Abstract
An unprecedented highly diastereoselective and enantioselective aldol reaction of α-alkyl azlactones and aliphatic aldehydes was achieved with cinchona alkaloid catalysts. To our knowledge, this reaction provides the first useful catalytic asymmetric access toward β-hydroxy-α-amino acids bearing alkyl substituents, which are structural motifs embedded in many natural products.
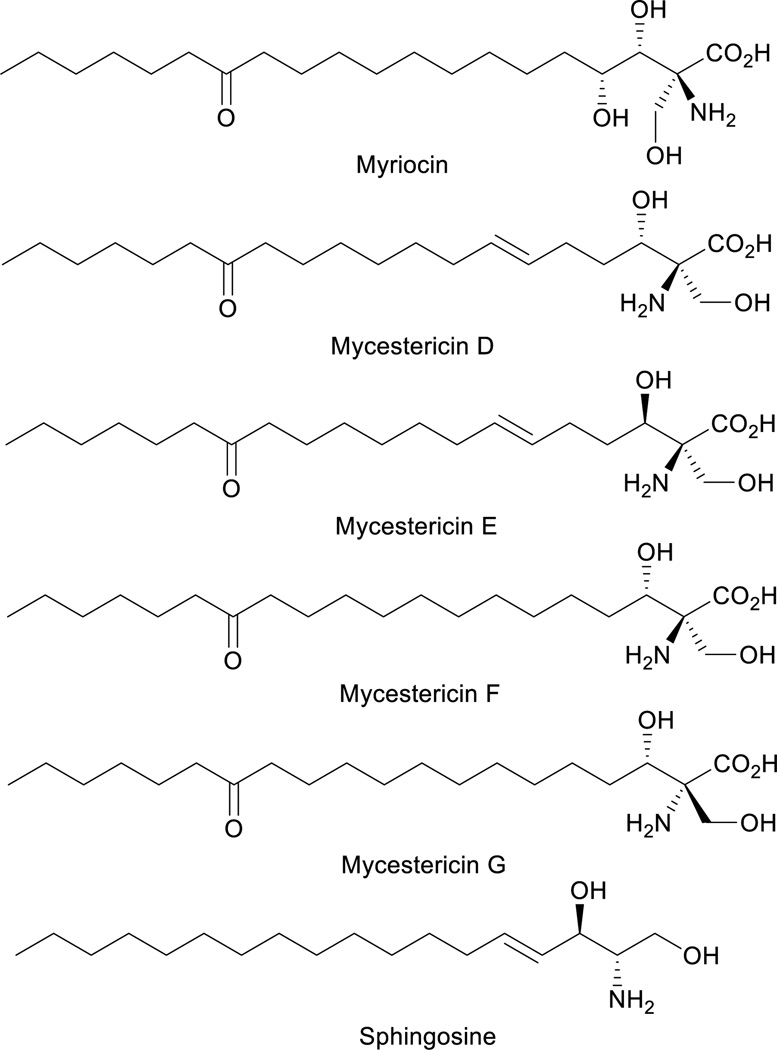
Optically active β-hydroxy-α-amino acids are an important class of amino acids as they are structural motifs in many biologically active natural products such as vancomycin1, katanosins2, cyclosporin3, myriocin4a–c, mycestericins4d–e, sphingosine and threonine (Figure 1). Furthermore, these amino acids are also useful chiral building blocks in organic synthesis as precursors to β-lactams5, β-halo-α-amino acids6, and aziridines7. A variety of catalytic asymmetric approaches for the synthesis of β-hydroxy-α-amino acids has been reported8–14. In a pioneering study8a, Ito, Hayahsi and coworkers reported a gold-catalyzed highly diastereoselective and enantioselective aldol reaction for the generation of β-hydroxy-α-amino acids containing tertiary α-carbons. Since then other groups have also reported asymmetric direct aldol reactions with chiral transition-metal catalysts8c–h, organocatalysts9 and aldolases10 for the synthesis of β-hydroxy-α-amino acids and their derivatives. In addition, Sharpless asymmetric aminohydroxylation11, transition-metal-catalyzed asymmetric hydrogenation12, palladium-catalyzed allylic alkylation13 and chiral phosphoric acid-catalyzed addition to oxocarbenium ion14 have been utilized to achieve the same goal.
Figure 1.
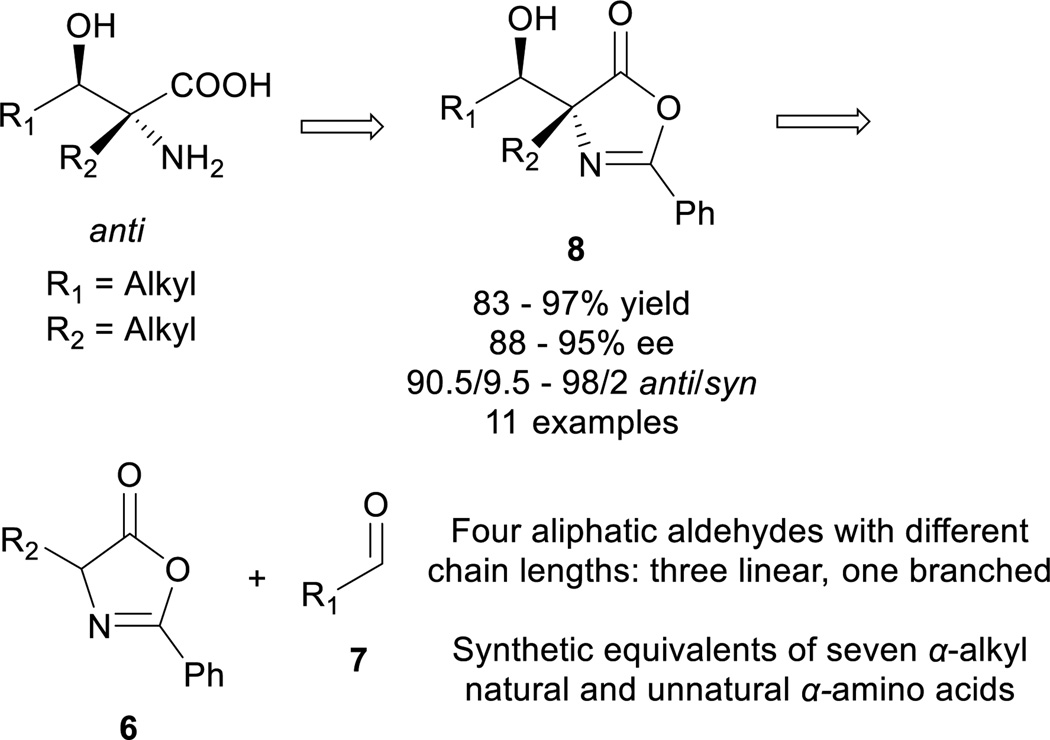
We became interested in the development of catalytic asymmetric synthesis of β-hydroxy-α-amino acids because biologically interesting natural products such as mycestericins contain a chiral β-hydroxy-α-amino acid motif that could not be constructed from existing catalytic asymmetric aldol reactions. In particular this motif presents both a tertiary β-stereocenter and a quaternary α-stereocenter with alkyl substituents. In principle, an efficient catalytic asymmetric aldol reaction of α-alkyl enolates or the equivalents with aliphatic aldehydes could provide a direct access to this structural motif15. However, to our knowledge, such an asymmetric transformation was not available. Herein, we report the first efficient catalytic asymmetric direct aldol reaction of α-alkyl azlactone 6 and aliphatic aldehyde 7 (Scheme 1), which provides, to our knowledge, the first useful asymmetric catalytic access toward β-hydroxy-α-amino acids bearing alkyl substituents at both the tertiary β-stereocenter and the quaternary α-stereocenter. The high anti-diastereoselectivity in combination with a broad substrate scope allows the reaction to complement existing methods to form a general strategy for the asymmetric synthesis of β-hydroxy-α-amino acids.
Scheme 1.
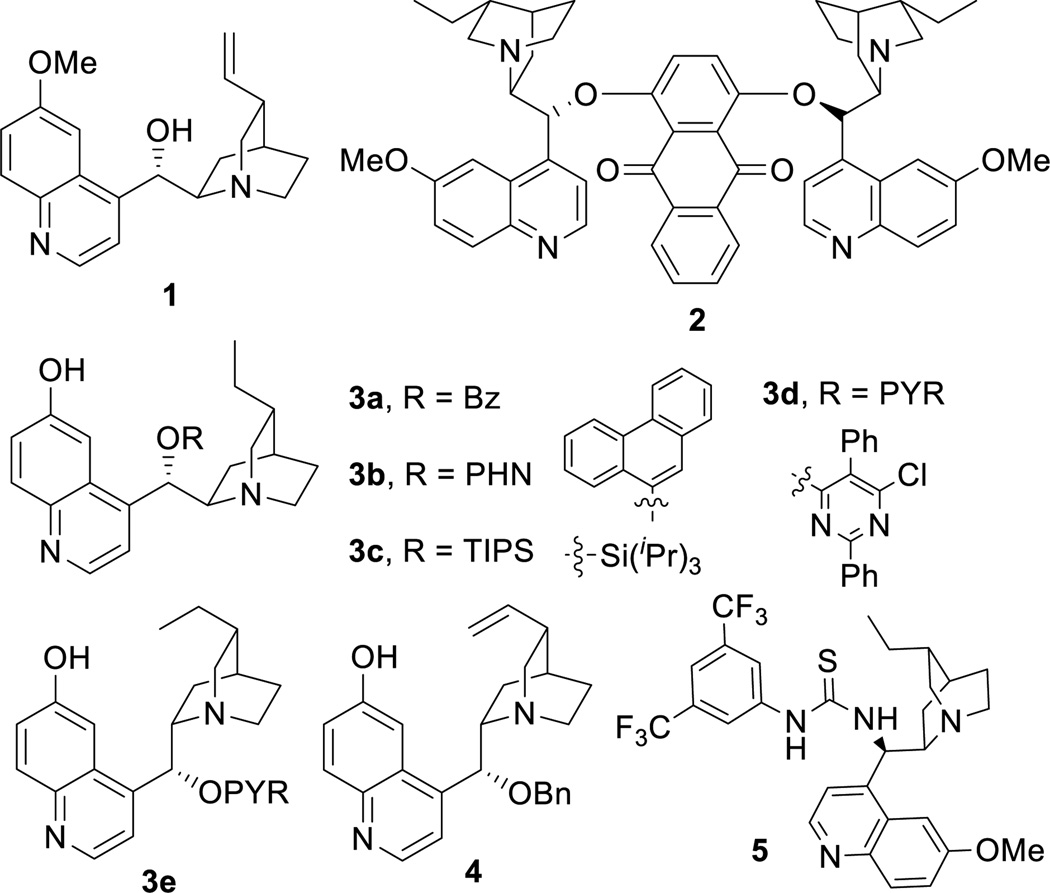
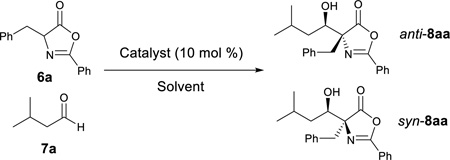
We initiated our study by reacting azlactone 6a and aldehyde 7a in the presence of a stoichiometric amount of triethylamine. After considerable experiments, we found that a reaction could be reasonably fast and clean at −20 °C in chloroform. We next investigated the possibility of promoting an asymmetric variant of this reaction with cinchona alkaloid-derived catalysts (Figure 2). Upon first screening of a series cinchona alkaloid derivatives, (entries 1–9, Table 1), we identified the 6’-OH cinchona alkaloid 3d as the most promising catalyst in terms of affording high diastereoselectivity and enantioselectivity (entry 6, Table 1). Catalyst 3e, the pseudo-enantiomer of 3d, gave comparable results with an expected reverse sense of asymmetric induction (entry 7, Table 1). Following these results, we carried out the 3-promoted aldol reaction in a variety of solvents with azlactone 6a at a significantly decreased concentration of 0.5 M (entry 10–15). We found that the reaction at the reduced concentration proceeded in higher diastereo- and enantioselectivity (entry 10 vs. 6). Moreover, the reaction in dichloromethane occurred in a slightly higher diastereoselectivity than and the same enantioselectivity as the reaction in chloroform (entries 11–10, Table 1). Both the diastereoselectivity and enantioselectivity afforded by catalyst 3d could be improved significantly when the reaction was performed at significantly reduced temperature and concentration (entry 16 vs 11), although a higher catalyst loading and an extended reaction time are required for the reaction to proceed to completion. Importantly, under these conditions, a highly diastereoselective and enantioselective aldol reaction was established to generate the desired aldol product 8aa in 92% isolated yield, 94% ee and 97.5/2.5 anti/syn ratio. It should be noted that no product resulted from the self-aldol reaction by aldehyde 7 was detected by NMR analysis.
Figure 2.
Table 1.
Catalytic Asymmetric Aldol Reaction of Azlactone 6a and aldehyde 7aa
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Catalyst | Solvent | Temp(°C) | Time | Conv. (%)c | ee (%)c, d | antilsync |
| 1 | 1 | CHCI3 (2 M) | −20 | 15 h | >95 | 29/22 | 43.5/56.5 |
| 2 | 2 | CHCI3 (2 M) | −20 | 15 h | 93 | 43/12 | 40.5/59.5 |
| 3 | 3a | CHCI3 (2 M) | −20 | 15 h | >95 | 57/25 | 81/19 |
| 4 | 3b | CHCI3 (2 M) | −20 | 15 h | >95 | 51/19 | 73/27 |
| 5 | 3c | CHCI3 (2 M) | −20 | 15 h | 92 | −7/18 | 71/29 |
| 6 | 3d | CHCI3 (2 M) | −20 | 15 h | >95 | 75/13 | 88/12 |
| 7 | 3e | CHCI3 (2 M) | −20 | 15 h | >95 | −73/−16 | 90/10 |
| 8 | 4 | CHCI3 (2 M) | −20 | 15 h | 91 | −64/−6 | 82/18 |
| 9 | 5 | CHCI3 (2 M) | −20 | 15 h | >95 | −71/22 | 66/34 |
| 10 | 3d | CHCI3 (0.5 M) | −20 | 34 h | 95 | 86/−11 | 91/9 |
| 11 | 3d | CH2CI2 (0.5 M) | −20 | 34 h | 93 | 86/−39 | 93.5/6.5 |
| 12 | 3d | PhCH3 (0.5 M) | −20 | 34 h | 80 | 48/−28 | 80/20 |
| 13 | 3d | THF (0.5 M) | −20 | 34 h | >95 | 50/−28 | 79.5/20.5 |
| 14 | 3d | Et2O (0.5 M) | −20 | 34 h | >95 | 53/−28 | 83/17 |
| 15 | 3d | CH3CN (0.5 M) | −20 | 34 h | >95 | 72/−18 | 88/12 |
| 16e, f | 3d | CH2CI2 (0.1 M) | −50 | 88 h | >95 (92)b | 94/ND | 97.5/2.5 |
Reactions were carried out with 0.1 mmol of 6a and 0.15 mmol of 7a.
Isolated yield.
Determined by chiral HPLC analysis.
ee (antilsyn).
10 mg of 4Å molecular sieves were added.
15 mol% 3d
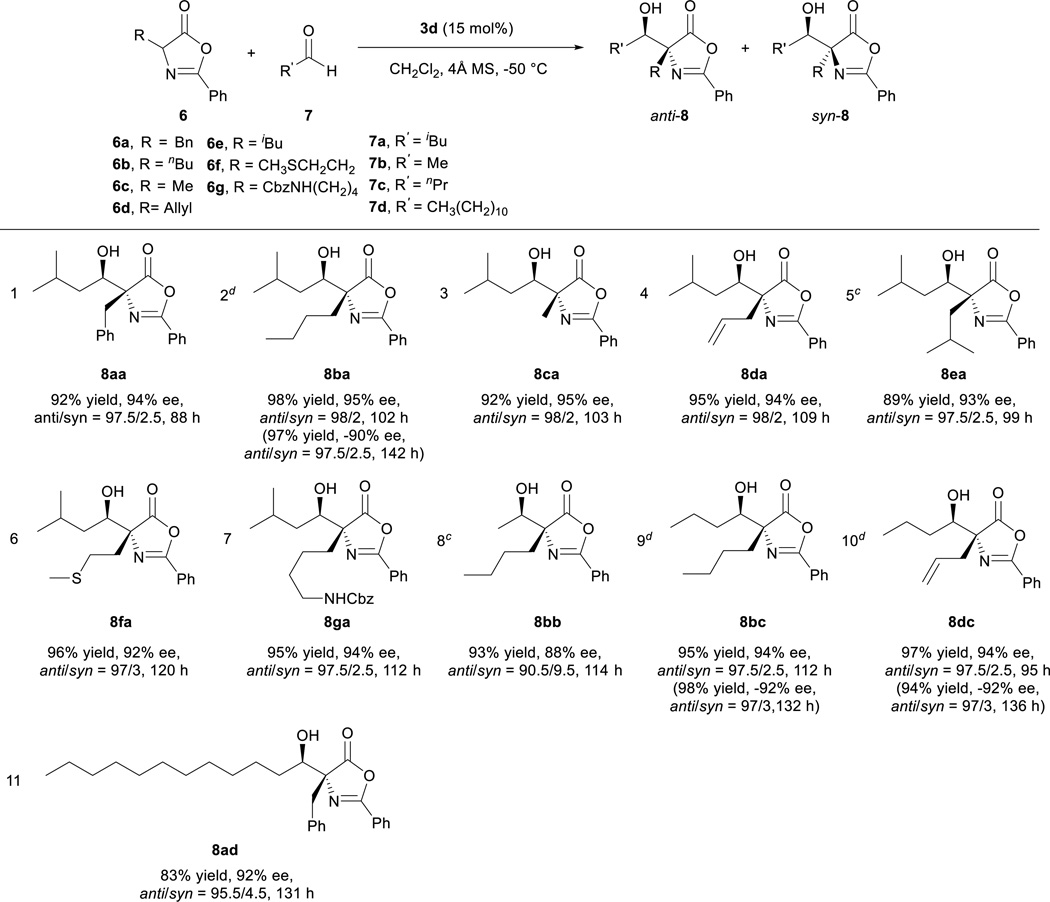
Applying the optimized reaction conditions for the model reaction we investigated the substrate scope of this asymmetric aldol reaction (Table 2). The reactions of aldehyde 7a and azlactones 6a–g bearing different α-alkyl substituents gave consistently excellent yields, enantioselectivity and anti-selective diastereoselectivity (entries 1–7, Table 2). The catalyst could also accommodate variations in aliphatic aldehydes as shown by its high efficiency in the promotion of asymmetric aldol reactions involving a series of aliphatic aldehydes (entries 8–11, Table 2). The tolerance of aldehyde 7d, which bears a linear C12 alkyl chain, is noteworthy. With catalyst 3e, the reaction provide equally efficient access to the other enantiomer of the aldol product, as shown in the formation of aldol adduct ent-8ba, ent-8bc and ent-8dc (entries 2, 9, 10, Table 2). As detailed in the supporting information, the relative and absolute configurations of aldol products 8 were determined by 1D NOESY experiment and a modified Mosher’s method, respectively16.
Table 2.
Unless noted, reactions were carried out with 0.1 mmol of 6, 0.15 mmol of 7, 0.015 mmol of 3d, 10 mg of 4Å molecular sleves in 1 mL of dichloromethane.
ee value and anti/syn ratio determined by chiral HPLC analysis.
0.2 mmol of 7b.
Results in parentheses obtained using 3e (15 mol%) as catalyst.
See Supporting Information for determination of relative and absolute configurations.
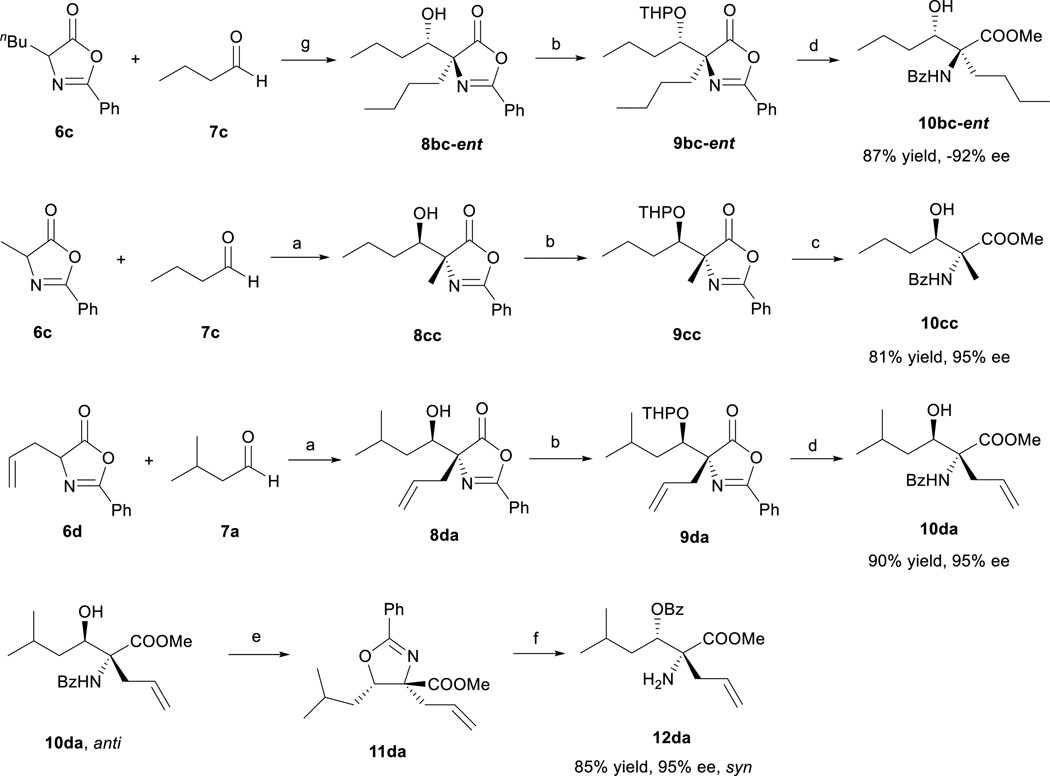
To demonstrate the potential synthetic utility of the chiral aldol adduct 8, ring opening transformations converting 8 into useful β-hydroxy-α-amino acid derivative 10 must be developed. We found that 8 were liable toward retro-aldol initiated decompositions under a variety of reaction conditions. After extensive experimental explorations, we were able to establish a high yield, three-step protocol to convert 8 into β-hydroxy-α-aminoester 10 (Scheme 2). Critical to the development of this useful conversion was the experimental discovery that the THP protected β-hydroxy-α-alkylazlactones 9, unlike 8, is inert toward retro-aldol decompositions17. It should be noted that the four-step enantioselective preparations of β-hydroxy-α-aminoester 10 from azlactones 6 and aldehydes 7 require only a single purification for the isolation of 10, both intermediates 8 and 9 were used for the next step without subjecting to purifications. To establish enantioselective access to all four stereoisomers of β-hydroxy-α-amino acid derivative 10, we developed a one-pot conversion of anti β-hydroxy-α-amino acid 10da into the corresponding syn β-hydroxy-α-amino acid syn-12da involving the treatment of anti-10da with thionyl chloride followed by HCl in THF (Scheme 2).
Scheme 2.
Transformation of aldol product 8a
aReagents and conditions: (a) 3d (15 mol%), CH2Cl2, 4Å MS, −50°C; (b) PPTS, DHP, CH2Cl2, rt; then K2CO3, Na2SO4, MeOH, rt; (c) 2N HCl, MeOH, rt; (d) HCl in MeOH (~1.25 M), rt; (e) SOCl2, THF, rt; (f) 2N HCl, THF, rt; (g) 3e (15 mol%), CH2Cl2, 4Å MS, −50°. PPTS = pyridinium p-toluenesulfonate; DHP = 3,4-dihydro-2H-pyran
Conclusions
In summary, we have developed a highly enantioselective and diastereoselective direct aldol reaction of α-alkyl azlactones with aliphatic aldehydes catalyzed by cinchona alkaloid catalysts 3d and 3e. To our knowledge, this is the first efficient asymmetric direct aldol reaction of azlactones and aliphatic aldehydes. Providing an efficient catalytic asymmetric access to β-hydroxy-α-amino acids bearing alkyl substituents at both the tertiary β-stereocenter and the quaternary α-stereocenter, this new catalytic asymmetric aldol reaction should find applications in natural product synthesis and medicinal chemistry18.
Supplementary Material
Acknowledgements
We are grateful for the generous financial support from National Institutes of General Medical Sciences (GM-61591).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures and characterization for new compounds are provided. See DOI: 10.1039/c000000x/
Notes and references
- 1.(a) Williams DH. Acc. Chem. Res. 1984;17:364. [Google Scholar]; (b) Harris CM, Kopecka H, Harris TM. J. Am. Chem. Soc. 1983;105:6915. [Google Scholar]; (c) Nagarajan R, Schabel AA, Occolowitz JL, Counter FT, Ott JL. J. Antibiot. 1988;41:1431. doi: 10.7164/antibiotics.41.1430. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kato T, Hinoo H, Terui Y, Kikuchi J, Shoji J. J. Antibiot. 1988;41:719. doi: 10.7164/antibiotics.41.719. [DOI] [PubMed] [Google Scholar]; (b) Shoji J, Hinoo H, Matsumoto K, Hattori T, Yoshida T, Matsuura S, Kondo E. J. Antibiot. 1988;41:713. doi: 10.7164/antibiotics.41.713. [DOI] [PubMed] [Google Scholar]; (c) Carr SA, Block E, Costello CE. J. Org. Chem. 1985;50:2854. [Google Scholar]
- 3.(a) Schreiber SL. Science. 1991;251:283. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]; (b) Evans DA, Weber AE. J. Am. Chem. Soc. 1986;108:6757. [Google Scholar]
- 4.(a) Fujita T, Inoue K, Yamamoto S, Ikumoto T, Sasaki S, Toyama R, Chiba K, Hoshino Y, Okumoto T. J. Antibiot. 1994;47:208. doi: 10.7164/antibiotics.47.208. [DOI] [PubMed] [Google Scholar]; (b) Fujita T, Inoue K, Yamamoto S, Ikumoto T, Sasaki S, Toyama R, Chiba K, Hoshino Y, Okumoto T. J. Antibiot. 1994;47:216. doi: 10.7164/antibiotics.47.216. [DOI] [PubMed] [Google Scholar]; (c) Sasaki S, Hashimoto R, Kiuchi M, Inoue K, Ikumoto T, Hirose R, Chiba K, Hoshino Y, Okumoto T, Fujita T. J. Antibiot. 1994;47:420. doi: 10.7164/antibiotics.47.420. [DOI] [PubMed] [Google Scholar]; (d) Fujita T, Hamamichi N, Kiuchi M, Matsuzaki T, Kitao Y, Inoue K, Hirose R, Yoneta M, Sasaki S, Chiba K. J. Antibiot. 1996;49:846. doi: 10.7164/antibiotics.49.846. [DOI] [PubMed] [Google Scholar]
- 5.(a) Lotz BT, Miller J. J. Org. Chem. 1993;58:618. [Google Scholar]; (b) Miller MJ. Acc. Chem. Res. 1986;19:49. [Google Scholar]
- 6.Pansare SV, Vederas JC. J. Org. Chem. 1987;52:4804. [Google Scholar]
- 7.Tanner D. Angew. Chem., Int. Ed. 1994;33:599. [Google Scholar]
- 8.For selected examples, see: Ito Y, Sawamura M, Hayashi T. J. Am. Chem. Soc. 1986;108:6405. Ito Y, Sawamura M, Shirakawa E, Hayashizaki K, Hayashi T. Tetrahedron. 1988;44:5253. Evans DA, Janey JM, Magomedov N, Tedrow JS. Angew. Chem., Int. Ed. 2001;40:1884. Kobayashi J, Nakamura M, Mori Y, Yamashita Y, Kobayashi S. J. Am. Chem. Soc. 2004;126:9192. doi: 10.1021/ja047597t. Willis MC, Cutting GA, Piccio VJ-D, Durbin MJ, John MP. Angew. Chem., Int. Ed. 2005;44:1543. doi: 10.1002/anie.200462125. Sladojevich F, Trabocchi A, Guarna A, Dixon DJ. J. Am. Chem. Soc. 2011;133:1710. doi: 10.1021/ja110534g. Yoshino T, Morimoto H, Lu G, Matsunaga S, Shibasaki M. J. Am. Chem. Soc. 2009;131:17082. doi: 10.1021/ja908571w. Trost BM, Miege F. J. Am. Chem. Soc. 2014;136:3016. doi: 10.1021/ja4129394.
- 9.For selected examples, see: Horikawa M, Busch-Peterson J, Corey EJ. Tetrahedron Lett. 1999;40:3843. Ooi T, Taniguchi M, Kameda M, Maruoka K. Angew. Chem., Int. Ed. 2002;41:4542. doi: 10.1002/1521-3773(20021202)41:23<4542::AID-ANIE4542>3.0.CO;2-3. Ooi T, Kameda M, Taniguchi M, Maruoka K. J. Am. Chem. Soc. 2004;126:9685. doi: 10.1021/ja048865q. Thayumanavan R, Tanaka F, Barbas CF., III Org. Lett. 2004;6:3541. doi: 10.1021/ol0485417. Li L, Klauber KG, Seidel D. J. Am. Chem. Soc. 2008;130:12248. doi: 10.1021/ja804838y. Chen W-B, Wu Z-J, Hu J, Cun L-F, Zhang X-M, Yuan W-C. Org. Lett. 2011;13:2472. doi: 10.1021/ol200724q.
- 10.For selected examples, see: Vassilev VP, Uchiyama T, Kajimoto T, Wong C-H. Tetrahedron Lett. 1995;36:4081. Kimura T, Vassilev VP, Shen G-J, Wong C-H. J. Am. Chem. Soc. 1997;119:11734. Gutierrez ML, Garrabou X, Agosta E, Servi S, Parella T, Joglar J, Clapés P. Chem. Eur. J. 2008;14:4647. doi: 10.1002/chem.200800031. Hernandez K, Zelen I, Petrillo G, Usón I, Wandtke CM, Bujons J, Joglar J, Parella T, Clapés P. Angew. Chem., Int. Ed. 2015;54:3013–3017. doi: 10.1002/anie.201411484.
- 11.For selected examples, see: Tao B, Schlingloff G, Sharpless KB. Tetrahedron Lett. 1998;39:2507. Morgan A, Masse CE, Panek JS. Org. Lett. 1999;1:1949. doi: 10.1021/ol9903032. Park H, Cao B, Joullié MM. J. Org. Chem. 2001;66:7223. doi: 10.1021/jo010482c.
- 12.For selected examples, see: Noyori R, Ikeda T, Ohkuma T, Widhalm M, Kitamura M, Takaya H, Akutagawa S, Sayo N, Saito T, Taketomi T, Kumobayashi H. J. Am. Chem. Soc. 1989;111:9134. Kuwano R, Okuda S, Ito Y. J. Org. Chem. 1998;63:3499. Mordant C, Dünkelmann P, Ratovelomanana-Vidal V, Genet JP. Eur. J. Org. Chem. 2004:3017. doi: 10.1039/b401631a. Mordant C, Dünkelmann P, Ratovelomanana-Vidal V, Genet JP. Chem. Commun. 2004:1296. doi: 10.1039/b401631a. Makino K, Goto T, Hiroki Y, Hamada Y. Angew. Chem., Int. Ed. 2004;43:882. doi: 10.1002/anie.200353072. Makino K, Hiroki Y, Hamada Y. J. Am. Chem. Soc. 2005;127:5784. doi: 10.1021/ja0432113.
- 13.(a) Trost BM, Ariza X. Angew. Chem., Int. Ed. 1997;36:2635. [Google Scholar]; (b) Trost BM, Lee CB. J. Am. Chem. Soc. 1998;120:6818. [Google Scholar]; (c) Trost BM, Lee CB. J. Am. Chem. Soc. 2001;123:12191. doi: 10.1021/ja0118338. [DOI] [PubMed] [Google Scholar]
- 14.Terada M, Tanaka H, Sorimachi K. J. Am. Chem. Soc. 2009;131:3430. doi: 10.1021/ja8090643. [DOI] [PubMed] [Google Scholar]
- 15.For total synthesis of Mycestericin D, E, F and G, see: Shibata K, Shingu K, Vassilev VP, Nishide K, Fujita T, Node M, Kajimoto T, Wong C-H. Tetrahedron Lett. 1996;37:2791. Fujita T, Hamamichi N, Matsuzaki T, Kitao Y, Kiuchi M, Node M, Hirose R. Tetrahedron Lett. 1995;36:8599. Nishide K, Shibata K, Fujita T, Kajimoto T, Wong C-H, Node M. Heterocycles. 2000;52:1191. Iwabuchi Y, Furukawa M, Esumi T, Hatakeyama S. Chem. Comm. 2001:2030. doi: 10.1039/b106471c. Berhal L, Takechi S, Kumagai N, Shibasaki M. Chem. Eur. J. 2011;17:1915. doi: 10.1002/chem.201002874. Fairhurst NWG, Mahon MF, Munday H R, Carbery DR. Org. Lett. 2012;14:756. doi: 10.1021/ol203300k.
- 16.Please see Supporting Information for details.
- 17.Miyashita M, Yoshikoshi A, Grieco PA. J. Org. Chem. 1977;42:3772. [Google Scholar]
- 18.Strader CR, Pearce CJ, Oberlies NH. J. Nat. Prod. 2011;74:900. doi: 10.1021/np2000528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.