Abstract
The process of amyloid formation by the normally soluble hormone islet amyloid polypeptide (IAPP) contributes to β-cell death in type-2 diabetes and in islet transplants. There are no clinically approved inhibitors of islet amyloidosis and the mode of action of existing inhibitors is not well understood. Resveratrol, a natural polyphenol, has been reported to inhibit amyloid formation by IAPP and by the Alzheimer's disease Aβ peptide. The mechanism of action of this compound is not known, nor is its mode of interaction with IAPP. In this study, we use a series of IAPP variants to examine possible interactions between resveratrol and IAPP. Fluorescence assays, transmission electron microscopy and mass spectrometry demonstrate that resveratrol is a much less effective inhibitor of IAPP amyloid formation than the polyphenol (-)-epigallocatechin 3-gallate (EGCG) and, unlike EGCG, does not significantly disaggregate preformed IAPP amyloid fibrils. Resveratrol is also shown to interfere with thioflavin T assays. His-18 mutants, a truncation mutant, mutants of each of the aromatic residues, and of Arg-11 of IAPP were examined. Mutation of His to Gln or Leu reduces the ability of resveratrol to inhibit amyloid formation by IAPP, as do mutations of Arg-11, Phe-15, or Tyr-37 to Leu, and truncation to form the variant Ac-IAPP8-37, which removes the first seven residues to eliminate Lys-1 and the N-terminal amino group. In contrast, replacement of Phe-23 with Leu has a smaller effect. The data highlights Phe-15, His-18 and Tyr-37 as important for IAPP:resveratrol interactions and are consistent with a potential role of the N-terminus, and Arg-11 in polypeptide:resveratrol interactions.
Keywords: Amylin, Islet Amyloid Polypeptide, Resveratrol, Polyphenol, Amyloid, Type 2 Diabetes
Introduction
Islet amyloidosis, caused by the pathological aggregation of human islet amyloid polypeptide (IAPP, amylin) in the pancreatic Islets of Langerhans contributes to p-cell dysfunction in type 2 diabetes.1-6 Amyloid formation by IAPP also plays a role in the failure of islet transplants, while the prevention of islet amyloidosis prolongs graft survival.7-9 IAPP is produced as a prohormone, is processed in parallel with insulin, and is stored in the insulin secretory granule, from which it is released by the same stimuli that lead to insulin secretion.10 The mature polypeptide is 37 residues long, contains an amidated C-terminus and a disulfide bridge between residue 2 and residue 7 (Figure 1). IAPP normally acts as a partner to insulin in glucose metabolism, but forms amyloid in type 2 diabetes.11 There are no clinically approved inhibitors of islet amyloidosis despite its therapeutic relevance and the mode of action of existing inhibitors of in vitro toxicity is not well understand.
Figure 1.
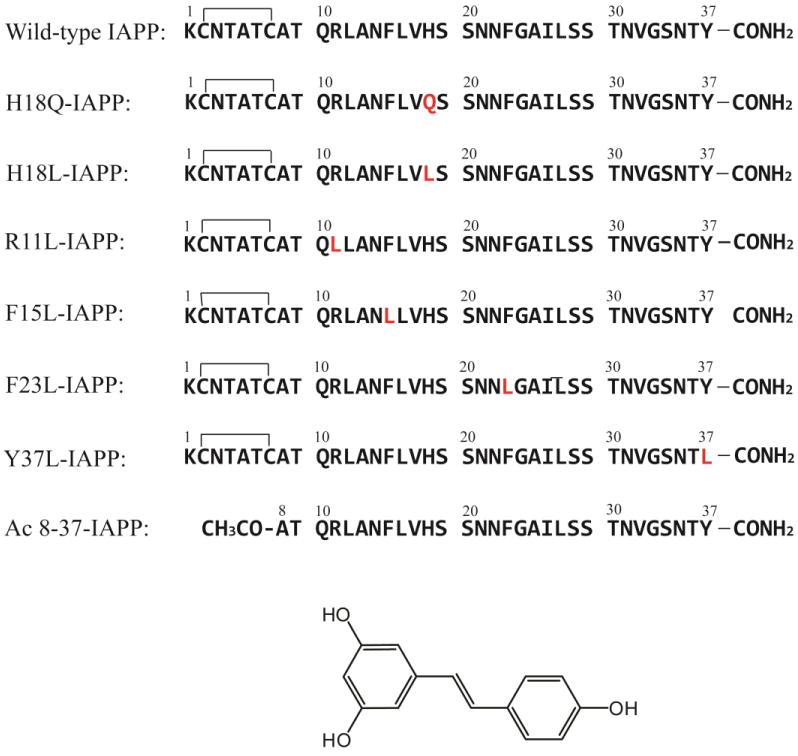
The primary sequence of IAPP and the IAPP variants studied here. The residues that differ from wild-type IAPP are highlighted in red. Wild-type IAPP and IAPP variants, with the exception of Ac 8-37-IAPP, all contain a disulfide bond between Cys-2 and Cys-7. All peptides contain an amidated C-terminus. The structure of resveratrol is shown at the bottom.
Polyphenols, a class of organic compounds with aromatic phenolic rings, have drawn particular attention as inhibitors of amyloid formation, including the inhibition of IAPP amyloid. For example, (-)-epigallocatechin 3-gallate (EGCG), the most abundant catechin in green tea, inhibits amyloid formation by Aβ, α-synuclein, IAPP and other polypeptides, and protects cultured p-cells against the toxic effects of human IAPP.12-19 EGCG is one of the most effective inhibitors of amyloid formation by IAPP known and disaggregates and remodels IAPP amyloid fibrils to smaller species.13,16,18 The compound is believed to divert amyloidogenic peptides into off-pathway aggregates that are incapable of further assembling to form amyloid.14,17,19 Resveratrol, a polyphenol present in red wine, has received considerable attention in the context of neurodegenerative diseases due to its anti-neuroinflammatory activity and because of its ability to inhibit amyloid formation by Aβ, the peptide linked to extracellular amyloid plaques in Alzheimer's disease (Figure 1).20-23 Resveratrol has been reported to inhibit IAPP amyloid formation and to protect against in vitro toxicity in cell culture, although it is not known if its ability to protect cells is due to the direct inhibition of interactions between IAPP toxic species and cells.24-26 Little is known about the mode of interaction of resveratrol with IAPP and its efficacy compared to EGCG. Indeed, little is known about the mechanism of any anti-IAPP amyloid agent.
Recent NMR studies, that made use of a non-physiological analogue of IAPP that lacks the normal amidated C-terminus, have led to the proposal that Lys-1 and His-18 are involved in the binding of resveratrol.27 The NMR spectra revealed that resonances from the side chain of His-18 exhibited the largest changes during a titration with resveratrol. On the basis of this work His-18 was proposed to be critical for resveratrol:IAPP interactions. Chemical shift changes for Lys-1 were also detected, suggesting that this residue could be a second site for resveratrol interactions. However, variants of IAPP with a free C-terminus behave differently to the physiological amidated form and assemble into amyloid on different time scales.28 Other work has highlighted the possible role of interactions between aromatic side chains and amyloid inhibitors,29 but this has not been examined for IAPP and resveratrol. П-cation interactions are important in stabilizing globular proteins and IAPP contains three or four positive charges depending on the pH: the N-terminus, Lys-1, Arg-11, and His-18. Thus, there is also the potential for interactions of the aromatic rings of polyphenols with the positively charged sites in IAPP. In this study, we compare the ability of resveratrol and EGCG to inhibit IAPP amyloid formation, examine the interaction of resveratrol with a series of IAPP variants designed to test the role of the aromatic residues and the possible role Arg-11, His-18 and the N-terminal disulfide bridged loop and critically examine the ability of resveratrol to remodel preformed amyloid fibrils.
Materials and Methods
Peptide synthesis, purification and sample preparation
Peptides were synthesized on a 0.1 or 0.25 mmol scale using 9-fluornylmethoxycarbonyl (Fmoc) chemistry on a CEM Liberty microwave peptide synthesizer. 5-(4′-fmoc-aminomethyl-3′, 5-dimethoxyphenol) valeric acid (Fmoc-PAL-PEG-PS) resin was used in order to incorporate an amidated C-terminus. Acetic anhydride was used to generate an acetylated N-terminus for the truncated 8-37 IAPP fragment. Fmoc protected pseudoproline dipeptide derivatives were incorporated at positions 9-10, 19-20, and 27-28 to facilitate the synthesis.30 β-branched residues, Arg, and all pseudoproline dipeptide derivatives were double coupled. A maximum temperature of 50 °C was used for the coupling of His and Cys in order to reduce the possibility of racemization.31 Peptides were cleaved from the resin by standard trifluoroacetic acid (TFA) methods. Crude peptides were partially dissolved in 20% acetic acid (v/v) and lyophilized. The dry peptide was redissolved in pure dimethyl sulfoxide (DMSO) at room temperature to promote the formation of the disulfide bond.32 Peptides were purified by reverse-phase HPLC using a Vydac or Proto 300 C18 preparative column (10 mm × 250 mm). A two buffer gradient system was used: buffer A consisted of 100% H2O and 0.045% HCl (v/v) and buffer B consisted of 80% acetonitrile, 20% H2O and 0.045% HCl. HCl was used as the counter ion because residual TFA can influence amyloid formation. Analytical HPLC was used to check the purity of peptides before use. MALDI-TOF mass spectrometry confirmed the correct molecular weight. IAPP, expected 3903.3, observed 3902.8; H18Q-IAPP, expected 3894.3, observed 3894.4; R11L-IAPP, expected 3860.3, observed 3860.9; H18L-IAPP, expected 3879.3, observed 3879.3; F15L-IAPP, expected 3871.3, observed 3872.0; F23L-IAPP, expected 3871.3, observed 3871.3; Y37L-IAPP, expected 3855.4, observed 3853.8; Ac 8-37 IAPP, expected 3225.5, observed 3225.1. Pure peptides were first dissolved in 100% hexafluoroisopropanol (HFIP) at a concentration of 1.6 mM and filtered to remove preformed aggregates. For kinetic studies, aliquots were lyophilized for 20∼24 h to remove HFIP. Resveratrol stock solutions were freshly prepared in pure DMSO.
Thioflavin-T fluorescence assays
Lyophilized peptides were dissolved in pH 7.4, 20 mM Tris buffer solution containing thioflavin-T and resveratrol at a final peptide concentration of 16 μM. 1% (v/v) DMSO was present in all solutions. Measurements were made at 25 °C using a Beckman Coulter DTX880 plate reader. An excitation wavelength of 430 nm and emission wavelength of 485 nm was used. For amyloid fibril remodeling studies, preformed fibrils were produced in advance. Thioflavin-T fluorescence was continuously monitored after the addition of resveratrol.
Transmission electron microscopy (TEM)
TEM was performed at the Life Science Microscopy Center at Stony Brook University. Aliquots removed from the fluorescence experiments were used for TEM analysis. 5 μL of peptide solution was placed on a carbon-coated Formvar 300 mesh copper grid for one minute and then negatively stained with saturated uranyl acetate for another minute.
Electrospray Ionization-mass spectrometry (ESI-MS)
Lyophilized peptides were dissolved in pH 7.4, 50:50 20 mM ammonium acetate: 20 mM ammonium bicarbonate buffer solution containing EGCG or resveratrol at a final peptide concentration of 32 μM. 1% (v/v) DMSO was present in all solutions. A Synapt HDMS quadrupole time-of-flight mass spectrometer (Micromass UK Ltd., Waters Corpn., Manchester, UK), equipped with a Triversa (Advion Biosciences, Ithaca, NY, USA) automated nano-ESI interface, was used for these analyses. A sampling cone voltage of 30 V was used, and an instrumental backing pressure of 2.0 mbar was applied to preserve protein-ligand interactions. Data were acquired over the range m/z 400–6,000.
Results and Discussion
The primary sequence of wild-type IAPP and the IAPP variants studied here are shown in Figure 1. The His-18 to Gln mutant (H18Q-IAPP) and the His-18 to Leu mutant (H18L-IAPP) allow us to test the role of His-18 in IAPP:resveratrol interactions. A Gln substitution was chosen because Gln has roughly similar volume and hydrophobicity as a neutral His residue, while Leu represents a more hydrophobic substitution. We used an IAPP fragment, residues 8-37 with an acetylated N-terminus (denoted Ac 8-37-IAPP), to test the importance of potential interactions with the positively charged Lys side chain and N-terminal amino group. This peptide is known to form amyloid with a morphology similar to that observed for wild-type IAPP.16 An Arg-11 to Leu mutant allows us to probe the role of this residue. Interactions between phenolic compounds and aromatic residues in amyloidogenic proteins have been suggested to be important for inhibitory effects and aromatic cation interactions can be energetically favorable.29,33 IAPP contains three aromatic residues, two Phe and one Tyr. We prepared three point mutants F15L-IAPP, F23L-IAPP and Y37L-IAPP to test the possible importance of interactions between resveratrol and aromatic amino acid side chains. The His and Arg mutants together with the truncation mutant allow us to probe potential π-cation interactions between the aromatic rings of the polyphenol and the positively charged sites in IAPP.
We first examined the stability of resveratrol at pH 7.4 as there have been differing reports on its stability in solution. NMR studies suggest that it can degrade in solution with a half-life on the order of 5 hours. We used ESI-MS to examine the effects of 24 hour incubation of the compound at pH 7.4. No change in the m/z ratio was observed and the value of (m+H), 229.04 corresponding to a molecular weight of 228.04, indicates that the compound had not degraded in this time frame (Supporting Information).41,42
The rate of amyloid formation was monitored initially using thioflavin-T fluorescence assays. Thioflavin-T is a small dye that undergoes an increase in fluorescent intensity upon binding to amyloid fibrils.34 Thioflavin-T can be used to monitor the kinetics of IAPP amyloid formation without altering the rate of aggregation under the conditions used.35 However, the use of the dye can be problematic for inhibition studies, since some compounds can compete for thioflavin-T binding or quench thioflavin T fluorescence and thereby bias the detection of amyloid fibrils.36,37 Resveratrol, the compound tested in these studies, has no significant absorption within the range of thioflavin-T fluorescence, but this does not mean that it will not interfere with thioflavin-T assays. One earlier study has shown that resveratrol reduces thioflavin-T fluorescence when it is added to preformed Aβ fibrils, but does not affect the thioflavin-T intensity of carboxymethylated κ-casein fibrils, suggesting that resveratrol might displace the bound thioflavin-T from Aβ fibrils.36 However, it is not known if resveratrol interferes with the binding of thioflavin-T to IAPP amyloid fibrils. Thus, complementary TEM studies were preformed to ensure that the results were not biased by interference between thioflavin-T and resveratrol.
Resveratrol prolongs the lag phase of IAPP amyloid formation, but interferes with thioflavin-T assays
We found that resveratrol slows amyloid formation by wild-type IAPP in a dose-dependent manner, but the effects are very modest (Figure 2A). 1:1 and 1:2 mixtures of wild-type IAPP with resveratrol exhibit a lag phase that is similar to that of wild-type IAPP in the absence of the inhibitor. The final thioflavin-T fluorescence intensity is greatly reduced, however, decreasing by approximately 30% and 60% respectively. TEM studies, described in detail below, show that at least part of this effect is due to the interference of resveratrol with thioflavin-T assays. The lag phase is increased by only a factor of 1.5 relative to the control for a 1:5 mixture of wild-type IAPP and resveratrol. The effect of resveratrol on the lag phase is very modest compared with that observed for EGCG, which essentially abolishes amyloid formation by IAPP at a 1:1 molar ratio, for experiments conducted at 16 μM IAPP.16,17 When the resveratrol concentration is increased to a 10-fold or a 20-fold excess, there is no detectable thioflavin-T fluorescence intensity. The decrease in fluorescence intensity upon the addition of resveratrol may be due to the formation of fewer fibrils, to the interference of resveratrol with thioflavin-T fluorescence, or due to effects of resveratrol on thioflavin-T binding. Visual inspection of TEM images recorded after 50 h of incubation for samples with a 10- and 20-fold excess of resveratrol contain fewer fibrils and those which are present appear to be thinner. In contrast, extensive mats of fibrils are observed at this time point when resveratrol is absent (Figure 2B-G). These observations confirm that resveratrol retards IAPP amyloid formation under the conditions examined here. However, large amounts of IAPP fibrils are observed in the 1:10 and 1:20 IAPP: resveratrol samples after 126 h of incubation even though there is no increase in thioflavin-T signal (Supporting Information). These data conclusively show that resveratrol interferes with thioflavin-T assays of IAPP amyloid formation.
Figure 2.
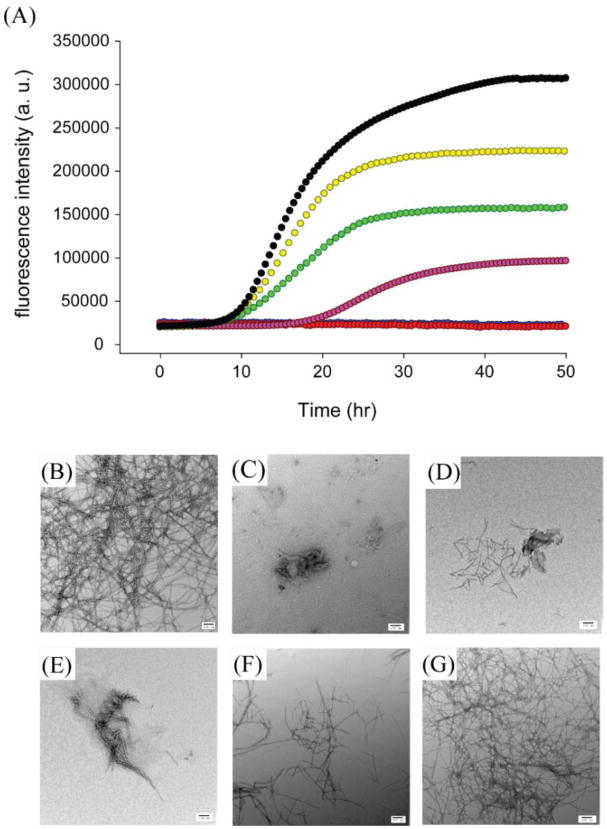
Resveratrol reduces the rate of amyloid formation by wild-type IAPP. (A) Thioflavin-T fluorescence monitored kinetic experiments for wild-type IAPP, black; wild-type IAPP and resveratrol at a 1:1 ratio, yellow; at a 1:2 ratio, green; at a 1:5 ratio, pink; at a 1:10 ratio, red; at a 1:20 ratio, blue. The red and blue curves overlap. TEM image of (B) wild-type IAPP after incubation for 50 h. TEM image of wild-type IAPP with resveratrol at a 1:20 ratio after incubation for (C) 42 h; (D) 50 h; (E) 66 h; (F) 96 h and (G) 126 h. Samples contained 16 μM IAPP in pH 7.4, 20 mM Tris buffer with 1% (v/v) DMSO. Scale bars represent 100 nm.
ESI-mass spectrometry reveals that resveratrol IAPP interactions are weaker than EGCG IAPP interactions
We conducted ESI-MS studies to further probe the interaction of resveratrol with IAPP. ESI-MS has been shown to be able to detect interactions between small molecules and monomeric and oligomeric IAPP.17 In the case of EGCG, ESI-MS revealed that the compound binds to monomeric IAPP (Figure 3), and perturbs self-association of the monomeric peptide into higher order amyloid assemblies.17 EGCG forms covalent Schiff base adducts with some amyloidogenic proteins, but this is not required for the inhibition of amyloid formation by IAPP.16-18 The ESI-MS data indicates, that under the conditions of these studies, EGCG interacts with IAPP but does not form a covalent complex. Quite different results were obtained when mixtures of resveratrol and IAPP were examined. In the case of resveratrol, binding to monomeric IAPP was not observed in the mass spectrum, despite the compound being present at a 20-fold molar excess over the peptide. It is clear from these data that EGCG binds either more favorably, more stably, or to a greater extent to monomeric IAPP than resveratrol. These results help to rationalize why EGCG is a much more effective inhibitor of IAPP amyloid formation than resveratrol.
Figure 3.
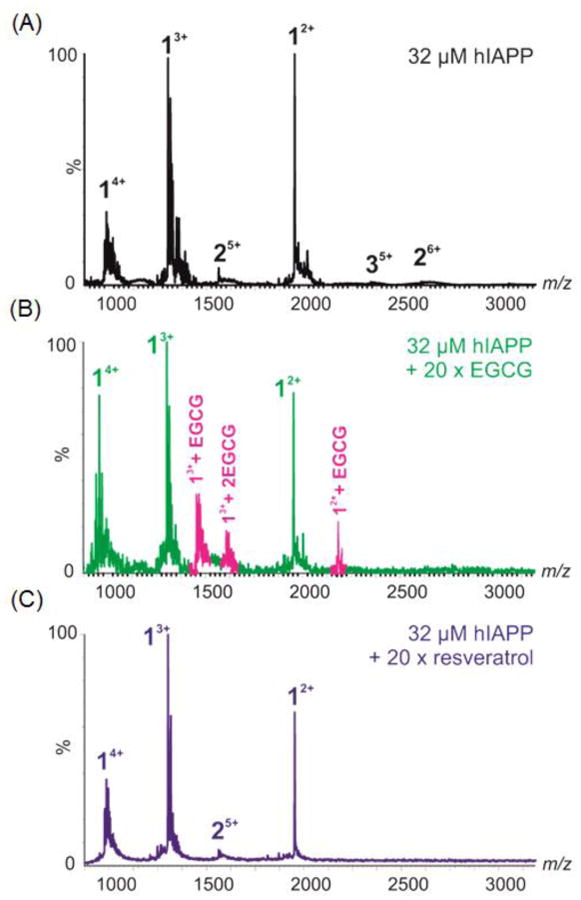
ESI-MS spectra showing A) wild-type IAPP alone (32 μM, 50:50 20 mM ammonium acetate: 20 mM ammonium bicarbonate buffer, pH 7.4), or in the presence of 640 μM EGCG (B) or resveratrol (C). Numbers adjacent to peaks denote oligomer order, with the positive charge state of each oligomer ions in superscript. Wild-type IAPP monomer exhibits +2, +3 and +4 charge states (labelled 1) and minor amounts of dimer (labelled 2). EGCG binds to both the +2 and +3 charge state ions of IAPP monomer, bound peaks coloured pink. No binding of resveratrol is observed to the IAPP monomer.
His-18 is important for resveratrol IAPP interactions and Arg-11 may play a role
We next investigated the effects of resveratrol on amyloid formation by two His-18 IAPP variants, H18Q-IAPP and H18L-IAPP. Both of these mutants have been shown to accelerate amyloid formation28. The compound had less effect on these mutants than it did on wild-type IAPP. 1:1 and 1:2 mixtures of H18Q-IAPP and resveratrol have similar lag times and display the same final fluorescence intensities as observed for H18Q-IAPP alone (Figure 4A). The lag phase of the 1:5 H18Q-IAPP resveratrol mixture is only slightly longer than the H18Q-IAPP control and the final intensity is around 30% lower than H18Q-IAPP alone. Addition of either 10- or 20-fold excess of resveratrol also had less effect on H18Q-IAPP aggregation than it did on wild-type IAPP. A 10-fold excess of resveratrol increased the lag phase of H18Q-IAPP by only a factor of 1.8 and dense mats of amyloid fibrils were observed in the TEM images (Figure 4B,C). Although the final thioflavin-T intensity of H18Q-IAPP in the presence of a 20-fold excess of resveratrol is greatly decreased, the kinetic curve is still sigmoidal with a lag phase only 2.4-fold longer than that observed for H18Q-IAPP alone. TEM images confirmed the presence of amyloid fibrils in these samples (Figure 4D). Collectively, the data show that mutation of His-18 significantly affects the ability of the resveratrol to modulate IAPP amyloid formation.
Figure 4.
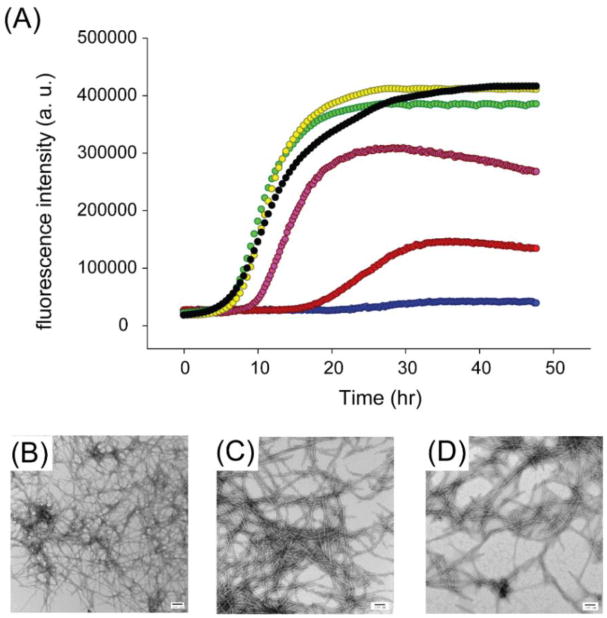
Resveratrol is not an effective inhibitor of amyloid formation by H18Q-IAPP. (A) Thioflavin-T monitored kinetic experiments for H18Q-IAPP, black; H18Q-IAPP and resveratrol at a 1:1 ratio, yellow; at a 1:2 ratio, green; at a 1:5 ratio, pink; at a 1:10 ratio, red; at a 1:20 ratio, blue. (B) TEM image of the amyloid fibrils formed by H18Q-IAPP. (C) TEM image of the 1:10 mixture of H18Q-IAPP and resveratrol. (D) TEM image for the 1:20 mixture of H18Q-IAPP and resveratrol. Samples were collected for TEM at 48 h and contained 16 μM IAPP in pH 7.4, 20 mM Tris buffer with 1% (v/v) DMSO. Scale bars represent 100 nm.
Resveratrol is even less effective at inhibiting amyloid formation by H18L-IAPP. The mutant forms amyloid more rapidly than wild-type IAPP, and the kinetic curves quickly reach a plateau for all conditions tested (Supporting Information). TEM images recorded for the 1:20 H18L-IAPP and resveratrol mixture revealed large amounts of amyloid fibrils. The mechanism of IAPP amyloid formation is not known in sufficient detail to offer an atomic level description of the consequences of the H18L mutant on amyloid formation by IAPP or on the binding of IAPP to resveratrol. Nevertheless, the H18L-IAPP data are consistent with the H18Q-IAPP results and with the proposed role of His-18 in resveratrol:IAPP interactions.27
We next examined the R11L mutant of IAPP. This polypeptide rapidly forms amyloid under the conditions of our studies with a lag time on the order of 0.9 hours in the absence of resveratrol compound to 10.0 hours for wild-type IAPP. The enhanced rate of aggregation can be rationalized by the existing structural modes of IAPP fibrils; they place Arg-11 in the first β-strand and the parallel, in register arrangement of the cross-β structure is expected to lead to electrostatic repulsions.43,44
A 5-fold addition of resveratrol increased the lag time of the R11L mutant by a factor of only 1.5 to 1.6, comparable to the effect observed for resveratrol on wild-type IAPP. The effect on the final fluorescence intensity is less, but as previously noted there is not a direct relationship between the intensity of a thioflavin-T assay and the amount of amyloid fibrils formed since the final intensity is related to the amount of thioflavin-T bound and its quantum yield. The increased thioflavin-T intensity allows the full curve to be monitored in the presence of a 20-fold excess of the compound. In this case, the lag time is increased only 2.7 to 2.8 fold relative to R11l-IAPP in the absence of resveratrol (Supporting Information). Comparative analysis of TEM images, described in subsequent sections, collected at twice and three times the value of t50, where t50 is the time required to reach 50% of the maximum thioflavin-T signal, suggests that the mutation does modulate the ability of resveratrol to inhibit IAPP amyloid formation.
The three aromatic residues in IAPP make different contributions to interactions with resveratrol
Wild-type IAPP contains three aromatic residues, Phe-15, Phe-23 and Tyr-37. Aromatic-aromatic interactions are not required for IAPP amyloid formation although their removal does slow the process,38,39 Aromatic interactions have been proposed to play an important role in amyloid formation and in IAPP:small molecule interactions,33 but it is not known whether aromatic interactions are important for resveratrol binding. The effects of single aromatic to Leu mutations on IAPP amyloid formation have been previously examined in the absence of resveratrol. All three single aromatic to Leu mutants formed amyloid fibrils and the mutations did not alter the fibril morphology.39 This collection of mutants provides a convenient tool to test the potential role of aromatic interactions in the interaction of IAPP with resveratrol.
Thioflavin-T fluorescence monitored kinetic curves were recorded for the set of aromatic to Leu mutants in the absence and in the presence of different amounts of resveratrol, and are displayed in Figure 5. The effects of resveratrol on amyloid formation by F15L-IAPP are similar to those observed for wild-type IAPP at the lower ratios of resveratrol, but are different at higher ratios (Figure 5A). The lag phase is slightly increased for the 1:5 mixture compared to the F15L-IAPP control. Only a few non-fibrillar aggregates were observed when wild-type IAPP is incubated at 42 h and at 50 h in the presence of a 20-fold excess of resveratrol (Figure 2). In contrast, amyloid fibrils, albeit ones that were shorter and thinner, were found in the 1:20 mixture of F15L-IAPP and resveratrol (Figure 5D,E). This indicates that the Phe-15 mutant affects IAPP: resveratrol interactions and suggests that Phe-15 plays a role in the interaction of resveratrol with IAPP. Different results were observed with the F23L mutant. This substitution did not significantly impact the ability of resveratrol to slow amyloid formation by IAPP, indicating that an aromatic residue at position 23 is not required for IAPP:resveratrol interactions (Figure 5B). Resveratrol slowed F23L-IAPP amyloid formation in a dose-dependent manner. The lag phase of F23L-IAPP increased when a 5-fold excess of resveratrol was present. TEM images recorded for the 1:20 mixture of F23L-IAPP and resveratrol at 96 h reveal that no detectable fibrils were formed, showing that resveratrol retards F23L-IAPP amyloid formation (Figure 5F,G). Further incubation for a total time of 142 hours revealed the presence of fibrils (Supporting Information). In contrast, resveratrol had no significant effect on Y37L-IAPP amyloid formation (Figure 5C). Although the final fluorescence intensity of the Y37L-IAPP kinetic experiments gradually decreased upon addition of resveratrol, the length of the lag phase was only 20% longer in the presence of a 10-fold excess of resveratrol. TEM images show that large amounts of Y37L-IAPP fibrils were found even at high ratios of resveratrol as early as 96 h (Figure 5H,I). The data show that Tyr-37 is important for resveratrol:IAPP interactions since mutation of this residue reduces the effect of the compound on IAPP amyloid formation. Mutation of Tyr-37 has a larger effect on the ability of the compound to slow IAPP amyloid formation than mutation of Phe-15 as judged by the t50 values (Supporting Information).
Figure 5.
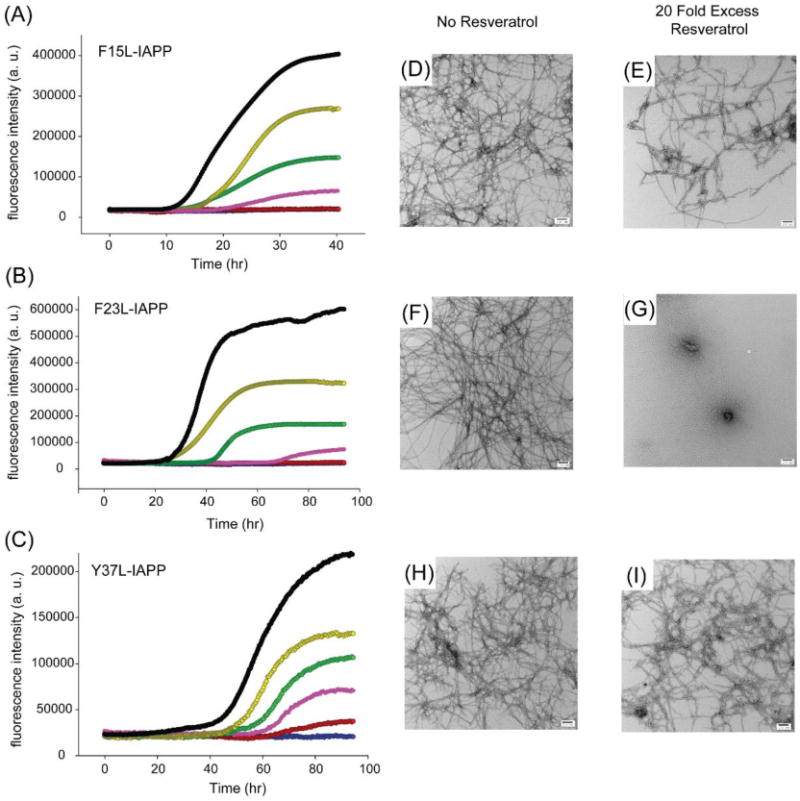
The three aromatic residues in IAPP interact differently with resveratrol. Thioflavin-T fluorescence monitored kinetic experiments for (A) F15L-IAPP, (B) F23L-IAPP and (C) Y37L-IAPP in the presence of resveratrol. Experiments were conducted at 25 °C, pH 7.4, 20 mM Tris buffer, 16 μM mutants and 1% (v/v) DMSO without stirring. Black peptide alone; Yellow, peptide with resveratrol at a 1:1 ratio; Green, peptide with resveratrol at a 1:2 ratio; Pink, peptide with resveratrol at a 1:5 ratio; Red, peptide with resveratrol at a 1:10 ratio; Blue, peptide with resveratrol at a 1:20 ratio. The red and blue curves overlap in (A) and (B). TEM image of (D) F15L-IAPP fibrils after 42 h; (E) F15L-IAPP fibrils with a 20-fold excess resveratrol after 42 h after 42 h; (F) F23L-IAPP fibrils and (G) F23L-IAPP fibrils with a 20-fold excess resveratrol. The F23L-IAPP samples were removed from the kinetic experiments at 96 h for TEM. (H) TEM image of Y37L-IAPP fibrils and (I) Y37L-IAPP fibrils with a 20-fold excess resveratrol. The Y37L-IAPP samples were removed from the kinetic experiments at 96 h for TEM. Scale bars represent 100 nm.
Resveratrol does not inhibit amyloid formation by Ac8-37- IAPP
Previous NMR studies suggested that Lys-1 could be a second binding site for resveratrol.16 We used a truncated acetylated variant of IAPP, Ac8-37-IAPP, which lacks the N-terminal seven residues to probe the role of the N-terminal region of the polypeptide. This variant has been used in mechanistic studies of IAPP amyloid formation. Resveratrol is not an inhibitor of amyloid formation by Ac 8-37-IAPP. A 10-fold excess of compound had no detectable effect on the lag phase, consistent with the proposed role of Lys-1 (Figure 6). Ac 8-37-IAPP quickly forms amyloid fibrils even when resveratrol is present in 10- or 20- fold excess. The results are consistent with the proposed role of the N-terminal region of IAPP in resveratrol IAPP interactions.
Figure 6.
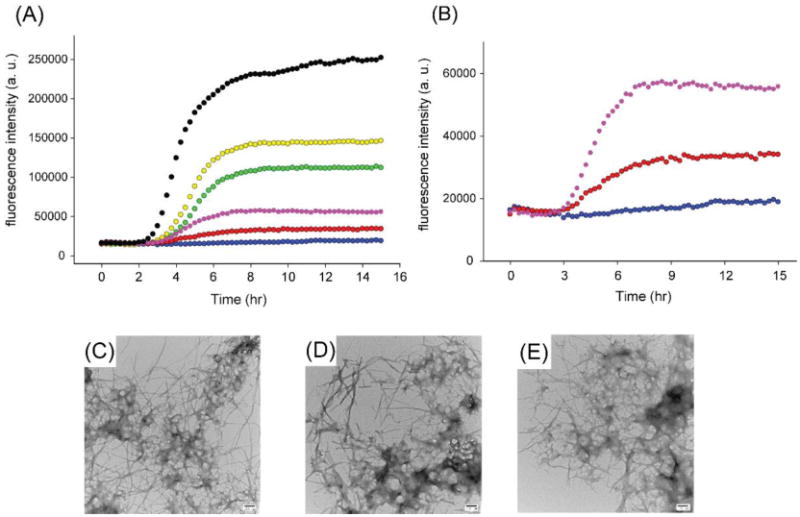
Resveratrol is not an effective inhibitor of amyloid formation by Ac 8-37-IAPP. (A) Thioflavin-T fluorescence monitored kinetic experiments for Ac 8-37-IAPP, Black; Ac 8-37-IAPP and resveratrol at a 1:1 ratio, Yellow; at a 1:2 ratio, Green; at a 1:5 ratio, Pink; at a 1:10 ratio, Red; at a 1:20 ratio, Blue. (B) An expansion of the data for Ac 8-37-IAPP with resveratrol at the 1:5, 1:10 and 1:20 ratios. TEM images of (C) Ac 8-37-IAPP alone; (D) Ac 8-37-IAPP incubated 1:5 with resveratrol and (E) Ac 8-37-IAPP incubated 1:20 with resveratrol. Samples were collected for TEM at 22 h. Samples contained 16 μM IAPP variant in pH 7.4, 20 mM Tris buffer with 1% (v/v) DMSO. Scale bars represent 100 nm.
TEM based competitive analysis of the effects of resveratrol on the IAPP mutants help to reveal the relative importance of different sites
We collected additional TEM images of all samples in the presence of a 20-fold excess of resveratrol at times corresponding to twice t50 and three times t50, where t50 is the time to reach 50% of the maximum thioflavin T fluorescence signal for each individual peptide in the absence of inhibitor. These correspond to wild-type t50= 16.5 h; H18Q-IAPP t50= 12.5 h; H18L-IAPP t50= 1.25 h; R11L-IAPP t50= 1.6 h; F15L-IAPP t50= 20.5 h; F23L-IAPP t50= 37.5 h; Y37L-IAPP t50= 60.5 h; Ac 8-37-IAPP t50= 4.25 h; under our conditions. The TEM data are shown in Figure 7 and confirm that mutation of Phe-15 or Tyr-37 or His-18 or Arg-11, or removal of Lys-1 and the N-terminal amino group affect the ability of resveratrol to reduce the rate of IAPP amyloid formation.
Figure 7.
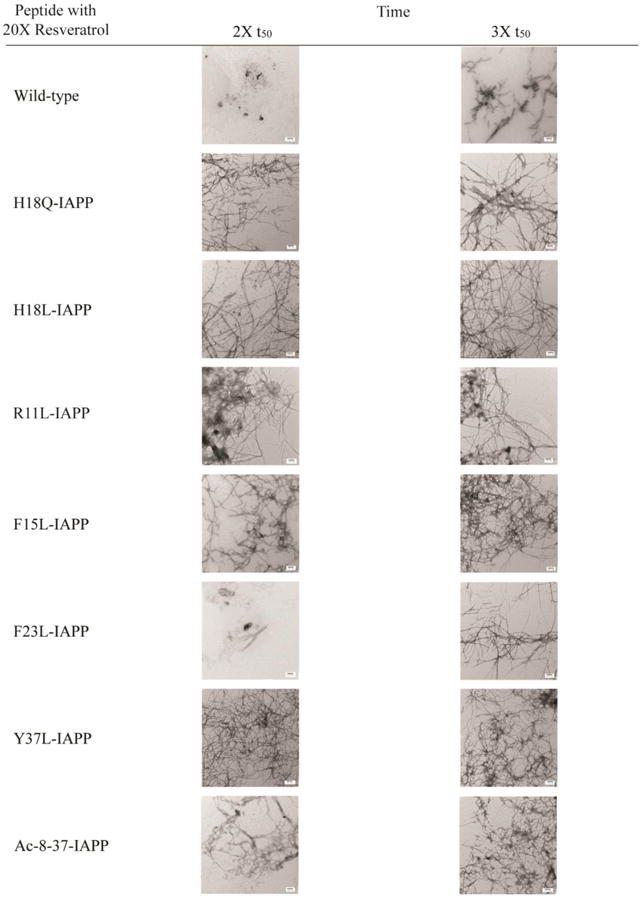
Summary of TEM data collected for each peptide in the presence of resveratrol. Images were collected at twice and 3-times the t50 value of the respective peptide in the absence of inhibitor. Samples contained 16 μM peptide and 320 μM resveratrol in pH 7.4, 20 mM Tris buffer with 1% (v/v) DMSO. Scale bars represent 100 nm.
Resveratrol does not effectively disaggregate IAPP fibrils
There are conflicting reports on the ability of polyphenols to disaggregate and remodel amyloid fibrils. Resveratrol has been shown to remodel amyloid fibrils formed by Ap.23 However, the ability of resveratrol to disaggregate amyloid fibrils formed by IAPP has not been examined. Figure 8 displays the results of studies to determine whether resveratrol is able to disaggregate IAPP fibrils. Resveratrol was added when IAPP amyloid formation reached a plateau (Figure 8A, black arrow), and TEM images were recorded before and directly after the addition of resveratrol (Figure 8B and 8C), as well as after 3 days and 6 days of incubation (Figure 8D and 8E). The thioflavin-T intensity drops rapidly after addition of resveratrol suggesting that the compound might interfere with thioflavin-T binding. A slower, additional, loss of signal occurs, but the thioflavin T intensity does not return to baseline even after 3 days of incubation. However, TEM images recorded at all time-points show that numerous amyloid fibrils were present in all samples and their morphology appears similar at the resolution of these experiments. The origin of the slow second phase of the time-dependent thioflavin-T signal is not understood, but the TEM data shows that resveratrol, does not disaggregate IAPP amyloid fibrils. In contrast, TEM studies have shown that EGCG disaggregates IAPP fibrils.16
Figure 8.
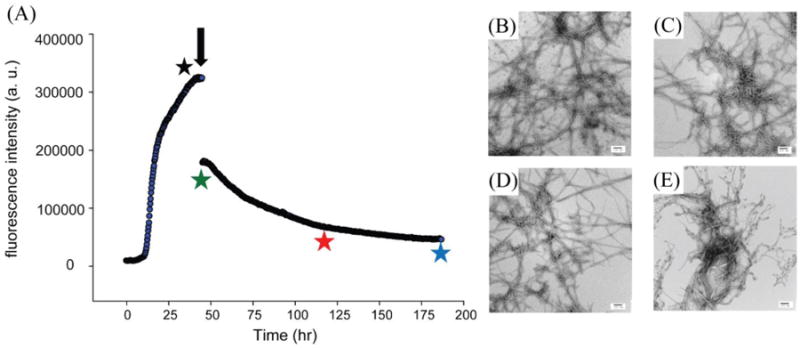
Resveratrol does not effectively remodel amyloid fibrils formed by wild-type IAPP. (A) Thioflavin-T monitored time course. Amyloid formation was allowed to proceed for 48 h and then resveratrol was added (black arrow). (B) TEM image recorded before adding resveratrol (black star). (C) TEM images recorded immediately after adding resveratrol (green star); (D) 3 days after addition of resveratrol (red star); (E) 6 days after addition of resveratrol (blue star). Experiments were conducted at 25 °C, 20 mM Tris buffer, 32 μM thioflavin-T and 16 μM IAPP. Resveratrol was added to a final concentration of 160 μM. Samples contained 1% (v/v) DMSO after the addition of resveratrol. Scale bars represent 100 nm.
Conclusions
The data presented here are consistent with previous studies24 which concluded that resveratrol slows, but does not prevent, human IAPP amyloid formation. The present work also shows that resveratrol is much less effective than EGCG and reveals that resveratrol interferes with thioflavin-T assays. In fact, this is a wide spread issue with thioflavin-T based assays of IAPP amyloid formation.36,37,40 The structure of EGCG and resveratrol are very different and there are several features that might contribute to their different effectiveness. EGCG is larger which may facilitate more extensive hydrophobic interactions with amino acid side chains. EGCG is also more extensively hydroxylated, containing three adjacent hydroxyl groups on two of its aromatic rings and two on the third. Structure/function studies have shown that the gallate ester group of EGCG contributes to its ability to inhibit IAPP amyloid formation as does the integrity of the trihydroxyl features.16 In addition, EGCG has been shown to form covalent linkages with some polypeptides,15 although this is not required for inhibition of IAPP amyloid formation and no cross linking was detected in our ESI-MS studies.16
Our data are consistent with previous NMR studies with a non-physiological variant of IAPP that lacks the amidated C-terminus. That work concluded that His-18 in IAPP makes contacts with resveratrol and the Lys-1 is a secondary binding site.27 The work presented here also reveals the importance of Phe-15 and Tyr-37 in IAPP: resveratrol interactions and the potential role of Arg-11. The observation that the vast majority of the mutations have modest effects on the ability of resveratrol to modulate IAPP amyloid formation is consistent with relatively nonspecific interactions between IAPP and the compound. The observed importance of Phe-15 and Tyr-37 is consistent with the hypothesis that aromatic residues are a possible site for inhibitor protein interactions, but this observation is not general for IAPP since EGCG effectively inhibited variants of IAPP that lacked aromatic residues.16
The present work also highlights the difficulty of using fluorescence-based thioflavin-T assays in inhibition studies. Even though small molecules may not absorb at the same wavelength as thioflavin-T fluorescence, they may alter the binding of thioflavin-T to amyloid fibrils, resveratrol is such a case. The use of complementary methods such as electron microscopy, atomic force microscopy, or mass spectrometry is highly recommended to support thioflavin-T data in inhibition studies.
In summary, we have shown that resveratrol retards IAPP amyloid formation, but does not completely abolish fibril formation under the conditions used here. Interactions between resveratrol and monomeric IAPP are not observed by ESI-MS, in contrast to the case with other polyphenols17, and changes in the lag time are modest. The study highlights the role of Arg-11, His-18, Phe-15, and Tyr-37, but indicates that Phe-23 is not as important for IAPP: resveratrol interactions. There is very little structural information available about pre-amyloid oligomers formed by IAPP and it is currently not possible to offer a structural explanation for the different effects observed at position-23 relative to those detected for residues 15 and 37. The mutations may modulate IAPP:resveratrol interactions by altering residues that make direct contacts with the compound, or they may exert their effects indirectly by modulating the properties of IAPP oligomers. Studies with the non-genetically coded amino acid p-cyano phenylalanine argue that the side chains of Phe-15, Phe-23 and Tyr-37 are all exposed to solvent during the lag phase of amyloid formation, suggesting that differential solvent accessible in pre-amyloid oligomers is not responsible for the different role of Phe-23.35 However, residues 15, 23, and 37 are in different environments in the amyloid fibrils. Residues 15 and 37 are part of the parallel β-sheet structure in high resolution models of IAPP amyloid fibrils, but Phe-23 is in a less well ordered loop which connects the N and C-terminal β-strands.43,44 It may be that the compound interacts with all of the aromatic residues, but interactions with Phe-15 and Tyr-37 have a larger impact on amyloid formation than interactions with Phe-23 since Phe-23 is not part of the core structure of the fibril. Another important feature that other IAPP amyloid inhibitors, such as EGCG and morin hydrate, have is the ability to remodel amyloid fibrils.16,40 Our TEM studies demonstrate that resveratrol does not disaggregate IAPP fibrils, even though it induces a decrease in thioflavin-T fluorescence.
Supplementary Material
Acknowledgments
We thank Professor David Green and members of the Raleigh, Radford and Ashcroft groups for helpful discussions.
Funding Sources: D.P.R. acknowledges support from the United States National Institutes of Health (GM078114). L.M.Y. is funded by a Biotechnology and Biological Sciences Research Council (BBSRC) CASE studentship (Grant Number BB/I015361/1) sponsored by Micromass UK Ltd/Waters Corpn, Manchester, UK. A.W. was supported, in part, by a GAANN fellowship from the United States Department of Education. The Synapt HDMS mass spectrometer was purchased with funds from the Biotechnology and Biological Sciences Research Council through its Research Equipment Initiative scheme (BB/E012558/1). S.E.R. also acknowledges funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013; 322408).
Abbreviations
- Ac 8-37-IAPP
a truncated acetylated variant of human islet amyloid polypeptide
- CD
circular dichroism
- EGCG
(-)-epigallocatechin 3-gallate
- F15L-IAPP
Phe-15 to Leu variant of human islet amyloid polypeptide
- F23L-IAPP
Phe-23 to Leu variant of human islet amyloid polypeptide
- H18Q-IAPP
His-18 to Gln variant of human islet amyloid polypeptide
- H18L-IAPP
His-18 to Leu variant of human islet amyloid polypeptide
- IAPP
human islet amyloid polypeptide
- NMR
nuclear magnetic resonance
- R11L-IAPP
Arg-11 to Leu variant of human islet amyloid polypeptide
- t50
time required for 50% of the total signal change in a kinetic experiment
- TEM
transmission electron microscopy
- Y37L-IAPP
Tyr-37 to Leu variant of human islet amyloid polypeptide
Footnotes
Notes: The authors declare no competing financial interests.
Supporting Information: A figure showing mass spectra of resveratrol after 24 hours of incubation in mass spectra buffer. A figure showing thioflavin-T monitored kinetic experiments for wild-type IAPP alone and wild-type IAPP in the presence of 20-fold excess resveratrol assayed from 0 h to 126 h. A figure showing thioflavin-T monitored kinetic experiments and TEM studies for H18L-IAPP and H18L-IAPP with resveratrol. Additional TEM image of a sample of F23L-IAPP with 20-fold excess of resveratrol collected after 142 h of incubation. A figure comparing the effects of different amounts of resveratrol on the t50 value for amyloid formation by wild-type IAPP and three aromatic to Leu point mutants. A figure showing the effect of different amounts of resveratrol on amyloid formation by R11L-IAPP. The figure includes thioflavin-T kinetic curves and TEM data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: A long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 2.Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: A critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 3.Konarkowska B, Aitken JF, Kistler J, Zhang SP, Cooper GJS. The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. Febs J. 2006;273:3614–3624. doi: 10.1111/j.1742-4658.2006.05367.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheng F, Marzban L. Cytotoxic effects of human islet amyloid polypeptide on primary islet alpha-cells and beta-cells: Implications for understanding the pathogenesis of type 2 diabetes. Diabetes. 2007;56:A410–A411. [Google Scholar]
- 5.Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocrol Rev. 2008;29:303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 7.Westermark GT, Westermark P, Nordin A, Tornelius E, Andersson A. Formation of amyloid in human pancreatic islets transplanted to the liver and spleen of nude mice. Ups J Med Sci. 2003;108:193–203. doi: 10.3109/2000-1967-113. [DOI] [PubMed] [Google Scholar]
- 8.Westermark GT, Westermark P, Berne C, Korsgren O Nordic Network Clin Islet, T. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359:977–9128. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 9.Potter KJ, Abedini A, Marek P, Klimek AM, Butterworth S, Driscoll M, Baker R, Nilsson MR, Warnock GL, Oberholzer J, Bertera S, Trucco M, Korbutt GS, Fraser PE, Raleigh DP, Verchere CB. Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proc Natl Acad Sci U S A. 2010;107:4305–4310. doi: 10.1073/pnas.0909024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn SE, Dalessio DA, Schwartz MW, Fujimoto WY, Ensinck JW, Taborsky GJ, Porte D. Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990;39:634–638. doi: 10.2337/diab.39.5.634. [DOI] [PubMed] [Google Scholar]
- 11.Young DA, Deems RO, Deacon RW, McIntosh RH, Foley JE. Effects of amylin on glucose-metabolism and glycogenolysis in vivo and in vitro. Am J Physiol. 1990;259:E457–E461. doi: 10.1152/ajpendo.1990.259.3.E457. [DOI] [PubMed] [Google Scholar]
- 12.Rezai-Zadeh K, Arendash GW, Hou HY, Fernandez F, Jensen M, Runfeldt M, Shytle RD, Tan J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Research. 2008;1214:177–187. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- 13.Meng FL, Abedini A, Plesner A, Verchere CB, Raleigh DP. The flavanol (-)-epigallocatechin 3-gallate inhibits amyloid formation by islet amyloid polypeptide, disaggregates amyloid fibrils, and protects cultured cells against IAPP-induced toxicity. Biochemistry. 2010;49:8127–8133. doi: 10.1021/bi100939a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, Wanker EE. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Pro Natl Acad Sci U S A. 2010;107:7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popovych N, Brender JR, Soong R, Vivekanandan S, Hartman K, Basrur V, Macdonald PM, Ramamoorthy A. Site specific interaction of the polyphenol EGCG with the SEVI amyloid precursor peptide PAP(248-286) J Phys Chem B. 2012;116:3650–3658. doi: 10.1021/jp2121577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao P, Raleigh DP. Analysis of the inhibition and remodeling of islet amyloid polypeptide amyloid fibers by flavanols. Biochemistry. 2012;51:2670–2683. doi: 10.1021/bi2015162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young LM, Cao P, Raleigh DP, Ashcroft AE, Radford SE. Ion mobility spectrometry-mass spectrometry defines the oligomeric intermediates in amylin amyloid formation and the mode of action of inhibitors. J Am Chem Soc. 2014;136:660–670. doi: 10.1021/ja406831n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palhano FL, Lee J, Grimster NP, Kelly JW. Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. J Am Chem Soc. 2013;135:7503–7510. doi: 10.1021/ja3115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, Engemann S, Pastore A, Wanker EE. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Strut Mol Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 20.Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savaskan E, Olivieri G, Meier F, Seifritz E, Wirz-Justice A, Muller-Spahn F. Red wine ingredient resveratrol protects from beta-amyloid neurotoxicity. Gerontology. 2003;49:380–383. doi: 10.1159/000073766. [DOI] [PubMed] [Google Scholar]
- 22.Marambaud P, Zhao HT, Davies P. Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 23.Ladiwala ARA, Lin JC, Bale SS, Marcelino-Cruz AM, Bhattacharya M, Dordick JS, Tessier PM. Resveratrol selectively remodels soluble oligomers and fibrils of amyloid A-beta into off-pathway conformers. J Biol Chem. 2010;285:24228–24237. doi: 10.1074/jbc.M110.133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra R, Sellin D, Radovan D, Gohlke A, Winter R. Inhibiting islet amyloid polypeptide fibril formation by the red wine compound resveratrol. Chembiochem. 2009;10:445–449. doi: 10.1002/cbic.200800762. [DOI] [PubMed] [Google Scholar]
- 25.Evers F, Jeworrek C, Tiemeyer S, Weise K, Sellin D, Paulus M, Struth B, Tolan M, Winter R. Elucidating the mechanism of lipid membrane-induced IAPP fibrillogenesis and its inhibition by the red wine compound resveratrol: A synchrotron X-ray reflectivity study. J Am Chem Soc. 2009;131:9516–9521. doi: 10.1021/ja8097417. [DOI] [PubMed] [Google Scholar]
- 26.Radovan D, Opitz N, Winter R. Fluorescence microscopy studies on islet amyloid polypeptide fibrillation at heterogeneous and cellular membrane interfaces and its inhibition by resveratrol. Febs Lett. 2009;583:1439–1445. doi: 10.1016/j.febslet.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 27.Wei L, Jiang P, Xu WX, Li H, Zhang H, Yan LY, Chan-Park MB, Liu XW, Tang K, Mu YG, Pervushin K. The molecular basis of distinct aggregation pathways of islet amyloid polypeptide. J Biol Chem. 2011;286:6291–6300. doi: 10.1074/jbc.M110.166678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tu LH, Serrano AL, Zanni MT, Raleigh DP. Mutational analysis of preamyloid intermediates: The role of His-Tyr interactions in islet amyloid formation. Biophys J. 2014;106:1520–1527. doi: 10.1016/j.bpj.2013.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porat Y, Mazor Y, Efrat S, Gazit E. Inhibition of islet amyloid polypeptide fibril formation: A potential role for heteroaromatic interactions. Biochemistry. 2004;43:14454–14462. doi: 10.1021/bi048582a. [DOI] [PubMed] [Google Scholar]
- 30.Abedini A, Raleigh DP. Incorporation of pseudoproline derivatives allows the facile synthesis of human IAPP, a highly amyloidogenic and aggregation-prone polypeptide. Org Lett. 2005;7:693–696. doi: 10.1021/ol047480+. [DOI] [PubMed] [Google Scholar]
- 31.Marek P, Woys AM, Sutton K, Zanni MT, Raleigh DP. Efficient microwave-assisted synthesis of human islet amyloid polypeptide designed to facilitate the specific incorporation of labeled amino acids. Org Lett. 2010;12:4848–4851. doi: 10.1021/ol101981b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abedini A, Singh G, Raleigh DP. Recovery and purification of highly aggregation-prone disulfide-containing peptides: Application to islet amyloid polypeptide. Ana Biochem. 2006;351:181–186. doi: 10.1016/j.ab.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 33.Porat Y, Abramowitz A, Gazit E. Inhibition of amyloid fibril formation by polyphenols: Structural similarity and aromatic interactions as a common inhibition mechanism. Chem Biol Drug Des. 2006;67:27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 34.Krebs MRH, Bromley EHC, Donald AM. The binding of thioflavin-T to amyloid fibrils: localisation and implications. J Struc Biol. 2005;149:30–37. doi: 10.1016/j.jsb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Marek P, Mukherjee S, Zanni MT, Raleigh DP. Residue-specific, realtime characterization of lag-phase species and fibril growth during amyloid formation: A combined fluorescence and IR study of p-cyanophenylalanine analogs of islet amyloid polypeptide. J Mol Biol. 2010;400:878–888. doi: 10.1016/j.jmb.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudson SA, Ecroyd H, Kee TW, Carver JA. The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. Febs J. 2009;276:5960–5972. doi: 10.1111/j.1742-4658.2009.07307.x. [DOI] [PubMed] [Google Scholar]
- 37.Meng FL, Marek P, Potter KJ, Verchere CB, Raleigh DP. Rifampicin does not prevent amyloid fibril formation by human islet amyloid polypeptide but does inhibit fibril thioflavin-T interactions: Implications for mechanistic studies beta-cell death. Biochemistry. 47:6016–6024. doi: 10.1021/bi702518m. [DOI] [PubMed] [Google Scholar]
- 38.Marek P, Abedini A, Song BB, Kanungo M, Johnson ME, Gupta R, Zaman W, Wong SS, Raleigh DP. Aromatic interactions are not required for amyloid fibril formation by islet amyloid polypeptide but do influence the rate of fibril formation and fibril morphology. Biochemistry. 2007;46:3255–3261. doi: 10.1021/bi0621967. [DOI] [PubMed] [Google Scholar]
- 39.Tu LH, Raleigh DP. Role of aromatic interactions in amyloid formation by islet amyloid polypeptide. Biochemistry. 2013;52:333–342. doi: 10.1021/bi3014278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noor H, Cao P, Raleigh DP. Morin hydrate inhibits amyloid formation by islet amyloid polypeptide and disaggregates amyloid fibers. Protein Sci. 2012;21:373–382. doi: 10.1002/pro.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trela BC, Waterhouse AL. Resveratrol: Isomeric molar absorptivities and stability. J Agr Food Chem. 1996;44(5):1253–7. [Google Scholar]
- 42.Pineda-Sanabria SE, Robertson IM, Sykes BD. Structure of trans-Resveratrol in Complex with the Cardiac Regulatory Protein Troponin C. Biochemistry. 2011;50(8):1309–20. doi: 10.1021/bi101985j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luca S, Yau WM, Leapman R, Tycko R. Peptide conformation and supramolecular organization in amylin fibrils: Constraints from solid-state NMR. Biochemistry. 2007;46(47):13505–22. doi: 10.1021/bi701427q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiltzius JJW, Sievers SA, Sawaya MR, Cascio D, Popov D, Riekel C, et al. Atomic structure of the cross-beta spine of islet amyloid polypeptide (amylin) Protein Sci. 2008;17(9):1467–74. doi: 10.1110/ps.036509.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


