Abstract
With no approved pharmacological treatment, non-alcoholic fatty liver disease (NAFLD) is now the most common cause of chronic liver disease in western countries and its worldwide prevalence continues to increase along with the growing obesity epidemic. Here we show that a high-fat high-sucrose (HFHS) diet, eliciting chronic hepatosteatosis resembling human fatty liver, lowers hepatic NAD+ levels driving reductions in hepatic mitochondrial content, function and ATP levels, in conjunction with robust increases in hepatic weight, lipid content and peroxidation in C57BL/6J mice. In an effort to assess the effect of NAD+ repletion on the development of steatosis in mice, nicotinamide riboside (NR), a precursor for NAD+ biosynthesis, was given to mice concomitant, as preventive strategy (NR-Prev), and as a therapeutic intervention (NR-Ther), to a HFHS diet. We demonstrate that NR prevents and reverts NAFLD by inducing a SIRT1- and SIRT3-dependent mitochondrial unfolded protein response (UPRmt), triggering an adaptive mitohormetic pathway to increase hepatic β-oxidation and mitochondrial complex content and activity. The cell-autonomous beneficial component of NR treatment was revealed in liver-specific Sirt1 KO mice (Sirt1hep−/−), while Apolipoprotein E-deficient (Apoe−/−) mice challenged with a high-fat high-cholesterol diet (HFC), affirmed the use of NR in other independent models of NAFLD. Conclusion: Our data warrant the future evaluation of NAD+ boosting strategies to manage the development or progression of NAFLD.
Keywords: non-alcoholic fatty liver disease (NAFLD), nicotinamide riboside (NR), sirtuins, poly(ADP-ribose) polymerases (PARPs), mitonuclear protein imbalance
NAFLD encompasses a disease spectrum that can progress from hepatic steatosis to non-alcoholic steatohepatitis (NASH), fibrosis, and finally cirrhosis (1), and is projected to become the most common indication leading to liver transplantation in the United States by 2030 (2). Unfortunately, there are few existing therapies for NAFLD and NASH (3, 4), which are also suboptimal, hence the urge to find new preventive and therapeutic strategies to manage these chronic hepatic diseases. Often alterations in lipid homeostasis, associated with obesity or insulin resistance, result in the increase of lipolysis in fat and delivery of free fatty acids to the liver, favoring hepatic lipogenesis (5). In addition, incomplete fatty acid (FA) oxidation in the context of mitochondrial dysfunction can also contribute to hepatic FA and lipid overload (reviewed in (6)). With prolonged existence, these alterations in lipid homeostasis can promote oxidative stress and lipid peroxidation in the liver, leading to elevated cytokine production, inflammation and fibrosis. It is this distinction that divides the appearance of a fatty liver with that of the appearance of NASH and eventually hepatocellular carcinoma (7, 8). As the generation of oxidative stress is one of the key mediators of this disease, the pursuit of treatments to improve the dysfunctional state of liver mitochondria has become a prime goal to alleviate the pathophysiology of NASH.
Recent evidence demonstrates that NAD+ repletion protects organisms from metabolic diseases, induced by genetic factors, diet or aging (9-14). These findings corroborate the role of sirtuins, many of which rely on NAD+ as a cosubstrate, in eliciting metabolic improvements in various tissues (reviewed in (15)). However, despite the rapidly advancing field, the role of NAD+ in the development and protection from NAFLD is still unspecified. Furthermore, the influence of NAD+ repletion on mice with preexisting liver hyperlipidemia and aberrant lipid metabolism has yet to be determined.
Several approaches have been recently explored to elevate NAD+ levels in vivo. This includes the use of natural NAD+ precursors, such as NR, that are readily converted to bioavailable NAD+ in metabolic tissues following dietary administration (16). In addition to NR, there are three other molecules described as root substrates for mammalian NAD+ biosynthetic pathways, including tryptophan, niacin and nicotinamide (15). Once NR is transported into cells by nucleoside transporters (17) it is phosphorylated by the NR kinases 1 and 2 (18). The phosphorylation of NR generates nicotinamide mononucleotide, an intermediate compound of NAD+ biosynthesis, which can also be intraperitoneally administered to animals to increase tissue NAD+ levels (11). This metabolite can then be converted to NAD+ through the action of NMN adenylyltransferase (NMNAT).
In this study, we demonstrate that a western diet, including high levels of fat and sucrose, could induce dysfunction in hepatic NAD+ homeostasis and contribute to the development of NAFLD, and that NAD+ repletion by feeding animals with a diet enriched in NR may prevent or reverse NAFLD by inducing the UPRmt to elicit a mitohormetic response. We further confirmed that NAD+ repletion can reverse liver mitochondrial dysfunction in HFC fed Apoe−/− mice, another well-known model of NAFLD (19).
Materials and Methods
Animal Experiments
Male C57BL/6J mice were purchased from Charles River and were housed under a 14hr light, 10hr dark cycle at 21-23°C and had ad libitum access to water and food throughout the experiment. From the age of 8 weeks, mice were separated into 4 groups of 10 animals. Animal cohorts were fed either a ‘Western’ high-fat and high-sucrose (HFHS) diet with 44.6% of kcal derived from fat (of which 61% is derived from saturated fatty acids) and 40.6% of kcal derived from carbohydrates (primarily sucrose 340 g/kg diet) (TD.08811, 45%kcal Fat Diet, Harlan Laboratories Inc., Madison, WI, USA) (20) or normal chow diet (CD). NR treated animals were fed with pellets containing vehicle (double distilled water; ddH20) or NR (400 mg/kg/day) for 2, 9 or 18 weeks. All animal experiments were carried according to national Swiss and EU ethical guidelines and approved by the local animal experimentation committee of the Canton de Vaud under license #2465.
Generation of Sirt1L2/L2 mice has been previously described (21). Liver-specific Sirt1 knockout mice were generated by breeding Sirt1L2/L2 mice with mouse albumin (Alb)-Cre mice (AlbcreTg/0) (22), both of which have been and backcrossed to C57BL/6J mice for ten generations. These mice lines were then further intercrossed to generate mutant AlbcreTg/0/Sirt1L2/L2 mice, which were termed Sirt1hep−/− mice. The following Sirt1hep−/− and Sirt1L2/L2 mice were fed with HFHS pellets containing vehicle (double distilled water; ddH20) or NR (400 mg/kg/day) for 14 weeks.
Six-week-old male Apoe−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and randomly assigned to modified AIN-93M diets (Dyets, Inc.) that included low fat (Apoe−/−/LF), high-fat and high-cholesterol (Apoe−/−/HFC), and HFC supplemented with NR (Apoe−/−/NR-Ther) (see Table S1). Seven-week-old mice were placed on either LF (4% fat by weight) or HFC (22% fat, 0.2% cholesterol by weight) diets for three weeks to induce aberrant lipid metabolism. At ten weeks of age, 500 mg/kg/day (mpk) of NR was supplemented to the assigned HFC diet group for seven weeks. All procedures concerning Apoe−/− mice were approved by the Animal Care and Use Committee (IACUC) at the University of Connecticut.
The procedures used for NR food preparation, along with in vivo mouse phenotyping and sacrifices are described in the Supporting Methods.
Primary Hepatocytes
Primary hepatocytes from Sirt1 floxed (Sirt1L2/L2) or Sirt3 floxed (Sirt3L2/L2) mice (described in (21) and (23), respectively) were isolated with Liberase Blendzyme (Roche) perfusion as described previously with minor modifications (24). Isolated hepatocytes were then plated in DMEM (Gibco; 4.5 g/l glucose) with 10% FBS and penicillin and streptomycin and maintained at 37°C in a 5% CO2 atmosphere. Four hours after plating, cells were transduced with Ad-GFP or an Ad-Cre virus at a MOI = 5 to generate matched Sirt1−/− or Sirt3−/− (loss-of-function) and Sirt1L2/L2 or Sirt3L2/L2 wild-type hepatocytes, respectively. Hepatocytes were then incubated overnight and before changing media. Primary hepatocytes were then treated with 1mM NR or vehicle (ddH20) for 24 hrs.
Histology and liver function
Preparation of histological tissue sections, staining procedures for Hematoxylin and eosin (H&E), Oil Red O, picrosirius red, cytochrome c oxidase activity, succinate dehydrogenase activity, CD45, Masson’s trichrome and blinded scoring for steatosis are described in the Supporting Methods. Mitochondrial function in fresh liver tissue was evaluated with high-resolution respirometry and citrate synthase activity, as also described in the Supporting Methods.
Quantification of NAD+ and ATP levels
NAD+ was extracted using acidic and alkaline extraction methods, respectively. Tissue NAD+ was analyzed with mass spectrometry as previously described (25). Total ATP content was measured by the CellTiter-Glo luminescent cell viability assays (Promega). Typically, the luminescence was recorded with a Victor X4 plate reader (PerkinElmer) and values are normalized by the total protein concentration determined using a Bradford assay.
Liver triglyceride, cholesterol and lipid peroxidation measurements
Hepatic lipids were extracted as performed previously (24). Triglyceride and cholesterol contents in hepatic lipid fractions were quantified using enzymatic assays (Roche). The byproduct of lipid peroxidation and a marker of oxidative stress, 4-Hydroxynonenal (HNE), was measured following the manufacturer’s protocol of the OxiSelect HNE-His Adduct ELISA Kit, (Cell Biolabs Inc., San Diego, CA, USA).
Identification of transcript correlations in mice and humans
Liver microarray data (Affymetrix Mouse Gene 1.0 ST) from a BXD mouse genetic reference population (26) were analyzed for transcript expression correlations between NAD+ regulating and β-oxidation genes using the GeneNetwork program (http://www.genenetwork.org). Raw microarray data are also publicly available on GEO (http://www.ncbi.nlm.nih.gov/geo) under the accession number GSE60149. Similarly, human microarray data was retrieved from the GSE48654 (27) and GSE9588 (28) GEO data sets.
Transcript and protein expression
Real-time quantitative polymerase chain reaction (RT-qPCR), mtDNA content, western immunoblotting and Blue-Native Page techniques are described in the Supplementary Methods.
Statistical Analysis
Statistical analysis was performed with Prism Software version 6.0 (GraphPad, La Jolla, CA). The significance of differences between two groups was determined by unpaired two-tailed Student t test. For comparison of multiple groups, we applied one-way ANOVA with a post-hoc Bonferroni test. Results are presented as mean ± SEM. A P value < 0.05 was considered significant.
Results
Regulation of NAD+ homeostasis and lipid metabolism
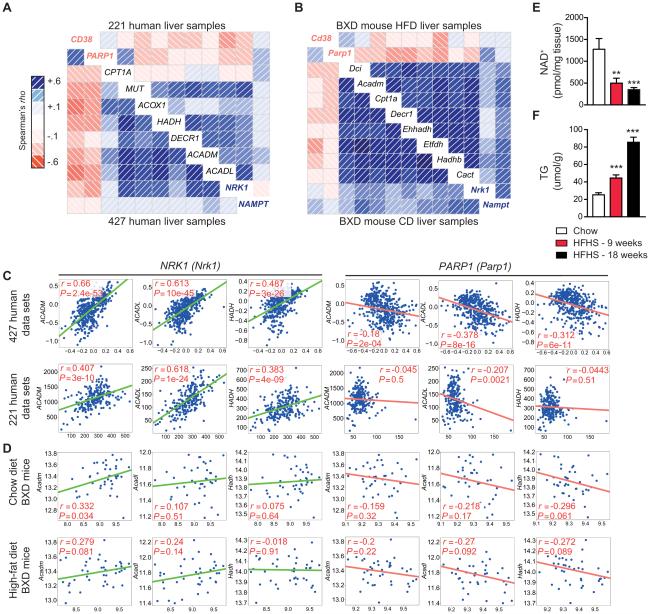
Exploring the potential for NAD+ dysfunction in the development and progression of NAFLD, we first evaluated gene expression patterns related to NAD+ homeostasis and lipid metabolism in two separate existing liver tissue data sets that include 221 (27) and 427 (28) normal human samples. These data sets conveyed a positive correlation between custom designed gene-sets for β-oxidation and genes involved in NAD+ biosynthesis (Fig. 1A, C). Specifically, transcripts for nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme for NAD+ salvage from nicotinamide (NAM), and NRK1 correlated positively to β-oxidation genes, suggesting a beneficial effect of NAD+-synthesis on NAFLD. In contrast, negative correlations were seen when compared to NAD+ consumers, such as poly ADP-ribose (PAR) polymerase 1 (PARP1), and the cADP-ribose synthase, CD38. These correlations were conserved in livers of genetically-diverse strains of mice from the BXD genetic reference population fed either chow or high-fat diets (described in (9, 26)) (Fig. 1B, D). A unique custom designed gene-set was also created for the comparison of matching genes between the human and mouse datasets (Fig. S1A). To experimentally corroborate this link observed in human and mice, we examined a mouse model of NAFLD (20). Mice given a HFHS diet displayed a progressive loss of liver NAD+ levels that was matched by stepwise increases in liver triglyceride levels, linking changes in NAD+ homeostasis to liver metabolism (Fig. 1E-F). Thus, NAD+ regulating enzymes, reflecting more NAD+ availability, appear to correlate with lipid metabolism in both human and mouse liver samples.
Fig. 1.
NAD+ regulating enzymes and NAD+ levels correlate with lipid metabolism in both humans and mice. Transcripts from NAD+-synthesis genes (shown in blue font), in contrast to NAD+-consuming genes (shown in red font), were positively correlated with regulators of β-oxidation using a custom designed data-set derived from (A) two human data sets, including 427 (28) and 220 (27) human liver samples or (B) two mouse data sets, including 42 BXD strains fed either chow or high-fat diets (9, 26). As seen on a correlogram, blue correlations are positive (red correlations are negative – intensity of the colors correlates with level of significance). NRK1 and NAMPT transcripts, in contrast to Parp1, were positively correlated to mitochondrial β-oxidation genes, ACADL, ACADM and HADH, in data sets from (A, C) 427 (28) and 220 (27) human liver samples or 42 BXD strains fed either (B, D) chow or high-fat diets (9, 26) Consistent with these findings in humans, we see reductions in hepatic (E) NAD+ levels, followed by increases in (F) hepatic triglyceride levels, in mice fed 9- to 18-weeks with a HFHS diet (n=5-10). *P < 0.05, **P < 0.001, ***P < 0.0001 compared to the CD cohort. Data are expressed as mean ± s.e.m. One-way ANOVA with a post-hoc Bonferroni test was used for all statistical analyses.
NAD+ repletion, as a therapeutic treatment, can revert indications of NASH in HFHS-fed mice
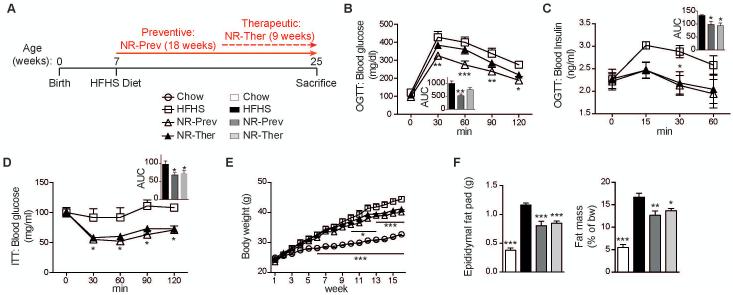
To evaluate the potential of NAD+ repletion to protect mice from the development of NAFLD, we compared 7-week old mice exposed to 18 weeks of HFHS diet to mice given the same diet supplemented with NR (400 mg/kg/day) from the onset of the HFHS diet (prevention mode) or 9 weeks after the start on the HFHS (therapeutic/intervention mode) (Fig. 2A). Importantly, both prevention and therapeutic approaches improved glucose tolerance (Fig. 2B-C) and insulin sensitivity (Fig. 2D) with concurrent reductions in body weight (Fig. 2E) and fat mass (Fig. 2F), each being frequently measured as secondary outcomes in NAFLD-related clinical trials.
Fig. 2.
Glucose intolerance and NAFLD-induced insulin sensitivity are reversed with NAD+ repletion. Phenotyping was performed on CD, HFHS, NR-Prev and NR-Ther cohorts following 5-18-weeks of treatment (NR dose: 400 mg/kg/day). (A) Schematic illustrating the 3 experimental groups; animals starting on a HFHS-diet at 7-weeks of age are given NR in a preventative (NR-Prev) mode for 18 weeks or 9 weeks in a therapeutic approach (NR-Ther) (NR: 400 mg/kg/day). Mice were sacrificed after a 4 hour fast. The control chow-diet regimen is not shown on the schematic. NR improved (B) glucose handling and (C) plasma insulin levels following an oral glucose tolerance test (OGTT) (insets show the area under the curve), measured following 15 weeks of diet (n=5; chow-diet regimen is not shown). NR also (D) improved insulin sensitivity during an insulin tolerance test (ITT), measured following 17 weeks of diet (n=5; chow-diet regimen is not shown), (E) and reduced body weight (n=8-10). Both cohorts treated with NR exhibited lower (F) whole body fat mass, as measured by EchoMRI following 18 weeks of diet, and epididymal fat mass at sacrifice, compared to the HFHS cohort (n=8-10). *P < 0.05, **P < 0.001, ***P < 0.0001 compared to the HFHS cohort. Data are expressed as mean ± s.e.m. One-way ANOVA with a post-hoc Bonferroni test was used for all statistical analyses. Male mice were used for these experiments.
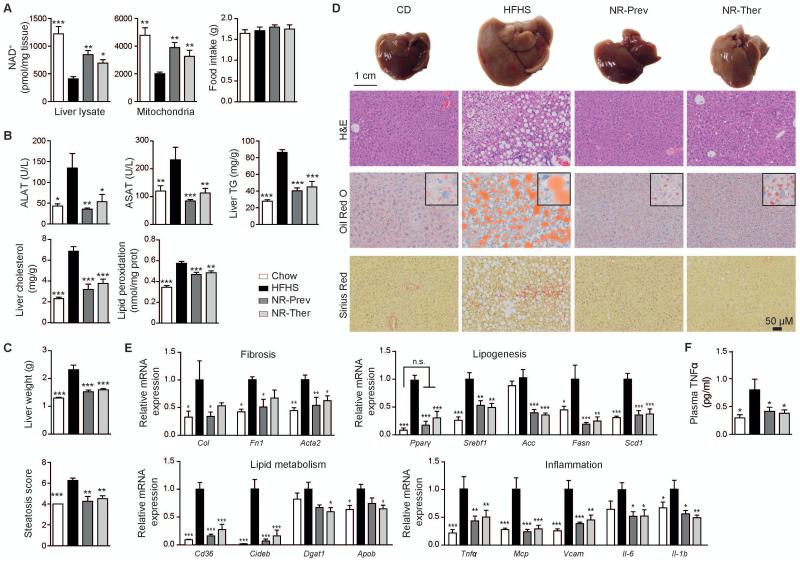
NR boosted whole liver lysate and mitochondrial NAD+ levels in HFHS fed animals from both cohorts, with each cohort consuming similar quantities of food (Fig. 3A). As a result, plasma elevations in alanine aminotransferase (ALAT) and aspartate aminotransferase (ASAT), indicators of liver damage, induced by a HFHS diet, were completely attenuated with NR (Fig. 3B). Hepatic triglyceride, cholesterol and lipid peroxidation levels were also reduced upon NR administration (Fig. 3B). Remarkably, the 9-week intervention, similar to the 18-week preventive treatment, reversed changes in liver weight that occurred after HFHS diet (Fig. 3C), indicating that even therapy initiated after the onset of the disease was beneficial. Furthermore, NR improved liver hematoxylin and eosin (H&E) histology scores for severity and extension of steatosis (Fig. 3C-D). Indications for increased lipid use with NR supplemented diet was evident after just 5 weeks of treatment with reductions in the respiratory exchange ratio (RER) during the dark and light cycles (Fig. S2A-C). The development of a pale white liver color, which occurs with increased lipid content or steatosis, was attenuated by NR, and was corroborated by reduced staining with oil-red-O for lipid content (Fig. 3D). NR also reduced hepatic fibrosis as indicated by less Picrosirius red and Masson’s trichrome staining (Fig. 3D and Fig. S3), substantiated by reduced transcript expression of Col, Fn1 and Acta2 (Fig. 3E). In line with this, although the observations were not linked to NAD+ metabolism, inhibition of PARP, a major consumer of NAD+, was shown to protect against chemically-induced liver fibrosis (29). In parallel, in NR treated animals there was a reduction in the HFHS-induced liver transcripts of the peroxisome proliferators-activated receptor γ (Pparγ), a mediator of lipogenesis (30) (reviewed in (31)), resulting in the reduction of Srebf1, Acc, Fasn and Scd1 mRNAs (Fig. 3E). NR treatment also lowered the expression of genes involved in lipid uptake (Cd36), triglyceride formation (Dgat1) and packaging for lipoprotein secretion (Cideb, Apob) (Fig. 3E). In agreement with the NR-mediated improvements in liver health and lipid content, there was also a reduction in the hepatic expression of inflammatory transcripts Tnfα, Mcp, Vcam, Il-6 and Il-1b (Fig. 3E). These observations were complimented by the attenuation of plasma TNFα levels (Fig. 3F) and the reduced infiltration of CD45 positive leukocytes into the liver with NR treatment (Fig. S3).
Fig. 3.
NAD+ repletion protects and reverses the development of NAFLD in HFHS-fed mice. Phenotyping was performed on CD, HFHS, NR-Prev and NR-Ther cohorts following 9-18-weeks of treatment (NR dose: 400 mg/kg/day). (A) Following sacrifice, we noted that NR elevated hepatic whole-tissue and mitochondrial NAD+ levels, with each cohort having consumed similar amounts of food (n=8-10). (B) NR reduced circulating ALAT and ASAT, indicators for liver damage (n=8-10), and improved liver triglyceride and cholesterol accumulation and lipid peroxidation (n=5). (C) NR lowered liver weight (n=8-10) and blinded H&E scores for severity and extension of steatosis. (D) Matching images of representative livers and liver sections stained with H&E, Oil Red O (lipid content appears red) and Picrosirius red (liver fibrosis represented by collagen stained red) (n=4-5) are also represented. (E) These functional and morphological changes are supported by the relative expression of genes associated with fibrosis, lipogenesis, lipid metabolism and inflammation (n=6). (F) Preventative and therapeutic treatments also attenuated HFHS-induced levels of plasma TNFα. *P < 0.05, **P < 0.001, ***P < 0.0001 compared to the HFHS cohort. Data are expressed as mean ± s.e.m. One-way ANOVA with a post-hoc Bonferroni test was used for all statistical analyses. Male mice were used for these experiments.
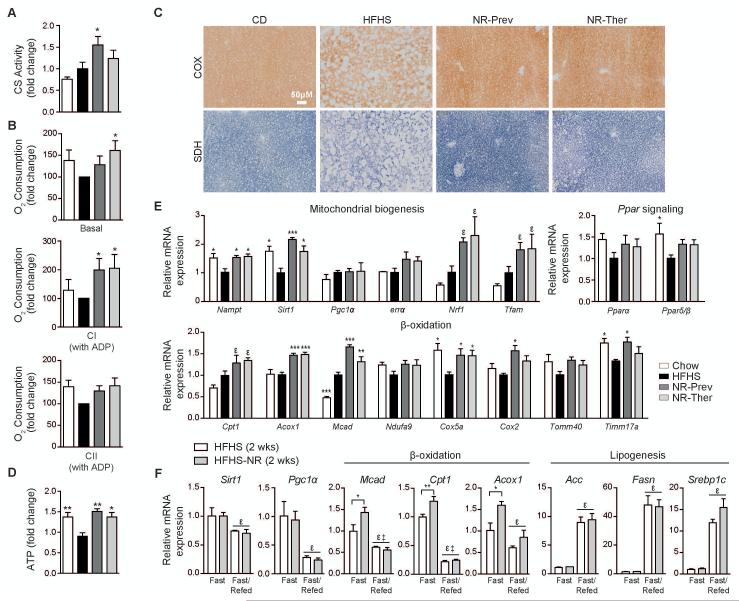
NR therapy reverses HFHS-induced mitochondrial dysfunction and increases transcripts for β-oxidation
The development of NAFLD, and the ensuing progression of liver cirrhosis, relies mainly on abnormal liver lipid accumulation. While increased lipid uptake and lipogenesis can lead to NAFLD, incomplete FA oxidation in the context of mitochondrial dysfunction also contributes to hepatic FA and lipid overload (20, 32) (reviewed in (6)). Although NR has been demonstrated to prevent diabesity in HFD-fed animals (16), the ability of NAD+ repletion to reverse liver damage, brought on my mitochondrial dysfunction, has yet to be shown. Here we demonstrate improvement in liver mitochondrial function by NR with increased citrate synthase (CS) activity and oxygen consumption rates (OCR) driven by complex I (and trending with complex II) in the presence of ADP (Fig. 4A-B). Additionally, cytochrome c oxidase (COX) and succinate dehydrogenase (SDH) activity was enhanced on liver sections following NR (Fig. 4C). Changes in citrate cycle activity and oxidative phosphorylation (OXPHOS) with NR led to a recovery in liver ATP concentrations compared to mice fed only a HFHS diet (Fig. 4D). These functional changes are supported by NR-induced increases in transcripts for Nampt, Sirt1, Nrf1 and Tfam, as regulators of mitochondrial biogenesis (Fig. 4E). The mRNA levels of Pparα and Pparδ/β, transcriptional activators of genes involved in β-oxidation and which protect against NAFLD (32-34), showed trends of reduction with a HFHS diet and recovery with NR (Fig. 4E). To analyze how PPARα, the predominant PPAR isoform in hepatocytes contributes to the beneficial effects of NR on β-oxidation and mitochondria biogenesis, we exposed AML12 hepatoma cells to siRNA for Pparα (Fig. S4). NR treatment of AML12 cells did not increase transcript levels of Pparα, yet upon knock-down of Pparα, the expression of Sirt1 showed a tendency towards a reduction along with the significant attenuation of the stimulatory effects of NR on β-oxidation genes, such as Mcad and Acox1, and the expression of Tfam, a crucial transcription factor for mitochondrial genes. Therefore, NR lost some of its ability to enhance β-oxidation and mitochondrial function when Pparα was absent.
Fig. 4.
Treatment with NR increases liver tissue respiratory capacity ex vivo and β-oxidation gene expression. Measurements were performed on liver samples from CD, HFHS, NR-Prev and NR-Ther cohorts following 9-18-weeks of treatment (NR dose: 400 mg/kg/day), and represented as fold changes compared to the HFHS group. NR increased (A) citrate synthase activity, (B) the basal OCR, CI-coupled driven OCR, in the presence of glutamate, pyruvate, malate and ADP, but was not significant for CII-coupled driven OCR, in the presence of succinate, although a trend was noted (n=6). (C) Histological sections show improvements in both COX and SDH activities in NR-treated groups compared to HFHS-fed mice (n=4), culminating in the attenuation of the decline in (D) ATP levels (n=5). (E) Increases were observed for mRNA transcript levels of genes regulating mitochondrial biogenesis, Pparα and Pparδ/β and genes regulating β-oxidation (n=6). (F) Liver gene transcript expression measurements were performed for Sirt1, Pgc-1 and genes associated with β-oxidation and lipogenesis in mice given 2-weeks of a HFHS or HFHS-NR diet (NR dose: 400 mg/kg/day). Starting at 8am the mice were fasted for 24hrs or fasted for 18hrs and refed for 6hrs. Data are represented as fold changes compared to the CD group. *P < 0.05, **P < 0.001, ***P < 0.0001 compared to the HFHS cohort and εP < 0.05 compared to the CD cohort. ε, P < 0.05, overall effect of treatment versus control mice, ‡, P < 0.05, interaction of each treatment versus control mice. Data are expressed as mean ± s.e.m. One-way ANOVA with a post-hoc Bonferroni test was used for statistical analyses of panels A, B, D and E. Two-way ANOVA with a post-hoc Holm-Sidak test was used for statistical analysis of panel F. Male mice were used for these experiments.
To investigate whether it was changes in β-oxidation or lipogenesis that initially led to the NR-mediated reduction of liver steatosis, we compared the activation of genes associated with β-oxidation or lipogenesis in NR-treated mice fed a HFHS diet for a short 2-week period, to avoid the confounding changes in mouse body weight seen after long-term HFHS diets. Following 2 weeks of diet, NR-treatment accentuated the elevation in transcripts for β-oxidation genes after 24 hours of fasting (Fig. 4F). In contrast, no difference in transcripts of lipogenesis genes were found in refed NR-treated animals. Consequently, in our long-term studies, NR-mediated reductions in lipogenesis transcripts (Fig. 3E) were not caused by the active repression of lipogenesis genes. Therefore, functional and molecular improvements in β-oxidation and OXPHOS upon NR treatment, instead of reduced lipogenesis, are responsible for the attenuation of NAFLD in HFHS animals and support the hypothesis that NAD+ repletion protects and enhances liver mitochondria during periods of FA overload.
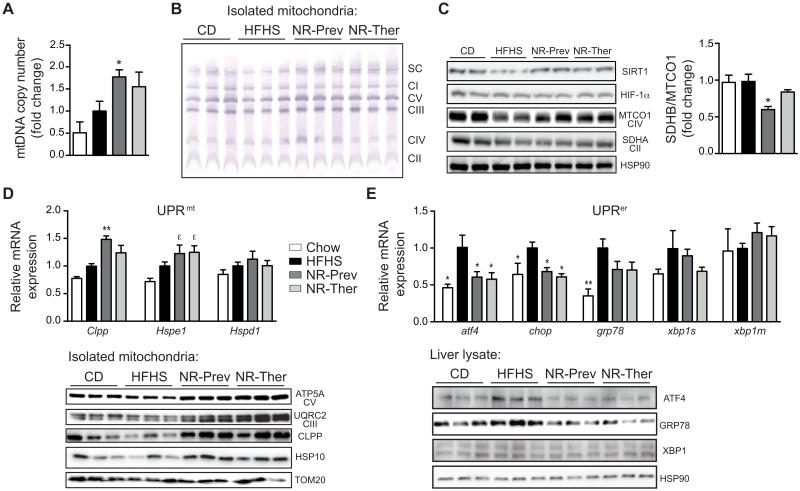
NAD+ repletion prompts mitonuclear protein imbalance, inducing the UPRmt markers CLPP and HSP10 in vivo, while reducing ER stress
Next we examined whether the severe hepatic lipid accumulation and respiratory defects that were improved by NR administration were the result of changes in mitochondrial content, complex formation, and UPRmt signaling. Recently, sirtuin-dependent mitochondrial changes were shown to rely, in part, on the induction of UPRmt, triggered by an imbalance in mitochondrial-versus nuclear-encoded mitochondrial proteins (21, 35). This mitonuclear imbalance activates a retrograde signal that induces a mitohormetic and adaptive nuclear response, ultimately repairing and improving mitochondrial function. These mitohormetic signals can attenuate the harmful repercussions of aging, inherited mitochondrial diseases or a high-fat diet on whole body metabolism (9, 21, 36). Mitochondrial DNA (mtDNA) abundance was elevated along with mitochondrial complexes and supercomplexes, as demonstrated by qPCR and Blue-native PAGE analyses (Fig. 5A-B). Interestingly, the protein ratio between SDHB, encoded by nDNA, and MTCO1, which is encoded by the mtDNA, was reduced following NR treatment, reflective of a mitonuclear protein imbalance (Fig. 5C) and reminiscent to similar changes in mitonuclear protein imbalance that we reported in other model systems (C. elegans (21) and muscle (9)) with NAD+ boosting strategies. Mitonuclear protein imbalance has previously been shown to trigger the UPRmt (35). In line with this premise, protein and transcript levels of CLPP and HSP10 (Hspe1), two UPRmt biomarkers (9, 21), were elevated in mice treated with NR (Fig. 5D). In contrast to NR-induced UPRmt, NR reduces endoplasmic reticulum stress. In fact an ER-specific unfolded protein response (UPRer) is activated during liver steatosis and occurs in NAFLD, viral hepatitis, and alcohol-induced liver injury (reviewed in (37)). Persistent activation of UPRer can cause ER stress-dependent alterations in lipid homeostasis and is suggested to contribute to steatosis. The activation of UPRer was confirmed in HFHS-fed animals by qPCR, demonstrating increased expression of the transcription factor ATF4, which transactivates the promoter of the binding immunoglobulin protein (BiP)/glucose-regulated protein 78 (GRP78) and the C/EBP homologous protein (CHOP), a pro-apoptotic transcription factor (Fig. 5E). Also at the protein level, ATF4 and GRP78 were induced (Fig. 5E). These transcript and protein markers of UPRer activity were all reduced following both NR treatment regimens (Fig. 5E).
Fig. 5.
Improvement in mitochondrial function driven by NAD+ and UPRmt induction and UPRer attenuation in vivo. (A) Mitochondrial abundance was higher in livers from mice treated with NR. Results are expressed as mitochondrial DNA amount (Cox2) relative to nuclear DNA (Hk2) (n=6). (B) This was matched by increases in mitochondrial complexes and supercomplexes, as evidenced by Blue-native PAGE of isolated liver mitochondria. (C) NR induced a mitonuclear protein imbalance, indicated by the reduced ratio between SDHB (nuclear encoded complex II protein) and MTCO1 (mtDNA encoded complex IV protein) expression, from whole liver extracts. (D) Activation of UPRmt by NR is demonstrated by increases in hepatic gene transcripts for Clpp and Hspe1 (HSP10), but not Hspd1 (HSP60) (n=6). These are matched by elevations in CLPP and HSP10 protein levels and the mitochondrial proteins ATP5A and UQCRC2, from isolated liver mitochondria, indicating higher expression of oxidative phosphorylation proteins per amount of mitochondria. (E) HFHS diet-induced transcripts involved in the UPRer, including atf4, chop, and grp78, were mostly reduced following NR treatments, as confirmed with ATF4 and GRP78 protein expression analysis. *P < 0.05, **P < 0.001, ***P < 0.0001 compared to the HFHS cohort and εP < 0.05 compared to the CD cohort. Data are expressed as mean ± s.e.m. One-way ANOVA with a post-hoc Bonferroni test was used for all statistical analyses. Male mice were used for these experiments.
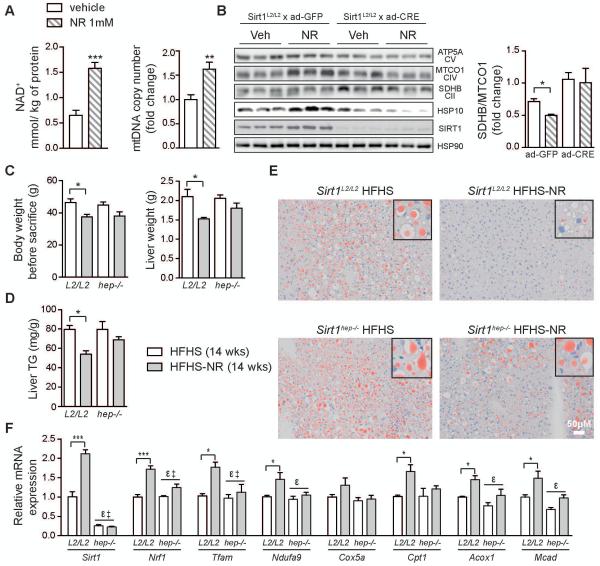
In vivo UPRmt-dependent activation of mitochondrial biogenesis requires SIRT1
Furthermore, we set out to determine whether NAD+ repletion in hepatocytes triggers mitochondrial improvements through a SIRT1- and/or SIRT3-dependent activation of UPRmt, as reported previously (21, 38). Treatment of primary hepatocytes with NR induced an elevation in NAD+ levels and mtDNA abundance, similar to the effect observed in vivo (Fig. 6A). The SIRT1-dependency of NR-induced mitochondrial biogenesis was first demonstrated in AML12 cells using the SIRT1 inhibitor, EX527 (Fig S5A). Moreover, primary hepatocytes from Sirt1L2/L2 mice, infected with an adenovirus virus expressing GFP (control) or Cre recombinase to induce a Sirt1 loss-of-function (LOF), showed a clear SIRT1-dependent reduction in the ratio of the nuclear-encoded SDHB and mitochondrial-encoded MTCO1 proteins (SDHB/MTCO1) resulting in a mitonuclear imbalance, which together with the elevation of HSP10, HSP60 and CLPP (data not shown) is indicative of the UPRmt (Fig. 6B). In addition to the Sirt1 LOF, which completely attenuated the UPRmt induction, we observed an analogous, but less robust effect, on primary hepatocytes from Sirt3L2/L2 mice in which Sirt3 LOF was induced with a similar strategy (Fig. S5B). Thus, the mitonuclear imbalance and activation of UPRmt, seen after boosting NAD+ levels in liver cells, are reliant on presence of either SIRT1 or SIRT3. We next evaluated the possible cell-autonomous contribution of SIRT1 in hepatocytes in mediating the effects of NR using a liver-specific SIRT1-deficient mouse line (Sirt1hep−/−). In these Sirt1hep−/− mice, the effect of NR on body and liver weights following 14 weeks on a HFHS diet were non-significant (Fig. 6C). This was matched by the reversal of the NR-mediated benefits in liver triglycerides and lipids in the Sirt1hep−/− mice (Fig. 6D-E). These results are further corroborated by clear reductions in NR’s efficacy to induce the expression of mitochondrial and β-oxidation associated genes (Fig. 6F). These data demonstrate that although NR may have some systemic effects that ameliorate the impact of a HFHS diet, SIRT1 in hepatocytes is essential for the NR-mediated liver benefits.
Fig. 6.
NR-mediated liver benefits arise from the liver-specific SIRT1-dependent activation of the UPRmt retrograde signaling. Primary hepatocytes cells treated with NR (1mM; 24hrs) show increased (A) cellular NAD+ levels and mitochondrial abundance. (B) Combining NR treatment of Sirt1L2/L2 hepatocytes with infection with an adenovirus expressing either Gfp or Cre attenuated mitochondrial protein expression upon Sirt1 loss-of-function, reflected by the quantified change in mitonuclear imbalance (SDHB/MTCO1 ratio). Increases in HSP10 are also shown to be dependent on SIRT1. (C) Body weight change, liver weight and (D) liver TG levels in liver-specific Sirt1hep−/− and SirT1L2/L2 mice fed a HFHS diet with NR for 14 weeks. (E) Matching images of representative liver sections stained with Oil Red O are also represented (n=5). (F) Relative changes in transcript levels of genes associated with mitochondrial biogenesis and β-oxidation (n=5) in mice of the indicated genotypes. *P < 0.05, **P < 0.001, ***P < 0.0001 compared to the HFHS cohort. ε, P < 0.05, overall effect of treatment or Sirt1hep−/− versus control mice, ‡, P < 0.05, interaction of each treatment versus control mice. Data are expressed as mean ± s.e.m. Two-way ANOVA with a post-hoc Holm-Sidak test was used for statistical analyses of panels B, C and F. Student’s t-test was used for statistical analysis of panel A. Male mice were used for these experiments.
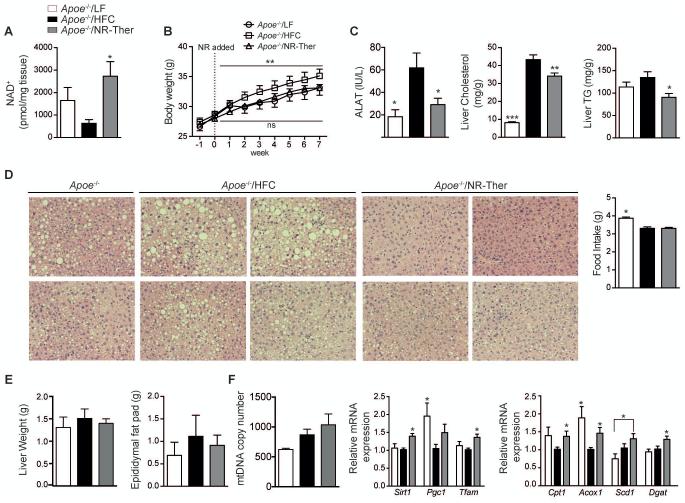
Reversal of tissue steatosis in the Apoe−/− mouse model of NAFLD is NAD+-dependent
To confirm the beneficial effects of NR, we repeated several phenotyping experiments on HFC fed Apoe−/− mice. Apoe−/− mice were challenged with 3 weeks of HFC diet then treated (in a therapeutic mode) with a HFC diet supplemented with NR for another 7 weeks (Apoe−/−/NR-Ther). Similar to HFHS fed C57BL/6J mice, HFC fed Apoe−/− mice treated therapeutically with NR exhibited increased hepatic NAD+ levels and reductions in body weight (Fig. 7A-B), circulating ALAT, and total hepatic cholesterol and triglyceride levels (Fig. 7C). NR also reduced hepatosteatosis in this model, as seen with H&E staining, which was not dependent on food intake (Fig. 7D; HFC cohorts). However, since liver weight and epididymal fat mass did not increase in Apoe−/− mice fed a HFC diet, there was no strong reduction in these parameters with NR (Fig. 7E). Yet, there was a trending increase in mtDNA abundance with NR treatment, that is consistent with increases in Sirt1, Tfam and β-oxidation gene expression (Fig. 7F). Thus, NR used as a therapeutic treatment consistently reverses indications of liver damage and mitochondrial dysfunction between two well-known models of NAFLD in mice.
Fig. 7.
Liver damage in Apoe−/− mouse model of NAFLD was reversed by NAD+ repletion. Apoe−/− mice were fed either a low-fat diet (Apoe−/−/LF) or given a HFC diet for 3 weeks that was either continued to induce NAFLD (Apoe−/−/HFC) or supplemented with NR (Apoe−/−/NR-Ther) for another 7 weeks (NR: 500 mg/kg/day). Mice were sacrificed after overnight fasting. (A) NR elevated hepatic NAD+ levels resulting in (B) reduced body weight. Elevated NAD+ levels reduced (C) circulating ALAT, an indicator for liver damage (n=8-10) and improved hepatic cholesterol and triglyceride accumulation. (D) NR led to less steatosis, as seen by H&E stained liver sections (n=8-10), with similar food intake between HFC fed animals (n=8-10). (E) NR showed a trend to reduce liver and epididymal fat pad weights. This resulted in (F) a trend to increase mtDNA abundance and in a significant elevation of the relative expression of genes associated with mitochondrial biogenesis and β-oxidation following NR (n=8-10). *P < 0.05, **P < 0.001, ***P < 0.0001 compared to the HFHS cohort. Data are expressed as mean ± s.e.m. One-way ANOVA with a post-hoc Bonferroni test was used for all statistical analyses. Male mice were used for these experiments.
Discussion
Although NAFLD is thought to arise from an imbalance between lipid uptake/synthesis and lipid oxidation/export in hepatocytes (6), relatively little is know about the role of mitochondria in this process. NAD+-mediated changes in sirtuin deacetylase activity have been previously associated with the induction of the expression of genes and the activation of proteins linked to improved mitochondrial function (reviewed in (39)). Supporting the importance of NAD+ in liver metabolism, we discovered a striking negative correlation between transcripts of NAD+-consuming genes, such as Cd38 and Parp1, and those involved in β-oxidation in liver biopsies from two large human cohorts and in livers from the mouse BXD genetic reference population. In contrast, NAD+ salvage enzyme transcripts, such as Nrk1 and Nampt were positively correlated to matching gene sets for β-oxidation in mice and humans. Correspondingly, mice that were fed a HFHS diet exhibited a progressive decline in liver NAD+ levels that were matched by an increased liver TG content. Overall, these findings demonstrate a conserved link in humans and mice between the expression of NAD+ consumption/biosynthesis genes and genes that regulate β-oxidation in the liver.
From this data we postulated that by boosting NAD+ levels in long-term HFHS-fed animals we could increase sirtuin-mediated UPRmt activation and mitochondrial biogenesis, while also reducing the UPRer stress response. In agreement, NR attenuated the severe mitochondrial dysfunction, typified by reduced mitochondrial content and function, present in the fatty livers of mice fed a long-term HFHS-diet. These robust effects of NR on mitochondrial function were reliant on the NAD+-mediated SIRT1- and SIRT3-induction of mitonuclear protein imbalance, triggering the UPRmt. Similar to previous reports linking the beneficial effects of NR to the UPRmt (9, 14, 21, 38, 40, 41), the activation of the UPRmt in the livers of these NR-treated animals maintained optimal mitochondrial function despite a chronic FA overload. Interestingly, NAD+ metabolism also appears to play a central role in alcohol-mediated liver damage (42), leading us to postulate that a proper NAD+ balance is essential for maintaining liver homeostasis.
Boosting NAD+ levels, by using NR as a preventive strategy, proved to avert the onset of NAFLD indicating that this vitamin B3 analog has potential as a nutraceutical or food supplement to protect liver function. More relevant for a therapeutic setting was our observation that NR administration was able to attenuate mitochondrial dysfunction and liver damage in mice with established NAFLD, as supported by the improvement of primary and secondary indicators of NAFLD. The therapeutic effect of NR was further confirmed in our study of Apoe−/− mice challenged with a HFC diet, as an independent model of NAFLD that is less-dependent on changes in body mass. Importantly, using Sirt1hep−/− mice we demonstrate that the beneficial effect of NR on NAFLD is reliant on a non-systemic component driven by the activation of SIRT1 specifically in hepatocytes.
Our results therefore indicate that NAD+ repletion prevented or reversed the phenotypes associated with NAFLD such as liver lipid accumulation, liver fibrosis, and the NAFLD molecular signature typified by increased PPARγ mRNA levels and its downstream transcripts linked to lipogenesis. However, the reduction in the activation of lipogenesis genes was shown to be secondary to mitochondrial activation and enhanced β-oxidation and OXPHOS. In parallel to the reversal of lipid accumulation and fibrosis, there were also significant reductions in liver and plasma inflammatory markers associated with NAFLD. Future work should examine how chronic lipid overload and the ensuing inflammation, a process associated with PARP activation (reviewed in (43)), are linked to the reduction in liver NAD+ levels.
In combination, our data show that the replenishment of depleted liver NAD+ stores in mouse models of NAFLD, by the administration of NR supplements, recovers liver NAD+-dependent SIRT1- and SIRT3-signaling to counteract the development of NAFLD. These beneficial effects of NR are largely mediated by the cell-autonomous effect of SIRT1 activation in the liver. Our bioinformatics data furthermore suggest that reduced NAD+ stores may also be a hallmark of human NAFLD and NASH, warranting further exploration of NAD+ replenishing strategies to treat these pervasive liver diseases.
Supplementary Material
Acknowledgements
KG is supported by a grant from the Geneva University Hospital, Switzerland. KJM is the recipient of a Heart and Stroke Foundation of Canada research fellowship award. This work was also supported by USDA Multi-state/Hatch CONS00916 to SIK and JL. JA is the Nestlé Chair in Energy Metabolism and his research is supported by EPFL, NIH (R01AG043930), Krebsforschung Schweiz / SwissCancerLeague (KFS-3082-02-2013), Systems X (SySX.ch2013/153) and SNSF (31003A-140780). C.C. is an employee of the Nestlé Institute of Health Sciences.
References
- 1.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science (New York, N.Y.) 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaker M, Tabbaa A, Albeldawi M, Alkhouri N. Liver transplantation for nonalcoholic fatty liver disease: new challenges and new opportunities. World J Gastroenterol. 2014;20:5320–5330. doi: 10.3748/wjg.v20.i18.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- 4.Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to treatment. Frontline gastroenterology. 2014;5:277–286. doi: 10.1136/flgastro-2013-100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 6.Begriche K, Massart J, Robin M-A, Bonnet F, Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology (Baltimore, Md.) 2013;58:1497–1507. doi: 10.1002/hep.26226. [DOI] [PubMed] [Google Scholar]
- 7.Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. Journal of hepatology. 2002;37:56–62. doi: 10.1016/s0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 8.Gentric G, Maillet V, Paradis V, Couton D, L'Hermitte A, Panasyuk G, Fromenty B, et al. Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. The Journal of clinical investigation. 2015;125:981–992. doi: 10.1172/JCI73957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pirinen E, Canto C, Jo YS, Morato L, Zhang H, Menzies KJ, Williams EG, et al. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 2014;19:1034–1041. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshino J, Mills KF, Yoon MJ, Imai S-i. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell metabolism. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsstrom S, Pasila L, et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med. 2014 doi: 10.1002/emmm.201403943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Weijer T, Phielix E, Bilet L, Williams EG, Ropelle ER, Bierwagen A, Livingstone R, et al. Evidence for a direct effect of the NAD+ precursor Acipimox on muscle mitochondrial function in humans. Diabetes. 2014 doi: 10.2337/db14-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerutti R, Pirinen E, Lamperti C, Marchet S, Sauve AA, Li W, Leoni V, et al. NAD(+)-Dependent Activation of Sirt1 Corrects the Phenotype in a Mouse Model of Mitochondrial Disease. Cell metabolism. 2014;19:1042–1049. doi: 10.1016/j.cmet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houtkooper RH, Canto C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell metabolism. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikiforov A, Dolle C, Niere M, Ziegler M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem. 2011;286:21767–21778. doi: 10.1074/jbc.M110.213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/s0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- 19.Tous M, Ferré N, Camps J, Riu F, Joven J. Feeding apolipoprotein E-knockout mice with cholesterol and fat enriched diets may be a model of non-alcoholic steatohepatitis. Molecular and cellular biochemistry. 2005;268:53–58. doi: 10.1007/s11010-005-2997-0. [DOI] [PubMed] [Google Scholar]
- 20.Verbeek J, Lannoo M, Pirinen E, Ryu D, Spincemaille P, Vander Elst I, Windmolders P, et al. Roux-en-y gastric bypass attenuates hepatic mitochondrial dysfunction in mice with non-alcoholic steatohepatitis. Gut. 2014 doi: 10.1136/gutjnl-2014-306748. [DOI] [PubMed] [Google Scholar]
- 21.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, Mottis A, et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jo Y-S, Ryu D, Maida A, Wang X, Evans RM, Schoonjans K, Auwerx J. Phosphorylation of the nuclear receptor co-repressor 1 by protein kinase B (PKB/Akt) switches its co-repressor targets in the liver. Hepatology (Baltimore, Md.) 2015 doi: 10.1002/hep.27907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Marcos PJ, Jeninga EH, Cantó C, Harach T, de Boer VCJ, Andreux P, Moullan N, et al. Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis. Scientific reports. 2012;2:425. doi: 10.1038/srep00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein S, Oosterveer MH, Mataki C, Xu P, Lemos V, Havinga R, Dittner C, et al. SUMOylation-Dependent LRH-1/PROX1 Interaction Promotes Atherosclerosis by Decreasing Hepatic Reverse Cholesterol Transport. Cell metabolism. 2014 doi: 10.1016/j.cmet.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 25.Yang T, Sauve AA. NAD metabolism and sirtuins: metabolic regulation of protein deacetylation in stress and toxicity. AAPS J. 2006;8:E632–643. doi: 10.1208/aapsj080472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Williams EG, Dubuis S, Mottis A, Jovaisaite V, Houten SM, Argmann CA, et al. Multilayered genetic and omics dissection of mitochondrial activity in a mouse reference population. Cell. 2014;158:1415–1430. doi: 10.1016/j.cell.2014.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tummala KS, Gomes AL, Yilmaz M, Graña O, Bakiri L, Ruppen I, Ximénez-Embún P, et al. Inhibition of De Novo NAD(+) Synthesis by Oncogenic URI Causes Liver Tumorigenesis through DNA Damage. Cancer cell. 2014;26:826–839. doi: 10.1016/j.ccell.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Schadt EE, Molony C, Chudin E, Hao K, Yang X, Lum PY, Kasarskis A, et al. Mapping the genetic architecture of gene expression in human liver. PLoS biology. 2008;6:e107. doi: 10.1371/journal.pbio.0060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukhopadhyay P, Rajesh M, Cao Z, Horvath B, Park O, Wang H, Erdelyi K, et al. Poly (ADP-ribose) polymerase-1 is a key mediator of liver inflammation and fibrosis. Hepatology. 2013 doi: 10.1002/hep.26763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasenfuss SC, Bakiri L, Thomsen MK, Williams EG, Auwerx J, Wagner EF. Regulation of steatohepatitis and PPARγ signaling by distinct AP-1 dimers. Cell metabolism. 2014;19:84–95. doi: 10.1016/j.cmet.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heikkinen S, Auwerx J, Argmann CA. PPARgamma in human and mouse physiology. Biochimica et biophysica acta. 2007;1771:999–1013. doi: 10.1016/j.bbalip.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakajima T, Kamijo Y, Tanaka N, Sugiyama E, Tanaka E, Kiyosawa K, Fukushima Y, et al. Peroxisome proliferator-activated receptor alpha protects against alcohol-induced liver damage. Hepatology (Baltimore, Md.) 2004;40:972–980. doi: 10.1002/hep.20399. [DOI] [PubMed] [Google Scholar]
- 33.Akiyama TE, Nicol CJ, Fievet C, Staels B, Ward JM, Auwerx J, Lee SS, et al. Peroxisome proliferator-activated receptor-alpha regulates lipid homeostasis, but is not associated with obesity: studies with congenic mouse lines. The Journal of biological chemistry. 2001;276:39088–39093. doi: 10.1074/jbc.M107073200. [DOI] [PubMed] [Google Scholar]
- 34.Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, Williams RW, et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497:451–457. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomes AP, Price NL, Ling AJY, Moslehi JJ, Montgomery MK, Rajman L, White JP, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. Journal of hepatology. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papa L, Germain D. SirT3 regulates the mitochondrial unfolded protein response. Molecular and cellular biology. 2014;34:699–710. doi: 10.1128/MCB.01337-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantó C, Menzies KJ, Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell metabolism. 2015 doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsström S, Pasila L, et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO molecular medicine. 2014;6:721–731. doi: 10.1002/emmm.201403943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraus D, Yang Q, Kong D, Banks AS, Zhang L, Rodgers JT, Pirinen E, et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508:258–262. doi: 10.1038/nature13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marmier S, Dentin R, Daujat-Chavanieu M, Guillou H, Bertrand-Michel J, Gerbal-Chaloin S, Girard J, et al. Novel role for carbohydrate responsive element binding protein in the control of ethanol metabolism and susceptibility to binge drinking. Hepatology (Baltimore, Md.) 2015 doi: 10.1002/hep.27778. [DOI] [PubMed] [Google Scholar]
- 43.Ba X, Garg NJ. Signaling mechanism of poly(ADP-ribose) polymerase-1 (PARP-1) in inflammatory diseases. The American journal of pathology. 2011;178:946–955. doi: 10.1016/j.ajpath.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.