Abstract
RNA interference is a conserved eukaryotic homology-dependent post-transcriptional gene silencing mechanism. The filamentous fungus Neurospora crassa is one of the first organisms used for RNAi studies. Quelling and Meiotic Silencing by Unpaired DNA (MSUD) are two RNAi related phenomena discovered in Neurospora and their characterizations have contributed significantly to our understanding of RNAi mechanisms in eukaryotes. More recently, a type of DNA damage-induced small RNA, microRNA-like small RNAs and Dicer-independent small silencing RNAs have been discovered in Neurospora crassa which can regulate gene expression. In addition, there are at least six different pathways responsible for the production of these small RNAs, indicating that this fungus is an important model system to study small RNA function and biogenesis. The RNAi studies in other filamentous fungi such as Cryphonectria paracitica and Aspergillus provide evidences that RNAi plays an important role in antiviral defense and RNAi mechanism is widely conserved in filamentous fungi, and RNAi has been commonly used as an efficient tool for studying the gene function. The discovery of the endogenous small RNAs from M. circinelloides further indicates the richness and complex of the RNAi field in eukaryotes.
Keywords: RNAi, quelling, meiotic silencing, microRNA, dicer-independent small RNAs, siRNA, qiRNA
Introduction
RNA interference (RNAi) is a conserved gene silencing mechanism at both post-transcriptional and transcriptional levels in eukaryotes, mediated by small silencing RNAs (sRNAs) which are small noncoding RNA molecules with various length of about 20-30 nucleotides [1-4]. RNAi includes such early phenomenal observations as co-suppression or post-transcriptional gene silencing (PTGS) in plants (1990), quelling in fungi (1992) and RNAi in animals (1998) [5-8]. In general, in the RNAi pathway, RNase III protein Dicer-like enzymes generate the small RNA duplexes from double-stranded RNA (dsRNA) precursors, then the small RNA duplexes are loaded onto the RNA-induced silencing complex (RISC) with an Argonaute family protein as the core catalytic component. Following the removal of the passenger strand of the small RNA duplex, the RISC is activated and uses the remaining strand as the guide to silence the targets [9-15]. The well known sRNAs functioning in RNAi pathways include at least the dicer-dependent microRNAs (miRNAs) and small interfering RNAs(siRNAs) and the dicer-independent small RNAs such as PIWI-interacting RNAs (piRNAs) (See reviews in [3-4,11,16-19]. miRNAs are processed from single-stranded RNA precursor transcripts containing hairpin structures, and can function through post-transcriptional gene silencing by mediating mRNA degradation or translational repression, or through transcriptional gene silencing involving DNA methylation or chromatin modifications. siRNAs are generated from dsRNA procursors, can act through post-transcriptional gene silencing pathways and transcriptional gene silencing pathways. piRNAs, found till now only in animals, derive mainly from repetitive elements, transposons and large piRNA clusters by matching only one DNA strand, and may function to protect germline integrity by targeting repetitive DNA and transposons involving in post-transcriptional gene silencing and transcriptional gene silencing pathways, though the functions of many piRNAs are still not known (See reviews in [3-4,11,16-19].
The filamentous fungus Neurospora crassa is one of the first eukaryotic model system for RNAi studies, and RNAi pathways in filamentous fungi have been mainly discovered from Neurospora crassa. As an excellent experimental model for more than 70 years, Neurospora crassa is in fact also a leading organism in RNAi studies, and studies on this simple fungus has been contributing greatly to the understanding of RNAi. Filamentous fungi include many important human and agricultural pathogens, antibiotics and other important fungal chemical producing groups, food producers such as mushrooms, symbiotics such as mycorrhizal fungi, nutrient recyclers and so on, which greatly influence our daily life. RNAi is widely present in filamentous fungi, and studies and applications of RNAi in filamentous fungi have not only been leading us to better understand how genes in these organisms function, but also been contributing significantly to find out more novel RNAi mechanisms. This review will focus on the discoveries and studies of the RNAi in Neurospora, then discuss the studies and the applications of RNAi in some other filamentous fungi.
Quelling/PTGS (or: RNAi in vegetative cells in Neurospora crassa)
Discovery of quelling
Quelling was discovered in Neurospora crassa by Macino group in 1992, the first transgene-induced gene silencing phenomenon described in fungi [6]. It is the second post-transcriptional gene silencing mechanism ever reported in eukaryotes, second to “co-suppression” in plants and followed by “RNAi” in animals [7]. Quelling was originally found by transforming exogenous albino-1 (al-1) or albino-3 (al-3) sequences into a typical orange wild type strain, which caused severe down-regulation of the endogenous al-1 or al-3 genes, two of the structural genes for biosynthesis of carotenoids, resulting in the albino (white) phenotype in up to 36% of the transformants [6,20-21]. This phenomenon is reversible spontaneously and progressively, as some of the albino transformants could revert back to wild type or intermediate phenotypes over a prolonged time of culture or after some subcultures, which was found to be correlated with the reduction of the copy number of the exogenous sequences integrated and accompanied by the increased steady-state mRNA levels of the corresponding endogenous genes [6]. Though quelled strains could contain only 1 to 2 ectopic integrated sequences of transgenes, both high copy number and a tandem arrangement of the transgenes seem to correlate with the successful triggering of quelling or more stable quelling [6,20-23]. It is interesting to notice that once quelling is relieved it cannot be triggered again in the reverted strains even in the presence of the ectopic sequences [6].
Quelling silences both the transgenes and the homologous endogenous genes in vegetative growth cells, with the minimum length requirement of the homologous transcribed region of 132 nt for sufficient quelling induction, though the promoter region itself is not required for and cannot induce quelling [6,20-21]. The silencing effect of quelling is posttranscriptional as the steady-state levels of the primary transcript (the precursor RNA) in quelled and non-quelled strains were unchanged though the stead-state levels of the mature mRNA of the silenced gene were reduced significantly in quelled strains [20].
al-1 mutation is generally recessive, i.e., albino phenotype should be generally observed in homokaryotic al-1 mutants but not in heterokaryotic al-1 mutants. However, many albino (quelled) transformants induced by trangenic al-1 were heterokaryons, and about 95% of the forced heterokaryons created from a homokaryon of the wild type al-1 and an albino homokaryotic transformant induced (quelled) by exogenous al-1 were albino [20]. These results demonstrate that silencing of al-1 by quelling is dominant in heterokaryons and is not nucleus-limited thus can act in-trans, indicating that quelling does not require ectopic pairing or DNA-DNA interactions and quelling is mediated by a diffusible trans-acting molecule [20]. This could be the first indication of the involvement of small RNAs in RNAi.
A transgene-derived sense RNA produced from promoterless al-1 transgenes was found to be accumulated in quelled strains but absent in the reverted strains, indicating that transcription of transgenes is required for quelling, though the amount of sense transcripts did not seem to be related to the quelling establishment [20]. The observation of the unexpected sense RNA of the transgenes, which was thought to be qualitatively different from mRNA and thus is aberrant, led to the hypothesis that production of aberrant RNA in the presence of transgenes causes the post-transcriptional gene silencing, where the aberrant RNA could be recognized by an RNA-dependent RNA polymerase to produce dsRNA and then further resulting in mRNA degradation [21,23-26]. This hypothesis was proposed before Fire and Mello's discovery of RNAi [7].
As mentioned above, introduction of al-1 transgenes could result in quelling up to 36%, i.e., still a large portion of transformants were not quelled, thus it is not sufficient for trangenes alone to induce quelling [6,26]. In fact, only transformants harbored duplicated sequences showed silencing, and high copy number and tandem insertions of the trangenes were usually correlated with quelling [21-23,26]. A stably quelled strain by transgenic al-1 was shown to contain tandem repeats of the transgenes [21]. These could indicate that tandem repeats of trangenes is a source for aberrant RNA [26].
Mechanism of quelling and dsRNA-induced RNAi in N. crassa
Taking advantage of a stably quelled strain by transgenic al-1 and using forward genetics approach by UV mutagenesis, Cogoni and Macino isolated 15 quelling defective mutants belonging to three distinct genetic loci qde-1, qde-2 and qde-3, which were defective in all tested transgene-induced gene silencing [21]. These three loci were further studied and the corresponding genes were cloned which encodes respectively three key components required for Quelling by Macino's group: QDE-1 (QUELLING DEFICIENT-1, an RNA-dependent RNA polymerase), QDE-2 (an Argonaute protein), QDE-3 (a RecQ DNA helicase homologous to the Werner/Bloom Syndrome proteins) [21,27-29]. Later small RNAs of about 25 nt were found to be specifically involved in quelling, and the production of these small RNAs required qde-1 and qde-3, but not qde-2 [30]. Genes for two partially redundant Dicer proteins DCL-1 (Dicer-like-1) and DCL-2 (Dicer-like-2) were further identified and characterized by reverse genetics thanks to the release of the whole genome sequence of N. crassa [31-32]. In 2007 Liu lab identified QIP, an exonuclease, as another key component required for RNAi [12]. Below more detailed information will be discussed about the identification and functions of these components.
Isolation of qdes and Production of dsRNA
qde-1 is the first gene ever cloned encoding a cellular component of the posttranscriptional gene silencing nechanism [27]. The qde-1 insertional mutant was identified by random insertional mutagenesis on a stable quelled al-1 transgenic strain as that mentioned above for identification of the three classes of mutants qde-1, qde-2 and qde-3, and the functional qde-1 gene was isolated from cosmids from N. crassa cosmid library, which rescued both the qde-1 insertional mutant and the UV-mutagenized mutant [21,27]. Since QDE-1 is homologous to an RdRP encoded by a tomato cDNA, the only homologue with known function at that time, the finding of QDE-1 provided the first experimental evidence that an RdRP is involved in PTGS, which supported the model that aberrant RNA produced directly from transgenes or resulted from the presence of transgenes could be used as the template by an RdRP to lead to the production of dsRNA and finally degradation of mRNA [23,27]. The wide presence of QDE-1 homologues in plants, fungi and C. elegans indicate that a conserved PTGS mechanism involving RdRP may exist in all these organisms [27].
Later it was known that qde-1 is homologous to sgs-2 in Arabidopsis and ego-1 in C. elegans, and both of them are required for RNAi [33-35]. The presence of an RdRP respectively to be required for quelling in fungi, PTGS in plants and RNAi in C. elegans (which further suggested that these three silencing phenomena are originated from one same silencing mechanism) in certain sense not only confirmed the hypothesis that in all these silencing phenomena an RdRP could play a role in the synthesis of complementary RNA and thus the production of dsRNA(a key intermediate component leading to specific RNA degradation) in the absence of direct dsRNA production from inverted repeat transgenes or exogenously provided dsRNA, but also indicated that an RdRP may function in the amplification of the dsRNA signal thus amplification of the silencing signal spreading from cell to cell [7-8,20,24,27,33,35-38]. Thus the finding of the first RdRP QDE-1 in RNA silencing somehow not only further supported such hypothesis in Fire and Mello's seminal RNAi discovery paper as the presence of a catalytic or amplification component in the silencing process, but could further provide more explainations to the observed silencing phenomena such as why the introduction of single stranded RNAs or transgenes could still induce silencing [7]. Now QDE-1 RdRP activity has been confirmed with its structure resolved, and it is the first cellular RNA-dependent RNA polymerase involved in posttranscriptional gene silencing that was shown to have RNA-dependent RNA polymerization activity in vitro [39-42]. It was originally postulated that the transcription of a transgene by RNA polymerase II produces an aberrant RNA, which is used as the substrate by QDE-1 to generate a dsRNA [42]. In fact, we know now aberrant RNA is not transcribed by common RNA polymerases (See below).
Back to early 2000, Cogoni noticed that the qde-2 gene required for quelling is homologous to the rde-1 gene required for the dsRNA interference in C. elegans, which provided the first evidence experimentally that dsRNA interference and trangene-induced PTGS have a common genetic component and indicated that they could evolve from the same ancestral mechanism, and dsRNA could be involved in quelling in fungi [29]. Soon after that, the identification and characterization of AGO1 from Arabidopsis, which is required for PTGS in plants and is homologous to QDE-2 (required for quelling) and RDE-1 (required for RNAi), provided another direct experimental evidence that PTGS in plants, quelling in fungi and RNAi in animals are related and could come from the same ancestral silencing mechanism [8]. Together with the requirement of an RdRP (SGS2, QDE-1 and EGO-1, respectively) mentioned above, PTGS, quelling and RNAi thus were linked together [8].
The isolation of the class of qde-3 mutants prompted the identification and characterization of qde-3 gene encoding a member of the RecQ DNA helicase family, which based on the homology could function in the quelling activation step through the DNA-DNA interaction between transgenes introduced or with an endogenous gene [28]. QDE-3 is another key protein discovered required for activation and maintenance of posttranscritional silencing, which was the first reported new function for a DNA helicase [28,43]. Though the exact role in quelling is still largely unknown, QDE-3 acts probably only upstream of the dsRNA formation, for introduction of constructs expressing dsRNA resulted in efficient gene silencing in qde-3 mutants [28,43]. QDE-3 itself and another RecQ-type DNA helicase RecQ-2 seem to play roles in DNA repair, which are not known to have any direct relationship with the quelling function [28,44-45]. OsRecQ1, a QDE-3 homologue in rice, was found to be required for RNA silencing induced the by introduction of inverted-repeat DNA, but not for RNA silencing induced by the introduction of constructs expressing dsRNA, which is similar to QDE-3 [46]. The levels of transcripts from the plasmid of the inverted-repeat DNA constructs were significantly decreased in the OsRecQ1 mutants compared to the wild type, indicating that OsRecQ1 is required for the efficient transcription from the plasmid construct producing inverted repeat RNA, which could be the aberrant RNA required for dsRNA formation [46]. This observation somehow may indicate or provide an experimental support for the hypothesis that QDE-3 may promote proper transcription of the transgenes or the production of aberrant RNA [46]. OsRecQ1 did not seem to influence the accumulation of some endogenous microRNAs tested and the production of the short interspersed nuclear element retroelement by small interfering RNA, which is similar to our observations of QDE-3 being not required for the production of endogenous small RNAs in Neurospora (see below), though rRecQ-1, a close homologue of QDE-3 in rats was reported to be correlated with piRNAs [42,46-47]. The efficiency of transgene induced quelling is usually around one third of the total transformants [6,42]. However, overexpression of the RdRP QDE-1 could dramatically elevate the quelling efficiency up to 92%, and furthermore, lower number of transgenes were needed to induce silencing and the quelled transformants were more stable despite progressive loss of tandemly repeated transgenes when QDE-1 was overexpressed; and the inverted-repeat constructs expressing dsRNA instead of transgenes can result in up to 80% quelling efficiency, all indicating that dsRNA production could be the limiting factor for quelling efficiency, and on the other hand, the activation and maintenance of transgene-induced silencing may depend on both the cellular amount of QDE-1 and the number of the transgenes integrated which could probably at the end influence the amount of dsRNA produced [42-43,48-49].
Though RNAi in different systems has been extensively studied, how are repetitive sequences first distinguished from endogenous genes before the dsRNA formation is not clear yet. It is believed that aberrant RNA (aRNA) synthesis and its specific recognition by RNA-dependent RNA polymerases to make double-stranded RNA are required for transgene induced RNAi, though the related mechanisms are not quite clear. In N. crassa, QDE-1 was proposed to specifically recognize and convert aRNA into dsRNA. By using tagged QDE-1 to immunopurify QDE-1-containing protein complexes, Nolan et al. demonstrated that QDE-1 interacts in the nucleus with RPA-1, the Neurospora homologue of the largest subunit of Replication Protein A which is a single-stranded DNA-binding protein important for DNA replication, repair and recombination [50]. Based on immunoprecipitation by the anti-FLAG antibody, FLAG QDE-1 was found enriched at the transgenic al-1 locus but not the unrelated, non-silenced endogenous actin gene, demonstrating that QDE-1 is recruited to the transgenic locus triggering silencing [50]. The accumulation of siRNAs is DNA synthesis dependent, as hydroxyurea treatment of mycelia to inhibit DNA replication abolished siRNAs accumulation [50]. Thus it was proposed that during replication transgenes are distinguished by QDE-1 interacting with RPA-1 from endogenous genes and then targeted for silencing via dsRNA production by QDE-1[50]. A question unanswered is how RPA-1 is guided to the transgenic sequences instead of the rest of the genome [50]. One speculation is that the RecQ DNA helicase QDE-3 may be involved in this process since the RPA and the homologues of QDE-3 in other organisms have been found to be related to preventing genome instability [50]. Biochemical analyses recently demonstrated that QDE-1 is both an RdRP and a DNA-dependent RNA polymerases (DdRP) (See below qiRNA part for detailes), further indicating that QDE-3 and RPA may facilitate QDE-1 to bind to transgenic region (probably QDE-3 could resolve the complex DNA structures at the transgenic locus created upon tandem integration, and RPA could recruit QDE-1 to the resolved transgenic ssDNA), and then QDE-1 could transcribe the transgenic DNA into aberrant RNA which will be further converted into dsRNA again by QDE-1, though much more work needs to be done to find direct in vivo evidence for this hypothesis [42,50-51].
Introduction of transgene constructs expressing intron-containing self-complementary RNA, i.e., direct expressed dsRNA, could successfully bypass quelling essential genes qde-1 and qde-3 to induce gene silencing with high efficiency and higher quelling stability, which further supports the notion that dsRNA is a necessary intermediate for quelling and QDE-1 and QDE-3 both function in the dsRNA production pathway [43]. On the other hand, functional qde-2 and dcl-1 dcl-2 were still needed for quelling under direct expression of dsRNA conditions, further demonstrating that dsRNA production is upstream of the dicer processing and the QDE-2 containing RISC formation and activation [43].
Generation of siRNA
Catalanotto et al. found in N. crassa both sense and antisense short RNAs 25-nt in length specifically accumulated in silenced transgenic strains, demonstrating that siRNAs are involved in quelling [30]. At this time, it was known that dsRNA can be processed by Dicer into 21 to 23 nt small RNAs in Drosophila and antisense small RNAs with size of about 25 nt were associated with PTGS in plants, and 21 nt small RNA duplexes could induce sequence-specific RNA degradation; moreover, siRNAs were found to be associated with RISC degrading homologous mRNAs of the silencing trigger, and AGO2, a member of Argonaute proteins was found to belong to the RISC nuclease [9,52-55]. As mentioned above, a member of Argonaute family proteins is required respectively for quelling in fungi, PTGS in plants and RNAi in animals, and thus a hypothesis at this time was that similar siRNA-directed mechanism could be present in all the three major branch of eukaryotes. Catalanotto et al. further found that though requiring functional qde-1 and qde-3, small RNA accumulation is not dependent on the quelling essential component functional qde-2, whereas QDE-2 copurified with siRNAs, indicating that QDE-2 could be a component of the siRNA-directed RISC [30]. Interestingly, besides those corresponding to transgenes, 25nt siRNAs coming from the vector of the transgene construct were also detected specifically in the silenced strains, suggesting that the chimeric transgenic transcripts (thus aberrant RNA) were recognized and converted into dsRNA by the quelling machinery including probably QDE-3 and QDE-1, and then were processed by dicer-like protein into siRNA [30].
In N. crassa Dicer-like proteins DCL-1 and DCL-2 process dsRNA into about 25 nt small RNAs in an energy dependent manner [31]. Double mutation of the two Dicer-like genes dcl-1 and dcl-2 completely abolished quelling and disrupted the processing of dsRNA into siRNA in vivo and in vitro, but both single mutants had similar quelling frequency to the wild type, and both could process dsRNA into short RNA about 25 nt, suggesting that the two Dicers are redundant, which could be why the two dicer genes escaped the earlier screening for quelling defective mutation [21,31]. However, the accumulation of siRNA was significantly reduced in the dcl-2 mutant compared to the level in the wild type, though that in the dcl-1 mutant was similar to that of the wild type, indicating the dsRNA processing activity is mostly dependent on DCL-2 [31]. These studies of the dicer function in Neurospora was the first demonstrating the involvement of Dicer in transgene induced gene silencing and supported the notion that the dsRNA produced by the cellular RdRP from transgenic transcripts is an essential silencing component [31].
Activation of RISC
QDE-2 is an Argonaute protein in N. crassa, which is among the earliest discovered protein family (QDE-2, AGO1 and RDE-1respectively) required for RNAi in fungi, animals and plants and linked together the three phenomena of co-suppression or PTGS in plants, quelling in fungi, and RNAi in animals as mentioned above [8,56]. QDE-2 is the core component of the RISC complex and is associated with siRNA [29-30]. QDE-2 and its slicer activity are required for gene silencing and the generation of single-stranded siRNA from siRNA duplexes in vivo [12]. Mutation of the qde-2 gene or the catalytic site of QDE-2 abolished the gene silencing and the single-stranded siRNA production. Wild type QDE-2 was associated with single-stranded siRNA, while the catalytic site mutated QDE-2 was only associated with siRNA duplex, indicating that in N. crassa RISC is loaded with double-stranded siRNA before the cleavage and removal of the passenger strand [12].
Another key component for quelling is QIP (QDE-2-interacting protein), an exonuclease domain containing protein which was identified by purifying QDE-2 biochemically from N. crassa [12]. At that time, it was known that exogenous or endogenous dsRNAs initiate the RNAi pathway conserved in plants, animals and fungi, which are then processed into about 20 to 25 nt siRNA duplexes; RISC is the RNAi effector complex with an Argonaute family protein as the catalytic core, and the siRNA duplex is loaded onto the RISC and is then separated with the passenger strand removed but the guide strand retained in the RISC and directing the mRNA target cleavage. It was found that Ago2 in Drosophila could bind and cleave the passenger strand of the siRNA dulex during RISC activation, and the embryo lysate from the Ago mutant could not produce single-stranded siRNA in vitro, indicating that the passenger strand removal is mediated by Argonaute family protein [57-59]. It was also proposed that an unknown factor may be involved in the removal of the cleaved passenger strand of siRNA duplex [57,60]. By utilizing a dsRNA expressing construct and a Myc-epitoped tagged QDE-2 and Myc-epitoped tagged QDE-2 (D664A), where one of the catalytic residues was mutated, Maiti et al. demonstrated in vivo that in N. crassa the Argonaute QDE-2 and its slicer activity are indispensible for single-stranded siRNA generation and effective silencing, which was supported by another paper at the same time showing similar function requirement of Ago2 cleavage activity during RISC activation in Drosophila [12,61]. Further experiments using Myc-His-epitope-tagged QDE-2, QIP, the QDE-2-interacting protein containing an exonuclease domain, was identified [12]. Mutation of the qip gene caused the accumulation of siRNA duplexes and impairment of gene silencing, and further analyses showed that QIP functioned as an exonuclease by removing the nicked passenger strand from the siRNA duplex in a QDE-2-dependent manner by interacting with QDE-2, which then results in RISC activation [12]. Thus QIP is not only an key player in PTGS promoting RISC activation by removing the nicked passenger strand from the siRNA duplex, it is probably the first reported exonuclease required for efficient RNAi in eukaryotes since especially the exogenuclease domain of QIP is required for its function [12]. Since PTGS is widely conserved in eukarytoes, similar function of QIP for RISC activation by removal of the passenger strand from siRNA duplexes may be present in other organisms. In Drosophila, an endoribonuclease C3PO was demonstrated biochemically to activate RISC by removing the cleavage products of siRNA passenger strand in an Ago2-dependent manner, which is similar to the function of QIP, indicating that similar function of QIP may be performed by different type of proteins in different systems [62]. The nick in duplex siRNA created by Ago2 cleavage could be the stimulator of C3PO RNase activity, indicating that the nuclease activity of QIP could be stimulated by the nicked siRNA duplex processed by QDE-2 [62].
Based on the above studies of QDE-2 and QIP, a model for the conserved RNAi pathway can be proposed like this [12]: dsRNA is processed into siRNA duplexes by dicer protein(s), which are loaded onto the RISC, then the Argonaute protein (QDE-2) cleaves the passenger strands of the siRNA duplexes, and an Argonaute associated protein (like the exonuclease QIP or other proteins with similar function) removes the nicked passenger strands with the guide strands remaining in the RISC complexes and activates the RISCs to silence mRNAs with homologue to the guide strands(See Figure 1).
Figure 1. A model for RNAi/PTGS pathways in vegetative cells in N. crassa.
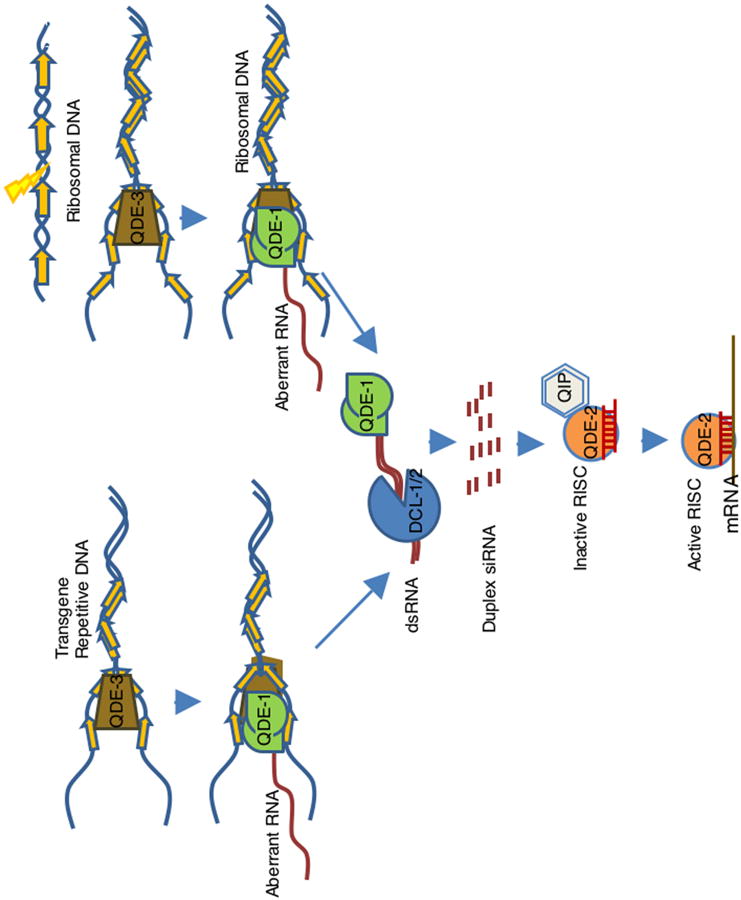
This model puts together (a) transgene-induced PTGS (quelling), (b) dsRNA-induced PTGS and (c) DNA-damage induced qiRNA pathway. Transgenes (quelling) or DNA-damage induce the synthesis of aberrant RNAs by the DdRP activity of QDE-1 facilitated by QDE-3 and RPA, where QDE-3 could probably resolve into ssDNA the complex DNA structures at the transgenic locus created upon tandem integration or caused by DNA damage, and RPA could recruit QDE-1 to the ssDNA. The aberrant RNA is then transcribed into dsRNAs by the RdRP activity of QDE-1. The Dicer proteins DCL-1 and DCL-2 cleave the dsRNAs, formed by QDE-1 or expressed directly from dsRNA-expressing constructs, into approximately 25 nt siRNA duplexes (or 20-21nt qiRNAs for DNA damage induced dsRNA), which are then loaded onto the RNA-induced silencing complex (RISC) containing QDE-2 and QIP. QDE-2 and QIPconvert the siRNA duplex into the mature siRNA, resulting in RISC activation to silence targets with homology to the siRNA. qiRNA bind to the core component of QDE-2, and play roles in response to DNA damage, but it is not known if qiRNAs function through the RISC containg QDE-2 and QIP.
Functions of RNAi in Neurospora
RNAi can play roles in genome defense against viruses and transposons, development regulation and chromosomal segregation [63-64] [3]. Quelling in Neurospora can function in silencing the transgenes by detecting and targeting the transgenetic DNA. Quelling can also act to repress the expression and expansion of transposons, for siRNAs against transposons were detected in N. crassa, and the transcript levels and copy number of the LINE1-like transposon Tad were significantly elevated in qde-2 mutants and Tad transcripts accumulated significantly in the dcl-1 dcl-2 double mutants [65-66].
RNAi is believed to be involved in heterochromation formation and/or DNA methylation in fission yeast, plants and animals, but no any of the known RNAi components in Neurospora, incuding three RdRPs (QDE-1, SAD-1 AND RRP-3), two Argonaute proteins (QDE-2 AND SMS-2), two dicer-like proteins (DCL-1 or SMS-3, DCL-2) and two RecQ helicases (QDE-3 and RecQ-2), is indispensable for either initiation or maintenance of heterochromatin nor DNA methylation [67]. Chicas et al. further demonstrated that transgenic siRNA production/quelling is not required for the histone H3 Lys9 methylation [65]. Thus RNAi thus far does not seem to function in heterochromation formation and DNA methylation in Neurospora. It was found that the transgenic al-1 locus was hypermethylated at LysH3 in both the silenced strain and strains with mutation background of qde-1, qde-2 and qde-3 rescpectively, but mutation of the histone Lys9H3 methyltransferase gene dim-5 caused lower efficiency of PTGS, and the silenced transformants lost rapidly the silenced phenotype and the integrated transgenic copies, and thus could not maintain the silencing [43]. The defect of dim-5 mutants in silencing seems to be only resulted from the failing to maintain the transgene in tandem, indicating DIM-5 may play a role in maintaining the transgene in tandem [65].
Recently Cecere and Cogoni reported that the rDNA gene copy numbers in the quelling mutants qde-1, qde-2 and qde-3 are all significantly reduced, indicating that quelling may play an important role in maintaining the rDNA locus integrity and stability [68].
A dsRNA-induced transcriptional program important for RNAi
Interestingly, Choudhary et al. showed that dsRNA production can significantly induce the expression of the key RNAi components qde-2 and dcl-2, while modestly induce the expression of qde-1, dcl-1 and qip [69]. dsRNA mediated induction of QDE-2, both transcriptionally and posttranscriptionally, is indispensable for efficient RNAi, and the posttranscriptional regulation is Dicer dependent, indicating that dicers or siRNA may play a role in QDE-2 accumulation.
The transcriptional responses were regulated by dsRNA instead of siRNA, as the transcriptional activation of dsRNA-activated genes (DRAGs) was present in the dcl double mutant, which abolished the siRNA production [69]. Genome-wide analysis by microarray and quantitative PCR analysis demonstrated that dsRNA could activate 60 genes including additional RNAi components and homologs of antiviral and interferon-stimulated genes. The latter indicates that the dsRNA induced activation of RNAi components is part of conserved ancient host defense response to counter against viral infection and retrotransposons [69]. The signaling pathway responsible for the dsRNA response is not clear yet in Neurospora. Since the key genes for RNAi including qde-2, dcl, and qde-1, are not required for dsRNA induced transcriptional activation, and Neurospora does not contain the well-known mammalian dsRNA sensors PKR and Toll-like receptor 3, a novel dsRNA-sensing and transcriptional activation pathway might be present instead in Neurospora [69].
The DNA damage-induced qiRNA and its relationship with Quelling
It was observed that the addition of histidine in the medium but not other amino acids significantly elevated the levels of qde-2 mRNA and QDE-2 protein [51]. Since histidine can cause DNA damage in Neurospora, other DNA damage agents such as ethyl methanesulphone (EMS), hydroxyurea andmethylmethanesulphonate were also tested which were shown to be able to induce QDE-2 expression. The induction of QDE-2 expression by these DNA damage regents including histidine dependends on the functional QDE-1, QDE-3 and the two Dicers. Moreover, QDE-2 levels were found increased in many DNA repair mutants compared to the wild type. Thus qde-2 expression can be induced by DNA damage [51]. On the other hand, since dsRNA can induce the expression of QDE-2 in a dicer dependent manner, and QDE-1 and QDE-3 are required for dsRNA generation from transgenes in quelling, thus the dependence of QDE-1, QDE-3 and Dicers for QDE-2 induction by the DNA damage regents suggest that DNA damage may cause dsRNA production endogenously which results in the induction of qde-2 expression [51,69](See also other related references above). These dsRNAs caused by DNA damage could then be processed into small RNAs by Dicers and bind to QDE-2 as those siRNAs produced in quelling.
Analysis of QDE-2-associated small RNAs using immunoprecipitated Myc-tagged QDE-2 expressed in a qde-2 mutant background did show that a novel class of 20 to 21 nt long small RNAs significantly induced by histidine or EMS are specifically associated with Myc-QDE-2. Levels of these small RNAs were very low under normal growth conditions while were induced robustly by treating with the DNA-damaging agents histidine, hydroxyurea or EMS. These small RNAs are shorter than the regular 25 nt quelling siRNAs and were named qiRNAs for the interaction with QDE-2 [51]. Sequencing analyses showed that qiRNAs originate mainly (about 86%) from the highly repetitive rDNA locus, have a strong 5′ uridine preference and also a 3′ preference for adenine, together with the specific association with QDE-2, indicating qiRNAs are not random nonspecific degradation products [51].
qiRNA production depends on QDE-1, QDE-3 and the Dicers, for no qiRNA was detected in the qde-1 and qde-3 single mutants and in the dcl-1 dcl-2 double mutants. qiRNAs are corresponding to both sense and antisense strands with similar numbers, and abundant long RNAs accumulated in the dcl-1 dcl-2 double mutant with DNA damage, indicating that qiRNAs are processed from long dsRNAs. Though DNA damage induces the expression of QDE-2, qiRNA production is not dependent on QDE-2. qiRNAs produced from the rDNA locus (the major qiRNA producing locus) match to not only mature rRNA regions, but also many external and internal transcribed spacer regions and the intergenic spacer regions, suggesting that qiRNAs originate from unconventional transcripts, i.e., qiRNAs require aberrant RNAs for biogenesis. qRT-PCR and northern blot analyses both demonstrated that the transcripts matching to both upstream and downstream of the transcribed rDNA region, which are aberrant RNA, were robustly induced in the wild type strain by DNA damage, while in the dicer double mutant, these aberrant transcripts accumulated abundantly with sizes from a few hundred nucleotides to about 2 kilobases, indicating that these aberrant transcripts are the precursors of the dsRNA. Treatment with a potent inhibitor (thiolutin) of RNA polymerases I,II and III could not inhibit the synthesis of aberrant RNA, further indicated that these DNA-damage induced transcripts are not conventional transcripts and do not require common RNA polymerases for the synthesis. The aberrant RNA was completely abolished in both the qde-3 mutant and qde-1ko mutant, which is a strong sign that the RecQ DNA helicase QDE-3 and the RdRP QDE-1are both indispensible for the synthesis of the DNA-damage-induced aberrant RNA [51]. Further studies using purified QDE-1 demonstrated that QDE-1 could generate full-length RNA products using either ssRNA or ssDNA as a template, and the products from ssDNA were mostly DNA/RNA hybrids [51]. And on the other hand, the common RNA polymerase inhibitor could not inhibit the RNA polymerase activity [51]. Thus QDE-1 is both an RdRP and a DdRP, indicating QDE-1 is the RNA polymerase to generate aRNA besides required for the production of dsRNA [51].
Besides the qde-3 mutant sensitive to DNA damaging agents mentioned above during qde-3 characterization and here lacking qiRNA production thus playing roles in DNA damage response, other RNAi mutants lacking qiRNA production such as qde-1 single mutant and dcl-1 dcl-2 double mutants were all shown to have increased sensitivity to DNA damage, all of which indicate that qiRNAs may function in the DNA-damage response by inhibiting protein translation, and RNAi/quelling in Neurospora may play roles in the DNA damage response [51].
In fact, both quelling and the DNA-damage-induced qiRNA pathway share all the key components such as QDE-1, QDE-2, QDE-3, Dicers and small RNAs (25 nt and 20-21nt respectively) and all need aberrant RNA production and dsRNA production, except the former is transgene induced and the latter is DNA damage induced, though it is not known if qiRNAs can function like transgene induced siRNAs to target the homologous mRNA through RISC with QIP to remove the nicked passenger strand for the activation. Based on the above, a common model can be proposed for transgene (and dsRNA, if expressed directly)-induced gene silencing and the DNA-damage-induced qiRNA pathway as below: Transgenes (quelling) or DNA-damage induce the synthesis of aberrant RNAs by the DdRP activity of QDE-1 facilitated by QDE-3 and RPA, where QDE-3 could probably resolve into ssDNA the complex DNA structures at the transgenic locus created upon tandem integration or caused by DNA damage, and RPA could recruit QDE-1 to the ssDNA. The aberrant RNA is then transcribed into dsRNAs by the RdRP activity of QDE-1. The Dicer proteins DCL-1 and DCL-2 cleave the dsRNAs, formed by QDE-1 or expressed directly from dsRNA-expressing constructs, into approximately 25 nt siRNA duplexes (or 20-21nt qiRNAs for DNA damage induced dsRNA), which are then loaded onto the RNA-induced silencing complex (RISC) containing QDE-2 and QIP. QDE-2 and QIP convert the siRNA duplex into the mature siRNA, resulting in RISC activation to silence targets with homology to the siRNA. qiRNA bind to the core component of QDE-2, and play roles in response to DNA damage, but it is not known if qiRNAs function through the RISC containg QDE-2 and QIP.
Meiotic Silencing by Unpaired DNA (MSUD)
Discovery of MSUD
Another PTGS mechanism discovered in N. crassa is the meiotic silencing by unpaired DNA (MSUD), which is similar to quelling but only present during meiosis [70-72]. N. crassa is generally haploid, though a transient diploid ascus cell is present when the two nuclei of opposite mating fuse (karyogamy) [73]. The diploid cell quickly goes through two rounds of meiosis and then one mitosis, resulting in eight ascospores each containing one nucleus [70,72-73]. MSUD functions in the first meiotic prophase by silencing all copies of the unpaired gene during the pairing of homologous chromosomes, though the silencing effects seem to be contained within the ascus (or asci) where the unpaired DNA is present [71-72]. In fact, MSUD originally was called meiotic transvection (or meiotic trans-sensing) though it was not clear yet at that time it is gene silencing related, referring the phenomenon that proper function of the ascospore maturation 1 gene asm-1+, i.e., the proper maturation of ascospores requires asm-1+ being in proximity or paired to its allelic counterpart in the transient diploid zygote [70-71,74-76]. Further experiments demonstrated that it is the absence of unpaired copies of asm-1+ is the real requirement for ascospore maturation, thus the name MSUD was the preferred name, describing the meiotic silencing by unpaired DNA of all the paired and unpaired homologues in the whole genome [71,76]. In the review of Kelly and Aramayo (2007), Meiotic trans-sensing and meiotic silencing are proposed to be two different though highly related mechanisms, with the trans-sensing scanning the presence or absence of the paired homologous genes and the presence of unpaired gene resulting in the meiotic silencing of all the copies present in the genome [77]. The view could be inferred by the observation that DNA methylation affected meiotic-sensing without influencing meiotic silencing [78].
Mechanism of MUSD
In order to figure out the silencing mechanism of MUSD, extensive screening for mutation by UV-irradiation passing through the cross in the presence of unpaired asm-1+ was performed [71-72]. Sad-1(suppressor of ascus dominance-1), a paralog of qde-1 is the first suppressor of MSUD identified, and its mutation resulting from either UV-irradiation, RIP or deletion successfully suppresses the MSUD [71-72]. SAD-1 shares high identities with RdRPs involved in post transcriptional silencing, such as SDE-1 from A. thaliana, EGO-1 from C. elegans and QDE-1 in N. crassa, indicating dsRNA synthesis may be involved in MSUD [71-72]. The requirement of the RdRP SAD-1for MSUD, and the silencing of paired copies as well as the unpaired copy itself by an unpaired DNA suggest that MSUD is a post transcriptional silencing [71].
Sms-2 (suppressor of meiotic silencing-2) was identified from the Neurospora genome encoding the paralog of the Argonaute QDE-2, and was later demonstrated to be required for MSUD by analyzing the sms-2 loss-of-function mutants [32,79].
sad-2, another dominant suppressor of meiotic silencing, is required for the proper location of SAD-1 which is important for SAD-1 function, and mutation of sad-2 suppresses MSUD [80-81]. SAD-1 and SAD-2 colocalize in the perinuclear region, and very likely interact each other physically in vivo based on the Bimolecular fluorescene complementation (BiFC) analysis [80-81]. SAD-2 localizes in the perinuclear region even in the absence of SAD-1 but not vice vesa [81]. Thus SAD-2 is a novel protein involved in RNA silencing, and may function by recruiting SAD-1 to the perinuclear region [80].
DCL-1, one of the two dicer-like proteins in N. crassa, also called SMS-3 (suppressor of meiotic silencing-3, though it is not a real dominant meiotic silencing suppressor, see below), is found to be required for MSUD [32,77,82]. A homozygous cross of dcl-1 deletion mutants is barren (but it is normal for dcl-2 deletion mutants), which is also true for the homozygous cross of the mutants of sad-1 and sad-2 respectively [71,80,82]. However, though asci were observed for the homozygous cross of both the sad-1 mutants and sad-2 mutants, no perithecium was even seen for dcl-1 mutants, indicating that the sexual development is defective at a much earlier stage for the dcl-1 mutant compared with the sad-1 and sad-2 mutants [82]. Mutants of sad-1 and sad-2 respectively function as dominant suppressors of meiotic silencing, but neither the dcl-1 deletion mutant, the dcl-2 deletion mutant, nor the dcl-1 dcl-2 double mutant could function as dominant suppressors of meiotic silencing [71,80,82]. By Performing the elegant experiments where dcl-1 could be expressed at an early stage in the sexual cycle but inactivated at later stages, Alexander et al. demonstrated that the dcl-1 deletion mutation, but not the dcl-2 deletion mutation, suppressed the silencing of unpaired hH1-gfp, thus suppressed the MSUD [82]. Interestingly, quelling relies on the redundant Dicers DCL-1 and DCL-2 with DCL-2 responsible for most of the siRNA production, but here MSUD requires the dcl-1 but not the dcl-2 for its function [82]. Thus different sets of proteins are required for MSUD and quelling respectively, supporting the notion that two separate RNAi pathways are present in N. crassa [32,83]. DCL-1, SMS-2, SAD-1 and SAD-2 were demonstrated to colocalize in the perinuclear region, an interesting place where siRNAs were shown to be accumulated, indicating that the perinuclear region is an active center for MSUD and the siRNAs might be involved in the MSUD [80,82]. On the other hand, the requirement of the RdRP SAD-1, the Argonaute protein SMS-2 and the Dicer DCL-1 indicates that dsRNA and small RNAs are involved in the meiotic silencing [77,82]. Lee et al. demonstrated that only the unpaired regions with homology to the reporter transcript could trigger the meiotic silencing of the reporter gene, with better silencing effects for the greater size and the greater homology of the unpaired region, and there was an unpairing –dependent loss of a reporter transcript correlated with the induction of meiotic silencing, which support the concept that MSUD is post-transcriptional [84]. Though much work needs to be done to interpret the functioning mechanism and silencing pathway, by comparing with Quelling, a simple model below may be proposed or MSUD [77,82-83](Figure 2): unpaired DNA is a signal to initiate the transcription of an aberrant RNA from an unpaired DNA region, and the RdRP SAD-1converts aberrant RNA into a dsRNA, which is processed by DCL-1 into small RNAs, and small RNAs are then loaded onto a RISC complex similar to that in Quelling with the Argonaute SMS-2 as the core component, which then performs the silencing function. SAD-2 may function in the MSUD pathway by recruiting SAD-1 to the proper location to perform its activity. The future finding of small RNAs associated with MSUD, especially whether there are SMS-2 binding small RNAs present during MSUD, will be a key to verify this model.
Figure 2. Meiotic silencing in N. crassa.
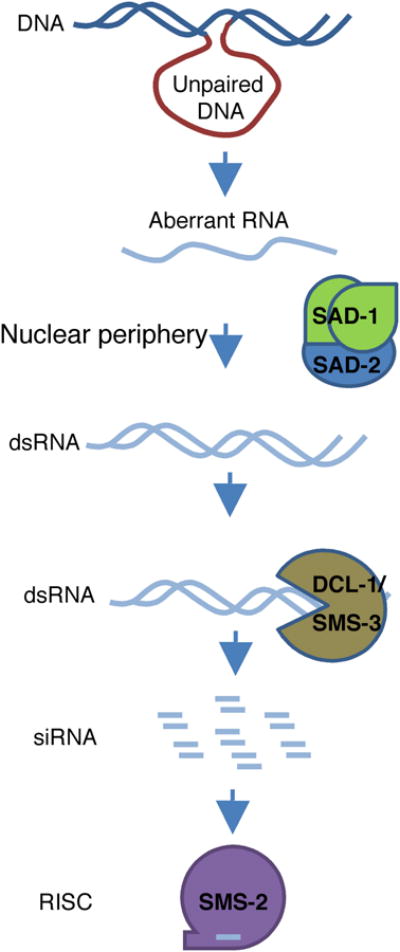
During the first meiotic prophase, an unpaired DNA is a trigger for the transcription of an aberrant RNA from an unpaired DNA region, and the RdRP SAD-1converts aberrant RNA into a dsRNA, which is processed by DCL-1 into small RNAs, and small RNAs are then loaded onto a RISC complex similar to that in Quelling with the Argonaute SMS-2 as the core component, which then perform the silencing function. SAD-2 may function in the MSUD pathway by recruiting SAD-1 to the proper location to perform its activity.
miRNA-like small RNA and Dicer-independnt sRNAs in N. crassa
miRNAs are small non-coding RNAs originated from single-stranded RNA precursor containing hairpin structures [64,85]. miRNAs have been found in animals, plants, and algae [86-92], and have been widely considered not present in the fungal kingdom. However, our recent studies have provided strong evidence that miRNA-like small RNAs are present in Neurospora and thus the miRNA mediated RNAi exist in filamentous fungi [93].
By analyzing the QDE-2-associated small RNAs produced under normal growth conditions, at least twenty-five potential miRNAs were discovered, named as microRNA-like small RNAs (milRNAs). For each milRNA, the vast majority of small RNA sequences match one arm of the hairpin (called the milRNA arm) and share a U at the 5′ ends. Furthermore, small RNAs matched to the complementary arm of the hairpin (named as milRNA*) were present but with much lower numbers. A 2nt 3′ overhang is present for some milRNA/milRNA* pairs indicating possible products of a Dicer-like enzyme. Though nearly all milRNAs from the milRNA arm share the same 5′ U position, two or more 3′ ends can be present, which is similar to the heterogeneity at 3′ termini of some miRNAs in other eukaryotes [94]. These small RNAs share many similarities with conventional miRNAs from animals and plants: these milRNAs come from highly specific stem-loop RNA precursors; most of the milRNAs require Dicer for the biogenesis; milRNAs may silence endogenous targets with imperfect complementarity like the way of the animal miRNA; and the milR-3 milRNA production mechanism is similar to plant miRNAs.
Diverse milRNA biogenesis pathways
The four loci producing most milRNAs (milR-1, milR-2, milR-3, and milR-4) are all in intergenic regions and each had at least 9,000 reads, but the sizes of the milRNA stem loop precursors are quite flexible: for milR-1, milR-3 and milR-4, the milRNA and milRNA* sequences are separated by more than 120 nt, but those are very close for milR-2. On the other hand, the size distributions of milRNAs from these four loci are also quite different, with bimodal for milR-1 and -4 and single peaks for milR-2 (25 nt) and milR-3 (19nt). All these results indicate that milRNAs from different loci may have different mechanisms of biogenesis.
Further analyses demonstrated that there are at least four different biogenesis mechanisms for milRs in Neurospora. The biogenesis of milR-3 milRNAs requires only Dicers for pre-milRNA and milRNA processing, which is like that for plant miRNAs; while milR-4 milRNAs biogenesis is partially Dicer-dependent. The milR-1 production however requires Dicers DCL-1/DCL-2, the Argonaute protein QDE-2 and the exonuclease QIP; whereas, the biogenesis of milR-2 milRNA requires QDE-2 but is completely independent of Dicer (See Figure 3).
Figure 3. Novel endogenous small RNA pathways under normal growth conditions (or microRNA-like RNAi pathways and disiRNA pathway?) in N. crassa.
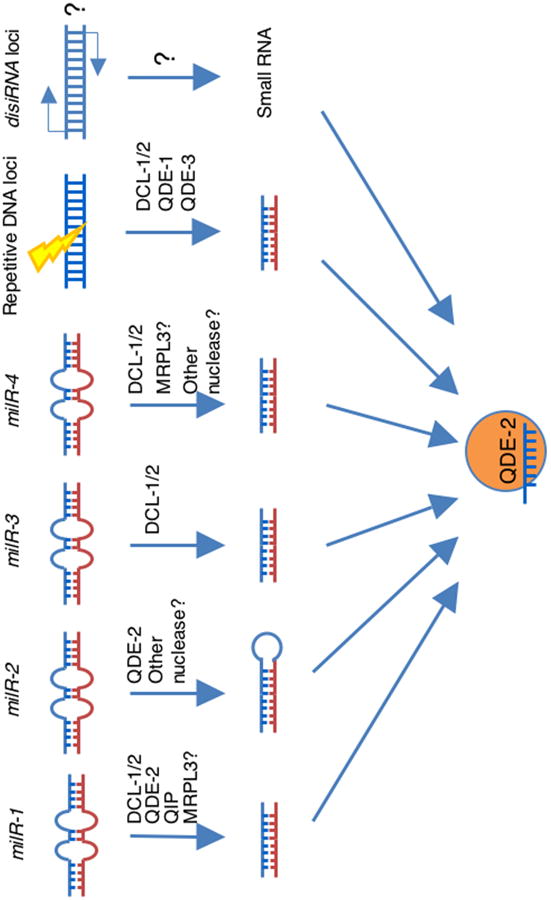
microRNA-like small RNAs are present in Neurospora with at least four different biogenesis mechanisms: (a) Dicer, QDE-2 and QIP-dependent milRNA-1; (b) Dicer-dependent milR-3; (c) Partial Dicer-dependent milR-4; (d) Dicer-independent, Argonaute QDE-2-dependent milR-2. As in aminals and plants, miRNA mediated RNAi pathway may be present in Neurospora, which is here exemplified by milRNA-1. milRNA-1 is first processed from primary milRNA precursor by Dicers into pre-milRNAs, which are further processed into mature milRNA-1s by QDE-2 and QIP. MRPL3 may involve both the pre- and mature milRNA-1 production. milRNA-1 can regulate gene expression and silence the complementary targets, probably via the similar mi-RISC-like complex as that in aminals and plants, with the Argaunote QDE-2 as the core component. Another novel RNAi pathway present in Neurospora could be the (e) Dicer-independent disiRNA pathway. disiRNAs are a novel class of QDE-2 associated small RNAs probably produced from dsRNAs, but their biogenesis do not dependent any known RNAi components.
Northern blot analyses and cloning showed that for milR-1 locus, the mature milRNAs are 9- and 24/25-nt, and pre-milRNAs are 33nt and 43 nt, pre-milRNA* species are about 33nt and 41nt respectively, and the pri-milRNA is the 170nt band which is indeed a transcript containing the predicted hairpin structure. The Dicers (mainly DCL-2, unpublished data) responsible for processing the pri-milRNA into pre-milRNAs, for all four smaller size products were gone while the level of the 170-nt product increased dramatically in the dcl-1dcl-2 double mutant dclDKO. The mature milRNAs species were gone in the qde-2 mutant, but they were not abolished in the qde-2 (D664A) mutant (no slicer activity of QDE-2 but retaining the dsRNA binding activity in this mutant); however, the production of mature milRNA was completely abolished both in the qip (encoding the QDE-2 interacting protein QIP) mutant and in the qip mutant expressing QIP with a point-mutation of the catalytic residue (H504A), but expression of the wild-type QIP completely rescued the mature milRNA production in the qip mutant. On the other hand, immunoprecipiation assays showed that Myc-QDE-2 could bind the short pre-milRNA (33nt) in the qip mutant, and bind also two mature milRNAs as well as the 33nt pre-milRNA in the wild-type strain. All these results suggest that QDE-2 binds to pre-milRNA (the 33nt pre-milRNA, which is probably the precursor of the mature milRNAs, with the 43nt longer pre-milRNA probably being an intermediate or by-product of pri-milRNA processing) and recruits the exonuclease QIP to process pre-milRNAs into mature milRNAs, where the catalytic activity of QIP is required.
The milR-2 locus forms perfect loop structure with both milRNA strand and milRNA* on the stem close to the loop. In fact, the 3′ ends of the mature milR-2 species (mostly around 25nt to 28 nt with the same 5′ start position) are on the loop. Northern blot analyses demonstrated three pre-milRNA species of about 33, 39, and 52 nt and a mature milRNA of∼25 nt. However, no any difference of the profiles of the pre-milRNAs and mature milRNA were observed in the dcl-1/dcl-2 double mutant dclDKO compared with the wild type, demonstrating that the biogenesis of milR-2 is Dicer-independent. Suprisingly, QDE-2 and its catalytic activity are instead required for the production of both pre-milRNAs and milRNA, for the two smaller species of pre-milRNAs disappeared but the 52-nt pre-milRNA accumulated abundantly in both the qde-2 and qde-2(D664A) mutants, with a RNA band about 2 nt larger than the wild-type mature milR-2 milRNA was observed in the mutants. For milR-2, qip mutation did not show significant influence compared with the wild type. Immunoprecipiation analysis showed that both the mature milRNA and the two shorter pre-milRNAs were associated with QDE-2. All these results suggest that the milR-2 pri-milRNA is first processed into the pre-milRNAs by an unknown nuclease(s), and then the Argonaute QDE-2 and its catalytic activity are required for processing the pre-milRNAs into mature milRNA. The mature milR-2 was still associated with Myc-QDE-2 in the qde-1 and dclDKO mutants suggesting that loading of milR-2 onto QDE-2 is also dicer-independent.
milR-2 is in fact the first known dicer-independent, but Argonaute-dependent microRNA-like small RNA for the biogenesis in eukaryotes. The discovery of dicer-independent milR-2 in Neurospora blurs the boundary of miRNA, as the general concept is that miRNAs are dicer-dependent. Quite interestingly, soon after our this discovery, two papers emerged on-line which both describe the same miR-451 as a dicer-independent but Argonaute-dependent miRNA [95-96]. miR-451 practically is very close to milR-2 both for the biogenesis and on the detailed structure, which further supports milR-2 is indeed a microRNA and our discovery of the presence of dicer-independent Argonaute-dependent miRNA in fungi.
At least four different mechanisms present for milRNA biogenesis in Neurospora, suggests that eukaryotic miRNA biogenesis involves mechanisms much more complex than the established pathways for plants and animals. The discovery of milRNAs in Neurospora supports the concept that miRNA and miRNA-like small RNAs exist in all major branches of eukaryotic organisms, and the milRNA related studies in Neurospora will broaden the miRNA studies in other eukaryotes. No highly homologous milR genes outside the Neurospora genus were found, suggesting individual milRs may have evolved independently from plant and animal miRNAs and the miRNAs in fungi may evolve rapidly, as some miRNAs in plants [97].
A novel RNAse III domain-containing protein MRPL3 invloves milRNA processing
The presence of Dicer-independent mechanisms for milRNA generation led us to look for proteins responsible for Dicer-independent nuclease activity. One protein (NCU08299) homologous to the yeast mitochondrial ribosomal protein MRPL3 in the Neurospora genome was noticed for its possession of a putative RNAse III domain and a dsRNA recognition motif, though the RNAse III domains of MRPL3 and its homologous proteins have little sequence similarity to those of Dicers and Drosha indicative of the presence of a different family of RNAse III domain proteins. Northern blot analyses by utilizing a heterokaryotic strain that carries both wild-type and mrpl3KO nuclei and a strain (dsmrpl3) in which mrpl3 is silenced by dsRNA demonstrated that the levels of the major mature milR-1 milRNA and the 33nt pre-milRNAs were significantly decreased in the mutants compared to the wild type, which indicates that MRPL3 is involved in the production of milR-1 pre-milRNAs and milRNAs by somehow collaborating with the Dicers. The mature milRNA levels of milR-4 in the mrpl3 mutants were also decreased to the levels similar to that in the dclDKO strain, where the milRNA production is partially dependent on Dicer. But the levels of milR-2 and milR-3 milRNAs and pre-milRNAs were not changed in the mrpl3 mutants compared to the wild type, indicative of some other nuclease(s) responsible for the processing pri-milR-2 to pre-milR-2s instead of MRPL3. In vitro pre-milR-4 processing activity was also significantly decreased in the silenced dsmrpl3 strain. All in all, these results show that MRPL3 is a novel protein responsible for the Dicer-independent processing of some milRNAs in Neurospora.
Function of milRNA
Introducing a Myc-tagged reporter construct with milR-1 milRNA complementary sequences into the wild-type and qde-2 mutant strains showed that the mRNA levels and protein levels of the reporter were all higher in the qde-2 mutants than in the wild-type strains, with the protein levels about 20 fold higher, supporting the conclusion that milR-1 expression leads to significant silencing of its complementary target. mRNA levels of several predicted targets of the milR miRNA are up-regulated in the dclDKO mutant, indicating milRNAs may regulate these genes. Furthermore, QDE-2 were found to specifically associate with these predicted miRNA targets but not with the control RNAs. All these show that milRNAs can regulate gene expression, suggesting miRNA mediated RNAi as that in animals and plants is present in fungi, though much work still needs to be done to understand the importance of these milRNAs to the growth and development.
DisiRNAs
Analyses of the Neurospora QDE-2-associated small RNAs by deep sequencing also revealed another novel type of small RNAs called dicer-independent small interfering RNAs (disiRNAs) [93]. This is a major group containing small RNAs symmetrically matched to both strands of DNA, which are averaged about 22nt long with a strong 5′ U preference. disiRNAs are derived from 50 loci not highly repetitive but including genes and intergenic regions with no apparent shared sequence motifs. Based on available EST data, nearly 80% of the disiRNA loci have overlapping sense and antisense transcripts, which suggests that these disiRNAs are likely processed from dsRNA made from naturally occurring complementary sense and antisense transcripts.
These disiRNAs do not depend QDE-1, QDE-2, nor QDE-3 for the biogenesis, and though they are very likely derived from dsRNA, their levels were not significantly changes in the dcl-1/dcl-2 double mutant dclDKO, so Dicers are not involved in their biogenesis. Moreover, neither the double mutation of the two Argonaute genes (qde-2 sms-2) altered the disiRNA levels, nor the mrpl3 mutants. These results indicate that, unlike animal piRNAs, an Argonaute-dependent maturation mechanism is not involved in disiRNA production. A heterokaryon or a knock-down strain by dsRNA for the RNT1 (an Rnase III domain containing protein in yeast) homolog in Neurospora did not show any defects on the disiRNA biogenesis. These small RNAs are not like qiRNAs either which are induced by DNA damage, derived from both strands of repetitive rDNA and dependent on QDE-1, QDE-3, and Dicers for the production [51]. None of the known RNAi pathway components in Neurospora influences the disiRNA production, thus an unknown novel small RNA biogenesis pathway may be present for disiRNAs. Whether these disiRNAs can function through certain RNAi pathway is not known, though all these small RNAs interact with QDE-2, the core component of the RISC, indicating that they might function via RNAi pathway (Figure 3).
RNAi in other filamentous fungi
RNAi is an antiviral defense mechanism in Cryphonectria paracitica
Except N. crassa, another excellent system for RNAi studies of filamentous fungi is the chestnut blight fungus Cryphonectria paracitica. Most significantly, C. parasitica can support the replication of members from five different RNA virus families, thus this fungus is an ideal model for studying the role of RNA silencing as an antiviral defense mechanism [98].
Though RNAi is well known in functioning as antiviral defense for plant viruses and animal viruses, no direct evidence was provided on this aspect for fungi until recently [2,99-102]. p29 is a papain-like protease encoded by the mycovirus Cryphonectria hypovirus 1 (CHV1), which is similar to the plant potyvirus-encoded suppressor of RNA silencing HC-Pro [103-106]. Using the CHV1-EP713/ C. parasitica system, Segers et al. demonstrated that p29 suppressed the hairpin RNA-induced silencing and reversed the established RNA silencing of GFP in C. parasitica; and p29 also suppressed both virus-induced and agroinfiltration-induced RNA silencing and systemic spread of silencing in GFP-expressing transgenic Nicotiana benthamiana [104]. These results suggest that the antiviral defense mechanism of RNA silencing is conserved in both fungi and plants [104]. Segers et al. further cloned dicer-like genes dcl-1 and dcl-2, which have high similarity to dcl-1 and dcl-2 in Neurospora respectively. Though the single mutants of dcl-1 and dcl-2 have no obvious phenotype compared to the wild type C. parasitica, and the single mutants of dcl-1 don't have phenotype difference compared to the wild type after the infection of hypovirus CHV1-EP713 or reovirus MyRV1-Cp9B21, the Δdcl-2 and Δdcl-1/Δdcl-2 mutant strains are highly susceptible to the infection. On the other hand, infecting the Δdcl-2 mutant by a hypovirus CHV1-EP713 mutant lacking the suppressor of RNA silencing p29 and the wild-type reovirus MyRV1-Cp9B21 showed elevated viral RNA levels compared to the wild type. Complementation of the Δdcl-2 strain with the dcl-2 wild type copy rescued the response to virus infection to the wild type level. These results provide an direct evidence that a fungal dicer can function to regulate virus infection and RNAi plays an important role in antiviral denfense in fungi [98,104]. This is further supported by the findings that the dicer gene dcl-2 is required for defective viral RNA production and recombinant virus vector RNA instability for hypovirus [107]; dcl-2 expression levels are significantly increased in response to viral infection, with further more increased expression when suppressor p29 is mutated in the virus, and dcl-2 processes hypovirus RNAs into virus-derived small interfering RNAs (vsRNAs) as part of an inducible RNA silencing antiviral response [107-108].
There are four Argonaute-like proteins (AGL1, AGL2, AGL3 and AGL4) in C. parasitica, but only AGL-2 is required for the antiviral defense response [109]. Similar to dcl-2, agl-2 also has an increase in transcription level in response to viral infection, though not so significant [109]. The agl2 gene is required for the increase of the virus-induced dcl2 transcription, and agl2 and dcl2 transcripts accumulated to much higher levels in response to infection by a mutant CHV1-EP713 hypovirus without p29 than to wild-type CHV1-EP713, further supporing that a virus-encoded RNA silencing suppressor suppress the RNA silencing components in fungi [109]. Like that in N. crassa, dsRNAs also induce significantly the expression of agl2 and dcl2 (to high levels similar to that in response to p29 mutated hypovirus), which could suggest that Quelling in N. crassa may also function as an antiviral silencing pathway [109].
RNAi plays a role for mycovirus defense in Aspergillus nidulans
Inverted repeat transgene -induced RNA silencing was successfully performed in Aspergillus nidulans, demonstrating that RNAi is present in this model filamentous fungus [110]. Interestingly, this fungus only has one intact Dicer and Argonaute respectively which are both required for RNAi, and lacks the N. crassa RdRP QDE-1 homolog though contains two RdRPs in the genome with none is required for the tested RNAi [110-111]. By investigating the relationship to RNAi of the three mycoviruses with stable infections of A. nidulans, Hammond and Keller demonstrated that the Aspergillus virus 1816 suppressed the inverted repeat transgene -induced RNA silencing, for the presence of this virus released the silencing phenotype and the corresponding siRNA was not detected. The mechanism for the suppression is not clear, which could involve an RNA silencing suppressor, if present in this virus. On the other hand, the virus 341-derived siRNA was detected robustly in an Argonaute mutant, showing that this virus is targeted and processed into siRNA by the RNA silencing machinery. All these results suggest that there is an antagonistic relationship between these mycoviruses and RNA silencing, and RNAi in A. nidulans functions in mycovirus defense [112].
RNAi and small RNA studies in Mucor circinelloides
In recent years, Mucor circinelloides has got tremendous attention on the studies of RNAi for its distance from other fungal models and the molecular tools conveniently available, with a transgene-induced RNA silencing pathway revealed and two dicer genes (dcl1 and dcl2) and two RdRP genes (rdrp1 and rdrp2) identified and characterized [113-116]. Self-replicative sense or inverted-repeat trangenes induced post-transcriptional gene silencing associated with both sense and antisense small RNAs, though two different size classes of small antisense siRNA of 21 nt and 25 nt respectively, were differentially accumulated during the vegetative growth of silenced transformants [114,116]. Secondary sense and antisense RNAs were detected, suggesting the amplification step is present for RNA silencing in this fungus [116]. DCL-2 is the major player in transgene-induced silencing and the production of the two classes of antisense siRNAs [114]. RdRP1 is also indicated to be involved in the transgene-induced silencing but not RdRP2 nor DCL-1 [113]. Most recently, by directly sequencing the cDNA libraries of short RNAs, four classes of endogenous short RNAs have been identified in this fungus matching to exons with fungction of regulating mRNA levels of many protein coding genes [113]. The biogenesis of the largest class of these exonic-siRNAs requires both RdRP1 and DCL-2, indicating RdRP1 converts the corresponding mRNA into dsRNA which is further processed by DCL2. A second group of exonic-siRNAs require DCL-2 and RdRP2 but not RdRP1 for the biogenesis. The third group requires both RdRP1 and RdRP2 for the biogenesis, though the two dicers seem to play redundant roles as these small RNAs are only down-regulated in the dicer double mutants. For the fourth group the small RNA production is majorly DCL-1 but not DCL-2-dependent, though both RdRPs are required for the biogenesis. Though some dicer-dependent endogeneous small RNAs were identified to map to transposons or repeats, no miRNA-like small RNA were found in this study, which could be explained by the following possibilities [113]: 1.miRNAs are not present in this fungus; 2. miRNAs are not expressed or not expressed abundantly under the test conditions; 3. miRNAs are usually Argonaute-associated, and deep sequencing of the Argonaute-binding small RNAs (enriched by Argonaute) under different growth conditions may help to answer if miRNAs are present in this fungus. The discovery of these endogenous small RNAs from M. circinelloides greatly enriches the understanding of RNAi in eukaryotes. The next question is to see if these small RNAs interact with rgonaute proteins, the core component of the RISC complex.
RNAi studies and application in other filamentous fungi
RNAi have been studied in some other filamentous fungi, especially after Fire and Mello's RNAi discovery [83]. A co-suppression phenomenon like that in plants was observed in Cladosporium fulvum in 1998 [83,117] and a homology-dependent silencing was observed in Schizophyllum commune in 1997 [118]. Since the seminal report of RNAi in 1998 [7], gene expression suppression by utilizing a dsRNA-expressing system has been found and successfully applied in many pathogenic and non-pathogenic fungi including Ascomycota, Basidiomycota, and Zygomycota, such as Magnaporthe oryzae [119], Sclerotinia sclerotiorum [120], Aspergillus fumigatus [121-124], Aspergillus oryzae [119,125], Aspergillus flavus [126] and Aspergillus parasiticus [126], Bipolaris oryzae [127], Colletotrichum lagenarium [128], Colletotrichum gloeosporioides [129], Coprinus cinereus [130-131], Fusarium solani [132], Fusarium graminearum[126], Fusarium verticillioides [133], Mucor circinelloides [115-116], Moniliophthora perniciosa [134], Histoplasma capsulatum [135-136], Cryptococcus neoformans [137], Schizophyllum commune [118,138], Coniothyrium minitans [139], Stagonospora nodorum [140], Ophiostoma floccosum and O. piceae [141], Botrytis cinerea [142] and so on, with some of them showing the involvement of dicer in the silencing and siRNA production, suggesting RNA silencing is conserved in most of fungal species [83,143].
As more and more fungal genomes being sequenced, homologues of RdRP, Argonaute and dicer proteins in various filamentous fungi from Ascomycota, Basidiomycota, and Zygomycota were identified though none was found in the basidiomycete Ustilago maydis [83,143]. Genes involved in the RNA silencing in the ascomycete fungi usually have a N. crassa ortholog, including three RdRPs, two Argonautes and two dicer-like proteins [83,143]. Basidiomycete fungi have similar numbers of the protein classes involved in RNAi to the ascomycetes, though with a wider diversity and more extensive gene expansion [83,143]. In the zygomycete Rhizopus oryzae, five RdRP genes were identified, indicative of more entensive gene expansion [83,143].
Perspectives.
Neurospora crassa not only has two famous well characterized RNAi pathways Quelling and Meiotic Silencing by Unpaired DNA, but also can generate endogenous small RNAs which could function in different RNA silencing pathways: DNA-damage induced qiRNA; Dicer, QDE-2 and QIP-dependent milRNA-1; Dicer-dependent milR-3; Partial Dicer-dependent milR-4; Dicer-independent, Argonaute QDE-2-dependent milR-2; Dicer-independent disiRNAs. With N. crassa is only one member of the millions of species, filamentous fungi are among the most diverse and populated groups of eukaryotes with tremendous impact on human daily life and the ecosystem balance. Since it is widely conserved in filamentous fungi, RNAi is and will play more important roles in understanding gene function and regulation, and RNAi itself may play important function in pathogenesis in pathogenic fungi, though much work needs to be done.
Though RNAi has been got enormous attentions and the most intense studies since its discovery, there are many questions still not resolved or even not touched, especially for the filamentous fungi. Even just in the model Neurospora, many obvious questions related to RNAi have not been answered: How aRNA is produced? Are microRNA-like small RNAs and disiRNAs widely present in filamentous fungi? What are the functions of those miroRNA-likes small RNAs and their biogenesis mechanisms? How are the disiRNAs produced and what importance do they have to the organism's growth and development? Are there (if so, what are they) SMS-2 interacting siRNAs present for MSUD? Being the most diversified and most populated group with various habitats of eukaryotes, filamentous fungi may have more unexploited fields in RNAi compared with other eukaryotes and RNAi may play more diverse roles in them.
References
- 1.Hannon GJ. RNA interference. Nature. 2002;418:244–51. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 2.Ding SW, Voinnet O. Antiviral immunity directed by small RNAs. Cell. 2007;130:413–26. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–55. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Napoli C, Lemieux C, Jorgensen R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell. 1990;2:279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romano N, Macino G. Quelling: transient inactivation of gene expression in Neurospora crassa by transformation with homologous sequences. Mol Microbiol. 1992;6:3343–53. doi: 10.1111/j.1365-2958.1992.tb02202.x. [DOI] [PubMed] [Google Scholar]
- 7.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 8.Fagard M, Boutet S, Morel JB, Bellini C, Vaucheret H. AGO1, QDE-2, and RDE-1 are related proteins required for post-transcriptional gene silencing in plants, quelling in fungi, and RNA interference in animals. Proc Natl Acad Sci U S A. 2000;97:11650–4. doi: 10.1073/pnas.200217597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–6. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 10.Jinek M, Doudna JA. A three-dimensional view of the molecular machinery of RNA interference. Nature. 2009;457:405–12. doi: 10.1038/nature07755. [DOI] [PubMed] [Google Scholar]
- 11.Siomi H, Siomi MC. On the road to reading the RNA-interference code. Nature. 2009;457:396–404. doi: 10.1038/nature07754. [DOI] [PubMed] [Google Scholar]
- 12.Maiti M, Lee HC, Liu Y. QIP, a putative exonuclease, interacts with the Neurospora Argonaute protein and facilitates conversion of duplex siRNA into single strands. Genes Dev. 2007;21:590–600. doi: 10.1101/gad.1497607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–9. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 14.Tomari Y, Zamore PD. Perspective: machines for RNAi. Genes Dev. 2005;19:517–29. doi: 10.1101/gad.1284105. [DOI] [PubMed] [Google Scholar]
- 15.Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- 16.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–39. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 17.Moazed D. Small RNAs in transcriptional gene silencing and genome defence. Nature. 2009;457:413–20. doi: 10.1038/nature07756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson T, Lin H. The biogenesis and function of PIWI proteins and piRNAs: progress and prospect. Annu Rev Cell Dev Biol. 2009;25:355–76. doi: 10.1146/annurev.cellbio.24.110707.175327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Liu Y. Diverse small non-coding RNAs in RNA interference Pathways. Methods in Molecular Biology (Goodchild, John edited) 2010 doi: 10.1007/978-1-61779-188-8_11. [DOI] [PubMed] [Google Scholar]
- 20.Cogoni C, Irelan JT, Schumacher M, Schmidhauser TJ, Selker EU, Macino G. Transgene silencing of the al-1 gene in vegetative cells of Neurospora is mediated by a cytoplasmic effector and does not depend on DNA-DNA interactions or DNA methylation. EMBO J. 1996;15:3153–63. [PMC free article] [PubMed] [Google Scholar]
- 21.Cogoni C, Macino G. Isolation of quelling-defective (qde) mutants impaired in posttranscriptional transgene-induced gene silencing in Neurospora crassa. Proc Natl Acad Sci U S A. 1997;94:10233–8. doi: 10.1073/pnas.94.19.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cogoni C, Romano N, Macino G. Suppression of gene expression by homologous transgenes. Antonie Van Leeuwenhoek. 1994;65:205–9. doi: 10.1007/BF00871948. [DOI] [PubMed] [Google Scholar]
- 23.Cogoni C, Macino G. Conservation of transgene-induced post-transcriptional gene silencing in plants and fungi. Trends in Plant Science. 1997;2:438–443. [Google Scholar]
- 24.Lindbo JA, Silva-Rosales L, Proebsting WM, Dougherty WG. Induction of a Highly Specific Antiviral State in Transgenic Plants: Implications for Regulation of Gene Expression and Virus Resistance. Plant Cell. 1993;5:1749–1759. doi: 10.1105/tpc.5.12.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baulcombe DC. RNA as a target and an initiator of post-transcriptional gene silencing in transgenic plants. Plant Mol Biol. 1996;32:79–88. doi: 10.1007/BF00039378. [DOI] [PubMed] [Google Scholar]
- 26.Cogoni C. Homology-dependent gene silencing mechanisms in fungi. Annu Rev Microbiol. 2001;55:381–406. doi: 10.1146/annurev.micro.55.1.381. [DOI] [PubMed] [Google Scholar]
- 27.Cogoni C, Macino G. Gene silencing in Neurospora crassa requires a protein homologous to RNA-dependent RNA polymerase. Nature. 1999;399:166–9. doi: 10.1038/20215. [DOI] [PubMed] [Google Scholar]
- 28.Cogoni C, Macino G. Posttranscriptional gene silencing in Neurospora by a RecQ DNA helicase. Science. 1999;286:2342–4. doi: 10.1126/science.286.5448.2342. [DOI] [PubMed] [Google Scholar]
- 29.Catalanotto C, Azzalin G, Macino G, Cogoni C. Gene silencing in worms and fungi. Nature. 2000;404:245. doi: 10.1038/35005169. [DOI] [PubMed] [Google Scholar]
- 30.Catalanotto C, Azzalin G, Macino G, Cogoni C. Involvement of small RNAs and role of the qde genes in the gene silencing pathway in Neurospora. Genes Dev. 2002;16:790–5. doi: 10.1101/gad.222402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catalanotto C, Pallotta M, ReFalo P, Sachs MS, Vayssie L, Macino G, Cogoni C. Redundancy of the two dicer genes in transgene-induced posttranscriptional gene silencing in Neurospora crassa. Mol Cell Biol. 2004;24:2536–45. doi: 10.1128/MCB.24.6.2536-2545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galagan JE, et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 2003;422:859–68. doi: 10.1038/nature01554. [DOI] [PubMed] [Google Scholar]
- 33.Mourrain P, et al. Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell. 2000;101:533–42. doi: 10.1016/s0092-8674(00)80863-6. [DOI] [PubMed] [Google Scholar]
- 34.Dalmay T, Hamilton A, Rudd S, Angell S, Baulcombe DC. An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell. 2000;101:543–53. doi: 10.1016/s0092-8674(00)80864-8. [DOI] [PubMed] [Google Scholar]
- 35.Smardon A, Spoerke JM, Stacey SC, Klein ME, Mackin N, Maine EM. EGO-1 is related to RNA-directed RNA polymerase and functions in germ-line development and RNA interference in C. elegans. Curr Biol. 2000;10:169–78. doi: 10.1016/s0960-9822(00)00323-7. [DOI] [PubMed] [Google Scholar]
- 36.Palauqui JC, Elmayan T, Pollien JM, Vaucheret H. Systemic acquired silencing: transgene-specific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J. 1997;16:4738–45. doi: 10.1093/emboj/16.15.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voinnet O, Vain P, Angell S, Baulcombe DC. Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell. 1998;95:177–87. doi: 10.1016/s0092-8674(00)81749-3. [DOI] [PubMed] [Google Scholar]
- 38.Kooter JM, Matzke MA, Meyer P. Listening to the silent genes: transgene silencing, gene regulation and pathogen control. Trends Plant Sci. 1999;4:340–347. doi: 10.1016/s1360-1385(99)01467-3. [DOI] [PubMed] [Google Scholar]
- 39.Makeyev EV, Bamford DH. Cellular RNA-dependent RNA polymerase involved in posttranscriptional gene silencing has two distinct activity modes. Mol Cell. 2002;10:1417–27. doi: 10.1016/s1097-2765(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 40.Salgado PS, Koivunen MR, Makeyev EV, Bamford DH, Stuart DI, Grimes JM. The structure of an RNAi polymerase links RNA silencing and transcription. PLoS Biol. 2006;4:e434. doi: 10.1371/journal.pbio.0040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laurila MR, Salgado PS, Makeyev EV, Nettelship J, Stuart DI, Grimes JM, Bamford DH. Gene silencing pathway RNA-dependent RNA polymerase of Neurospora crassa: yeast expression and crystallization of selenomethionated QDE-1 protein. J Struct Biol. 2005;149:111–5. doi: 10.1016/j.jsb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Fulci V, Macino G. Quelling: post-transcriptional gene silencing guided by small RNAs in Neurospora crassa. Current Opinion in Microbiology. 2007;10:199–203. doi: 10.1016/j.mib.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Goldoni M, Azzalin G, Macino G, Cogoni C. Efficient gene silencing by expression of double stranded RNA in Neurospora crassa. Fungal Genet Biol. 2004;41:1016–24. doi: 10.1016/j.fgb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Pickford A, Braccini L, Macino G, Cogoni C. The QDE-3 homologue RecQ-2 co-operates with QDE-3 in DNA repair in Neurospora crassa. Curr Genet. 2003;42:220–7. doi: 10.1007/s00294-002-0351-6. [DOI] [PubMed] [Google Scholar]
- 45.Kato A, Akamatsu Y, Sakuraba Y, Inoue H. The Neurospora crassa mus-19 gene is identical to the qde-3 gene, which encodes a RecQ homologue and is involved in recombination repair and postreplication repair. Curr Genet. 2004;45:37–44. doi: 10.1007/s00294-003-0459-3. [DOI] [PubMed] [Google Scholar]
- 46.Chen H, et al. OsRecQ1, a QDE-3 homologue in rice, is required for RNA silencing induced by particle bombardment for inverted repeat DNA, but not for double-stranded RNA. Plant J. 2008;56:274–86. doi: 10.1111/j.1365-313X.2008.03587.x. [DOI] [PubMed] [Google Scholar]
- 47.Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, Kingston RE. Characterization of the piRNA complex from rat testes. Science. 2006;313:363–7. doi: 10.1126/science.1130164. [DOI] [PubMed] [Google Scholar]
- 48.Forrest EC, Cogoni C, Macino G. The RNA-dependent RNA polymerase, QDE-1, is a rate-limiting factor in post-transcriptional gene silencing in Neurospora crassa. Nucleic Acids Res. 2004;32:2123–8. doi: 10.1093/nar/gkh530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ziv C, Yarden O. Gene Silencing for Functional Analysis: Assessing RNAi as a Tool for Manipulation of Gene Expression. 2010:77–100. doi: 10.1007/978-1-60761-611-5_6. eds. [DOI] [PubMed] [Google Scholar]
- 50.Nolan T, Cecere G, Mancone C, Alonzi T, Tripodi M, Catalanotto C, Cogoni C. The RNA-dependent RNA polymerase essential for post-transcriptional gene silencing in Neurospora crassa interacts with replication protein A. Nucleic Acids Res. 2008;36:532–8. doi: 10.1093/nar/gkm1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee HC, Chang SS, Choudhary S, Aalto AP, Maiti M, Bamford DH, Liu Y. qiRNA is a new type of small interfering RNA induced by DNA damage. Nature. 2009;459:274–7. doi: 10.1038/nature08041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 53.Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286:950–2. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- 54.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 55.Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–50. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- 56.Catalanotto C, Azzalin G, Macino G, Cogoni C. Transcription: Gene silencing in worms and fungi. Nature. 2000;404:245–245. doi: 10.1038/35005169. [DOI] [PubMed] [Google Scholar]
- 57.Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell. 2005;123:607–20. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 58.Rand TA, Petersen S, Du F, Wang X. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell. 2005;123:621–9. doi: 10.1016/j.cell.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 59.Miyoshi K, Tsukumo H, Nagami T, Siomi H, Siomi MC. Slicer function of Drosophila Argonautes and its involvement in RISC formation. Genes Dev. 2005;19:2837–48. doi: 10.1101/gad.1370605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haley B, Zamore PD. Kinetic analysis of the RNAi enzyme complex. Nat Struct Mol Biol. 2004;11:599–606. doi: 10.1038/nsmb780. [DOI] [PubMed] [Google Scholar]
- 61.Kim K, Lee YS, Carthew RW. Conversion of pre-RISC to holo-RISC by Ago2 during assembly of RNAi complexes. RNA. 2007;13:22–9. doi: 10.1261/rna.283207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, et al. C3PO, an Endoribonuclease That Promotes RNAi by Facilitating RISC Activation. Science. 2009;325:750–753. doi: 10.1126/science.1176325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waterhouse PM, Wang MB, Lough T. Gene silencing as an adaptive defence against viruses. Nature. 2001;411:834–42. doi: 10.1038/35081168. [DOI] [PubMed] [Google Scholar]
- 64.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 65.Chicas A, Forrest EC, Sepich S, Cogoni C, Macino G. Small interfering RNAs that trigger posttranscriptional gene silencing are not required for the histone H3 Lys9 methylation necessary for transgenic tandem repeat stabilization in Neurospora crassa. Mol Cell Biol. 2005;25:3793–801. doi: 10.1128/MCB.25.9.3793-3801.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nolan T, Braccini L, Azzalin G, De Toni A, Macino G, Cogoni C. The post-transcriptional gene silencing machinery functions independently of DNA methylation to repress a LINE1-like retrotransposon in Neurospora crassa. Nucleic Acids Res. 2005;33:1564–73. doi: 10.1093/nar/gki300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freitag M, Lee DW, Kothe GO, Pratt RJ, Aramayo R, Selker EU. DNA methylation is independent of RNA interference in Neurospora. Science. 2004;304:1939. doi: 10.1126/science.1099709. [DOI] [PubMed] [Google Scholar]
- 68.Cecere G, Cogoni C. Quelling targets the rDNA locus and functions in rDNA copy number control. BMC Microbiol. 2009;9:44. doi: 10.1186/1471-2180-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choudhary S, Lee HC, Maiti M, He Q, Cheng P, Liu Q, Liu Y. A double-stranded-RNA response program important for RNA interference efficiency. Mol Cell Biol. 2007;27:3995–4005. doi: 10.1128/MCB.00186-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aramayo R, Metzenberg RL. Meiotic transvection in fungi. Cell. 1996;86:103–13. doi: 10.1016/s0092-8674(00)80081-1. [DOI] [PubMed] [Google Scholar]
- 71.Shiu PK, Raju NB, Zickler D, Metzenberg RL. Meiotic silencing by unpaired DNA. Cell. 2001;107:905–16. doi: 10.1016/s0092-8674(01)00609-2. [DOI] [PubMed] [Google Scholar]
- 72.Shiu PK, Metzenberg RL. Meiotic silencing by unpaired DNA: properties, regulation and suppression. Genetics. 2002;161:1483–95. doi: 10.1093/genetics/161.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Springer ML. Genetic control of fungal differentiation: the three sporulation pathways of Neurospora crassa. Bioessays. 1993;15:365–74. doi: 10.1002/bies.950150602. [DOI] [PubMed] [Google Scholar]
- 74.Aramayo R, Peleg Y, Addison R, Metzenberg R. Asm-1+, a Neurospora crassa gene related to transcriptional regulators of fungal development. Genetics. 1996;144:991–1003. doi: 10.1093/genetics/144.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cogoni C. Unifying homology effects. Nat Genet. 2002;30:245–6. doi: 10.1038/ng0302-245. [DOI] [PubMed] [Google Scholar]
- 76.Kasbekar DP. Sex and the single gene: meiotic silencing by unpaired DNA. J Biosci. 2002;27:633–5. doi: 10.1007/BF02708370. [DOI] [PubMed] [Google Scholar]
- 77.Kelly WG, Aramayo R. Meiotic silencing and the epigenetics of sex. Chromosome Res. 2007;15:633–51. doi: 10.1007/s10577-007-1143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pratt RJ, Lee DW, Aramayo R. DNA methylation affects meiotic trans-sensing, not meiotic silencing, in Neurospora. Genetics. 2004;168:1925–35. doi: 10.1534/genetics.104.031526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee DW, Pratt RJ, McLaughlin M, Aramayo R. An argonaute-like protein is required for meiotic silencing. Genetics. 2003;164:821–8. doi: 10.1093/genetics/164.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shiu PK, Zickler D, Raju NB, Ruprich-Robert G, Metzenberg RL. SAD-2 is required for meiotic silencing by unpaired DNA and perinuclear localization of SAD-1 RNA-directed RNA polymerase. Proc Natl Acad Sci U S A. 2006;103:2243–8. doi: 10.1073/pnas.0508896103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bardiya N, Alexander WG, Perdue TD, Barry EG, Metzenberg RL, Pukkila PJ, Shiu PK. Characterization of interactions between and among components of the meiotic silencing by unpaired DNA machinery in Neurospora crassa using bimolecular fluorescence complementation. Genetics. 2008;178:593–6. doi: 10.1534/genetics.107.079384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alexander WG, Raju NB, Xiao H, Hammond TM, Perdue TD, Metzenberg RL, Pukkila PJ, Shiu PK. DCL-1 colocalizes with other components of the MSUD machinery and is required for silencing. Fungal Genet Biol. 2008;45:719–27. doi: 10.1016/j.fgb.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 83.Nakayashiki H. RNA silencing in fungi: Mechanisms and applications. FEBS Letters. 2005;579:5950–5957. doi: 10.1016/j.febslet.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 84.Lee DW, Seong KY, Pratt RJ, Baker K, Aramayo R. Properties of unpaired DNA required for efficient silencing in Neurospora crassa. Genetics. 2004;167:131–50. doi: 10.1534/genetics.167.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ambros V, et al. A uniform system for microRNA annotation. RNA. 2003;9:277–9. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochromatic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 87.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–8. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 88.Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294:862–4. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 89.Llave C, Kasschau KD, Rector MA, Carrington JC. Endogenous and silencing-associated small RNAs in plants. Plant Cell. 2002;14:1605–19. doi: 10.1105/tpc.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molnar A, Schwach F, Studholme DJ, Thuenemann EC, Baulcombe DC. miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii. Nature. 2007;447:1126–9. doi: 10.1038/nature05903. [DOI] [PubMed] [Google Scholar]
- 91.Zhao T, Li G, Mi S, Li S, Hannon GJ, Wang XJ, Qi Y. A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes Dev. 2007;21:1190–203. doi: 10.1101/gad.1543507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grimson A, et al. Early origins and evolution of microRNAs and Piwi-interacting RNAs in animals. Nature. 2008;455:1193–7. doi: 10.1038/nature07415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee HC, et al. Diverse Pathways Generate MicroRNA-like RNAs and Dicer-Independent Small Interfering RNAs in Fungi. Mol Cell. 2010 doi: 10.1016/j.molcel.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruby JG, Jan C, Player C, Axtell MJ, Lee W, Nusbaum C, Ge H, Bartel DP. Large-scale sequencing reveals 21U-RNAs and additional microRNAs and endogenous siRNAs in C. elegans. Cell. 2006;127:1193–207. doi: 10.1016/j.cell.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 95.Cifuentes D, et al. A Novel miRNA Processing Pathway Independent of Dicer Requires Argonaute2 Catalytic Activity. Science. 2010 doi: 10.1126/science.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cheloufi S, Dos Santos CO, Chong MM, Hannon GJ. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010 doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen X. Small RNAs and their roles in plant development. Annu Rev Cell Dev Biol. 2009;25:21–44. doi: 10.1146/annurev.cellbio.042308.113417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Segers GC, Zhang X, Deng F, Sun Q, Nuss DL. Evidence that RNA silencing functions as an antiviral defense mechanism in fungi. Proc Natl Acad Sci U S A. 2007;104:12902–6. doi: 10.1073/pnas.0702500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li HW, Ding SW. Antiviral silencing in animals. FEBS Lett. 2005;579:5965–73. doi: 10.1016/j.febslet.2005.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang XH, Aliyari R, Li WX, Li HW, Kim K, Carthew R, Atkinson P, Ding SW. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–4. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li F, Ding SW. Virus counterdefense: diverse strategies for evading the RNA-silencing immunity. Annu Rev Microbiol. 2006;60:503–31. doi: 10.1146/annurev.micro.60.080805.142205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aliyari R, et al. Mechanism of induction and suppression of antiviral immunity directed by virus-derived small RNAs in Drosophila. Cell Host Microbe. 2008;4:387–97. doi: 10.1016/j.chom.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suzuki N, Maruyama K, Moriyama M, Nuss DL. Hypovirus papain-like protease p29 functions in trans to enhance viral double-stranded RNA accumulation and vertical transmission. J Virol. 2003;77:11697–707. doi: 10.1128/JVI.77.21.11697-11707.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Segers GC, van Wezel R, Zhang X, Hong Y, Nuss DL. Hypovirus papain-like protease p29 suppresses RNA silencing in the natural fungal host and in a heterologous plant system. Eukaryot Cell. 2006;5:896–904. doi: 10.1128/EC.00373-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suzuki N, Chen B, Nuss DL. Mapping of a hypovirus p29 protease symptom determinant domain with sequence similarity to potyvirus HC-Pro protease. J Virol. 1999;73:9478–84. doi: 10.1128/jvi.73.11.9478-9484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choi GH, Pawlyk DM, Nuss DL. The autocatalytic protease p29 encoded by a hypovirulence-associated virus of the chestnut blight fungus resembles the potyvirus-encoded protease HC-Pro. Virology. 1991;183:747–52. doi: 10.1016/0042-6822(91)91004-z. [DOI] [PubMed] [Google Scholar]
- 107.Zhang X, Nuss DL. A host dicer is required for defective viral RNA production and recombinant virus vector RNA instability for a positive sense RNA virus. Proc Natl Acad Sci U S A. 2008;105:16749–54. doi: 10.1073/pnas.0807225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang X, Segers GC, Sun Q, Deng F, Nuss DL. Characterization of hypovirus-derived small RNAs generated in the chestnut blight fungus by an inducible DCL-2-dependent pathway. J Virol. 2008;82:2613–9. doi: 10.1128/JVI.02324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun Q, Choi GH, Nuss DL. A single Argonaute gene is required for induction of RNA silencing antiviral defense and promotes viral RNA recombination. Proc Natl Acad Sci U S A. 2009;106:17927–32. doi: 10.1073/pnas.0907552106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hammond TM, Keller NP. RNA silencing in Aspergillus nidulans is independent of RNA-dependent RNA polymerases. Genetics. 2005;169:607–17. doi: 10.1534/genetics.104.035964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hammond TM, Bok JW, Andrewski MD, Reyes-Dominguez Y, Scazzocchio C, Keller NP. RNA silencing gene truncation in the filamentous fungus Aspergillus nidulans. Eukaryot Cell. 2008;7:339–49. doi: 10.1128/EC.00355-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hammond TM, Andrewski MD, Roossinck MJ, Keller NP. Aspergillus mycoviruses are targets and suppressors of RNA silencing. Eukaryot Cell. 2008;7:350–7. doi: 10.1128/EC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nicolas FE, et al. Endogenous short RNAs generated by Dicer 2 and RNA-dependent RNA polymerase 1 regulate mRNAs in the basal fungus Mucor circinelloides. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.de Haro JP, Calo S, Cervantes M, Nicolas FE, Torres-Martinez S, Ruiz-Vazquez RM. A single dicer gene is required for efficient gene silencing associated with two classes of small antisense RNAs in Mucor circinelloides. Eukaryot Cell. 2009;8:1486–97. doi: 10.1128/EC.00191-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nicolas FE, de Haro JP, Torres-Martinez S, Ruiz-Vazquez RM. Mutants defective in a Mucor circinelloides dicer-like gene are not compromised in siRNA silencing but display developmental defects. Fungal Genet Biol. 2007;44:504–16. doi: 10.1016/j.fgb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 116.Nicolas FE, Torres-Martinez S, Ruiz-Vazquez RM. Two classes of small antisense RNAs in fungal RNA silencing triggered by non-integrative transgenes. EMBO J. 2003;22:3983–91. doi: 10.1093/emboj/cdg384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hamada W, Spanu PD. Co-suppression of the hydrophobin gene HCf-1 is correlated with antisense RNA biosynthesis in Cladosporium fulvum. Mol Gen Genet. 1998;259:630–8. doi: 10.1007/s004380050857. [DOI] [PubMed] [Google Scholar]
- 118.Schuurs TA, Schaeffer EA, Wessels JG. Homology-dependent silencing of the SC3 gene in Schizophyllum commune. Genetics. 1997;147:589–96. doi: 10.1093/genetics/147.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kadotani N, Nakayashiki H, Tosa Y, Mayama S. RNA silencing in the phytopathogenic fungus Magnaporthe oryzae. Mol Plant Microbe Interact. 2003;16:769–76. doi: 10.1094/MPMI.2003.16.9.769. [DOI] [PubMed] [Google Scholar]
- 120.Erental A, Harel A, Yarden O. Type 2A phosphoprotein phosphatase is required for asexual development and pathogenesis of Sclerotinia sclerotiorum. Mol Plant Microbe Interact. 2007;20:944–54. doi: 10.1094/MPMI-20-8-0944. [DOI] [PubMed] [Google Scholar]
- 121.Mouyna I, Henry C, Doering TL, Latge JP. Gene silencing with RNA interference in the human pathogenic fungus Aspergillus fumigatus. FEMS Microbiol Lett. 2004;237:317–24. doi: 10.1016/j.femsle.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 122.Khalaj V, Eslami H, Azizi M, Rovira-Graells N, Bromley M. Efficient downregulation of alb1 gene using an AMA1-based episomal expression of RNAi construct in Aspergillus fumigatus. FEMS Microbiol Lett. 2007;270:250–4. doi: 10.1111/j.1574-6968.2007.00680.x. [DOI] [PubMed] [Google Scholar]
- 123.Henry C, Mouyna I, Latge JP. Testing the efficacy of RNA interference constructs in Aspergillus fumigatus. Curr Genet. 2007;51:277–84. doi: 10.1007/s00294-007-0119-0. [DOI] [PubMed] [Google Scholar]
- 124.Bromley M, Gordon C, Rovira-Graells N, Oliver J. The Aspergillus fumigatus cellobiohydrolase B (cbhB) promoter is tightly regulated and can be exploited for controlled protein expression and RNAi. FEMS Microbiol Lett. 2006;264:246–54. doi: 10.1111/j.1574-6968.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- 125.Yamada O, Ikeda R, Ohkita Y, Hayashi R, Sakamoto K, Akita O. Gene silencing by RNA interference in the koji mold Aspergillus oryzae. Biosci Biotechnol Biochem. 2007;71:138–44. doi: 10.1271/bbb.60405. [DOI] [PubMed] [Google Scholar]
- 126.McDonald T, Brown D, Keller NP, Hammond TM. RNA silencing of mycotoxin production in Aspergillus and Fusarium species. Mol Plant Microbe Interact. 2005;18:539–45. doi: 10.1094/MPMI-18-0539. [DOI] [PubMed] [Google Scholar]
- 127.Moriwaki A, Ueno M, Arase S, Kihara J. RNA-mediated gene silencing in the phytopathogenic fungus Bipolaris oryzae. FEMS Microbiol Lett. 2007;269:85–9. doi: 10.1111/j.1574-6968.2006.00606.x. [DOI] [PubMed] [Google Scholar]
- 128.Nakayashiki H, Hanada S, Nguyen BQ, Kadotani N, Tosa Y, Mayama S. RNA silencing as a tool for exploring gene function in ascomycete fungi. Fungal Genet Biol. 2005;42:275–83. doi: 10.1016/j.fgb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 129.Chague V, Maor R, Sharon A. CgOpt1, a putative oligopeptide transporter from Colletotrichum gloeosporioides that is involved in responses to auxin and pathogenicity. BMC Microbiol. 2009;9:173. doi: 10.1186/1471-2180-9-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Namekawa SH, Iwabata K, Sugawara H, Hamada FN, Koshiyama A, Chiku H, Kamada T, Sakaguchi K. Knockdown of LIM15/DMC1 in the mushroom Coprinus cinereus by double-stranded RNA-mediated gene silencing. Microbiology. 2005;151:3669–78. doi: 10.1099/mic.0.28209-0. [DOI] [PubMed] [Google Scholar]
- 131.Walti MA, Villalba C, Buser RM, Grunler A, Aebi M, Kunzler M. Targeted gene silencing in the model mushroom Coprinopsis cinerea (Coprinus cinereus) by expression of homologous hairpin RNAs. Eukaryot Cell. 2006;5:732–44. doi: 10.1128/EC.5.4.732-744.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ha YS, Covert SF, Momany M. FsFKS1, the 1,3-beta-glucan synthase from the caspofungin-resistant fungus Fusarium solani. Eukaryot Cell. 2006;5:1036–42. doi: 10.1128/EC.00030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tinoco ML, Dias BB, Dall'astta RC, Pamphile JA, Aragao FJ. In vivo trans-specific gene silencing in fungal cells by in planta expression of a double-stranded RNA. BMC Biol. 2010;8:27. doi: 10.1186/1741-7007-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Caribe dos Santos AC, et al. dsRNA-induced gene silencing in Moniliophthora perniciosa, the causal agent of witches' broom disease of cacao. Fungal Genet Biol. 2009;46:825–36. doi: 10.1016/j.fgb.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 135.Rappleye CA, Engle JT, Goldman WE. RNA interference in Histoplasma capsulatum demonstrates a role for alpha-(1,3)-glucan in virulence. Mol Microbiol. 2004;53:153–65. doi: 10.1111/j.1365-2958.2004.04131.x. [DOI] [PubMed] [Google Scholar]
- 136.Bohse ML, Woods JP. RNA interference-mediated silencing of the YPS3 gene of Histoplasma capsulatum reveals virulence defects. Infect Immun. 2007;75:2811–7. doi: 10.1128/IAI.00304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu H, Cottrell TR, Pierini LM, Goldman WE, Doering TL. RNA interference in the pathogenic fungus Cryptococcus neoformans. Genetics. 2002;160:463–70. doi: 10.1093/genetics/160.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Jong JF, Deelstra HJ, Wosten HA, Lugones LG. RNA-mediated gene silencing in monokaryons and dikaryons of Schizophyllum commune. Appl Environ Microbiol. 2006;72:1267–9. doi: 10.1128/AEM.72.2.1267-1269.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gong X, Fu Y, Jiang D, Li G, Yi X, Peng Y. L-arginine is essential for conidiation in the filamentous fungus Coniothyrium minitans. Fungal Genet Biol. 2007;44:1368–79. doi: 10.1016/j.fgb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 140.Solomon PS, Jorgens CI, Oliver RP. Delta-aminolaevulinic acid synthesis is required for virulence of the wheat pathogen Stagonospora nodorum. Microbiology. 2006;152:1533–8. doi: 10.1099/mic.0.28556-0. [DOI] [PubMed] [Google Scholar]
- 141.Tanguay P, Bozza S, Breuil C. Assessing RNAi frequency and efficiency in Ophiostoma floccosum and O. piceae. Fungal Genet Biol. 2006;43:804–12. doi: 10.1016/j.fgb.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 142.Patel RM, van Kan JA, Bailey AM, Foster GD. RNA-mediated gene silencing of superoxide dismutase (bcsod1) in Botrytis cinerea. Phytopathology. 2008;98:1334–9. doi: 10.1094/PHYTO-98-12-1334. [DOI] [PubMed] [Google Scholar]
- 143.Nakayashiki H, Kadotani N, Mayama S. Evolution and diversification of RNA silencing proteins in fungi. J Mol Evol. 2006;63:127–35. doi: 10.1007/s00239-005-0257-2. [DOI] [PubMed] [Google Scholar]


