Abstract
Objective
Ocular palatal tremor typically develops after a breach in the Guillian-Mollaret triangle. We herein describe a variant of this syndrome in which dystonia is also present, hence called, here, ocular palatal tremor plus dystonia.
Methods
We assessed eye-head movements and dystonia in six patients with ocular palatal plus dystonia.
Results
Among six patients with ocular palatal tremor two had focal dystonia, three had multifocal dystonia, and one had generalized dystonia. The dystonia affected the upper extremities and neck in four patients, the lower extremities in three and the face in two. Three out of four cervical dystonia patients had head tremor. Two patients also had speech involvement. Lack of correlation between eye and head oscillations suggested that head oscillations were not compensatory or secondary to the eye oscillations and vice versa.
Conclusions
We describe a novel variant of ocular palatal tremor with dystonia. We speculate that in such variant the dystonia is possibly could be a result of abnormal cerebellar outflow in patients with a breach in Guillain-Mollaret triangle.
Keywords: cerebellum, oscillation, nystagmus, inferior olive, brainstem
Introduction
The syndrome of ocular palatal tremor (OPT) is a unique manifestation following lesions involving the Guillian-Mollaret triangle. The Guillian-Mollaret triangle is comprised of the projection fibers from the deep cerebellar nuclei, which pass around the red nucleus to synapse with the contralateral inferior olive. Olivary fibers then project back to the deep cerebellar nuclei1. Breach in the continuity of the Guillian-Mollaret triangle results in pseudohypertrophy and spontaneous rhythmic discharges from the inferior olive2–6. Such activity is the basis for the coarse, dysconjugate, and irregular eye oscillations associated with rhythmic palate movements that typify OPT5, 7.
Progressive ataxia palatal tremor syndrome is a variant of OPT where the eye and palate oscillations are accompanied by ataxia but without a focal lesion compromising the integrity of the Guillian-Mollaret triangle8. Occasionally the patients with lesions of Guillian-Mollaret triangle with pseudohypertrophy of the inferior olive have pure eye nystagmus without involvement of the palate or vice versa 7.
We here describe a series of patients with novel OPT variant where eye and palate oscillations were associated with various forms of dystonia. We refer to this variant as ocular palatal tremor plus dystonia (OPTD).
Methods
Six patients with OPTD were examined between July 2011 and June 2014. Three patients were diagnosed at Emory University, two at Case Western Reserve University, and one at the Cleveland Clinic. All subjects provided written informed consent before participating in the study. A neurological examination was videotaped for all six patients. Dystonia was quantitatively assessed with the motor component of Burke Fahn Marsden rating scale 9. Eye and head movement were quantitatively assessed in three patients.
Results
All patients had pseudohypertrophy of the inferior olive associated with focal lesions interrupting the Guillian-Mollaret triangle. Figure 1 depicts an example of inferior olive hypertrophy in T2-weighted brain MRI. Table 1 summarizes the etiology of the lesions affecting Guillian-Mollaret triangle. Clinical examination in all patients revealed coarse, irregular, and dysconjugate oscillations of the eyes. Video 1 depicts example from one patient. One patient had gaze position dependent torsional and vertical eye oscillations, two patients had eye oscillations along the horizontal and vertical axis and in two patients the oscillations were present in all three axes. The waveforms are coarse, irregular, and disconjugate. Consistent with the characteristics of OPT, the eye oscillations eye oscillation frequency was 3.4 ± 1.6 Hz in horizontal, 3.5 ± 2.3 Hz in vertical, and 3.8 ± 1.7 Hz in torsional axis. There was no evidence of change in the characteristics of oscillations after removal of visual fixation, but they attenuated during ocular convergence. Palatal tremor was present in all but one patient.
Figure 1.
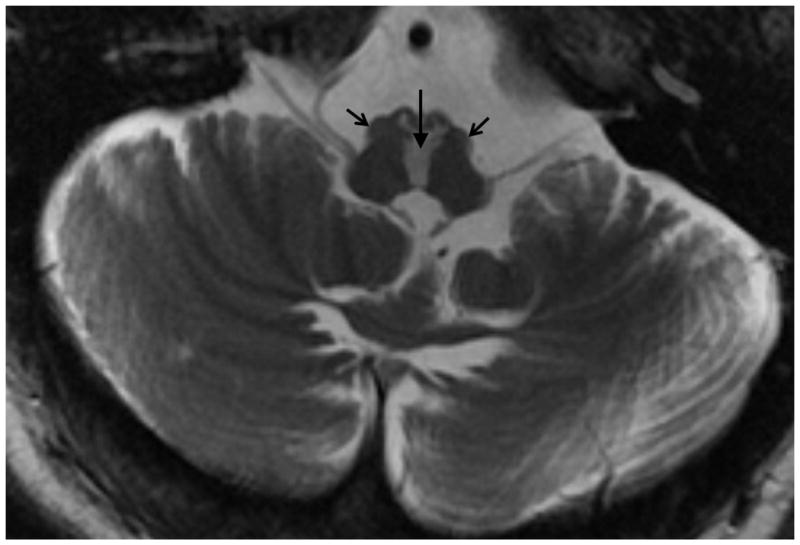
An example of T2-weighted brain MRI showing bilateral inferior olive hypertrophy in a patient with OPTD. Open arrows show hypertrophied inferior olive, while hyper dense midline area shown by filled vertical arrow shows injury to input fibers to the inferior olive.
Table 1.
The table summarizes pertinent clinical features of six patients with OPTD.
| Patient | Age/Gender | History | Nystagmus | Palate tremor | Dystonia | Other movement disorders | Pharmacothera py |
|---|---|---|---|---|---|---|---|
| 1 | 48/F | Resection of deep cerebellar mass | Torsional and vertical nystagmus on slight left gaze, nystagmus increases with further eccentric left gaze, no nystagmus on straightahead gaze | Yes | Torticollis (mild) Right hand (mild), both feet (moderate) | Lip tremor, Speech tremor | Baclofen, Clonazepam, Botulinum toxin |
| 2 | 46/M | Hemorrhagic stroke in pons and midbrain due to unknown etiology | Coarse irregular dysconjugate eye oscillatiions in horizontal, and vertical directions. | Yes | Left laterocollis (mild), right torticollis (mild), right hand | Head tremor | Memantine |
| 3 | 26/M | Pliocytic astrocytoma status post radioablation, hemorrhage | Horizontal and vertical pendular nystagmus in straight-ahead and eccentric gaze | No | Low face dystonia | Upper extremity ataxia | Leviteracetam (for history of seizure) |
| 4 | 42/F | Infarction of brainstem, left cerebellar hemisphere, superior vermis, left superior cerebellar peduncle, thalamus, and left occipital cortex due to vertebral artery dissection. | Irregular, coarse, disconjugate oscillations of both eyes in all three axes, more prominent nystagmus in vertical axis. | Yes | Torticollis (mild), Right hand dystonia (mild). | Both appendicular ataxia, mild head tremor, peri-oral twiches | Memantine, gabapentin, clonazepam |
| 5 | 56/M | Pontine stroke | Prominent horizontal and mild torsional nystagmus in primary gaze. No change in eccentric gaze, enucleation of the left eye. | Yes | Moderate to severe torticollis, mild retrocollis, mild shoulder elevation | Upper appendicular ataxia. | Botulinum toxin. |
| 6 | 48/M | Pontine hemorrhagic stroke after AVM rupture and fall. | Horizontal, vertical, and torsional coars, irregular, disconjugate nystagmus | Yes | Moderate to severe torticollis, mild laterocollis, moderate bilateral hand dystonia, bilateral foot dystonia. | memantine |
Dystonia occurred in all six patients (Table 1). Dystonia had a variable distribution and wide range of severity (Table 1). Two patients had focal dystonia, three had multifocal dystonia, and one patient had generalized dystonia. The face was involved in two patients and one had blepharospasm. Speech was dysarthric in two patients but it was difficult to characterize because of apparent combination of ataxic and dystonic dyarthria. None of our patients had trunk dystonia. Appendicular and cervical dystonia were most common; both types were present in four patients. The upper extremities were involved in all four patients, while the lower extremity was also involved in three of them. All four patients with cervical dystonia had torticollis, three also had laterocollis, and two had retrocollis. Three patients with cervical dystonia also had head tremor. The frequency of horizontal head oscillations was 4.0 ± 2.3 Hz, while vertical oscillations were 3.6 ± 2.4 Hz. Differences in frequency of eye and head oscillations supported for the lack of temporal correlation between eye and head oscillations. These results suggested that head oscillations were not compensatory or causative for the eye oscillations. The results also suggest that eye oscillations were not compensatory physiological eye movements, such as vestibulo-ocular reflex evoked by the head oscillations, but they were part of the phonemoenology of ocular palatal tremor plus dystonia. The Burke Fahn Marsde rating scale in our patients ranged from 6 to 90 (Table 2).
Table 2.
This table summarizes total and subcategory values of Burke Fahn Marsden dystonia rating scale from six patients with OPTD.
| Patient # | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Eye | 0 | 0 | 4.5 | 0 | 0 | 0 |
| Mouth | 0 | 0 | 1.5 | 0.5 | 0 | 2 |
| Speech/swallowing | 2 | 0 | 0 | 1 | 0 | 16 |
| Neck | 0 | 6 | 0 | 4 | 8 | 8 |
| Right arm | 12 | 0 | 0 | 6 | 0 | 16 |
| Left arm | 12 | 1 | 0 | 6 | 0 | 16 |
| Trunk | 0 | 0 | 0 | 0 | 0 | 0 |
| Right leg | 9 | 0 | 0 | 9 | 0 | 16 |
| Left leg | 9 | 0 | 0 | 9 | 0 | 16 |
| Total | 44 | 7 | 6 | 35.5 | 8 | 90 |
The burden of dystonia on the activity of daily living was also variable when compared to other manifestations of OPTD such as vertigo, diplopia, oscillopsia, and ataxia. The three patients, who were most concerned about dystonia, were the least cognizant of the eye movement deficits. In these cases the primary reason for their clinic visit was dystonia; ocular and palatal tremor were incidental findings discovered during neurological examination. One patient was primarily affected by diplopia and vertigo. Although moderate, dystonia was least concerning to her. The subjective disability due to dystonia and diplopia appeared to be equal in one patient. One patient was confined to bed and was ventilator dependent due to brainstem hemorrhage. Oscillopsia and generalized dystonia contributed significant burden to his overall morbidity. The lesions of Guillian-Mollaret triangle had acute onset in three patients, making it possible to estimate the latency of onset of dystonia in these patients. In one patient, dystonia started within in two months after the lesion, in another dystonia occurred after six months, and the third had a latency of several years. The onset of dystonia in first two patients paralleled that of eye oscillations.
Two of the three patients who primarily presented for the symptoms related to their dystonia were treated with botulinum toxin injections. Two of three patients who were most concerned for oscillopsia were treated with memantine, while one was treated with combination of memantine, gabapentin, and clonazepam. These treatments had modest benefit for the oscillopsia.
Discussion
We describe a novel OPT variant that includes dystonia (OPTD) in six patients. All patients had typical lesions causing a breach in the Guillian-Mollaret triangle and pseudohypertrphy of inferior olive. The head oscillations were not compensatory or adaptive response to suppress eye nystatgmus as seen in spasmus nutans or infantile nystagmus syndrome10–13. The eye oscillations were not physiological phenomenon, the vestibuloocular reflex, in response to head oscillations14–16. None of the patients had structural lesions affecting basal ganglia on the MRI scans. It is therefore possible that dystonia in OPTD patients is merely a coincidence. The alternate suggestion is that dystonia in OPTD could be related to alterations in the cerebellar output17–21. Controversial role of cerebellum in etiology of dystonia is a significant caveat to the cerebellar theory for dystonia in OPTD22, 23. We will first outline pathophysiology of OPT, and then discuss the speculated mechanism for dystonia as a variant of OPT.
Elegant experiments have demonstrated that disruption of the Guillian-Mollaret triangle leads to pseudohypertrophy and abnormal increase in soma-somatic gap junctions in the inferior olive 2–6. The electrotronic coupling through these gap junctions leads to increased synchronous output from the inferior olive. Typical signs of OPT such as eye and palate oscillations develop as the inferior olive becomes enlarged5, 24. A dual-mechanism theory for OPT suggested that synchronized inferior olive oscillations are altered by maladaptive discharges from the cerebellum7. The action potentials from the inferior olivary neurons travel into climbing fibers and give rise to complex spikes in the Purkinje cells. The collaterals of climbing fibers also projects to the deep cerebellar nuclei and then also to the Purkinje neurons, this time via granular cell layer. Hence the synchronized oscillation signal from the inferior olive is received twice by the Purkinje neurons, once through the climbing fibers and then after a small delay through the parallel fiber and granular cell layer. Such redundant and randomized timed delayed signals to the Purkinje neurons cause maladaptive learning 7. This phenomenon induces an independent cerebellar signal that distorts the output of inferior olive as it passes through the cerebellum7. We speculate that distorted cerebellar output in patients with OPT might cause dystonia, hence the variant OPTD 17–21, 23.
The syndrome of OPT was reported in form of several studies and case reports in past. To our knowledge this is the first description of dystonia in OPT patients. Two of the previous large studies on OPT by one of the authors for this study (AS) included several cases of OPT patients – most from two of the nation’s leading eye movement clinics and laboratories 7, 25. Only one of these 21 patients had dystonia. Therefore the reason for lack of report of dystonia in previous literature with OPT could be extremely rare incidence of OPTD. It is also possible that dystonia had a delayed onset, and was considered a “separate” entity in previous literature. The four of the six patients were seen in Emory movement disorders clinic for dystonia, hence there was a clear selection bias. Nevertheless, the goal of this study is not to delineate the epidemiology of OPTD, but to describe it as a new syndromic entity co-occurring with the OPT.
The severity of dystonia was mild in some patients with OPTD. Such mild form of dystonia can be expected in an OPT variant called progressive ataxia and palatal tremor 8. We did not consider the diagnosis of progressive ataxia and palatal tremor in any of our patients because all of them had primary (acquired) lesion causing breach in Guillian-Molaret triangle, hence, explaining the basis for the conventional form of OPT. As a consequence further investigations for the etiologies of progressive ataxia and palatal tremor was not justified.
In conclusion, we describe a novel variant of OPT that includes dystonia (OPTD). We speculate that the dystonia in OPTD is more than just an epiphenomenon. It can be explained through contemporary concept of abnormal cerebellar output as a cause of OPT and dystonia. Future experiments and animal models are needed to support our hypothetical scheme describing the pathophysiology of OPTD. Future studies will also delineate the epidemiology and incidence of OPTD.
Footnotes
Author roles:
Aasef G. Shaikh: Research conception, organization, execution, data collection, statistical analysis, writing manuscript and editing manuscript.
Fatema F. Ghasia; H.A. Jinnah, Mahlon R. DeLong, Stewart A. Factor, and Alan Freeman: Research organization, execution, statistical analysis, data collection, editing manuscript.
Financial disclosures for the previous 12 months: None
Disclosures:
Funding sources and Conflict of Interest: This work was supported by Dystonia Medical Research Foundation Clinical Fellowship grant (AS), Knights Templar Eye Foundation grant (FG), Research to Prevent Blindness grant (FG), Blind Children’s Center grant (FG), the Dystonia Coalition NIH NS065701 (HAJ), and the Sartain Lanier Family Foundation (SAF). Authors thank Dr. John Leigh for collegial support and help with technical equipment. Dr. Leigh was supported by NIH EY06717.
Reference List
- 1.Guillain G, Mollaret P. Deux cas de moclonies synchrones et rhythmes velopharyngo-laryngo-oculo-diaphragmatiques. Reviews in Neurology. 1931;2:545–566. [Google Scholar]
- 2.Koeppen AH, Barron KD, Dentinger MP. Olivary hypertrophy: histochemical demonstration of hydrolytic enzymes. Neurology. 1980;30:471–480. doi: 10.1212/wnl.30.5.471. [DOI] [PubMed] [Google Scholar]
- 3.Sperling MR, Herrmann C., Jr Syndrome of palatal myoclonus and progressive ataxia: two cases with magnetic resonance imaging. Neurology. 1985;35:1212–1214. doi: 10.1212/wnl.35.8.1212. [DOI] [PubMed] [Google Scholar]
- 4.Birbamer G, Gerstenbrand F, Aichner F, et al. MR-imaging of post-traumatic olivary hypertrophy. Functional neurology. 1994;9:183–187. [PubMed] [Google Scholar]
- 5.Deuschl G, Toro C, Valls-Sole J, Zeffiro T, Zee DS, Hallett M. Symptomatic and essential palatal tremor. 1. Clinical, physiological and MRI analysis. Brain : a journal of neurology. 1994;117( Pt 4):775–788. doi: 10.1093/brain/117.4.775. [DOI] [PubMed] [Google Scholar]
- 6.Goyal M, Versnick E, Tuite P, et al. Hypertrophic olivary degeneration: metaanalysis of the temporal evolution of MR findings. AJNR American journal of neuroradiology. 2000;21:1073–1077. [PMC free article] [PubMed] [Google Scholar]
- 7.Shaikh AG, Hong S, Liao K, et al. Oculopalatal tremor explained by a model of inferior olivary hypertrophy and cerebellar plasticity. Brain : a journal of neurology. 2010;133:923–940. doi: 10.1093/brain/awp323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuel M, Torun N, Tuite PJ, Sharpe JA, Lang AE. Progressive ataxia and palatal tremor (PAPT): clinical and MRI assessment with review of palatal tremors. Brain. 2004;127:1252–1268. doi: 10.1093/brain/awh137. [DOI] [PubMed] [Google Scholar]
- 9.Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35:73–77. doi: 10.1212/wnl.35.1.73. [DOI] [PubMed] [Google Scholar]
- 10.Carl JR, Optican LM, Chu FC, Zee DS. Head shaking and vestibulo-ocular reflex in congenital nystagmus. Investigative ophthalmology & visual science. 1985;26:1043–1050. [PubMed] [Google Scholar]
- 11.Gresty M, Halmagyi GM, Leech J. The relationship between head and eye movement in congenital nystagmus with head shaking: objective recordings of a single case. The British journal of ophthalmology. 1978;62:533–535. doi: 10.1136/bjo.62.8.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gresty MA, Barratt HJ, Page NG, Ell JJ. Assessment of vestibulo-ocular reflexes in congenital nystagmus. Annals of neurology. 1985;17:129–136. doi: 10.1002/ana.410170205. [DOI] [PubMed] [Google Scholar]
- 13.Gresty MA, Ell JJ. Spasmus nutans or congenital nystagmus? Classification according to objective criteria The British journal of ophthalmology. 1981;65:510–511. doi: 10.1136/bjo.65.7.510-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaski D, Saifee TA, Buckwell D, Bronstein AM. Ocular tremor in Parkinson’s disease is due to head oscillation. Movement disorders : official journal of the Movement Disorder Society. 2013;28:534–537. doi: 10.1002/mds.25342. [DOI] [PubMed] [Google Scholar]
- 15.Leigh RJ, Martinez-Conde S. Tremor of the eyes, or of the head, in Parkinson’s disease? Movement disorders : official journal of the Movement Disorder Society. 2013;28:691–693. doi: 10.1002/mds.25478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaikh AG, Reich S, Zee DS. Pseudonystagmus--clinical features and quantitative characteristics. Nature reviews Neurology. 2010;6:519–523. doi: 10.1038/nrneurol.2010.103. [DOI] [PubMed] [Google Scholar]
- 17.Neychev VK, Gross RE, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiology of disease. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:7825–7833. doi: 10.1523/JNEUROSCI.22-17-07825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prudente CN, Pardo CA, Xiao J, et al. Neuropathology of cervical dystonia. Experimental neurology. 2013;241:95–104. doi: 10.1016/j.expneurol.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raike RS, Pizoli CE, Weisz C, van den Maagdenberg AM, Jinnah HA, Hess EJ. Limited regional cerebellar dysfunction induces focal dystonia in mice. Neurobiology of disease. 2012;49C:200–210. doi: 10.1016/j.nbd.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoons E, Tijssen MA. Pathologic changes in the brain in cervical dystonia pre- and post-mortem - a commentary with a special focus on the cerebellum. Experimental neurology. 2013;247:130–133. doi: 10.1016/j.expneurol.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Sadnicka A, Hoffland BS, Bhatia KP, van de Warrenburg BP, Edwards MJ. The cerebellum in dystonia - help or hindrance? Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2012;123:65–70. doi: 10.1016/j.clinph.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Lehericy S, Tijssen MA, Vidailhet M, Kaji R, Meunier S. The anatomical basis of dystonia: current view using neuroimaging. Movement disorders : official journal of the Movement Disorder Society. 2013;28:944–957. doi: 10.1002/mds.25527. [DOI] [PubMed] [Google Scholar]
- 24.Kim JS, Moon SY, Choi KD, Kim JH, Sharpe JA. Patterns of ocular oscillation in oculopalatal tremor: imaging correlations. Neurology. 2007;68:1128–1135. doi: 10.1212/01.wnl.0000258665.37827.f6. [DOI] [PubMed] [Google Scholar]
- 25.Shaikh AG, Thurtell MJ, Optican LM, Leigh RJ. Pharmacological tests of hypotheses for acquired pendular nystagmus. Annals of the New York Academy of Sciences. 2011;1233:320–326. doi: 10.1111/j.1749-6632.2011.06118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


