Abstract
Background
Major depressive disorder (MDD) is a debilitating disorder characterized by widespread brain abnormalities. The literature is mixed as to whether or not white matter abnormalities are associated with MDD. This study sought to examine fractional anisotropy (FA) in white matter tracts in individuals with MDD using diffusion tensor imaging (DTI).
Methods
139 participants with MDD and 39 healthy controls (HC) in a multi-site study were included. DTI scans were acquired in 64 directions and FA was determined in the brain using four methods: region of interest (ROI), tract-based spatial statistics, and diffusion tractography. Diffusion connectometry was used to identify white matter pathways associated with MDD.
Results
There were no significant differences when comparing FA in MDD and HC groups using any method. In the MDD group, there was a significant relationship between depression severity and FA in the right medial orbitofrontal cortex, and between age of onset of MDD and FA in the right caudal anterior cingulate cortex using the ROI method. There was a significant relationship between age of onset and connectivity in the thalamocortical radiation, inferior longitudinal fasciculus, and cerebellar tracts using diffusion connectometry.
Conclusions
The lack of group differences in FA and connectometry analysis may result from the clinically heterogenous nature of MDD. However, the relationship between FA and depression severity may suggest a state biomarker of depression that should be investigated as a potential indicator of response. Age of onset may also be a significant clinical feature to pursue when studying white matter tracts.
Keywords: depression, brain imaging/neuroimaging, mood disorders, diffusion tensor imaging, white matter tracts, connectometry, fractional anisotropy, multi-site study
Introduction
Major depressive disorder (MDD) is a debilitating psychiatric disorder that is highly prevalent in the US population. MDD is characterized by a variable combination of affective and cognitive symptoms, and not surprisingly is associated with widespread structural and functional brain differences. In particular, there has been a great deal of attention focused on circuits connecting frontal and subcortical brain regions [1–3]. Evidence suggests that MDD is associated with structural deficits in midline frontal brain regions, particularly in anterior cingulate cortex (ACC), medial orbitofrontal cortex (MOFC) and dorsomedial prefrontal cortex (DMPFC) [2; 4]. Abnormal circuits connecting these frontal regions with subcortical regions are hypothesized to underlie the neurobiology of depression [1], leading to a “disconnection syndrome” resulting from white matter deficits [5; 6]. In other words, a “disconnection syndrome” occurs when frontal and subcortical brain regions cannot effectively communicate, which is hypothesized to lead to the manifestation of depressive symptoms. One way to examine this phenomenon is to study white matter tracts, which are essential for communication between different brain regions.
Diffusion tensor imaging (DTI) can be used to characterize white matter tracts in the human brain in vivo. Fractional anisotropy (FA) is a DTI-derived measure reflecting the directionality of water diffusion, which is used as an indication of the location and strength of white matter tracts. FA in certain regions has been reported to be lower in MDD suggesting white matter fiber disruption or disorganization. For example, low FA in MDD has been reported in white matter adjacent to frontal [7–9], temporal [8], parietal [8; 9], and cingulate cortices [10]. Low FA has also been reported in white matter tracts, including the inferior longitudinal fasciculus [11; 12], superior longitudinal fasciculus [9; 13], internal capsule [10; 12; 14; 15], external capsule [14; 15] and corpus callosum [13–15]. We recently reported extensive white matter deficits in frontal medial brain regions in MDD compared to healthy controls [16], mirroring findings reported by a recent meta-analysis [17]. However, not all studies find group differences in FA between MDD and healthy controls [18; 19] and one study reported low FA only in those with melancholic MDD [20].
Using diffusion tractography methods, it is possible to examine FA in individual tracts to determine which tracts may be affected in MDD, rather than discrete brain regions. Song and colleagues [21] found lower FA in the right solitary tract of MDD subjects than HC subjects. However, another study reported lower FA in MDD in only one of seven tracts investigated (the parahippocampal cingulum) and only in male subjects [22]. Most studies do not find a relationship between FA and clinical characteristics [3; 7; 8; 11; 14; 15; 18; 21]. However, two studies showed a negative correlation between depression severity and FA in the left anterior limb of the internal capsule [10; 12].
There are several limitations in the DTI literature that need to be addressed. First, there is only one group that has reported DTI data with a large MDD sample [N=95; 3; 21]. Other studies report on samples ranging from 14 [e.g., 8] to 45 MDD subjects [e.g., 12; 13]. Second, about half of the studies in the literature used 13 or fewer directions [e.g., 7; 19; 23], and only one group used 61 directions [13]. FA can vary depending on the number of directions [24], and a minimum of 30 unique directions has been shown to provide adequate resolution for estimating FA [25]. Third, some studies report data that was not corrected for multiple comparisons [e.g., 7; 8; 19; 26] or report a small cluster size [e.g., 11]. Finally, most studies use only one method to examine FA, either ROI [e.g., 7], VBA [e.g., 8; 27], TBSS [e.g., 13; 19] or tractography [e.g., 3; 28].
To address shortcomings in previous studies, we examined FA in the largest MDD sample to date using 64-direction DTI, using multiple approaches: ROI, TBSS, and probabilistic tractography. Additionally, differences in white matter were explored using diffusion connectometry [29], which aimed to map the trajectories of affected tracts [30; 31]. Each technique brings a unique contribution to this investigation. Namely, the ROI approach is focused on a small number of a priori brain regions based on the literature. A further advantage of the ROI approach is that the data is obtained in DTI space, so there is no warping of the brain to fit a template. TBSS is a voxel based approach to examine white matter tracts. Although the data is derived from a standardized template, this approach allows for a broad investigation of the entire brain. Probabilistic tractography is another focused technique that allows investigators to identify specific tracts by identifying seed ROIs and estimating how many tracts extend from those ROIs. The benefit of this approach is that one can examine white matter indices along a specific tract, rather than discrete parts of the brain that are assessed using ROI and TBSS approaches. Finally, diffusion connectometry differs from probabilistic tractography in that probabilistic tractography defines connectivity by the number of "tracks" or "streamlines," whereas connectometry uses density of diffusing spins. Conceptually, probabilistic tractography aims to find a difference in tracks, whereas connectometry tracks the difference in voxels that have substantial correlation with the study variable, aiming to identify the entire affected section.
By addressing all the limitations in the literature, we expect the results from this study to provide more definitive results regarding the possibility of white matter deficits in MDD. We hypothesized that MDD controls would have lower FA in white matter tracts in the frontal cortex than healthy controls. Although there is no supportive literature to develop a specific hypothesis about the connectometry analysis, we expect to identify white matter deficits in the frontal cortex in MDD compared to healthy controls which is consistent with the FA literature.
Materials & Methods
Subjects
Participants were enrolled in the EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care for Depression, NIMH 1U01 MH092250, http://embarc.utsouthwestern.edu/) project at four sites. These sites are the University of Texas Southwestern Medical Center (TX), University of Michigan (UM), Massachusetts General Hospital (MG), and Columbia University Medical Center (CU). The EMBARC study was designed to measure treatment outcomes. However, the current study is a preliminary analysis aimed at comparing baseline neuroimaging data in depressed and healthy controls. The Institutional Review Board for all four sites approved the protocol, and subjects gave written informed consent.
One hundred and thirty nine subjects who met Diagnostic and Statistical Manual of Mental Disorders [DSM-IV-TR; 32] criteria for a current major depressive episode (MDE) in context of MDD and 39 healthy controls (HCs) were included. Overall, there were 166 MDD subjects enrolled in the EMBARC study at the time of this analysis. Twenty seven MDD subjects were not included in the analysis for the following reasons: they did not complete baseline imaging (N=10), had poor quality images due to significant head motion (N=4) and poor segmentation (N=11). Therefore, there were 139 MDD subjects remaining for analysis. As for the HCs, there were 40 HC subjects enrolled in the EMBARC study at that time of this analysis. One HC was not included due to significant head motion.
MDD and HCs were recruited by flyers, advertisements, and posting on websites. Participants who were potentially eligible were scheduled for an evaluation visit where study eligibility was assessed through psychiatric and medical history, chart review, clinical interview, physical examination, routine blood tests, pregnancy test, and urine toxicology. All participants were between the ages of 18 and 65. MDD participants had to meet DSM-IV-TR criteria for a current MDE (early onset, before 30 years) and have a minimum score of 14 on the Quick Inventory of Depressive Symptoms [QIDS-SR16; 33]. MDD participants were excluded if they had a lifetime history of bipolar disorder or psychosis, current drug or alcohol abuse/dependence, or a current primary diagnosis of obsessive-compulsive disorder. Patients on psychoactive medication underwent a three-week medication washout prior to neuroimaging. The following current comorbid diagnoses were reported in the MDD group: panic disorder (N=15), obsessive-compulsive disorder (N=4), social phobia (N=23), PTSD (N=11), generalized anxiety disorder (N=19), anxiety disorder, not otherwise specified (N=5), bulimia nervosa (N=2), or other diagnosis (N=2). HCs were included if they did not have a lifetime history of a mood, anxiety, or psychotic disorder.
Clinical measures
Diagnoses were based on the Structured Clinical Interview for DSM-IV Patient Version [SCID-IV-I/P; 34]. The Hamilton Depression Rating Scale [HAM-D; 35] and the Quick Inventory of Depressive Symptoms [QIDS-SR16; 33] assessed clinician- and self-rated depression severity, respectively. Scores from clinical measures administered at the evaluation visit are reported.
Image acquisition
All participants underwent a magnetic resonance imaging (MRI) scan at one of the participating sites. Each site had a different 3 Tesla MRI scanner. Two sites operated Philips scanners (TX and UM), one site operated a GE scanner (CU), and one site operated a Siemens scanner (MG). Structural MRIs were acquired with following parameters: TR (repetition time): 5.9 to 8.2 ms, TE (echo time): 2.4 to 3.7 ms, Flip Angle: 9° to 12°, slice thickness: 1 mm, FOV (field of view): 256 mm × 256 mm, voxel dimensions: 1 mm × 1 mm × 1 mm, acquisition matrix: 240 x 240, 256 × 243 or 256 × 256, acceleration factor: 2, and 174–178 sagittal slices. Diffusion images were acquired using a single-shot EPI (echo planar imaging) sequence. Scan parameters were as follows: TR=8310–9500 ms, TE=95–96.3 ms, Flip Angle 90 degrees, slice thickness=2.5 mm, FOV=240 x 240 mm, voxel dimensions 2.5 mm× 2.5 mm × 2.5 mm or 1.9 mm× 1.9 mm × 2.5 mm, acquisition matrix=96 x 96, b value = 1000 s/mm2, and 64 collinear directions with 1 or 5 non-weighted images. DTI scan time was approximately 10 minutes.
Image processing
Diffusion Tensor Imaging (DTI)
All T1 and DTI images were transferred to and processed at Stony Brook University. Each DTI image was run through a series of quality assurance tests for common artifacts, including ghost, ring, slice-wise intensity, venetian blind, and gradient-wise motion artifacts [36]. Diffusion images were then corrected for distortion induced by gradient coils and simple head motion using the eddy current correction routine within FSL (FMRIB Software Library, http://www.fmrib.ox.ac.uk/fsl/). FSL’s Brain Extraction Tool (BET) removed non-brain tissue from the image. Following this, Camino [http://web4.cs.ucl.ac.uk/research/medic/camino/pmwiki/pmwiki.php] was used to estimate FA computing the least-squares-fit diffusion tensor with non-linear optimization using a Levenburg-Marquardt algorithm, constrained to be positive by fitting its Cholesky decomposition [37; 38].
Region of Interest (ROI) Determinations
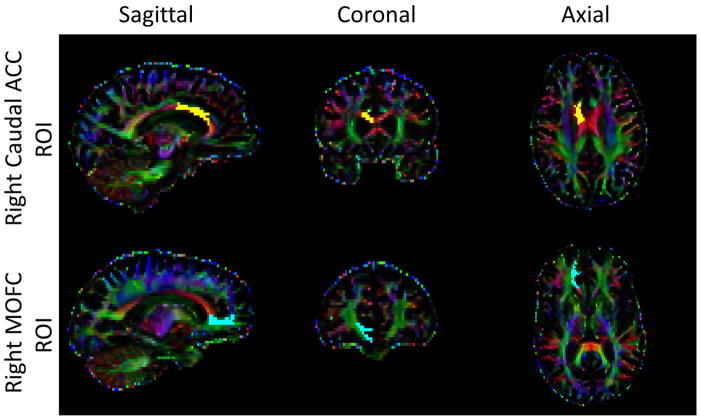
Anatomical T1 images were cropped and corrected for image non-uniformity using the Bias Field Corrector in Advanced Normalization Tools (ANTS; http://www.picsl.upenn.edu/ANTS/). ROIs were created using the cortical parcellation tool based on the Desikan-Killiany atlas [39]. Each white matter voxel was labeled on the basis of the nearest cortical voxel [40]. Then, the DTI image was co-registered to the cropped T1 image using ANTS, and the inverse transformation was applied to the cortical map in order to place ROIs into DTI space for analysis. Finally, mean FA values in white matter were computed for each ROI. In order to reduce the number of comparisons, the following bilateral ROIs in the frontal cortex were chosen a priori based on reported white matter deficits in frontal cortical regions in the MDD literature: medial orbital frontal cortex (MOFC), superior frontal cortex (SFC), rostral anterior cingulate cortex (rACC), and caudal anterior cingulate cortex [cACC; 2; 6]. Figure 1 shows the location of the right cACC and the right MOFC ROIs. We renamed the SFC label “dorsomedial prefrontal cortex” or “DMPFC” to be consistent with the literature and provide a more descriptive name for the region.
Figure 1.
Sagittal, coronal, and axial view of the right caudal ACC ROI (top) and the right MOFC ROI (bottom) in a representative subject.
Tract-Based Spatial Statistics (TBSS) analysis
TBSS analysis [41] of the FA diffusion maps included several steps. Individual FA maps were non-linearly aligned to a common FMRIB58 FA template. Then the aligned FA maps were averaged to obtain a study mean FA map, and a white matter skeleton was computed on the mean FA map. The white matter skeleton was then thresholded (FA≥0.2) to maintain only WM voxels. Each individual FA map was projected onto the common FA skeleton to obtain the individual FA skeleton, on which a voxel-wise analysis was performed to examine differences between the two populations.
Tractography and Weighted FA
Probabilistic tractography was performed using FMRIB’s Diffusion Toolbox [FDT; 42]. This algorithm computes probabilistic streamlines through each voxel by repetitive sampling from the principal diffusion directions. The outcome of this method is the probability of connections from the seed (medial orbitofrontal cortex) to all targets in the brain. The MOFC was chosen based on the finding that FA in this region was significantly correlated with depression severity. The algorithm was run with a tract curvature threshold of 0.2 mm, maximum number of steps per sample equal to 2000, length of each step equal to 0.5 mm, and 5000 samples. In order to calculate the (weighted) average FA within the defined tracts, the voxel-based FA measures were multiplied by the probability of connection at each voxel and divided by the sum of probabilities. The two outcomes of interest were weighted average FA and number of tracts. The tractography algorithm failed in 16 MDD subjects, therefore data on 123 MDD subjects is reported. Additionally, 2 MDD subjects were removed for the “Total number of tracts” analysis due to extremely high values (>7 million tracts).
Diffusion MRI Connectometry
We conducted diffusion MRI connectometry [29] to further explore group difference. The diffusion data were reconstructed using q-space diffeomorphic reconstruction [43], with a diffusion sampling length ratio of 1.25. The output resolution was 2 mm. A multiple regression model including age, site of data collection, and sex was used to calculate the T-score of the difference between control subjects and patients. A deterministic fiber tracking algorithm [44] was used to connect local fiber directions with T-score greater than 2.0, and tracts with connected length greater than 40 mm were collected. This cutoff was determined by the lower bound of the uncinated fasciculus length [45], which is used to estimate the minimum length of a major tract. To estimate the false discovery rate, a total of 1000 randomized permutations were applied to the group label in order to obtain the null distribution of the tract length. In the MDD group, clinical measures (i.e., depression severity and age of onset of depression) were used in a multiple regression model to determine their relationship to connectometry results. The regression model included site of data collection, age, and sex. The analysis was conducted using publicly available software DSI Studio (http://dsistudio.labsolver.org).
Statistical analysis
Data were statistically evaluated using IBM SPSS Statistics (SPSS Inc., Chicago, Illinois, USA, Version 22.0). Comparisons of demographic and clinical variables were performed with a univariate analysis of covariance (ANCOVA) for continuous variables and chi-square analyses for categorical variables with group (HC vs. MDD) as the between-subjects factor with site as a covariate. For the ROI analyses, ANCOVAs were performed with site, age and sex included as covariates. For the TBSS analyses, we used the general linear model (GLM) method with the permutation-based non-parametric testing included in the FSL tools. Significance threshold was set to p<0.05 corrected for family-wise errors (FWE) using the Threshold-Free Cluster Enhancement (TFCE) method in FSL’s randomise tool. Using TFCE, FWEs are corrected using the null-distribution of the maximum test statistic across the image [46]. The group comparison was adjusted for (mean centered) age, site and sex. Only clusters with a minimum of 50 voxels were considered significant. For the tractography analyses, an ANCOVA was used to compare MDD and HP groups with site, age and sex as covariates. For number of tracts, MOFC seed size was included as a covariate. Partial correlations between clinical and diffusion measures were performed in the MDD group using Spearman’s Rho for ordinal variables (HAM-D 17 and QIDS), variables that were not normally distributed (Duration of MDE), and variables that were converted into categorical categories (Number of MDEs; 1=1–2 episodes, 2=3–4 episodes, 3=5–8 episodes, 4=9–20 episodes and 5=24+ episodes. Each category included approximately 15–20% of the sample). Pearson correlation coefficient was used for Age of Onset correlations. All correlations included age, site and sex as covariates. Bonferroni correction was used to account for multiple correlations (0.05/50 = 0.001).
Results
Demographics and Clinical Measures
Demographic and clinical data are presented in Table 1. There were no significant differences in age (F(1,175)=0.12,p=0.73) or years of education (F(1,170)=0.46,p=0.50) between HC and MDD groups There were no significant differences in sex (χ2(1)=0.006,p=0.94), race (χ2(4)=1.25,p=0.87), ethnicity (χ2(1)=1.71,p=0.19), or handedness (χ2(2)=0.24,p=0.88) between the HC and MDD groups. Additionally, there was no group differences when comparing sex, race, ethnicity, or handedness by site (all p-values > 0.05), with the exception that TX has significantly younger HCs compared to the MDD group (F(1,64)=7.75,p=0.007). As expected, the MDD group scored higher than the HC group on the HAM-D (F(1,169)=551.05,p<0.001).
Table 1.
Demographic and clinical variables. Data are presented as mean ± standard deviation unless otherwise specified.
| HC (N=39) | MDD (N=139) | ||
|---|---|---|---|
| Demographic Variables | |||
|
| |||
| Sex, N (%) Male | 14 (36%) | 49 (35%) | |
| Age, years | Mean | 37.0 ± 14.6 | 37.2 ± 13.1 |
| Range | 18 – 65 | 18 – 65 | |
| Race, N (%) Caucasian | 13 (33%) | 43 (31%) | |
| Ethnicity, N (%) Hispanic | 3 (8%) | 22 (16%) | |
| Handedness, N (%) Right | 32 (82%) | 118 (85%) | |
| Education, years | Mean | 15.2 ± 2.3 | 14.8 ± 2.5 |
| Range | 10 – 20 | 8 – 21 | |
|
| |||
| Clinical Variables | |||
|
| |||
| Age of onset of MDE | Mean | - | 15.8 ± 6.2 |
| Range | 2 – 30 | ||
| Number of MDEs | Median | - | 6 |
| Range | 1 – 120 | ||
| Length of Current Episode, months | Mean | - | 37.6 ± 63.0 |
| Range | 1 – 400 | ||
| HAM-D 17-Item*** | Mean | 0.7 ± 0.9 | 18.7 ± 4.5 |
| Range | 0 – 3 | 10 – 32 | |
| QIDS-SR16*** | Mean | 1.4 ± 1.3 | 18.3 ± 2.8 |
| Range | 0 – 4 | 14 – 27 | |
p<0.001 vs. HC.
Abbreviations: HC=healthy control, MDD=major depressive disorder, MDE=major depressive episode, HAM-D=Hamilton Rating Scale for Depression, QIDS-SR16=Quick Inventory of Depressive Symptoms.
ROI & TBSS Analyses
Table 2 presents mean FA in each of the bilateral ROIs investigated (cACC, rACC, MOFC, and DMPFC). There were no significant differences in mean FA in ROIs when comparing the HC and MDD groups. There were no clusters identified by the TBSS analysis that differentiated the two groups.
Table 2.
Fractional anisotropy (FA) in white matter parcellated regions of interest (ROIs) using the Desikan Killiany atlas in FreeSurfer. F-test includes age, site and sex as covariates. Data are presented as mean ± standard deviation.
| ROI | R/L | HC (N=39) | MDD (N=139) | F-test |
|---|---|---|---|---|
| Caudal ACC | R | 0.62 ± 0.04 | 0.62 ± 0.05 | F(1,173)=0.20, p=0.66 |
| L | 0.61 ± 0.04 | 0.61 ± 0.05 | F(1,173)=0.02, p=0.88 | |
|
| ||||
| Rostral ACC | R | 0.58 ± 0.05 | 0.59 ± 0.05 | F(1,173)=0.21, p=0.65 |
| L | 0.55 ± 0.04 | 0.54 ± 0.04 | F(1,173)=0.50, p=0.48 | |
|
| ||||
| Medial Orbital Frontal | R | 0.43 ± 0.03 | 0.43 ± 0.03 | F(1,173)=0.79, p=0.38 |
| L | 0.41 ± 0.04 | 0.40 ± 0.03 | F(1,173)=1.52, p=0.22 | |
|
| ||||
| Dorsomedial Prefrontal | R | 0.48 ± 0.03 | 0.49 ± 0.03 | F(1,173)=0.67, p=0.41 |
| L | 0.49 ± 0.03 | 0.49 ± 0.03 | F(1,173)=0.39, p=0.54 | |
Abbreviations: ACC=anterior cingulate cortex, HC=healthy control, L=left, MDD=major depressive disorder, R=right.
Tractography and Weighted FA
Probabalistic tractography was used to examine white matter tracts extending from the right MOFC in light of the correlations reported below. Table 3 presents the means for the three outcome measures from the tractography: weighted FA, total number of tracts, and seed size. There were no significant differences in these outcome measures when comparing the HC and MDD groups.
Table 3.
Outcome measures from probabilistic tractography using the right MOFC as the seed and all voxels in the brain as the target. F-test includes age, site and sex as covariates. For total number of tracts, seed size is also included as a covariate. Data are presented as mean ± standard deviation.
| HC (N=39) | MDD (N=123)* | F-test | |
|---|---|---|---|
| Weighted FA | 0.32 ± 0.03 | 0.32 ± 0.03 | F(1,157)=0.32, p=0.57 |
| Total number of tracts | 2,041,410 ± 575,536 | 2,030,248 ± 523,381 | F(1,155)=0.12, p=0.73 |
Two subjects were removed for the “Total number of tracts” analysis due to extremely high values (>7 million tracts).
Abbreviations: FA=fractional anisotropy, HC=healthy control, MDD=major depressive disorder.
Diffusion MRI Connectometry
We used connectometry to identify white matter pathways associated with MDD [29]. The density of diffusing spins at various orientations were calculated and regressed against MDD and HC groups, with age, sex, and site included in the regression model. The results did not show any white matter pathways that were significantly associated with MDD.
Clinical Correlations
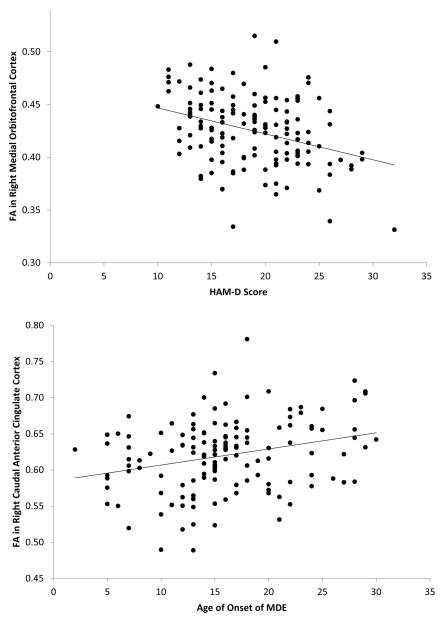
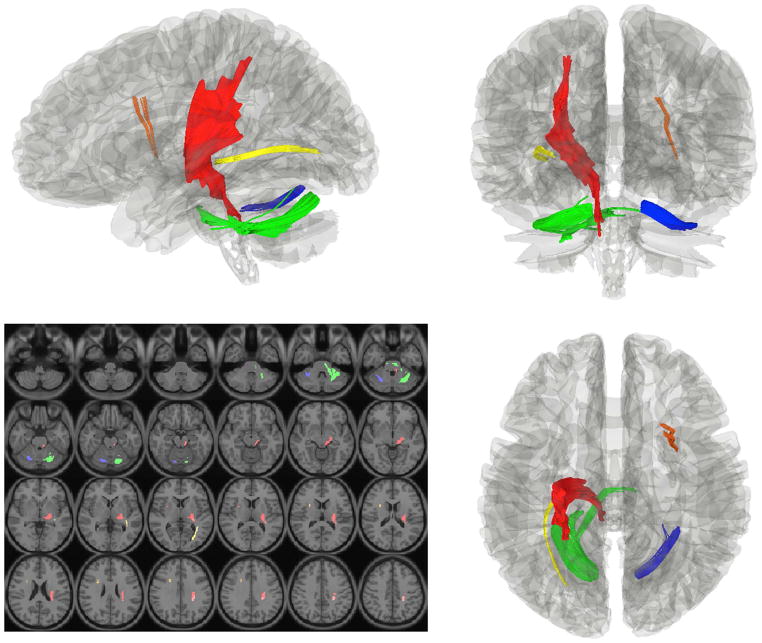
In order to test the relationship between FA and clinical severity, correlations were performed between DTI and clinical measures in the MDD group (Table 4). There was a significant correlation between FA in the right MOFC and HAM-D scores (p<0.001) and FA in the right cACC and age of onset (p=0.003; Figure 2). There were no significant correlations between tractography outcome measures and clinical measures at the p≤0.001 level. Diffusion MRI connectometry was also used to identify white matter pathways associated with depression severity and age of onset in the MDD group. The analysis showed a significant negative correlation (FDR=0.02) between age of onset of depression and connectivity in four fiber pathways: 1) left sensory pathway through the posterior limb of the internal capsule, 2) right thalamocortical radiation, 3) left inferior longitudinal fasciculus, and 4) bilateral cerebellar tracts (Figure 3). The connectometry analysis showed no significant correlation between the MOFC and HAM-D scores (FDR>0.05).
Table 4.
Partial correlation coefficients for the association between FA in white matter parcellated ROIs, tractography outcome measures, and clinical measures in the MDD group. Age, site and sex are included as covariates.
| ROI Analysis | R/L | HAM-D | QIDS-SR16 | Age of Onset | Length of MDE (months) | Number of MDEs |
|---|---|---|---|---|---|---|
|
| ||||||
| Caudal ACC | R | −0.10 | 0.004 | 0.25** | −0.003 | −0.07 |
| L | 0.04 | 0.00 | 0.10 | 0.01 | 0.03 | |
|
| ||||||
| Rostral ACC | R | −0.17 | −0.04 | 0.07 | 0.03 | −0.09 |
| L | 0.04 | 0.00 | 0.05 | 0.01 | 0.03 | |
|
| ||||||
| Medial Orbital Frontal | R | −0.29*** | −0.17 | 0.23 | −0.06 | 0.02 |
| L | −0.20 | −0.10 | 0.15 | −0.12 | 0.03 | |
|
| ||||||
| Dorsomedial Prefrontal | R | −0.24 | 0.02 | 0.21 | −0.08 | 0.09 |
| L | −0.18 | 0.01 | 0.13 | −0.05 | 0.14 | |
|
| ||||||
| Tractography | ||||||
|
| ||||||
| Weighted FA in Tract Extending from the Medial Orbital Frontal Seed | R | −0.18 | −0.09 | 0.02 | 0.06 | −0.03 |
|
| ||||||
| # of Tracts Extending from the Medial Orbital Frontal Seed | R | −0.09 | 0.23 | 0.03 | 0.03 | 0.01 |
p=0.003,
p<0.001 indicates a significant correlation.
Abbreviations: ACC=anterior cingulate cortex, FA=fractional anisotropy, HAM-D=Hamilton Rating Scale for Depression, HP=healthy participant, L=left, MDD=major depressive disorder, MDE=major depressive episode, QIDS-SR16=Quick Inventory of Depressive Symptoms, ROI=regions of interest, R=right.
Figure 2.
Top: Scatterplot depicting the relationship between fractional anisotropy (FA) in the right medial orbitofrontal cortex (MOFC) and Hamilton Depression Rating Scale scores (HAM-D) in the major depressive disorder (MDD) group. Bottom: Scatterplot depicting the relationship between FA in the right caudal anterior cingulate cortex (cACC) and age of onset of depression in the MDD group.
Figure 3.
Connectometry results showing significant negative correlations between age of onset of depression in the MDD group and white matter density in the following tracts: 1) left sensory pathway through the posterior limb of the internal capsule (red), 2) right thalamocortical radiation (orange), 3) left inferior longitudinal fasciculus (yellow), and 4) bilateral cerebellar tracts (blue and green). DTI image slices are presented in radiologic convention, and the tract images are presented in neurological convention.
Discussion
Prevailing theories of MDD include impaired connections in the brain linking cortical and subcortical regions [47–49]. The current DTI study examined white matter tracts in MDD in one of the largest samples published to date, using a 64-direction protocol. Using multiple methods to examine FA we found no difference in FA comparing individuals with MDD and healthy controls. This is at odds with a previous paper where we reported decreased FA in MDD in several frontal cortex regions [16]. One main difference between the two studies is that the HC group was significantly younger than the MDD group in our previous paper. FA decreases in adulthood [50] making this a potentially confounding variable. Although we used age as a covariate in all of our analyses, it is possible that variability due to age could not be completely regressed out [51]. The literature on DTI in MDD has been mixed, both the findings and the methods used to examine white matter, making it difficult to draw any concrete conclusions. The current study had groups well matched on all demographic variables, had a larger sample, and had a higher resolution DTI scan than our previous study, strengthening our confidence in our findings.
Of significant interest, we found a relationship between FA in the right MOFC and depression severity, such that low FA was related to high depression scores. The MOFC is thought to be central to reward processing in the brain [52; 53]. It is also uniquely situated to integrate sensory and emotional information through its connections with visual, auditory [54], and limbic structures [1]. There have been only two other DTI papers that reported a relationship between FA and depression severity in adult-onset MDD [10; 12]. Both groups found that FA in the anterior limb of the internal capsule was negatively correlated with depression severity. One study of late-life depression found that FA in the MOFC was negatively correlated with depression severity [55], and another found a positive correlation between MOFC white matter lesion density and depression severity [56], supporting our finding. DTI studies that did not find such a relationship between clinical measures and FA had a small sample size, whereas the studies reporting a relationship had 25 MDD [10] and 45 MDD subjects [12]. When analyzing data from each of the four EMBARC sites separately, only two of the sites showed a significant correlation between the right MOFC and depression severity. These two sites had the largest samples. It is possible that studies with a small sample size limit the range of depression severity scores, making it unlikely to find such a relationship. Although two DTI studies with large samples did not find a significant correlation between FA and depression severity [3; 21], these studies were focused on FA in specific locations [21] or broad network based measures of FA [3].
Age of depression onset was also significantly related to FA in the right cACC using the ROI method, and to white matter density in the thalamocortical radiation (connecting the thalamus and caudal ACC, see Figure 2) using diffusion connectometry. This is the first study to report a significant relationship between age of onset of depression and DTI measures; other groups do not find a significant relationship [11; 18; 21]. A recent study showed that individuals with pediatric-onset MDD had a smaller genu of the corpus callosum compared to healthy controls, whereas corpus callosum volume was not different when comparing adult-onset MDD and healthy controls [57]. It is possible that suffering from depression while white matter growth is still in progress (i.e., throughout childhood and adolescence) may have a deleterious effect on continued development. There have been a number of studies that support the notion that early life stress adversely affects brain development [58; 59], which is consistent with this finding. It is important to note that age of depression onset was reported as early as 2 years of age in some MDD subjects. Limitations associated with retrospective recall is a significant factor to consider, although we found comparable results when limiting age of onset to a minimum of 10 years (r=0.24, p=0.017). Additionally, the significance of the relationship between RA in the right cACC using the ROI method (p=0.003) was just below our strict significance cut-off of p<0.001. This finding should be interpreted with caution.
The other three tracts identified using the diffusion connectometry method are important for sensory processing. The internal capsule facilitates the communication of sensory information to the cortex, the inferior longitudinal fasciculus connects the orbitofrontal and occipital cortices, and cerebellar tracts provide proprioceptive information about the limbs to the cerebellum. There have been some studies that report low FA in these tracts in MDD [10–12; 14; 15], no previous study reported a significant correlation between these tracts and age of onset of MDD. Future studies are needed to confirm this relationship and to expand on the clinical and behavioral correlates underlying this relationship.
There is increasing support for evaluating dimensional variables, rather than categorically grouping individuals based on heterogeneous symptoms, in order to identify biomarkers associated with psychiatric symptoms [60]. Therefore, the findings from the current study lend support for a relationship between depression severity, age of onset of depression, and white matter integrity. The main goal of the current study is to find moderators of treatment outcome, which doesn’t require a biomarker to distinguish controls and MDD subjects. In fact, it is likely that it will be difficult to find a strong biomarker linked to depression as an overarching construct that describes a clinically heterogeneous disorder. Since an important goal of this research is to find endophenotypes or biomarkers that are useful for diagnostic and prognostic purposes, as well as potential biomarkers for personalized treatment, we are hopeful that the current findings point in an interesting direction for further examination.
Conclusions
Overall, the findings from the current study should be considered a benchmark for future work, taking great care in the recruitment of a large number of participants who are well matched, and the acquisition of high-resolution data. Future studies should investigate dimensional characteristics within depression samples that may be linked to DTI measures in specific white matter tracts that could provide a connection between behavioral and biological measures. Parsing the clinically heterogeneous groups of depressed individuals, such as isolating those with early-onset depression, will be of significant value moving forward in order for us to understand how depression may be impacting the development of white matter connections in the brain.
Supplementary Material
Acknowledgments
We thank the study participants and the entire staff from each of the sites for their time and effort from the very onset of this study. This research was supported by a grant from the National Institute of Mental Health (U01-MH092250).
Footnotes
Conflict of interest statement: There are no conflicts of interest or financial disclosures related to this work.
References
- 1.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sexton CE, Mackay CE, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry. 2009;66(9):814–23. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Korgaonkar MS, Fornito A, Williams LM, Grieve SM. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol Psychiatry. 2014;76(7):567–74. doi: 10.1016/j.biopsych.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 4.aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. CMAJ. 2009;180(3):305–13. doi: 10.1503/cmaj.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao Y, Huang X, Wu Q, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2012;37(4):110180. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Ma N, Li Z, et al. Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Res. 2007;1168:124–8. doi: 10.1016/j.brainres.2007.06.094. [DOI] [PubMed] [Google Scholar]
- 8.Ma N, Li L, Shu N, et al. White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. Am J Psychiatry. 2007;164(5):823–6. doi: 10.1176/ajp.2007.164.5.823. [DOI] [PubMed] [Google Scholar]
- 9.Wu F, Tang Y, Xu K, et al. Whiter matter abnormalities in medication-naive subjects with a single short-duration episode of major depressive disorder. Psychiatry Res. 2011;191(1):80–3. doi: 10.1016/j.pscychresns.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu X, Wang X, Xiao J, et al. Altered white matter integrity in first-episode, treatment-naive young adults with major depressive disorder: a tract-based spatial statistics study. Brain Res. 2011;1369:223–9. doi: 10.1016/j.brainres.2010.10.104. [DOI] [PubMed] [Google Scholar]
- 11.Versace A, Almeida JR, Quevedo K, et al. Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol Psychiatry. 2010;68(6):560–7. doi: 10.1016/j.biopsych.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou K, Huang X, Li T, et al. Alterations of white matter integrity in adults with major depressive disorder: a magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33(6):525–30. [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy ML, Carballedo A, Fagan AJ, et al. Neurotrophic tyrosine kinase polymorphism impacts white matter connections in patients with major depressive disorder. Biol Psychiatry. 2012;72(8):663–70. doi: 10.1016/j.biopsych.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Guo WB, Liu F, Chen JD, et al. Altered white matter integrity of forebrain in treatment-resistant depression: A diffusion tensor imaging study with tract-based spatial statistics. Prog Neuropsychopharmacol Biol Psychiatry. 2012 doi: 10.1016/j.pnpbp.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Guo WB, Liu F, Xue ZM, et al. Altered white matter integrity in young adults with first-episode, treatment-naive, and treatment-responsive depression. Neurosci Lett. 2012 doi: 10.1016/j.neulet.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 16.Olvet DM, Peruzzo D, Thapa-Chhetry B, et al. A diffusion tensor imaging study of suicide attempters. J Psychiatr Res. 2014;51:60–7. doi: 10.1016/j.jpsychires.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao Y, Huang X, Wu Q, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38(1):49–56. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abe O, Yamasue H, Kasai K, et al. Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiatry Res. 2010;181(1):64–70. doi: 10.1016/j.pscychresns.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Kieseppa T, Eerola M, Mantyla R, et al. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J Affect Disord. 2010;120(1–3):240–4. doi: 10.1016/j.jad.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Korgaonkar MS, Grieve SM, Koslow SH, et al. Loss of white matter integrity in major depressive disorder: evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum Brain Mapp. 2011;32(12):2161–71. doi: 10.1002/hbm.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song YJ, Korgaonkar MS, Armstrong LV, et al. Tractography of the brainstem in major depressive disorder using diffusion tensor imaging. PLoS One. 2014;9(1):e84825. doi: 10.1371/journal.pone.0084825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ugwu ID, Amico F, Carballedo A, et al. Childhood adversity, depression, age and gender effects on white matter microstructure: a DTI study. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0769-x. [DOI] [PubMed] [Google Scholar]
- 23.Steele JD, Bastin ME, Wardlaw JM, Ebmeier KP. Possible structural abnormality of the brainstem in unipolar depressive illness: a transcranial ultrasound and diffusion tensor magnetic resonance imaging study. J Neurol Neurosurg Psychiatry. 2005;76(11):1510–5. doi: 10.1136/jnnp.2004.057612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giannelli M, Cosottini M, Michelassi MC, et al. Dependence of brain DTI maps of fractional anisotropy and mean diffusivity on the number of diffusion weighting directions. Journal of Applied Clinical Medical Physics. 2010;11(1):176–190. doi: 10.1120/jacmp.v11i1.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med. 2004;51(4):807–15. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- 26.Wu YH, Gau SS, Lo YC, Tseng WY. White matter tract integrity of frontostriatal circuit in attention deficit hyperactivity disorder: Association with attention performance and symptoms. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blood AJ, Iosifescu DV, Makris N, et al. Microstructural abnormalities in subcortical reward circuitry of subjects with major depressive disorder. PLoS One. 2010;5(11):e13945. doi: 10.1371/journal.pone.0013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korgaonkar MS, Cooper NJ, Williams LM, Grieve SM. Mapping inter-regional connectivity of the entire cortex to characterize major depressive disorder: a whole-brain diffusion tensor imaging tractography study. Neuroreport. 2012;23(9):566–71. doi: 10.1097/WNR.0b013e3283546264. [DOI] [PubMed] [Google Scholar]
- 29.Yeh FC, Tang PF, Tseng WY. Diffusion MRI connectometry automatically reveals affected fiber pathways in individuals with chronic stroke. Neuroimage Clin. 2013;2:912–21. doi: 10.1016/j.nicl.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abhinav K, Yeh FC, Pathak S, et al. Advanced diffusion MRI fiber tracking in neurosurgical and neurodegenerative disorders and neuroanatomical studies: A review. Biochim Biophys Acta. 2014;1842(11):2286–2297. doi: 10.1016/j.bbadis.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Abhinav K, Yeh FC, El-Dokla A, et al. Use of diffusion spectrum imaging in preliminary longitudinal evaluation of amyotrophic lateral sclerosis: development of an imaging biomarker. Front Hum Neurosci. 2014;8:270. doi: 10.3389/fnhum.2014.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- 33.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 34.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 35.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Z, Wang Y, Gerig G, et al. In: Quality control of diffusion weighted images. Liu BJ, Boonn WW, editors. 2010. pp. 86280J–76280J-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander DC, Barker GJ. Optimal imaging parameters for fiber-orientation estimation in diffusion MRI. Neuroimage. 2005;27(2):357–67. doi: 10.1016/j.neuroimage.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Jones DK, Basser PJ. "Squashing peanuts and smashing pumpkins": how noise distorts diffusion-weighted MR data. Magn Reson Med. 2004;52(5):979–93. doi: 10.1002/mrm.20283. [DOI] [PubMed] [Google Scholar]
- 39.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Salat DH, Greve DN, Pacheco JL, et al. Regional white matter volume differences in nondemented aging and Alzheimer's disease. Neuroimage. 2009;44(4):1247–58. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 42.Behrens TE, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34(1):144–55. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeh FC, Tseng WY. NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage. 2011;58(1):91–9. doi: 10.1016/j.neuroimage.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 44.Yeh FC, Verstynen TD, Wang Y, et al. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One. 2013;8(11):e80713. doi: 10.1371/journal.pone.0080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasan KM, Iftikhar A, Kamali A, et al. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 47.Friedman DP, Aggleton JP, Saunders RC. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the Macaque brain. J Comp Neurol. 2002;450(4):345–65. doi: 10.1002/cne.10336. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466(1):48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 49.Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10(4):420–30. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 50.Lebel C, Gee M, Camicioli R, et al. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340–52. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- 51.Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110(1):40–8. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- 52.Hare TA, O'Doherty J, Camerer CF, et al. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28(22):5623–30. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55(1):11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- 55.Nobuhara K, Okugawa G, Sugimoto T, et al. Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2006;77(1):120–2. doi: 10.1136/jnnp.2004.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacFall JR, Payne ME, Provenzale JE, Krishnan KR. Medial orbital frontal lesions in late-onset depression. Biol Psychiatry. 2001;49(9):803–6. doi: 10.1016/s0006-3223(00)01113-6. [DOI] [PubMed] [Google Scholar]
- 57.Kemp A, MacMaster FP, Jaworska N, et al. Age of onset and corpus callosal morphology in major depression. J Affect Disord. 2013;150(2):703–6. doi: 10.1016/j.jad.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 58.Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48(8):778–90. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- 59.Cohen RA, Grieve S, Hoth KF, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59(10):975–82. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 60.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.