Abstract
The blood-brain barrier (BBB) consists of highly specialized cells including brain microvascular endothelial cells, astrocytes, microglia, pericytes, and neurons, which act in concert to restrict the entry of pathogens, immune cells, and soluble molecules into the central nervous system (CNS). If pathogens manage to cross the BBB and establish infection within the CNS, the BBB can open in a regulated manner to allow leukocyte transmigration into the CNS so that microbes, infected cells, and debris can be cleared. This review highlights how different inflammatory cytokines or signaling pathways disrupt or enhance BBB integrity in a way that regulates entry of neurotropic viruses into the CNS.
INTRODUCTION
Neurotropic viruses that trigger encephalitis are a significant cause of morbidity and mortality globally, resulting in clinical phenotypes that range in severity from mild cognitive impairment and memory loss to permanent central nervous system (CNS) damage and death [1,2]. However, most patients who are infected at peripheral sites with neurotropic viruses never develop evidence of CNS infection [3]. Since neuroinvasion occurs in only a small minority of infected patients, it is thought that host-pathogen interactions and immune system responses in peripheral organs and at the blood-brain barrier (BBB) prevent viruses from gaining access to and establishing infection within the CNS.
Structure of the BBB
In 1885, Paul Ehrlich noted that dyes administered intravenously into animals failed to enter the CNS, although his initial interpretation was that the dye did not stain the brain because of altered affinity rather than a physical barrier [4]. In 1909, Goldmann theorized the existence of a structural barrier in the brain because intravenously injected trypan blue dye failed to enter the CNS [5]. It was not until the mid-20th century when electron microscopy studies revealed the ultrastructural characteristics that define the BBB [6,7]. Brain microvascular endothelial cells (BMECs) line post-capillary venules and function as the primary structural component of the BBB. BMECs closely associate with the foot processes of astrocytes, which secrete soluble factors that promote tight junctions and barrier integrity, pericytes, which regulate angiogenesis, vessel integrity, and blood flow, and microglia, which release cytokines and matrix metalloproteinases (MMP) in response to pathogen-associated stimuli [8-10]. Cytokines and other inflammatory mediators secreted by these supporting cells regulate tight junctions composition and the opening and closing of the BBB (see below).
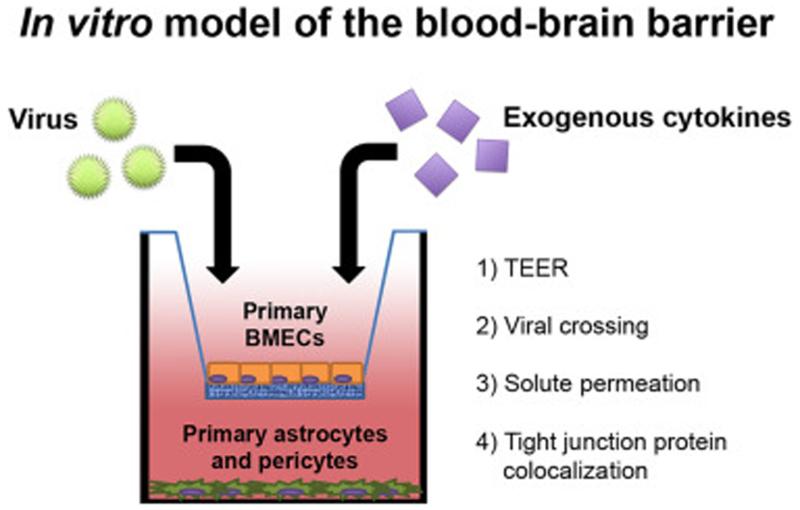
The use of in vitro models of the BBB in combination with in vivo studies in small animals has advanced our understanding of the cellular and molecular mechanisms that prevent viruses from disseminating into the CNS. Many studies that have defined the contributions of cytokines to BMEC permeability have used in vitro transwell systems in which BMECs are cultured over supporting astrocytes and/or pericytes (Figure 1). Colocalization of tight and adherens junction proteins is evaluated by immunofluorescence confocal microcopy, and barrier integrity is quantitated by measuring trans-endothelial electrical resistance or transit of virus/solutes across a BMEC monolayer [11,12]. In vivo assessment of BBB integrity primarily relies on the measurement of dye (e.g., fluorescein) or protein (e.g., immunoglobulin) permeation into the CNS [13].
Figure 1. In vitro model of the BBB.
Primary brain microvasulcar endothelial cells (BMECs) are cultured in a transwell above primary astrocytes and/or primary pericytes. Virus and/or exogenous cytokines are added to the upper chamber followed by measurement of trans-endothelial electrical resistance (TEER), virus crossing, and/or permeation of solutes into the lower chamber. BMECs also can be examined by immunofluoresence and confocal microscopy to assess expression and colocalization of tight junction proteins, which regulate permeability across BMEC monolayers.
Viral crossing of the BBB
Many viruses infect the CNS including retroviruses, morbillivirus, picornaviruses, rhabdoviruses, flaviviruses, bunyaviruses, alphaviruses, and coronaviruses, among others [14]. With the exception of rabies virus, most neuroinvasive viruses enter the CNS in a small subset of infected individuals. The host mechanisms that restrict viruses from crossing into the CNS vary depending on the route of entry of the particular virus.
The pathways by which individual neurotropic viruses enter the CNS have been difficult to demonstrate with precision. Viruses invade the CNS by either directly crossing the BBB or by circumventing the BBB via non-hematogenous routes of entry [15]. Pathways of direct viral transit across the BBB include: (1) Spread of viruses across BMEC tight junctions due to high levels of viremia and inflammation [12]; (2) Direct infection of BMECs and transport of nascently generated viruses across basolateral membranes [16]; and (3) A “Trojan horse” pathway in which infected leukocytes in the blood migrate across the BBB to seed the CNS with infectious virus [17,18]. This can occur in the context of inflammation-directed diapedesis or as part of tissue surveillance, which occurs at low levels at baseline. Some viruses can circumvent the BBB entirely by utilizing non-hematogenous routes of entry into the CNS. These mechanisms include: (1) Retrograde axonal transport of virions from peripheral nerves into the CNS [19]; and (2) Infection of the olfactory epithelium followed by transit of virus into the CNS across the cribriform plate and infection of cells in the olfactory bulb [20]. When considered together, these five pathways of entry into the CNS are not mutually exclusive and may vary depending on the immune context or specific virus. It is plausible that more than one pathway may be used by certain viruses. For example, Venezuelan equine encephalitis virus first invades the CNS via the cribriform plate, which subsequently triggers a delayed opening of the BBB that may allow a second wave of viral neuroinvasion directly across the BBB [20].
Young mice are more susceptible to viral encephalitis than adult mice, an observation that was described nearly 80 years ago [21]. Vulnerability of young mice to neurotropic viruses may occur in part as a result of BBB breakdown during viral infection, which has been demonstrated by intravenous injection of dye and measurement of permeation into the CNS [12]. This phenomenon is not limited to animal models, since human neonates and children also are more susceptible to many forms of viral encephalitis [1]. Age-dependent effects on viral neuroinvasion can also be seen in the context of viruses that are not classically considered neurotropic. For example, old world alphaviruses including chikungunya virus (CHIKV) typically cause inflammatory arthritis and only rarely cause neurological disease in adults. In the 2006 epidemic of CHIKV on La Reunion Island, there were multiple cases of neuroinvasive CHIKV infection in neonates, which resulted in microcephaly, flaccid paralysis, cerebral palsy, seizure disorders, and even death [22-25]. Some have speculated that young animals have an immature BBB despite the fact that tight junctions and other relevant structural components observed in adult animals are present early in development [26,27]. Young animals may be more vulnerable to viral encephalitis as a result of a combination of host factors, with BBB breakdown being just one of the variables.
Modulators of BBB integrity
Host factors regulate BBB integrity both to prevent pathogen invasion into the CNS and if necessary, to enable leukocyte transmigration after a neuroinvasive infection is established. The balance and type of cytokines and their cumulative effects at the BBB are complex and regulated by multiple signaling pathways and cell types, including BMECs, astrocytes, and pericytes.
(a) Type I and Type III interferons
Interferon (IFN)-α/β signaling through the type I IFN receptor (IFNAR1/IFNAR2) activates an antiviral program that up-regulates IFN-stimulated genes (ISGs), which antagonize viral replication in many cell types. Recent studies have shown that activation of viral RNA-sensing pathways in BMECs leads to Rac-1-dependent tight junction formation and enhanced BBB integrity to prevent viruses from transiting across BMEC monolayers [12]. The discovery that type I IFNs promote BBB integrity may explain the efficacy of IFN-β in the treatment of multiple sclerosis, an autoimmune disease characterized by BBB breakdown and unchecked migration of autoreactive T cells into the brain and spinal cord. IFN-λ, a type III IFN, also stabilizes tight junctions and limits viral crossing into the CNS of mice infected with flaviviruses. Animals lacking the IFN-λreceptor (IFNLR1/IL10β) exhibited earlier entry of West Nile virus (WNV) into the brain despite similar levels of viral replication in peripheral organs compared to wild-type animals, and treatment of WNV-infected mice with pegylated IFN-λ protected animals against virus neuroinvasion and lethality [13]. Type I and type III IFNs exert their barrier-tightening effects in BMECs through independent receptors but via a common non-canonical STAT1-independent signaling pathway that requires Rac1 activation and actin cytoskeletal reorganization [12,13].
Enveloped viruses that display phosphatidylserine on their viral membranes interact with Gas6 and Protein S, which bind to the TAM receptor tyrosine kinases (Tyro3, Axl, and Mertk) on endothelial cells [28,29]. All three TAM receptors contribute to BBB integrity in the context of different pathological conditions, with Tyro3 and Mertk having the most prominent effects. Mice lacking Mertk exhibit enhanced BBB permeability during infection with two unrelated encephalitic viruses, WNV and La Crosse encephalitis virus [30]. Tyro3 signaling promoted BBB integrity in the context of cerebral vascular occlusion [31], whereas signaling via Mertk on endothelial cells and possibly microglia was more important during viral infection [30]. Cooperative signaling via Mertk and the type I IFN receptor rapidly enhanced BMEC barrier integrity, suggesting that circulating TAM receptor ligands (Gas6 and Protein S) can amplify the barrier-stabilizing effects of type I IFN at the BBB.
Viral antagonism of type I IFN signaling is an established immune evasion mechanism, and some viruses may use this strategy to facilitate neuroinvasion. For example, mouse hepatitis virus (MHV), a coronavirus, perturbs the BBB by antagonizing IFN-β production, which resulted in decreased expression of junctional proteins ZO-1, VE-cadherin, and occludin [32].
(b) Pro-inflammatory cytokines and chemokines
In contrast to the barrier-tightening effects of type I and type III IFNs, the pro-inflammatory cytokines TNF-α, IL-6, IL-1β and IFN-λ disrupt BBB integrity. Daniels et al. demonstrated that BMEC-intrinsic expression of these pro-inflammatory cytokines at the BBB during WNV infection results in a loss of tight junction integrity [12]. Treatment of BMECs with TNF-α or IL-1β activated the RhoA/Rho kinase pathway, which disrupted tight junctions and enhanced permeability of BMEC monolayers in vitro. Consistent with a role for pro-inflammatory cytokines in causing BBB breakdown, mice lacking toll-like receptor 3 (TLR3) exhibited diminished cytokine (e.g., TNF-α, and IL-6) production systemically and enhanced BBB integrity during WNV infection, which partially protected mice from lethal WNV infection. TNF-α produced during infection of BMECs also up-regulated expression of the adhesion molecules ICAM-1, VCAM-1, and E-selectin, which facilitate leukocyte adhesion to BMECs and coincided with diminished TEER values measured across BMEC monolayers using an in vitro model of the BBB [33]. Thus, pro-inflammatory cytokines produced in BMECs during viral infection can enhance leukocyte recruitment as well as promote BBB breakdown.
Chemokine effects on BBB integrity have been demonstrated in the context of studies with HIV-1. Expression of the chemokine CCL2 may enhance migration of HIV-1-infected leukocytes across the BBB [34,35]. Treatment of BMEC monolayers with CCL2 triggered sequestration of endothelial β-catenin, which disrupted adherens junctions and transiently opened the endothelial cell barrier [36]. CXCL12 promotes lymphocyte transmigration across BMEC monolayers, providing a possible “Trojan horse” pathway for HIV-1 entry into the CNS. Migration of HIV-1-infected monocytes into the CNS leads to infection and replication in microglia as well as non-productive infection of astrocytes [35]. HIV infection of astrocytes may further disrupt BBB integrity if cytokines and/or chemokines that promote leukocyte trafficking into the CNS are induced [37].
Newer, more complex co-culture systems using multiple cell types (e.g., BMECs, astrocytes, and pericytes) have started to yield more mechanistic insight into the contributions of pericytes and astrocytes as regulators of BBB permeability. For example, in a co-culture system of pericytes with BMECs, Japanese encephalitis virus (JEV) infection of pericytes triggered secretion of IL-6, which resulted in degradation of ZO-1 in BMECs and endothelial barrier disruption [38]. WNV infection of astrocytes in vitro resulted in production of matrix metalloproteinases (MMP) that degrade tight junctions and promote BBB breakdown [39]. The relevance of MMPs during WNV infection is supported by the observation that degradation of tight junction proteins correlated with expression of MMPs in vivo [40]. The contribution of MMP9 in WNV neuroinvasion was established by the discovery that MMP9−/− mice were resistant to WNV infection [41]. In addition to the release of MMPs that degrade tight junctions, astrocytes also produce cytokines that contribute to BBB breakdown. For example, JEV infection of astrocytes triggered release of IL-6 and VEGF, which alter endothelial cell junctions by causing proteasomal degradation of ZO-1 [42]. Consistent with these in vitro data using mouse models, JEV infection in non-human primates causes activation of astrocytes, pericytes, and BMECs [43], all of which may contribute to the BBB breakdown that is seen clinically.
Microbiome and the BBB
The gastrointestinal microbiome recently was identified as a modulator of BBB integrity. Occludin and claudin-5 expression on BMECs in vivo is diminished in germ-free mice, and enhanced BBB permeability in germ-free mice can be reversed in response to administration of sodium butyrate, a chemical produced by Clostridium tyrobutyricum [44]. Remarkably, colonization of germ-free mice with only Clostridium tyrobutyricum was sufficient to restore BBB integrity [44], although this finding does not exclude a role for other gastrointestinal commensals in regulating BBB integrity. Since disruption of the BBB occurred in germ-free mice of all ages, colonization of the gut with normal flora early in life may contribute to age-dependent properties of the BBB during viral infection, although this has yet to be demonstrated. Studies are needed to define how changes in gut microbiota affect BBB permeability in the context of neuroinvasive viral infections.
Summary
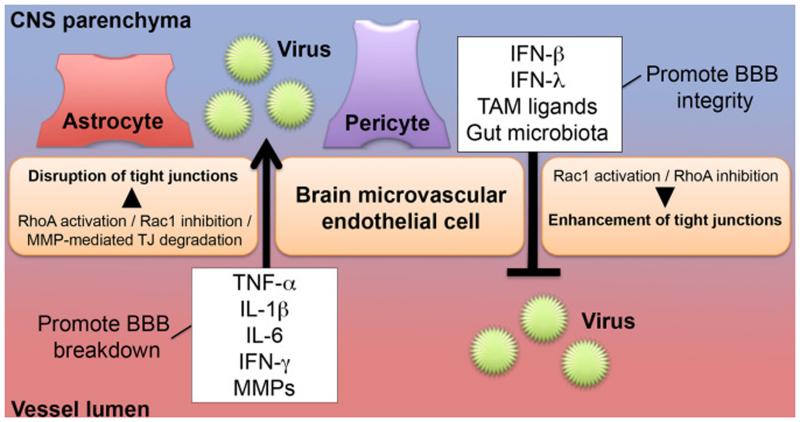
The multicellular nature of the BBB makes the study of neuroinvasive viral infections a complex and intriguing field of study (Figure 2). The use of complementary in vivo and in vitro models will continue to define molecular mechanisms by which proteins, cell types, and microbes affect viral neuroinvasion at the BBB. Furthermore, the field is only beginning to define how factors such as age, gastrointestinal microbiota, and immune signaling interact to affect entry into the CNS of neurotropic viruses. These studies may yield new therapeutic strategies for modulating BBB integrity and viral entry into the CNS, which also might be relevant for autoimmune diseases in which BBB penetration by leukocytes contributes to disease pathogenesis.
Figure 2. Regulation of BBB integrity during viral infection.
Pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6, IFN-γ which are expressed either luminally or abluminally) act to open the BBB by causing breakdown of tight junctions. Astrocytes, pericytes, BMECs, leukocytes, and/or other cell types may produce these cytokines. MMPs disrupt BBB integrity during viral infection by directly degrading tight junctions. Collectively, the pro-inflammatory cytokines and MMPs facilitate viral crossing into the CNS parenchyma. By contrast, tight junction stabilization occurs in response to signals by type I IFNs (e.g., IFN-β) or type III IFN (IFN-λ). TAM receptor ligands induce signals that augment this effect by cooperating with type I IFN to activate Rac1, rearrange actin filaments, and stabilize endothelial tight junctions. Gut microbiota enhance BBB integrity as well, although its mechanistic role during neuroinvasive viral infection is yet to be defined.
HIGHLIGHTS.
Neurotropic viruses invade the CNS via several routes including direct transit across the blood-brain barrier (BBB).
Pro-inflammatory cytokines promote BBB breakdown during viral infection.
IFN-β, IFN-λ, and TAM receptor signaling enhance BBB integrity.
Gastrointestinal microbiota modulate BBB function.
ACKNOWLEDGEMENTS
This work was supported by NIH grants U19 AI083019 and R01 AI101400. J.J.M. was supported by an NIH training grant, T32-AR007279 and a Rheumatology Research Foundation Scientist Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gaensbauer JT, Lindsey NP, Messacar K, Staples JE, Fischer M. Neuroinvasive Arboviral Disease in the United States: 2003 to 2012. Pediatrics. 2014;134:e642–e650. doi: 10.1542/peds.2014-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stahl J-P, Mailles A. What is new about epidemiology of acute infectious encephalitis? Current Opinion in Neurology. 2014;27:337–341. doi: 10.1097/WCO.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 3.Barzon L, Pacenti M, Sinigaglia A, Berto A, Trevisan M, Palù G. West Nile virus infection in children. Expert Review of Anti-infective Therapy. 2015:1–14. doi: 10.1586/14787210.2015.1083859. [DOI] [PubMed] [Google Scholar]
- 4.Ehrlich P. Das sauerstufbudurfnis des organismus, in Eine Farbenanalytische Studie. Hirschwald, Berlin: 1885. [Google Scholar]
- 5.Goldmann E. Vitalfarbung am zentralnervensystem. Abhandl Konigl preuss Akad Wiss. 1913;1:1–60. [Google Scholar]
- 6.van Breemen VL, Clemente CD. Silver deposition in the central nervous system and the hematoencephalic barrier studied with the electron microscope. The Journal of Biophysical and Biochemical Cytology. 1955;1:161–166. doi: 10.1083/jcb.1.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. The Journal of Cell Biology. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia L, Ju T, Westmuckett A, An G, Ivanciu L, McDaniel JM, Lupu F, Cummings RD, McEver RP. Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. The Journal of Cell Biology. 2004;164:451–459. doi: 10.1083/jcb.200311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkins BT, Davis TP. The Blood-Brain Barrier/Neurovascular Unit in Health and Disease. Pharmacological Reviews. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 10.Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of Microglia in Central Nervous System Infections. Clinical Microbiology Reviews. 2004;17:942–964. doi: 10.1128/CMR.17.4.942-964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda M, Nakagawa S, Hayashi K, Kitagawa N, Tsutsumi K, Nagata I, Niwa M. Adrenomedullin Improves the Blood–Brain Barrier Function Through the Expression of Claudin-5. Cellular and Molecular Neurobiology. 2006;26:109–118. doi: 10.1007/s10571-006-9028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Daniels BP, Holman DW, Cruz-Orengo L, Jujjavarapu H, Durrant DM, Klein RS. Viral Pathogen-Associated Molecular Patterns Regulate Blood-Brain Barrier Integrity via Competing Innate Cytokine Signals. mBio. 2014;5 doi: 10.1128/mBio.01476-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Lazear HM, Daniels BP, Pinto AK, Huang AC, Vick SC, Doyle SE, Gale M, Klein RS, Diamond MS. Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Science Translational Medicine. 2015;7:284ra259–284ra259. doi: 10.1126/scitranslmed.aaa4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swanson PA, II, McGavern DB. Viral diseases of the central nervous system. Current Opinion in Virology. 2015;11:44–54. doi: 10.1016/j.coviro.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koyuncu OO, Hogue IB, Enquist LW. Virus Infections in the Nervous System. Cell host & microbe. 2013;13:379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dittmar S, Harms H, Runkler N, Maisner A, Kim KS, Schneider-Schaulies J. Measles Virus-Induced Block of Transendothelial Migration of T Lymphocytes and Infection-Mediated Virus Spread across Endothelial Cell Barriers. Journal of Virology. 2008;82:11273–11282. doi: 10.1128/JVI.00775-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Tapia D, Loiacono CM, Kleiboeker SB. Replication of West Nile virus in equine peripheral blood mononuclear cells. Veterinary Immunology and Immunopathology. 2006;110:229–244. doi: 10.1016/j.vetimm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Verma S, Lo Y, Chapagain M, Lum S, Kumar M, Gurjav U, Luo H, Nakatsuka A, Nerurkar VR. West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: Transmigration across the in vitro blood-brain barrier. Virology. 2009;385:425–433. doi: 10.1016/j.virol.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C-S, Yao Y-C, Lin S-C, Lee Y-P, Wang Y-F, Wang J-R, Liu C-C, Lei H-Y, Yu C-K. Retrograde Axonal Transport: a Major Transmission Route of Enterovirus 71 in Mice. Journal of Virology. 2007;81:8996–9003. doi: 10.1128/JVI.00236-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schäfer A, Brooke CB, Whitmore AC, Johnston RE. The Role of the Blood-Brain Barrier during Venezuelan Equine Encephalitis Virus Infection. Journal of Virology. 2011;85:10682–10690. doi: 10.1128/JVI.05032-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabin AB, Olitsky PK. Influence of host factors on neuroinvasiveness of vesicular stomatitis virus: I. Effect of age on the invasion of the brain by virus instilled in the nose. The Journal of Experimental Medicine. 1937;66:15–34. doi: 10.1084/jem.66.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajapakse S, Rodrigo C, Rajapakse A. Atypical manifestations of chikungunya infection. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2010;104:89–96. doi: 10.1016/j.trstmh.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Lemant J, Boisson V, Winer A, Thibault L, André H, Tixier F, Lemercier M, Antok E, Cresta MP, Grivard P, et al. Serious acute chikungunya virus infection requiring intensive care during the reunion island outbreak in 2005–2006*. Critical Care Medicine. 2008;36:2536–2541. doi: 10.1097/CCM.0b013e318183f2d2. [DOI] [PubMed] [Google Scholar]
- 24.Wielanek AC, Monredon JD, Amrani ME, Roger JC, Serveaux JP. Guillain-Barre Syndrome Complicating a Chikungunya Virus Infection. Neurology. 2007;69:2105–2107. doi: 10.1212/01.wnl.0000277267.07220.88. [DOI] [PubMed] [Google Scholar]
- 25.Singh S, Manimunda S, Sugunan A, Sahina Vijayachari P. Four cases of acute flaccid paralysis associated with chikungunya virus infection. Epidemiology & Infection. 2008;136:1277–1280. doi: 10.1017/S0950268807009739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saunders NR, Dreifuss J-J, Dziegielewska KM, Johansson PA, Habgood MD, Møllgård K, Bauer H-C. The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Frontiers in Neuroscience. 2014;8:404. doi: 10.3389/fnins.2014.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saunders NR, Liddelow SA, Dziegielewska KM. Barrier Mechanisms in the Developing Brain. Frontiers in Pharmacology. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meertens L, Carnec X, Lecoin Manuel P, Ramdasi R, Guivel-Benhassine F, Lew E, Lemke G, Schwartz O, Amara A. The TIM and TAM Families of Phosphatidylserine Receptors Mediate Dengue Virus Entry. Cell Host & Microbe. 2012;12:544–557. doi: 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharyya S, Zagórska A, Lew Erin D, Shrestha B, Rothlin Carla V, Naughton J, Diamond Michael S, Lemke G, Young John AT. Enveloped Viruses Disable Innate Immune Responses in Dendritic Cells by Direct Activation of TAM Receptors. Cell Host & Microbe. 2013;14:136–147. doi: 10.1016/j.chom.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Miner J, Daniels B, Shrestha B, Proenca-Modena J, Lew E, Lazear H, Gorman M, Lemke G, Klein R, Diamond M. The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nature Medicine. 2015 doi: 10.1038/nm.3974. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu D, Wang Y, Singh I, Bell RD, Deane R, Zhong Z, Sagare A, Winkler EA, Zlokovic BV. Protein S controls hypoxic/ischemic blood-brain barrier disruption through the TAM receptor Tyro3 and sphingosine 1-phosphate receptor. Blood. 2010;115:4963–4972. doi: 10.1182/blood-2010-01-262386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Bleau C, Filliol A, Samson M, Lamontagne L. Brain Invasion by Mouse Hepatitis Virus Depends on Impairment of Tight Junctions and Beta Interferon Production in Brain Microvascular Endothelial Cells. Journal of Virology. 2015;89:9896–9908. doi: 10.1128/JVI.01501-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roe K, Orillo B, Verma S. West Nile Virus-Induced Cell Adhesion Molecules on Human Brain Microvascular Endothelial Cells Regulate Leukocyte Adhesion and Modulate Permeability of the In Vitro Blood-Brain Barrier Model. PLoS ONE. 2014;9:e102598. doi: 10.1371/journal.pone.0102598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR. Breaking down the barrier: The effects of HIV-1 on the blood–brain barrier. Brain Research. 2011;1399:96–115. doi: 10.1016/j.brainres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eugenin EA, Clements JE, Zink MC, Berman JW. Human Immunodeficiency Virus Infection of Human Astrocytes Disrupts Blood–Brain Barrier Integrity by a Gap Junction-Dependent Mechanism. The Journal of Neuroscience. 2011;31:9456–9465. doi: 10.1523/JNEUROSCI.1460-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts TK, Eugenin EA, Lopez L, Romero IA, Weksler BB, Couraud P-O, Berman JW. CCL2 Disrupts the Adherens Junction: Implications for Neuroinflammation. Laboratory investigation; a journal of technical methods and pathology. 2012;92:1213–1233. doi: 10.1038/labinvest.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams R, Dhillon NK, Hegde ST, Yao H, Peng F, Callen S, Chebloune Y, Davis RL, Buch SJ. Proinflammatory cytokines and HIV-1 synergistically enhance CXCL10 expression in human astrocytes. Glia. 2009;57:734–743. doi: 10.1002/glia.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Chen C-J, Ou Y-C, Li J-R, Chang C-Y, Pan H-C, Lai C-Y, Liao S-L, Raung S-L, Chang C-J. Infection of Pericytes In Vitro by Japanese Encephalitis Virus Disrupts the Integrity of the Endothelial Barrier. Journal of Virology. 2014;88:1150–1161. doi: 10.1128/JVI.02738-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verma S, Kumar M, Gurjav U, Lum S, Nerurkar VR. Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology. 2010;397:130–138. doi: 10.1016/j.virol.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roe K, Kumar M, Lum S, Orillo B, Nerurkar VR, Verma S. West Nile virus-induced disruption of the blood–brain barrier in mice is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases. Journal of General Virology. 2012;93:1193–1203. doi: 10.1099/vir.0.040899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang P, Dai J, Bai F, Kong K-F, Wong SJ, Montgomery RR, Madri JA, Fikrig E. Matrix Metalloproteinase 9 Facilitates West Nile Virus Entry into the Brain. Journal of Virology. 2008;82:8978–8985. doi: 10.1128/JVI.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Chang C-Y, Li J-R, Chen W-Y, Ou Y-C, Lai C-Y, Hu Y-H, Wu C-C, Chang C-J, Chen C-J. Disruption of in vitro endothelial barrier integrity by Japanese encephalitis virus-Infected astrocytes. Glia. 2015;63:1915–1932. doi: 10.1002/glia.22857. [DOI] [PubMed] [Google Scholar]
- 43.Myint KSA, Kipar A, Jarman RG, Gibbons RV, Perng GC, Flanagan B, Mongkolsirichaikul D, Van Gessel Y, Solomon T. Neuropathogenesis of Japanese Encephalitis in a Primate Model. PLoS Neglected Tropical Diseases. 2014;8:e2980. doi: 10.1371/journal.pntd.0002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Kundu P, et al. The gut microbiota influences blood-brain barrier permeability in mice. Science Translational Medicine. 2014;6:263ra158–263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]