Abstract
Compensation is a critical process for the unbiased analysis of flow cytometry data. Numerous compensation strategies exist, including the use of bead-based products. The purpose of this study was to determine whether beads, specifically polystyrene microspheres (PSMS) compare to the use of primary leukocytes for single color based compensation when conducting polychromatic flow cytometry. To do so, we stained individual tubes of both PSMS and leukocytes with panel specific antibodies conjugated to fluorochromes corresponding to fluorescent channels FL1-FL10. We compared the matrix generated by PSMS to that generated using peripheral blood mononuclear cells (PBMC). Ideal for compensation is a sample with a discrete negative and brightly positive population. We demonstrate that PSMS display autofluorescence properties similar to PBMC. When comparing PSMS to PBMC for compensation PSMS yielded more evenly distributed and discrete negative and positive populations to use for compensation. We analyzed three donors’ PBMC stained with our ten-color T cell subpopulation panel using compensation generated by PSMS vs. PBMC and detected no significant differences in the population distribution. Panel specific antibodies bound to PSMS represent an invaluable valid tool to generate suitable compensation matrices especially when sample material is limited and/or the sample requires analysis of dynamically modulated or rare events.
Keywords: Compensation, Polystyrene Microspheres, Flow Cytometry, Immunophenotyping, Polychromatic, Beads, Leukocytes
INTRODUCTION
With the advent of fluorescein and rhodamine centered two-color flow cytometry, eliminating spectral overlap using compensation has become a necessary requirement for data analysis(1). Perhaps the most well-known two-color flow cytometry analysis requiring compensation is fluorescein isothiocyanate (FITC) and phycoerythrin (PE). As the number of fluorochromes used in multi-color flow cytometry increases, so does the need for appropriate compensation (2-5). Consequently, a multitude of theories and strategies exist for suitable compensation (6-10). Of consensus is that single color compensation controls are required for experimental setup (11,12). Single color compensation that hinges upon the use of cells from sample material can be problematic as more often than not sample material may be limited and heterogeneous. Importantly, cells display a wide variance in background fluorescence (13). Widely accepted is the use of beads coated with antibody capture sites. This allows for a bright binding of even the most dynamically regulated antigens. Beads have a smaller error in their distribution of background fluorescence, allowing for precise spillover computation. However, many commercially available beads introduce false negative background and do not recognize a diverse amount of host isotypes (14).
Polystyrene microspheres (PSMS) are antibody-capture beads made of polystyrene, a petroleum based plastic made of the monomer styrene. PSMS are 3.0-3.4 microns in size. Recognizing all mouse and rat isotypes, most hamster isotypes, and rabbit polyclonal IgG, PSMS can be used for single color compensation in situations where cell samples are limited. Negative, uncoated PSMS provide background fluorescence similar to unstained cells across the different excitation/emission wavelength combinations. Many clinical studies use precious sample material as single color controls for compensation such that their consumption in compensation could limit the analytical power of flow cytometry. We sought to determine whether PSMS can be used as a substitute for cells for compensation of spectral overlap of ten fluorochromes in flow cytometry analysis. To do so we compared single color controls from PSMS incubated with antibodies from our ten color panel to single color controls from primary human leukocytes (peripheral blood mononuclear cells, PBMC) incubated with the same antibodies. We then applied the compensation matrices generated from PSMS and PBMC, respectively to three donors’ PBMC.
MATERIALS AND METHODS
Experiment overview
In this work we compare PSMS to cells to be used for compensation for Immunophenotyping of primary human leukocytes in a clinical study. We designed in our lab a T cell subpopulation panel that would allow us to stratify T cell subsets based on cell surface markers to be used ultimately for sorting and RNA extraction. We designed this panel based on the following parameters: antigens of interest, antibody with conjugated fluorochrome availability and the expected brightness/frequency of antigen on the target cell population. As this panel will ultimately be used for patient material, specifically PBMC and tumor-infiltrating lymphocytes, it was important that we develop a system that will limit the use of our sample material.
Flow sample and specimen description
T cell subpopulation panel
Cell surface markers were chosen based on their ability to discriminate subsets in the T cell population of humans. The panel consists of the following antibodies: TCRαβ FITC (Becton Dickinson, Franklin Lakes, NJ), TCRγδ PE (Becton Dickinson, Franklin Lakes, NJ), CD25 ECD (IOTest, Beckman Coulter, Brea, CA), CD4 PERCP (Becton Dickinson, Franklin Lakes, NJ), CCR6 PE-Cy7 (Biolegend, San Diego, CA), CD45RO APC (Becton Dickinson, Franklin Lakes, NJ), IL-23R AF700 (R&D Systems, Minneapolis, MN), CD3 APC-AF750 (IOTest, Beckman Coulter, Brea, CA), CD8 Pacific Blue (IOTest, Beckman Coulter, Brea, CA), or CD45 Krome Orange (IOTest, Beckman Coulter, Brea, CA), corresponding to fluorescent channels FL1-FL10, respectively, for the generation of a compensation matrix.
Polystyrene microspheres preparation and staining
Each bottle of PSMS (VersaComp Antibody capture beads, Beckman Coulter, Brea, CA) contains approximately 10 × 106 PSMS/ml. To generate single color controls one 50ul drop (5 × 105) of coated PSMS and one 50ul drop (5 × 105) of uncoated PSMS for a total of 1×106 PSMS were added per tube. Each tube was stained per manufacturer’s recommendation for ≤ 1×106 cells with a different antibody from the T cell subpopulation panel for twenty minutes at room temperature in the dark. Tubes containing PSMS were washed in two milliliters of phosphate buffered saline (PBS; Sigma-Aldrich, St Louis, MO) containing 1% fetal calf serum (FCS; HyCloneTM, Thermo Scientific, Logan, UT) and centrifuged at 400g for five minutes. Excess buffer was then decanted leaving the PSMS pellet in a residual volume of 100 microliters. Tubes were briefly vortexed and then assayed for expression of cell surface marker antibodies.
PBMC preparation and staining
PBMC were isolated from the donors’ blood using a density gradient medium (Lymphoprep; Greiner Bio-One, NC). After which the cells were washed in PBS (PBS; Sigma-Aldrich, St Louis, MO) and frozen in medium containing 50% FCS (HyClone™, Thermo Scientific, Logan, UT), 40% RPMI 1640 (Sigma-Aldrich, St Louis, MO) and 10% DMSO and placed at −80 C in a Nalgene “Mr. Frosty” freezing container (Thermo Scientific, Waltham, MA). Prior to staining, cells were thawed in a 37°C water bath, followed by two washes with PBS for 5min at 400g. Cells were then aliquotted into 1×106 cells per condition. PBMC were prepared and stained individually (single color tubes) with antibodies from the T cell subpopulation panel as per manufacturer’s test recommendations.
Instrument and data analysis details
Flow Cytometry Analyzer and Data Analysis Software
All samples were run on the Gallios Flow Cytometer with the following 3 laser, 10 color configuration: Blue (488nm laser) – fluorescent channel 1 (FL1): 525/30 band pass filter/window width (BP), FL2: 575/30 BP, FL3: 620/30 BP, FL4: 670 long pass filter (LP), FL5: 775 LP, Red (638nm laser) - FL6: 660/20 BP, FL7: 725/20 BP, FL8: 775 LP and Violet (405nm Laser) - FL9: 450/50 BP, FL10: 550/40 BP, Beckman Coulter, Brea, CA). All samples were run with the same voltage settings. PSMS were identified on the basis of forward and side scatter area (FSC-A and SSC-A), FSC-A vs time of flight (singlet identification) and back-gating for the antigen of interest for each channel. PBMC were identified based on back-gating of the CD45 and CD3 positive cells with intermediate size (FSC-A) and low granularity (SSC-A). Upon selection of these gates the rest of the data were then analyzed post-acquisition using the Kaluza software (Beckman Coulter, Brea, CA). The two compensation matrices generated from 1) PSMS and individual T cell subpopulation markers antibodies and 2) PBMC and individual T cell subpopulation markers were applied post-acquisition in Kaluza on three donors’ PBMC stained with all of the antibodies in the T cell subpopulation panel.
Statistical analysis
Percent gated, median and standard deviation were calculated using Kaluza. Statistical analysis was performed with GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla California USA, www.graphpad.com). Differences were tested by Student’s t-test as appropriate. All reported P values are two-tailed.
RESULTS
PSMS share similar physical properties to PBMC
In order to investigate whether the use of PSMS would be capable of replacing cell sample for single color compensation setup, it was necessary to characterize the microspheres. PSMS were prepared and stained with single color conjugates reflecting the markers of interest in the T cell subpopulation panel, specifically: TCRαβ FITC, TCRγδ PE, CD25 ECD, CD4 PERCP, CCR6 PECy7, CD45RO APC, IL-23R AF700, CD3 APC-AF750, CD8 Pacific Blue, and CD45 Krome Orange. Finally, PBMC were prepared and stained with individual markers of the T cell subpopulation panel. Using the same cytometer voltages, compensation was manually determined stepwise moving from FL1-FL10 on a Gallios 3 laser/10 detector flow cytometer for PSMS stained with markers in the T cell subpopulation panel. Compensation was then cleared and manually optimized using PBMC stained with individual markers in the T cell subpopulation panel. In particular, we compared each fluorescent channel pairwise to the nine other channels. Upon analyzing the bivariate plots of the fluorochrome combinations x and y medians were adjusted such that they were on average within +/− 0.2 standard deviations.
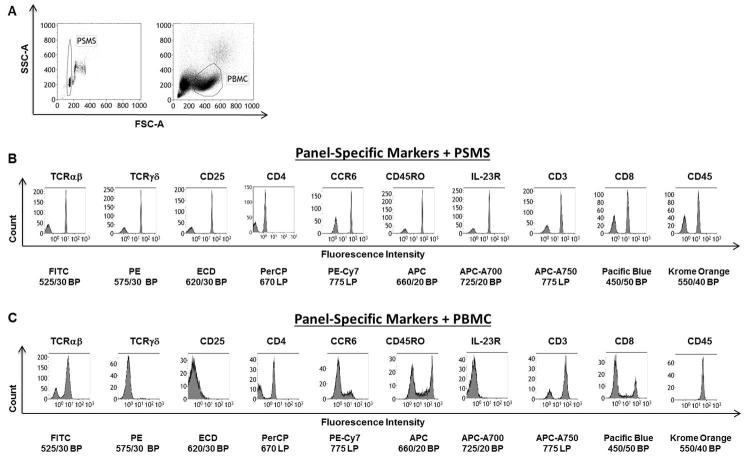
PSMS can be located using the same parameters as PBMC. The same photomultiplier tube (PMT) voltages were kept constant. 3-3.4 microns in size PSMS are just smaller than lymphocytes (4-10 microns) (15). Figure 1A shows the results of ungated acquisition of PSMS and PBMC using the same settings. Aggregated PSMS and dead cells and debris respectively were excluded on the basis of forward and side scatter (Figure 1A).
Figure 1.
PSMS yield more discrete negative and positive populations across all ten fluorescent channels
Figure 1B displays single parameter histograms yielded from analysis of PSMS stained with panel antibodies. The same is shown for PBMC (Figure 1C). Based on these single parameter histograms plots percent negative and percent positive populations were selected for each fluorescent channel. The exact regions included to make these calculations along with representative data can be found in supplementary figures 1 and 2, for PSMS and PBMC, respectively. The percent gated are summarized in Table 1. On average PSMS possessed more even distribution of percent positive and negative events as coated and uncoated PSMS respectively ensured the presence of both populations. Compensating using PBMC was more challenging due to the dynamic range of expression of the antigen on PBMC. This was especially true for staining corresponding to fluorescent channels FL2 (ECD: 620/30 BP), 3 (PE 575/30 BP), and 7 (APC-A700 725/20 BP), corresponding to the antibodies TCRγδ PE, CD25 ECD, IL-23R AF700. Gamma delta T cells represent a very small fraction of the PBMC population; hence identifying a population to use for compensation can be difficult. This is amplified when dealing with hard to obtain specimens for which sample is limited. Markers CD25 (FL3) and IL-23R (FL7) displayed no clear degree of separation between positive and negative cells, but instead have a gradient of expression (Figure 1C). These are extremely difficult to compensate as one may not be able to determine whether such a result is due to poor titration/antibody function or if the sample being used to set up compensation is dim/negative for that particular antigen. On the contrary, PSMS stained with the same antibodies consistently yielded discrete positive and negative positive populations with high enough frequency to be readily used for compensation (Figure 1B, Table 1).
Table 1.
Population distribution PSMS vs. PBMC
| PSMS | PBMC | |||
|---|---|---|---|---|
| % negative | % positive | % negative | % positive | |
| FL1: FITC 525/30 BP | 44.76 | 55.24 | 21.05 | 78.95 |
| FL2: PE 575/30 BP | 38.69 | 61.31 | 98.58 | 1.42 |
| FL3: ECD 620/30 BP | 41.5 | 58.5 | 98.59 | 1.41 |
| FL4: PERCP 670 LP | 38.61 | 61.39 | 50.24 | 49.76 |
| FL5: PE-Cy7 775 LP | 49.15 | 50.85 | 77.9 | 22.1 |
| FL6: APC 660/20 BP | 30.21 | 69.79 | 48.41 | 51.59 |
| FL7: APC-A700 725/20 BP | 31.76 | 68.24 | 99.68 | 0.31 |
| FL8: APC-A750 775 LP | 34.97 | 65.03 | 21.19 | 78.83 |
| FL9: PacBlue 450/50 BP | 41.35 | 58.65 | 69.3 | 30.62 |
| FL10: Krome Orange 550/40 BP | 38.94 | 61.06 | 0.27 | 99.73 |
Background autofluorescence of PSMS are comparable to PBMC
Next we compared the autofluorescent properties of PSMS when compared to PBMC (Table 2). To assess this we calculated the median of the negative population of univariate histogram plots for each of the fluorescent channels. The geometric mean of fluorescence intensity (MFI) is often used as a representative number for a population of cells. However, median provides a more accurate reflection of the fluorescent intensity of a population as a whole as most flow cytometry data is not normally distributed. Hence we chose the median fluorescence intensity (MedFI) as a way to quantify autofluorescence. For PSMS the autofluorescence of the negative population for PSMS ranged from 0.14-2.05. PBMC MedFI ranged from 0.12-3.13. Paired two tailed T test indicated that there was no significant difference between the autofluorescence between PSMS and PBMC for FL1- FL10 (Table 2). With a range equivalent to or lower than PBMC, PSMS do not contribute to the compensation spillover calculation; allowing one to apply the compensation matrix to PBMC.
Table 2.
Autofluorescence of PSMS vs. PBMC
| X Median of % negative | ||
|---|---|---|
| PSMS | PBMC | |
| FL1: FITC 525/30 BP | 0.23 | 0.52 |
| FL2: PE 575/30 BP | 0.76 | 0.93 |
| FL3: ECD 620/30 BP | 0.29 | 0.28 |
| FL4: PERCP 670 LP | 0.14 | 0.12 |
| FL5: PE-Cy7 775 LP | 2.05 | 1.14 |
| FL6: APC 660/20 BP | 1.35 | 3.13 |
| FL7: APC-A700 725/20 BP | 0.77 | 0.46 |
| FL8: APC-A750 775 LP | 1.59 | 1.97 |
| FL9: Pacific Blue 450/50 BP | 0.73 | 0.84 |
| FL10: Krome Orange 550/40 BP | 0.45 | 1.62 |
Lastly before applying our matrices to three donors for analysis we assessed a parameter referred to as the stain index. Stain index (SI) takes into account the spread of the percent negative histogram peak, unlike simpler calculations like Signal: Noise (S: N) that just look at the fold increase of positive over negative. The formula for SI is the difference between the X median of the positive and negative population peaks on a histogram plot divided by two times the standard deviation of the negative peak. On average the stain index was higher for PSMS compared to PBMC (Table 3). The one exception was FL4, corresponding to the dim fluorochrome PerCP (Table 3).
Table 3.
Stain index (SI)
| PSMS | PBMC | |
|---|---|---|
| FL1: FITC 525/30 BP | 50.8 | 14.1 |
| FL2: PE 575/30 BP | 44.4 | 22.3 |
| FL3: ECD 620/30 BP | 78.4 | 4.3 |
| FL4: PERCP 670 LP | 1.4 | 18.3 |
| FL5: PE-Cy7 775 LP | 51.2 | 10.1 |
| FL6: APC 660/20 BP | 61.4 | 49.2 |
| FL7: APC-A700 725/20 BP | 38.9 | 9.0 |
| FL8: APC-A750 775 LP | 28.0 | 40.3 |
| FL9: Pacific Blue 450/50 BP | 28.9 | 89.9 |
| FL10: Krome Orange 550/40 BP | 20.1 | 24.8 |
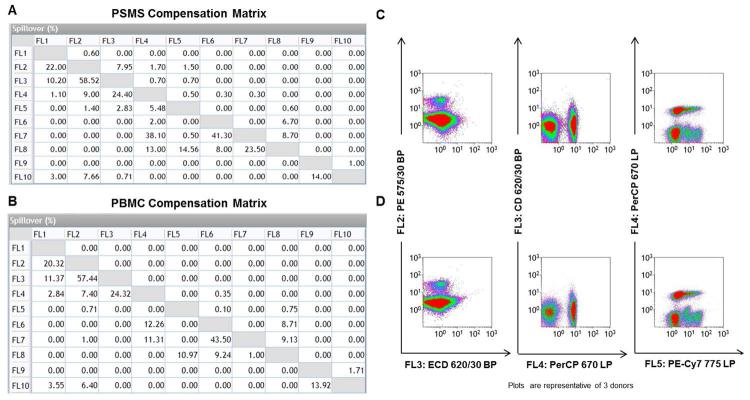
PSMS vs PBMC yield two different compensation matrices
As a result of the two compensation series, two separate compensation matrices were generated (Figure 2A & 2B). There were minimal differences in compensation between the two matrices. The highest compensation variability often occurs between these pairs: FL2 (PE) vs. FL3 (ECD), FL3 (ECD) vs. FL4 (PerCP), and FL4 (PerCP) vs. FL5 (PE-Cy7). In order to better understand the differences in these compensations matrices, a sample of PBMC were processed and stained with all the antibodies in the T cell subpopulation panel. The representative dot plots of PBMC stained with the multi-color T cell subpopulation panel using the Kaluza® software are shown in figures 2C and 2D.
Figure 2.
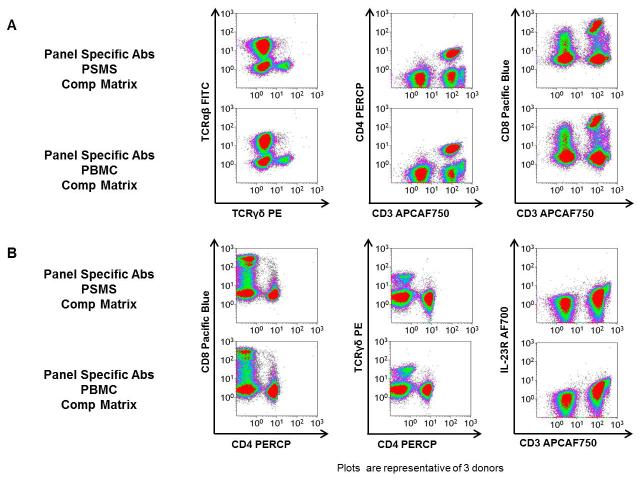
PSMS are able to effectively compensate data from three donors stained with our Ten-color T cell subpopulation panel
We next analyzed the effect these two different compensation matrices had on a variety of T cell subpopulations. After exhaustive study over the entirety of the T cell subpopulation panel, no obvious differences were found regardless of which compensation matrix was applied to the PBMC stained with the multi-color T cell subpopulation panel. In Figure 3, the following cell populations were evaluated: TCRαβ, TCRγδ, CD4 T cells, CD8 T cells, and IL-23R+ CD3+ cells (Figures 3A & 3B). Regardless of which series of compensation tubes were used for single color setup, the PBMC stained with the T cell subpopulation displayed no evidence of significant over- or under-compensation.
Figure 3.
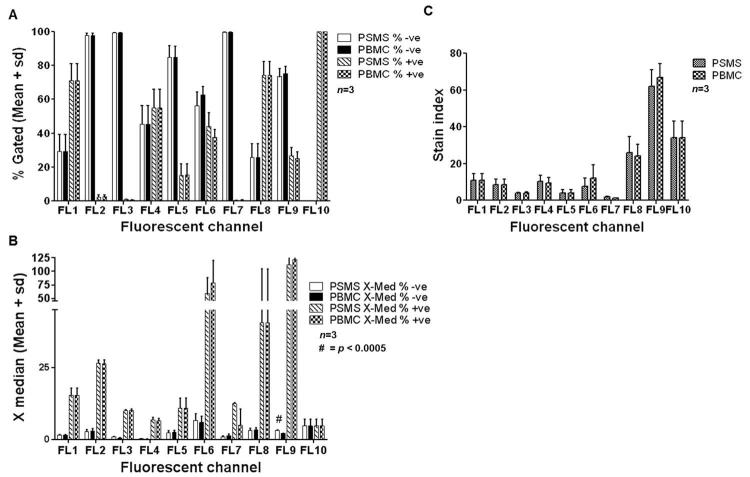
To determine the effects of compensating with PSMS versus PBMC we studied three donors in which we compared the data on a global scale looking at the percent negative and positive populations (Figure 4A), the MedFI of both of these populations (Figure 4B) and stain index (Figure 4C). For all three parameters (autofluorescence, population distribution and stain index) no major significant differences were detected when using either PSMS or PBMC compensation matrices (Figure 4A - 4C). The only significant difference detected was the X MedFI of the negative fraction of FL9 (Pacific Blue 450/50 BP), on average the X MedFI of data using the PBMC based compensation was 1.07 lower than data compensated using PSMS. While this difference was observed it is important to note that this difference did not affect the population distribution of the sample set (Figure 4A). There were large differences in the standard deviation of FL6 (APC 660/20 BP) and FL8 (Pacific Blue 450/50 BP) MedFI of the positive cell population. However, this result was independent of the compensation used and reflects donor variability. Perhaps the most striking difference between PSMS and PBMC compensation matrices were between FL7 (APC-A700 725/20 BP) and FL8 (APC-A750 775 LP). This is important to note as FL7 corresponds to IL23R APC-A700 staining. This staining using PBMC did not yield a distinct positive and negative fraction thus making compensation difficult.
Figure 4.
PSMS bind intracellular staining antibodies as well as isotypes from different species
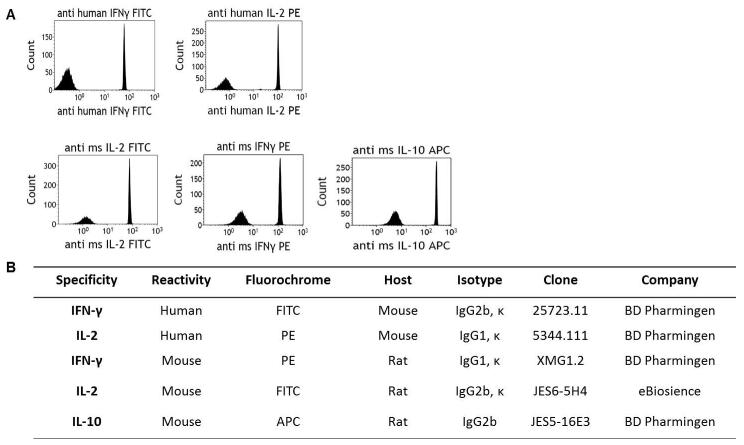
While we focused on cell surface markers for our T cell subpopulation panel, we also tested the ability of PSMS to bind antibodies designed for intracellular staining as this could serve as another useful tool for PSMS. Intracellular staining requires permeabilization of the cell membrane and often 4 hrs. of stimulation for detection. We stained PSMS with both human and murine antibodies against the immunomodulatory cytokines, IFN-gamma, IL-2 and IL-10 (Figure 5A). The antibodies used consisted of both different host species as well as isotypes (Figure 5B). Results demonstrate that PSMS were able to bind all of these antibodies and serve as a compensation option not only for rare samples and dynamically expressed events, but also in lieu of intracellular stained cells (Figure 5A).
Figure 5.
DISCUSSION
Our findings demonstrate that PSMS closely reflect the background auto-fluorescence of primary human leukocytes. As seen in Figure 1, PSMS and PBMC had a similarly defined negative peak. Secondly, utilizing panel-specific antibodies is appropriate for staining PSMS. PSMS were able to bind a variety of antibodies with different hosts and isotypes (Supplementary Figure 3). No significant differences the compensation percentages required were similar across both matrices. Furthermore, the two compensation matrices resulted in no obvious over- or under-compensation of critical cell populations. Altogether, we conclude that the use of PSMS is a valid tool for single color compensation setup.
The PSMS used to conduct these experiments (VersaComp antibody capture beads, Beckman Coulter, Brea, CA) are just one example of a number of products on the market. Similar products include the ABC Bead kit and ArC™ Amine Reactive Compensation Bead Kit (Invitrogen) and Ultracomp eBeads (eBioscience) and have previously been used for compensation (16,17).
PSMS similar to all methods are not without concerns for compensation. No method is without limitations. There are inherent differences within the machine and day to day variability which often leads to a spreading effect even in properly compensated data. Further there are interactions that can sometimes not be predicted from single color controls. To address these issues it may be necessary to use fluorescence minus one (FMO) stained cells in order to identify true populations versus artifacts (14,18). PSMS are similar to leukocytes in size. Many bead based platforms and tools for flow cytometry are optimized for leukocytes. When assaying larger cells, such as tumor cells it may be important to use cells initially to define appropriate ranges for PMT voltages and then use these cytometer setting to run the PSMS (14). PSMS tend to stain as a bright as or brighter than the antigen of interest. This is ideal; however this will need to be verified. In cases where PSMS are less bright than cells sample it will need to be initially tested whether this has an effect on compensation before routinely using PSMS. Of particular concern in our panel was the low stain index observed by PSMS bound to CD4 PERCP in FL4: 670 LP. Fortunately this difference did not have a significant impact on the percentages of populations within our sample set (Figure 4A). To test what could be the source of this difference we first examined lot to lot variation. Upon using a new vial and lot of CD4 PerCP (Becton Dickinson, Franklin Lakes, NJ) the stain index increased from 1.4 to 60.7 (Supplementary Figure 4). Other options include using a different fluorochrome. We selected PerCP as it was included as a cocktail mixture in an intracellular staining panel (panel not shown); however PE Cy5.5 is an alternate option which stains more brightly on PSMS (data not shown).
For our ten color T cell subpopulation panel we used only direct flow cytometry in which all antibodies were directly conjugated to a particular fluorochrome. With smaller panels it is possible, with careful selection of species reactivity, order of antibody addition and washes to use indirect flow cytometry staining. Consequently, we decided to test whether PSMS could be used for compensation of samples stained using indirect flow cytometry, in which a primary unconjugated Ab and a secondary fluorochrome conjugated antibody are used. To test this we stained both PSMS and PBMC with either the secondary conjugated antibody, rat anti-mouse IgG1 PE (RAM IgG1 PE, Becton Dickinson, Franklin Lakes, NJ) or mouse (IgG1) anti-human CD28 primary unconjugated antibody (Becton Dickinson, Franklin Lakes, NJ) followed by RAM IgG1 PE secondary antibody. As we expected PSMS were able to bind the secondary alone. We also observed a sharp clean signal from the use of a primary and secondary with our PSMS. This signal as anticipated was amplified when compared to using secondary alone. The MedFI of the %positive peak and SI of PSMS with secondary only or primary and secondary were 129.31, 136.6 and 249.27, 282.3, respectively. As expected our human T cells stained with secondary alone served as a negative control. When using primary and secondary antibodies T cells stained nicely for CD28. More importantly, PSMS stained with primary and secondary antibodies were as bright as T cells (Supplementary Figure 5).
With single color controls recorded compensation can often be applied post-acquisition. A feature which is rarely utilized is the use of auto-compensation to generate a compensation matrix from single color controls to be applied to a data set. We decided to test how the compensation matrices would differ when using the Kaluza 1.2 auto-compensation features (Supplementary Figure 6). We opted for the positive-negative auto-compensation gating setting to conduct our analysis. While the final compensation applied to the mixed tubes from both PSMS and PBMC single color controls were easy to generate the process did require some manual adjustments. In particular it was necessary that we manually adjust the “dim vs. bright” or % negative vs. % positive regions. It is also important to note that while we had difficulty manually compensating the data in FL7: IL23R APC-A700 725/20 BP using PBMC, due to the very small population of IL23R positive cells, auto-compensation values of channels FL7 vs. FL8 were closer to the results we saw when using manual compensation and PSMS (Supplementary Figure 6).
CONCLUSION
Other multi-parameter based cytometry platforms, such as mass cytometry (CyTOF) (19-23) exist. However, to date flow cytometry remains the gold standard for multiparameter single cell analysis. In the clinical setting flow cytometry plays a critical role in immunophenotyping especially in the context of diagnosis of hematological malignancies, such as leukemia and lymphoma. PSMS provide a resource for compensation when it is unfeasible to run single color controls of cell sample. PSMS are a useful tool for rare or dynamically expressed events. Further PSMS can be used to set up compensation for intracellular staining platforms. Bead-based platforms for compensation have been in existence since the late 1990s (18). Perhaps more widespread recognition of such tools will aid in proper compensation in multicolor flow analysis.
Supplementary Material
Acknowledgments
Research was supported by the Alliance for Cancer Gene Therapy (ACGT, Inc), Alex’s Lemonade Stand Pediatric Cancer Foundation (ALSF) and Stand Up To Cancer St. Baldrick1s Pediatric Dream Team Translational Research Grant (SU2C-AACR-DT1113). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. This research is also supported in part by Award Numbers T32GM088129 and 5T32HL092332 from the National Institute Of General Medical Sciences and National Heart, Lung and Blood Institute, respectively. This project was supported by the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the NIH (AI036211, CA125123, and RR024574). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of General Medical Sciences, National Heart, Lung and Blood Institute or the National Institutes of Health. NA, MH and TB have patent applications in the field of T-cell and gene-modified T-cell therapy for cancer. KC and JN, authors listed on this manuscript, are employed by Beckman Coulter, Inc. which supply the polystyrene microspheres used in this manuscript. TB, MH and NA are consumers of Beckman Coulter, Inc. products, which include the polystyrene microspheres. However, neither party has received any direct compensation for the contents of this work.
LITERATURE CITED
- 1.Herzenberg LA, Parks D, Sahaf B, Perez O, Roederer M, Herzenberg LA. The history and future of the fluorescence activated cell sorter and flow cytometry: a view from Stanford. Clin Chem. 2002;48:1819–27. [PubMed] [Google Scholar]
- 2.Stewart CC, Stewart SJ. Four color compensation. Cytometry. 1999;38:161–75. doi: 10.1002/(sici)1097-0320(19990815)38:4<161::aid-cyto3>3.3.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin BE, Baumgarth N, Bigos M, Roederer M, De Rosa SC, Altman JD, Nixon DF, Ottinger J, Oxford C, Evans TG. Nine-color flow cytometry for accurate measurement of T cell subsets and cytokine responses. Part I: Panel design by an empiric approach. Cytometry A. 2008;73A:400–10. doi: 10.1002/cyto.a.20555. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLaughlin BE, Baumgarth N, Bigos M, Roederer M, De Rosa SC, Altman JD, Nixon DF, Ottinger J, Li J, Beckett L. Nine-color flow cytometry for accurate measurement of T cell subsets and cytokine responses. Part II: Panel performance across different instrument platforms. Cytometry A. 2008;73A:411–20. doi: 10.1002/cyto.a.20556. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Njemini R, Onyema OO, Renmans W, Bautmans I, De Waele M, Mets T. Shortcomings in the application of multicolour flow cytometry in lymphocyte subsets enumeration. Scand J Immunol. 2014;79:75–89. doi: 10.1111/sji.12142. [DOI] [PubMed] [Google Scholar]
- 6.Bagwell CB, Adams EG. Fluorescence spectral overlap compensation for any number of flow cytometry parameters. Ann N Y Acad Sci. 1993;677:167–84. doi: 10.1111/j.1749-6632.1993.tb38775.x. [DOI] [PubMed] [Google Scholar]
- 7.Bayer J, Grunwald D, Lambert C, Mayol JF, Maynadie M. Thematic workshop on fluorescence compensation settings in multicolor flow cytometry. Cytometry B Clin Cytom. 2007;72B:8–13. doi: 10.1002/cyto.b.20153. [DOI] [PubMed] [Google Scholar]
- 8.Novo D, Gregori G, Rajwa B. Generalized unmixing model for multispectral flow cytometry utilizing nonsquare compensation matrices. Cytometry A. 2013;83A:508–20. doi: 10.1002/cyto.a.22272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugar IP, Gonzalez-Lergier J, Sealfon SC. Improved compensation in flow cytometry by multivariable optimization. Cytometry A. 2011;79A:356–60. doi: 10.1002/cyto.a.21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Rodijnen NM, Pieters M, Hoop S, Nap M. Data-driven compensation for flow cytometry of solid tissues. Adv Bioinformatics. 2011;2011:184731. doi: 10.1155/2011/184731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tung JW, Heydari K, Tirouvanziam R, Sahaf B, Parks DR, Herzenberg LA, Herzenberg LA. Modern flow cytometry: a practical approach. Clin Lab Med. 2007;27:453–68. v. doi: 10.1016/j.cll.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Rosa SC, Herzenberg LA, Herzenberg LA, Roederer M. 11-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat Med. 2001;7:245–8. doi: 10.1038/84701. [DOI] [PubMed] [Google Scholar]
- 13.Hulspas R, O'Gorman MR, Wood BL, Gratama JW, Sutherland DR. Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry B Clin Cytom. 2009;76B:355–64. doi: 10.1002/cyto.b.20485. [DOI] [PubMed] [Google Scholar]
- 14.Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001;45:194–205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Polliack A, Lampen N, Clarkson BD, De Harven E, Bentwich Z, Siegal FP, Kunkel HG. Identification of human B and T lymphocytes by scanning electron microscopy. J Exp Med. 1973;138:607–24. doi: 10.1084/jem.138.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmerlin L, Donnenberg VS, Donnenberg AD. Rare event detection and analysis in flow cytometry: bone marrow mesenchymal stem cells, breast cancer stem/progenitor cells in malignant effusions, and pericytes in disaggregated adipose tissue. Methods Mol Biol. 2011;699:251–73. doi: 10.1007/978-1-61737-950-5_12. [DOI] [PubMed] [Google Scholar]
- 17.Mittag A, Tarnok A. Basics of standardization and calibration in cytometry--a review. J Biophotonics. 2009;2:470–81. doi: 10.1002/jbio.200910033. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YZ, Kemper C, Bakke A, Haugland RP. Novel flow cytometry compensation standards: internally stained fluorescent microspheres with matched emission spectra and long-term stability. Cytometry. 1998;33:244–8. doi: 10.1002/(sici)1097-0320(19981001)33:2<244::aid-cyto20>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Lai L, Ong R, Li J, Albani S. A CD45-based barcoding approach to multiplex mass-cytometry (CyTOF) Cytometry A. 2015 doi: 10.1002/cyto.a.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao Y, Liu R, Shin MS, Trentalange M, Allore H, Nassar A, Kang I, Pober JS, Montgomery RR. CyTOF supports efficient detection of immune cell subsets from small samples. J Immunol Methods. 2014;415:1–5. doi: 10.1016/j.jim.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G, Weng NP. Analyzing the phenotypic and functional complexity of lymphocytes using CyTOF (cytometry by time-of-flight) Cell Mol Immunol. 2012;9:322–3. doi: 10.1038/cmi.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung RK, Utz PJ. Screening: CyTOF-the next generation of cell detection. Nat Rev Rheumatol. 2011;7:502–3. doi: 10.1038/nrrheum.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjornson ZB, Nolan GP, Fantl WJ. Single-cell mass cytometry for analysis of immune system functional states. Curr Opin Immunol. 2013;25:484–94. doi: 10.1016/j.coi.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leipold MD, Maecker HT. Mass cytometry: protocol for daily tuning and running cell samples on a CyTOF mass cytometer. J Vis Exp. 2012;e4398 doi: 10.3791/4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Abbasi F, Ornatsky O, Cole KD, Misakian M, Gaigalas AK, He HJ, Marti GE, Tanner S, Stebbings R. Human CD4+ lymphocytes for antigen quantification: characterization using conventional flow cytometry and mass cytometry. Cytometry A. 2012;81A:567–75. doi: 10.1002/cyto.a.22060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.