Abstract
Chronic obstructive pulmonary disorder (COPD) is characterized by enhanced chronic airway and lung inflammatory responses to noxious particles or gases. It is a major unmet medical need worldwide and, in western society, it is strongly associated with cigarette smoke (CS) exposure. CS-induced inflammation is believed to be a key immune driver in the pathogenesis of COPD. Since the concept of inflammasome was first introduced nearly a decade ago, the inflammasome has been increasingly recognized as a central player in innate immune and inflammatory responses. In addition, studies have emerged that demonstrate mitochondrial innate immune signaling plays important roles in CS-induced inflammasome activation, pulmonary inflammation and tissue remodeling responses. Here, recent discoveries on inflammasome activation and mitochondrial biology and their role in COPD pathogenesis are reviewed. In addition, the current limitations of our understanding on this theme and future research directions are discussed.
Keywords: chronic obstructive pulmonary disease (COPD), mitochondria, inflammasome, MAVS, NLRX1
1. Introduction
Chronic obstructive pulmonary disease (COPD) encompasses several clinical syndromes, most notably emphysema and chronic bronchitis1. As noted in the definition, COPD is characterized by airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases2. COPD tissues are characterized by chronic inflammation, mucus metaplasia, alveolar destruction and structural cell apoptosis3–5. The inflammation in COPD lung tissues is believed to be causally related to emphysema development and other pathologic alterations in the lungs that worsen with disease progression5–7. In addition, over the past several years, the understanding of COPD has evolved from it being a disease affecting the lungs to it being a complex systemic disorder with multiple extrapulmonary comorbidities. For example, patients with COPD die primarily from coronary artery disease, lung cancer and stroke, which are all related to smoking, in addition to respiratory failure with/without infection8,9. They are also at greater risk of diabetes, peripheral artery disease, skeletal muscle dysfunction, osteoporosis and chronic kidney disease. Giving the growing evidence that chronic systemic inflammation might be the common pathway linking these comorbidities, it has been proposed that COPD could be considered as part of a “chronic systemic inflammatory syndrome” (reviewed in reference10). Overall, cigarette smoke (CS)-induced/associated inflammation is believed to be a key driver of the pathogenesis in lung tissues as well as in other organs from COPD patients.
Recent understanding of inflammasome(s) as a central player in innate immune and inflammatory responses has begun to shed light on the underlying mechanism(s) of CS-induced/associated inflammation. Accordingly, the roles of inflammasomes in COPD pathogenesis are increasingly being explored. In addition, the significance of mitochondrial molecules on the regulation of CS-induced inflammasome activation has been highlighted recently11. Here, the current status of our understanding of the functional roles of inflammasome and its activation mechanisms are reviewed in the context of COPD pathogenesis. Specifically, recent discoveries are summarized about the functional role of novel mitochondrial molecules that have been identified as critical players in the development of COPD. In addition, the limitations of our understanding and remaining unsolved questions regarding will be discussed.
2. Inflammasome as a Central Player in Inflammatory Responses: Implications in COPD Pathogenesis
Inflammation is a fundamental response of the innate immune system to noxious stimuli12. Since the concept of inflammasomes was introduced nearly a decade ago, inflammasomes have been increasingly recognized as a central player in innate immune and inflammatory responses in settings of infection and sterile inflammation (reviewed in the reference13). Inflammasomes are multimolecular complexes whose essential components consist of a sensor protein, the adapter protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), and the inflammatory protease caspase-1. Conformational changes in the sensor protein lead to their assembly into a platform for the activation of pro-inflammatory caspase-1. Inflammasome-activated caspase-1 is then used for the activation of pro-inflammatory cytokines IL-1β and IL-18, and release of these cytokines results in the recruitment of effector cell populations important in the immune response and tissue repair. Currently, the identified inflammasome sensor proteins include the nucleotide binding domain and leucine-rich-repeat-containing (NLR) proteins such as NLRP1, NLRP3, NLRC4, NLRP6 and NAIP5, as well as the DNA-sensing complex of absent in melanoma 2 protein (AIM2)14. During infection or injury, inflammasomes are directly or indirectly activated by a wide array of pathogen-associated molecular patterns (PAMP) or danger-associated molecular patterns (DAMP) and, under normal circumstances, activation of the inflammasomes culminates in the resolution of infection or inflammation and contributes to homeostatic processes. However, in a pathologic context, perpetuation of inflammasome activation occurs and this leads to a variety of chronic inflammatory disorders such as metabolic disorders, tumorigenesis and autoimmune disorders (reviewed in references13–15). In this regard, our group and others reported that CS activates caspase-1, a crucial component of inflammasome complex and its downstream target molecules, IL-1β and IL-1816,17. In addition, a recent study demonstrated that markers of activation of the ATP-NLRP3 inflammasome pathway are upregulated in patients with COPD, suggesting this pathway might play important roles in the pathogenesis of COPD18. The increase of IL-1 β and IL-18, target cytokines of inflammasome activation, in COPD patients has also been demonstrated previously in many studies (reviewed in references19,20). These studies taken together suggest that inflammasome activation and the resultant activation of proinflammatory cytokines IL-1β and IL-18 make a significant contribution to the pathogenesis of COPD.
3. Mitochondrial Regulation of Inflammasome Activation
Traditionally, mitochondria have been best known as biosynthetic and bioenergetics organelles. Recently, however, the role of mitochondria was expanded; mitochondria is also considered as signaling organelles with critical roles in cell death, differentiation, innate immunity and metabolism21,22. Accordingly, our understanding of mitochondrial role in human health as well as various disorders has fundamentally evolved21,23. Indeed, the roles of mitochondria on inflammasome regulation have been explored in multiple studies (reviewed in references24–26). A detailed discussion in this topic is beyond of the scope of this review. Instead, this review will focus on specific mitochondrial molecules that have direct implications in COPD pathogenesis.
3.1 Mitochondrial Antiviral Signaling Protein (MAVS)
MAVS was initially identified while studying antiviral innate immune recognition and signaling. Originally, Toll-like receptors (TLRs) 3, 7, 8 and 9 were known to recognize distinct types of virus-derived nucleic acids and to activate signaling cascades that result in the induction of type I IFNs27. Later, however, the existence of TLR-independent pathways that are highly effective in providing antiviral innate immunity were discovered; this included the retinoic acid inducible gene I (RIG-I)-like RNA helicase (RLH) pathway to be identified as an intracellular sensor for viral nucleic acids28. Following the identification of RLH signaling, in 2005, MAVS was defined as a key signal integrator of the RLH pathway and currently, a variety of evidence highlight the essential role of MAVS in this major antiviral signaling pathway29,30. Further studies extended the functional roles of MAVS beyond the induction of antiviral and inflammatory responses, revealing that MAVS interacts with a number of novel molecules with roles in apoptosis, mitochondrial dynamics, autophagy and proteasome degradation31. In this regard, it is interesting that MAVS played a critical role in the exaggerated production of IL-18 production as well as pulmonary inflammatory and remodeling responses that are observed after CS and respiratory virus or viral pathogen associated molecular pattern (PAMP) co-exposures32. The synergistic enhanced activation of IL-18 in murine lungs after CS and viral PAMP co-exposure was largely dependent on MAVS, suggesting that MAVS plays an important role in CS-induced inflammasome activation32.
3.2 MAVS as a Critical Adaptor Molecule of NLRP3 Inflammasome Activation
Indeed, in a recent seminal publication, MAVS was identified as an important adaptor molecule for NLRP3 activation33. Subramamian and colleagues performed a series of elegant experiments to demonstrate that MAVS is required for optimal NLRP3 inflammasome activity and mediates recruitment of NLRP3 to the mitochondria, promoting production of IL-1β33. Interestingly, the researchers observed that the MAVS-dependent optimal recruitment of NLRP3 to mitochondria was only with the administration of some NLRP3 stimulants such as nigericin and ATP. Other NLRP3 stimulants, including alum and monosodium urate crystal, activated NLRP3 inflammasome via a pathway that is mainly independent of the MAVS molecule. They differentiated these stimulants as non-crystalline (nigericin and ATP) and crystalline (alum and monosodium urate) NLRP3 stimulants. Furthermore, another study demonstrated that both MAVS and ASC form functional prion-like fibers through their respective death domains to propagate downstream signaling, indicating that prion-like polymerization is a conserved mechanism in innate immunity and inflammation34.
3.3 Nucleotide Binding Domain and Leucine-rich-repeat-containing Protein X1 (NLRX1)
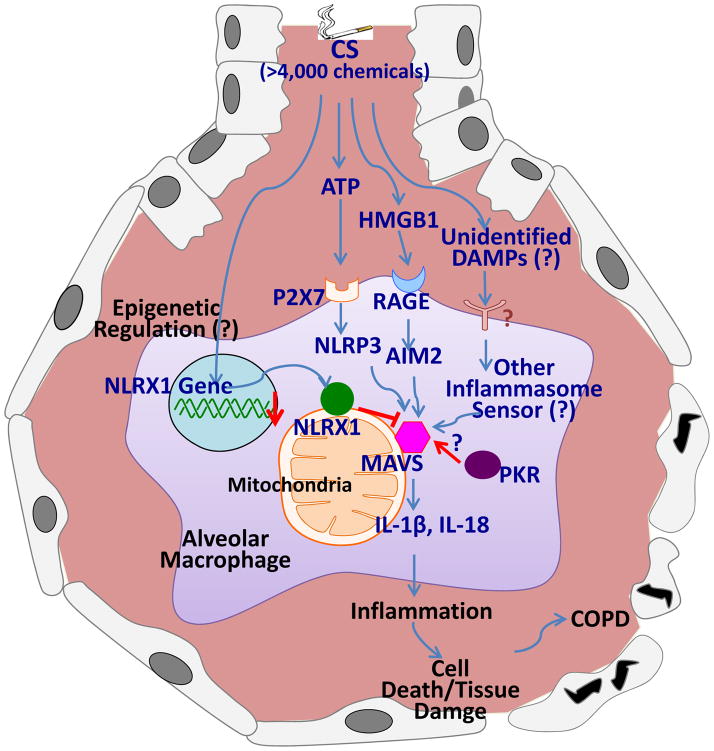
NLRX1 is the member of the NLR family of pattern recognition receptors (PRRs) that has a unique N-terminal domain, which account for the letter “X’ in its acronym. It contains a mitochondrial targeting sequence and biochemical analyses demonstrate that NLRX1 localizes to the mitochondria35. Strikingly, NLRX1 represents the first and so far only example of a pattern recognition receptor (PRR) family member that targets this cellular location, therefore indicating NLRX1’s potential role in establishing a fundamental link between mitochondrial functions and innate immunity30. Initially, it was identified to function as a negative regulator of RIG-I signaling by targeting the RIG-I downstream MAVS36. Consistent with these in vitro knockdown data, in vivo experiments of virus infection of NLRX1-deficient mice confirmed that NLRX1 is a negative regulator of MAVS37. Furthermore, structural and functional characterization of the RNA-binding element of the NLRX1 molecule has also been described38. It is important to note, however, that this molecule, NLRX1, is such a novel molecule that previously unrecognized roles of NLRX1 are continuously being identified. For example, NLRX1 was recently identified to negatively regulate TLR-induced NF-κB signaling by targeting TRAF6 and IκB kinase (IKK)39. In another recent publication, NLRX1 was identified to interact with mitochondrial Tu translation elongation factor (TUFM) and promote autophagy40. It is important to note that there is also some controversy about the exact function of NLRX1 and its localization on/in mitochondrial compartments30,41,42. For example, a different group who also generated NLRX1−/− mice and employed in vitro and in vivo experiments observed no significant difference in RLH signaling with or without NLRX143. Another group reported that NLRX1 is located in the mitochondria matrix where the NLRX1 interacts with UQCRC2, a matrix-facing protein of the respiratory chain complex III and regulates ROS generation35. Interestingly, our recent publication has demonstrated, for the first time, that NLRX1 was significantly suppressed after CS exposure and CS-induced activation of inflammasomes and the consequent interleukin (IL)-18 activation; pulmonary inflammation; and emphysematous destruction were exaggerated in the absence of NLRX111. On the contrary, these responses were significantly ameliorated with lentiviral overexpression of NLRX1 in vivo. Furthermore, the exaggerated CS-induced COPD-like phenotype observed in NLRX1 null mutant (−/−) mice was significantly ameliorated in NLRX1 and MAVS double mutant (NLRX1−/−/MAVS−/−) mice, suggesting that MAVS is functioning as a critical downstream molecule of NLRX1 signaling in a murine COPD model11. The importance of NLXR1 was also evident in clinical studies. In three independent human COPD cohorts, the expression of NLRX1 was suppressed in the lungs of COPD patients and this NLRX1 suppression exhibited strong correlation with increased degree of airflow limitation, a hallmark of COPD11. Taken together, these observations allow us to hypothesize that NLRX1 is a critical mediator that inhibits CS-induced pulmonary inflammation and remodeling responses via its regulation of MAVS function (Fig. 1).
Figure 1.
Hypothetical mechanism of cigarette smoke-induced inflammasome activation and its contribution to the COPD pathogenesis. Cigarette smoke (CS) containing more than 4,000 chemicals may stimulate multiple danger-associated molecular patterns (DAMPs)-mediated pathways. These multiple pathways might converge on mitochondrial antiviral signaling protein (MAVS). MAVS-dependent inflammasome activation results in the activation of proinflammatory cytokines, IL-1β and IL-18, where Protein kinase R (PKR) may play as an important regulatory role. Nucleotide binding domain and leucine-rich-repeat-containing protein X1 (NLRX1) may mediate a critical inhibitory role on the MAVS-mediated activation of inflammasomes. Please see the main text for the explanation in detail.
3.4. NLRX1 and Alveolar Macrophages (AMs)
Macrophages are essential for pulmonary host defense through their capacity to survey the exposed airways and regulate innate and adaptive immunity (reviewed in Ref.44,45). The pulmonary macrophage system consists of several different populations that are found in anatomically distinct compartments that include the airways, alveolar spaces (alveolar macrophages (AMs)), and resident lung tissue. AMs constitute over 90% of the pulmonary macrophage population46, constantly encountering inhaled substances due to their exposed position in the alveolar lumen. As known, AMs are considered to be major effector cells in innate host defense against inhaled irritants by virtue of their phagocytic ability47. Therefore, it is vital that resident AMs are kept in a relatively quiescent state with inherent active suppression mechanisms of inflammation in response to the harmless inhaled antigens to prevent collateral damage to lung tissue48.
There is a large body of evidence implicating AMs in the pathogenesis of COPD44. AMs are activated by CS and other irritants to release inflammatory mediators. AMs also secrete elastolytic enzymes (proteases), including matrix metalloprotease (MMP)-2, MMP-9, MMP-12, cathepsin K, L, and S in response to irritants and infection, which together are responsible for the destruction of lung parenchyma. Indeed, AM is the major cell population where IL-18 is increased in the murine lungs after CS exposure, as well as in lungs from active smokers when compared to those from non-smoker controls16. In this regard, it is intriguing that AM was major cell population in which NLRX1 is most prominently expressed in the pulmonary system of mice as well as of humans11. In addition, chronic CS exposure induced significant suppression of NLRX1 expression in AMs in the murine COPD model11.
Taken together, it is plausible to speculate that NLRX1 may play as an essential inhibitor of inflammation to keep AMs in a quiescent homeostatic state in the lung. The suppression of NLRX1 may be a key mechanism of the CS-induced activation of AMs and the resultant activation of inflammasome and the production of active IL-18 in these cell population. It is also possible to speculate that the suppression of NLRX1 might be a key characteristic of distinct AM subpopulations present in COPD. It has been increasingly clear that different macrophage subpopulations exist in the inflamed lung. Although the existence of such subpopulations has been implicated in COPD, the importance of these subpopulations is still largely unknown. Thus, there is a need to better characterize distinct AM subpopulations present in COPD and their relative contribution to disease pathology, as their highly plastic nature offer a therapeutic opportunity to reprogram macrophages to facilitate restoration of lung homeostasis. Further studies on these questions will provide us better understanding of AMs and their critical roles in COPD pathogenesis.
4. Mechanism(s) of Inflammasome Activation in COPD
4.1. Which DMAP(s) and PAMP(s) Drive Inflammasome Activation in COPD?
As noted above, inflammasomes are directly or indirectly activated by a wide array of DAMPs or PAMPs. It is not surprising that most studies about the inflammasome and COPD have focused on DAMPs and their recognition mechanisms for the development of COPD when we look at the definition of DAMP. DAMP was originally proposed to explain how the immune system works to distinguish between self and non-self danger signals in response to sterile inflammation in the absence of pathogens. DAMP is now defined as the endogenous molecules released or secreted from cells in response to injury, cell death and other stress responses49–51. DAMPs can be derived from any compartment of the cell including the nucleus and cytoplasm (e.g., mitochondria, endoplasmic reticulum, and lysosome). Among them, protein DAMPs include endogenous proteins, such as HMGB1, HSPs, the S100 family of calcium binding proteins, serum amyloid A, and histones, whereas other non-protein sources of DAMPs include ATP, uric acid, heparin sulfate, DNA (genomic and mitochondrial DNA), and RNA.
ATP may play a role as DAMP in the context of COPD pathogenesis. In humans, chronic smokers had elevated ATP concentrations in bronchoalveolar lavarge (BAL) flluids (BALF) compared with never-smokers and ATP concentrations from BALF correlated negatively with lung function and positively with BALF neutrophil counts18. Furthermore, airway macrophages from patients with COPD responded with an increased secretion of proinflammatory and tissue-degrading mediators after ATP stimulation18. Animal studies demonstrated that CS-induced neutrophilia and the increase of markers of inflammasome activation in the lungs were attenuated via the P2X7-NLRP3-caspase1/11 axis-dependent pathway, suggesting that P2X7, a receptor of ATP and its downstream NLRP3 inflammasome pathway plays a role in CS-induced inflammasome activation17,52. However, another animal-based study failed to demonstrate that the NLRP3-Caspase1-IL-1β axis is responsible for the CS-induced inflammation53. It is important to note that all these animal studies utilized acute or subacute CS-induced murine inflammation model and thus, the knowledge obtained from these animal studies might not be as directly relevant to the CS-mediated chronic inflammation and injury responses seen in human COPD.
There is evidence that high-mobility group box 1 (HMGB1), a nuclear protein that is released during inflammation and repair, and interact with the receptor for advanced glycation end products (RAGE), may play an important role in COPD pathogenesis. BALF levels of HMGB1 were higher in smokers with COPD than in smokers and never-smokers54. In addition, HMGB1 correlated positively with IL-1β and negatively with clinical variables of lung functional decline. Furthermore, RAGE was overexpressed in the airway epithelium and smooth muscle of patients with COPD and it co-localized with HMGB1. Overall, these data suggest that elevated HMGB1 expression in COPD airways may sustain inflammation and remodeling through its interaction with IL-1β and its receptor, RAGE54. Another study demonstrated also that HMGB1 levels in peripheral airways were elevated in smokers without COPD, as compared with non-smokers, and the HMGB1 levels were further augmented in COPD patients55.
4.2. Multiple DAMPs and Multiple Inflammasome Sensors in COPD?
It is possible that multiple DAMPs including the DAMPs discussed above and other DAMPs yet to be recognized in the context of COPD pathogenesis may act in an additive or synergistic manner. It is tempting to speculate that CS-induced stress and injury responses may evoke multiple DAMPs from immune cells as well as structural cells especially when one consider that CS contains more than 4,000 chemicals. These multiple DAMPs may play complex roles in the regulation of not only inflammasome activation, but also in various cellular biologic responses such as cell death and tissue remodeling responses in the development of COPD. In this regard, it is interesting that Protein kinase R (PKR) activity is integral to inflammasome assembly and activation where PKR physically interacts with multiple inflammasome components, including NLRP3, NLRP1, NLRC4 and AIM2, and broadly regulates inflammasome activation56. We demonstrated that PKR could be activated by CS exposure in vivo in CS-induced murine COPD model32. Thus, CS-induced activation of PKR might exert a broad role in regulating CS-induced inflammasomes activation via multiple pathways. There has been no study yet to define the direct role of PKR on CS-induced inflammasomes activation.
If multiple DAMPs play distinct roles in the development of COPD and these DAMPs are recognized by each of the specific inflammasome sensor molecules, leading into inflammasome activation via various pathways, then, how can the NLRX1/MAVS signaling play such a dominant role in CS-induced inflammasome activation? The mechanism of the biological action of MAVS on inflammasome regulation may provide important clues. As noted above, MAVS has multiple functions such as antiviral signaling, activation of NLRP3 inflammasome, apoptosis and others. The fundamental actionable mechanism of MAVS on these diverse biological phenomena is their role as key adaptor molecule to convey the multiple signals described. Therefore, it might be true that multiple pathways from various DAMPs are converged into MAVS, where MAVS is the key adaptor molecule for CS-induced inflammasome activation, whereas NLRX1 plays as a crucial inhibitor of MAVS-mediated downstream signaling (Fig. 1). The exact mechanism by which inflammasomes are activated by CS exposure is still unclear and awaits the identification of sensor proteins and other regulators and/or interacting partners to fully elucidate the mechanisms of their action. Further studies are warranted to explore these intriguing and fundamental questions.
5. Central Role of Mitochondria in Inflammasome Activation, Oxidant injury and Apoptosis: Implication for COPD Pathogenesis
A number of major theories on COPD pathogenesis have been promulgated. Initially, since the 1960s, the protease/anti-protease hypothesis dominated the thinking in the area of COPD. This concept built up to the idea that the increase in protease burden is derived from inflammatory cells (hence the inflammation hypothesis of COPD pathogenesis). In addition, there is the apoptosis hypothesis, which proposes that cellular injury is a primary event in the pathogenesis of pulmonary COPD and emphysema57,58. Furthermore, for a long time, the exaggerated production of ROS and the resulting oxidant injury has been postulated to be the major events in the pathogenesis of COPD59. However, these different hypotheses are not separate entities nor mutually exclusive. Rather, each of these concepts is believed to represent one of multifaceted biological processes that are involved in the development of COPD. Recent studies on mitochondrial biology have suggested that COPD pathogenesis could be explained by a unified concept if it is focused from the mitochondrial perspective. As discussed above, a variety of host danger signals during tissue damage responses that activate the inflammasome and lead to the secretion of pro-inflammatory cytokines such as IL-1β and IL-18, are interpreted at the level of mitochondria which interacts to engage the inflammatory and apoptosis or cell death pathways. This idea is not so surprising when we consider that the mitochondria perform diverse yet interconnected functions, contributing to cellular stress responses such as autophagy and apoptosis. Indeed, mitochondrial dysfunction has recently emerged as a key factor in a myriad of diseases23. Therefore, when combined with the recent scientific discoveries which link mitochondrial dysfunction, inflammasome activation, ROS imbalance and apoptosis, one can readily appreciate how mitochondrial dysfunction or dysregulation of mitochondrial molecules could contribute to the pathogenesis of COPD.
6. Conclusions
In this review, with the recent understanding of mitochondrial molecules involved in inflammasomes activation, we have discussed a previously unidentified novel pathway of NLRX1/MAVS-mediated inflammasomes signaling which might play a crucial role in the development of COPD (Fig. 1). As discussed, the functional roles of inflammasome and the underlying mechanisms of the inflamassome activation in the context of COPD pathogenesis have not yet been adequately elucidated. Furthermore, questions remain how the mitochondria-related molecules regulate inflammasome activation in COPD. We believe that these evolving concepts open new options to better understand the pathogenesis of COPD. We hope that the discussion presented here will stimulate further research to explore the roles as well as the underlying mechanisms of mitochondria and their molecules in the COPD pathogenesis.
Acknowledgments
Funding Sources: This work was supported by Flight Attendance Medical Research Institute (FAMRI) Grant #113258 (MJK), FAMRI Grant #182165 (CDC), Connecticut Department of Public Health Grant # 2013-0196 (MJK), and NIH Grant # R56HL119511 (MJK).
Footnotes
COMPETITING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Senior RM, Shapiro SD. Chronic obstructive pulmonary disease: Epidemiology, pathophysiology, and pathogenesis. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, Kaiser LR, Senior RM, editors. Fishman’s Pulmonary Diseases and Disorders. New York, NY: McGraw-Hill, Inc; 1998. pp. 659–81. [Google Scholar]
- 2.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 3.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–51. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31:1334–56. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]
- 5.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 6.Bhalla DK, Hirata F, Rishi AK, Gairola CG. Cigarette smoke, inflammation, and lung injury: a mechanistic perspective. Journal of toxicology and environmental health Part B, Critical reviews. 2009;12:45–64. doi: 10.1080/10937400802545094. [DOI] [PubMed] [Google Scholar]
- 7.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–59. doi: 10.1146/annurev.pathol.4.110807.092145. [DOI] [PubMed] [Google Scholar]
- 8.McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62:411–5. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anthonisen NR, Connett JE, Enright PL, Manfreda J. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med. 2002;166:333–9. doi: 10.1164/rccm.2110093. [DOI] [PubMed] [Google Scholar]
- 10.Nussbaumer-Ochsner Y, Rabe KF. Systemic manifestations of COPD. Chest. 2011;139:165–73. doi: 10.1378/chest.10-1252. [DOI] [PubMed] [Google Scholar]
- 11.Kang MJ, Yoon CM, Kim BH, et al. Suppression of NLRX1 in chronic obstructive pulmonary disease. J Clin Invest. 2015;125:2458–62. doi: 10.1172/JCI71747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.An inflammatory assemblage. Nat Immunol. 2012;13:320. doi: 10.1038/ni.2268. [DOI] [PubMed] [Google Scholar]
- 13.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–86. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 14.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–35. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Kang MJ, Homer RJ, Gallo A, et al. IL-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J Immunol. 2007;178:1948–59. doi: 10.4049/jimmunol.178.3.1948. [DOI] [PubMed] [Google Scholar]
- 17.Eltom S, Stevenson CS, Rastrick J, et al. P2X7 receptor and caspase 1 activation are central to airway inflammation observed after exposure to tobacco smoke. PLoS One. 2011;6:e24097. doi: 10.1371/journal.pone.0024097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lommatzsch M, Cicko S, Muller T, et al. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:928–34. doi: 10.1164/rccm.200910-1506OC. [DOI] [PubMed] [Google Scholar]
- 19.Dima E, Koltsida O, Katsaounou P, et al. Implication of Interleukin (IL)-18 in the pathogenesis of chronic obstructive pulmonary disease (COPD) Cytokine. 2015;74:313–7. doi: 10.1016/j.cyto.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Brusselle GG, Provoost S, Bracke KR, Kuchmiy A, Lamkanfi M. Inflammasomes in respiratory disease: from bench to bedside. Chest. 2014;145:1121–33. doi: 10.1378/chest.13-1885. [DOI] [PubMed] [Google Scholar]
- 21.Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406–17. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–12. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–59. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tschopp J. Mitochondria: Sovereign of inflammation? Eur J Immunol. 2011;41:1196–202. doi: 10.1002/eji.201141436. [DOI] [PubMed] [Google Scholar]
- 25.Gurung P, Lukens JR, Kanneganti TD. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends in molecular medicine. 2015;21:193–201. doi: 10.1016/j.molmed.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012;13:780–8. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- 27.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 28.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–7. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 29.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–82. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Arnoult D, Soares F, Tattoli I, Girardin SE. Mitochondria in innate immunity. EMBO Rep. 2011;12:901–10. doi: 10.1038/embor.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belgnaoui SM, Paz S, Hiscott J. Orchestrating the interferon antiviral response through the mitochondrial antiviral signaling (MAVS) adapter. Curr Opin Immunol. 2011;23:564–72. doi: 10.1016/j.coi.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Kang MJ, Lee CG, Lee JY, et al. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest. 2008;118:2771–84. doi: 10.1172/JCI32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153:348–61. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai X, Chen J, Xu H, et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156:1207–22. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnoult D, Soares F, Tattoli I, Castanier C, Philpott DJ, Girardin SE. An N-terminal addressing sequence targets NLRX1 to the mitochondrial matrix. J Cell Sci. 2009;122:3161–8. doi: 10.1242/jcs.051193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore CB, Bergstralh DT, Duncan JA, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–7. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 37.Allen IC, Moore CB, Schneider M, et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011;34:854–65. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong M, Yoon SI, Wilson IA. Structure and functional characterization of the RNA-binding element of the NLRX1 innate immune modulator. Immunity. 2012;36:337–47. doi: 10.1016/j.immuni.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia X, Cui J, Wang HY, et al. NLRX1 negatively regulates TLR-induced NF-kappaB signaling by targeting TRAF6 and IKK. Immunity. 2011;34:843–53. doi: 10.1016/j.immuni.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei Y, Wen H, Yu Y, et al. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity. 2012;36:933–46. doi: 10.1016/j.immuni.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parvatiyar K, Cheng G. NOD so fast: NLRX1 puts the brake on inflammation. Immunity. 2011;34:821–2. doi: 10.1016/j.immuni.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdul-Sater AA, Said-Sadier N, Lam VM, et al. Enhancement of reactive oxygen species production and chlamydial infection by the mitochondrial Nod-like family member NLRX1. J Biol Chem. 2010;285:41637–45. doi: 10.1074/jbc.M110.137885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rebsamen M, Vazquez J, Tardivel A, Guarda G, Curran J, Tschopp J. NLRX1/NOD5 deficiency does not affect MAVS signalling. Cell Death Differ. 2011;18:1387. doi: 10.1038/cdd.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Frontiers in immunology. 2014;5:435. doi: 10.3389/fimmu.2014.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–95. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van oud Alblas AB, van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979;149:1504–18. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franke-Ullmann G, Pfortner C, Walter P, Steinmuller C, Lohmann-Matthes ML, Kobzik L. Characterization of murine lung interstitial macrophages in comparison with alveolar macrophages in vitro. J Immunol. 1996;157:3097–104. [PubMed] [Google Scholar]
- 48.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170:499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 50.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 51.Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–42. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eltom S, Belvisi MG, Stevenson CS, et al. Role of the inflammasome-caspase1/11-IL-1/18 axis in cigarette smoke driven airway inflammation: an insight into the pathogenesis of COPD. PLoS One. 2014;9:e112829. doi: 10.1371/journal.pone.0112829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pauwels NS, Bracke KR, Dupont LL, et al. Role of IL-1alpha and the Nlrp3/caspase-1/IL-1beta axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur Respir J. 2011;38:1019–28. doi: 10.1183/09031936.00158110. [DOI] [PubMed] [Google Scholar]
- 54.Ferhani N, Letuve S, Kozhich A, et al. Expression of high-mobility group box 1 and of receptor for advanced glycation end products in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:917–27. doi: 10.1164/rccm.200903-0340OC. [DOI] [PubMed] [Google Scholar]
- 55.Kanazawa H, Tochino Y, Asai K, Ichimaru Y, Watanabe T, Hirata K. Validity of HMGB1 measurement in epithelial lining fluid in patients with COPD. European journal of clinical investigation. 2012;42:419–26. doi: 10.1111/j.1365-2362.2011.02598.x. [DOI] [PubMed] [Google Scholar]
- 56.Lu B, Nakamura T, Inouye K, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–4. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuder RM, Petrache I, Elias JA, Voelkel NF, Henson PM. Apoptosis and emphysema: the missing link. Am J Respir Cell Mol Biol. 2003;28:551–4. doi: 10.1165/rcmb.F269. [DOI] [PubMed] [Google Scholar]
- 58.Taraseviciene-Stewart L, Voelkel NF. Molecular pathogenesis of emphysema. J Clin Invest. 2008;118:394–402. doi: 10.1172/JCI31811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacNee W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:50–60. doi: 10.1513/pats.200411-056SF. [DOI] [PubMed] [Google Scholar]