, Hajizadeh R, et al. Multiple Mycobacterium antigens induce interferon-gamma production from sarcoidosis peripheral blood mononuclear cells. Clin Exp Immunol. 2007;150(3):460–468. doi: 10.1111/j.1365-2249.2007.03510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards KM, Cynamon MH, Voladri RK, et al. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2001;164(12):2213–2219. doi: 10.1164/ajrccm.164.12.2106093. [DOI] [PubMed] [Google Scholar]
- 32.Rigel NW, Gibbons HS, McCann JR, McDonough JA, Kurtz S, Braunstein M. The Accessory SecA2 System of Mycobacteria Requires ATP Binding and the Canonical SecA1. J Biol Chem. 2009;284(15):9927–9936. doi: 10.1074/jbc.M900325200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braunstein M, Espinosa BJ, Chan J, Belisle JT, Jacobs WR., Jr SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol Microbiol. 2003;48(2):453–464. doi: 10.1046/j.1365-2958.2003.03438.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen ES, Wahlstrom J, Song Z, et al. T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol. 2008;181(12):8784–8796. doi: 10.4049/jimmunol.181.12.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pathan AA, Wilkinson KA, Klenerman P, et al. Direct ex vivo analysis of antigen-specific IFN-gammasecreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol. 2001;167(9):5217–5225. doi: 10.4049/jimmunol.167.9.5217. [DOI] [PubMed] [Google Scholar]
- 36.Oswald-Richter KA, Culver DA, Hawkins C, et al. Cellular responses to mycobacterial antigens are present in bronchoalveolar lavage fluid used in the diagnosis of sarcoidosis. Infect Immun. 2009;77(9):3740–3748. doi: 10.1128/IAI.00142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersen P. The T cell response to secreted antigens of Mycobacterium tuberculosis. Immunobiology. 1994;191(4–5):537–547. doi: 10.1016/S0171-2985(11)80460-2. [DOI] [PubMed] [Google Scholar]
- 38.Andersen P, Askgaard D, Ljungqvist L, Bentzon MW, Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991;59(4):1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Essone PN, Chegou NN, Loxton AG, et al. Host cytokine responses induced after overnight stimulation with novel M. tuberculosis infection phase-dependent antigens show promise as diagnostic candidates for TB disease. PLoS One. 2014;9(7):e102584. doi: 10.1371/journal.pone.0102584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Beer FC, Nel AE, Gie RP, Donald PR, Strachan AF. Serum amyloid A protein and C-reactive protein levels in pulmonary tuberculosis: relationship to amyloidosis. Thorax. 1984;39(3):196–200. doi: 10.1136/thx.39.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinozuka N, Kasamatsu N, Seto T, Yasui T, Nakamura A, Hashizume I. A fatal case of pulmonary non-tuberculous mycobacteriosis with reactive AA amyloidosis. Nihon Kokyuki Gakkai Zasshi. 2007;45(8):636–642. [PubMed] [Google Scholar]
- 42.McAdam KP, Foss NT, Garcia C, et al. Amyloidosis and the serum amyloid A protein response to muramyl dipeptide analogs and different mycobacterial species. Infect Immun. 1983;39(3):1147–1154. doi: 10.1128/iai.39.3.1147-1154.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen ES, Moller DR. Etiologic role of infectious agents. Semin Respir Crit Care Med. 2014;35(3):285–295. doi: 10.1055/s-0034-1376859. [DOI] [PubMed] [Google Scholar]
- 44.Chen ES, Moller DR. Etiologies of Sarcoidosis. Clin Rev Allergy Immunol. 2015 doi: 10.1007/s12016-015-8481-z. [DOI] [PubMed] [Google Scholar]
- 45.Haggmark A, Hamsten C, Wiklundh E, et al. Proteomic profiling reveals autoimmune targets in sarcoidosis. Am J Respir Crit Care Med. 2015;191(5):574–583. doi: 10.1164/rccm.201407-1341OC. [DOI] [PubMed] [Google Scholar]
- 46.Wahlstrom J, Dengjel J, Persson B, et al. Identification of HLA-DR-bound peptides presented by human bronchoalveolar lavage cells in sarcoidosis. J Clin Invest. 2007;117(11):3576–3582. doi: 10.1172/JCI32401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh A, Dey AB, Mohan A, Mitra DK. Programmed death-1 receptor suppresses gamma-IFN producing NKT cells in human tuberculosis. Tuberculosis (Edinb ) 2014;94(3):197–206. doi: 10.1016/j.tube.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Singh A, Mohan A, Dey AB, Mitra DK. Inhibiting the programmed death 1 pathway rescues Mycobacterium tuberculosis-specific interferon gamma-producing T cells from apoptosis in patients with pulmonary tuberculosis. J Infect Dis. 2013;208(4):603–615. doi: 10.1093/infdis/jit206. [DOI] [PubMed] [Google Scholar]
- 49.Henao-Tamayo M, Irwin SM, Shang S, Ordway D, Orme IM. T lymphocyte surface expression of exhaustion markers as biomarkers of the efficacy of chemotherapy for tuberculosis. Tuberculosis (Edinb ) 2011;91(4):308–313. doi: 10.1016/j.tube.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colley DG, Sasser LE, Reed AM. PD-L2+ dendritic cells and PD-1+ CD4+ T cells in schistosomiasis correlate with morbidity. Parasite Immunol. 2005;27(1–2):45–53. doi: 10.1111/j.1365-3024.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 51.Braun NA, Celada LJ, Herazo-Maya JD, et al. Blockade of the programmed death-1 pathway restores sarcoidosis CD4(+) T-cell proliferative capacity. Am J Respir Crit Care Med. 2014;190(5):560–571. doi: 10.1164/rccm.201401-0188OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patsoukis N, Sari D, Boussiotis VA. PD-1 inhibits T cell proliferation by upregulating p27 and p15 and suppressing Cdc25A. Cell Cycle. 2012;11(23):4305–4309. doi: 10.4161/cc.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5(230):ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheppard KA, Fitz LJ, Lee JM, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574(1–3):37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 55.Kato T, Nishida T, Ito Y, Murase M, Murata M, Naoe T. Correlations of programmed death 1 expression and serum IL-6 level with exhaustion of cytomegalovirus-specific T cells after allogeneic hematopoietic stem cell transplantation. Cell Immunol. 2014;288(1–2):53–59. doi: 10.1016/j.cellimm.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 56.Dirks J, Tas H, Schmidt T, et al. PD-1 analysis on CD28(-) CD27(-) CD4 T cells allows stimulationindependent assessment of CMV viremic episodes in transplant recipients. Am J Transplant. 2013;13(12):3132–3141. doi: 10.1111/ajt.12480. [DOI] [PubMed] [Google Scholar]
- 57.Sester U, Presser D, Dirks J, Gartner BC, Kohler H, Sester M. PD-1 expression and IL-2 loss of cytomegalovirus-specific T cells correlates with viremia and reversible functional anergy. Am J Transplant. 2008;8(7):1486–1497. doi: 10.1111/j.1600-6143.2008.02279.x. [DOI] [PubMed] [Google Scholar]
- 58.Yonemaru M, Kasuga I, Kusumoto H, et al. Elevation of antibodies to cytomegalovirus and other herpes viruses in pulmonary fibrosis. Eur Respir J. 1997;10(9):2040–2045. doi: 10.1183/09031936.97.10092040. [DOI] [PubMed] [Google Scholar]
- 59.Rottoli P, Bianchi Bandinelli ML, Rottoli L, Zazzi M, Panzardi G, Valensin PE. Sarcoidosis and infections by human lymphotropic viruses. Sarcoidosis. 1990;7(1):31–33. [PubMed] [Google Scholar]
- 60.Sheu J, Saavedra AP, Mostaghimi A. Rapid response of tattoo-associated cutaneous sarcoidosis to minocycline: case report and review of the literature. Dermatol Online J. 2014;20(8) [PubMed] [Google Scholar]
- 61.Steen T, English JC. Oral minocycline in treatment of cutaneous sarcoidosis. JAMA Dermatol. 2013;149(6):758–760. doi: 10.1001/jamadermatol.2013.2977. [DOI] [PubMed] [Google Scholar]
- 62.Baba K, Yamaguchi E, Matsui S, et al. A case of sarcoidosis with multiple endobronchial mass lesions that disappeared with antibiotics. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23(1):78–79. [PubMed] [Google Scholar]
- 63.Miyazaki E, Ando M, Fukami T, Nureki S, Eishi Y, Kumamoto T. Minocycline for the treatment of sarcoidosis: is the mechanism of action immunomodulating or antimicrobial effect? Clin Rheumatol. 2008;27(9):1195–1197. doi: 10.1007/s10067-008-0903-3. [DOI] [PubMed] [Google Scholar]
- 64.Takemori N, Nakamura M, Kojima M, Eishi Y. Successful treatment in a case of Propionibacterium acnes-associated sarcoidosis with clarithromycin administration: a case report. J Med Case Rep. 2014;8:15. doi: 10.1186/1752-1947-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tercelj M, Stopinsek S, Ihan A, et al. In vitro and in vivo reactivity to fungal cell wall agents in sarcoidosis. Clin Exp Immunol. 2011;166(1):87–93. doi: 10.1111/j.1365-2249.2011.04456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. JAMA Dermatol. 2013;149(9):1040–1049. doi: 10.1001/jamadermatol.2013.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Drake W, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30(3):201–211. [PMC free article] [PubMed] [Google Scholar]
- 68.Swigris JJ, Olson AL, Huie TJ, et al. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183(11):1524–1530. doi: 10.1164/rccm.201010-1679OC. [DOI] [PMC free article] [PubMed] [Google Scholar]