Abstract
Enzymes involved in de novo production of guanosine triphosphate (GTP) have been recently revealed as integral components of melanoma progression through modulation of the activity of small GTPases. Here, we discuss the biology and therapeutic implications of these findings.
Guanosine triphosphate (GTP), through its effects on GTP-binding proteins, is arguably the most important small molecule regulator of cellular processes in both healthy cells and for maintenance of transformed phenotypes. Importantly, levels of enzymes regulating nucleotide metabolism, including metabolism of guanylates are often changed in human cancers. 1–5
G-proteins, including the ones that are often constitutively activated in cancers (e.g. RAS, RAC1, RHOA, CDC42), act as “molecular switches” that fluctuate between guanosine diphosphate (GDP)-bound (inactive) and GTP-bound (active) states. This cycling is controlled by two types of regulatory proteins: GTPase-Activating Proteins (GAPs) promote the hydrolysis of G-protein-bound GTP molecules, thus converting a G-protein into its inactive GDP-bound state. Guanosine exchange factors (GEFs) release GDP from the G-protein, so it can bind GTP and become active again (Figure 1). However, very little is known about whether changes in intracellular GTP levels affect GTPases activity.
Figure 1. Inhibition of guanylate biosynthesis enzymes limits tumor invasion and growth.
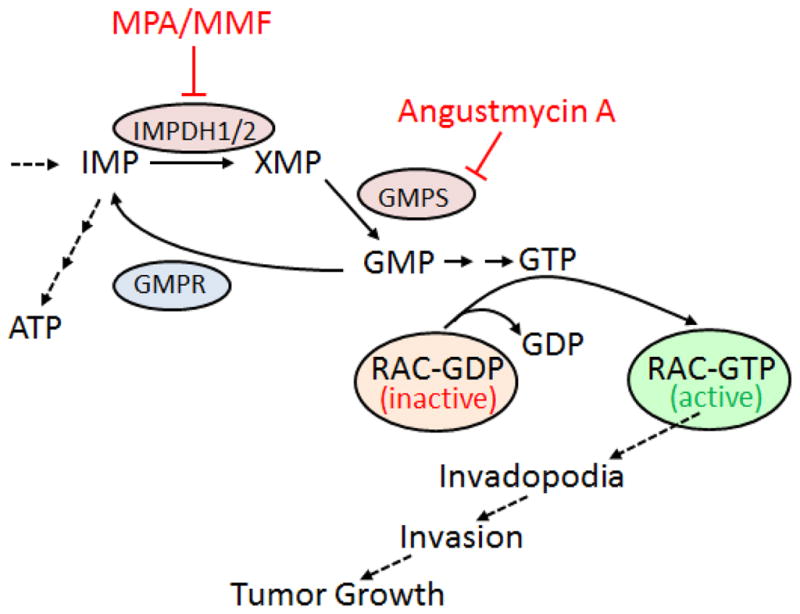
Simplified schematic of guanylate metabolic pathway and the proposed model. Enzymes are shown by ovals. Inhibitors of the pathway are indicated. IMP, inositol monophosphate; XMP, xanthosine monophosphate; GMP, guanosine monophosphate; GDP, guanosine diphosphate; GTP, guanosine triphosphate; ATP, adenosine triphosphate; IMPDH, inositol monophosphate dehydrogenase; GMPS, GMP synthase; GMPR, GMP reductase; MPA, mycophenolic acid; MMF, mycophenolate mofetil.
Notably, two recent works from our group have highlighted a previously unrecognized role played by guanylate metabolism enzymes in GTPases activation and tumor cell invasion. In our previous report, we established that guanosine monophosphate (GMP) reductase (GMPR, a key enzyme in guanylate metabolism (Figure 1)) is a melanoma metastasis suppressor, 3 directly linking its activity to suppression of GTP levels and subsequent inhibition of RAC1 and formation of invadopodia. In our current work, we identified GMP synthase (GMPS, another key enzyme in guanylate metabolism and a functional antagonist of GMPR (Figure 1)) as a driver of melanoma invasion.1
Most intriguingly, the variations in GTP amounts detected upon manipulation of GMPR levels were relatively modest; however they translated into a disproportionate effect on the activation status of RAC1 (and of RHOA and RHOC, to a lesser extent). Pharmacological targeting of other key guanylate biosynthesis enzymes, including inosine monophosphate dehydrogenases (IMPDH, Figure 1) led to similar results.
These findings raise an obvious question: why is GTPase activity affected if the intracellular concentration of GTP, albeit reduced, is still many-fold higher than that required for saturation of these GTPases? One intriguing hypothesis is that, similarly to ATP, 6,7 GTP is not homogeneously distributed throughout the cell but rather fractionated in a gradient. Moreover, information about intracellular GTP levels comes from methodologies that cannot detect local GTP changes in the cell, such as HPLC. Therefore, it is conceivable that spatiotemporal variation in GTP distribution throughout the cell may result in significant localized drops in GTP concentrations, ultimately affecting activity of GTPases (as well as other GTP-binding proteins).
The importance of GTP pools for tumor progression is further supported by the finding that alterations in the levels of guanylate metabolism enzymes seems to be a widespread mechanism adopted by multiple types of cancer cells. 3,8–10 Therefore, a more in depth understanding of guanylate metabolism is likely to lead to the discovery of novel therapeutic targets and/or drugs. One such example is our current finding that Angustmycin A, a potent antibiotic produced by fungi Streptomyces hygroscopius and a selective inhibitor of GMPS, reduces the invasion of metastatic melanoma cells in vitro and their growth as xenografts in vivo.1
Angustmyicin A (also known as decoyinine) is a nucleoside that was isolated for the first time in the mid-1950s. In one study, researcher evaluated the possible immunosuppressive properties of angustmycin A due to its convergence of action with mycophenolic acid (MPA), an immunosuppressing agent that works via inhibition of IMPDH, the enzyme acting upstream of GMPS (Figure 1). No significant activities were found. In a single follow-up study, analogues of angustmycin A showed minimal effects on cancer cells growth inhibition and, to the best of our knowledge, no further evaluations on its anti-tumor activities were done until our current work. Interestingly, MPA (in the form of its salt mycophenolate mofetil, MMF) was demonstrated to suppress growth of human tumor cell xenografts in mice. 8,9 However, our experiments demonstrated that Angustmycin A possesses higher anti-melanoma activity than MMF in in vivo settings. These findings underline the importance of guanylate metabolism enzymes for melanoma progression and identify a novel target for anti-melanoma therapy.
References
- 1.Bianchi-Smiraglia A, et al. Pharmacological targeting of guanosine monophosphate synthase suppresses melanoma cell invasion and tumorigenicity. Cell death and differentiation. 2015 doi: 10.1038/cdd.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mannava S, et al. Direct role of nucleotide metabolism in C-MYC-dependent proliferation of melanoma cells. Cell Cycle. 2008;7:2392–2400. doi: 10.4161/cc.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wawrzyniak JA, et al. A purine nucloetide biosynthesis enzyme guanosine monophosphate reductase is a suppressor of melanoma invasion. Cell Reports. 2013;5:493–507. doi: 10.1016/j.celrep.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannava S, et al. Depletion of deoxyribonucleotide pools is an endogenous source of DNA damage in cells undergoing oncogene-induced senescence. The American journal of pathology. 2013;182:142–151. doi: 10.1016/j.ajpath.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannava S, et al. Ribonucleotide reductase and thymidylate synthase or exogenous deoxyribonucleosides reduce DNA damage and senescence caused by C-MYC depletion. Aging. 2012;4:917–922. doi: 10.18632/aging.100512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imamura H, et al. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15651–15656. doi: 10.1073/pnas.0904764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Horssen R, et al. Modulation of cell motility by spatial repositioning of enzymatic ATP/ADP exchange capacity. The Journal of biological chemistry. 2009;284:1620–1627. doi: 10.1074/jbc.M806974200. [DOI] [PubMed] [Google Scholar]
- 8.Domhan S, et al. Molecular mechanisms of the antiangiogenic and antitumor effects of mycophenolic acid. Molecular cancer therapeutics. 2008;7:1656–1668. doi: 10.1158/1535-7163.MCT-08-0193. [DOI] [PubMed] [Google Scholar]
- 9.Dun B, et al. Mycophenolic acid inhibits migration and invasion of gastric cancer cells via multiple molecular pathways. PloS one. 2013;8:e81702. doi: 10.1371/journal.pone.0081702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arozarena I, et al. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]


