Abstract
The results of recent clinical trials using novel immunotherapy strategies such as immune checkpoint blockade and adoptive T-cell therapy approaches including CAR T-cell therapy have clearly established immunotherapy as an important modality for the treatment of cancer besides the traditional approaches of surgery, radiotherapy, and chemotherapy or targeted therapy. However, to date immunotherapy has been shown to induce durable clinical benefit in only a fraction of the patients. The use of combination strategies is likely to increase the number of patients that might benefit from immunotherapy. Indeed, over the last decade, the characterization of multiple immune resistance mechanisms used by the tumor to evade the immune system and the development of agents that target those mechanisms has generated a lot of enthusiasm for cancer immunotherapy. But a critical issue is to determine how best to combine such agents. This review will focus on novel immunotherapy agents currently in development and discuss strategies to develop and personalize combination cancer immunotherapy strategies.
Keywords: Cancer, immunotherapy, personalized, T cells, immune checkpoint
1. Cancer immunotherapy agents
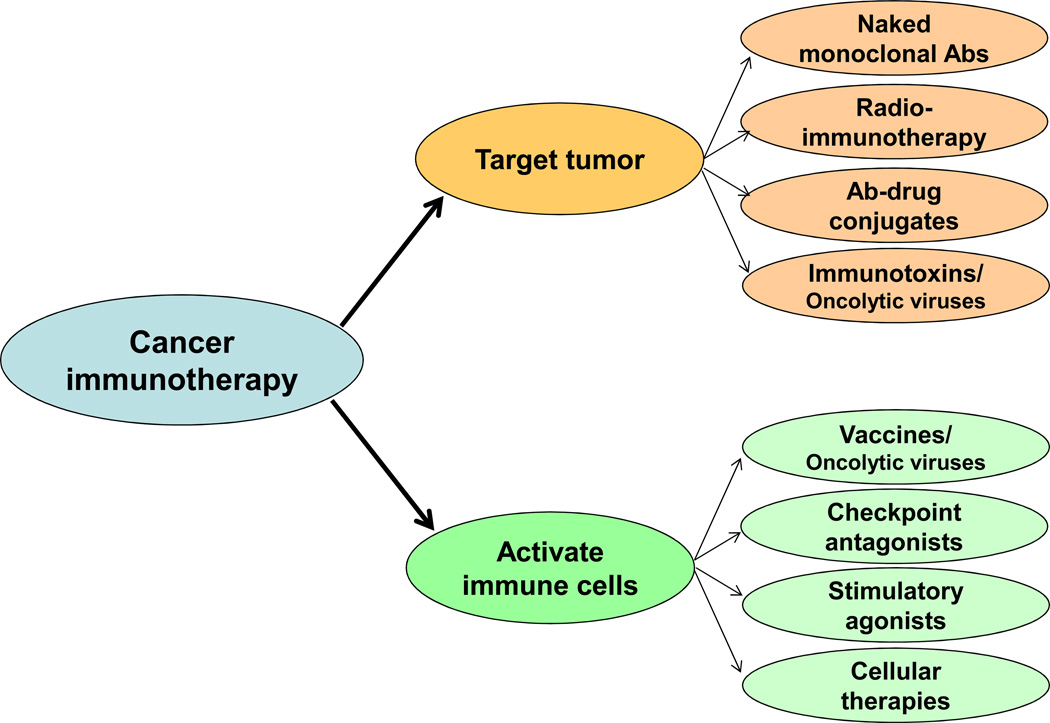
The potential role of the immune system to prevent or control cancer has been recognized over 100 years ago. However, cancer immunotherapy has become a reality only within the last two decades. Two broad strategies are currently in development for cancer immunotherapy: agents that target the tumor directly and agents that activate immune cells that in turn target the tumor (Fig. 1).
Figure 1. Categories of cancer immunotherapy agents.
Cancer immunotherapy agents may be broadly categorized into those that target the tumor and those that activate immune cells. Sub-classes of agents within each group are shown. Ab, antibody.
i. Immunotherapy agents targeting the tumor
Direct targeting of the tumor has largely focused on development of monoclonal antibodies. Although Paul Ehrlich first proposed the “magic bullet hypothesis” in 1897 (Ehrlich, 1906), the use of antibodies as “magic bullets” became feasible only after the development of the hybridoma technology by Kohler and Milstein in 1975 (Kohler and Milstein, 1975). The US Food and Drug Administration (FDA) approved the first monoclonal antibody for the treatment of cancer, rituximab, in 1997 (McLaughlin et al., 1998). In the ensuing years, many monoclonal antibodies were approved for the treatment of various cancers including B-cell malignancies, breast cancer, colon cancer, and others. The “naked” monoclonal antibodies such as rituximab and trastuzumab induced tumor cell death via both Fc dependent and independent mechanisms (Weiner et al., 2010). The Fc dependent mechanisms include antibody-dependent cell-mediated cytotoxicity (ADCC) mediated by NK cells and macrophages, antibody-dependent cellular phagocytosis (ADCP) mediated by macrophages, and complement-dependent cytotoxicity (CDC). The Fc independent mechanisms include induction of direct apoptosis following the binding of the antibody to its receptor or by blocking receptor-ligand interactions, for example growth factor signaling mediated via receptors such as HER2 on tumor cells.
The naked monoclonal antibodies whether used alone or in combination with traditional chemotherapy agents have improved the overall response rates, complete remission rates, and progression-free and overall survival in multiple cancers including breast cancer, colon cancer, lymphomas, and others (Cheson and Leonard, 2008; Van Cutsem et al., 2009; Vogel et al., 2002; Weiner et al., 2010). To further improve their efficacy, monoclonal antibodies were conjugated to radioisotopes such as Iodine-131 or Yttrium-90 to generate radioimmunotherapy agents (Kraeber-Bodere et al., 2014) or cytotoxic molecules such as monomethyl auristatin E or emtansine to generate antibody-drug conjugates (Younes et al., 2010; Zolot et al., 2013). Such conjugated monoclonal antibodies facilitated targeted delivery of radioisotopes and cytotoxic molecules to the tumor and were shown to be more effective than the corresponding naked monoclonal antibodies. In addition, radioimmunotherapy agents could also induce tumor killing of non-targeted cells in the vicinity via “crossfire” effect. Another strategy to target the tumor is to use immunotoxins where an immune molecule such as a cytokine is conjugated to a toxin. The cytokine binds to its receptor on the surface of tumor cell and delivers the toxin into the cell following receptor-mediated endocytosis. Denileukin diftitox is an immunotoxin that was approved by the FDA for the treatment of recurrent cutaneous T-cell lymphoma (Olsen et al., 2001). It is a fusion protein consisting of interleukin-2 conjugated to diphtheria toxin. Interleukin-2 targets its receptor CD25 on the malignant cells and diphtheria toxin inhibits intracellular protein synthesis and leads to cell death following its delivery into the cell.
In summary, naked monoclonal antibodies as well as monoclonal antibodies conjugated to radioisotopes or cytotoxic molecules and immunotoxins have already been shown to be very effective in multiple cancers (Fig. 1). Over the last decade, many novel cell surface targets have been identified in a variety of cancers and development of monoclonal antibodies against such targets is underway. In addition to targeting novel molecules, refinements in antibody engineering technology and conjugation to novel radioisotopes and cytotoxic molecules are expected to further enhance the therapeutic efficacy of monoclonal antibodies against cancer in the future.
ii. Agents that activate immune cells
Although strategies that activate immune cells have been developed and tested in parallel with tumor-targeting monoclonal antibodies, most of the early agents that were tested to activate immune cells have not shown good efficacy. However, the successful results reported recently with the use of novel immunotherapies that induce and/or enhance the activity of tumor-specific T cells have generated renewed enthusiasm for development of agents that activate immune cells to treat cancer. These approaches include cancer vaccines, oncolytic viruses, immune checkpoint antagonists, stimulatory agonists, and various forms of cellular therapies (Fig. 1).
Therapeutic cancer vaccines
The goal of therapeutic cancer vaccines is to induce antitumor T cells by immunizing patients against tumor-associated or tumor-specific antigens. Although therapeutic cancer vaccines have been evaluated for over two decades, the FDA to date has approved only one therapeutic cancer vaccine, sipuleucel-T. Sipuleucel-T was approved for the treatment of asymptomatic or minimally symptomatic metastatic castrate resistant prostate cancer. It consists of autologous CD54+ leukocytes activated with a fusion protein of prostatic acid phosphatase conjugated with GM-CSF. In a randomized phase 3 clinical trial, it improved overall survival of subjects with asymptomatic or minimally symptomatic castration-resistant prostate cancer by about 4 months compared with placebo in the control group (Kantoff et al., 2010). Other cancer vaccines that have shown clinical benefit in randomized phase 3 trials include a melanoma vaccine with gp100 peptide and a lymphoma vaccine with idiotype protein (Schuster et al., 2011; Schwartzentruber et al., 2011). However, the degree of clinical benefit induced by the therapeutic vaccines has been modest in all three phase 3 trials. This may be because most therapeutic cancer vaccines to date have used tumor-associated antigens as antigenic material. It is likely that vaccination with tumor-associated antigens induces only low to moderate affinity T cells because of self-tolerance mechanisms. In contrast, tumor-specific antigens such as mutated proteins or peptides might be recognized as foreign by the immune system and lead to induction of high affinity T cells and robust antitumor immunity (Cohen et al., 2015; Gros et al., 2014; Robbins et al., 2013; Tran et al., 2014). With the advent of next generation sequencing and improvements in bioinformatic algorithms that predict immunogenicity of the mutated genes, it might be possible to generate personalized cancer vaccines that are likely to be much more potent than the traditional vaccines that used tumor-associated antigens. Indeed, this personalized cancer vaccination strategy was shown to induce robust antitumor immune responses and/or tumor rejection in mice and humans (Carreno et al., 2015; Gubin et al., 2014).
Immune checkpoint antagonists
As opposed to therapeutic cancer vaccines that induce new antitumor T cells following immunization, immune checkpoint antagonists act by enhancing the function of preexisting antitumor T cells (Fig. 2). Immune checkpoint blockade is based on the notion that antitumor T cells are naturally generated against tumor antigens in most cancers. Indeed, recent studies show that such T cells are induced against mutated neo-antigens and are likely to be high affinity T cells (Cohen et al., 2015; Gros et al., 2014; Robbins et al., 2013; Tran et al., 2014). However, they may be rendered ineffective in the tumor microenvironment due to a number of immune resistance mechanisms collectively referred to as immune checkpoints that lead to immune escape by the tumor (Pardoll, 2012; Śledzińska, 2015). These checkpoints may arise from either physiologic mechanisms that have been co-opted by the tumor or alternatively may be the result of genetic aberrations within the tumor. Blocking the immune checkpoints enhances the function of antitumor T cells and skews the balance in the tumor microenvironment from immune resistance to immune destruction of the tumor.
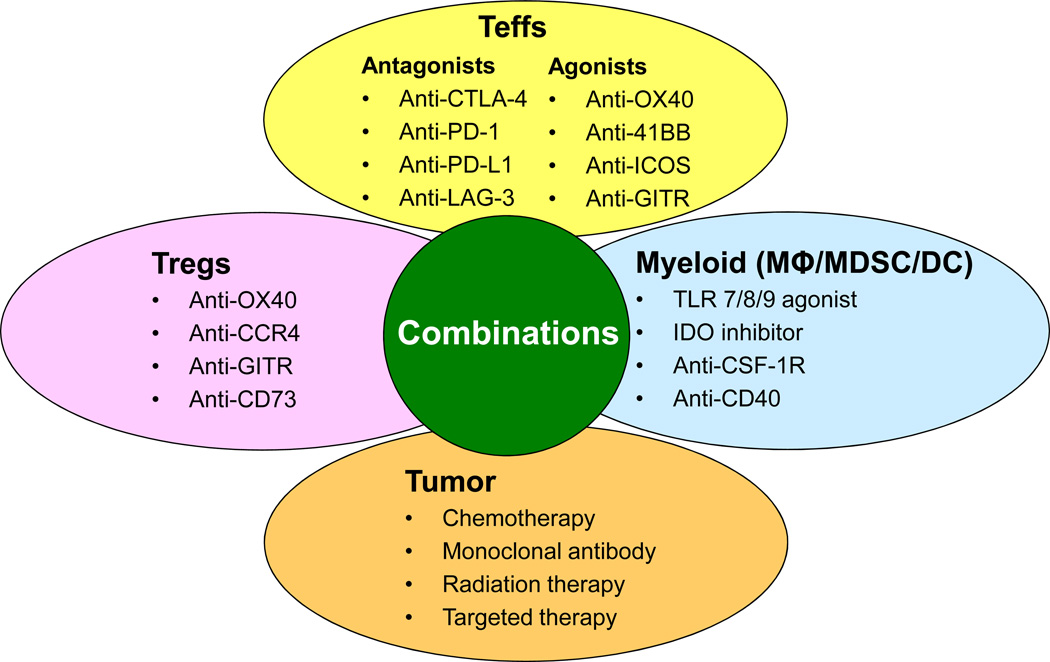
Figure 2. Combination immunotherapy strategies to enhance function of effector T cells (Teffs).
Examples of agents targeting tumor or immune cells in the tumor microenvironment that may be used in combinations to enhance function of antitumor Teffs are shown. Certain chemotherapeutic agents such as doxorubicin and radiation therapy may cause immunogenic tumor cell death and cause release of tumor neo-antigens in an inflammatory microenvironment and promote Teff activation. Monoclonal antibodies and targeted therapies against the tumor may also induce a ‘vaccine-like’ effect by inducing immunogenic tumor cell death. Agonistic antibodies targeting co-stimulatory molecules or antagonistic antibodies targeting co-inhibitory molecules on Teffs could be used alone or in combination to augment Teff function. Agents targeting regulatory T cells (Tregs) may either inhibit their immunosuppressive function or cause depletion of Tregs from the tumor microenvironment. Agents targeting myeloid cells including macrophages (MΦ), myeloid derived suppressor cells (MDSC), and dendritic cells (DC) may either skew their polarization to inflammatory state that promotes Teff activation or cause their depletion from the tumor microenvironment. Combination therapies with these agents are expected to be complementary and/or synergistic and may significantly improve clinical efficacy and outcome of cancer immunotherapy in the future.
Following activation, T cells upregulate a number of coinhibitory receptors on their cell surface that regulate their function to prevent uncontrolled immune responses that might lead to immune mediated damage of normal tissues. Over the last few years, many such receptors have been identified including CTLA-4, PD-1, TIM-3, BTLA, LAG-3, and others. Once the antigen has been eliminated as might happen in acute infections, the T cells down-regulate these inhibitory receptors and a subset of the T cells persist as memory T cells. However, in cancer and chronic infections, due to chronic antigenic stimulation these inhibitory receptors may be overexpressed and remain upregulated on T cells and lead to impaired T cell function or even state of T cell exhaustion (Blackburn et al., 2009; Day et al., 2006; Wherry, 2011). Blocking interactions between such receptors and their ligands can enhance and/or restore the function of exhausted T cells. This approach to treat cancer was first shown in a mouse model by Jim Allison and colleagues using a monoclonal antibody against CTLA-4 (Leach et al., 1996). Subsequently, ipilimumab, the monoclonal antibody against human CTLA-4 was developed and was shown to induce durable clinical remissions in about a fifth of patients with metastatic melanoma (Hodi et al., 2010). Targeting CTLA-4 has been shown to augment function effector T cells and may also lead to depletion of immunosuppressive regulatory T cells (Tregs) in the tumor microenvironment (Peggs et al., 2009; Quezada et al., 2006; Simpson et al., 2013). More recently, monoclonal antibodies against PD-1 (nivolumab and pembrolizumab) or their ligand PD-L1 were similarly shown to enhance function of antitumor T cells and induce durable clinical remissions in many cancers including melanoma, lung cancer, renal cell cancer, colon cancer, lymphomas, bladder cancer, etc (Ansell et al., 2015; Brahmer et al., 2012; Hamid et al., 2013; Lesokhin AM, 2014; Topalian et al., 2012). Additional agents targeting other coinhibitory receptors are currently in various stages of preclinical and clinical development.
Stimulatory agonists
Another strategy to enhance the function of T cells is to provide activating signals to costimulatory receptors that are also induced upon T cell activation. These include 4-1BB (CD137), OX-40 (CD134), GITR (CD357), CD27, and others (Fig. 2) (Mellman et al., 2011). Mouse studies show that agonistic monoclonal antibodies targeting these molecules could enhance the function of effector T cells (Teffs) and lead to tumor eradication. In addition, targeting OX-40 and GITR has also been shown to impair function and/or deplete the immunosuppressive Tregs (Bulliard et al., 2013; Houot and Levy, 2009; Marabelle et al., 2013; Voo et al., 2013). Agents targeting myeloid cells such as macrophages, myeloid derived suppressor cells (MDSC), and dendritic cells may also be used to promote effector T cell activation (Fig. 2). Several of these agents are currently being evaluated in clinical trials.
Bispecific antibodies (also called as bi-specific T-cell engagers) have also been used to stimulate and redirect specificity of T cells. These are synthesized by linking the single-chain variable fragment (scFv) constructs of two antibodies that bind to different antigens. One antibody is targeted to a molecule on the tumor and the other triggers an activating receptor on T cell in the vicinity. Blinatumomab is an FDA approved bispecific antibody for treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (Przepiorka et al., 2015). It targets CD19 on surface of tumor cells and activates T cells via CD3 to exert their cytotoxic activity on the target B cell. Bispecific antibodies targeting other surface receptors in various cancers including EGFR, HER2, GP100, MET, GPA33, CD123, and others are in clinical development (Weiner, 2015).
Oncolytic viruses
Oncolytic viruses are natural or genetically altered viruses that preferentially infect and replicate in tumor cells, and lead to immunogenic tumor cell death. Following oncolysis, the release of danger signals and tumor antigens results in antigen uptake and activation of dendritic cells that in turn induce adaptive antitumor immunity. Thus, oncolytic viruses lead to tumor killing both by targeting the tumor directly but also indirectly by acting as therapeutic cancer vaccines leading to induction of antitumor T cells (Bartlett et al., 2013). T-VEC is an oncolytic immunotherapy that is furthest along in clinical development. It is derived from herpes simplex virus type-1 that has been engineered to selectively replicate within tumors and to produce GM-CSF to enhance systemic antitumor immune responses. In a randomized phase 3 clinical trial in subjects with advanced melanoma, the overall response rate was 26% with T-VEC compared to the ORR of 6% with GM-CSF in the control group. In addition, there was a trend towards improved overall survival in the experimental group (Johnson et al., 2015).
Cellular therapies
Various forms of adoptive T cell therapies also look extremely promising for the treatment of cancer. In vitro expanded tumor-infiltrating lymphocyte (TIL) therapy induced ORR of >50% and durable responses lasting many years in patients with metastatic melanoma (Geukes Foppen, 2015; Rosenberg, 2012; Rosenberg et al., 2011). Redirected T cell therapy where T cells are transduced with a T-cell receptor (TCR) that specifically recognizes a HLA-peptide complex with the peptide derived from a tumor-associated antigen such as NY-ESO-1 or MART-1 have also induced durable responses in a significant proportion of patients with metastatic melanoma and synovial cell sarcoma (Karpanen, 2015; Morgan et al., 2006; Robbins et al., 2011). Another form of redirected T cells therapy uses chimeric antigen receptor (CAR) T cells. These T cells are manufactured by transducing a CAR construct that consists of an extracellular domain, an antibody in the form of scFV to recognize a cell surface receptor on the tumor cell (e.g. CD19 on tumor B cells) and an intracellular domain to activate T cells via CD3ζ and a costimulatory domain such as CD28 or 4-1BB. CD19 CAR T cell therapies have induced durable remissions in a high percentage of patients with refractory B-cell lymphomas, chronic lymphocytic leukemia, and acute lymphoblastic leukemia (Kalos et al., 2011; Kochenderfer et al., 2012; Kochenderfer et al., 2010; Porter et al., 2011; Whilding, 2015). CAR T cell therapies targeting cell surface molecules in various other malignancies are in development (June et al., 2015).
2. Determinants of immune-responsiveness of tumors
Many factors likely affect the responsiveness of tumors to cancer immunotherapy agents. These factors may be broadly categorized into either intrinsic or extrinsic to the tumor.
i. Tumor intrinsic factors
Emerging evidence indicates that the antitumor immune response that is spontaneously induced is targeted against mutated neo-antigens (Cohen et al., 2015; Gros et al., 2014; Robbins et al., 2013; Tran et al., 2014). This endogenous antitumor immune response is probably triggered when the resident dendritic cells (DC) take up the mutated proteins released upon tumor cell death. The DCs then present the processed antigens to prime and activate naïve T cells in the draining lymph nodes. The activated T cells traffic back to the tumor and mediate tumor eradication (Chen and Mellman, 2013). A critical element necessary for induction of these antitumor immune responses is the presence of immunogenic neo-antigens in the tumor. An analysis of nonsynonymous mutations in melanoma and lung cancer tumors indicated that only a minute fraction of the mutated proteins induce immune responses and might serve as neo-antigens. For example, in one study of approximately 400 candidate neo-epitopes, only two peptides induced antigen-specific T cells as detected by MHC tetramer positive populations among tumor-infiltrating lymphocytes (van Rooij et al., 2013). Similar results were found in other studies (Linnemann et al., 2015; Rizvi et al., 2015; Robbins et al., 2013; Snyder et al., 2014). While the mutation load generally increases the chance of immunogenic neo-antigens in a tumor, it appears that mutation load alone is not enough. But rather the neoepitope as defined by the pattern of a four-amino acid sequence spanning each point mutation might be important to determine immunogenicity of the neo-antigen. Interestingly, neoepitopes that were homologous to epitopes from microbial antigens were found to be more likely to be immunogenic (Snyder et al., 2014). Furthermore, presence of such neoepitopes was associated with long-term clinical benefit after immune checkpoint blockade therapy. Thus, the mutational landscape in each tumor is an important determinant of endogenous antitumor immune response and responsiveness to immune checkpoint inhibitor therapy.
Mutations in the tumor could also lead to immune escape by the tumor. For example, inactivating mutations of β2-microglobulin may lead to aberrant loss of MHC class I expression and impaired recognition of tumor by CD8 effector T cells (Challa-Malladi et al., 2011). Inactivating mutations of costimulatory molecules such as CD58 may lead to impaired killing by NK cells (Challa-Malladi et al., 2011). Genetic aberrations may also lead to overexpression of inhibitory ligands such as PD-L1 and PD-L2 as seen in Hodgkin lymphoma and primary mediastinal large B-cell lymphoma (Ansell et al., 2015; Greaves and Gribben, 2013). Other factors such as defects in antigen processing have also been described. Thus, factors intrinsic to the tumor largely driven by mutations or other genetic aberrations could affect the natural immunogenicity as well as responsiveness to therapies that enhance T and/or NK cell effector functions.
ii. Tumor extrinsic factors
The composition and spatial organization of the stromal cells in the tumor microenvironment may also have major impact on response to cancer immunotherapy agents. While effector T cells mediate antitumor function, other cells in the tumor microenvironment including tumor-associated macrophages, MDSC, Tregs, cancer-associated fibroblasts, tolerogenic dendritic cells, and mesenchymal stromal cells promote tumor growth either directly by providing growth and survival signals or indirectly by promoting immune escape via suppression of effector T cells (Zou, 2005). The number and composition of these stromal cells varies significantly between various cancers and also between individuals with the same cancer. This variation may be driven by cross talk between the tumor and stromal cells as well as by mutations or other genetic aberrations in each tumor.
Tumors may produce chemokines such as CCL17 and CCL22 that preferentially mediate recruitment of immunosuppressive Tregs via CCR4 (Curiel et al., 2004; Rawal et al., 2013; Yang et al., 2006b). In contrast, an inflammatory phenotype of the tumor and presence of CXCL9 and CXCL10, which engage CXCR3 on effector T cells was associated with increased numbers of CD8+ T cells (Dengel et al., 2010; Harlin et al., 2009). Tumors may also express cytokines or other molecules that skew polarization of naïve T cells into Tregs (Yang et al., 2006a; Zou, 2005, 2006). Mutations in CREBBP were associated with decreased MHC class II expression and decreased infiltration of CD4+ T cells (Green et al., 2015). Macrophages may exhibit pro or antitumor effects depending on whether they are M2 or M1 phenotypes, respectively (Biswas and Mantovani, 2010). Tumor-associated macrophages generally tend to be of M2 phenotype likely due to polarization by IL-4, IL-10, IL-13, and/or M-CSF in the tumor microenvironment. M2 macrophages promote formation of denser stroma and lead to poor T cell infiltration. In addition, M2 macrophages cause an immunosuppressive milieu by promoting recruitment of Tregs through production of CCL22 (Biswas and Mantovani, 2010; Zou, 2005, 2006). Myeloid-derived suppressor cells are immature myeloid-lineage cells that can also be immunosuppressive in the tumor microenvironment and can suppress effector T cell function and induce differentiation of Tregs (Gabrilovich et al., 2012; Marvel and Gabrilovich, 2015). MDSC mediate suppression of effector T cells by modulating L-arginine metabolism through arginase and also by nitrosylation of surface proteins on infiltrating T cells, including the T-cell receptor (Gabrilovich et al., 2012; Marvel and Gabrilovich, 2015).
The composition of the tumor microenvironment has been shown to impact clinical outcome in a variety of cancers. In most cancers, infiltration with CD8+ T cells has been shown to positively correlate with survival as illustrated in colorectal and breast cancer (Galon et al., 2006; Mahmoud et al., 2011; Tosolini et al., 2011). A high ratio of CD8+ T cells to Tregs in the ovarian cancer tumor microenvironment has been associated with a better response rates and outcome (Curiel et al., 2004; Zhang et al., 2003). In contrast, infiltration with macrophages and Tregs is generally associated with inferior outcome (Biswas and Mantovani, 2010; Curiel et al., 2004; Farinha et al., 2005; Sanchez-Espiridion et al., 2012). In addition to stromal cells in the tumor microenvironment, host factors such as polymorphisms of immune regulatory genes may also affect clinical outcome after therapy with immunomodulatory agents.
3. Developing personalized and combinatorial cancer immunotherapy strategies
As described above, many cancer immunotherapy agents have recently been shown to induce durable clinical remissions in a variety of cancers. But, only a fraction of the patients appear to respond when these agents are used as monotherapy. Early results from use of combination immunotherapy strategies blocking multiple immune resistance mechanisms show that a greater proportion of patients may benefit with combination therapies. For example, combination therapy with nivolumab and ipilimumab induced responses in over half the patients with relapsed melanoma as compared with 10–30% with single agents although combination therapy was associated with higher incidence of immune-related adverse events (Wolchok et al., 2013). Emerging data from preclinical studies also suggest that combination strategies are likely to significantly improve responses and possibly cures in many cancers (Curran et al., 2010; Houot et al., 2009; Houot and Levy, 2009; Kohrt et al., 2011; Kohrt et al., 2014; Marabelle et al., 2013). Combinations of agents that enhance effector T cell function with agents that suppress immunosuppressive elements such as MDSC, Tregs, and macrophages in the tumor microenvironment may be complementary and possibly synergistic (Fig. 2).
Immunomodulatory agents may also be combined with traditional therapies such as chemotherapy and radiation therapy. Certain chemotherapy agents such as cyclophosphamide, doxorubicin, and oxaliplatin have been shown to induce immunogenic cell death and activate antitumor T cell immune responses (Kroemer et al., 2013; Michaud et al., 2011; Sistigu et al., 2014). Combination therapy with low doses of such chemotherapy agents with immune checkpoint inhibitors or other immunomodulatory agents could improve clinical efficacy. The abscopal effect induced by radiotherapy, where irradiation to a local tumor induces regression of metastatic cancer presumably via activation of immune system, may also be enhanced by combination with immunomodulatory agents such as immune checkpoint inhibitors (Postow et al., 2012) or toll-like receptor ligands to activate dendritic cells (Brody et al., 2010; Kim et al., 2012). In a recent study, Kolstad and colleagues showed that combining radiotherapy with intranodal injection of low-dose rituximab, immature dendritic cells, and GM-CSF induces systemic CD8+ T cell immunity and regression of disseminated follicular lymphoma (Kolstad et al., 2015). Together, these studies demonstrate that immunotherapeutioc agents can be combined with conventional chemotherapeutic agents and radiation therapy to enhance antitumor immunity and improve clinical outcome.
However, a key question is to determine which patients need combination strategies and what combinations are best in any given patient. Studies in metastatic melanoma showed that approximately a fifth of the patients may be cured with four infusions of ipilimumab monotherapy and similar trend has been observed with nivolumab and pembrolizumab monotherapy (Hamid et al., 2013; Hodi et al., 2010; Topalian et al., 2012). These results suggest that some patients achieve durable benefit with monotherapy and may not need combination immunotherapy. There is an urgent need to develop biomarkers to identify such patients to preselect them for monotherapy in order to avoid the toxicities associated with combination immunotherapy. It is also important to develop biomarkers to determine the optimal combination therapies appropriate for patients that do need combination therapy. Identification of such predictive biomarkers could lead to personalized cancer immunotherapy strategies that in turn can improve efficacy, reduce toxicity, and reduce costs of therapy.
Expression of PD-L1 on tumor or stromal cells has been suggested as a potential predictive biomarker for therapy with anti-PD-1 antibody therapy (Pardoll, 2012; Taube et al., 2012; Topalian et al., 2012). This is based on the notion that PD-L1 is expressed as an adaptive resistance mechanism in response to an antitumor immune response mediated by effector T cells. In other words, PD-L1 expression probably identified patients that have a preexisting antitumor immune response and therefore are likely to respond to immune checkpoint inhibitor therapy. Consistent with this, melanoma and lung cancer patients with PD-L1 expression in their tumors at baseline had a significantly higher response rate than patients without PD-L1 expression after therapy with anti-PD-1 antibody (Brahmer et al., 2012; Garon et al., 2015; Topalian et al., 2012). Another alternative to identify such patients is to measure the baseline endogenous antitumor T cell response. This may be accomplished by counting effector T cells by immunohistochemistry (Tumeh et al., 2014) or by determining the magnitude of the antitumor T cell response by gene signature score of effector T cells (Westin et al., 2014). Both of these biomarkers have also been associated with improved clinical outcome after anti-PD-1 antibody therapy.
While determining PD-L1 expression or measuring effector T cell content might indicate what tumors are immunogenic and will respond to anti-PD-1 antibody therapy, they do not provide any insights into what combination immunotherapies might be appropriate for the vast majority of patients that are not likely to respond. Identification of additional biomarkers that can measure both tumor intrinsic and extrinsic factors described above and determine the dominant immune resistance mechanisms in each tumor are needed to develop rational and individualized combination immunotherapy strategies. The “immunoscore” immunohistochemical analysis described by Galon et al (Angell and Galon, 2013) and novel bioinformatic analyses of gene expression profiling data such as “CIBERSORT” (Newman et al., 2015) to enumerate immune and stromal cell subsets in the tumor microenvironment might be helpful in this respect.
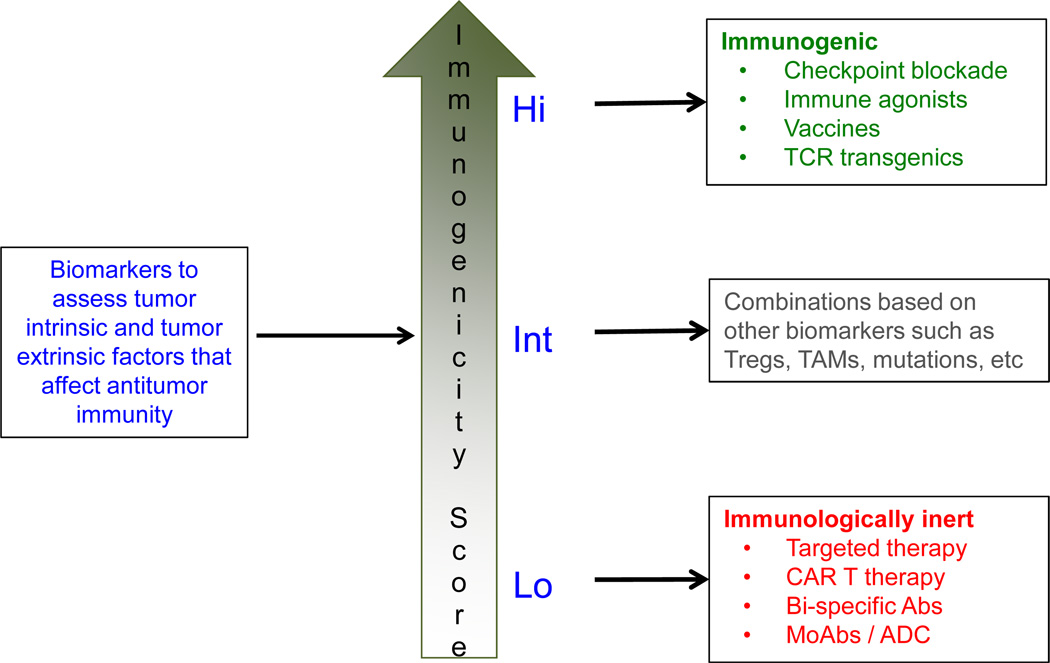
Eventually, with the appropriate biomarkers it might be possible to categorize tumors based on their immunogenicity score for treatment planning (Fig. 3). Tumors that are “highly immunogenic” may be treated with single cancer immunotherapy agents such as immune checkpoint inhibitors or neo-antigen vaccines whereas at the other end of the spectrum tumors may be “immunologically inert” and may need to be primarily treated with targeted therapies, monoclonal antibodies, antibody-drug conjugates, or re-directed T-cell therapies such as CAR T cell therapy or bi-specific antibodies. However, most tumors are likely to be of “intermediate immunogenicity” and such tumors may need to be treated with combination immunotherapy strategies based on the dominant immune resistance mechanisms that might be mediating immune escape.
Figure 3. Strategy for personalized cancer immunotherapy.
A potential strategy for development of personalized cancer immunotherapy is to establish the baseline ‘immunogenicity score’ of each tumor using a panel of biomarkers that assess both the tumor intrinsic and tumor extrinsic factors that affect antitumor immunity. Tumors with ‘high score’ may be highly immunogenic and may be treated with monotherapy with one of the cancer immunotherapy agents. Tumors that have ‘low score’ may be immunologically inert and may need to be primarily treated with targeted therapies, monoclonal antibodies, or re-directed T cell therapies such as CAR T cell therapy or b-specific antibodies. Tumors with ‘intermediate immunogenicity score’ may need to be treated with combination immunotherapy strategies and the precise combination approach may be determined by the dominant immune resistance mechanism elucidated by the biomarker analysis.
4. Conclusions and future directions
In summary, the demonstration of durable clinical remissions with a variety of immunotherapy agents has clearly established immunotherapy as an important treatment modality for most cancers. Many more immunomodulatory agents targeting novel molecules and cell types are in development and entering clinical trials. In addition, recent data suggests that many novel targeted therapies may also have immunomodulatory effects. In the future, further improvements in clinical outcomes and cures are expected with the use of combination immunotherapy strategies with or without targeted therapies analogous to combination chemotherapy strategies that have been shown to be more effective than therapy with single agents in many cancers. However, identification of robust predictive biomarkers that can accurately measure determinants of immune-responsiveness of tumors are needed to guide development and personalization of combination immunotherapy strategies. It is anticipated that personalized cancer immunotherapy strategies will improve efficacy, reduce toxicity, and reduce costs of therapy.
Highlights.
Many immunotherapy agents are in development for the treatment of cancer.
Immune checkpoint inhibitors and adoptive T-cell therapies have already been shown to induce durable clinical remissions in a variety of cancers.
Combination strategies are likely to further improve efficacy of cancer immunotherapy.
Identification of biomarkers may lead to development of rational combination strategies and personalized cancer immunotherapy approaches.
Acknowledgements
This work was supported by research grants to SSN from the Leukemia and Lymphoma Society (P-QFC-3068-14), National Institutes of Health (R01CA155143), Cancer Prevention and Research Institute of Texas (RP150316), and The University of Texas MD Anderson Cancer Center’s Moon Shots Program.
SSN has received research support from Bristol-Myers Squibb (BMS), Merck, and Kite Pharma.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: VS has nothing to declare.
Author Contributions: VS and SSN wrote the article.
References
- Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Current opinion in immunology. 2013;25:261–267. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. The New England journal of medicine. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y, Guo ZS. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer. 2013;12:103. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature immunology. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature immunology. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK, Wapnir I, Tibshirani RJ, Levy R. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–4332. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, Brogdon JL. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. The Journal of experimental medicine. 2013;210:1685–1693. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH, Mardis ER, Linette GP. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challa-Malladi M, Lieu YK, Califano O, Holmes AB, Bhagat G, Murty VV, Dominguez-Sola D, Pasqualucci L, Dalla-Favera R. Combined genetic inactivation of beta2-Microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer cell. 2011;20:728–740. doi: 10.1016/j.ccr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Leonard JP. Monoclonal Antibody Therapy for B-Cell Non-Hodgkin's Lymphoma. New England Journal of Medicine. 2008;359:613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- Cohen CJ, Gartner JJ, Horovitz-Fried M, Shamalov K, Trebska-McGowan K, Bliskovsky VV, Parkhurst MR, Ankri C, Prickett TD, Crystal JS, Li YF, El-Gamil M, Rosenberg SA, Robbins PF. Isolation of neoantigen-specific T cells from tumor and peripheral lymphocytes. The Journal of clinical investigation. 2015 doi: 10.1172/JCI82416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Dengel LT, Norrod AG, Gregory BL, Clancy-Thompson E, Burdick MD, Strieter RM, Slingluff CL, Jr, Mullins DW. Interferons induce CXCR3-cognate chemokine production by human metastatic melanoma. J Immunother. 2010;33:965–974. doi: 10.1097/CJI.0b013e3181fb045d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich P. J. New York: Wiley & Sons; 1906. Collected studies on immunity. [Google Scholar]
- Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K, Klasa R, Voss N, Connors JM, Gascoyne RD. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL) Blood. 2005;106:2169–2174. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature reviews. Immunology. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, Investigators K. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England journal of medicine. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- Geukes Foppen MH, Donia M, Svane IM, Haanen JBAG. Tumor-infiltrating lymphocytes for the treatment of metastatic cancer. Molecular Oncology. 2015 doi: 10.1016/j.molonc.2015.10.018. (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121:734–744. doi: 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MR, Kihira S, Liu CL, Nair RV, Salari R, Gentles AJ, Irish J, Stehr H, Vicente-Duenas C, Romero-Camarero I, Sanchez-Garcia I, Plevritis SK, Arber DA, Batzoglou S, Levy R, Alizadeh AA. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E1116–E1125. doi: 10.1073/pnas.1501199112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, Hanada K, Almeida JR, Darko S, Douek DC, Yang JC, Rosenberg SA. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. The Journal of clinical investigation. 2014;124:2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, Lam SS, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee HG, Melief CJ, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. The New England journal of medicine. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houot R, Goldstein MJ, Kohrt HE, Myklebust JH, Alizadeh AA, Lin JT, Irish JM, Torchia JA, Kolstad A, Chen L, Levy R. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood. 2009;114:3431–3438. doi: 10.1182/blood-2009-05-223958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houot R, Levy R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood. 2009;113:3546–3552. doi: 10.1182/blood-2008-07-170274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DB, Puzanov I, Kelley MC. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy. 2015;7:611–619. doi: 10.2217/imt.15.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Science translational medicine. 2015;7:280ps287. doi: 10.1126/scitranslmed.aaa3643. [DOI] [PubMed] [Google Scholar]
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science translational medicine. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England journal of medicine. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- Karpanen T, Olweus J. T-cell receptor gene therapy – ready to go viral? Molecular Oncology. 2015 doi: 10.1016/j.molonc.2015.10.006. (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Gratzinger D, Harrison C, Brody JD, Czerwinski DK, Ai WZ, Morales A, Abdulla F, Xing L, Navi D, Tibshirani RJ, Advani RH, Lingala B, Shah S, Hoppe RT, Levy R. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood. 2012;119:355–363. doi: 10.1182/blood-2011-05-355222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan DA, Morgan RA, Laurencot C, Rosenberg SA. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M, Nathan DA, Lanier BJ, Morgan RA, Rosenberg SA. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, Muller A, Pachynski R, Czerwinski D, Coutre S, Chao MP, Chen L, Tedder TF, Levy R. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood. 2011;117:2423–2432. doi: 10.1182/blood-2010-08-301945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, Fuseri N, Bonnafous C, Czerwinski D, Rajapaksa A, Waller E, Ugolini S, Vivier E, Romagne F, Levy R, Blery M, Andre P. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014;123:678–686. doi: 10.1182/blood-2013-08-519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolstad A, Kumari S, Walczak M, Madsbu U, Hagtvedt T, Bogsrud TV, Kvalheim G, Holte H, Aurlien E, Delabie J, Tierens A, Olweus J. Sequential intranodal immunotherapy induces antitumor immunity and correlated regression of disseminated follicular lymphoma. Blood. 2015;125:82–89. doi: 10.1182/blood-2014-07-592162. [DOI] [PubMed] [Google Scholar]
- Kraeber-Bodere F, Bodet-Milin C, Rousseau C, Eugene T, Pallardy A, Frampas E, Carlier T, Ferrer L, Gaschet J, Davodeau F, Gestin JF, Faivre-Chauvet A, Barbet J, Cherel M. Radioimmunoconjugates for the treatment of cancer. Seminars in oncology. 2014;41:613–622. doi: 10.1053/j.seminoncol.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annual review of immunology. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- Lesokhin AMAS, Armand P, et al. Preliminary Results of a Phase I Study of Nivolumab (BMS-936558) in Patients with Relapsed or Refractory Lymphoid Malignancies. Blood (ASH Annual Meeting Abstracts) 2014 Abstract 291. [Google Scholar]
- Linnemann C, van Buuren MM, Bies L, Verdegaal EM, Schotte R, Calis JJ, Behjati S, Velds A, Hilkmann H, Atmioui DE, Visser M, Stratton MR, Haanen JB, Spits H, van der Burg SH, Schumacher TN. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med. 2015;21:81–85. doi: 10.1038/nm.3773. [DOI] [PubMed] [Google Scholar]
- Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, Rajapaksa R, Green MR, Torchia J, Brody J, Luong R, Rosenblum MD, Steinman L, Levitsky HI, Tse V, Levy R. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. The Journal of clinical investigation. 2013;123:2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. The Journal of clinical investigation. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. Journal of Clinical Oncology. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, Tailler M, Menger L, Vacchelli E, Galluzzi L, Ghiringhelli F, di Virgilio F, Zitvogel L, Kroemer G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen E, Duvic M, Frankel A, Kim Y, Martin A, Vonderheid E, Jegasothy B, Wood G, Gordon M, Heald P, Oseroff A, Pinter-Brown L, Bowen G, Kuzel T, Fivenson D, Foss F, Glode M, Molina A, Knobler E, Stewart S, Cooper K, Stevens S, Craig F, Reuben J, Bacha P, Nichols J. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. The Journal of experimental medicine. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman RA, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S, Wolchok JD. Immunologic correlates of the abscopal effect in a patient with melanoma. The New England journal of medicine. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przepiorka D, Ko CW, Deisseroth A, Yancey CL, Candau-Chacon R, Chiu HJ, Gehrke BJ, Gomez-Broughton C, Kane RC, Kirshner S, Mehrotra N, Ricks TK, Schmiel D, Song P, Zhao P, Zhou Q, Farrell AT, Pazdur R. FDA Approval: Blinatumomab. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:4035–4039. doi: 10.1158/1078-0432.CCR-15-0612. [DOI] [PubMed] [Google Scholar]
- Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. The Journal of clinical investigation. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal S, Chu F, Zhang M, Park HJ, Nattamai D, Kannan S, Sharma R, Delgado D, Chou T, Lin HY, Baladandayuthapani V, Luong A, Vega F, Fowler N, Dong C, Davis RE, Neelapu SS. Cross talk between follicular Th cells and tumor cells in human follicular lymphoma promotes immune evasion in the tumor microenvironment. Journal of immunology. 2013;190:6681–6693. doi: 10.4049/jimmunol.1201363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, Samuels Y, Rosenberg SA. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19:747–752. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Science translational medicine. 2012;4:127ps128. doi: 10.1126/scitranslmed.3003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Espiridion B, Martin-Moreno AM, Montalban C, Medeiros LJ, Vega F, Younes A, Piris MA, Garcia JF. Immunohistochemical markers for tumor associated macrophages and survival in advanced classical Hodgkin's lymphoma. Haematologica. 2012;97:1080–1084. doi: 10.3324/haematol.2011.055459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, Winter JN, Flowers CR, Nikcevich DA, Sotomayor EM, McGaughey DS, Jaffe ES, Chong EA, Reynolds CW, Berry DA, Santos CF, Popa MA, McCord AM, Kwak LW. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol. 2011;29:2787–2794. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, Kendra KL, White RL, Gonzalez R, Kuzel TM, Curti B, Leming PD, Whitman ED, Balkissoon J, Reintgen DS, Kaufman H, Marincola FM, Merino MJ, Rosenberg SA, Choyke P, Vena D, Hwu P. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. The New England journal of medicine. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. The Journal of experimental medicine. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, Fend L, Hannani D, Aymeric L, Ma Y, Niso-Santano M, Kepp O, Schultze JL, Tuting T, Belardelli F, Bracci L, La Sorsa V, Ziccheddu G, Sestili P, Urbani F, Delorenzi M, Lacroix-Triki M, Quidville V, Conforti R, Spano JP, Pusztai L, Poirier-Colame V, Delaloge S, Penault-Llorca F, Ladoire S, Arnould L, Cyrta J, Dessoliers MC, Eggermont A, Bianchi ME, Pittet M, Engblom C, Pfirschke C, Preville X, Uze G, Schreiber RD, Chow MT, Smyth MJ, Proietti E, Andre F, Kroemer G, Zitvogel L. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- Śledzińska A, Menger L, Bergerhoff K, Peggs KS, Quezada SA. Negative immune checkpoints on T lymphocytes and their relevance to cancer immunotherapy. Molecular Oncology. 2015 doi: 10.1016/j.molonc.2015.10.008. (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. The New England journal of medicine. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Science translational medicine. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, Parkhurst MR, Yang JC, Rosenberg SA. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. The New England journal of medicine. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJ, Behjati S, Hilkmann H, El Atmioui D, Nieuwland M, Stratton MR, Kerkhoven RM, Kesmir C, Haanen JB, Kvistborg P, Schumacher TN. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31:e439–e442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- Voo KS, Bover L, Harline ML, Vien LT, Facchinetti V, Arima K, Kwak LW, Liu YJ. Antibodies targeting human OX40 expand effector T cells and block inducible and natural regulatory T cell function. Journal of immunology. 2013;191:3641–3650. doi: 10.4049/jimmunol.1202752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 2015;15:361–370. doi: 10.1038/nrc3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature reviews. Immunology. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, Romaguera J, Hagemeister F, Fanale M, Samaniego F, Feng L, Baladandayuthapani V, Wang Z, Ma W, Gao Y, Wallace M, Vence LM, Radvanyi L, Muzzafar T, Rotem-Yehudar R, Davis RE, Neelapu SS. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. The lancet oncology. 2014;15:69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nature immunology. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Whilding LM, Maher J. CAR T-cell immunotherapy: the path from the by-road to the freeway? Molecular Oncology. 2015 doi: 10.1016/j.molonc.2015.10.012. (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. The New England journal of medicine. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006a;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Attenuation of CD8(+) T-cell function by CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin's lymphoma. Cancer Res. 2006b;66:10145–10152. doi: 10.1158/0008-5472.CAN-06-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, Forero-Torres A. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. The New England journal of medicine. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England journal of medicine. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- Zolot RS, Basu S, Million RP. Antibody-drug conjugates. Nat Rev Drug Discov. 2013;12:259–260. doi: 10.1038/nrd3980. [DOI] [PubMed] [Google Scholar]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nature reviews. Immunology. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]