Abstract
Objectives
Genital HPV infection in men causes benign and cancerous lesions, the incidence of which differs by age. The goal of this work was to comprehensively evaluate incidence and clearance of individual HPV genotypes among men by age group.
Methods
HIV-negative men ages 18–70 with no history of anogenital cancer were recruited for the HPV Infection in Men (HIM) Study. Participants completed clinical exams and questionnaires every six months for up to ~4 years. Genital specimens underwent HPV genotyping, with associations between age and HPV assessed using Cox analyses.
Results
4085 men were followed for a median of 48.6 months (range: 0.3–94.0). Significantly lower HPV incidence rates were observed among the oldest age group (55–70 years) for grouped high-risk (incidence rate ratio [IRR]=0.71), HPV16 (IRR=0.54), grouped low-risk (IRR=0.74), and HPV6 (IRR=0.57) infections compared to men ages 18–24. However, incidence of the grouped 9-valent HPV vaccine types remained constant across the lifespan. Likelihood of HPV6 and HPV16 clearance remained constant until age 54, then increased significantly for men ages 55–70 (adjusted hazard ratio [AHR]=1.92 and 1.65, respectively).
Conclusions
Men remain susceptible to HPV infections throughout their lifespan, highlighting the need for prevention efforts with long-lasting duration.
Keywords: Human papillomavirus, Natural history, Incidence, Clearance, Age-specific, HIM Study
1. Background
Several cancers of the anogenital tract in men are caused by human papillomavirus (HPV) types 16 and 18, including approximately 50% of penile cancers [1,2]. The difference in age patterning of different HPV-related diseases suggests that genital HPV natural history in men varies by age and HPV type. Penile cancer rates vary greatly by ethnicity and country, with Brazil having among the highest penile cancer rates in the world (~8/100,000) [3]. Although current penile cancer rates are relatively low in Denmark and The Netherlands, both countries have reported trends of rising incidence, as have other European countries [4,5]. While low rates of penile cancer are observed in the U.S. (~1/100,000), incidence is approximately twofold higher among Hispanics compared to non-Hispanics [6,7]. Unlike cervical cancer, which occurs at a median age of ~45 years, diagnosis of penile cancer occurs at older ages, with a median age of ≥60 years. In contrast to penile cancer, condyloma (genital warts), primarily caused by HPV types 6 and 11, occur at younger ages, with peak incidence in early adulthood (late twenties) among men [8].
In Latin American studies, cervical HPV prevalence and incidence appear to follow a bimodal age pattern [9–11]. In contrast to these findings in women, no overall association has been found between age and grouped HPV infection incidence and prevalence among men [12,13]. However, studies to date have not been large enough to examine the age patterning of HPV infection incidence and duration by HPV genotype. We hypothesized that cancer-causing HPV types follow a different pattern with age in men than do HPV types that cause condyloma, and that this pattern is different than what has been reported among women.
2. Methods
2.1. Study design and population
A prospective analysis was conducted within the HPV Infection in Men (HIM) Study, an ongoing natural history study of HPV infection among 4085 healthy men in the U.S. (Tampa, Florida), Brazil (São Paulo), and Mexico (Cuernavaca). HIM Study participants 18–70 years of age were recruited between 2005 and 2009. Men were eligible to participate if they had no prior diagnosis of anogenital cancer, genital warts, or HIV infection at their baseline visit. Participants were enrolled into the prospective component of the study upon completion of their first follow-up visit (two weeks post-baseline). Additional follow-up visits occurred every six months thereafter for up to ~4 years. Details of the HIM Study have been previously published [13]. Less than 1% of HIM Study participants reported receiving >1 dose of an HPV vaccine post-licensure (after 2009). Participants provided written informed consent, and procedures were approved by the following human subjects committees: University of South Florida, Ludwig Institute for Cancer Research, Centro de Referência e Treinamento em Doencas Sexualmente Transmissiveis e AIDS (CRT-DST/AIDS), and Instituto Nacional de Salud Pública de México.
2.2. Specimen collection and testing
Clinical examinations were conducted at each visit. Using prewetted Dacron swabs, genital specimens were collected from the coronal sulcus/glans penis, penile shaft, and scrotum [13] and combined into one sample per participant before being stored at −80 °C. At a central laboratory, DNA was extracted from the samples (QIAamp DNA Blood Mini Kit, Qiagen), followed by PCR and HPV genotyping. Samples that tested positive for β-globin or at least one HPV genotype were considered adequate and were included in the analysis (overall β-globin positivity 98%). The Linear Array assay (Roche Diagnostics) was utilized for detection of 37 HPV genotypes classified as high-risk (oncogenic; HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) or low-risk (non-oncogenic; HPV 6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 66, 67, 69, 70, 71, 72, 73, 81, 82, 82 subtype IS39, 83, 84, and 89 [formerly CP6108]) [14].
2.3. Statistical analysis
Genotype-specific HPV infections were reported individually and by groups (“any”, “high-risk”, “low-risk”, and “9-valent vaccine”). The “any” group included those positive for ≥1 of the 37 HPV genotypes. HPV infections with single or multiple high-risk types were classified as high-risk. Similarly, HPV infections with at least one low-risk HPV type were classified as low-risk, and HPV infections with at least one of the genotypes targeted by the newly licensed 9-valent HPV vaccine (HPV 6, 11, 16, 18, 31, 33, 45, 52, or 58) were included in the “9-valent vaccine” group.
For individual and grouped HPV incidence analyses, only the first acquired infection was considered for a given HPV type or group. Incidence analyses included only men who tested negative for a given individual HPV type or grouped HPV infection category at baseline. Cumulative risk of HPV incidence was estimated using the Kaplan–Meier (KM) method. Associations between age and HPV incidence were assessed using Cox proportional hazards (PH) regression. Baseline factors included in modeling are shown in Table 1. For each individual or grouped HPV incidence, backward selection methods were used to facilitate variable selection for the multivariable models. Design variables (age and country) and those variables found to be statistically significant by backward selection methods (race, circumcision status, total and recent numbers of sexual partners, smoking status, education, and marital status) were included in the final multivariable models.
Table 1.
Distribution of baseline demographic and behavioral characteristics by age group.
| Characteristics | Overall (N=4085) | Age group
|
||||
|---|---|---|---|---|---|---|
| 18–24 (N=1231) | 25–34 (N=1245) | 35–44 (N=1082) | 45–54 (N=351) | 55–70 (N=176) | ||
| Country of residence, N (%) | ||||||
| US | 1326 (32.5) | 734 (59.6) | 209 (16.8) | 210 (19.4) | 99 (28.2) | 74 (42.0) |
| Brazil | 1410 (34.5) | 261 (21.2) | 509 (40.9) | 462 (42.7) | 123 (35.0) | 55 (31.3) |
| Mexico | 1349 (33.0) | 236 (19.2) | 527 (42.3) | 410 (37.9) | 129 (36.8) | 47 (26.7) |
| Race, N (%) | ||||||
| White | 1818 (44.5) | 668 (54.3) | 460 (36.9) | 445 (41.1) | 144 (41.0) | 101 (57.4) |
| Black | 636 (15.6) | 152 (12.3) | 210 (16.9) | 180 (16.6) | 69 (19.7) | 25 (14.2) |
| Asian/pacific islander | 112 (2.7) | 81 (6.6) | 13 (1.0) | 16 (1.5) | 1 (0.3) | 1 (0.6) |
| Other/mixed race | 1334 (32.7) | 248 (20.1) | 506 (40.6) | 403 (37.2) | 129 (36.8) | 48 (27.3) |
| Refused | 185 (4.5) | 82 (6.7) | 56 (4.5) | 38 (3.5) | 8 (2.3) | 1 (0.6) |
| Education (years), N (%) | ||||||
| <12 | 905 (22.2) | 142 (11.5) | 324 (26.0) | 295 (27.3) | 95 (27.1) | 49 (27.8) |
| 12 | 1086 (26.6) | 340 (27.6) | 347 (27.9) | 301 (27.8) | 79 (22.5) | 19 (10.8) |
| 13–15 | 1035 (25.3) | 565 (45.9) | 178 (14.3) | 176 (16.3) | 71 (20.2) | 45 (25.6) |
| 16 | 785 (19.2) | 161 (13.1) | 305 (24.5) | 212 (19.6) | 69 (19.7) | 38 (21.6) |
| ≥17 | 249 (6.1) | 16 (1.3) | 82 (6.6) | 93 (8.6) | 35 (10.0) | 23 (13.1) |
| Refused | 25 (0.6) | 7 (0.6) | 9 (0.7) | 5 (0.5) | 2 (0.6) | 2 (1.1) |
| Marital status, N (%) | ||||||
| Single | 1833 (44.9) | 1066 (86.6) | 500 (40.2) | 205 (18.9) | 48 (13.7) | 14 (8.0) |
| Married | 1391 (34.1) | 73 (5.9) | 437 (35.1) | 580 (53.6) | 189 (53.8) | 112 (63.6) |
| Cohabiting | 484 (11.8) | 80 (6.5) | 212 (17.0) | 148 (13.7) | 39 (11.1) | 5 (2.8) |
| Divorced/separated/widowed | 355 (8.7) | 6 (0.5) | 87 (7.0) | 144 (13.3) | 74 (21.1) | 44 (25.0) |
| Refused | 22 (0.5) | 6 (0.5) | 9 (0.7) | 5 (0.5) | 1 (0.3) | 1 (0.6) |
| Current smoker, N (%) | ||||||
| No | 3105 (76.0) | 978 (79.4) | 888 (71.3) | 825 (76.2) | 264 (75.2) | 150 (85.2) |
| Yes | 961 (23.5) | 247 (20.1) | 350 (28.1) | 253 (23.4) | 86 (24.5) | 25 (14.2) |
| Refused | 19 (0.5) | 6 (0.5) | 7 (0.6) | 4 (0.4) | 1 (0.3) | 1 (0.6) |
| Circumcision, N (%) | ||||||
| No | 2570 (62.9) | 582 (47.3) | 929 (74.6) | 746 (68.9) | 220 (62.7) | 93 (52.8) |
| Yes | 1515 (37.1) | 649 (52.7) | 316 (25.4) | 336 (31.1) | 131 (37.3) | 83 (47.2) |
| HPV status at baseline | ||||||
| Positive | 2135 (52.3) | 544 (44.2) | 725 (58.2) | 588 (54.3) | 185 (52.7) | 93 (52.8) |
| Negative | 1950 (47.7) | 687 (55.8) | 520 (41.8) | 494 (45.7) | 166 (47.3) | 83 (47.2) |
| Lifetime number of female sex partners, N (%) | ||||||
| 0–1 | 719 (17.6) | 339 (27.5) | 171 (13.7) | 153 (14.1) | 40 (11.4) | 16 (9.1) |
| 2–9 | 1621 (39.7) | 588 (47.8) | 517 (41.5) | 365 (33.7) | 103 (29.3) | 48 (27.3) |
| 10–49 | 1273 (31.2) | 240 (19.5) | 414 (33.3) | 400 (37.0) | 149 (42.5) | 70 (39.8) |
| ≥50 | 228 (5.6) | 21 (1.7) | 59 (4.7) | 92 (8.5) | 29 (8.3) | 27 (15.3) |
| Refused | 244 (6.0) | 43 (3.5) | 84 (6.7) | 72 (6.7) | 30 (8.5) | 15 (8.5) |
| Number of recent female sex partners (within past six months), N (%) | ||||||
| 0 | 1229 (30.1) | 347 (28.2) | 349 (28.0) | 347 (32.1) | 115 (32.8) | 71 (40.3) |
| 1 | 1654 (40.5) | 514 (41.8) | 496 (39.8) | 420 (38.8) | 154 (43.9) | 70 (39.8) |
| 2 | 525 (12.9) | 163 (13.2) | 171 (13.7) | 138 (12.8) | 39 (11.1) | 14 (8.0) |
| ≥3 | 528 (12.9) | 183 (14.9) | 173 (13.9) | 135 (12.5) | 23 (6.6) | 14 (8.0) |
| Refused | 149 (3.6) | 24 (1.9) | 56 (4.5) | 42 (3.9) | 20 (5.7) | 7 (4.0) |
| Lifetime number of male anal sex partners, N (%) | ||||||
| 0–1 | 3619 (88.6) | 1124 (91.3) | 1088 (87.4) | 940 (86.9) | 313 (89.2) | 154 (87.5) |
| 2–9 | 232 (5.7) | 57 (4.6) | 67 (5.4) | 77 (7.1) | 17 (4.8) | 14 (8.0) |
| ≥10 | 162 (4.0) | 39 (3.2) | 61 (4.9) | 44 (4.1) | 14 (4.0) | 4 (2.3) |
| Refused | 72 (1.8) | 11 (0.9) | 29 (2.3) | 21 (1.9) | 7 (2.0) | 4 (2.3) |
| Number of recent male anal sex partners (within past months), N (%) | ||||||
| 0 | 3832 (93.8) | 1151 (93.5) | 1149 (92.3) | 1023 (94.5) | 336 (95.7) | 173 (98.3) |
| ≥1 | 244 (6.0) | 78 (6.3) | 92 (7.4) | 57 (5.3) | 14 (4.0) | 3 (1.7) |
| Refused | 9 (0.2) | 2 (0.2) | 4 (0.3) | 2 (0.2) | 1 (0.3) | 0 (0.0) |
P values were derived using Monte Carlo estimation of the exact Pearson chi-squared test and were significant (P < 0.05) for all variables except lifetime number of male anal sex partners. Data reflect responses collected at baseline.
HPV clearance was defined as two consecutive negative test results following a positive test, excluding infections detected for the first time at a participant’s final visit and prevalent infections. For grouped HPV clearance analyses, we adjusted for within-subject correlation, as men may have been infected with multiple HPV types within a defined group (e.g., positive for both HPV 16 and HPV 18, which are both considered high-risk). The median time to HPV clearance (median duration) among men with an incidently detected infection was estimated using the KM method for individual HPV infections, with the clustered KM method used for grouped HPV infections [15]. To model the associations between age and HPV clearance, we employed Cox PH regression with and without the robust covariance matrix estimator [16] for grouped and individual HPV clearance, respectively. The same variable selection procedure described above was applied in building multivariable clearance models, using data collected at the baseline visit. Although we did not include prevalent HPV infections in clearance estimates, we included baseline HPV status as a covariate in our clearance models, as it has been shown to significantly impact HPV clearance estimates for subsequent incidently detected HPV infections [17].
Participant characteristics were compared across age groups using the Monte Carlo estimation of the exact Pearson chi-square test. Age, our primary factor of interest, was primarily treated as a categorical variable with five age groups: 18–24, 25–34, 35–44, 45–54, and 55–70 years. KM curves of HPV incidence and clearance for these five age groups were also generated.
To thoroughly investigate the association between age and HPV endpoints, we used Cox modeling and the same set of covariates, treating age in the following ways: (1) as a categorical variable with three pre-specified age groups, (2) as a categorical variable with five pre-specified age groups, and (3) as a continuous variable.
All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). All statistical tests were two-sided and considered significant at α=0.05.
3. Results
A total of 4085 men were followed for a median of 48.6 (range: 0.3–94.0) months. Demographic and behavioral characteristics are reported in Table 1. Overall, the majority of participants were ages 18–34 (60.6%), white (44.5%), non-smokers (76.0%), and uncircumcised (62.9%). Socio-demographic characteristics and sexual behaviors differed significantly by age group for all variables examined except lifetime number of male anal sex partners. For example, lifetime number of female sex partners reported was highest among the oldest age groups and number of recent female sex partners was lowest among the oldest age groups. However, 8% of men in the oldest age group (55–70 years) report having three or more female sexual partners within the last six months, indicating that older men within this study remain sexually active.
HPV infection incidence rates per 1000 person-months (Table 2) for “any HPV” remained stable across age groups (Table 2), while incidence rates for high-risk infections were significantly lower among men ages 35–44 and ages 55–70, compared to the youngest age group (ages 18–24). Incidence of grouped low-risk HPV infections peaked at ages 25–34 (24.5/1000 person months) and declined by 27% among those ages 55–70 years, where the incidence rate was significantly lower (17.9/1000 person months). HPV infection incidence of the 9-valent HPV vaccine types was fairly constant across the lifespan, with a 24% non-significant decline from ages 18–24 to 55–70 years; however, a significantly lower incidence was observed among men ages 35–44 compared to those ages 18–24 years. Incidence rates for individual HPV types followed different patterns depending on genotype. HPV 16 incidence declined linearly with age, with a 46% significant drop in incidence comparing men in the youngest and oldest age groups. Among individual low-risk HPV genotypes, the highest HPV 6 incidence rates were found among men in the youngest two age groups (18–34), after which incidence decreased significantly by 43% among men 55–70 years.
Table 2.
Incidence rates of genital HPV infection among men in the HIM Study (N=4032).
| Ages 18–24
|
Ages 25–34
|
Ages 35–44
|
Ages 45–54
|
Ages 55–70
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Infec- tions |
Incidence Rate (95% CI)a |
No. Infec- tions |
Incidence Rate (95% CI)a |
IRRb(95% CI)a | No. Infec- tions |
Incidence Rate (95% CI)a |
IRRb(95% CI)a | No. Infec- tions |
Incidence Rate (95% CI)a |
IRRb(95% CI)a | No. Infec- tions |
Incidence Rate (95% CI)a |
IRRb(95% CI)a | |
| Any HPV | 412 | 30.7 (27.8–33.8) | 350 | 30.5 (27.4–33.8) | 0.99 (0.86–1.15) | 324 | 29.5 (26.4–32.9) | 0.96 (0.83–1.11) | 102 | 25.4 (20.7–30.8) | 0.83 (0.67–1.03) | 50 | 23.9 (17.8–31.5) | 0.78 (0.58–1.04) |
| HR HPV | 421 | 19.6 (17.8–21.6) | 404 | 17.7 (16.0–19.6) | 0.90 (0.79–1.04) | 377 | 16.2 (14.6–17.9) | 0.83 (0.72–0.95) | 124 | 16.1 (13.4–19.1) | 0.82 (0.67–1.00) | 51 | 13.9 (10.3–18.2) | 0.71 (0.53–0.95) |
| HPV 16 | 183 | 5.0 (4.3–5.8) | 176 | 4.0 (3.5–4.7) | 0.80 (0.65–0.98) | 120 | 3.0 (2.5–3.6) | 0.60 (0.48–0.76) | 43 | 3.3 (2.4–4.4) | 0.66 (0.47–0.92) | 19 | 2.7 (1.6–4.3) | 0.54 (0.34–0.87) |
| HPV 18 | 91 | 2.2 (1.8–2.7) | 95 | 1.9 (1.6–2.4) | 0.86 (0.65–1.15) | 49 | 1.1 (0.8–1.4) | 0.50 (0.35–0.71) | 22 | 1.5 (1.0–2.3) | 0.68 (0.43–1.09) | 8 | 1.1 (0.5–2.2) | 0.50 (0.24–1.03) |
| HPV 31 | 68 | 1.6 (1.2–2.0) | 66 | 1.3 (1.0–1.7) | 0.81 (0.58–1.14) | 44 | 1.0 (0.7–1.3) | 0.63 (0.43–0.91) | 13 | 0.9 (0.5–1.5) | 0.56 (0.31–1.02) | 8 | 1.1 (0.5–2.1) | 0.69 (0.33–1.43) |
| HPV 33 | 21 | 0.5 (0.3–0.7) | 18 | 0.4 (0.2–0.6) | 0.80 (0.43–1.50) | 16 | 0.4 (0.2–0.6) | 0.80 (0.42–1.53) | 7 | 0.5 (0.2–1.0) | 1.00 (0.43–2.35) | 2 | 0.3 (0.0–0.9) | 0.60 (0.14–2.56) |
| HPV 39 | 128 | 3.2 (2.7–3.8) | 96 | 2.0 (1.6–2.5) | 0.63 (0.48–0.81) | 64 | 1.5 (1.2–1.9) | 0.47 (0.35–0.63) | 21 | 1.5 (0.9–2.2) | 0.47 (0.30–0.74) | 9 | 1.3 (0.6–2.4) | 0.41 (0.21–0.80) |
| HPV 45 | 66 | 1.5 (1.2–1.9) | 84 | 1.7 (1.4–2.1) | 1.13 (0.82–1.56) | 79 | 1.8 (1.4–2.3) | 1.20 (0.87–1.66) | 21 | 1.5 (0.9–2.2) | 1.00 (0.61–1.63) | 5 | 0.7 (0.2–1.6) | 0.47 (0.19–1.16) |
| HPV 51 | 208 | 5.7 (4.9–6.5) | 159 | 3.6 (3.0–4.1) | 0.63 (0.51–0.78) | 130 | 3.2 (2.7–3.8) | 0.56 (0.45–0.70) | 41 | 3.0 (2.2–4.1) | 0.53 (0.38–0.74) | 16 | 2.4 (1.3–3.8) | 0.42 (0.25–0.70) |
| HPV 52 | 131 | 3.3 (2.7–3.9) | 122 | 2.6 (2.1–3.1) | 0.79 (0.62–1.01) | 101 | 2.4 (2.0–2.9) | 0.73 (0.56–0.94) | 34 | 2.4 (1.7–3.4) | 0.73 (0.50–1.06) | 17 | 2.6 (1.5–4.1) | 0.79 (0.48–1.31) |
| HPV 58 | 82 | 1.9 (1.5–2.4) | 66 | 1.4 (1.0–1.7) | 0.74 (0.53–1.02) | 54 | 1.2 (0.9–1.6) | 0.63 (0.45–0.89) | 23 | 1.6 (1.0–2.4) | 0.84 (0.53–1.34) | 7 | 1.0 (0.4–2.0) | 0.53 (0.24–1.14) |
| HPV 59 | 168 | 4.4 (3.8–5.1) | 142 | 3.1 (2.6–3.7) | 0.70 (0.56–0.88) | 99 | 2.4 (1.9–2.9) | 0.55 (0.43–0.70) | 36 | 2.7 (1.9–3.7) | 0.61 (0.43–0.88) | 16 | 2.4 (1.3–3.8) | 0.55 (0.33–0.91) |
| LR HPV | 443 | 24.2 (22.0–26.5) | 396 | 24.5 (22.1–27.0) | 1.01 (0.88–1.16) | 324 | 21.4 (19.1–23.8) | 0.88 (0.77–1.02) | 111 | 20.5 (16.9–24.7) | 0.85 (0.69–1.04) | 54 | 17.9 (13.4–23.3) | 0.74 (0.56–0.98) |
| HPV 6 | 142 | 3.7 (3.1–4.4) | 163 | 3.7 (3.2–4.3) | 1.00 (0.80–1.25) | 98 | 2.4 (1.9–2.9) | 0.65 (0.50–0.84) | 26 | 1.9 (1.3–2.8) | 0.51 (0.34–0.78) | 15 | 2.1 (1.2–3.5) | 0.57 (0.33–0.97) |
| HPV 11 | 40 | 0.9 (0.7–1.3) | 56 | 1.1 (0.8–1.5) | 1.22 (0.81–1.83) | 37 | 0.8 (0.6–1.1) | 0.89 (0.57–1.39) | 6 | 0.4 (0.1–0.9) | 0.44 (0.19–1.05) | 6 | 0.8 (0.3–1.8) | 0.89 (0.38–2.10) |
| HPV 53 | 146 | 3.7 (3.1–4.3) | 174 | 3.9 (3.4–4.5) | 1.05 (0.85–1.31) | 112 | 2.7 (2.3–3.3) | 0.73 (0.57–0.93) | 44 | 3.4 (2.4–4.5) | 0.92 (0.66–1.29) | 23 | 3.6 (2.3–5.4) | 0.97 (0.63–1.51) |
| HPV 61 | 90 | 2.2 (1.7–2.7) | 147 | 3.2 (2.7–3.8) | 1.45 (1.12–1.89) | 124 | 3.0 (2.5–3.6) | 1.36 (1.04–1.79) | 43 | 3.2 (2.3–4.2) | 1.45 (1.01–2.09) | 17 | 2.5 (1.4–4.0) | 1.14 (0.68–1.91) |
| HPV 62 | 164 | 4.2 (3.6–4.9) | 164 | 3.7 (3.2–4.4) | 0.88 (0.71–1.09) | 149 | 3.9 (3.3–4.6) | 0.93 (0.74–1.16) | 49 | 3.9 (2.9–5.1) | 0.93 (0.67–1.28) | 26 | 4.3 (2.8–6.3) | 1.02 (0.68–1.55) |
| HPV 66 | 160 | 4.1 (3.5–4.8) | 144 | 3.1 (2.6–3.7) | 0.76 (0.60–0.95) | 113 | 2.7 (2.2–3.3) | 0.66 (0.52–0.84) | 29 | 2.1 (1.4–3.1) | 0.51 (0.34–0.76) | 15 | 2.2 (1.2–3.5) | 0.54 (0.32–0.91) |
| HPV 84 | 226 | 6.3 (5.5–7.2) | 172 | 4.0 (3.4–4.6) | 0.63 (0.52–0.77) | 128 | 3.3 (2.7–3.9) | 0.52 (0.42–0.65) | 55 | 4.3 (3.2–5.6) | 0.68 (0.51–0.92) | 23 | 3.6 (2.3–5.4) | 0.57 (0.37–0.88) |
| HPV 89c | 223 | 5.9 (5.2–6.7) | 173 | 3.9 (3.4–4.6) | 0.66 (0.54–0.81) | 140 | 3.5 (3.0–4.2) | 0.59 (0.48–0.73) | 49 | 3.7 (2.7–4.9) | 0.63 (0.46–0.85) | 18 | 2.6 (1.6–4.2) | 0.44 (0.27–0.71) |
| HPV 9vd | 359 | 14.1 (12.7–15.7) | 378 | 13.4 (12.0–14.8) | 0.95 (0.82–1.10) | 310 | 11.2 (10.0–12.5) | 0.79 (0.68–0.92) | 108 | 11.8 (9.7–14.2) | 0.84 (0.67–1.04) | 53 | 10.7 (8.0– | 0.76 (0.57–1.01) |
Note: HR: high-risk, LR: low risk, IRR: incidence rate ratio, CI: confidence interval. Although 37 high- and low-risk HPV types are evaluated using the Roche Linear Array method, data are only shown for individual types included in the 9-valent HPV vaccine (HPV 6, 11, 16, 18, 31, 33, 45, 52, 58) and those that had incidence rates > 3.0 in any age group.
Incidence rate is estimated as the number of cases per 1000 person-months.
IRRs were calculated using 18–24 years as the referent age group, with significant values indicated in bold.
Formerly HPV CP 6108.
One or more of the 9-valent HPV vaccine types.
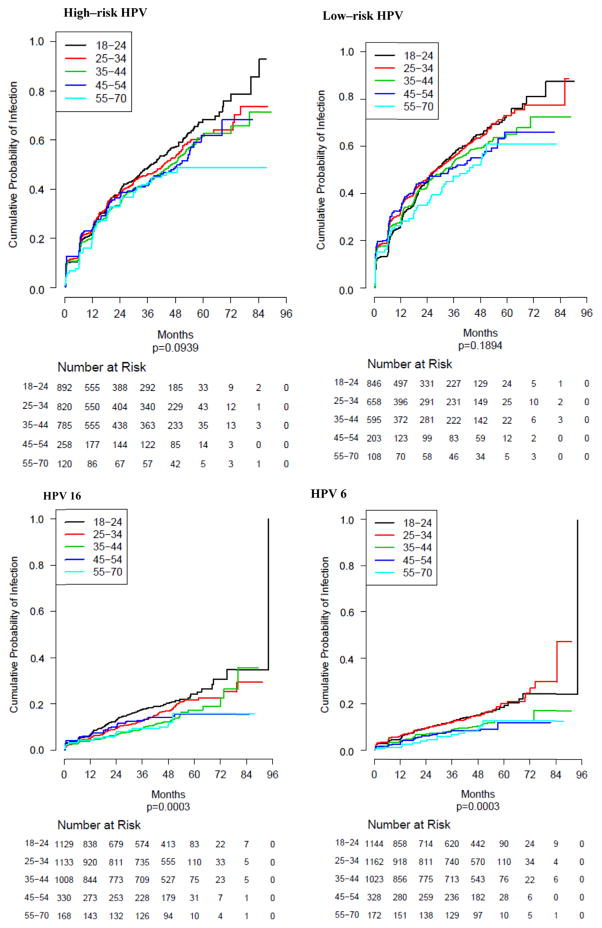
Fig. 1 presents the KM curves for HPV acquisition across all age groups over the follow-up period. Overall, significant differences in HPV 6 and 16 acquisition were observed by age group. For both HPV types, younger men (ages 18–34) had higher rates of infection acquisition.
Fig. 1.
Kaplan–Meier analyses for incidence of grouped (A) high-risk and (B) low-risk HPV infections and of type-specific infections with (C) HPV 16 and (D) HPV 6, stratified by five age groups (18–24, 25–34, 35–44, 45–54, 55–70 years). Significant differences in incidence by age were observed for individual HPV types 6, 16, 18, 39, 51, 59, 61, 67, 71, and 84. Incidence did not vary significantly by age within any of the grouped HPV infection categories or among infections with other individual HPV types.
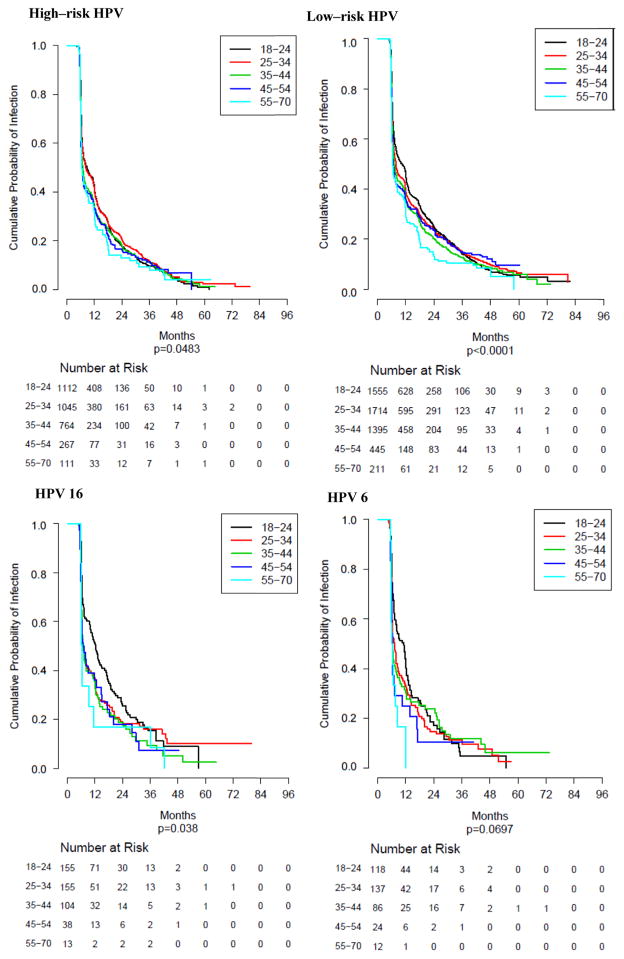
Significant age-related decreases in HPV infection median duration (time to clearance) across the five age groups (Table 3; Fig. 2) were observed for all grouped infections (any HPV, low-risk HPV, high-risk HPV, and 9v HPV). Significant declines in duration were also observed for individual high-risk HPV types 16 and 51 and individual low-risk HPV types 40, 62, 73, and 82. Of these, all had the highest median duration among the youngest age groups (18–24 and 25–34 years) with the exception of HPV 82, for which median duration was higher in the oldest age group (55–70 years). Fig. 2 presents KM curves for high risk, low risk, HPV 16 and HPV 6 infection HPV duration by age group. While significant differences were observed for high and low risk HPV time to clearance by age group, the differences appear minimal. In contrast, significant differences in HPV 16 time to clearance with larger separation by age group was observed, with time to clearance longer among the younger age groups.
Table 3.
Median duration of incident HPV infection among men in the HIM Study by age (N=4085).
| Median duration, months (95% CI)
|
p value* | |||||
|---|---|---|---|---|---|---|
| Ages 18–24 | Ages 25–34 | Ages 35–44 | Ages 45–54 | Ages 55–70 | ||
| Any HPV | 9.4 (8.3–11.3) | 7.9 (7.5–8.5) | 7.0 (6.8–7.4) | 6.6 (6.4–7.1) | 6.4 (6.2–7.4) | <0.001 |
| High-risk HPV | 8.7 (7.6–10.4) | 8.3 (7.4–10.1) | 6.9 (6.5–7.4) | 6.6 (6.3–7.4) | 6.4 (6.2–8.1) | 0.048 |
| HPV 16 | 12.2 (9.8–14.7) | 6.8 (6.3–8.0) | 6.9 (6.2–8.1) | 7.0 (6.0–12.5) | 6.3 (5.8–11.5) | 0.038 |
| HPV 18 | 8.1 (6.5–11.9) | 8.0 (6.2–11.9) | 8.5 (6.2–11.6) | 6.2 (5.9–7.4) | 6.8 (5.7-NE) | 0.865 |
| HPV 31 | 6.9 (6.4–12.1) | 10.5 (6.7–12.4) | 6.7 (6.0–10.5) | 6.4 (5.7-NE) | 6.4 (5.7-NE) | 0.839 |
| HPV 33 | 6.2 (5.9–12.0) | 6.0 (5.7–6.9) | 7.3 (5.8–12.3) | 6.6 (5.5-NE) | 12.2 (NE-NE) | 0.892 |
| HPV 35 | 6.9 (6.2–12.0) | 14.5 (7.1–23.1) | 8.3 (6.2–17.8) | 12.5 (6.4-NE) | 24.7 (18.1-NE) | 0.474 |
| HPV 39 | 8.9 (7.1–12.0) | 13.4 (7.2–22.8) | 12.2 (7.2–15.2) | 8.6 (6.0–18.2) | 9.6 (6.0–18.2) | 0.267 |
| HPV 45 | 6.2 (6.1–6.7) | 7.8 (6.2–12.6) | 6.2 (6.0–6.7) | 10.7 (6.8–16.1) | 9.0 (6.0-NE) | 0.070 |
| HPV 51 | 12.1 (8.0–14.0) | 11.7 (7.9–16.3) | 7.8 (6.5–11.8) | 6.4 (6.0–8.1) | 6.4 (5.7–17.2) | 0.032 |
| HPV 52 | 8.8 (6.4–13.1) | 7.3 (6.4–10.6) | 6.5 (6.0–6.9) | 6.7 (6.0–17.1) | 9.2 (5.9–13.2) | 0.337 |
| HPV 56 | 6.9 (6.3–8.5) | 11.6 (6.4–13.7) | 6.2 (5.9–9.8) | 6.0 (5.6–6.6) | 6.2 (5.7–12.4) | 0.092 |
| HPV 58 | 11.5 (7.3–12.8) | 12.5 (11.2–17.4) | 7.7 (6.0–12.6) | 6.2 (5.9–26.6) | 5.9 (5.1-NE) | 0.173 |
| HPV 59 | 11.3 (7.0–12.5) | 6.6 (6.1–8.2) | 6.7 (6.0–9.3) | 6.5 (5.8–12.5) | 6.2 (5.7–7.3) | 0.322 |
| HPV 68 | 7.1 (6.3–7.7) | 6.7 (6.1–8.2) | 6.8 (6.0–8.5) | 6.0 (5.7–6.4) | 8.4 (5.7-NE) | 0.406 |
| Low-risk HPV | 10.4 (8.7–11.9) | 7.9 (7.3–8.3) | 7.1 (6.8–7.7) | 6.6 (6.4–7.4) | 6.4 (6.2–8.0) | <0.001 |
| HPV 6 | 11.0 (7.8–12.2) | 7.1 (6.2–8.3) | 6.2 (6.0–7.8) | 6.3 (6.0–7.1) | 6.3 (5.7–8.4) | 0.070 |
| HPV 11 | 7.8 (6.2–18.0) | 11.5 (6.4–12.9) | 6.2 (6.0–6.9) | 6.9 (5.6-NE) | 7.2 (5.5-NE) | 0.236 |
| HPV26 | 6.7 (6.0–9.9) | 6.3 (5.6–11.7) | 6.9 (5.8–11.7) | 5.7 (5.5-NE) | NE | 0.058 |
| HPV 40 | 12.4 (7.2–15.6) | 7.6 (6.2–12) | 7.8 (6.2–17.3) | 5.9 (5.5–6.4) | 6.4 (5.7-NE) | 0.021 |
| HPV 42 | 11.7 (7.4–14.6) | 8.7 (6.4–12.3) | 6.8 (6.0–12.4) | 12.0 (5.3-NE) | 6.2 (5.1-NE) | 0.166 |
| HPV 53 | 9.9 (6.5–12.4) | 7.3 (6.6–8.8) | 7.4 (6.1–10.7) | 7.5 (6.1–18.2) | 15.7 (6.8–18.1) | 0.901 |
| HPV 54 | 12.2 (8.5–17.6) | 8.0 (6.6–12) | 8.2 (6.4–13.6) | 6.4 (6.0–31.5) | 6.4 (5.5–10.3) | 0.071 |
| HPV 55 | 12.0 (7.0–18.7) | 6.7 (6.1–11.5) | 7.3 (6.5–11.7) | 6.8 (6.0–24.4) | 6.1 (5.7-NE) | 0.666 |
| HPV 61 | 13.4 (7.6–17.6) | 7.1 (6.6–11.2) | 8.0 (6.8–12.2) | 6.3 (5.9–12.0) | 7.9 (5.5–12.2) | 0.145 |
| HPV 62 | 12.3 (8.3–18.6) | 7.8 (6.7–12.4) | 6.9 (6.4–8.0) | 8.8 (6.4–12.5) | 6.4 (6.0–11.0) | 0.046 |
| HPV 64 | 6.2 (5.6-NE) | 5.9 (5.3–6.2) | 6.1 (5.5-NE) | 6.2 (5.5-NE) | 9.0 (6.0-NE) | 0.967 |
| HPV 66 | 12.0 (7.6–16.2) | 11.2 (6.7–12.6) | 8.1 (6.6–11.9) | 7.1 (6.0–11.9) | 6.0 (5.6–17.4) | 0.248 |
| HPV 67 | 6.3 (6.0–7.1) | 6.3 (6.0–6.9) | 7.6 (6.0–13.4) | 6.3 (6.0-NE) | 5.8 (5.5-NE) | 0.111 |
| HPV 69 | 7.8 (5.5-NE) | 7.9 (5.6-NE) | 5.9 (5.7–13.4) | 5.5 (5.3-NE) | 5.8 (NE-NE) | 0.097 |
| HPV 70 | 7.0 (6.3–11.3) | 6.8 (6.3–11.1) | 6.7 (6.0–11.3) | 6.8 (6.0-NE) | 6.7 (5.3–42.2) | 0.903 |
| HPV 71 | 7.5 (6.0–17.7) | 11.5 (6.8–23.1) | 12.2 (6.2–23.0) | 6.3 (5.8–18.5) | 16.1 (6.7-NE) | 0.974 |
| HPV 72 | 6.2 (5.9–8.1) | 6.4 (6.1–7.4) | 6.4 (6.0–12.0) | 6.0 (5.9–17.5) | 6.0 (5.7–17.8) | 0.918 |
| HPV 73 | 9.2 (6.8–13.6) | 10.1 (6.4–12.9) | 6.7 (6.0–16.9) | 6.1 (6.0–6.4) | 6.0 (5.7-NE) | 0.031 |
| HPV 81 | 6.4 (6.2–9.4) | 8.0 (6.9–12) | 8.4 (6.6–12.0) | 6.8 (5.9–7.7) | 10.8 (5.7-NE) | 0.659 |
| HPV 82 | 6.5 (6.1–8.9) | 6.9 (5.9–15.2) | 6.5 (5.9–7.7) | 5.7 (5.5-NE) | 12.4 (11.7-NE) | 0.018 |
| HPV 82sa | 9.9 (6.0–17.9) | 13.2 (6.0–23.7) | 12.2 (6.7–15.6) | 6.2 (6.0-NE) | 18.2 (5.7-NE) | 0.438 |
| HPV 83 | 6.7 (6.2–9.9) | 11.3 (7.2–14.5) | 6.9 (6.2–11.7) | 24.6 (11.5-NE) | 6.2 (5.7-NE) | 0.007 |
| HPV 84 | 12.5 (9.9–19.8) | 12 (7.6–17.7) | 6.8 (6.2–8.3) | 6.6 (6.1–12.8) | 6.6 (6.0–18.3) | 0.078 |
| HPV 89b | 12.3 (7.8–16.2) | 7.9 (6.6–11.4) | 7.1 (6.4–11.8) | 7.6 (6.0–16.8) | 6.0 (5.7–25.7) | 0.148 |
| HPV 9vc | 9.3 (7.8–11.3) | 7.8 (7.1–8.8) | 6.5 (6.2–6.9) | 6.7 (6.3–7.7) | 6.4 (6.2–7.6) | 0.014 |
Note: 95% CI=95% Confidence interval. NE=Non-estimable.
HPV 82 subtype IS39.
Formerly HPV CP6108.
One or more of the 9-valent HPV vaccine types (6, 11, 16, 18, 31, 33, 45, 52, 58).
P values for grouped infections and type-specific infections were derived from Kaplan–Meier curves of HPV infection clearance (Fig. 2) comparing the five age groups across the entire follow-up period. Values in bold denote statistical significance.
Fig. 2.
Kaplan–Meier analyses for incidence of grouped (A) high-risk and (B) low-risk HPV infections and of type-specific infections with (C) HPV 16 and (D) HPV 6, stratified by five age groups (18–24, 25–34, 35–44, 45–54, 55–70 years). Significant differences in age for clearance were observed for grouped “any HPV,” “low-risk HPV,” and “HPV 9v” infections, as well as for type-specific infections with HPV 6, 51, 54, and 66. Clearance did not vary significantly by age for any of the other grouped or type-specific infections analyzed.
3.1. HPV infection incidence: associations with age
We examined the associations between age and genital HPV incidence using multiple approaches, considering age as a categorical variable (three and five pre-specified age groups) and as a continuous variable (Table 4). All three approaches were consistent in demonstrating significantly lower HPV incidence with age for grouped high-risk infections, HPV 16, and HPV 6 infections.
Table 4.
Effect of age on HPV infection incidence among men.
| High-risk HPV
|
Low-risk HPV
|
HPV 16
|
HPV 6
|
|||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | AHR (95% CI) | HR (95% CI) | AHR (95% CI) | HR (95% CI) | AHR (95% CI) | HR (95% CI) | AHR (95% CI) | |
| Three age groups | ||||||||
| 18–30 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 31–44 | 0.91 (0.81–1.01) | 0.87 (0.76–0.99) | 0.96 (0.85–1.08) | 0.97 (0.84–1.13) | 0.69 (0.57–0.83) | 0.64 (0.53–0.78) | 0.77 (0.63–0.94) | 0.75 (0.61–0.92) |
| 45–70 | 0.84 (0.71–1.00) | 0.82 (0.67–0.99) | 0.86 (0.72–1.02) | 0.82 (0.66–1.01) | 0.67 (0.51–0.88) | 0.61 (0.46–0.80) | 0.56 (0.40–0.78) | 0.60 (0.43–0.84) |
| Five age groups | ||||||||
| 18–24 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 25–34 | 0.92 (0.81–1.06) | 0.85 (0.72–1.00) | 1.05 (0.91–1.2) | 0.91 (0.77–1.08) | 0.82 (0.67–1.01) | 0.78 (0.62–0.98) | 1.03 (0.82–1.29) | 0.99 (0.78–1.26) |
| 35–44 | 0.86 (0.74–0.98) | 0.76 (0.64–0.92) | 0.92 (0.80–1.06) | 0.84 (0.70–1.02) | 0.61 (0.49–0.77) | 0.56 (0.43–0.72) | 0.67 (0.52–0.87) | 0.66 (0.50–0.87) |
| 45–54 | 0.85 (0.70–1.04) | 0.76 (0.59–0.96) | 0.90 (0.73–1.11) | 0.76 (0.58–0.98) | 0.67 (0.48–0.94) | 0.61 (0.43–0.86) | 0.55 (0.36–0.84) | 0.58 (0.38–0.89) |
| 55–70 | 0.74 (0.55–0.99) | 0.68 (0.49–0.94) | 0.80 (0.60–1.06) | 0.70 (0.51–0.98) | 0.57 (0.36–0.92) | 0.49 (0.30–0.79) | 0.60 (0.35–1.02) | 0.65 (0.38–1.12) |
| Continuous | ||||||||
| 18–70 | 0.94 (0.90–0.99) | 0.92 (0.86–0.98) | 0.96 (0.91–1.00) | 0.88 (0.83–0.93) | 0.84 (0.78–0.91) | 0.81 (0.74–0.88) | 0.83 (0.76–0.91) | 0.84 (0.76–0.92) |
Note: HR=hazard ratio; AHR=adjusted hazard ratio; CI=confidence interval. Associations between age and HPV incidence were assessed using Cox proportional hazards regression. Design variables (country and age) were forced into multivariable models. Race, circumcision status, total and recent numbers of female and male sexual partners, smoking status, education, and marital status were all included in multivariable modeling. Baseline measures were used for all variables. Bold values denote statistical significance in multivariable models.
3.2. HPV infection clearance: associations with age
Overall, when treating age as a categorical variable, likelihood of grouped high-risk HPV infection clearance was significantly greater among men ages 45 and older, although the magnitude of the difference was low (17–18% increased likelihood of clearance compared to younger men). Likelihood of clearing HPV 6 and 16 infections remained constant across the lifespan when age was treated as a continuous variable or categorized into tertiles. HPV 6 and 16 clearance was significantly higher only when age was categorized into quintiles, with men in the oldest age group (55–70 years) having a 92% and 65% higher likelihood of infection clearance, respectively (Table 5).
Table 5.
Effect of age on HPV infection clearance among men.
| High-risk HPV
|
Low-risk HPV
|
HPV 16
|
HPV 6
|
|||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | AHR (95% CI) | HR (95% CI) | AHR (95% CI) | HR (95% CI) | AHR (95% CI) | HR (95% CI) | AHR (95% CI) | |
| Three age groups | ||||||||
| 18–30 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 31–44 | 0.98 (0.91–1.05) | 1.03 (0.95–1.11) | 0.96 (0.91–1.02) | 1.05 (0.98–1.12) | 0.98 (0.82–1.16) | 0.97 (0.81–1.16) | 0.96 (0.79–1.16) | 0.99 (0.81–1.20) |
| 45–70 | 1.13 (1.00–1.26) | 1.17 (1.04–1.32) | 0.98 (0.89–1.08) | 1.07 (0.96–1.19) | 1.37 (1.06–1.77) | 1.35 (1.04–1.76) | 1.00 (0.71–1.41) | 1.06 (0.75–1.50) |
| Five age groups | ||||||||
| 18–24 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 25–34 | 0.92 (0.85–1.00) | 1.00 (0.92–1.09) | 0.96 (0.89–1.03) | 1.09 (1.00–1.18) | 1.04 (0.86–1.25) | 1.02 (0.84–1.23) | 1.07 (0.88–1.3) | 1.09 (0.89–1.35) |
| 35–44 | 0.96 (0.88–1.05) | 1.04 (0.95–1.15) | 0.97 (0.90–1.04) | 1.13 (1.03–1.23) | 1.02 (0.82–1.26) | 1.01 (0.81–1.25) | 0.86 (0.68–1.09) | 0.91 (0.71–1.16) |
| 45–54 | 1.09 (0.95–1.26) | 1.18 (1.02–1.37) | 0.96 (0.86–1.07) | 1.11 (0.98–1.26) | 1.33 (0.98–1.81) | 1.30 (0.95–1.78) | 0.84 (0.55–1.27) | 0.91 (0.59–1.40) |
| 55–70 | 1.08 (0.89–1.30) | 1.15 (0.95–1.39) | 0.99 (0.85–1.16) | 1.18 (1.00–1.40) | 1.65 (1.05–2.60) | 1.65 (1.06–2.56) | 1.87 (1.42–2.48) | 1.92 (1.34–2.74) |
| Continuous | ||||||||
| 18–70 | 1.02 (0.99–1.05) | 1.05 (1.01–1.08) | 0.99 (0.96–1.02) | 1.04 (1.00–1.07) | 1.07 (0.99–1.16) | 1.07 (0.99–1.15) | 1.00 (0.91–1.09) | 1.02 (0.93–1.12) |
Note: HR=hazard ratio; AHR=adjusted hazard ratio; CI=confidence interval. Associations between age and HPV incidence were assessed using Cox proportional hazards regression. Design variables (country, age, and HPV positivity at enrollment) were forced into multivariable models. Race, circumcision status, total and recent numbers of female and male sexual partners, smoking status, education, and marital status were all included in multivariable modeling. Baseline measures were used for all variables. Bold values denote statistical significance in multivariable models.
4. Discussion
Despite differences in ages at which men develop genital warts and penile cancer, rates of detection of incident genital HPV infection in this multinational cohort of men remained relatively stable across age groups, with different patterns observed across various HPV types. Risk of incident infection detection was lower for high-risk HPV, low-risk HPV, and individual HPV types 6 and 16 among older men. Likelihood of clearing HPV 16 and 6 infections remained fairly constant across the lifespan and was only higher among men in the oldest age group (55–70 years). It remains unclear whether risk of progression to malignant and benign lesions differs by age at which the infection was acquired. Although we observed lower rates of incident infections at older ages, there was continued detection of apparently new infections, and duration of these detected infections was consistent across most age groups. Altogether, it appears that men remain at risk for prevalent genital HPV infection across the lifespan. To our knowledge, this is the first study to provide a comprehensive view of incident HPV detection and duration by HPV genotype and age over a relatively long follow-up period among men.
Previous studies have shown a bimodal age pattern of HPV infection prevalence and incidence in women, with peaks near age 20 and between ages 45–50 in Latin American and other populations [9–11,18,19]. However, studies from other geographical regions have not observed these trends [20,21]. The observed differences between study populations may be explained by the variation in HPV natural history by country or by differences in sexual and other behavioral factors between populations. The increase in HPV incidence at older ages in Latin American women has been postulated to result from decreased immune response over time, enabling latent infections to reactivate or new infections to persist for longer periods [22]. Results presented here suggest an additional hypothesis in which women in the oldest age group might acquire a new infection from their male partner, as incident infection detection, although lower in older men, was high among men of all ages in the U.S. and Latin America. While number of recent partners declined with age, men in this study remained sexually active across all age groups, potentially leading to the introduction of new infections throughout the lifespan. Furthermore, recent reports have demonstrated low rates of seroconversion following natural HPV infection in men [23–25], perhaps explaining in part the apparently continued risk of incident HPV detection in men across the lifespan.
Duration of HPV infections significantly declined with age for all grouped infections, as well as for a number of type-specific infections, including HPV 16. Given the six-month sampling intervals for HIM Study participants, duration estimates represent maximum infection duration. However, these findings are consistent with studies among women that demonstrate shorter duration of newly acquired infections with age [26]. When prevalent HPV infections were considered, duration was highest among older women, similar to what we have previously demonstrated among men [13].
Although genital HPV 16 and other high-risk HPV infection incidence declines with age, older men continue to acquire new infections, albeit at half the rate of the youngest men, and these infections continue to persist. These data correspond to the older median ages of penile cancer diagnoses (≥60 years) and emphasize the need for prevention interventions to have continued efficacy in later years, especially given the low HPV seroconversion rates among men. As the HPV vaccine has been demonstrated to prevent genital warts and anal cancer in men [27], it is of interest for future studies to determine whether similar efficacy occurs against other cancers in men, such as penile cancer, and for sufficient longevity (following adolescent vaccination) to cover the entire period of HPV susceptibility.
Notable strengths of this study include the size and multinational nature of this male cohort, the long duration of follow-up, and the use of multiple modeling strategies to assess associations with age and HPV infection. Our methodologies were similar to those employed in studies of female cohorts, providing the opportunity to compare differences in trends between genders. Additionally, the use of a computer-assisted self-interview (CASI) at each visit was likely to reduce bias associated with answering highly sensitive questions, such as those related to sexual behavior. Although the HIM Study is not a population-based study, the demographics of the men included at each of the three international clinical sites are similar to the underlying population of men ages 18–70 years in their respective communities [13] and, on average, they have a similar sexual history and behavior to a nationally representative group of men in the U.S. [13,28]. As such, the incident HPV detection rates observed in this study likely represent those one would observe in the population at large. However, potential limitations that should be considered include a possible lack of external validity due to the methods of recruitment and extended length of follow-up, which may result in a study cohort that is not reflective of the general population of the participating countries. This work also utilized only baseline data for demographic and behavioral factors, potentially limiting the interpreted effect of time-varying covariates, such as sexual behavior. However, baseline sexual behaviors, such as the number of sexual partners in the past 3–6 months, reflected patterns that continued through successive six-month follow-up visits (data not shown). Additionally, estimates of HPV incidence and duration were based on visits that occurred approximately every six months and therefore do not reflect changes in infection status that occurred during the intervening months. Furthermore, despite the use of highly sensitive HPV DNA detection methods at each study visit, infections deemed as “incident” may actually represent reactivation of past infections, infections that were prevalent at baseline but below the limits of detection, or presence of virus on the skin surface that does not represent true infection. Current laboratory methods do not distinguish reactivated latent infections from those that are newly acquired.
In summary, the data presented here show that the age patterning of incident genital HPV detection and duration in men remains relatively constant across the lifespan. Given the higher incidence of genital warts in young men and of penile cancers in older men, it is therefore likely that other factors influence the association between age and HPV-related diseases. Additionally, while most HPV-related diseases in men are caused by HPV 16 (cancer) and 6 (genital warts), transmission of other high-risk HPV types from men to women elevates risk of pre-cancer and cancer in female partners. As such, the consistently high detection of incident infections and duration of high-risk HPV types in men across the lifespan support the need for effective interventions to prevent infection and disease in both genders.
Acknowledgments
Funding
The HIM Study infrastructure was supported by the National Cancer Institute (Grant number R01 CA098803 to A.R.G.). S.L.S. was supported by the National Cancer Institute (R25T CA147832 and K05 CA181320).
The authors would like to thank the HIM Study teams and participants in the U.S. (Moffitt Cancer Center, Tampa, FL), Brazil (Centro de Referência e Treinamento em DST/AIDS, Fundação Faculdade de Medicina Instituto do Câncer do Estado de São Paulo, Ludwig Institute for Cancer Research, São Paulo), and Mexico (Instituto Mexicano del Seguro Social, Instituto Nacional de Salud Pública, Cuernavaca). The authors also acknowledge contributions from the Moffitt Tissue Core and the Moffitt Biostatistics Core (supported by National Cancer Institute Cancer Center Support Grant 5P30 CA07629216 to T. Sellers).
Footnotes
Conflicts of interest
ARG receives research funding from Merck and is a member of their Speaker’s Bureau. LLV and ARG are consultants of Merck for HPV vaccines. None of the other authors have conflicts of interest to report.
References
- 1.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J, Bray F, Plummer M, Franceschi S. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 2.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 3.Rossari JR, Vora T, Gil T. Advances in penile cancer management. Curr Opin Oncol. 2010;22:226–235. doi: 10.1097/CCO.0b013e3283376ac0. [DOI] [PubMed] [Google Scholar]
- 4.Baldur-Felskov B, Hannibal CG, Munk C, Kjaer SK. Increased incidence of penile cancer and high-grade penile intraepithelial neoplasia in Denmark 1978–2008: a nationwide population-based study. Cancer Causes Control. 2012;23:273–280. doi: 10.1007/s10552-011-9876-7. [DOI] [PubMed] [Google Scholar]
- 5.Graafland NM, Verhoeven RH, Coebergh JW, Horenblas S. Incidence trends and survival of penile squamous cell carcinoma in the Netherlands. Int J Cancer. 2011;128:426–432. doi: 10.1002/ijc.25355. [DOI] [PubMed] [Google Scholar]
- 6.Barnholtz-Sloan J, Patel N, Rollison D, Kortepeter K, MacKinnon J, Giuliano A. Incidence trends of invasive cervical cancer in the United States by combined race and ethnicity. Cancer Causes Control. 2009;20:1129–1138. doi: 10.1007/s10552-009-9317-z. [DOI] [PubMed] [Google Scholar]
- 7.Patel NR, Rollison DE, Barnholtz-Sloan J, Mackinnon J, Green L, Giuliano AR. Racial and ethnic disparities in the incidence of invasive cervical cancer in Florida. Cancer. 2009;115:3991–4000. doi: 10.1002/cncr.24427. [DOI] [PubMed] [Google Scholar]
- 8.Anic GM, Lee JH, Stockwell H, Rollison DE, Wu Y, Papenfuss MR, Villa LL, Lazcano-Ponce E, Gage C, Silva RJ, Baggio ML, Quiterio M, Salmeron J, Abrahamsen M, Giuliano AR. Incidence and human papillomavirus (HPV) type distribution of genital warts in a multinational cohort of men: the HPV in men study. J Infect Dis. 2011;204:1886–1892. doi: 10.1093/infdis/jir652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazcano-Ponce E, Herrero R, Munoz N, Cruz A, Shah KV, Alonso P, Hernandez P, Salmeron J, Hernandez M. Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer. 2001;91:412–420. doi: 10.1002/1097-0215(20010201)91:3<412::aid-ijc1071>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Molano M, Posso H, Weiderpass E, van den Brule AJ, Ronderos M, Franceschi S, Meijer CJ, Arslan A, Munoz N. Prevalence and determinants of HPV infection among Colombian women with normal cytology. Br J Cancer. 2002;87:324–333. doi: 10.1038/sj.bjc.6600442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrero R, Hildesheim A, Bratti C, Sherman ME, Hutchinson M, Morales J, Balmaceda I, Greenberg MD, Alfaro M, Burk RD, Wacholder S, Plummer M, Schiffman M. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000;92:464–474. doi: 10.1093/jnci/92.6.464. [DOI] [PubMed] [Google Scholar]
- 12.Smith JS, Gilbert PA, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of human papillomavirus infection in males: a global review. J Adolesc Health. 2011;48:540–552. doi: 10.1016/j.jadohealth.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Giuliano AR, Lee JH, Fulp W, Villa LL, Lazcano E, Papenfuss MR, Abrahamsen M, Salmeron J, Anic GM, Rollison DE, Smith D. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377:932–940. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens – Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 15.Ying Z, Wei LJ. The Kaplan–Meier estimate for dependent failure time observations. J Multivar Anal. 1994;50:17–29. [Google Scholar]
- 16.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 17.Schabath MB, Villa LL, Lin HY, Fulp WJ, Akogbe GO, Abrahamsen ME, Papenfuss MR, Lazcano-Ponce E, Salmeron J, Quiterio M, Giuliano AR. Racial differences in the incidence and clearance of human papilloma virus (HPV): the HPV in men (HIM) study. Cancer Epidemiol Biomark Prev. 2013;22:1762–1770. doi: 10.1158/1055-9965.EPI-13-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muñoz N, Mendez F, Posso H, Molano M, van den Brule AJ, Ronderos M, Meijer C, Munoz A. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190:2077–2087. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 19.Hamlin-Douglas LK, Coutlee F, Roger M, Franco EL, Brassard P. Prevalence and age distribution of human papillomavirus infection in a population of Inuit women in Nunavik, Quebec. Cancer Epidemiol Biomarkers Prev. 2008;17:3141–3149. doi: 10.1158/1055-9965.EPI-08-0625. [DOI] [PubMed] [Google Scholar]
- 20.Sukvirach S, Smith JS, Tunsakul S, Munoz N, Kesararat V, Opasatian O, Chichareon S, Kaenploy V, Ashley R, Meijer CJ, Snijders PJ, Coursaget P, Franceschi S, Herrero R. Population-based human papillomavirus prevalence in Lampang and Songkla, Thailand. J Infect Dis. 2003;187:1246–1256. doi: 10.1086/373901. [DOI] [PubMed] [Google Scholar]
- 21.Matos E, Loria D, Amestoy GM, Herrera L, Prince MA, Moreno J, Krunfly C, van den Brule AJ, Meijer CJ, Munoz N, Herrero R. Prevalence of human papillomavirus infection among women in Concordia, Argentina: a population-based study. Sex Transm Dis. 2003;30:593–599. doi: 10.1097/01.OLQ.0000085181.25063.6C. [DOI] [PubMed] [Google Scholar]
- 22.Herrero R, Castle PE, Schiffman M, Bratti MC, Hildesheim A, Morales J, Alfaro M, Sherman ME, Wacholder S, Chen S, Rodriguez AC, Burk RD. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1796–1807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- 23.Mooij SH, Landen O, van der Klis FR, van der Sande MA, de Melker HE, Xiridou M, van Eeden A, Heijman T, Speksnijder AG, Snijders PJ, Schim van der Loeff MF. HPV seroconversion following anal and penile HPV infection in HIV-negative and HIV-infected MSM. Cancer Epidemiol Biomarkers Prev. 2014;23:2455–2461. doi: 10.1158/1055-9965.EPI-14-0199. [DOI] [PubMed] [Google Scholar]
- 24.Giuliano AR, Viscidi R, Torres BN, Ingles DJ, Sudenga SL, Villa LL, Baggio ML, Abrahamsen M, Quiterio M, Salmeron J, Lazcano-Ponce E. Seroconversion following anal and genital HPV infection in men: the HIM study. Papillomavirus Res. 2015 doi: 10.1016/j.pvr.2015.06.007. http://dx.doi.org/10.1016/j.pvr.2015.06.007i (in press) [DOI] [PMC free article] [PubMed]
- 25.Edelstein ZR, Carter JJ, Garg R, Winer RL, Feng Q, Galloway DA, Koutsky LA. Serum antibody response following genital {alpha}9 human papillomavirus infection in young men. J Infect Dis. 2011;204:209–216. doi: 10.1093/infdis/jir242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, Solomon D, Guillen D, Alfaro M, Morales J, Hutchinson M, Katki H, Cheung L, Wacholder S, Burk RD. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102:315–324. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Jr, Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R, Chang YH, Ferris D, Rouleau D, Bryan J, Marshall JB, Vuocolo S, Barr E, Radley D, Haupt RM, Guris D. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011;364:401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaturvedi AK, Graubard BI, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, Gillison ML. NHANES 2009–2012 findings: association of sexual behaviors with higher prevalence of oral oncogenic human papillomavirus infections in U.S. men. Cancer Res. 2015;75:2468–2477. doi: 10.1158/0008-5472.CAN-14-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]