Abstract
Background
Monocytes play a central role in HIV neuropathogenesis, but there are limited data on monocyte subsets and markers of monocyte activation in perinatally HIV-infected children.
Objective
To determine the relationship between monocyte subsets, the sCD163 monocyte activation marker, and neuropsychological performance among perinatally HIV-infected children initiating antiretroviral therapy (ART).
Methods
ART-naïve children from the PREDICT study were categorised into two groups: those on ART for ≥24 weeks (ART group, n =201) and those untreated (no ART group, n =79). This analysis used data from the baseline and week 144 including sCD163 and frequencies of activated monocytes (CD14+/CD16+/HLA-DR+), perivascular monocytes (CD14+/CD16+/CD163+ and CD14low/CD16+/CD163+), and neuropsychological testing scores: Verbal and Performance Intelligence Quotient (VIQ and PIQ), Beery Visuomotor Integration (VMI) and Children’s Color Trails 2 (CT2).
Results
Baseline demographic and HIV disease parameters were similar between groups. The median age was 6 years, CD4 was 20% (620 cells/mm3), and HIV RNA was 4.8 log10. By week 144, the ART vs the no ART group had significantly higher CD4 (938 vs 552 cells/mm3) and lower HIV RNA (1.6 vs 4.38 log10 copies/mL, P <0.05). sCD163 declined in the ART vs no ART group (median changes −2533 vs −159 ng/mL, P <0.0001). Frequencies of all monocyte subsets declined in the treated but not the untreated group (P <0.05). Higher CD14+/CD16+/HLA-DR+ percentage was associated with higher VIQ, Beery VMI and CT2 scores. Higher percentages of CD14+/CD16+/CD163+ and CD14low/CD16+/CD163+ were associated with higher CT2 and VIQ, respectively.
Conclusion
ART significantly reduced sCD163 levels and frequencies of activated and perivascular monocytes. Higher frequencies of these cells correlated with better neuropsychological performance suggesting a protective role of monocyte-macrophage immune activation in perinatal HIV infection in terms of neuropsychological function.
Keywords: HIV, ART, children, immune activation, monocyte, sCD163, neurocognition, neuropsychological testing
Introduction
HIV exerts deleterious effects on the growing brains of children infected perinatally as evidenced by lower neuropsychological test scores compared to uninfected peers [1,2]. Cognition, language, psychomotor function and attention deficits could affect children’s learning ability with potential long-lasting adverse neurobehaviour consequences as they age into adolescence [3,4]. Antiretroviral therapy (ART) can prevent and reverse some neurodevelopmental damage caused by HIV, particularly when treatment is initiated early [1,5].
Several lines of evidence support the central role of monocytes in trafficking HIV into the brain [6,7]. High turnover of monocytes from the bone marrow and plasma biomarkers for monocyte activation are correlated with HIV neuropathogenesis in adults [8,9]. Several populations of blood monocytes are of interest. The CD14+/16+ monocytes are highly infected by HIV, with particular involvement of activated monocytes (CD14+/CD16+/HLA-DR+) and non-classical monocytes (CD14low/CD16+) [7,10,11]. Furthermore, CD14+/CD16+ monocytes that express CD163 are presumed to be precursors of perivascular macrophages that are associated with brain pathology in simian immunodeficiency virus (SIV) and HIV [12]. CD163 is a scavenger receptor for haemoglobin–haptoglobin complexes, and following activation of monocytes and macrophages, soluble CD163 (sCD163) is cleaved from the cell surface into the circulation. Levels of sCD163 are associated with cognitive impairment in HIV-positive adults, and seronegative adults and children with liver injury, vascular inflammation, and other inflammatory conditions [13–16].
There are limited data on the dynamic of monocyte subsets and sCD163 in perinatally HIV-infected children on ART, and the associations between these markers and neuropsychological performance in children are unknown. Therefore, the objectives of this analysis are to: (1) assess the effects of ART on sCD163 levels and frequencies of monocyte subsets (CD14+/CD16+/HLA-DR+, CD14+/CD16+/CD163+, CD14low/CD16+/CD163+); and (2) assess the relationship between sCD163 and monocyte subsets with neuropsychological performance.
Material and methods
Study design and population
This analysis utilised data from the Pediatric Randomized Early versus Deferred Initiation in Cambodia and Thailand study (the PREDICT study, clinicaltrials.gov identification number NCT00234091) that randomised 300 ART-naïve HIV-infected children in Thailand (n =180) and Cambodia (n =120) to early (ART initiation at CD4 15–24%) vs deferred treatment (ART initiation at CD4 <15%) for 144 weeks [17]. The study was approved by the Thai and Cambodian National and local institutional review boards. Caregivers gave consent, and children gave assent according to local ethics requirements.
For this analysis, children were grouped by their ART status. The ART group included children who had at least 24 weeks of ART during the period of 144 weeks of follow-up. This group combined children who initiated ART at baseline in the early ART arm and those in the deferred arm who initiated ART after the baseline visit when their CD4 declined below 15%. The no ART group included children in the deferred arm who did not initiate ART during the 144 weeks of the study because they were able to maintained CD4 above 15%.
Monocytes phenotyping and soluble CD163
Soluble CD163 was assayed at baseline and week 144 by enzyme-linked immunoassay using the Macro163 kit (IQ Products and Trillium Diagnostics, Bangor, Maine, USA). The flow cytometry was completed according to Pediatric AIDS Clinical Trials Group procedures at US National Institute of Allergy and Infectious Diseases certified laboratories with rigorous quality assurance programs as published elsewhere [18]. Data from three monocyte subsets from week 0 and week 144 were included in this analysis including CD14+/CD16+/HLA-DR+ (activated monocytes), CD14+/CD16+/CD163+ (perivascular monocytes believed to be precursors of brain perivascular macrophages), and non-classical monocytes that express CD163 (CD14low/CD16+/CD163+) [7,11]. The Forward Scatter (FSC) and Side Scatter (SSC) were gated to first select total monocyte population (R1 gate). The CD14low/CD16+ monocyte subset was then identified (R2 gate) and CD14low/CD16+/CD163+ monocytes were separated. Antibodies used for flow cytometry were all from Becton Dickinson: anti-CD14 PerCP (clone 61D3), anti-CD16 PE (clone CB16) and anti-CD163 FITC (clone GHI/61).
Neuropsychological testing
A brief battery of cognitive measures was administered based on age of the participants and cultural application. Verbal and Performance Intelligence Quotients (VIQ and PIQ) were derived from the Thai versions of the Wechsler Intelligence Scale for Children III for ages 6–17 (WISC III) and the Wechsler Preschool and Primary Scale of Intelligence III (WIPSI III) for ages 2–7.25. These two measures were only administered to children in Thailand as versions were not available for Cambodia. Children from both Thailand and Cambodia were administered the Beery Visual Motor Integration (VMI) to assess visual-motor coordination, and Color Trails 2 was administered to assess psychomotor speed, executive function and visual scanning [2]. The neuropsychological testing was initiated as part of a substudy after the main study commenced; therefore, the first assessment was completed at a median of 36 (IQR 0–60) weeks after enrolment. Subsequently children performed the neuropsychological tests every 24 weeks until week 144.
Statistical analysis
Statistical analysis was conducted with Stata version 13 (Statacorp, College Station, TX, USA) and graphs were constructed using GraphPad Prism version X (GraphPad Software Inc, San Diego, CA, USA). Participants’ socio-demographic characteristics were described as median [interquartile range (IQR)] or n (%). Neuropsychological test scores that were standardised according to US controls were described as mean [standard deviation (SD)]. Changes in neuropsychological test scores from baseline to week 144 between children who started ART were calculated as the mean difference in change scores (95% confidence interval) between ART and no ART groups [19]. Formal comparison of the change in monocyte percentages and sCD163 concentrations from baseline to week 144 between the ART and no ART groups, and children in the early and deferred arms, were completed using the Wilcoxon rank sum test. Spearman’s rank correlation coefficient was used to correlate sCD163 concentration and monocyte percentages with neurocognitive test scores at week 144.
Results
Cohort characteristics
All children had been infected with HIV perinatally and were ART-naïve at baseline. The ART group included 201 children treated with ART for at least 24 weeks. These children were either randomised to the early ART arm and initiated ART at baseline (n =142) or they were in the deferred arm and initiated ART when CD4 declined below 15% (n =59). This latter group started ART at a median (IQR) of 61 (29–76) weeks into the study. Seventy-eight children in the deferred arm did not start ART because they maintained their CD4 above 15% and are included here as the ‘no ART’ group. The ART and no ART groups had similar demographic and HIV disease characteristics at baseline (Table 1). The median age of the cohort was 6 years. There were slightly more males than females and more Thais than Cambodians. Most had mild HIV symptoms with a median CD4 of 20% and HIV RNA of 4.8 log10 copies/mL. The majority came from low-income families with caregivers who had less than secondary school education. By week 144, the ART group had significantly higher CD4% and count, and lower HIV RNA. Most (92%) achieved HIV RNA below 50 copies/mL. Their median duration of ART was 144 (IQR 119–144) weeks. The most common regimen used (68%) was zidovudine (AZT)/lamivudine (3TC)/nevirapine (NVP). The other regimens were AZT/3TC/lopinavir/ritonavir(r) (LPV/r) in 12%, AZT/3TC/efavirenz (EFV) in 6% and abacavir (ABC)/3TC/NVP in 6%.
Table 1.
Characteristics of children, by ART status at week 144
| Characteristic | Total (n=279) | ART (n=201) | No ART (n=78) |
|---|---|---|---|
| Week 0 | |||
| Age (years) | 6 (3–8) | 6 (3–8) | 6 (4–8) |
| Male:Female | 165(59):114(41) | 109(54):92(46) | 56(72):22(28) |
| Thai:Cambodian | 167(60):112(40) | 116(58):85(42) | 51(65):27(34) |
| CDC | |||
| N | 4 (1) | 3 (1) | 1 (1) |
| A | 170 (61) | 124 (62) | 46 (59) |
| B | 105 (38) | 74 (37) | 31 (40) |
| CD4% | 20 (17–23) | 19 (17–22) | 21 (18–25) |
| CD4 cell count (cells/mm3) | 620 (449–851) | 610 (425–833) | 694 (543–876) |
| HIV RNA log10 copies/mL | 4.80 (4.35–5.00) | 4.92 (4.70–5.00) | 4.51 (4.01–4.89) |
| Income | |||
| Very low | 26 (9) | 19 (9) | 7 (9) |
| Low | 130 (47) | 93 (46) | 37 (47) |
| Average | 72 (26) | 51 (25) | 21 (27) |
| Above average | 4 (1) | 2 (1) | 2 (3) |
| Unknown | 47 (17) | 36 (18) | 11 (14) |
| Primary caregiver | |||
| Parent | 173 (62) | 127 (63) | 46 (59) |
| Other | 98 (35) | 69 (34) | 29 (37) |
| Unknown | 8 (3) | 5 (3) | 3 (4) |
| Education of primary caregiver | |||
| None | 39 (14) | 28 (14) | 11 (14) |
| Elementary | 122 (44) | 83 (41) | 39 (50) |
| Secondary/vocational school | 92 (33) | 68 (34) | 23 (29) |
| Bachelor’s degree or higher | 17 (6) | 15 (7) | 2 (3) |
| Unknown | 9 (3) | 6 (3) | 3 (4) |
| VIQ* | 72 (12.2) n=154 | 72 (12.0) n=106 | 74 (12.7) n=48 |
| PIQ* | 79 (12.6) n=156 | 78 (12.1) n=107 | 80 (13.6) n=49 |
| Beery VMI* | 85 (16.3) n=271 | 84 (15.9) n=196 | 88 (17.1) n=75 |
| Color Trail 2 standard score* | 78 (16.4) n=158 | 79 (16.6) n=107 | 77 (16.2) n=55 |
| sCD163 (ng/mL) | 3713 (2665–4650) n=237 | 3806 (2710–4900) n=174 | 3334 (2399–4555) n=63 |
| %CD14+/CD16+/HLA-DR+ | 10.3 (5.4–16.2) n=141 | 10.6 (6.9–16.2) n=105 | 6.7 (4.5–17.0) n=36 |
| %CD14+/CD16+/CD163+ | 4.8 (2.5–16.2) n=137 | 5.3 (2.9–9.3) n=101 | 3.75 (1.7–7.65) n=36 |
| %CD14low/CD16+/CD163+ | 18.7 (9.1–24.5) n=121 | 18.6 (8.3–25.1) n=90 | 20.2 (12.0–24.2) n=31 |
| Week 144 | |||
| Duration of ART | N/A | 144 (119–144) | N/A |
| CD4% | 30 (24–35) | 32 (27–37) | 21 (17–25) |
| CD4 cell count (cells/mm3) | 833 (595–1103) | 938 (749–1182) | 552 (436–711) |
| HIV RNA log10 copies/mL | 1.70 (1.60–3.53) | 1.60 (1.60–1.70) | 4.38 (3.85–4.72) |
| % with HIV RNA <50 copies/mL | 184 (66) | 184 (92) | 0 (0) |
| VIQ | 71 (11.9) n=113 | 70 (11.5) n=77 | 73 (12.5) n=36 |
| PIQ | 83 (14.5) n=114 | 83 (13.9) n=78 | 84 (16.0) n=36 |
| Beery | 86 (14.1) n=267 | 85 (14.6) n=192 | 88 (12.3) n=75 |
| Color Trail 2 standard score | 86 (14.9) n=156 | 85 (14.9) n=105 | 89 (14.8) n=51 |
| sCD163 (ng/mL) | 1468 (784–2792) n=181 | 1049 (648–1724) n=127 | 3348 (2269–4419) n=54 |
| %CD14+/CD16+/HLA-DR+ | 5.8 (3.0–10.0) n=277 | 4.8 (2.5–8.7) n=199 | 7.85 (5.2–12.8) n=78 |
| %CD14+/CD16+/CD163+ | 3.0 (1.3–5.1) n=277 | 2.9 (1.2–4.8) n=199 | 3.55 (1.4–7.1) n=78 |
| %CD14low/CD16+/CD163+ | 11.3 (5.31–19.9) n=181 | 10.95 (5.29–19.9) n=127 | 12.9 (5.6–18.8) n=54 |
Data presented as median (IQR) or n (%), except for neurocognitive test data which is standardised and presented as mean (SD)
At time of the first neuropsychological testing: a median of 36 (IQR 0–60) weeks from enrolment
CDC: Center for Disease Control and Prevention clinical staging
Neuropsychological performances did not differ between the two groups at the baseline assessment. Both groups exhibited VIQ and PIQ scores approximately two standard deviations below US norms with no significant differences by ART status. Similarly, the groups did not differ significantly on the Beery VMI or the Color Trails 2 test. By week 144, there were no differences in the mean changes from first test to week 144 between the ART vs no ART groups for all tests.
sCD163 and monocyte subsets
Figure 1 illustrates the sCD163 levels and frequencies of monocyte subsets between week 0 and week 144 for the ART and no ART groups. A significant decline in sCD163 at week 144 was evident in the ART group (median change −2533 mg/mL, IQR −3774 to −1702 ng/mL) compared to the no ART group (median change −159 ng/mL, IQR −971 to 711 ng/mL, P <0.0001).
Figure 1. Soluble CD163 (sCD163) and monocyte subsets at baseline and week 144 in children who are on ART vs those not on ART.
(a) sCD163 levels; (b) Frequency of CD14+/CD16+/HLA-DR+ cells; (c): Frequency of CD14+/CD16+/CD163+ cells: (d) Frequency of CD14low/CD16+/CD163+ cells
NB: There were 201 children in the ART group (●) and 78 in the no ART group (▲)
The frequency of the CD14+/CD16+/HLA-DR+ showed a greater reduction after ART (median change −6.1%, IQR −9.6 to −2.1) vs no ART (median change −1.7%, IQR −9.9 to 3.5, P<0.001) as did the CD14+/CD16+/CD163+ cell subset (median changes of −3.3%, IQR −5.6 to −0.4 for ART group vs −1.5%, IQR −4.4 to 1.6 for no ART group, P=0.03). In the CD14low/CD16+/CD163+ monocyte subset, the median change from baseline was negative in the ART group (−3.4, IQR −10.7 to 5.31) and positive in the no ART group (2.4, IQR −8.0 to 6.56), but the difference in change between groups was not significant (P>0.05).
In the ART group, the changes in these markers in the subset of children who initiated ART at a higher CD4 (early arm) vs those who had CD4 decline prompting ART initiation (deferred arm) were similar, and not significantly different between treatment arms. Median change in sCD163 was −2521 (−3774 to −1729) ng/mL in the early arm and −2787 (−4494 to −1050) ng/mL in the deferred arm. Median change in CD14+/CD16+/HLA-DR+ monocyte frequency was −6.0% (IQR −9.6% to −1.7%) in the early arm and −6.4% (−9.6% to −3.1%) in the deferred arm. Median change in the frequency of CD14+/CD16+/CD163+ monocytes was −2.9% (IQR −5.8% to 0%) in the early arm vs −3.6% (−5.1% to −0.8%) in the deferred arm, and the median change in the frequency of CD14low/CD16+/CD163+ monocytes was −2.0% (IQR −8.6% to 6.4%) in the early and −9.6% (−20.6% to 2.5%) in the deferred arm.
Relationship between sCD163 and monocyte subsets with neuropsychological performance
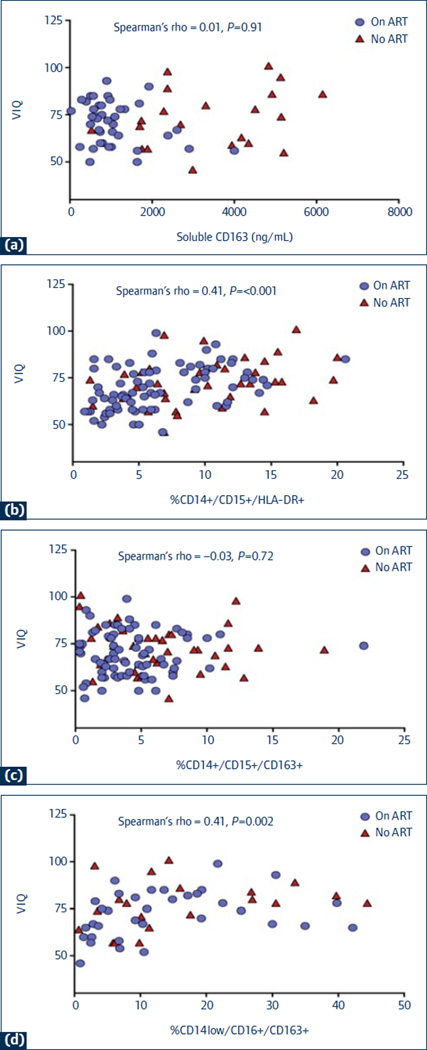
The relationships between sCD163 and monocyte subsets with neuropsychological performance were examined at week 144 (Figure 2). Higher frequency of CD14+/CD16+/HLA-DR+ cells was associated with higher VIQ (Spearman’s rho=0.41, P<0.001), Beery VMI performance (Spearman’s rho=0.16, P=0.01), and Color Trails 2 scores (Spearman’s rho=0.16, P=0.05). Higher CD14+/CD16+/CD163+ percentages were also associated with higher Color Trails 2 performance (Spearman’s rho=0.21, P=0.01). Finally, higher percentage of CD14low/CD16+/CD163+ cells correlated with higher VIQ scores (Spearman’s rho=0.41, P=0.002). For each of the neuropsychological tests, there was no other statistically significant association with sCD163 level or frequency of monocyte subsets.
Figure 2. The associations between verbal intelligence quotient (VIQ) scores and sCD163 and monocyte subsets at week 144.
(a) VIQ vs sCD163 levels; (b) VIQ vs frequency of CD14+/CD16+/HLADR+ cells; (c) VIQ vs frequency of CD14+/CD16+/CD163+ cells; (d) VIQ vs. frequency of CD14low/CD16+/CD163+ cells.
NB: There were 201 children in the ART group (●) and 78 in the no ART group (▲)
Discussion
Our study observed significant reductions in markers of monocyte activation after ART. The sCD163 level and frequencies of CD14+/CD16+/HLA-DR+ and CD14+/CD16+/CD163+ cells were significantly lower in the treated children compared to the untreated group. The PREDICT trial provided a unique opportunity to evaluate sCD163 longitudinally and monocyte subsets before and after ART, as well as assess their relationships to neuropsychological testing in well-characterised children who had high CD4 cell counts and no advanced HIV disease [2,17]. Importantly, the untreated group were children ≥5 years old who maintained a relatively high CD4 count (median 552 cells/mm3) throughout the 3-year study, and many would not have been eligible for ART under the current World Health Organization guideline [20]. This suggests persistent monocyte activation in untreated children who are long-term non-progressors.
sCD163 is a marker of monocyte activation and the levels are reduced by ART. However, in one study, sCD163 levels only returned to normal in adults treated in early infection and not in ‘late treaters’ [14]. Our children were on ART regimens considered highly penetrable into the central nervous system (CNS) compartment (CNS penetration effectiveness scores of 9 to 10) [21]. Nevertheless, the levels of sCD163 in both the treated and untreated groups in our study were higher than that previously reported in a group of 144 US children with HIV (median 487 ng/mL). The reasons could be the longer period of viral suppression of about 10 years or more in that study and the differences in the manufactured test kits [22]. In a study of virally suppressed adults, a median sCD163 level of 1401 ng/mL was observed amongst those with HIV-associated neurocognitive disease (HAND) [13]. Our untreated children had levels that were 3-times higher whereas the treated children had similar levels to the adults with HAND. Long-term follow-up will be required to understand the effects of persistent monocyte activation on health outcomes in the children in our study.
The study by Burdo et al. identified significant associations between sCD163 levels and impaired performances in the cognitive domains of learning and executive function [13]. We did not see a correlation between sCD163 and any of the neuropsychological testing, which could be due to several reasons. First, our neuropsychological battery did not test specifically for learning abilities and executive function was measured in only one test. Secondly, we used continuous neuropsychological testing scores instead of impairment classifications because normative reference samples do not exist for Thai and Cambodian children.
Presence of CD163-expressing macrophages has been documented in the perivascular lesions of encephalitic brains from SIV-infected macaques, implicating them in SIV neuropathogenesis [23]. Studies of autopsied brain tissues from HIV-infected adults have illustrated the role of CNS inflammation in HIV neuropathogenesis [24,25]. CD163 and HLA DR-expressing macrophages and microglial infiltration were observed in well controlled HIV, and to a larger extent, in those with encephalitis, suggesting that CNS inflammation occurs throughout the spectrum of HIV disease severity [25]. High frequencies of circulating monocytes are associated with HIV brain complications in children and adults [9,26]. Studies in adults showed infection of CD14+/CD16+ monocytes correlates with HAND [27]. Brain NAA/Cr, an imaging marker for neuronal injury, also inversely correlated with circulating CD14low/CD16+ monocytes [28]. The frequency of CD14+/CD16+/HLA-DR+ cells declines after successful ART in adults but data in children are lacking [14]. There are also very few data on the effects of ART on frequencies of CD14+/CD16+/CD163+ and CD14low/CD16+/CD163+ cells [14]. In our study we observed reductions in all three monocyte populations in the ART group but not in the untreated group. Unexpectedly, we observed an association between higher frequencies of the activated monocytes and better performance on several neuropsychological measures (VIQ, Beery and Color Trails 2). Similarly, we observed positive associations between CD14low/CD16+/CD163+ with VIQ and CD14+/CD16+/CD163+ with Color Trails 2. Higher frequency of activated CD8+ T cells (CD8+/CD38+/HLA-DR+) has been shown to correlate with better Full Scale IQ scores in extensively treated perinatally HIV-infected children [29]. In contrast to the poor prognostic implication of activation in adults [30,31], it is conceivable that children living with HIV since birth have adapted to a state of immune activation. Some have postulated that an activation-dependent mechanism could help children cope with high viral turnover, and better survival was observed in children with high frequency of activated CD8+ T cells [19,32]. It is also possible that the associations, although statistically significant, could have limited clinical relevance.
In summary, in two groups of children with relatively high CD4 cell counts, we observed the benefit of ART in lowering sCD163, a marker of monocyte activation. sCD163 levels were not predictive of neuropsychological testing scores. The activated and perivascular monocyte populations also declined in frequency after ART. Higher frequencies of these cells were associated with better performance on several neuropsychological measures, suggesting a possible protective role of monocyte activation in perinatal HIV infection.
Acknowledgements
The PREDICT study is sponsored by National Institute of Allergy and Infectious Disease (NIAID), Grant number U19 AI053741, http://Clinical trial.gov identification number NCT00234091. Antiretoviral drugs for PREDICT are provided by GlaxoSmithKline (AZT, 3TC), Boehringer Ingelheim (NVP), Merck (EFV), Abbott (RTV) and Roche (NFV). The flow cytometry was funded by the National Institute of Allergy and Infectious Diseases grant R01AI075408-02 (JA). Funding to conduct the sCD163 assays was provided through TREAT Asia/amfAR, The Foundation for AIDS Research, with support from the US National Institutes of Health’s National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907).
PREDICT Study group
CIP TH001: HIV Netherlands Australia Thailand (HIV-NAT) Research Collaboration, Thai Red Cross AIDS Research Center, Bangkok, Thailand: Kiat Ruxrungtham, Jintanat Ananworanich, Thanyawee Puthanakit, Chitsanu Pancharoen, Torsak Bunupuradah, Stephen Kerr, Theshinee Chuenyam, Sasiwimol Ubolyam, Apicha Mahanontharit,Tulathip Suwanlerk, Jintana Intasan,Thidarat Jupimai, Primwichaya Intakan, Tawan Hirunyanulux, Praneet Pinklow, Kanchana Pruksakaew,Oratai Butterworth, Nitiya Chomchey, Chulalak Sriheara, Anuntaya Uanithirat, Sunate Posyauattanakul, Thipsiri Prungsin, Pitch Boonrak, Waraporn Sakornjun, Tanakorn Apornpong, Jiratchaya Sophonphan, Ormrudee Rit-im, Nuchapong Noumtong, Noppong Hirunwadee, Chaiwat Ungsedhapand, Chowalit Phadungphon, Wanchai Thongsee, Orathai Chaiya, Augchara Suwannawat, Threepol Sattong, Niti Wongthai, Kesdao Nantapisan, Umpaporn Methanggool, Narumon Suebsri, Chris Duncombe, Taksin Panpuy, Chayapa Phasomsap, Boonjit Deeaium, Pattiya Jootakarn, Suteeraporn Pinyakorn
CIP TH003: Bamrasnaradura Infectious Diseases Institute, Nonthaburi,Thailand: Jurai Wongsawat, Rujanee Sunthornkachit, Visal Moolasart, Natawan Siripongpreeda, Supeda Thongyen, Piyawadee Chathaisong, Vilaiwan Prommool, Duangmanee Suwannamass, Simakan Waradejwinyoo, Nareopak Boonyarittipat, Thaniya Chiewcharn, Sirirat Likanonsakul, Chatiya Athichathana, Boonchuay Eampokalap, Wattana Sanchiem
CIP TH004: Srinagarind Hospital, Khon Kaen University, Khon Kaen, Thailand: Pope Kosalaraksa, Pagakrong Lumbiganon, Chulapan Engchanil, Piangjit Tharnprisan, Chanasda Sopharak, Viraphong Lulitanond, Samrit Khahmahpahte, Ratthanant Kaewmart, Prajuab Chaimanee, Mathurot Sala, Thaniita Udompanit, Ratchadaporn Wisai, Somjai Rattanamanee, Yingrit Chantarasuk, Sompong Sarvok, Yotsombat Changtrakun, Soontorn Kunhasura, Sudthanom Kamollert
CIP TH005: Queen Savang Vadhana Memorial Hospital, Chonburi, Thailand: Wicharn Luesomboon, Pairuch Eiamapichart, Tanate Jadwattanakul, Isara Limpet-ngam, Daovadee Naraporn, Pornpen Mathajittiphun, Chatchadha Sirimaskul, Woranun Klaihong, Pipat Sittisak, Tippawan Wongwian, Kansiri Charoenthammachoke, Pornchai Yodpo.
CIP TH007: Nakornping Hospital, Chiang Mai, Thailand: Suparat Kanjanavanit, Maneerat Ananthanavanich, Penpak Sornchai, Thida Namwong, Duangrat Chutima, Suchitra Tangmankhongworakun, Pacharaporn Yingyong, Juree Kasinrerk, Montanee Raksasang, Pimporn Kongdong, Siripim Khampangkome, Suphanphilat Thong-Ngao, Sangwan Paengta, Kasinee Junsom, Ruttana Khuankaew M, Parichat Moolsombat, Duanpen Khuttiwung, Chanannat Chanrin.
CIP TH009: Chiangrai Regional Hopsital, Chiang Rai, Thailand: Rawiwan Hansudewechakul, Yaowalak Jariyapongpaiboon, Chulapong Chanta, Areerat Khonponoi, Chaniporn Yodsuwan, Warunee Srisuk, Pojjavitt Ussawawuthipong, Yupawan Thaweesombat, Polawat Tongsuk, Chaiporn Kumluang, Ruengrit Jinasen, Noodchanee Maneerat, Kajorndej Surapanichadul, Pornpinit Donkaew.
CIP TH010: National Pediatric Hospital, Phnom Penh, Cambodia: Saphonn Vonthanak, Ung Vibol, Sam Sophan, Pich Boren, Kea Chettra, Lim Phary, Toun Roeun, Tieng Sunly, Mom Chandara, Chuop Sokheng, Khin Sokoeun,Tuey Sotharin.
CIP TH011: Social Health Clinic, Phnom Penh, Cambodia: Saphonn Vonthanak, Ung Vibol, Vannary Bun, Somanythd Chhay Meng, Kea Chettra, Sam Phan, Wuddhika In vong, Khuon Dyna.
CIP TH012: Prapokklao Hospital, Chantaburi, Thailand: Chaiwat Ngampiyaskul, Naowarat Srisawat, Wanna Chamjamrat, Sayamol Wattanayothin, Pornphan Prasertphan, Tanyamon Wongcheeree, Pisut Greetanukroh, Chataporn Imubumroong, Pathanee Teirsonsern.
Footnotes
| Steering committee: Praphan Phanuphak, David A Cooper, John Kaldor, Mean Chhi Vun, Saphonn Vonthanak, Kiat Ruxrungtham. |
| Primary endpoint review committee: Carlo Giaquinto, Mark Cotton, Rangsima Lolekha. |
| Clinical events review committee: Virat Sirisanthana, Kulkanya Chokephaibulkit, Piyarat Suntarattiwong. |
| Data safety monitoring board members: Paul Volberding (Chair), Shrikant Bangdiwala, Kruy Lim, NM Samuel, David Schoenfeld, Annette Sohn, Suniti Solomon, Panpit Suwangool, Ruotao Wang, Fujie Zhang, Laurie Zoloth, Dennis O Dixon. |
| National Institutes of Health: Lawrence Fox, Akinlolu O Ojumu, Jane E Bupp, Michael Ussery, Neal T Wetherall, Pim Brouwers, Lynne M Mofenson. |
| Advisors: Matthew Law, William T Shearer, Victor Valcour, Rober Paul, Kovit Pattanapanyasat, Natthaga Sakulploy, Janet M McNicholl. |
| ViiV Healthcare and GlaxoSmithKline: Wendy Snowden, Navdeep K Thoofer. |
| Boehringer Ingelheim: Manuel Distel. |
| Abbott: Annette S Meints, Adawan Methasate. |
| Roche: Matei Popescu, Aeumporn Srigritsanapol. |
| Merck: Suchai Kitsiripornchai. |
Disclaimer
The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or the Department of Defense.
References
- 1.Brahmbhatt H, Boivin M, Ssempijja V, et al. Neurodevelopmental benefits of antiretroviral therapy in Ugandan children aged 0–6 years with HIV. J Acquir Immune Defic Syndr. 2014;67:316–322. doi: 10.1097/QAI.0000000000000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puthanakit T, Ananworanich J, Vonthanak S, et al. Cognitive function and neurodevelopmental outcomes in HIV-infected Children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the PREDICT neurodevelopmental study. Pediatr Infect Dis J. 2013;32:501–508. doi: 10.1097/INF.0b013e31827fb19d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith R, Wilkins M. Perinatally acquired HIV infection: long-term neuropsychological consequences and challenges ahead. Child Neuropsychol. 2015;21:234–268. doi: 10.1080/09297049.2014.898744. [DOI] [PubMed] [Google Scholar]
- 4.Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc. 2013;16:18603. doi: 10.7448/IAS.16.1.18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laughton B, Cornell M, Grove D, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS. 2012;26:1685–1690. doi: 10.1097/QAD.0b013e328355d0ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kallianpur KJ, Valcour VG, Lerdlum S, et al. HIV DNA in CD14+ reservoirs is associated with regional brain atrophy in patients naive to combination antiretroviral therapy. AIDS. 2014;28:1619–1624. doi: 10.1097/QAD.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DW, Anastos K, Morgello S, et al. JAM-A and ALCAM are therapeutic targets to inhibit diapedesis across the BBB of CD14+CD16+ monocytes in HIV-infected individuals. J Leukocyte Biol. 2015;97:401–412. doi: 10.1189/jlb.5A0714-347R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdo TH, Soulas C, Orzechowski K, et al. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer-Smith T, Bell C, Croul S, et al. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008;14:318–326. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer-Smith T, Croul S, Sverstiuk AE, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 11.Kim WK, Corey S, Alvarez X, Williams K. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukocyte Biol. 2003;74:650–656. doi: 10.1189/jlb.0503207. [DOI] [PubMed] [Google Scholar]
- 12.Williams KC, Corey S, Westmoreland SV, et al. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burdo TH, Weiffenbach A, Woods SP, et al. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS. 2013;27:1387–1395. doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazankov K, Moller HJ, Lange A, et al. The macrophage activation marker sCD163 is associated with changes in NAFLD and metabolic profile during lifestyle intervention in obese children. Pediatr Obes. 2015;10:226–233. doi: 10.1111/ijpo.252. [DOI] [PubMed] [Google Scholar]
- 16.Tantawy AA, Adly AA, Ismail EA. Soluble CD163 in young sickle cell disease patients and their trait siblings: a biomarker for pulmonary hypertension and vaso-occlusive complications. Blood Coagulat Fibrinol. 2012;23:640–648. doi: 10.1097/MBC.0b013e3283573a42. [DOI] [PubMed] [Google Scholar]
- 17.Puthanakit T, Saphonn V, Ananworanich J, et al. Early versus deferred antiretroviral therapy for children older than 1 year infected with HIV (PREDICT): a multicentre, randomised, open-label trial. Lancet Infect Dis. 2012;12:933–941. doi: 10.1016/S1473-3099(12)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ananworanich J, Apornpong T, Kosalaraksa P, et al. Characteristics of lymphocyte subsets in HIV-infected, long-term nonprogressor, and healthy Asian children through 12 years of age. J Allergy Clini Immunol. 2010;126:1294–1301. doi: 10.1016/j.jaci.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Martino M, Rossi ME, Azzari C, et al. Different meaning of CD38 molecule expression on CD4+ and CD8+ cells of children perinatally infected with human immunodeficiency virus type 1 infection surviving longer than five years. Pediatr Res. 1998;43:752–758. doi: 10.1203/00006450-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 20.WHO. World Health Organization (WHO); 2013. Jun 30, Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [PubMed] [Google Scholar]
- 21.Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010;18:45–55. [PMC free article] [PubMed] [Google Scholar]
- 22.Persaud D, Patel K, Karalius B, et al. Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr. 2014;168:1138–1146. doi: 10.1001/jamapediatrics.2014.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerngross L, Lehmicke G, Belkadi A, Fischer T. Role for cFMS in maintaining alternative macrophage polarization in SIV infection: implications for HIV neuropathogenesis. J Neuroinflamm. 2015;12:58. doi: 10.1186/s12974-015-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelman BB, Chen T, Lisinicchia JG, et al. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PloS One. 2012;7:e46178. doi: 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tavazzi E, Morrison D, Sullivan P, et al. Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection. Curr HIV Res. 2014;12:97–110. doi: 10.2174/1570162x12666140526114956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Ramon S, Bellon JM, Resino S, et al. Low blood CD8+ T-lymphocytes and high circulating monocytes are predictors of HIV-1-associated progressive encephalopathy in children. Pediatrics. 2003;111:E168–E175. doi: 10.1542/peds.111.2.e168. [DOI] [PubMed] [Google Scholar]
- 27.Shiramizu B, Ananworanich J, Chalermchai T, et al. Failure to clear intra-monocyte HIV infection linked to persistent neuropsychological testing impairment after first-line combined antiretroviral therapy. J Neurovirol. 2012;18:69–73. doi: 10.1007/s13365-011-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell JH, Burdo TH, Autissier P, et al. Minocycline inhibition of monocyte activation correlates with neuronal protection in SIV neuroAIDS. PloS One. 2011;6:e18688. doi: 10.1371/journal.pone.0018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapetanovic S, Aaron L, Montepiedra G, et al. T-cell activation and neurodevelopmental outcomes in perinatally HIV-infected children. AIDS. 2012;26:959–969. doi: 10.1097/QAD.0b013e328352cee7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68:396–404. doi: 10.1097/QAI.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lok JJ, Hunt PW, Collier AC, et al. The impact of age on the prognostic capacity of CD8+ T-cell activation during suppressive antiretroviral therapy. AIDS. 2013;27:2101–2110. doi: 10.1097/QAD.0b013e32836191b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlesinger M, Peters V, Jiang JD, et al. Increased expression of activation markers on CD8 lymphocytes in children with human immunodeficiency virus-1 infection. Pediatr Res. 1995;38:390–396. doi: 10.1203/00006450-199509000-00020. [DOI] [PubMed] [Google Scholar]