Abstract
Rho GTPases activated by GDP/GTP exchange factors (GEFs) play key roles in the developing and adult nervous system. Kalirin-7 (Kal7), the predominant adult splice form of the multifunctional Kalirin RhoGEF, includes a PDZ binding domain and localizes to the postsynaptic side of excitatory synapses. In vitro studies demonstrated that overexpression of Kal7 increased dendritic spine density whereas reduced expression of endogenous Kal7 decreased spine density. To evaluate the role of Kal7 in vivo, mice lacking the terminal exon unique to Kal7 were created. Mice lacking both copies of the Kal7 exon (Kal7KO) grew and reproduced normally. Golgi impregnation and electron microscopy revealed decreased hippocampal spine density in Kal7KO mice. Behaviorally, Kal7KO mice showed decreased anxiety-like behavior in the elevated zero maze and impaired acquisition of a passive avoidance task, but normal behavior in open field, object recognition and radial arm maze tasks. Kal7KO mice were deficient in hippocampal long-term potentiation. Western blot analysis confirmed the absence of Kal7 and revealed compensatory increases in larger Kalirin isoforms. PSDs purified from the cortices of Kal7KO mice showed a deficit in Cdk5, a kinase known to phosphorylate Kal7 and play an essential role in synaptic function. The early stages of excitatory synaptic development proceeded normally in cortical neurons prepared from Kal7KO mice, with decreased excitatory synapses apparent only after 21 days in vitro. Expression of exogenous Kal7 in Kal7KO neurons rescued this deficit. Kal7 plays an essential role in synaptic structure and function, affecting a subset of cognitive processes.
Keywords: dendritic spine, hippocampus, Golgi method, LTP, passive avoidance, anxiety, PSD, cdk5
Introduction
Alterations in the placement, prevalence and structure of dendritic spines, the site of most glutamatergic, excitatory endings in the brain, play a critical role in the synaptic plasticity underlying learning and memory (Yuste and Bonhoeffer, 2001;Knott et al., 2006;Craig et al., 2006;Craig and Kang, 2007;Dunaevsky and Mason, 2003;Ehlers, 2002;Araya et al., 2007;McAllister, 2007). Spines change in response to stimuli producing long term potentiation or long term depression (Mezey et al., 2004;Geinisman et al., 2000). In vivo, learning tasks, memory tasks, electroconvulsive shock and exposure to hormones, cocaine or ethanol alter spine morphology and function (Li et al., 2004;Jelks et al., 2007;McAllister, 2007;Fukazawa et al., 2003;Lisman and Raghavachari, 2006;Knott et al., 2006;Norrholm et al., 2003;Robinson et al., 2001). Inherited conditions associated with mental retardation and neurologic/psychiatric abnormalities are often associated with spine abnormalities and have revealed key roles for small GTP binding proteins of the Rho family (Meredith et al., 2000;Norrholm and Ouimet, 2000;Ramakers, 2000;Hill et al., 2006;Tabuchi et al., 2007).
Variations in spine size and shape affect signal transmission by AMPA and NMDA receptors localized to the post-synaptic density (PSD). The PSD is a complex, 1.1 gigadalton molecular machine, with its many components arrayed in a tightly controlled but dynamic manner; proteomic approaches (Elias et al., 2006;Beique et al., 2006;Collins et al., 2006;Sheng and Hoogenraad, 2007) reveal the presence of 300 copies of PSD-95 and 15 to 20 tetrameric NMDA and AMPA receptors in the typical cortical PSD. Kalirin-7(Kal7) is the only Rho GDP/GTP exchange factor (RhoGEF) identified in purified PSDs (Sheng and Hoogenraad, 2007;Collins et al., 2006;Penzes et al., 2001). Cyclin dependent kinase 5 (Cdk5), a Ser/Thr kinase localized to the PSD and known to phosphorylate several proteins critical to synaptic function (Zhang et al., 2008;Morabito et al., 2004;Cheung and Ip, 2007;Cheung et al., 2006), also phosphorylates Kal7, altering its effects on spine morphology (Xin et al., 2008).
Kal7 is co-localized with PSD-95, AMPA and NMDA receptors at excitatory synapses on the dendritic spines of CA1 hippocampal pyramidal neurons and the dendritic shafts of hippocampal GABAergic interneurons (Ma et al., 2008). Expression of Kal7 increases during the time of maximal synaptogenesis in the hippocampus (Ma et al., 2003;Ma et al., 2008). In vitro studies point to an important role for Kal7 in synaptic function. Exogenous Kal7 caused spine formation in aspiny GABAergic interneurons while decreased expression of endogenous Kal7 caused the loss of excitatory synapses (Ma et al., 2008). The interactions of Kal7 with PDZ domain proteins such as AF-6 (Xie et al., 2008) and PSD-95 (Penzes et al., 2001), coupled with the actions of the Sec14p, spectrin-like and GEF domains it shares with the larger isoforms of Kalirin (Schiller et al., 2008;Schiller et al., 2005), all contribute to its actions.
Since in vitro studies do not always accurately indicate in vivo function (Chubykin et al., 2007;Tabuchi et al., 2007;Varoqueaux et al., 2006), we engineered a mouse lacking the unique exon that defines Kal7. Despite the existence of 69 RhoGEFs (Rossman et al., 2005), lack of this isoform of Kalirin results in a decreased number of dendritic spines and excitatory synapses. In addition to morphological changes, mice lacking Kal7 and ΔKal7 (Fig. 1A) exhibit decreased anxiety-like behavior, deficits in hippocampal-dependent learning and diminished long term potentiation. The decreased levels of Cdk5 found in PSDs purified from Kal7KO mice may contribute to many of these deficits.
Fig. 1. Kal7 knockout strategy.
A. The major isoforms of Kalirin are shown; alternative splicing of three different 3′-untranslated regions generates Kal7, Kal8 and Kal9. Use of a promoter located in the intron preceding exon 11 (which encodes spectrin repeat 5) yields ΔKal7. Sec14p, homologous to yeast Sec14p; SH3, Src homology 3 domain; DH, Dbl homology domain; Kin, Ser/Thr protein kinase domain. B. The gene targeting approach used to eliminate the Kal7 exon, a 0.9 kb 3′-terminal exon situated between exons 33 and 34, is shown (not to scale); lox-p, neomycin-resistance, frt sites and primers are indicated. Product A/B had the expected sequence. C. The genomic product created by the action of FRT (frt) and Cre (lox-p) recombinase is shown; PCR primers used to detect excision are shown; product A/E exhibited the correct sequence. D. PCR genotyping of progeny from a Kal7+/CKO cross with C57BL/6 mouse (CKO mice) and from a Kal7+/CKO cross with a female expressing Cre recombinase in the germ cells (KO mice). E. PCR genotyping of pups from a Kal7+/KO × Kal7+/KO mating; under the conditions used, primer pair A/E only yielded product from the knockout allele; controls are shown to right of molecular weight ladder. F. Kal7+/KO and Kal7KO mice were born in the expected Mendelian ratio when Kal7+/KO mice were mated (N=377 animals). G. Western blot analysis of cerebral cortex from wildtype and Kal7KO adult mice. SDS lysates (30 μg protein) were analyzed using Kal7-specific antibody (JH2959), with tubulin and synaptophysin as loading controls. H. Growth curve for male Kal7KO mice and wildtype littermate controls (n=7–15 at each timepoint).
Methods
Creation of the Kal7 conditional and total null mice
The basic strategy for creation of the conditional knockout is diagramed in Fig. 1B. The Kal7 exon, which encodes the 20 amino acids unique to Kal7 and ΔKal7, is 0.9 kb in length and is flanked by introns which are 18 kb (upstream) and 36 kb (downstream). Lox-p sites were introduced 1.7 kb upstream (nt 34155267, mm9, July 2007) and 2.0 kb downstream of the Kal7 exon (924 nt) (nt 34150670). The neomycin resistance cassette was used in cell selection and was removed by passage through flipper female mice (Farley et al., 2000). The conditional knockout (Kal7CKO/+) was bred for 10 generations into C57Bl/6 females from Jackson Labs. To remove the Kal7 exon, Kal7CKO/+ males were bred with Hprt-Cre females (Jackson # 004302) and the progeny were then bred into the C57Bl/6 background; these mice lack 4.6 kb of chromosome 16 and retain the lox-p sequence (ATAACTTCGTATAATGTATGCTATACGAAGTTAT; Fig. 1C). Experiments reported here used mice back-crossed into C57Bl/6 for three to seven generations; Kal7KO/+ and Kal7KO mice were compared to littermate controls. Screening of genomic DNA prepared from tail snips utilized primers A (AATAAAATTACTCAAGCCACTTCCAGTC), B (GGACATTTGCATGACATTGAGTCTAAAG) and E (TGTTCATACAGCTGTCTGGGG) (Fig. 1B, C). PCR conditions: 94°C, 2.5 min; 55°C,1 min; 72°C, 32 sec; 40 cycles. Products encompassing the lox-p sites and the products of Flp and Cre recombinase were of the predicted sizes; DNA sequence analysis of excised bands confirmed their identities. The Kal7CKO mice were created in the University of Connecticut Gene Targeting and Transgenic Facility.
Antibodies used in these studies
Antisera described previously include polyclonal antisera to the Kalirin spectrin domain (JH2582), Kal7 COOH-terminal end (affinity-purified JH2958; JH2959; specific for the COOH-terminal portion of the 20 amino acid residues unique to Kal7 (Ma et al., 2008)), Kal12 COOH-terminal end (JH3225) and monoclonal antibodies to the Kal7 COOH-terminal end (20D8; specific for the NH2-terminal portion of the 20 amino acid residues unique to Kal7), PSD-95, and myc (Ma et al., 2008), and a polyclonal to Trio (CT233) (McPherson et al., 2005). Commercial antibodies to bassoon, Vglut1, GluR1, GluR2, NR1, GAD65, GAD65/67, VGAT, MAP2, and GFP were as described (Ma et al., 2008). The following commercial antibodies were also used: synaptophysin (Clone svp-38), δ-catenin (C2989) and actin (A2066) (Sigma, St. Louis, MO), NR2B (clone N59/20, NeuroMab, Davis, CA), Shank3 (cloneN69/46, NeuroMab), neuroligins-1 and -2 (Synaptic Systems, Gottingen, Germany), βIII-tubulin (Covance, Berkeley, CA), GFAP, CaMKIIα (MAB3119) and Cre-recombinase (Chemicon, Temecula, CA), α-adaptin, dynamin and clathrin (BD Transduction Labs, San Jose, CA), BiP (Affinity BioReagents, Golden, CO), Rac1 (23A8, Millipore, Temecula CA), Cdk5 and PP1 (Santa Cruz, Santa Cruz CA).
Western blot analyses and Rac activation assays
For analysis of total protein, freshly dissected tissue or cells were sonicated in SDS lysis buffer and heated for 5 min at 95°C (Ma et al., 2008). Subcellular fractionation was used to prepare samples enriched in ER/Golgi, cytosol, synaptosomal membranes, synaptic vesicles and synaptosomal cytosol (Huttner et al., 1983). Purified PSDs were prepared using a modification of published procedures (Carlin et al., 1980). PSDs removed from the interface of the 1.0/1.2 M sucrose layers of an equilibrium gradient were pelleted and then solubilized by incubation for 30 min at 4°C with 0.5% TX-100, 10 mM Hepes, pH 7.4. The identity of various Kalirin proteins was established using differential antibody reactivity plus comigration with recombinant ΔKal7, Kal7, Kal9, and Kal12 (produced in pEAK-Rapid cells; (Schiller et al., 2008)). ΔKal7 expressed in insect cells using the Baculovirus expression system was purified using Talon resin (Schiller et al., 2008). Protein concentrations were determined using bicinchoninic acid (Pierce) with bovine serum albumin as the standard.
For measurement of activated Rac, cerebral cortex (50 mg wet weight) was homogenized in 10 volumes 10 mM HEPES, 320 mM sucrose, 10 mM MgCl2, 1 mM EDTA, 1 mM NaF, pH 7.4 containing 10 μg/ml GST-Pak-CRIB and protease inhibitor mix. Following removal of debris (1000 × g for 5 min), Rac activation in the total lysate was assessed by adding ¼ vol 5X MLB (Schiller et al., 2008) and allowing each sample to tumble with 15 μl glutathione Sepharose 4B resin for 1 h at 4°C. Beads were washed twice with 1XMLB containing 8% glycerol and bound proteins were eluted with boiling Laemmli sample buffer. Rac was visualized using monoclonal antibody from Upstate. Crude synaptosomes were prepared by differential centrifugation (Carlin et al., 1980); P2 was resuspended in 1XMLB containing 10 μg/ml GST-Pak-CRIB and allowed to tumble at 4°C for 20 min before centrifugation at 100,000×g for 15 min; this supernatant was bound to glutathione Sepharose 4B resin and washed as described above. Positive and negative controls were prepared as described (Xin et al., 2008).
Immunocytochemistry
Dissociated neurons
Immunocytochemical staining of neuronal cultures was performed as described (Ma et al., 2003;Ma et al., 2008). Neurons were generally fixed for 20 min in 4% paraformaldehyde in PBS or with anhydrous methanol (−20°C). For visualization of synaptic proteins including PSD-95, NR1, Vglut1, GluR1, VGAT, and Kal7, cells were fixed with methanol for 12 min at −20°C, except where indicated. Primary antibodies were visualized with appropriate secondary antibodies as described (Ma et al., 2008).
Tissue sections
Immunohistochemical staining of tissue sections from perfusion-fixed mice has been described (Ma et al., 2001;Ma et al., 2002). Briefly, animals were perfused transcardially with 4% formaldehyde/0.1 M Na phosphate buffer, pH 7.4, under deep anesthesia with Ketamine. Following fixation, brains were postfixed in 4% paraformaldehyde for 6 hours. Sections (10 μm) through dorsal hippocampus were cut using a cryostat and immunostained with appropriate antibodies as described (Ma et al., 2001).
Golgi staining
Animals were perfused transcardially with 2% formaldehyde/1% glutaraldehyde (in 0.1 M Na phosphate buffer, pH 7.4) under deep anesthesia with Ketamine (n=4, Wt; n=4, Kal7KO). Following fixation, brains were removed and half brains were placed in 4% paraformaldehyde overnight. Brains were then sectioned through dorsal hippocampus at 100 μm on a vibratome and prepared for Golgi impregnation as described (Norrholm and Ouimet, 2000). The other halves of the brains were processed for electron microscopy as described below.
Electron microscopy
Half brains prepared as described above were postfixed in 2% glutaraldehyde for 2 hours. Brains were then sectioned at 50 μm on a vibratome and the sections were embedded in Polybed epoxy resin (Polysciences, Warrington, PA). Ultrathin sections (50 nm) from hippocampal CA1 stratum radiatum were cut with a Reichert Ultracut E ultramicrotome and collected on copper grids and counterstained with uranyl acetate and lead citrate. Sections were examined in a JEOL 100CX transmission electron microscope at 80 kV accelerating voltage. For quantification of PSDs, images were taken at 8,000x magnification. All measurements were performed independently by two researchers who were blind to the genotype of the animals.
Behavioral and physiological testing
Initial data
Animals were group housed in the UCHC animal facility with a 12 hour light/dark cycle (lights on 0700 – 1900). All behavior experiments were done in accordance with UCHC IACUC and NIH procedures for animal care. Simple physiological observations were performed on all mice, including body weight over time and general observations such as lack of obvious tremor, normal gait and ability to navigate/climb in the home cage. For behavioral studies, male littermates between 2 and 4 months of age were tested during the light phase. All animals were handled daily for at least five days before behavioral testing to minimize experimenter-induced stress during testing. With the exception of passive avoidance testing, all behavioral tests were carried out in dim lighting in the Scoville Neurobehavioral Suite at UCHC. For all experiments, animals were allowed to habituate to the room for one hour before testing. Some animals were re-used for behavioral tests, but great care was taken to ensure that the less stressful tests preceded those that were more stressful (Elevated zero → Open Field → Object recognition → Radial arm → Passive avoidance). Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, IL) with one-way ANOVA or repeated measures ANOVA to assess differences between groups.
Open Field
Spontaneous locomotor activity was measured using a PAS Open Field system (San Diego Instruments, San Diego, CA). Briefly, animals were placed in the center of a 15″ × 15″ plexiglas chamber and their horizontal ambulatory activity was recorded by photocell beam breaks. Animals were allowed to move freely about the chamber for an hour for a single testing session.
Elevated Zero Maze
The zero maze (San Diego Instruments) was made of white plastic with two “closed” quadrants consisting of 6″ tall walls on either side and two “open” quadrants with ¼″ walls. At the beginning of testing, each animal was placed in a closed quadrant facing it. Behavior was monitored by a blinded experienced scorer for the next five minutes. Time spent in the open, open entries (all four paws entering the open arm), stretch attends (stretching of forepaws into the open arm before retraction into the closed arm) and head dips (dipping the head over the open arm to the crux of the mandible) were scored for each animal. Entry into a new quadrant was scored only when all four paws of the animal left the previous quadrant.
Passive Avoidance
Mice were tested using a two compartment box with footshock grid (San Diego Instruments). On the training day, each animal was placed in the box with lights off and the interchamber door closed. After a five second habituation, the house lights were turned on only in the side where the mouse had been placed and the door was opened. When the mouse crossed to the darkened side, the door was closed and a 0.3 mA × 2 sec scrambled footshock was delivered. Mice were left in the shocked environment for 30 sec before being returned to their homecage. For the test trial, animals were brought back to the room after the appropriate interval and run through the same protocol, but without footshock. For both training and testing, the latency to cross to the dark was measured using photocells in the boxes. Shock sensitivity was assessed (Hawasli et al., 2007) with cohorts of previously shocked and naïve animals. Animals were placed in the box and given 2 sec shocks ranging from 0.05–0.5mA. A blinded scorer examined videotape and recorded the lowest shock that elicited vocalization, flinching and jumping.
Radial Arm Maze
The radial arm maze was used to test both working and reference memory. The maze (San Diego Instruments) consisted of a 5.25″ central platform with eight arms (2″ wide × 6″ high) radiating from the platform. Extensive intramaze (distinct geometric shapes on walls) and extramaze (objects of varying shape and size present over arms) cues were provided throughout the testing procedures. Animals were food deprived for 5 days prior to the start of the experiment and were maintained at 85% of their free-feeding weight throughout training. Mice were first group habituated (3–4 mice at a time) to the maze, where reward pellets (small pieces of Cocoa Puffs cereal, General Mills Inc, Golden Valley, Minnesota) were scattered throughout. The mice were then given an individual habituation session with similarly scattered rewards. During testing, the animals were placed in the maze and confined to the center platform using a white cylinder for 15 sec. After the 15 sec acclimation, the cylinder was removed and the animals were given free run of the maze. Throughout the training period, two arms separated by 135° were baited with a hidden food pellet. The baited arms were varied between animals but remained consistent for each subject. Mice were allowed to explore the maze freely for four minutes or until both rewards were obtained. A blinded observer watched and scored throughout each session. Reference memory errors were scored when an animal entered an unbaited arm or when a baited arm was entered but the reward was not obtained. Working memory errors were re-entries into arms within a trial. Two cohorts of animals were used for this experiment. In the first (4 Wt, 6 KO), animals were given one trial per day for the first seven days and then three trials per day for the remaining days. Animals in the second cohort (5 Wt, 7 KO) were given two trials on the first day and three trials on each successive day. These differences in training procedure produced no difference in acquisition curves, so data were combined for the purpose of analysis.
Object Recognition
Testing was performed in a large, clean cage normally used for housing rats. At the start of the training day, each mouse was placed into this novel environment, which contained no objects other than bedding, and allowed to habituate for five minutes. After habituation, the animals were briefly placed in a holding cage while two identical objects were placed at either end of the cage (50 ml Falcon tubes or small Leggo objects). Mice were allowed three minutes to explore the two objects before being returned to their home cages. Twenty four hours later, the animals were again habituated to the empty cage for five minutes. When they were returned to the cage for the test, one of the objects was the same as the previous day (Object A) and the other was a novel object (Object B – the object not used previously). Animals were again allowed three minutes to explore the cage and objects. Both sessions were videotaped and scored by an experienced blinded observer. Object exploration was strictly defined as direct nasal contact with the object.
Cell culture
Hippocampal or cortical cultures from genotyped newborn mice (P1) were prepared as described (Ma et al., 2008); PCR genotyping allows same day culturing of Wt and KO neurons from Kal7KO/+ × Kal7KO/+ matings. Briefly, hippocampi or cortices were digested with 0.25% trypsin for 25 min at 37°C. Dissociated cells were plated in Neurobasal A medium containing 7% heat-inactivated horse serum, maintained at 37°C in 5% CO2. Three hours later, plating medium was replaced with fresh medium containing only 3% horse serum (heat inactivated), 2% B27 supplement, 0.5mM glutamine, 25 μM glutamate, 25 units/ml penicillin, 25 μg/ml streptomycin. Three days after plating, the culture medium was exchanged with maintenance medium [Neurobasal A medium containing 2% B27 supplement, 0.5mM glutamine, 25 units/ml penicillin, 25 μg/ml streptomycin]. Thereafter, half of the medium was replaced twice a week for up to 4 weeks. Nucleofection of freshly dissociated neurons was performed at P1 as described (Ma et al., 2008). Adenovirus encoding Cre recombinase (Iowa Gene Transfer Vector Core (http://www.uiowa.edu/~gene/) was used where indicated.
Electrophysiological analysis of Kal7 null mice
Slice Preparation
Mice of 4–5 weeks of age were sacrificed by decapitation and their brains quickly removed in ice-cold artificial cerebral spinal fluid solution (ACSF; in mM): 125 NaCl, 26 NaHCO3, 10 glucose, 2.3 KCl, 2 CaCl2, 2 MgSO4, 1.26 KH2PO4 (aerated with 95% O2 and 5%CO2; pH=7.3; 310 mOsm/kg) (Zhou et al., 2008). Coronal slices, 300 μm thick, were allowed to incubate at room temperature for at least 1 hour before recordings.
Whole Cell Recordings
Slices were transferred to a recording chamber heated to 32°C and perfused with aerated ACSF with the addition of 50 μM Picrotoxin (PTX; Sigma-Aldrich) to isolate excitatory neurotransmission. Recording pipettes with 3–5 MO tips were filled with internal solution containing (in mM): 135 KGluconate, 10 HEPES, 10 PCreatine, 3 Na2ATP, 2 MgCl2, 0.3 Na2GTP (pH=7.3; 285 mOsm/kg) (Zhou et al., 2008). Cells were patched under visual guidance using infrared differential interference contrast optics. Data were collected using a Multiclamp 700B amplifier (Axon Instruments/Molecular Devices Sunnyvale, CA) and recorded/analyzed using pClamp 9.2 software (Axon Instruments).
Intrinsic Membrane Properties
Integrity of each patch was assessed through a series of negative and positive steps in current clamp mode (20 × 100 pA steps, −600 pA to +1300 pA; 100 msec steps). The three initial negative steps were used to calculate membrane resistance; single exponential curve fits yielded tau (τ) and capacitance. Action potential threshold was defined as the point of voltage inflection for the first spike fired in the lowest current step. Inter-spike interval (ISI) was assessed in steps eliciting 3 or more spikes and defined as time between peaks of the last two spikes within a step.
Spontaneous Glutamatergic Activity
We recorded spontaneous Excitatory Post Synaptic Currents (EPSCs) while voltage clamped at −70 mV for 1 minute for each cell. Events observed in the full minute were analyzed using MiniAnalysis (Synaptosoft Inc, Chapel Hill, NC). Two outlier cells from each group were excluded from statistical analysis; their event frequency values were far greater than 2 standard deviations above their group mean.
Long Term Potentiation
Bipolar tungsten electrodes (World Precision Instruments, Sarasota, FL) were placed in the stratum radiatum to stimulate Schaffer collateral axons. EPSCs were recorded in voltage clamp mode at −70 mV, with stimulation strength adjusted to obtain 20–50 pA EPSCs at baseline. Test pulses were applied at 0.1 Hz to establish averaged baseline (10–25 pulses) and post-LTP induction EPSC values (25–50 pulses, recorded within 15 min after LTP induction). EPSC amplitudes were measured as the absolute difference between baseline and peak inward current within 50 ms after the stimulus artifact. A small subset of cells was recorded in the absence of PTX, and included in the analysis because their electrophysiological measures were consistent. LTP induction was initiated using Theta Burst Pairing in voltage clamp mode and always within 15 minutes after achieving whole cell configuration. LTP induction consisted of 15 4-pulse bursts at 100 Hz separated by 200 ms intervals, and each burst was paired with a 50 msec depolarizing voltage step to 0 mV. Cells that required greater than 200 μA of stimulation or showed LTP smaller than 120% were excluded from analyses (Kauer and Malenka, 2007). Statistical comparisons were made using Student’s T-test.
Image analysis and quantification
Images captured using a Zeiss LSM510 Meta confocal microscope were analyzed as described (Ma et al., 2003;Ma et al., 2008). For quantification of spine density and synaptic clusters, a stack of images (Z step, 0.2 μm) was acquired using a 63X objective (3.0 digital zoom factor) and dendrites were visualized in 3-dimensions. For analysis of synaptic marker colocalization, single plane images through the brightest point were used. For analysis of Golgi staining, a stack of images (Z step, 0.2 μm) was acquired using a Nikon TE 300 microscope with a 100X objective and a Hamamatsu camera; dendrites were visualized in 3-dimensions using Open Lab and Volocity software (Improvision, Waltham, MA). For each experiment, all images were taken with identical settings under the same conditions. Spine density and synaptic clusters were counted after images were calibrated and thresholds were set to ensure that all interesting structures were included in the analysis. Quantifications were performed using Metamorph (Universal Imaging, West Chester, PA) and were limited to dendrites within 100 μm of the soma in culture, or 100–150 μm in tissue slices. Data are presented as average ± SEM. Statistical analyses were performed with JMP6 software (SAS Institute, Cary NC) using one way analysis of variance (ANOVA) followed by Dunnett’s test or Student’s T-test to assess statistical significance between groups; *p < 0.05 or **p<0.01 was considered statistically significant. All quantifications were performed independently by two investigators blind to genotype.
Results
Design and characterization of Kal7 null (Kal7KO) mice
The targeting scheme shown in Fig. 1 was used to establish two independent lines of mice with lox-p sequences flanking the single exon unique to Kal7 and ΔKal7. The polymerase chain reaction (PCR) schemes for screening mouse genomic DNA before and after Cre-recombinase mediated excision of the Kal7 exon are outlined in Fig. 1B–E. Sequence analysis of the lox-p PCR product (primers A+B) and the knockout allele PCR product (primers A+E) confirmed that lox-p insertion and DNA excision occurred as expected (not shown). Neuronal cultures prepared from conditional null mice (Kal7CKO/CKO) were infected with adenovirus encoding Cre-recombinase or GFP in order to verify successful excision of the Kal7 exon (Fig. 1D). Male Kal7+/CKO mice were mated with female mice expressing Cre-recombinase, producing Kal7+/KO heterozygote mice, with a normal Kal7 allele from the mother and a null allele from the father (Fig. 1E). After several back-crosses into the C57Bl/6 background, Kal7+/KO heterozygote matings yielded wildtype, Kal7+/KO and Kal7KO mice in the expected Mendelian ratio with no differences in survival (Fig. 1F). Western analysis of cortical extracts from Kal7KO mice demonstrated complete loss of Kal7 and ΔKal7 (Fig. 1G). Growth curves for wildtype and Kal7KO mice were identical (Fig. 1H).
Hippocampal histology and spine density in adult male wildtype and Kal7KO mice
In Wt mice, Kal7 is at the postsynaptic side of virtually all excitatory synapses in the cortex and hippocampus (Fig. S1). Low resolution images of the brain were obtained using cresyl violet stained sections from wildtype and Kal7KO mice (Fig. S2). The gross anatomy of the hippocampus and cortex, regions rich in Kal7/ΔKal7, and the cerebellum, a region with very little Kal7/ΔKal7 (not shown), did not differ between wildtype and Kal7KO mice. To compare spine density and morphology, Golgi impregnation techniques were utilized, revealing a deficit in spine density along the apical dendrites of CA1 pyramidal neurons in Kal7KO mice (Fig. 2A, B).
Fig. 2. Neuronal architecture.
A–C. Golgi staining showed a decrease in spine density in apical dendrites of CA1 pyramidal neurons from 6–7 week old Kal7KO mice; Wt, n=4 mice; Kal7KO, n=4 mice. Three sections (100 μm) through the dorsal hippocampus were used per animal and at least two neurons were analyzed for each section. Spines on apical dendrites in the stratum radiatum (SR), 100–150 μm from the soma, were counted; SO, stratum oriens. At least 500 spines were counted for each neuron.
In the Kal7KO mice, linear spine density dropped to 85% of wildtype values (Fig. 2C); spines in the knockout animals were smaller than those from wildtype animals (not shown). Tissue sections from the hippocampus of wildtype and Kal7KO mice of the same age were also examined immunocytochemically. Consistent with the decrease in spine density seen with the Golgi method, Kal7KO tissue showed a slight decrease in Vglut1 (excitatory presynaptic marker) and PSD-95 (postsynaptic marker) staining in the neuropil, with no change in GAD65 (inhibitory presynaptic marker) staining (not shown).
Normal Gross Development and Physiology in Kal7KO Mice
From the time of birth, Kal7KO mice exhibited no gross developmental or physiological deficits. Kal7KO animals bred successfully with other Kal7KO animals, with no difference in average litter size when compared to Wt pairs, and Kal7KO pups suckled normally. Adult Kal7KO animals had a normal gait, lacked any sign of tremor, had a normal righting reflex, had normal leg splay when lifted by the tail and displayed normal climbing behavior in the home cage. In a wire hang test to measure grip strength, both wildtype and Kal7KO animals hung suspended from a wire for three minutes without failure. To measure their spontaneous locomotor activity, Wt, Kal7KO/+ and Kal7KO mice were monitored for 60 minutes in an open field chamber. Genotype had no effect on total locomotor activity (Fig. S3). Additionally, mice of all three genotypes exhibited a similar decrease in locomotor activity across the 60 minute session (data not shown).
Decreased anxiety-like behaviors in Kal7KO mice
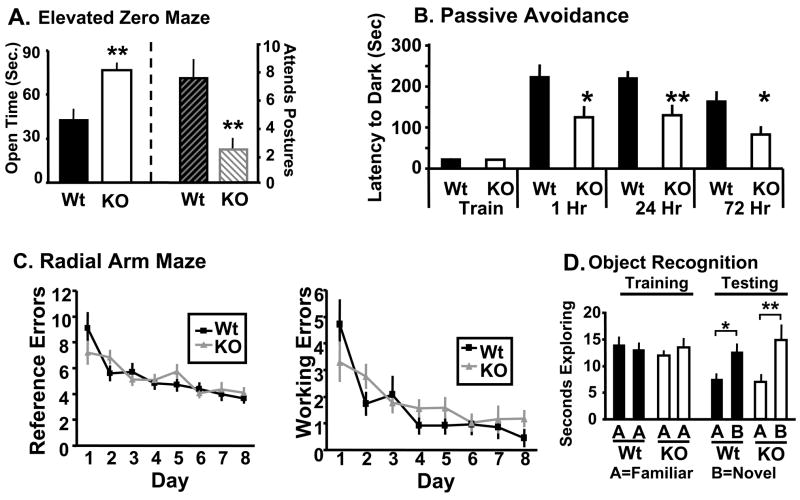
Given the apparent deficits in synaptic connectivity in Kal7KO mice, baseline anxiety-like behavior was assessed to determine if the morphological changes observed lead to behavioral changes (Pogorelov et al., 2005). Wildtype and Kal7KO mice underwent a one time elevated zero maze test. When compared to wildtype mice, Kal7KO mice spent significantly longer in the open arm of the elevated zero maze (Fig. 3A). Additionally, Kal7KO mice exhibited fewer stretch attend postures as they extended from the closed into the open area (Fig. 3A) and a greater number of open arm entries (not shown). Data from the open field test (Fig. S3) demonstrated that these effects were not simply due to hyperactivity in response to a novel environment. Stretch attend postures are generally thought to represent hesitation as the mouse tries to avoid any unseen threat in the exposed area. The fact that the Kal7KO mice made more open entries with fewer stretch attend postures indicates less anxiety about entering a potentially threatening area. Given the greater time spent in the open arms and the decreased hesitation to enter the open areas, it is clear that Kal7KO mice have a significantly decreased level of anxiety-like behavior at baseline.
Fig. 3. Behavioral deficits in Kal7KO mice.
A. In the elevated zero maze, Kal7KO mice spent more time in the open arm (p=0.003; ANOVA) and displayed fewer stretch attend postures (p=0.011; ANOVA) [N=14Wt; 9KO] than Wt mice. B. In a passive avoidance task, Kal7KO and Wt mice exhibited no differences in training times (p=0.895; ANOVA); compared to Wt mice, Kal7KO mice showed decreased latency to enter the dark, shock-paired side at 1 (p=0.03; ANOVA), 24 (p=0.006; ANOVA) and 72 (p=0.02; ANOVA) hr after the training session. C. In a radial arm maze acquisition task, both Wt (N=9) and Kal7KO (N=13) animals made an equal number of reference and working errors initially and throughout the learning sessions: Left, reference errors, F(1,20)=0.02; p = 0.888, repeated measures two-way ANOVA; Right, working errors, F(1,20)=0.54; p = 0.469, repeated measures two-way ANOVA. D. Both Wt (N=11) and Kal7KO (N=7) mice spent an equal amount of time exploring a novel object in an object recognition task (p=0.364; Fisher’s LSD).
Impaired contextual fear learning in Kal7KO mice
Passive avoidance conditioning was used to determine if Kal7KO mice exhibited altered abilities in a hippocampus-dependent learning task; in this paradigm, lasting context-fear association can be imparted with a single training session (Duvarci et al., 2005). During the training session, wildtype and Kal7KO animals showed the same latency to cross to the dark side of the chamber (Fig. 3B), whereupon they received a single footshock. When animals were tested one hour after conditioning, Kal7KO animals exhibited a significantly decreased latency to cross to the footshock-paired side. This decrease in latency was also seen in animals that were tested 24 hours and 72 hours after conditioning. This pattern of behavior indicates that Kal7KO animals had difficulty with the initial learning of the association rather than a simple deficit in long term consolidation of the association. To ensure that the shock was being interpreted similarly by Kal7KO and wildtype mice, a shock sensitivity test was performed. Both Wt and Kal7KO mice showed identical thresholds to vocalize, flinch and jump in response to a scrambled footshock (Fig. S3). Taken together, these experiments indicate that Kal7 is necessary for contextual fear memory, which is dependent on both the hippocampus and amygdala (Pare et al., 2004).
Kal7KO mice exhibit normal learning in a radial arm maze task as well as normal object recognition
From the passive avoidance experiments, it was clear that Kal7KO mice were deficient in learning in response to an aversive stimulus. We next examined their ability to acquire a repeated-trial appetitively motivated task that was also dependent on the hippocampus. For this task, Kal7KO and wildtype mice were food deprived to 85% of their free-feeding body weight and trained to locate food rewards in a radial arm maze. By baiting only two arms, we were able to examine both long term (reference) memory and short term (working) memory. Each mouse received 23 trials in the maze and all mice decreased the number of reference and working memory errors made over the course of the training (Effect of Day F(1,20)=13.65; p=0.001, repeated measures two-way ANOVA). The Kal7KO mice made the same number of reference (Fig. 3C left) and working (Fig. 3C right) errors as the wildtype controls. Our experimental design allowed the animals free reign of the maze, likely leading to more exploratory reference errors, causing the number of errors to plateau at a higher level than reported with some other experimental designs (Hung et al., 2008;Bannerman et al., 2008). Regardless of this, animals of both genotypes showed significant improvement across days, with similar learning curves and similar final levels of responding. These data indicate that Kal7 is not necessary for this type of appetitive repeated-trial hippocampal conditioning.
To further test the ability of Kal7KO animals to learn hippocampal dependent tasks, an object recognition test was performed (Fig. 3D). There was no difference between genotypes in level of object exploration on the training day. Animals of each genotype showed significantly increased exploration of the novel object on day 2, and there was no significant difference between genotypes. These data indicate that Kal7KO mice are able to form a stable contextual hippocampal dependent memory in a non-aversive context.
Electrophysiological analysis of Kal7KO mice
In order to assess the functional consequences of Kal7 knockout, we performed electrophysiological recordings from acute slice preparations. First we evaluated passive membrane properties. Capacitance is a measure of cell surface area and dendritic spines contribute 40–70% of the total surface area of hippocampal CA1 pyramidal cells (Mainen et al., 1996;Harris and Stevens, 1989;Inoue et al., 2001). Given the reduction in spine density observed in the CA1 pyramidal neurons of Kal7KO mice (Fig. 2), we expected to see slightly reduced capacitance values for Kal7KO vs. Wt neurons. Hyperpolarizing and depolarizing current steps were applied to patched CA1 hippocampal neurons to provide measures of membrane resistance and tau so that capacitance could be calculated (Fig. 4A). On average, Kal7KO neurons had higher input resistance (Wt: 122 ± 7 MO, Kal7KO: 147 ± 5 MO; p=0.008; 32 Wt neurons and 29 KO neurons) and lower membrane capacitance (Wt: 66 ± 3 pF, Kal7KO: 57 ± 2 pF; p=0.028); time constants did not differ (Wt: 7.6 ± 0.3 ms; KO: 8.1 ± 0.3 ms; p=0.19). The resting potential and action potential threshold for knockout and Wt pyramidal neurons were identical (Fig. S4A). In knockout neurons, the number of action potentials fired during weak depolarizing current steps was slightly increased (Fig. S4B) while latency to fire the first action potential following step initiation was decreased (Fig. S4C); both results are consistent with the decreased membrane capacitance (decreased surface area) observed in Kal7KO neurons. There was no significant difference in interspike interval between the two groups. Since the CA1 pyramidal neurons of Kal7KO mice have fewer dendritic spines, we assessed spontaneous excitatory post-synaptic currents (EPSCs) (Fig. 4B); as predicted, spontaneous EPSC frequency was significantly decreased in Kal7KO neurons. No difference was observed in amplitude (Fig. S4D).
Fig. 4. Electrophysiological deficits.
A. Representative traces from Wt and Kal7KO CA1 pyramidal neurons of membrane responses to positive and negative 200 pA somatic current injections. B. Composite spontaneous EPSC frequency (N= 24 Wt, 18 KO neurons; p=0.023; left) and LTP (N= 7 Wt, 13 KO neurons; p=0.0079; right) data. C. Averaged traces of EPSCs recorded from single CA1 pyramidal neurons from Wt and Kal7KO mice before and within 15 minutes following theta burst pairing.
We went on to compare plastic properties of Wt and Kal7KO synapses. EPSCs, evoked via Schaffer collateral stimulation, were of similar amplitude and required similar stimulation strengths in Wt and Kal7KO neurons (Fig. S4E, F). Taken with the lack of difference in spontaneous EPSC amplitude, these data suggest normal strength of basal synaptic transmission in Kal7KO neurons. In order to measure Long Term Potentiation (LTP), EPSCs were examined before and after applying a Theta Burst Pairing paradigm (TBP). A single TBP trial resulted in significant LTP that was maintained for at least 1 hour in both Wt and Kal7KO slices (Fig. 4C). LTP was quantified by comparing EPSC amplitudes within 15 min after the TBP trial to averaged baseline EPSC amplitudes. In comparison to Wt neurons, Kal7KO neurons showed markedly blunted LTP (Fig. 4B, C). This deficiency in LTP may correlate with the decreased ability to retain behavioral training (Fig. 3), as LTP is typically considered to be one cellular correlate of learning.
Kal7 and Kal7 are reduced to half in Kal7+/KO mice and eliminated in Kal7KO mice
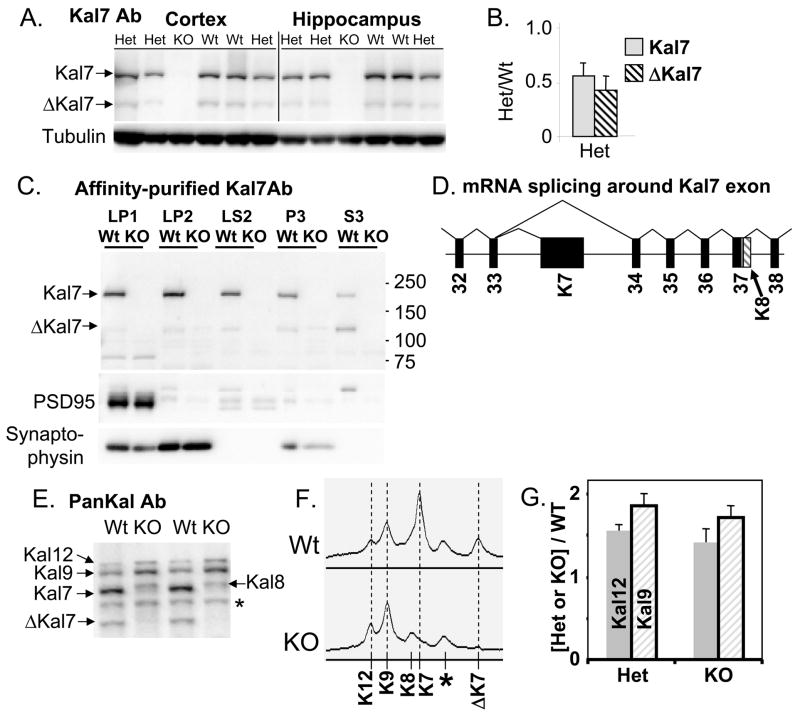
Antisera specific for the sequence encoded by the Kal7 exon were used to evaluate the success of the targeting strategy (Fig. 5A). In both cortex and hippocampus, levels of Kal7 and ΔKal7 were reduced to approximately 50% of Wt levels in Kal7+/KO mice (Fig. 5B); no compensatory increase in Kal7 expression was observed. In the Kal7KO mice, cross-reactive material the size of Kal7 and ΔKal7 was eliminated (Fig. 5A). Differential centrifugation was used to prepare fractions enriched in synaptsomal membranes (LP1), synaptic vesicles (LP2), synaptosomal cytosol (LS2), endoplasmic reticulum/Golgi (P3) and cytosol (S3). Two independent polyclonal antisera specific for the C-terminus of Kal7/Kal7 confirmed its elimination in the Kal7KO mice (Fig. 5C; one shown).
Fig. 5. Biochemical verification of Kal7KO.
A. Total SDS lysates (20 μg protein) prepared from the parietal cortices and hippocampi of Wt, Het and Kal7KO mice were analyzed for Kal7 and βIII tubulin. B. Levels of Kal7 and ΔKal7 in Kal7+/KO mice were compared to levels in Wt mice. C. Parietal cortices from Wt and Kal7KO mice were separated into crude subcellular fractions. Equal amounts of protein (5 μg) from each fraction were analyzed using Kal7-specific antibody (JH2959); similar results were obtained with affinity-purified Kal7-specific antibody (JH2958). The success of the fractionation was verified by visualizing synaptophysin and PSD-95. D. The splicing pattern for the region around the Kal7-specific exon is depicted; introns are not drawn to scale. Kal8 transcripts retain part of the adjacent intron (hatched), including a small protein coding region (gray) followed by a poly-A addition signal and a poly-A tract (Johnson et al., 2000). E. SDS lysates (20 μg) prepared from parietal cortices of Wt and Kal7KO mice were visualized with a pan-Kalirin antibody, JH2582; *, nonspecific band. F. Scans of gels were aligned, revealing the presence of Kal8 in Kal7KO lysates; *, nonspecific band. G. Levels of Kal12 and Kal9 in Het and KO mice were compared to Wt mice; n=7.
Transcripts encoding Kal9 and Kal12 are generated by splicing Kalirin exon 33 to exon 34, eliminating the exon that encodes the unique COOH-terminus and 3′-untranslated region of Kal7 (McPherson et al., 2002) (Fig. 5D). The genomic region excised in the Kal7KO mouse included the polyA addition site (McPherson et al., 2002). To explore the possibility that transcripts that would normally encode Kal7 generated Kal8, Kal9 and Kal12 in Kal7KO mice, we used pan-Kalirin antibodies to visualize these isoforms (Fig. 5E). Levels of Kal7 and Kal7 were not detectable as expected in Kal7KO mice, but levels of Kal9 and Kal12 increased. Scans of these gels confirmed the appearance of a protein the size of Kal8 (Fig. 5F) and quantification revealed an increase of about 50% in levels of Kal9 and Kal12 in both Kal7+/KO and Kal7KO mouse cortex (Fig. 5G). Although Kal7/ΔKal7 account for about 75% of the pan-Kalirin signal in wildtype samples, the increased levels of Kal8, Kal9 and Kal12 in Kal7KO cortex mean that pan-Kalirin levels normalized to tubulin dropped by only about 25% in the knockouts (to 74 ± 5% of wildtype; N=7).
Biochemical analysis of Kal7KO mice
We first examined levels of cell-type specific markers in SDS lysates prepared from cerebral cortex. Neuron-specific βIII-tubulin, glutamic acid decarboxylase (GAD; a marker for inhibitory neurons) and glial fibrillary acidic protein (GFAP; a marker for astrocytes) were unaltered (Fig. 6A). Proteins abundant in presynaptic terminals (synaptophysin, α-adaptin and clathrin) were unchanged, as was BiP, a major endoplasmic reticulum marker common to all cells. Levels of known Kalirin interactors were then assessed (Fig. 6B); levels of PSD-95, a Kal7/ΔKal7 specific interactor (Penzes et al., 2001) and Rac1, a Kal7 substrate, were unaltered. Cdk5, which phosphorylates Thr1590 in Kal7/ΔKal7, and PP1, which reverses this modification (Xin et al., 2008), were unaltered. Levels of dynamin, an interactor with the IgFnIII domain of Kalirin (Xin et al, submitted) were unaltered. Levels of several postsynaptic proteins, AMPA receptor subunits GluR1 and GluR2, and NMDA receptor subunits NR1, NR2B were unaltered in Kal7KO mice (Fig. 6C); levels of neuroligin-1, another component of the PSD, were unaltered. Finding normal levels of these markers in tissue lysates is consistent with the limited deficits observed. The significant alterations observed in Golgi impregnation profiles (Fig. 2), behavioral parameters (Fig. 3) and electrophysiological properties (Fig. 4) led us to focus on the ultrastructure of synapses in Kal7KO vs. Wt mice.
Fig. 6. Western blots.
Parietal cortex SDS lysates were analyzed (5, 10 or 20 μg protein). A. Levels of the indicated cell type, presynaptic and subcellular organelle markers were quantified after normalization to βIII tubulin and setting the average Wt intensity to 1.0. Representative gels are shown. The graphs show the average ± standard error of the mean for at least 6 independent pairs of wildtype and Kal7KO mice. B. Levels of known Kalirin interactors were tested in the same manner. C. Glutamate receptor subunits and neuroligin-1 (NL1) were tested as in A.
Hippocampal ultrastructure in adult male Wt and Kal7KO mice
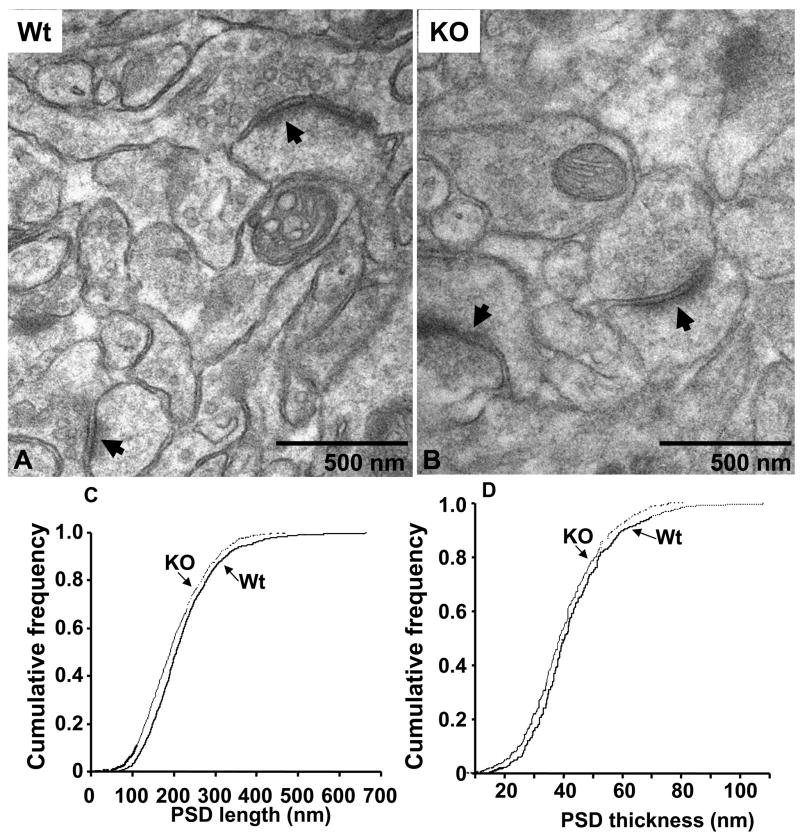
Synapses on the apical dendrites of CA1 hippocampal pyramidal neurons in Wt and Kal7KO neurons were compared (Fig. 7A, B). Despite the total absence of Kal7, synaptic structures of normal appearance were plentiful in Kal7KO tissue. Postsynaptic densities aligned with presynaptic endings full of small synaptic vesicles were prevalent in Wt and Kal7KO tissue. The formation of spines is clearly possible in the total absence of Kal7. The length and thickness of the PSD were measured in wildtype and Kal7KO neurons (Fig. 7C, D). Compared to wildtype mice, there was a small, but significant decrease in both the length and thickness of the PSD in Kal7KO mice (Fig. 7C, 7D; p<0.005, Kolmogorov-Smirnov, 800+ PSDs measured for each plot). Even a change of this magnitude can be of significance in synaptic transmission (Harris and Stevens, 1989;Hung et al., 2008). The synapses present in micrographs from the CA1 region of wildtype and Kal7KO tissue were counted (arrows in Fig. 7A, B). The number of synapses (defined as a PSD with apposed presynaptic ending containing vesicles) in Kal7KO mice dropped by about a third compared to wildtype mice (from 0.53 ± 0.02/μm2 to 0.31 ± 0.02/μm2; synapses in 100 μm2 were quantified for 5 images of each genotype; p<0.005, Student’s T-test). Although larger in magnitude, the change in ultrastructurally identified synapse number is consistent with the decrease in linear spine density observed using Golgi impregnation (Fig. 2). Our cell culture studies demonstrated that Kal7 shRNA and antisense treatments produced spine-like structures lacking presynaptic terminals; if a similar phenomenon occurs in vivo, synapse number as measured by EM would decrease more than spine number as measured by Golgi staining (Ma et al., 2003;Ma et al., 2008).
Fig. 7. Normal synapses are formed in Kal7KO mice.
A Representative electron micrographs of hippocampal CA1 striatum radiatum synapses from adult Wt and Kal7KO mice. Complete synapses (PSDs apposed to presynaptic endings with vesicles) were readily identified in Kal7KO tissue. PSD length and thickness were measured. Cumulative frequency distributions of PSD length (C) and thickness (D) from Wt (solid line) and Kal7KO (dashed line) mice are shown. Both PSD length (C) and thickness (D) were decreased in Kal7KO mice. At least 800 randomly selected synapses from each of four Wt and four Kal7KO mice were measured by observers blind to genotype. Differences are significant by Kolmogorov-Smirnov, p<0.005.
Biochemical characterization of purified PSDs from Kal7KO mice
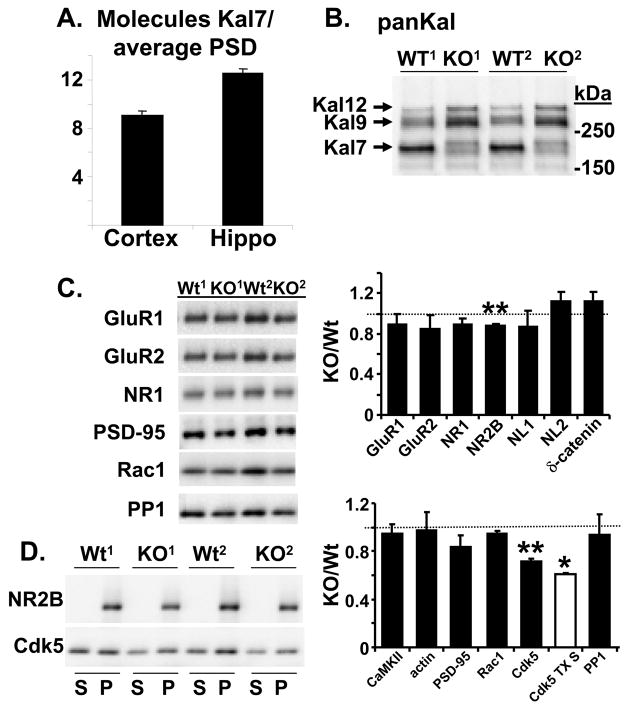
While the GEF domain of Kal7 clearly has a catalytic role, its spectrin-repeat region suggests that Kalirin might serve a structural role similar to that of spectrin itself (Schiller et al., 2008). To evaluate this possibility, we determined the average number of Kal7 molecules present per PSD (Fig. 8A). The PSD purification protocol optimized for wildtype mouse cortex and hippocampus worked well for tissue from Kal7KO mice (Fig. S5). Using purified ΔKal7 as a standard, we determined that the average cortical or hippocampal PSD contained only 9 –12 molecules of Kal7. With hundreds of copies of PSD-95, Shank and Homer per PSD, a structural role for Kal7 seems unlikely (Elias et al., 2006;Beique et al., 2006;Collins et al., 2006;Sheng and Hoogenraad, 2007;Sugiyama et al., 2005;Cheng et al., 2006).
Fig. 8. PSD preparations from Wt and Kal7KO mice.
A. Purified ΔKal7 was used to quantify the amount of Kal7 in the average 1.1 gigadalton PSD (Collins et al., 2006;Sheng and Hoogenraad, 2007). Recombinant purified ΔKal7 was quantified by making a dilution series and comparing Coomassie Blue staining to a bovine serum albumin standard of known concentration. Western blot signals, using affinity-purified Kal7 antibody, for the ΔKal7 standard were used to construct a standard curve to quantify Kal7 in the PSD samples. B. Use of the pan-Kalirin antibody revealed that PSDs (5 μg protein) prepared from Kal7KO mice had increased levels of Kal9 and Kal12 compared to PSDs prepared from Wt mice. C. Left. PSDs (5 μg protein) purified from the cortices of 2 sets of Wt and Kal7KO mice were blotted for AMPA and NMDA receptor subunits, neuroligins 1 and 2, δ-catenin and for the Kal7 interactors PSD-95, Rac1, and PP1. Right. Both the Triton-soluble (S) and insoluble PSD (P) fractions were blotted for NR2B and Cdk5. D. Data from 3 sets of Wt and Kal7KO PSDs were averaged (n = 2 for Cdk5 TX S); *, p<0.05; **, p<0.01.
A pan-Kalirin antibody was used to compare the Kalirin content of PSDs purified from wildtype and Kal7KO animals (Fig. 8B). Although Kal7 was absent, the amount of Kal9 and Kal12 in Kal7KO PSDs increased compared to Wt PSDs (up 50 ± 22% and 79 ± 21% over wildtype, respectively), indicating that the PDZ binding motif at the COOH-terminus of Kal7 is not the only means of localizing Kalirin to the PSD. In non-neuronal cells, both Sec14p mediated interactions with phosphatidylinositides and the spectrin-repeat region contribute to the association of Kal7 with membranes (Schiller et al., 2008). To determine whether the absence of Kal7 diminished Rac activation, we assayed crude tissue homogenates and the synaptosomal fraction (P2) for Rac1-GTP using the Pak-CRIB pulldown assay (Schiller et al., 2008). No difference in Rac1 activation was detected in either fraction under baseline conditions (Fig. S5C). Investigation of specific signaling pathways may be required to reveal the effect of a lack of Kal7 on Rac activation.
Since Kal7KO neurons were deficient in LTP, we examined levels of AMPA receptor subunits GluR1 and GluR2 and NMDA receptor subunits NR1 and NR2B in purified cortical PSDs (Fig. 8C). A significant decrease in the level of NR2B was observed, with no significant change in GluR1, GluR2 or NR1 (Fig. 8D). Levels of neuroligin-2, which is associated with inhibitory synapses (Chubykin et al., 2007;Varoqueaux et al., 2006) and δ-catenin, a neuron-specific catenin which binds synaptic scaffolding proteins and affects AMPA receptor trafficking (Ochiishi et al., 2008;Kosik et al., 2005), were unchanged. Further studies will be required to determine whether surface levels of AMPA and NMDA receptors and receptor trafficking are altered.
We next looked at levels of several Kal7 interactors. Neither levels of CaMKIIα, which has been reported to phosphorylate Kalirin (Xie et al., 2007), nor actin were changed (Fig. 8C). Levels of PSD-95, which binds the COOH-terminus of Kal7, and Rac1, a Kalirin substrate, were unaltered (Fig. 8C). The most striking change observed was a decrease in the level of Cdk5 (Fig. 8C, D), which phosphorylates Kal7 at Thr1590 (Xin et al., 2008). Since Cdk5 associates with membranes via its interactions with p35 and p39, we examined Cdk5 levels in the TX-100 wash used to prepare PSDs; levels of Cdk5 were also reduced in this fraction (Fig. 8C, D). Cdk5 regulates the actions of a wide variety of post-synaptic proteins (Hawasli et al., 2007;Zhang et al., 2008;Morabito et al., 2004) and its loss may contribute to the phenotype observed in Kal7KO mice. Levels of PP1, which dephosphorylates P-Thr1590 of Kal7 (Xin et al., 2008) were unchanged.
The late stages of synaptogenesis are deficient in Kal7KO neurons in culture
We next asked whether the differences in spine density seen in Kal7KO mice in vivo could be reproduced in vitro. Dissociated cortical neurons prepared from P1 wildtype and Kal7KO littermates were examined after 7, 14, 21 or 28 days in vitro (DIV) (Fig. 9 and Fig. S6). At each time point, replicate cultures were stained for MAP2, a dendritic marker, and Vglut1; one culture was stained for Kal7 and the other for PSD-95. Images from DIV21 are shown in Fig. 9 and images from DIV7 and DIV14 are shown in Fig. S6. The MAP2 staining patterns were similar in wildtype and Kal7KO neurons at all time points, but dendritic morphology has not yet been quantified. As expected, Kal7KO cultures lacked any detectable staining for Kal7 at all time points. At DIV28, Vglut1 staining along wildtype dendrites was almost perfectly aligned with Kal7 staining (Fig. 9A). Vglut1 positive clusters still abutted the dendritic shafts of Kal7KO neurons (Fig. 9B, C). Quantification revealed a decrease of 28% in the density of Vglut1 positive clusters along the dendrites of DIV28 Kal7KO neurons (Fig. 9G, H). Vglut1 staining was paired with PSD-95 in order to determine whether glutamatergic presynaptic endings were contacting dendritic spines (Fig. 9D–F). In DIV28 wildtype neurons, almost every Vglut1 cluster was apposed to a PSD-95-positive cluster. The number of Vglut1-PSD-95 clusters along the dendrites of Kal7KO neurons dropped by 39% and Vglut1 clusters not apposed to PSD-95 clusters became apparent (Fig. 9E, F, H; 0.99 Vglut alone/10μm in Kal7KO; p=0.003 vs. Wt; green stars). These data are in remarkably good agreement with the decrease in the number of synapses in vivo in the ultrastructural analyses (Fig. 7).
Fig. 9. Decreased number of synapses in the mature Kal7KO neurons in culture.
Cortical cultures were prepared from P1 littermate wildtype and Kal7KO mice. Replicate cultures were fixed at DIV7 (Fig. S5A), DIV14 (Fig. S5B), DIV21 (images not shown) and DIV28 (A–F). One set of cultures was visualized with antibodies specific to Vglut1, MAP2 and Kal7 (A–C); the other set was visualized with antibodies to Vglut1, MAP2 and PSD-95 (D–F). Clusters of Vglut1, Kal7 and PSD-95 staining were quantified by observers blinded to genotype. Differences in number of Vglut1-only clusters (excitatory presynaptic terminals; green stars) or number of excitatory synapses (Vglut1-PSD-95 clusters; red circles) between wildtype and Kal7KO mice were only detected at DIV28 (G–H); n=9–12 for Wt, n=10–12 for KO. Scale bar, 5 μm.
Younger cultures were examined to determine when these differences between Kal7KO and wildtype neurons became apparent (Fig. S6). Similar numbers of Vglut1-PSD-95 clusters were found along the dendrites of wildtype and Kal7KO neurons at DIV7; Kal7 was not yet detectable in wildtype neurons (Fig. S6A). For both wildtype and Kal7KO neurons, the number of dendritic Vglut1 clusters increased substantially by DIV14 (Fig. S6B, C). In wildtype neurons, Kal7 staining localized to the postsynaptic side of a few Vglut1 positive synapses. As at DIV7, neither the number of Vglut1 clusters nor the number of Vglut1-PSD-95 clusters was altered in Kal7KO neurons (Fig. S6C). At DIV21, Kal7 was detected in most excitatory synapses in wildtype cultures. However, Kal7KO neurons and wildtype neurons still had the same number of Vglut1 clusters and Vglut1-PSD-95 synapses (Fig. S6C). The linear density of Vglut1 clusters along the dendrites of Kal7KO neurons did not increase after DIV14 (Fig. 9G), while wildtype neurons formed more synapses between DIV14 and DIV28 (p=0.032, Wt vs. KO at DIV28).
Rescue of Kal7KO neurons
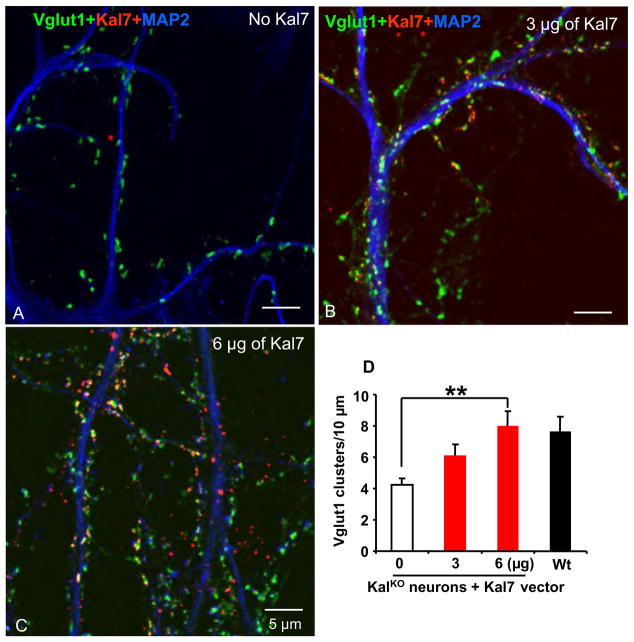
We next asked whether the deficit in dendritic spines observed in Kal7KO neurons could be reversed by introduction of exogenous Kal7. DIV1 cortical neurons from Kal7KO mice were transfected with two different amounts of vector encoding Kal7; control neurons were not transfected (Fig. 10). At DIV28, expression of Kal7 caused a DNA dose-dependent increase in the number of Vglut1 positive clusters along the dendrites of Kal7KO neurons (Fig. 10B–D). The presynaptic Vglut1 terminals were closely aligned with postsynaptic clusters of Kal7 (Fig. 10B, C). The Vglut1 clusters were aligned with PSD-95, NR1 and GluR1 positive clusters (not shown).
Fig. 10. Exogenous Kal7 restores synapse number in Kal7KO neurons.
At the time of plating, cortical neurons from Kal7KO mice were transfected by nucleofection with a vector encoding Kal7; control cells were not transfected. At DIV28, Kal7 (red), Vglut1 (green) and MAP2 (blue) were visualized as in Fig. 9. A. Kal7-staining was absent from control neurons; Vglut1 clusters were apparent along MAP2 positive dendrites. In Kal7KO neurons transfected with 3 (B) or 6 (C) μg of Kal7 vector, Vglut1 clusters were often apposed to clusters of Kal7. The reduced number of excitatory presynaptic Vglut1 terminals in KO mice was rescued with exogenous Kal7 in a DNA concentration-dependent manner (D). N=11 Wt, n=10–12 for Kal7KO.
Discussion
Kal7 plays an essential role in neuronal function in vivo
A series of in vitro studies demonstrated that Kal7 is necessary for the formation and maintenance of dendritic spines (Ma et al., 2003;Ma et al., 2008). We show here that mice lacking Kal7 have deficits severe enough to affect synaptic transmission and specific behaviors. Shank1 and Kal7 are essential for dendritic spine formation in vivo and in culture, but Kal7KO produces a greater drop in hippocampal spine density than Shank1 (15% vs. 6%) (Hung et al., 2008), making Kal7 an important model for human disease. Mental retardation syndromes are often associated with dysregulation of dendritic spines (Benarroch, 2007;Laumonnier et al., 2007). For example, Patau Syndrome (trisomy 13), Down Syndrome (trisomy 21) and Fragile X all have alterations in the number and shape of dendritic spines (Kaufmann and Moser, 2000;Grossman et al., 2006;Govek et al., 2004). Understanding why a subset of behaviors is affected by loss of Kal7 while other learning tasks are not affected should provide new insights into signaling.
Comparing in vitro and in vivo phenotypes
As observed for other PSD protein knockout mice (Varoqueaux et al., 2006;Migaud et al., 1998;Elias et al., 2006), the phenotype of the Kal7KO mouse is less severe than would have been predicted from in vitro studies. Several factors may contribute to this difference. Most importantly, alternative splicing allows the larger isoforms of Kalirin to accumulate in Kal7KO neurons. Lacking the Kal7 exon, the splicing machinery associated with Kalirin transcripts would be expected to splice exon 33 to exon 34, creating the increased levels of Kal8, Kal9 and Kal12 in total homogenates (Fig. 5 E–H). The other targeting strategy considered, insertion of a stop codon within the Kal7 exon preceding the PDZ binding motif, would have generated a truncated product, complicating analysis in a different manner. Since the Sec14p and spectrin-like repeats target Kalirin to membranes, the 9 copies of Kal7 present at the “typical” synapse in wildtype mice may be replaced by 6 copies of these larger Kalirin isoforms, which could help preserve excitatory synapses and alter synaptic function in a region- and context-specific manner.
Recent studies assigning an essential role for Kal7 in activity-dependent activation of the Rac and N-cadherin/afadin pathways utilized an shRNA directed against the spectrin repeat region (nt 1229–1250; spectrins 2–3) (Xie et al., 2008;Xie et al., 2007), reducing expression of all major forms of Kalirin, not just Kal7. Although this approach led to the conclusion that Kal7 regulates the GluR1 content of pyramidal neuron dendritic spines and AMPA receptor mediated synaptic transmission (Xie et al., 2007), the GluR1 content of PSDs purified from Kal7KO neurons is indistinguishable from wildtype. Selective elimination of Kal7 using antisense or shRNA targeted to its unique 3′-untranslated region (Ma et al., 2008) produced a more profound decrease in spine density (~ 2-fold) than observed in cultures prepared from Kal7KO mice. A Kal7-specific shRNA would not be expected to increase levels of the larger kalirin isoforms; in addition, breakdown products generated from Kal7 transcripts targeted by the shRNA could contribute to the effects observed (Kim and Rossi, 2008).
Spine and Plasticity Analysis
Ultrastructural analyses demonstrated a substantial decrease in the number of synapses (PSD with presynaptic terminal) in CA1 hippocampal neurons from Kal7KO mice. Our cell culture data showed a similar decline in synapses assessed as VGlut1/PSD-95 clusters (Fig. 9). Golgi staining revealed a less dramatic decrease in linear spine density. While Vglut1-positive terminals in wildtype neurons usually align with PSD-95 clusters, this strict association is lost in Kal7KO neurons (Fig. 9H). Neither unpaired spine-like structures nor unpaired presynaptic endings would be counted by EM analysis. The PSDs in Kal7KO mice were narrower and thinner than in wildtype mice. These changes in PSD dimensions were consistent and comparable to changes seen in Shank1KO mice (Hung et al., 2008).
Tracking the development of synapses in cortical cultures prepared from Kal7KO mice was revealing. Both in vivo and in culture, synapses begin to form before Kal7 is expressed. Synaptic development proceeded normally for 21 days in the absence of Kal7; VGlut1 positive presynaptic endings contacted dendritic shafts aligned with PSD-95 clusters. While spine density increased between DIV21 and DIV28 in wildtype neurons, no increase was observed in Kal7KO neurons; it is not clear what distinguishes the spines formed between P21 and P28. Kal7 may play an essential role in the maturation and/or maintenance of dendritic spines, as proposed for the neuroligins (Varoqueaux et al., 2006). Consistent with the conclusion that Kal7 has a role late in synaptic development, no differences in LTP were apparent when slices from P21 and younger mice were examined. When synaptic plasticity was tested in Kal7KO mice older than P28, deficits were apparent (Fig. 4).
Although PSD length and width were reduced in Kal7KO mice, the effect was small, consistent with our inability to see dramatic changes in PSD content of a number of proteins. Indeed, similar results have been seen with other PSD-protein knockouts. Ablation of PSD-95 caused no change in spine volume, but made spines longer and thinner and decreased the number of AMPA receptors (Elias et al., 2006;Beique et al., 2006). Knockout of Shank1, which caused a decrease in spine number and PSD size, decreased levels of GKAP and Homer and weakened basal synaptic transmission, leaving synaptic plasticity unaltered (Hung et al., 2008).
Potential Mechanisms
Determining how the absence of Kal7 causes these changes in spine number and synaptic function will require detailed analysis of the deficits. Kal7KO PSDs contained less Cdk5 and less NR2B than wildtype PSDs. Cdk5 plays a complex and important role in postsynaptic signaling and architecture (Cheung and Ip, 2007;Cheung et al., 2006;Benavides and Bibb, 2004) and the decrease in Cdk5 may contribute to many of the changes observed. For example, the GEF activity of Kal7 is increased following Cdk5-catalyzed phosphorylation of Thr1590 (Xin et al., 2008). Although Kal7 with either a T1590A or T1590D mutation caused spine formation when expressed in rat cortical neurons, spine morphologies differed (Xin et al., 2008). Based on the fact that dominant negative Cdk5 blocked the ability of Kal7 to affect PC12 cell morphology, this interaction appears to play a critical role in Kalirin function.
Like Kal7, the Cdk5/p35 complex affects Rac/Pak signaling (Nikolic et al., 1998), which alters cytoskeletal dynamics in dendritic spines (Tashiro et al., 2000). Through its effects on ephrins, the decreased Cdk5 in Kal7KO PSDs may contribute to the dearth of dendritic spines. Cdk5 plays a role in EphA-dependent spine retraction (Fu et al., 2007) while Kal7 plays a role in EphB-dependent spine maturation (Penzes et al., 2003). Cdk5 phosphorylates PSD-95, regulating its clustering (Morabito et al., 2004), which might contribute to the changes in PSD size and shape seen in Kal7KO mice. Importantly, Cdk5 plays a role in the phosphorylation of NR2B, resulting in its stabilization in the membrane (Zhang et al., 2008). The decreased levels of Cdk5 in Kal7KO PSDs may cause the decreased levels of NR2B. Further studies are underway to determine how Kal7 and Cdk5 interact to contribute to the changes observed in Kal7KO mice.
Kal7 is essential for specific memory processes
Given the spine changes seen in mental retardation and the role of dendritic spines in memory formation (Benarroch, 2007), it is not surprising that Kal7KO animals exhibit learning deficits. While numerous knockout mice have demonstrated deficits in hippocampal learning processes, few have revealed disparities in appetitive spatial and aversive contextual hippocampal-dependent learning paradigms (Hung et al., 2008;Bach et al., 1995). It has been posited that different mechanisms underlie single-trial versus more gradual repetitively learned tasks (Hung et al., 2008;Bach et al., 1995). The Kal7KO mice were normal in non-aversive tests of learning and memory (object recognition, radial arm maze), but abnormal in tests of anxiety and fear learning (elevated zero maze, passive avoidance). Perhaps specific proteins or signaling pathways are essential for the formation/function of synapses necessary for particular types of learning (e.g. fear learning) but not others (e.g. spatial learning). The hypothesis that specific synapses or molecular pathways underlie different forms of hippocampal memory was put forth previously (Bach et al., 1995), but has received little further investigation. Future behavioral and neurochemical mapping studies of Kal7KO and Kal7CKO mice will help clarify the role of different spine proteins in different types of learning.
Kal7 plays a key role in synaptic plasticity and in human psychiatric conditions
Kal7 is the only RhoGEF specifically trafficked to the PSD (Sheng and Hoogenraad, 2007) and the only RhoGEF identified in the complex of NR2B associated proteins (Collins et al., 2006). Decreased levels of Kal7 were observed in postmortem cortices from schizophrenics and Alzheimer Disease patients (Youn et al., 2007;Hill et al., 2006;Tataki et al., 2005). In addition to the present findings, preliminary studies with Kal7KO mice revealed aberrant responses to drugs of abuse (Kiraly et al., unpublished) and additional deficits in synaptic plasticity and cognitive function (Kiraly and Gaier, unpublished). Kal7KO mice have now emerged as a valuable model for understanding synaptic malfunctions and human psychiatric disorders.
Acknowledgments
We thank Darlene D’Amato for making the Neuropeptide Lab run. We especially thank Dr. Srdjan Antic, who gave generously of his time and talents in getting the electrophysiological studies going, and Dr. Fouad Lemtiri-Chlieh, who participated in exploratory electrophysiological studies. We especially thank Dr. Caiying Guo of the University of Connecticut Gene Targeting and Transfer Facility for enormous help in constructing these mouse lines, and Maya Yankova of the University of Connecticut EM Facility for beautiful electron micrographs. Supported by grants from the National Institutes of Health: R01-DA15464 (BAE), R21-18274 (REM), R01-DA16791 (ESL) and T32-NS41224 (DDK and EDG).
Reference List
- Araya R, Nikolenko V, Eisenthal KB, Yuste R. Sodium channels amplify spine potentials. Proc Natl Acad Sci U S A. 2007;104:12347–12352. doi: 10.1073/pnas.0705282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach ME, Hawkins RD, Osman M, Kandel ER, Mayford M. Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the theta frequency. Cell. 1995;81:905–915. doi: 10.1016/0092-8674(95)90010-1. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Niewoehner B, Lyon L, Romberg C, Kohr G, Rawlins JNP. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. J Neurosci. 2008;28:3623–2630. doi: 10.1523/JNEUROSCI.3639-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci U S A. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Rho GTPases: role in dendrite and axonal growth, mental retardation, and axonal regeneration. Neurology. 2007;68:1315–1318. doi: 10.1212/01.wnl.0000259588.97409.8f. [DOI] [PubMed] [Google Scholar]
- Benavides DR, Bibb JA. Role of cdk5 in drug abuse and plasticity. Ann N Y Acad Sci. 2004;1025:335–344. doi: 10.1196/annals.1316.041. [DOI] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86:831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Hoogenraad CC, Rush J, Ramm E, Sheng M, Peng J. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006;5:1158–1170. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Fu AKY, Ip NY. Synaptic roles of cdk5: implications in higher cognitive functions and neurodegenerative diseases. Neuron. 2006;50:13–18. doi: 10.1016/j.neuron.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Ip NY. The roles of cyclin-dependent kinase 5 in dendrite and synapse development. Biotech J. 2007;2:949–957. doi: 10.1002/biot.200700056. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MO, Husi H, Yu L, Brandon JM, Choudhary JS, Grant SGN. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J Neurochem. 2006;97(Suppl 1):16–23. doi: 10.1111/j.1471-4159.2005.03507.x. [DOI] [PubMed] [Google Scholar]
- Craig AM, Graf ER, Linhoff MW. How to build a central synapse: clues from cell culture. Trends Neurosci. 2006;29:8–20. doi: 10.1016/j.tins.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Kang YH. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaevsky A, Mason CA. Spine motility: a means toward an end? Trends Neurosci. 2003;26:155–160. doi: 10.1016/S0166-2236(03)00028-6. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K, Ledoux JE. Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Molecular morphogens for dendritic spines. Trends Neurosci. 2002;25:64–67. doi: 10.1016/s0166-2236(02)02061-1. [DOI] [PubMed] [Google Scholar]
- Elias GM, Funke L, Stein V, Grant SGN, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- Fu WY, Chen Y, Sahin M, Zhao XS, Shi S, Greenberg ME, Ip NY. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nature Neurosci. 2007;10:67–75. doi: 10.1038/nn1811. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Disterhoft JF, Gundersen HJG, Mcechron MD, Persina IS, West MJ. Remodeling of hippocampal synapses after hippocampus-dependent associative learning. J Comp Neurol. 2000;417:49–59. [PubMed] [Google Scholar]
- Govek EE, Newey SE, Akerman CJ, Cross JR, Van der Veken L, Van Aelst L. The X-linked mental retardation protein oligophrenin-1 is required for dendritic spine morphogenesis. Nature Neurosci. 2004;7:364–372. doi: 10.1038/nn1210. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Aldridge GM, Weiler IJ, Greenough WT. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci. 2006;26:7151–7155. doi: 10.1523/JNEUROSCI.1790-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Cooper DC, Bibb JA. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- Hung AY, Futai K, Sala C, Valtschanoff JG, Weinberg RJ, Sheng M. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Hashimoto Y, Kudo Y, Miyakawa H. Dendritic attenuation of synaptic potentials in the CA1 region of rat hippocampal slices detected with an optical method. Eur J Neurosci. 2001;13:1711–1721. doi: 10.1046/j.0953-816x.2001.01550.x. [DOI] [PubMed] [Google Scholar]
- Jelks KB, Wylie R, Floyd CL, McAllister AK, Wise P. Estradiol targets synaptic proteins to induce glutamatergic synapse formation in cultured hippocampal neurons: critical role of estrogen receptor-alpha. J Neurosci. 2007;27:6903–6913. doi: 10.1523/JNEUROSCI.0909-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RC, Penzes P, Eipper BA, Mains RE. Isoforms of kalirin, a neuronal Dbl family member, generated through use of different 5′- and 3′-ends along with an internal translational initiation site. J Biol Chem. 2000;275:19324–19333. doi: 10.1074/jbc.M000676200. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nature Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kim DH, Rossi JJ. RNAi mechanisms and applications. Biotechniques. 2008;44:613–616. doi: 10.2144/000112792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nature Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Donahue CP, Israely I, Liu X, Ochiishi T. delta-catenin at the synaptic-adherens junction. Trends Cell Biol. 2005;15:172–178. doi: 10.1016/j.tcb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Cuthbert PC, Grant SGN. The role of neuronal complexes in human X-linked brain diseases. Am J Hum Genet. 2007;80:205–220. doi: 10.1086/511441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Greengard P, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Raghavachari S. A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Science STKE 2006. 2006:re11. doi: 10.1126/stke.3562006re11. [DOI] [PubMed] [Google Scholar]
- Ma XM, Huang JP, Wang Y, Eipper BA, Mains RE. Kalirin, a multifunctional Rho GEF, is necessary for maintenance of hippocampal pyramidal neuron dendrites and dendritic spines. J Neurosci. 2003;23:10593–10603. doi: 10.1523/JNEUROSCI.23-33-10593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Johnson RC, Mains RE, Eipper BA. Expression of Kalirin, a Neuronal GDP/GTP Exchange Factor of the Trio Family, in the Central Nervous System of the Adult Rat. J Comp Neurol. 2001;429:388–402. doi: 10.1002/1096-9861(20010115)429:3<388::aid-cne3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Ma XM, Mains RE, Eipper BA. Plasticity in hippocampal peptidergic systems induced by repeated electroconvulsive shock. Neuropsychopharmacology. 2002;27:55–71. doi: 10.1016/S0893-133X(02)00284-1. [DOI] [PubMed] [Google Scholar]
- Ma XM, Wang Y, Ferraro F, Mains RE, Eipper BA. Kalirin-7 Is an Essential Component of both Shaft and Spine Excitatory Synapses in Hippocampal Interneurons. J Neurosci. 2008;28:711–724. doi: 10.1523/JNEUROSCI.5283-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Carnevale NT, Zador AM, Claiborne BJ, Brown TH. Electrotonic architecture of hippocampal CA1 pyramidal neurons based on three-dimensional reconstructions. J Neurophysiol. 1996;76:1904–1923. doi: 10.1152/jn.1996.76.3.1904. [DOI] [PubMed] [Google Scholar]
- McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson CE, Eipper BA, Mains RE. Genomic organization and differential expression of Kalirin isoforms. Gene. 2002;284:41–51. doi: 10.1016/s0378-1119(02)00386-4. [DOI] [PubMed] [Google Scholar]
- McPherson CE, Eipper BA, Mains RE. Multiple novel isoforms of Trio are expressed in the developing rat brain. Gene. 2005;347:125–135. doi: 10.1016/j.gene.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Meredith GE, DeSouza IE, Hyde TM, Tipper G, Wong ML, Egan MF. Persistent alterations in dendrites, spines, and dynorphinergic synapses in the nucleus accumbens shell of rats with neuroleptic-induced dyskinesias. J Neurosci. 2000;20:7798–7806. doi: 10.1523/JNEUROSCI.20-20-07798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey S, Doyere V, De Souza I, Harrison E, Laroche S, Stewart MG. Long-term synaptic morphometry changes after induction of long-term potentiation and long-term depression in the dentate gyrus of awake rats are not simply mirror phenomena. Eur J Neurosci. 2004;19:2310–2318. doi: 10.1111/j.0953-816X.2004.03334.x. [DOI] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Morrison JH, O’Dell TJ, Grant SGN. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Morabito MA, Sheng M, Tsai L-H. Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J Neurosci. 2004;24:865–876. doi: 10.1523/JNEUROSCI.4582-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–198. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Ouimet CC. Chronic fluoxetine administration to juvenile rats prevents age-associated dendritic spine proliferation in hippocampus. Brain Res. 2000;883:205–215. doi: 10.1016/s0006-8993(00)02909-7. [DOI] [PubMed] [Google Scholar]
- Ochiishi T, Futai K, Okamoto K, Kameyama K, Kosik KS. Regulation of AMPA receptor trafficking by delta-catenin. Mol Cell Neurosci. 2008 doi: 10.1016/j.mcn.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, Mains RE, Eipper BA. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–242. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Pogorelov VM, Insco ML, Caron MG, Wetsel WC. Novelty seeking and stereotypic activation of behavior in mice with disruption of the Dat1 gene. Neuropsychopharmacology. 2005;30:1818–1831. doi: 10.1038/sj.npp.1300724. [DOI] [PubMed] [Google Scholar]
- Ramakers GJA. Rho proteins and the cellular mechanisms of mental retardation. Am J Med Genet. 2000;94:367–371. doi: 10.1002/1096-8628(20001023)94:5<367::aid-ajmg4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine Self-Administration Alters the Morphology of Dendrites and Dendritic Spines in the Nucleus Accumbens and Neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on Rho GTPases with guanine nucleotide-exchange factors. Nature Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Schiller MR, Blangy A, Huang JP, Mains RE, Eipper BA. Induction of lamellipodia by Kalirin does not require its guanine nucleotide exchange factor activity. Exp Cell Res. 2005;307:402–417. doi: 10.1016/j.yexcr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Schiller MR, Ferraro F, Wang Y, Ma XM, McPherson CE, Sobota JA, Schiller NI, Mains RE, Eipper BA. Autonomous functions for the Sec14p/spectrin-repeat region of Kalirin. Exp Cell Res. 2008 doi: 10.1016/j.yexcr.2008.05.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Kawabata I, Sobue K, Okabe S. Determination of absolute protein numbers in single synapses by a GFP-based calibration technique. Nat Methods. 2005;2:677–684. doi: 10.1038/nmeth783. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cerebral Cortex. 2000;10:927–938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- Tataki M, Paek M, Kamiya A, Balkissoon R, Tomoda T, Penzes P, Sawa A. Disrupted-in-schizophrenia-1 (DISC1) binds to Kalirin-7 and modulates its function as a GDP/GEP exchange factor. Soc Neurosci Abstracts. 2005:674.9. [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Xie Z, Photowala H, Cahill ME, Srivastava DP, Woolfrey KM, Shum CY, Huganir RL, Penzes P. Coordination of synaptic adhesion with dendritic spine remodeling by AF-6 and Kalirin-7. J Neurosci. 2008;28:6079–6091. doi: 10.1523/JNEUROSCI.1170-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Surmeier J, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Wang Y, Ma XM, Rompolas P, Keutmann HT, Mains RE, Eipper BA. Regulation of kalirin by cdk5. J Cell Sci. 2008;121:2601–2611. doi: 10.1242/jcs.016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn HS, Jeoung MK, Koo YB, Ji H, Markesbery WR, Ji I, Ji TH. Kalirin is under-expressed in Alzheimer’s Disease hippocampus. J Alzheimers Disease. 2007;11:385–397. doi: 10.3233/jad-2007-11314. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Zhang S, Edelmann L, Liu J, Crandall JE, Morabito MA. Cdk5 regulates the phosphorylation of 1472 NR2B and the surface expression of NMDA receptors. J Neurosci. 2008;28:415–424. doi: 10.1523/JNEUROSCI.1900-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou WL, Yan P, Wuskell JP, Loew LM, Antic SD. Dynamics of action potential backpropagation in basal dendrites of prefrontal cortical pyramidal neurons. Eur J Neurosci. 2008;27:923–936. doi: 10.1111/j.1460-9568.2008.06075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]