SUMMARY
Aims
Brain ischemia activates astrocytes in a process known as astrogliosis. Although this process has beneficial effects, excessive astrogliosis can impair neuronal recovery. Polyinosinic–polycytidylic acid (Poly IC) has shown neuroprotection against cerebral ischemia–reperfusion injury, but whether it regulates reactive astrogliosis and glial scar formation is not clear.
Methods
We exposed cultured astrocytes to oxygen–glucose deprivation/reoxygenation (OGD/R) and used a rat middle cerebral artery occlusion (MCAO)/reperfusion model to investigate the effects of Poly IC. Astrocyte proliferation and proliferation-related molecules were evaluated by immunostaining and Western blotting. Neurological deficit scores, infarct volumes and neuroplasticity were evaluated in rats after transient MCAO.
Results
In vitro, Poly IC inhibited astrocyte proliferation, upregulated Toll-like receptor 3 (TLR3) expression, upregulated interferon-β, and downregulated interleukin-6 production. These changes were blocked by a neutralizing antibody against TLR3, suggesting that Poly IC function is TLR3-dependent. Moreover, in the MCAO model, Poly IC attenuated reactive astrogliosis, reduced brain infarction volume, and improved neurological function. In addition, Poly IC prevented MCAO-induced reductions in soma size, dendrite length, and number of dendritic bifurcations in cortical neurons of the infarct penumbra.
Conclusions
By ameliorating astrogliosis-related damage, Poly IC is a potential therapeutic agent for attenuating neuronal damage and promoting recovery after brain ischemia.
Keywords: Astrogliosis, Cerebral ischemia–reperfusion, Poly IC, TLR3, TLR4
Introduction
Astrocytes are the most abundant cell type in the mammalian central nervous system (CNS). They are essential for maintaining a homeostatic environment in the CNS and for supporting normal neuronal function. All forms of brain injury can induce astrocyte activation known as reactive astrogliosis [1,2]. Astrogliosis is characterized by hypertrophic morphology, cell proliferation, and a rapid increase in glial fibrillary acidic protein (GFAP) expression. These morphologic changes are accompanied by secretion of many cytokines and growth factors. Although this reactive process is thought to be beneficial in certain pathologic conditions, excessive and persistent astrogliosis with compact glial scar formation inhibits interaction between neurons and obstructs neuronal axon regeneration, an effect that is detrimental to recovery from CNS injuries [3]. In this regard, moderating astrogliosis at the subacute stage might promote brain repair after injuries such as cerebral ischemia and trauma [4].
Toll-like receptors (TLRs) recognize distinct pathogen-associated molecular patterns as well as damage-associated molecular patterns and play an important role in immune and inflammatory responses. Reports show that TLRs, especially TLR2 and TLR4, trigger deleterious inflammatory effects in cerebral ischemia–reperfusion (I/R) injury by activating the TLR/myeloid differentiation factor 88 (MyD88)/NF-kB pathway [5]. By contrast, preconditioning with ligands of TLR4 or TLR2 attenuated brain ischemic injuries [6–12], possibly by modulating TLR-related inflammatory signals or inducing production of neuroprotective cytokines such as transforming growth factor (TGF)-β and interleukin (IL)-10.
Unlike all other Toll-like receptors, TLR3 ultimately signals through the MyD88-independent pathway to activate interferon (IFN) regulatory factors and generate type I interferons. Although TLR3 deficiency in mice does not alter outcome after stroke [9], reports have shown that TLR3 activation by ligand polyinosinic–polycytidylic acid (Poly IC) is associated with neuroprotection against brain ischemic injury [13–17]. However, the mechanistic details remain unknown. It has been shown that Poly IC-conditioned medium reduces proliferation of cultured human astrocytes under normoxic conditions [18], but it is unclear whether Poly IC regulates reactive astrogliosis and glial scar formation under postischemic conditions; thus, we do not know whether Poly IC affects ischemic outcome. To address this issue, we used ischemic models in vitro and in vivo to detect the effect of Poly IC on astrogliosis and determine its underlying mechanism.
Materials and Methods
Animals
Postnatal and adult male Sprague-Dawley rats were provided by the Center for Experimental Animals, Tongji Medical College, Huazhong University of Science and Technology. Animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Huazhong University of Science and Technology and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Primary Cortical Astrocyte Culture
Primary astrocyte cultures were prepared from cortex of 1-day-old neonatal rats as reported previously [19]. Briefly, the cerebral cortices were digested with 0.25% trypsin, and the dissociated cells were plated in flasks at a density of 1 × 106 viable cells/mL. Cells were cultured in DMEM/F-12 medium supplemented with 20% FBS, 100 mg/mL streptomycin, and 100 U/mL penicillin at 37°C in a 5% CO2, humidified incubator. The culture medium was changed every 2–3 days. On day 14 in vitro, the confluent cultures were incubated with 5 mM L-leucine methyl ester and shaken at 200 rpm with a thermostatic shaker for 6 h to reduce microglial cell contamination. Then, astrocytes that adhered to the flasks were collected, suspended in medium to a density of 1–2 × 105 cells/mL, and seeded on tissue culture plates or dishes coated with poly-D-lysine (100 μg/mL). We determined the purity of astrocytes by immunofluorescent staining with mouse anti-GFAP (Epitomics, Cambridge, UK). Counterstaining with DAPI was used to indicate nuclei. Results showed that more than 95% were GFAP-positive cells. All experiments were performed on cultures at 21–22 days in vitro.
Oxygen–Glucose Deprivation/Reoxygenation
We subjected astrocyte cultures to oxygen–glucose deprivation/reoxygenation (OGD/R) to simulate ischemia in vitro [20,21]. Briefly, cultured astrocytes were incubated in serum- and glucose-free DMEM/F12 medium in an anaerobic acrylic jar that was continuously supplied with a mixture of 95% N2 and 5% CO2. After 6-h OGD, the cells were transferred to standard culture conditions for an additional 24 h as the reoxygenation period. Poly IC (1 and 10 μg/mL) or normal saline was applied to the media at the beginning of reoxygenation. Cultures supplied with standard medium and maintained in normoxic conditions served as controls.
Assessment of Cell Viability and Proliferation
Cleavage of the tetrazolium dye MTT into formazan is used as an indication of cell viability [22]. In short, 0.5 mg/mL MTT was added into each well and incubated at 37°C for 4 h. Then, the blue reaction product, formazan, was solubilized in 100 μL dimethyl sulfoxide. The absorbance value at 570 nm was determined with a microplate reader. Each experiment was carried out in triplicate, and the results were expressed as percentages of control values. To assess cell proliferation, we incubated astrocytes with 10 μM 5-ethynyl-2′-deoxyuridine (EdU) during OGD or reperfusion [23]. Cells were then fixed and permeabilized, and EdU that had been incorporated into newly synthesized DNA was detected with the fluorescent azide probe (Cell-Light, EdU Alexa Fluor 567 Imaging Kit, Ruibo, Guangzhou). In addition, we labeled the cells with fluorescent Hoechst 33342 to assess the total cell number. Three random fields for each coverslip were scanned with a 40× objective, and EdU-positive cells were counted and presented as a percentage of the total cell number. Each experiment was carried out in triplicate and the results expressed as mean ± SD.
In vivo I/R Model
Transient brain ischemia was induced by the middle cerebral artery occlusion (MCAO) model as described previously [24–26]. In brief, rats were anesthetized with chloral hydrate and placed on a heating pad to maintain the body temperature at 37°C during surgery. A 3-0 monofilament nylon suture with a rounded tip was advanced from the external carotid artery into the internal carotid artery until it blocked the origin of the middle cerebral artery. Occlusion was confirmed by a reduction in regional cerebral blood flow to 15–20% of baseline as recorded by a laser Doppler flowmeter (MoorVMS-LDF, Axminster, UK). After 90 min of occlusion, the suture was withdrawn to restore blood flow. The wound was sutured, and the rat was allowed to recover from anesthesia before being returned to its cage. Sham-operated rats underwent the same procedure but without arterial occlusion. Animals were excluded from the study if laser Doppler flow metry indicated unsuccessful MCAO or if they died prematurely.
Poly IC (1.25 mg/kg) or normal saline was administrated intraperitoneally at the onset of reperfusion and at 1, 3, and 5 days after reperfusion. Two investigators blinded to groups evaluated the neurological deficits of each rat at 7 days of reperfusion using the Zea-Longa method [26] After neurological deficit evaluation, the rats were anesthetized, and the brains were removed and cut into seven 2-mm coronal slices. Then, the slices were incubated in 2% 2, 3, 5-triphenyltetrazolium chloride monohydrate (TTC) at 37°C for 10–20 min, followed by 4% paraformaldehyde fixation. The brain slices were photographed, and the infarct area was analyzed by NIH ImageJ software. The total infarct volume was calculated by integration of the infarct areas in sequential 2-mm-thick brain sections [27]. To control for the interference of brain edema, the infarcted volume was corrected by subtracting the volume of ipsilateral hemisphere that was not infarcted from the contralateral hemisphere volume and expressing it as a percentage of the contralateral hemisphere volume [28].
Immunocytochemistry for GFAP and Neurocan Expression
Brain sections and astrocytes cultured on coverslips were immunostained for GFAP or neurocan as previously described [19,29,30]. The sections or cells were permeabilized with 0.1% Triton X-100 for 1 h at 37°C after formaldehyde fixation. Mouse anti-GFAP (1:200; Epitomics) or goat antineurocan (1:100, Santa Cruz Biotechnology, Dallas, TX, USA) primary antibody was applied in 5% bovine serum albumin (BSA) overnight at 4°C. After repeated washes, the cells or brain sections were incubated with FITC-conjugated secondary antibody in 5% BSA for 1 h at 37°C. The coverslips or tissue sections were mounted on glass microscope slides and photographed under fluorescent microscopy (Olympus BX51, Olympus Optical, Tokyo, Japan).
Western Blot Analysis for GFAP, TLR3, and TLR4 Protein Expression
Total protein was isolated from the cultured astrocytes or ischemic cortex after various treatments. Equal amounts of protein (30 μg) per lane were separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membranes. Membranes were incubated with mouse anti-GFAP antibody (1:1000; Epitomics), goat anti-actin antibody (1:500; Santa Cruz Biotechnology), rabbit anti-TLR3 antibody (1:1000; Abcam, Cambridge, UK), rabbit anti-TLR4 antibody (1:1000; Abcam), or mouse anti-GAPDH antibody (1:500; Santa Cruz Biotechnology) at 4°C overnight, followed by incubation with secondary antibodies for 2 h at room temperature. The blots were scanned and quantified with ImageJ software, and the protein levels were normalized to actin or GAPDH.
Western Blot Analysis for Neurocan Secreted by Astrocytes
Identical amounts of supernatant (500 μL) from each astrocyte sample were condensed using Amicon Ultra centrifugal filters (100 kD, Millipore, Billerica, MA, USA) as described previously. After protein concentration was determined, the protein samples were digested with 0.02 U/mL chondroitinase ABC (Sigma, St. Louis, MO, USA). Equal amounts of protein were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. Goat anti-neurocan (1:500, Santa Cruz Biotechnology) and horseradish peroxidase-conjugated anti-goat IgG were used to detect the neurocan expression.
Golgi Staining
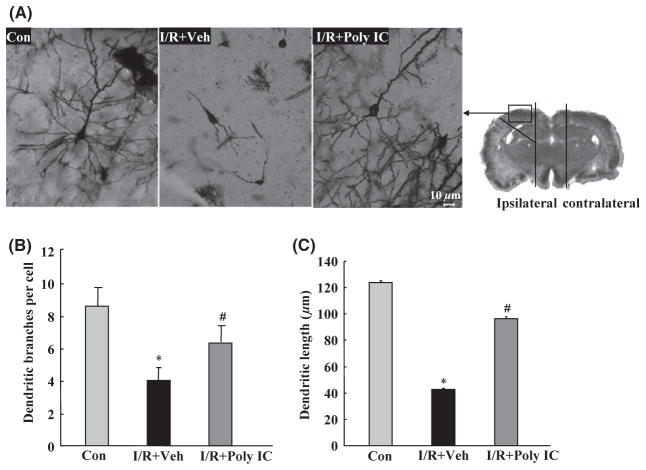
Seven days after ischemia, three animals from each group were deeply anesthetized, perfused transcardially with normal saline solution, and then fixed with 4% paraformaldehyde. The brains were removed and stained by the modified Golgi-Cox method [31,32]. Coronal sections of 50 μm thickness were obtained with a vibratome (Campden Instruments, MA752, Leicester, UK). A Zeiss fluorescence microscope with 40 × objective was used to obtain images under differential interference contrast. Ischemic penumbral regions were defined as described previously [33]. The midline between the two hemispheres was identified and then two longitudinal cuts were made at approximately 2 mm from the midline. A transverse diagonal cut at approximately the “2 o’clock” position (as shown in Figure 6) was made to separate the core from the penumbra in the adjacent cortex. We studied the golgi-impregnated neurons in the region of ischemic penumbra and measured dendritic branching and length using NIH ImageJ software [31].
Figure 6.
Effects of Poly IC (1.25 mg/kg) on neuronal morphology in the ischemic cortex of rats 7 days after focal cerebral ischemia/reperfusion (I/R). (A) Representative photomicrograph of Golgi-Cox-impregnated cortical neurons in the ischemic cortex from various treatment groups. Scale bar = 10 μm. (B) Quantitative analysis of dendritic branch numbers in the ischemic cortex of rats after I/R. (C) Quantitative analysis of dendritic length in the ischemic cortex of rats after I/R. Data are presented as the mean ± SEM. n = 3 per group. *P < 0.05 versus sham group; #P < 0.05 versus I/R+vehicle (Veh) group.
Statistical Analysis
In vitro data are expressed as mean ± SD, and in vivo data are expressed as mean ± SEM. Statistical analysis of the results was carried out by one-way ANOVA with SPSS. Ranked data of neurological deficit scores were analyzed by the nonparametric Kruskal–Wallis test. Differences were considered significant at P < 0.05.
Results
Effects of Poly IC on Astrocyte Proliferation After OGD/R
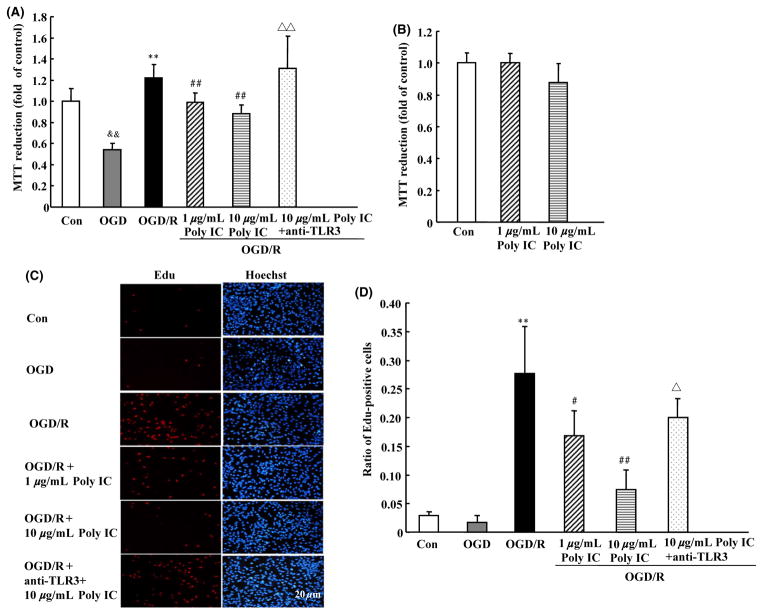
Compared with normoxic control, exposure of astrocytes to 6-h OGD resulted in a significant decrease in MTT reduction, indicating a decrease in cell viability. However, 6-h OGD followed by 24-h reoxygenation caused a significant increase in MTT reduction, indicating astrocyte activation after OGD/R. Poly IC treatment (1–10 μg/mL) dose dependently reduced OGD/R-induced astrocyte activation (Figure 1A). Various concentrations of Poly IC had no effect on astrocyte viability under normoxic conditions, as indicated by MTT reduction (Figure 1B). We examined changes in EdU-positive cells to further evaluate astrocyte proliferation. Edu labeling revealed an increase in astrocyte proliferation after 24 h of reoxygenation; the EdU-positive cell population was approximately 150% that in the control group. Poly IC treatment also inhibited astrocyte activation and proliferation, as indicated by a decline in the percentage of EdU-positive cells as compared with that in the OGD/R group (Figures 1C and D). The effects of Poly IC on astrocyte viability and proliferation were blocked by a TLR3 neutralizing antibody (Figures 1C, D).
Figure 1.
Poly IC inhibits oxygen–glucose deprivation/reoxygenation (OGD/R)-induced astrocyte proliferation. Cells were exposed to OGD for 6 h followed by reoxygenation for 24 h. Poly IC (1 and 10 μg/mL) or saline was applied to the media at the beginning of reoxygenation. (A and B) Cell viability was measured by the MTT assay. The control group (Con) was grown in standard medium under normoxic conditions. (C) Cell proliferation was assayed by EdU immunostaining. Scale bar = 20 μm. (D) Statistical analysis of the percentage of EdU-positive astrocytes in each group. Results are expressed as mean ± SD and are from three independent experiments. &&P < 0.01 versus control group; **P < 0.01 versus OGD group; #P < 0.05, ##P < 0.01 versus OGD/R group; ΔP < 0.05, ΔΔP < 0.01 versus 10 μg/mL Poly IC-treated group.
Effect of Poly IC on Astrocyte Morphology and GFAP Expression After OGD/R Injury
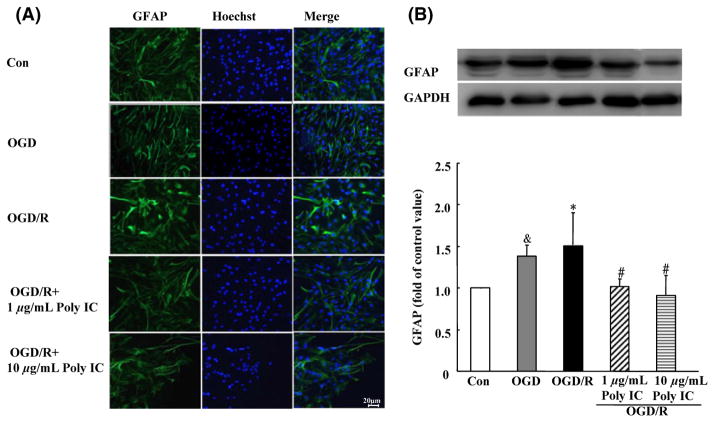
Astrocytes in the control group had a flat, polygonal morphology, whereas in the OGD group, the cells were clustered and stellate, with extended processes. At 24 h after reoxygenation, the astrocytes showed a hypertrophic morphology with increased GFAP immunoreactivity (Figure 2A). In addition, Western blot analysis showed that GFAP expression was upregulated in the OGD/R group compared with that in the OGD group. When treated with Poly IC (1–10 μg/mL), the cells became flat and small. Moreover, Poly IC dose dependently reduced GFAP expression in astrocytes after OGD/R injury compared with that in the vehicle-treated astrocytes (Figure 2B).
Figure 2.
Poly IC attenuates astrogliosis-associated overexpression of GFAP. (A) Double staining with anti-GFAP (green) and Hoechst (blue) in astrocytes after oxygen–glucose deprivation (OGD), OGD and 24 h reperfusion (OGD/R), or OGD/R + 1 or 10 μg/mL Poly IC. Scale bar = 20 μm. (B) Immunoblot analysis of GFAP expression. Values are expressed as mean ± SD and are from three independent experiments. &P < 0.05 versus control group; *P < 0.05 versus OGD group; #P < 0.05 versus OGD/R group.
Effect of Poly IC on Astrocytic Neurocan Expression and Secretion After OGD/R
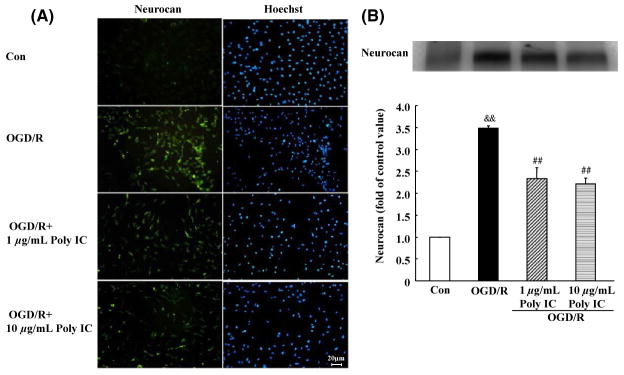
To further evaluate whether Poly IC inhibits glial scar formation, we examined the expression of neurocan, one of the main scar-associated markers. Based on immunocytochemistry results, the expression of neurocan did not change significantly after OGD compared with that of control cells. However, the expression of neurocan was markedly upregulated after 24 h of reoxygenation (Figure 3A). Poly IC treatment (1–10 μg/mL) downregulated neurocan expression compared with that of the vehicle-treated group. Western blot analysis of secreted neurocan levels in the conditioned medium showed similar results. OGD/R caused a significant increase in neurocan secretion that was reduced by Poly IC treatment (Figure 3B).
Figure 3.
Poly IC attenuates astrogliosis-associated overexpression of neurocan. (A) Double staining with antineurocan (yellow) and Hoechst (blue) in astrocytes after oxygen-glucose deprivation (OGD), OGD and 24 h reperfusion (OGD/R), or OGD/R + 1 or 10 μg/mL Poly IC. Scale bar = 20 μm. (B) Immunoblot analysis of neurocan secretion in conditioned medium of astrocytes (band at 130 kD). Results are expressed as a percentage of control OD value and are from three independent experiments. &&P < 0.01 versus control group; ##P < 0.01 versus OGD/R group.
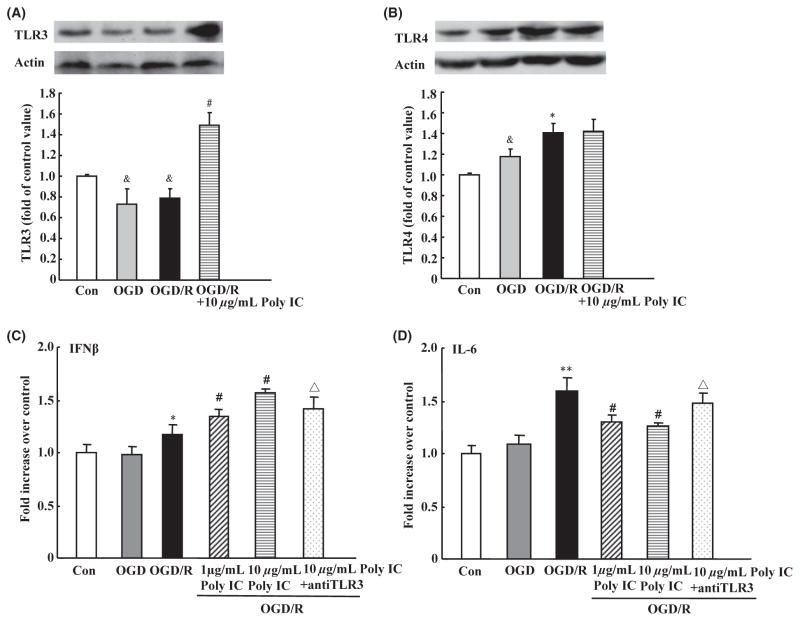
Effect of Poly IC on the Expression of TLR3 and TLR4 and Secretion of Cytokines After Astrocyte OGD/R Injury
After OGD/R, astrocytes exhibited a decrease in TLR3 expression but an increase in TLR4 expression. Poly IC (10 μg/mL) treatment of OGD/R astrocytes induced a significant increase in TLR3 expression (Figure 4A) but did not affect TLR4 expression (Figure 4B). Reactive astrocytes might secrete cytokine IL-6, which is associated with astrogliosis through a positive autocrine loop, and another cytokine, IFN-β, was able to suppress glial scar formation [34–37]. ELISA showed that IL-6 level in the medium was increased, but that IFN-β level did not change after OGD/R compared with levels in the normoxic control group. Treatment with different concentrations of Poly IC decreased IL-6 secretion and increased IFN-β secretion compared with that in the vehicle-treated group. However, the actions of Poly IC were neutralized by addition of an anti-TLR3 antibody (Figure 4C and D).
Figure 4.
Effects of Poly IC on astrocytic TLR3 and TLR4 expression and cytokine release after oxygen-glucose deprivation/reoxygenation (OGD/R). (A and B) Immunoblot analysis of TLR3 and TLR4 expression. (C and D) IFN-β and IL-6 levels were measured in astrocyte media by ELISA. Values are expressed as the mean ± SD and are from three independent experiments. &P < 0.05 versus control group; *P < 0.05, **P < 0.01 versus OGD group; #P < 0.05 versus OGD/R group; ΔP < 0.05 versus 10 μg/mL Poly IC-treated group.
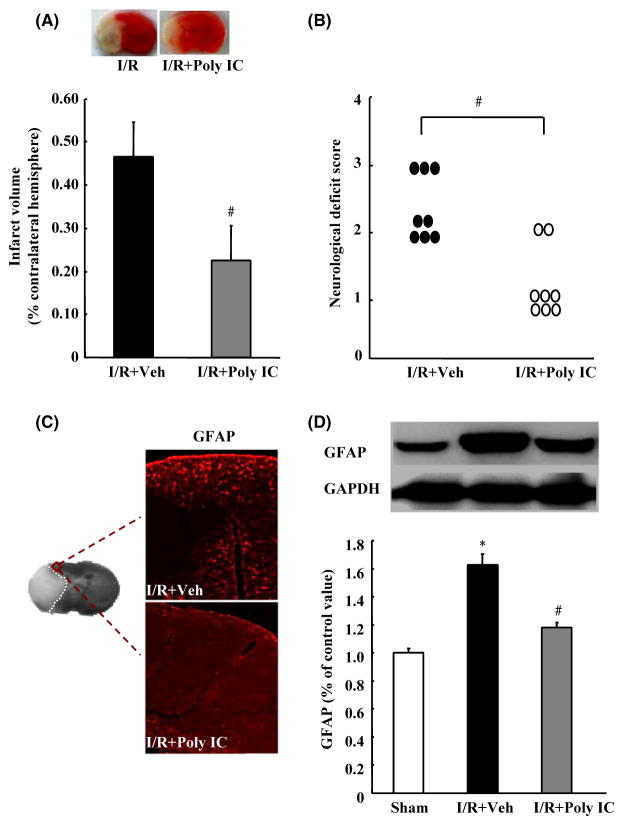
Effects of Poly IC on Brain Ischemic Injury and Astrocyte Proliferation After Transient MCAO in Rats
To observe the protective effects of Poly IC against cerebral I/R injury in vivo, we injected rats intraperitoneally with Poly IC (1.25 mg/kg) or normal saline at the onset of reperfusion and at 1, 3, and 5 days after reperfusion. TTC staining on day 7 postreperfusion showed that Poly IC treatment significantly reduced the infarct volume compared with that in the vehicle-treated group (Figure 5A). Poly IC treatment also significantly ameliorated neurological dysfunction at the same time point (Figure 5B). Furthermore, GFAP immunolabeling and Western blot analysis revealed increased expression of GFAP in the ischemic ipsilateral brain compared with that in the sham-operated group, but decreased GFAP expression after Poly IC treatment (Figure 5C and D).
Figure 5.
Poly IC attenuates cerebral ischemia–reperfusion (I/R)-induced brain damage and astrocyte proliferation in rats. Poly IC (1.25 mg/kg) or normal saline was administrated by intraperitoneal injection at the onset of reperfusion and at 1, 3, and 5 days after cerebral I/R (I/R+Poly IC and I/R+vehicle [Veh], respectively). (A) Representative 2,3,5-triphenyltetrazolium chloride-stained brain sections (2 mm thick) and quantification of the total infarct volume at 7 days postreperfusion. (B) Neurological function of rats after I/R injury, as evaluated by the Zea-Longa method. (C) Representative images of GFAP immunostaining in the ischemic penumbra. (D) Immunoblot analysis of GFAP expression. Results are expressed as mean ± SEM; n = 6–8 per group. *P < 0.05 versus sham group; #P < 0.05 versus I/R+Veh group.
Effects of Poly IC on Dendritic Damage After Cerebral I/R Injury in Rats
Focal cerebral I/R in rats caused significant reductions in soma size (Figure 6A), number of dendritic bifurcations (Figure 6B), and apical and basilar dendrite length (Figure 6C) of cortical neurons in the penumbra of the ischemic brain. These changes were prevented by Poly IC treatment.
Discussion
In this study, 6-h OGD followed by 24 h of reoxygenation induced cultured astrocytes to exhibit the typical manifestation of a glial scar, as evidenced by increased cell viability and increased EdU-positive astrocytes. OGD/R also upregulated the expression of GFAP and neurocan. Notably, TLR3 agonist Poly IC depressed activation and proliferation of astrocytes. We found corresponding results after a focal cerebral ischemia in rats. Treatment of rats with Poly IC significantly suppressed astrogliosis around the infarct tissue.
Several days to 1 week or even longer after ischemia is regarded as the subacute phase of ischemic injury. During this time, astrocytes are activated, proliferated, and secreted various extracellular matrix molecules [20,38]. The period of 16–24 h after reoxygenation of astrocytes that have undergone OGD in vitro parallels the in vivo subacute phase of cerebral ischemia [20,21]. Indeed, we observed scar-forming activity at 24 h. Results from our models demonstrating astrogliosis after I/R in vivo and in vitro are consistent with those of previous reports [4,20,21,39]. Astrocytes are thought to protect the penumbra during the acute phase of brain ischemia by shielding neurons from oxidative stress [40]. However, reactive gliosis has inhibitory effects on CNS regeneration and functional recovery when it is not resolved within the early chronic stage after injury [41,42].
Our results showed that TLR3 ligand Poly IC depressed astrocyte activation and proliferation caused by 6-h OGD and 24-h reoxygenation. In addition, Poly IC administration after cerebral I/R in rats suppressed astrogliosis in the peri-infarct zone and improved recovery after stroke. These findings are in line with reports that Poly IC preconditioning reduces cerebral ischemia injury in vivo and in vitro [14–16,19] and that Poly IC treatment after stroke exerts therapeutic effects [13]. The neuroprotective effects of Poly IC were associated with regulation of cerebral I/R inflammatory injury. Our results demonstrate that the therapeutic effects of Poly IC against cerebral ischemia may occur partly through inhibition of glial scar formation.
To determine the underlying mechanism of Poly IC-mediated inhibition of astrocyte proliferation, we measured astrocytic expression of TLR3 and TLR4 and release of cytokines after OGD/R injury. We observed an increase in the expression of TLR3 and IFN-β, accompanied by a decrease in the expression of IL-6. IFN-β is a type I interferon that displays multiple biological effects and acts as a cell cycle regulator [43,44]. It has shown neuroprotective effects that are associated with reduced pro-inflammatory cytokine expression and increased anti-inflammatory cytokine expression in sites of injury. Recently, it was reported that IFN-β gene delivery suppresses astrocyte activation and glial scar formation after spinal cord injury [45,46]. In contrast to IFN-β, IL-6 is thought to contribute to astrogliosis in response to brain injuries [34,35]. In addition, increased concentration of IL-6 in cerebrospinal fluid is correlated with poor stroke outcome [47]. Reactive astrocytes can induce IL-6 secretion that in turn leads to further astrocyte activation in a positive autocrine feedback loop [37]. Continuous IL-6 application in vivo via macroencapsulation of IL-6-expressing COS-7 cells induced massive astrogliosis, and increased IL-6 expression in transgenic mice caused reactive astrocytosis [34,36]. Downregulation of the IL-6/IL-6 receptor signaling pathway in astrocytes by IL-6 siRNA reduced OGD/reoxygenation-induced astrogliosis [21]. Here, we found that IFN-β was increased in astrocytes, whereas IL-6 was decreased, after Poly IC treatment, indicating that Poly IC might suppress astrocyte proliferation by enhancing the expression of IFN-β and lessening the expression of IL-6. Those actions of Poly IC were blocked by TLR3-neutralizing antibody, indicating that Poly IC inhibited OGD/R-induced astrocyte proliferation in a TLR3-dependent manner. Once stimulated, TLR3 is known to activate TIR domain-containing adaptor inducing IFN-β (TRIF) adaptor protein, which in turn leads to production of antiinflammatory molecules such as IFN-β and IL-12. TLR3 activation can modulate the TLR4/NF-kB signaling pathway and thus may inhibit generation of proinflammatory cytokines such as TNF-α and IL-6 [6,13,19,48].
Studies in experimental spinal cord injury models showed that TLR4 expression is increased in the gliosis lesion site and that TLR4 mutant mice exhibit sustained locomotor deficits and increased demyelination, astrogliosis, and macrophage activation [45,49]. In addition, it was suggested that TLR4 signaling in the spinal cord is required for exogenous IFN-β to suppress glial scar formation [45,49]. These data suggest that TLR4 is important for regulating gliosis as well as inflammatory processes after CNS injury. In our study, we also found increased expression of TLR4 in activated astrocytes. Although it has been reported that Poly IC downregulates TLR4 expression and its downstream signaling molecules [13,48], our results showed no effect of Poly IC on TLR4 expression in activated astrocytes.
Results from our study and previous reports indicate that TLR4, as well as TLR3, is important for modulating inflammation and gliosis after CNS injury. We hypothesize that Poly IC inhibition of I/R-induced astrocyte proliferation is mediated by upregulation of the TLR3/IFN-β signaling pathway and concurrent downregulation of IL-6 production, although the detailed mechanism has not been explored. Our study demonstrates that TLR3 ligand Poly IC reduces astrogliosis-associated injuries, thus improving functional recovery after ischemia in rats. This is the first study to show that the therapeutic effect of Poly IC involves inhibition of astrogliosis after I/R injury and that this reduction in astrogliosis may be mediated in part by regulation of the TLR3 signaling pathway. Additional research will be needed to identify the exact role of TLR3 activation after cerebral ischemia, likely in transgenic animals such as TLR3 conditional knockout mice.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 81001432, No. 81001425, NO. 81173038), and NIH (R01AT007317, R01NS078026). We thank Claire Levine for help with this manuscript.
Footnotes
Disclosure
The authors declare no conflict of interest.
References
- 1.Pekny M, Wilhelmsson U, Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett. 2014;565:30–38. doi: 10.1016/j.neulet.2013.12.071. [DOI] [PubMed] [Google Scholar]
- 2.Haupt C, Witte OW, Frahm C. Temporal profile of connexin 43 expression after photothrombotic lesion in rat brain. Neuroscience. 2007;144:562–570. doi: 10.1016/j.neuroscience.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 3.Pekny M, Pekna M. Astrocyte reactivity and reactive astrogliosis: Costs and benefits. Physiol Rev. 2014;94:1077–1098. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Z, Zhang Q, Yu Z, et al. Inhibiting cell cycle progression reduces reactive astrogliosis initiated by scratch injury in vitro and by cerebral ischemia in vivo. Glia. 2007;55:546–558. doi: 10.1002/glia.20476. [DOI] [PubMed] [Google Scholar]
- 5.Hamanaka J, Hara H. Involvement of Toll-like receptors in ischemia-induced neuronal damage. Cent Nerv Syst Agents Med Chem. 2011;11:107–113. doi: 10.2174/187152411796011312. [DOI] [PubMed] [Google Scholar]
- 6.Vartanian KB, Stevens SL, Marsh BJ, Williams-Karnesky R, Lessov NS, Stenzel-Poore MP. LPS preconditioning redirects TLR signaling following stroke: TRIF-IRF3 plays a seminal role in mediating tolerance to ischemic injury. J Neuroinflammation. 2011;8:140. doi: 10.1186/1742-2094-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens SL, Ciesielski TM, Marsh BJ, et al. Toll-like receptor 9: A new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008;28:1040–1047. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung PY, Stevens SL, Packard AE, et al. Toll-like receptor 7 preconditioning induces robust neuroprotection against stroke by a novel type I interferon-mediated mechanism. Stroke. 2012;43:1383–1389. doi: 10.1161/STROKEAHA.111.641522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyakkoku K, Hamanaka J, Tsuruma K, et al. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 2010;171:258–267. doi: 10.1016/j.neuroscience.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 10.Hua F, Ma J, Ha T, et al. Preconditioning with a TLR2 specific ligand increases resistance to cerebral ischemia/reperfusion injury. J Neuroimmunol. 2008;199:75–82. doi: 10.1016/j.jneuroim.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 12.Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 13.Wang PF, Fang H, Chen J, et al. Polyinosinicpolycytidylic acid has therapeutic effects against cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via TLR3. J Immunol. 2014;192:4783–4794. doi: 10.4049/jimmunol.1303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan LN, Zhu W, Li C, Xu XL, Guo LJ, Lu Q. Toll-like receptor 3 agonist Poly I: C protects against simulated cerebral ischemia in vitro and in vivo. Acta Pharmacol Sin. 2012;33:1246–1253. doi: 10.1038/aps.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Packard AE, Hedges JC, Bahjat FR, et al. Poly-IC preconditioning protects against cerebral and renal ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2012;32:242–247. doi: 10.1038/jcbfm.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gesuete R, Packard AE, Vartanian KB, et al. Poly-ICLC preconditioning protects the blood-brain barrier against ischemic injury in vitro through type I interferon signaling. J Neurochem. 2012;123(Suppl 2):75–85. doi: 10.1111/j.1471-4159.2012.07946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borysiewicz E, Doppalapudi S, Kirschman LT, Konat GW. TLR3 ligation protects human astrocytes against oxidative stress. J Neuroimmunol. 2013;255:54–59. doi: 10.1016/j.jneuroim.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- 19.Pan LN, Zhu W, Li Y, et al. Astrocytic Toll-like receptor 3 is associated with ischemic preconditioning-induced protection against brain ischemia in rodents. PLoS ONE. 2014;9:e99526. doi: 10.1371/journal.pone.0099526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang R, Zhang X, Zhang J, et al. Oxygen-glucose deprivation induced glial scar-like change in astrocytes. PLoS ONE. 2012;7:e37574. doi: 10.1371/journal.pone.0037574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Q, Li Y, Shen L, et al. Bone marrow stromal cells reduce ischemia-induced astrocytic activation in vitro. Neuroscience. 2008;152:646–655. doi: 10.1016/j.neuroscience.2007.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Zhuang H, Dore S. Heme oxygenase 2 is neuroprotective against intracerebral hemorrhage. Neurobiol Dis. 2006;22:473–476. doi: 10.1016/j.nbd.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Chehrehasa F, Meedeniya AC, Dwyer P, Abrahamsen G, Mackay-Sim A. EdU, a new thymidine analogue for labelling proliferating cells in the nervous system. J Neurosci Methods. 2009;177:122–130. doi: 10.1016/j.jneumeth.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Zan L, Zhang X, Xi Y, et al. Src regulates angiogenic factors and vascular permeability after focal cerebral ischemia-reperfusion. Neuroscience. 2014;262:118–128. doi: 10.1016/j.neuroscience.2013.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan T, Venkat P, Ye X, et al. HUCBCs increase angiopoietin 1 and induce neurorestorative effects after stroke in T1DM rats. CNS Neurosci Ther. 2014;20:935–944. doi: 10.1111/cns.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Q, Xia N, Xu H, et al. Betulinic acid protects against cerebral ischemia-reperfusion injury in mice by reducing oxidative and nitrosative stress. Nitric Oxide. 2011;24:132–138. doi: 10.1016/j.niox.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Wu H, Wu T, Li M, Wang J. Efficacy of the lipid-soluble iron chelator 2,2′-dipyridyl against hemorrhagic brain injury. Neurobiol Dis. 2012;45:388–394. doi: 10.1016/j.nbd.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Yu L, Jiang C, Chen M, Ou C. Bone marrow mononuclear cells exert long-term neuroprotection in a rat model of ischemic stroke by promoting arteriogenesis and angiogenesis. Brain Behav Immun. 2013;34:56–66. doi: 10.1016/j.bbi.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu T, Wu H, Wang J. Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain. J Neuroinflammation. 2011;8:22. doi: 10.1186/1742-2094-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tulsulkar J, Shah ZA. Ginkgo biloba prevents transient global ischemia-induced delayed hippocampal neuronal death through antioxidant and anti-inflammatory mechanism. Neurochem Int. 2013;62:189–197. doi: 10.1016/j.neuint.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Zhao Y, Zhang X, et al. Loss of sorting nexin 27 contributes to excitatory synaptic dysfunction by modulating glutamate receptor recycling in Down’s syndrome. Nat Med. 2013;19:473–480. doi: 10.1038/nm.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li CJ, Lu Y, Zhou M, et al. Activation of GABAB receptors ameliorates cognitive impairment via restoring the balance of HCN1/HCN2 surface expression in the hippocampal CA1 area in rats with chronic cerebral hypoperfusion. Mol Neurobiol. 2014;50:704–720. doi: 10.1007/s12035-014-8736-3. [DOI] [PubMed] [Google Scholar]
- 33.Ashwal S, Tone B, Tian HR, Cole DJ, Pearce WJ. Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke. 1998;29:1037–1046. discussion 1047. [PubMed] [Google Scholar]
- 34.Tilgner J, Volk B, Kaltschmidt C. Continuous interleukin-6 application in vivo via macroencapsulation of interleukin-6-expressing COS-7 cells induces massive gliosis. Glia. 2001;35:234–245. doi: 10.1002/glia.1088. [DOI] [PubMed] [Google Scholar]
- 35.Marz P, Heese K, Dimitriades-Schmutz B, Rose-John S, Otten U. Role of interleukin-6 and soluble IL-6 receptor in region-specific induction of astrocytic differentiation and neurotrophin expression. Glia. 1999;26:191–200. doi: 10.1002/(sici)1098-1136(199905)26:3<191::aid-glia1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 36.Fattori E, Lazzaro D, Musiani P, Modesti A, Alonzi T, Ciliberto G. IL-6 expression in neurons of transgenic mice causes reactive astrocytosis and increase in ramified microglial cells but no neuronal damage. Eur J Neurosci. 1995;7:2441–2449. doi: 10.1111/j.1460-9568.1995.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 37.Dietrich PY, Walker PR, Saas P. Death receptors on reactive astrocytes: A key role in the fine tuning of brain inflammation? Neurology. 2003;60:548–554. doi: 10.1212/01.wnl.0000042049.74547.7f. [DOI] [PubMed] [Google Scholar]
- 38.Anderson MF, Blomstrand F, Blomstrand C, Eriksson PS, Nilsson M. Astrocytes and stroke: Networking for survival? Neurochem Res. 2003;28:293–305. doi: 10.1023/a:1022385402197. [DOI] [PubMed] [Google Scholar]
- 39.Qu WS, Wang YH, Ma JF, et al. Galectin-1 attenuates astrogliosis-associated injuries and improves recovery of rats following focal cerebral ischemia. J Neurochem. 2011;116:217–226. doi: 10.1111/j.1471-4159.2010.07095.x. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Lundkvist A, Andersson D, et al. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- 41.Wanner IB, Deik A, Torres M, et al. A new in vitro model of the glial scar inhibits axon growth. Glia. 2008;56:1691–1709. doi: 10.1002/glia.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sukumari-Ramesh S, Alleyne CH, Jr, Dhandapani KM. Astrogliosis: A target for intervention in intracerebral hemorrhage? Transl Stroke Res. 2012;3:80–87. doi: 10.1007/s12975-012-0165-x. [DOI] [PubMed] [Google Scholar]
- 43.Kaynor C, Xin M, Wakefield J, Barsoum J, Qin XQ. Direct evidence that IFN-beta functions as a tumor-suppressor protein. J Interferon Cytokine Res. 2002;22:1089–1098. doi: 10.1089/10799900260442511. [DOI] [PubMed] [Google Scholar]
- 44.Borden EC, Sen GC, Uze G, et al. Interferons at age 50: Past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishimura Y, Natsume A, Ito M, et al. Interferon-beta delivery via human neural stem cell abates glial scar formation in spinal cord injury. Cell Transplant. 2013;22:2187–2201. doi: 10.3727/096368912X657882. [DOI] [PubMed] [Google Scholar]
- 46.Ito M, Natsume A, Takeuchi H, et al. Type I interferon inhibits astrocytic gliosis and promotes functional recovery after spinal cord injury by deactivation of the MEK/ERK pathway. J Neurotrauma. 2009;26:41–53. doi: 10.1089/neu.2008.0646. [DOI] [PubMed] [Google Scholar]
- 47.Ahmad M, Dar NJ, Bhat ZS, et al. Inflammation in ischemic stroke: Mechanisms, consequences and possible drug targets. CNS Neurol Disord Drug Targets. 2014;13:1378–1396. doi: 10.2174/1871527313666141023094720. [DOI] [PubMed] [Google Scholar]
- 48.Jiang W, Sun R, Wei H, Tian Z. Toll-like receptor 3 ligand attenuates LPS-induced liver injury by down-regulation of toll-like receptor 4 expression on macrophages. Proc Natl Acad Sci U S A. 2005;102:17077–17082. doi: 10.1073/pnas.0504570102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J Neurochem. 2007;102:37–50. doi: 10.1111/j.1471-4159.2007.04524.x. [DOI] [PubMed] [Google Scholar]