Abstract
Oncolytic paramyxoviruses include some strains of Measles, Mumps, Newcastle disease, and Sendai viruses. All these viruses are well equipped for promoting highly specific and efficient malignant cell death, which can be direct and/or immuno-mediated. A number of proteins that serve as natural receptors for oncolytic paramyxoviruses are frequently overexpressed in malignant cells. Therefore, the preferential interaction of paramyxoviruses with malignant cells rather than with normal cells is promoted. Due to specific genetic defects of cancer cells in the interferon (IFN) and apoptotic pathways, viral replication has the potential to be promoted specifically in tumors. Viral mediation of syncytium formation (a polykaryonic structure) promotes intratumoral paramyxo-virus replication and spreading, without exposure to host neutralizing antibodies. So, two related processes: efficient intratumoral infection spread as well as the consequent mass malignant cell death, both are enhanced. In general, the paramyxoviruses elicit strong anticancer innate and adaptive immune responses by triggering multiple danger signals. The paramyxoviruses are powerful inducers of IFN and other immuno-stimulating cytokines. These viruses efficiently promote anticancer activity of natural killer cells, dendritic cells, and cytotoxic T lymphocytes. Moreover, a neuraminidase (sialidase), a component of the viral envelope of Newcastle Disease, Mumps, and Sendai viruses, can cleave sialic acids on the surface of malignant cells thereby unmasking cancer antigens and exposing them to the immune system. These multiple mechanisms contribute to therapeutic efficacy of oncolytic paramyxovi-ruses and are responsible for encouraging results in preclinical and clinical studies.
INTRODUCTION
Metastatic cancer remains largely an incurable disease which requires development of novel therapeutic strategies. While the accumulating knowledge on the molecular basis of cancer offers new potential targets for anticancer drugs, there is an alternative approach that relies on mechanisms developed through the millions of years of human coexistence with viruses. The viruses emerge as promising instruments against cancer. Substantial selectivity of infection and replication in cancer cells is characteristic of many viruses, and their therapeutic efficiency and safety can be dramatically improved by genetic manipulations.
Paramyxoviruses represent a well-studied model of oncolytic viruses with promising therapeutic potentials. The antitumor efficacy by oncolytic paramyxoviruses appears to be associated with three properties of the viruses: (i) selective replication in tumor cells, (ii) oncolytic properties by the viruses per se; and (iii) immunostimulatory capacity of the viruses. Studies on viral oncolysis mediated by paramyxoviruses have revealed some properties that collectively contribute to strong anticancer effects. Some of these properties are common to many oncolytic viruses and some are specific to oncolytic paramyxoviruses only. All these properties are described below.
BIOLOGY OF PARAMYXOVIRUSES
Paramyxoviruses (members of the Paramyxoviridae family) are associated with a number of diseases in animals and humans, such as measles virus (MV), mumps, and several respiratory infections. Sendai virus (SeV) affects mice and some other rodents. Newcastle disease virus (NDV) is associated with a contagious disease affecting many domestic and wild species of birds. SeV and NDV were not found to be associated with any serious human diseases, while MV and mumps are well-studied human pathogens. Few representatives of paramyxoviruses NDV, SeV as well as attenuated vaccine strains of MV and mumps viruses were tested as oncolytic agents in multiple model experiments and in a few clinical trials.
Paramyxoviruses enter the life cycle with binding of their attachment protein to an appropriate cell-surface receptor. There could be either direct fusion of the envelope with plasma membrane1assisted by the F protein, which is activated by the interaction with sialic acid–containing surface glycoproteins,2 or the virus can enter the cell through the endocytic route with fusion occurring in acidic conditions inside endosomes.3 As a result, the nucleocapsid containing viral genome is released into the cytosol where viral replication takes place.
Viral RNA-dependent RNA polymerase transcribes the genes into mRNAs, which are then translated into structural and nonstructural proteins. The transcription starts from a single promoter located at the 3′ end of the genome and then may either terminate within specified regions between each viral gene or proceed further downstream. This mode of transcription is responsible for the observed product polarity in which the genes closest to the 3′ end of the genome are expressed more abundantly than their downstream counterparts.1
This mechanism is a simple and effective way for transcription level regulation to generate the necessary balance of viral products. A concentration of the most abundantly synthesized nucleoprotein determines the time at which RNA-dependent RNA polymerase switches from gene transcription to genome replication. Replication involves synthesis of full-length positive-strand RNAs, which are then transcribed into the progeny genomic minus-strand RNAs. The maturing virions finally gain their envelopes with the membrane-trapped viral glycoproteins by budding through the outer membrane. New virions can then infect other cells and enter new life cycles. An alternative pathway for spreading viral infection involves fusion of infected cells with their neighbors and formation of syncytia.4 Viral fusion proteins used by the virus to enter the cell are exposed to the cell surface of infected cells inducing fusion with plasma membranes of neighboring cells. Therefore, a single virion can potentially infect and kill dozens of cells.
THREE LEVELS OF CANCER-SPECIFIC INFECTION AND SPREADING FOR PARAMYXOVIRUSES
Receptors for paramyxoviruses are frequently overexpressed in malignant cells
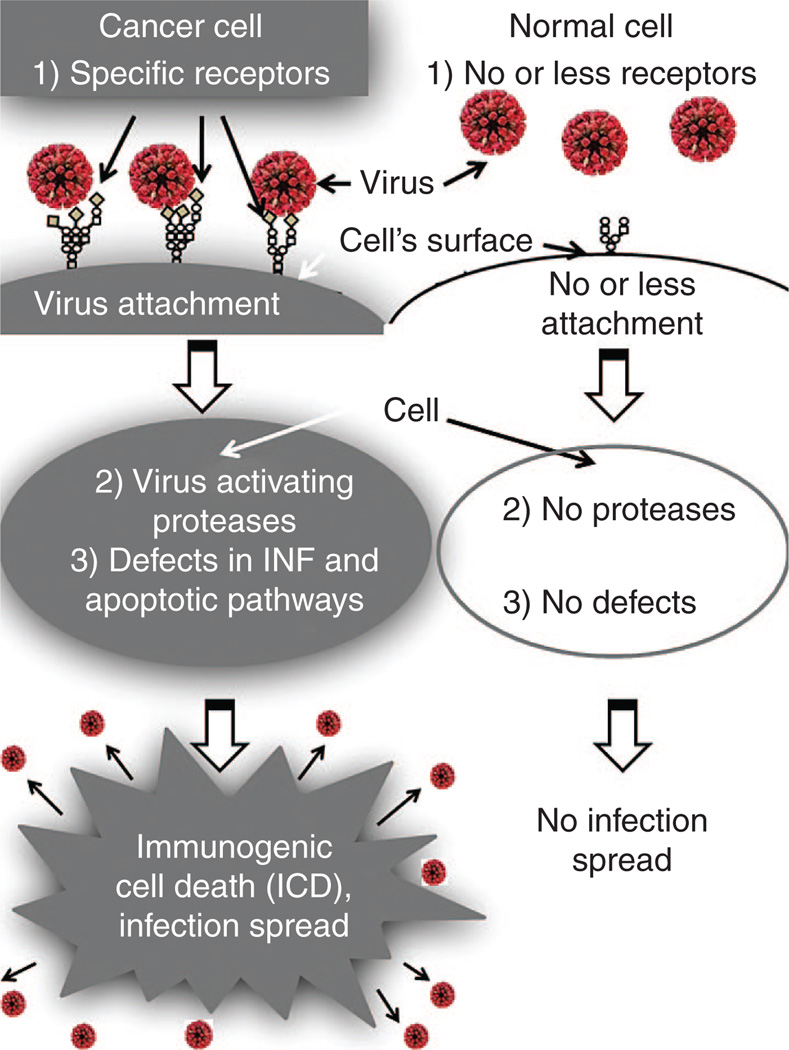
A first level of specificity for cancer cells is related to overexpression of specific receptors for oncolytic viruses (Figure 1). NDV, mumps, and SeV use sialic acid–containing sialoglycoproteins as the cell surface receptors.5,6 The abundant presence of sialoglycoproteins on the surface of cancer cells7,8 most likely promotes preferential association of the virus with malignant rather than with normal cells and contributes to their selective cytolytic effect in primary tumors and in metastases. Perhaps high sensitivity to SeV mediated cell death of sialic acid–rich prostate carcinoma cells, in comparison with normal prostate epithelium, is explained by SeV’s preferential association with malignant cells.9
Figure 1.
Three levels of cancer-specific infection spread for paramyxoviruses. (1) First level of virus specificity for cancer cells is related to overexpression of specific receptors for paramyxoviruses. Sialic acids residues in a form of sialoglycoproteins serve as receptors for NDV, mumps, and SeV. These sialoglycoproteins are frequently overexpressed in malignant cells. CD46 and nectin 4 serve as natural receptors for attenuated strain of MV, and they are also frequently overexpressed in malignant cells. (2) Second level of virus specificity is related to cancer cells’ specific proteases. The replication cycles of paramyxoviruses require protease cleavage of viral glycoproteins for productive cell enter. Desirable proteases for oncolytic viruses that can activate them are expressed preferentially by cancer cells. For example, matrix metalloproteinases are overexpressed in almost all human cancer cells. (3) Third level of virus specificity is related to cancer cells’ specific genetic defects that allow viral replication. During the process of malignization, a cancer cell accumulates many genetic changes. Along with mutations that promote accelerated proliferation and invasion, many cancerous cells are losing abilities to produce interferon (IFN) and to respond to IFN by apoptotic pathway induction. Such abnormalities make these cells highly susceptible to viral infection. So, oncolytic paramyxoviruses due to abundance of cancer-specific receptors and due to cancer-specific genetic defects are more likely spreading among malignant cells rather than among normal cells.
The attenuated measles virus (Edmonston strain) (MVES) uses the CD46 receptor, which is a regulator of complement activation that is universally expressed in all nucleated human cells but is often overexpressed in tumor cells.10 MVES can kill cells that overexpress this receptor without significant cytopathic effect against nontransformed cells expressing low receptor levels.11 Nectin 4 has recently been identified as the additional receptor for MV.12 It is a member of adhesion receptors of the immunoglobulin superfamily localized to the adherents’ junctions of epithelial cells. Nectin 4 can be considered as a tumor cell marker for breast, lung, and ovarian cancers,13–15suggesting that it can be partially responsible for the selectivity of measles virus toward cancer cells. Table 1 summarizes information related to viral receptors and their overexpression in malignant cells.
Table 1.
Oncolytic paramyxoviruses and their receptors overexpression in cancer cells
| Virus | Receptor | Receptor overexpression |
|---|---|---|
| Newcastle disease virus (NDV) | Syalic acid containing molecules2 | Invasive and metastatic cancers8 |
| Sendai virus (SeV) | ||
| Mumps | ||
| Measles virus Edmonston strain (MVES) | CD46 (ref. 11) | Breast and ovarian cancers and other malignancies157,158 |
| Nectin 4 (refs. 12,159) | Breast, lung, and ovarian cancers13,14,160 |
Virus activation through cancer-specific proteases
A second level of cancer cells’ specificity is related to cancer cells’ specific proteases (Figure 1). The replication cycles of enveloped viruses, such as paramyxoviruses, require protease cleavage of viral glycoproteins for productive cell entry.16 Desirable proteases in cancer cells for oncolytic viruses should be expressed mainly by cancer cells. Such proteases exist; they are represented by matrix metalloproteinases.17 These molecules are endopeptidases that are overexpressed in almost all human cancer cells.18–20 It is still unknown whether these proteases can activate wild-type viruses. However, a recombinant Sendai virus,21 as well as MV constructs22,23 that are selectively activated in human tumor cells expressing matrix metalloproteinases, has been created.
Genetic defects of cancer cells
A third level of cancer cells’ specificity is related to their specific genetic defects which allow viral replication (Figure 1). During the process of malignization, a cancer cell accumulates many genetic changes. Along with mutations that promote accelerated proliferation and invasion, many cancerous cells lose their ability to produce and respond to interferon (IFN) by apoptotic pathway induction.24Such abnormalities make these cells highly susceptible to viral infection.25 So, due to the abundance of cancer-specific receptors, cancer-specific genetic defects, and proteases, oncolytic paramyxoviruses are more likely going to spread among malignant than among normal cells.
ONCOLYSIS MEDIATED BY DIRECT CANCER CELL KILLING THROUGH FORMATION OF SYNCYTIUM
Paramyxoviruses have a mechanism of virus infection spread without the release of mature virus particles from the cells. The mechanism includes fusion of infected cells with their noninfected neighbors. The fusion results in the formation of a syncytium, a large polykaryonic conglomerate originating from many cells. A single infected cell could trigger up to 50–100 neighboring cells to fuse together and to form a syncytium.26 The process of cell fusion allows the virus to minimize exposure to host neutralizing antibodies. Usually, the syncytia survive no longer than 4–5 days.
Syncytium death is associated with nuclear fusion, premature chromosome condensation, and autophagic degeneration, accompanied by release of vesicles reminiscent of exosomes (syncytiosomes). “Dying syncytia produce significantly more syncytiosomes than normal cells or cells killed by irradiation, freeze thaw, or osmotic shock. These syncytiosomes also load dendritic cells (DCs) for antigen presentation more effectively than exosomes from cells dying by other mechanisms.”27
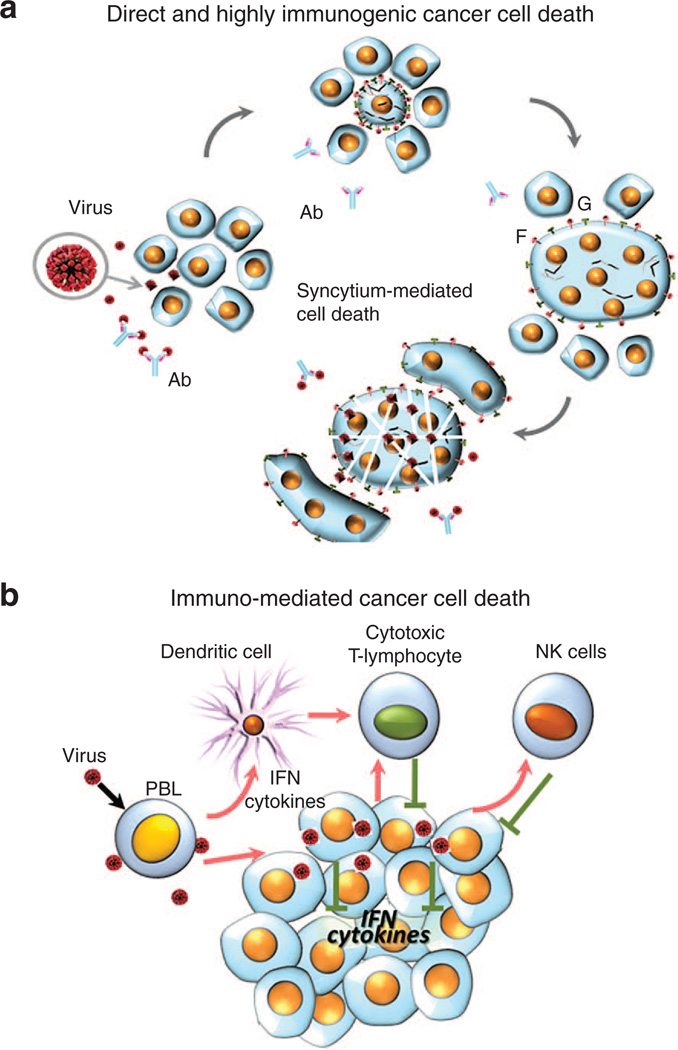
Viral fusogenic membrane glycoprotein expression causes syncytia development and consequent cellular immunogenic death,4,28 which can contribute to the efficiency of viral oncolysis. Consequently, the ability of viruses to induce the formation of syncytia might correlate with their oncolytic potential. This hypothesis is supported by some experimental evidence. The fusion protein of NDV was introduced into an oncolytic strain of vesicular stomatitis virus (VSV), with significant enhancement of oncolytic activity of the virus against multifocal hepatocellular carcinoma in the livers of immunocompetent rats.29 Similarly, the oncolytic activity of a strain of replication-competent herpes simplex virus (HSV) was significantly increased after the introduction of a hyperfusogenic glycoprotein from the gibbon ape leukemia virus.30 The fusogenic potential could be further increased by the introduction of amino acid substitutions. A novel NDV variant harboring an L289A substitution within the F gene possesses enhanced fusion and oncolytic activities against rat hepatocellular carcinoma cells, both in vitro and in immunocompetent rats.31 A mutant of SV5 paramyxovirus that harbors a glycine-to-alanine substitution in the fusion protein was hyperfusogenic and displayed an enhanced oncolytic activity.32 Remarkably, even plasmid vectors25,33,34 or replication-deficient viruses35–37 expressing the fusogenic membrane glycoproteins are capable of promoting significant tumor regression, suggesting that cell fusion and syncytia formation substantially contribute to the oncolytic activity of paramyxoviruses. So, ability to kill cancer cells directly through formation of syncytium is an important property of oncolytic paramyxoviruses (Figure 2a).
Figure 2.
Direct or immuno-mediated cancer cells death. (a) Direct and highly immunogenic cancer cell death through formation of syncytia. Virus-infected cells start expressing viral fusion protein (F) at their cell surface that forces fusion of infected and surrounding noninfected cells and formation of large polykaryonic structures known as syncytia. The syncytia support continuous viral replication and further fusion with new uninfected cells. Eventually, the syncytia die. The syncytia formation allows viral infection to spread even in the presence of high titers of neutralizing antiviral antibodies. This type of cell death assists in viral oncolysis. Proteolytic cleavage of viral protein F0 is needed to activate it and transform it into F1. Only F1 can promote cell fusion into syncytia. For some representatives of paramyxoviruses, F0 that is expressed in infected human cells is fusion incompetent because of the absence of proteolytic cleavage to activate F-protein (F0) and transform it to F1. Therefore, syncytia formation could not expand in tumor tissues, unless they express a protease that could perform appropriate proteolytic cleavage. (b) Immuno-mediated cancer cell death. Paramyxoviruses stimulate innate and adaptive immunity that targets cancer cells. Malignant cells are frequently unable to react to virus infection by producing INF. They are also frequently unable to respond to INF by apoptotic pathway induction. In response to virus infection, normal cells are able to produce INF. Notably, virus induced INF is synthesized by macrophages, dendritic, endothelial and tumor stromal cells. Moreover, in response to virus infection, tumor stromal cells also secrete numerous cytokines that elicit a strong antitumor reaction by attracting macrophages, monocytes, natural killer (NK) cells and other components of the innate immune system. In addition, IFNs and cytokines stimulate dendritic cells (DCs) to educate cytotoxic T-lymphocytes to target tumor cells.
SUMMARY OF FACTORS THAT AFFECT VIRUS PRODUCTION LEVEL IN MALIGNANT CELL
It is noticeable from the sections presented above that the factors potentially influencing paramyxovirus production levels in cancer cells are virus and cancer cell specific. So, a paramyxovirus representative and a cancer cell have to complement each other to achieve effective virus-mediated tumor destruction. A particular virus receptor should be overexpressed in a particular malignant cell for efficient virus attachment. A malignant cell should have genetic defects in IFN and apoptotic pathways that allow viral replication in this cell. A malignant cell should overexpress a specific protease for efficient viral particle maturation. The F protein of the paramyxovirus utilized should have a suitable proteolytic cleavage site for the malignant cell-specific protease to enable virus processing and maturation. The viral F protein should be able to induce malignant cell fusion (syncytium formation) that allows paramyxoviruses to minimize exposure to host neutralizing antibodies and maximize yield of infectious particles. Through genetic engineering, it is possible to create viral constructs with higher affinity to malignant cells receptor (described below in the section Improving tumor-specific targeting), constructs with better proteolytic processing abilities by cancer cells specific matrix metalloproteinases and constructs with better cancer cell fusing activities (described in the section Optimization of syncytia formation and F protein cleavage site). However, it is very difficult to optimize all the factors in one genetic construct for most efficient malignant cells attachment, replication, and particles maturation but perhaps it will be accomplished in future.
ONCOLYSIS MEDIATED BY PARAMYXOVIRUS-INDUCED STIMULATION OF THE ANTITUMOR IMMUNE RESPONSE
In addition to the direct killing of infected cancer cells, viral infection elicits systemic responses that strongly contribute to viral oncolysis. These responses include stimulation of antiviral innate immunity, such as the production of IFNs, other cytokines, and the activation of natural killer (NK) cells. Also, viral infection elicits adaptive immune responses that not only act to contain the infection by targeting the released viral particles and the infected cells, but also assist in the exposure of cancer cells to the antigen-presenting cells leading to activation of the anticancer immune response (Figure 2b).
Enhancement of cancer cell immunogenicity
Paramyxoviruses, including oncolytic paramyxoviruses, often modulate genes related to antigen presentation and enhance the immunogenicity of cancer cells. For example, it has been known that NDV upregulates human lymphocyte antigens (HLA) class I and II as well as costimulatory molecule intercellular adhesion molecule 1 in human cancer cells.38
Contribution of danger signals
Oncolytic viruses could play a crucial role in tumor rejection by helping to overcome immune suppression mediated by the tumor microenvironment. Perhaps, to some degree, anticancer immune activation can occur even without replication of a virus in a tumor mass. It is likely the antitumor immune response can be triggered even if the virus is injected into skin or intratumoral.39 By its nature, the virus acts as a danger signal, which is able to recruit and activate professional antigen-presenting cells such as DCs. DCs act as part of both innate and adaptive immunity and can prime adaptive T cell immune responses against specific, tumor-associated antigens (TAAs).40 Paramyxoviral fusogenic membrane glycoproteins promote syncytia formation that triggers particular efficient TAA presentation by DC.27
In general, DC activation can be induced by endogenous and exogenous danger signals.41 In current terms, the endogenous signals are damage-associated molecular patterns (DAMPs) and exogenous danger signals are pathogen-associated molecular patterns (PAMPs) molecules. DAMPs and PAMPs act to initiate immune responses to infection, pathological processes, and therapeutic processes such as oncolytic virotherapy.42–44Cancer cells can evade the immune system by conditioning their microenvironment until viruses overcome the immunosuppressive environment. They produce immunosuppressive cytokines and recruit immunosuppressive cells, such as T regulatory cells (T-regs), myeloid derived suppressor cells, and M2 macrophages to maintain immune tolerance within the tumor.45Acting on both cancer and immune cells, oncolytic paramyxoviruses elicit potent adaptive immunity against specific tumor antigens and thus against cancer cells. These viruses operate as immuno-adjuvants to overcome the immunosuppressive tumor microenvironment, as cell-surface exposed viral antigens and products of dying cells provide potent danger signals. As a result, immunogenic cell death (ICD) of cancer cells gets promoted. It may resemble apoptosis, necrosis, pyroptosis, or autophagic cell death.46,47 DAMPs include the calreticulin (ecto-CRT) and heat-shock proteins (HSP70, HSP90) exposed on the cell surface, high-mobility group box 1 (HMGB1) protein, ATP, and uric acid released from dying cells. This release happens during mid to late phases of apoptosis, necrosis, or autophagy.
Introduction of PAMPs to cancer cells through virus infection is related to the previously described processes known as artificial heterogenization48 or xenogenization49 meaning that pathogens increase cancer cells visibility for immune system. The danger signals activate DCs, which cross-present TAAs to cluster of differentiation (CD) 8+ T cells for potent antitumor immunity. By this mechanism, MV induces ICD in human melanoma.50 Following ICD of cancer cells infected with MV vaccine, the apoptotic bodies are ingested by DCs, which mature and then produce high levels of IFN-α, and cross-present TAAs, leading to a generation of tumor-specific CD8+ cytotoxic T cells.51,52
Role of autophagy
Autophagy is a well-regulated process that mediates degradation and recycling of cellular proteins, organelles, as well as pathogens.53Autophagy plays important roles in both innate and adaptive immunity.54,55 A number of paramyxoviruses, including MV,56 NDV,57and SeV,53 have been shown to induce autophagy in infected cancer or normal cells. As mentioned earlier, death of syncytia is associated with autophagy.58 Interestingly, the engagement of CD46, a ubiquitous human surface receptor, which is able to bind several different pathogens including MV, is sufficient to induce autophagy.59Autophagy enhances tumor immunogenicity not only by releasing DAMPs but also by promoting an antigen cross-presentation from cancer cells to DCs and then to T cells. Cross-presentation of TAAs by DCs is promoted by the nonapoptotic (most likely autophagic) cell death induced by paramyxoviral fusogenic membrane glycoproteins.27
Inhibition of the IFN response in target cells
One of the most important defense mechanism a cell has against viral infection is induction of IFNs and IFN-inducible proteins, resulting in suppression of protein synthesis and establishment of an antiviral state. However, viruses have evolved diverse strategies to evade or antagonize the IFN antiviral response proteins. Paramyxoviruses encode and use a number of proteins, which facilitate escape from cellular defense by blocking IFN production or/and signaling. NDV and mumps viruses use V- protein,60,61 SeV uses a family of C-protein,61 V-protein,62 and MV uses V-protein,63P-protein,64 and C-protein65 to block the IFN pathway.
During a process of malignization, a tumor cell genetically changes and gets rid of functions that serve the needs of the whole organism. Along with mutations that promote accelerated proliferation and invasion, the IFNs signaling is often compromised through a homozygous loss of the chromosome 9p21 region, which encodes type I IFN genes.24 In malignant cells, virus may not affect IFN signaling as this pathway is already broken. However, sometimes during the genetic engineering process, it is necessary to minimize the potential impact of viral construct on healthy cells; therefore, viral genes, which block the IFN pathway, are deleted or attenuated.66
Stimulation of the IFN response in peripheral blood leukocytes
Paramyxoviruses are known to be strong inducers of IFNs in human peripheral blood leukocytes (HPBLs). This property that has been used in biotechnology: the Sendai virus was chosen from among many other viruses for industrial-scale production of IFN from human leukocytes.67 In HPBLs, SeV behaves as a potent inducer of IFN-α68; it induces at least nine different IFN-α species.69 In HPBLs, SeV also stimulates the production of IFN-γ.70 Similarly, the NDV stimulates the production of IFN-α from several gene isoforms.71 The IFN secretion can be induced by the viral Hemagglutinin-Neuraminidase (HN-protein or HN), a process independent of the double-stranded RNA (dsRNA) response, and by dsRNA replicative intermediates.71,72Perhaps, the existence of two alternative parallel mechanisms of IFN induction explains the high IFN stimulating capacity of NDV72,73 and other paramyxoviruses.
IFNs exert complex systemic effects that make them a useful means for adjuvant therapy of cancer.74,75 IFNs have been used in therapeutic schemes for the treatment of metastatic melanoma, renal carcinoma, Kaposi sarcoma, bladder carcinoma, hairy-cell leukemia, etc. The significant prolongation of a disease-free survival has now been largely validated by the combined analyses of multi-institutional trials and by the subsequent studies that have included meta-analyses.76 IFNs can act systemically by regulating the immune response through the activation of DCs, cytotoxic T cells, and NK cells. IFNs markedly increase the major histocompatibility complex (MHC) class I and class II-dependent antigen presentation that causes increased presentation of tumor-specific antigens by malignant and professional antigen-presenting cells. This presentation stimulates antitumor activity and proliferation of cytotoxic T lymphocytes.77–79
The responses to IFNs are dysfunctional in many malignancies making the direct effects of IFNs less efficient. However, IFNs can affect tumor growth indirectly, through suppression of angiogenesis by altering the stimuli from tumor cells and by inhibiting endothelial cells. The degree of inhibition correlates with the reduced tumor vascularization and the consequent retardation of tumor growth.76,80,81 The treatment with IFNs may also affect the viability of cancer stem cells that are highly resistant to chemo- and radiotherapy and are responsible for the disease relapses. Most likely, IFNs affect tumor vasculature and disrupt the vascular niche of stem cells, as it was found in murine xenografts of human glioma.75 It is suggested that IFN-γ triggered by ultraviolet (UV) inactivated SeV is responsible for lung metastasis suppression in melanoma murine model.82
Stimulation of cytokines
In HPBLs, SeV was shown to stimulate the synthesis of interleukins (IL) 2, 6, 8, macrophage inflammatory protein-1 (MIP-1) α, MIP-1-β, regulated on activation normal T cell expressed and secreted (RANTES) proteins, and monocyte chemotactic protein −1 (MCP-1).68,70 Live or UV-irradiated SeV can stimulate the IL-6 release in treated animals.83 In DCs, incubation with SeV results in the IL-6 secretion; the fusion protein (F) was found to be responsible for the effect.84 An injection of an UV-inactivated SeV into established mouse renal cell carcinoma tumors resulted in the secretion of chemokine C-X-C motif chemokine 10 (CXCL10) by infiltrating DCs.85NDV was also shown to induce secretion of chemokine (C-C motif) ligand 5 (CCL5) (also known as RANTES) and CXCL10 from infected human cancer cells.38 The CXCL10 was found to have an antitumor activity through the attraction of monocytes, macrophages, NK cells, T cells, and DCs. The CXCL10 also promotes T cells adhesion to endothelial cells and the inhibition of angiogenesis.86 CCL5 and CXCL10 are T helper type 1 (Th1) chemokines and can attract activated Th1 and TCL cells into tumor nodules to exert their cytotoxicity on cancer cells.87 In HPBLs, incubation with NDV also stimulates the secretion of cytokines such as tumor necrosis factor α (TNF-α)88,89 and TNF-related apoptosis-inducing ligand (TRAIL).71,89 It appears that the induction of TRAIL is due to a single viral HN protein.71,90 NDV induces the secretion of TRAIL in monocytes and NDV kills various human tumor cell lines following the stimulation of TRAIL receptors 2 (TRAIL-R2).90 Macrophages can also be stimulated by incubation with NDV leading to upregulation of a set of macrophage-specific genes and secretion of TNF-α.91
Stimulation of NK cells
NK cells constitute a type of cytotoxic lymphocytes that bridge the innate and adaptive branches of the immune system. These cells widely interact with other components of the immune system affecting the antigen-specific T and B cell responses.92 The NK cells participate in the early control against virus infection and in tumor immune surveillance. These cells are responsible for a fast protective effect due to their ability to destroy abnormal or stressed cells that lack antigen-specific cell surface receptors and MHC. The NK cells do not require activation in order to kill cells, which are not expressing “self” markers of the MHC class I.
The NK cells can be activated for most efficient NK-mediated cell lysis. NK cells display natural cytotoxicity receptors that are responsible for their activation. Among these receptors are NK protein 46 (NKp46) and NK protein 44 (NKp44). Hemagglutinin neuraminidase (HN) protein of paramyxoviruses directly interacts with two receptors types: NKp44 and NKp46, and this interaction triggers the NK activation and NK-mediated cell lysis.93–96 In particular mumps viral HN protein mediates NKp46 activation of NK cells, and this activation results in tumor rejection via NK-DC crosstalk.97 Studies with the UV-inactivated SeV virus also highlight the important role of NK cells in virus-mediated oncolysis. In a mouse renal cancer model, UV-inactivated SeV produced strong anticancer effect, which was compromised by coinjection of an NK-depleting anti-mono-sialo-tetra-hexosyl-ganglioside 1 (GM1) antibody.85
Stimulation of DCs
DCs are professional antigen-presenting cells that can efficiently stimulate innate as well as adaptive immune responses against various pathogens and cancer cells. After sensing a virus or other pathogens, the DCs enter a maturation program and became competent for education of naive T cells.
The ex vivo infection of DCs by recombinant SeV can induce DCs maturation and activation in only 1 hour.98 Treatment with activated DCs harboring different variants of recombinant SeV can dramatically improve the survival of animals inoculated with cells of malignant melanoma,82,99 colon carcinoma,100 squamous cell carcinoma,101 hepatocellular carcinoma, neuroblastoma, and prostatic cancer.102The administration of such DCs before tumor inoculation has demonstrated a preventive effect against lung metastasis of neuroblastoma103 and prostate adenocarcinoma.104 Another response of DC cells to virus infection is inhibition of immunosuppression mediated by the regulatory T-cells. In experiments with the UV-inactivated SeV, carbohydrates of the viral fusion (F) protein are recognized by an unknown receptor(s) on DC cells leading to the nuclear factor kappa-light-chain-enhancer (NF-kB) activation of B cells and secretion of IL-6.84
Stimulation of tumor-specific cytotoxic T-cell mediated activity
Cell surface binding of NDV can stimulate the tumor-specific cytotoxic CD8+ T-cell (CTL) response and increase CD4+ T-helper cells’ activity even in the absence of an antiviral T-cell response and cell infection.105 The activation and infiltration of tumor sites with CTLs is the result of complex effects of virus triggered immune system, including local and systemic release of IFNs and other cytokines. Interestingly, UV-inactivated NDV was as active in promoting the tumor-specific CTL response as the replication competent NDV. It was found that this effect is mediated by the presence on the tumor cell surface of functional viral HN molecules that have affinity to plasma membranes and therefore can mediate a strong adhesion of the infected cells to CTLs.105 Since the HN proteins of many paramyxoviruses are homologous, it is likely that the HN protein, regardless of whether it derives from NDV or other related paramyxoviruses, can activate both NK and CTL responses.
Viral sialidase activity of the HN protein
Metastatic cancer cells often express a high density of sialic acid-rich glycoproteins that increase the invasive potential.106 The overexpression of sialic acid on the surfaces creates a negative charge on cell membranes, leading to repulsion between cells that promote metastases by helping cancer cells entry into the blood stream. Indeed, the ability of tumor cells to metastasize correlates with the abundance of sialic acids on the surface of many types of malignant cells.106–108 The extent of cell surface poly-sialylation was suggested as a marker characterizing the differentiation status of thyroid and small-cell lung carcinoma cells.109
A more detailed analysis reveals qualitative differences in sialic acids on the surface of cells displaying different degrees of invasive-ness. While in normal human colon cells, as well as in adenomas and several carcinomas of different grades, an alpha 2, 3-linked sialic acid is detected, in the highly malignant variants, alpha 2, 6-linked sialic acids are also present. It was found that the malignant progression was associated with the de novo expression of an alpha 2,6 sialyl-transferase, which transforms the alpha 2,3-linked sialic acid into the 2,6-linked sialic acid.110 The increase in alpha 2,6 sialylation coincident with tumor progression is detected for hepatocellular111and colon carcinomas.112
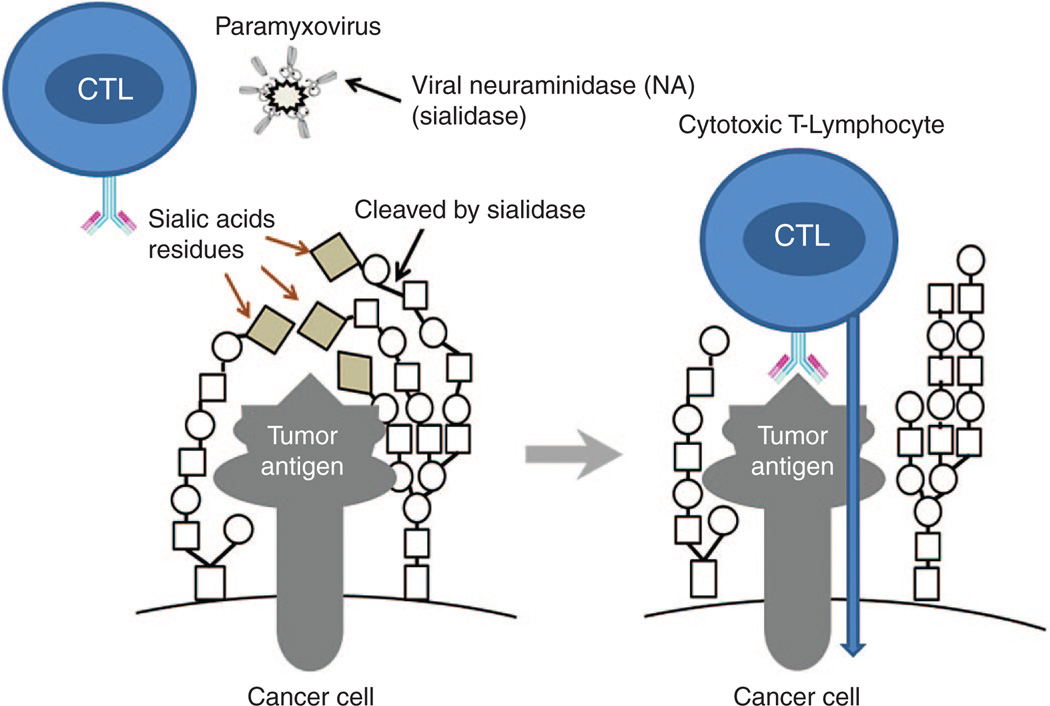
Inhibitors of the sialylation process decrease the malignancy of cancer cells and now are considered as candidate drugs against metastatic cancer.113 One of the possible mechanisms linking the increased sialylation with malignant phenotype is the creation of a thick “coat” on the cell surface that hides cancer antigens and provides an escape of malignant cells from the immunosurveillance. For example, it is found that the tumor specific Lewis antigen of malignant medullary thyroid cancer can be masked from the immune system by both alpha 2,3- and alpha 2,6-linked sialic acid residues.114 Removing some sialic acid residues from the surface of malignant cells by sialidase can unmask cancer-specific antigens and make cells visible to the immune system (Figure 3). The removal of sialic acids from tumor cells is associated with a reduced growth potential, an activation of NK cells, and a secretion of IFN-γ.115
Figure 3.
A hypothetical role of viral sialidase in activation of cytotoxic T-lymphocyte response against cancer cells. The removal of sialic acids may unmask hidden tumor antigens and make them more visible by the immune system.
The HN proteins present in SeV, NDV, and some other paramyxoviruses possess both an erythrocyte agglutination and a neuraminidase (sialidase) activities.116,117 Neuraminidase is capable of cleaving and removing sialic acid residues from the surface of malignant cells leading to a dramatic increase in their ability to induce the T-cell response.118
SUMMARY OF ANTICANCER IMMUNO-TRIGGERING ABILITIES OF ONCOLYTIC PARAMYXOVIRUSES
A number of molecular mechanisms are responsible for the anti-cancer immuno-triggering abilities of oncolytic paramyxoviruses. One of them is related to viral modulation of gene expression related to antigen presentation that results in enhancement of the immunogenicity of cancer cells. Another is related to induction of autophagy in infected cancer cell that also enhances cancer cells immunogenicity. Viral sialidase activity of paramyxovirus HN protein might also contribute to improvement of cancer cell visibility for immune system by unmasking cancer antigens from a “thick coat” of sialic acid polymers. In addition, oncolytic paramyxoviruses stimulate anticancer NK cells and tumor-specific cytotoxic T cell– mediated activity. They also trigger activation of DCs, which are responsible for better cancer antigen presentation.
Strong immune activation by paramyxoviruses is a double edged sword. On one hand, it triggers strong anticancer immunity. On the other hand, it can inhibit replication of paramyxoviruses and trigger their fast clearance from a patient’s body. So, perhaps a panel of different oncolytic viruses that represents different families instead of one particular virus should be used in a sequential way for maximizing a chance of complete remission during a course of oncolytic virotherapy.
COMPARISON OF ONCOLYTIC PARAMYXOVIRUSES WITH OTHER ONCOLYTIC VIRUSES
Many oncolytic viruses that belong to various viral families are currently being tested in clinical trials.17,119 It is becoming clear that oncolytic effects can be produced by most animal viruses having quite different genome organizations: from small positive-strand RNA-containing picornaviruses,120–123 small double-stranded (ds) RNA reoviruses,124 small ds-DNA parvoviruses,125 to medium-size ds-DNA adeno-126,127 and herpes viruses,128 negative-strand RNA rhabdo-129 and paramyxoviruses73,130 as well as very large poxviruses.131,132 The high degree of diversity among these agents suggests that while there could be common mechanisms of oncolysis characteristic to all viruses, certain specific mechanisms could be associated with particular groups of viruses.
Are there any advantages in using paramyxoviruses versus representatives of other viral families as oncolytic agents? We believe that the ability to trigger syncytium formation is a great advantage of oncolytic paramyxoviruses. As mentioned earlier (see section Oncolysis mediated by direct cancer cell killing through formation of syncytium), paramyxoviruses have a mechanism of spreading infection without the release of mature virus particles from cells. This includes fusion of infected cells with their noninfected neighbors. The fusion results in the formation of a syncytium from 50–100 neighboring cells (Figure 2).26 The ability of the viruses to induce the formation of syncytia correlates with their oncolytic potential. Most likely, syncytia formation contributes to the efficiency of viral oncolysis because it allows extra rounds of viral replication without any exposure to host neutralizing antibodies. Moreover, malignant cells death through syncytia fusion is highly immunogenic and resembles autophagy (see section “role of autophagy”). Fused tumor cells secrete an abundance of “syncytiosomes,” which are exosome-like vesicles that highly efficiently present TAA via MHC molecules.27,33 In addition, cross-presentation of TAAs by DCs is strongly promoted by paramyxoviral fusogenic membrane glycoproteins.27
Another potential unique advantage of some paramyxoviruses (Mumps, NDV, and SeV) is the sialidase (neuraminidase) activity of their HN protein which has the potential to remove sialic residues from the surface of tumor cells (see section Viral sialidase activity of the HN protein). Such removal is associated with reduced growth potential of malignant cells, activation of NK cells, and secretion of IFN-γ.115
FUTURE PERSPECTIVES
Improving tumor-specific targeting
NDV, SeV, and Mumps viruses can target a variety of cancers because they use sialic acids residues as their cell receptors. However, these viruses are also able to bind sialic acid residues on normal cells. So, antibodies that diminish viral binding to normal cells and increase viral binding to malignant cells could be very useful for improving target specificity. Such bi-specific antibodies have been developed for NDV. These antibodies indeed increase NDV target tumor cells specificity in animal model experiments.133
A number of studies for increasing target specificity were also performed with MVES. Even though CD46 and nectin 4 receptor binding already provides some malignant cell target specificity for MVES, it could be further increased through variable routes described below.
A series of studies on retargeting oncolytic strains of MVES employed viral attachment hemagglutinin (H) protein,134 which is responsible for the receptor recognition and the binding specificity.135 To kill interaction of the H-protein with CD46, the region responsible for the binding is mutagenized, and a variety of ligands that bind to selected targets of potential host cells are introduced. The ligands successfully tested in this system include the antigen-binding peptides (single-chain antibodies) against epidermal growth factor receptor (EGFR; synonyms ErbB-1; HER1 in humans),136–138 HER-2/neu,139 folate receptor α,140 CD20,141 CD38,137a peptide echistatin that binds to integrins,142 and cytokine IL-13.143
Optimization of syncytia formation and F protein cleavage site
Ability of paramyxoviruses to form syncytia increases viral oncolytic potency. The F protein of paramyxoviruses is responsible for viral fusion with the cell membrane. It is also responsible for viral spread from cell to cell via formation of syncytia. The F protein cleavage site is a determinant of virulence for different NDV viral strains.144,145 The introduction of the multibasic cleavage site into the F protein allows this protein to be cleaved and to be activated by a broad range of proteases. To increase oncolytic potency of one NDV strain, a poly-basic cleavage site was introduced into the F protein to generate a new site. While the resultant virus exhibited only an intermediate virulence phenotype based on a mean death time in embryonated eggs, the virus formed large syncytia and was enhanced in its replication in cancer cells, leading to enhanced oncolytic effects in various animal tumor models.146–152
Similar findings were shown when the F protein of the NDV La Sota strain was modified in an analogous fashion.144,153 “The fusogenic and oncolytic activity of the rNDV/F3aa strain was further enhanced by a point mutation in the F protein at residue 289 from leucine to alanine, generating rNDV/F3aa (L289A). In an immunocompetent liver tumor rat model, administration of the mutant virus via hepatic arterial infusion resulted in improved syncytia formation and necrosis. This improvement in syncytia formation caused a significant 20% prolongation of survival.”31,146
Introduction of specific cleavage sites into the F protein allows utilization of tumor tissue-specific proteases, as demonstrated with SeV.21,154,155 Introduction of a ubiquitously recognized cleavage-motive into the F protein of SeV enables its continuous spread in malignant human tissues. A tumor-specific spreading of such recombinant SeV without evidence of bio-distribution into nonmalignant tissues66 was found in a hepatoma xenograft animal model. Such viral constructs represent candidates for clinical trials.
Combination with autologous cell vaccines: a personalized oncolytic viral immunotherapy
“A therapeutic, autologous, antigen-presenting cell vaccine (Sipuleucel-T) was recently approved by the US Food and Drug Administration for metastatic prostate cancer, after demonstration of prolongation of overall survival in a randomized, double-blind, placebo-controlled study”.156 Combination of autologous vaccine with an oncolytic paramyxovirus could provide extra benefit for specific antitumor immune stimulation. The use of autologous tumor tissue for development of a personalized oncolytic viral immunotherapy should be further exploited with oncolytic paramyxoviruses.
Individual viral sensitivity test
There is a growing interest in personalized cancer therapy, which involves identifying those treatments which may work best for an individual’s cancer. Individual viral sensitivity testing should involve testing an individual’s cancer cells in the laboratory to see which virus demonstrate the best response. It therefore could provide guidance about which oncolytic virus representative may be best for the individual in clinical practice.
CONCLUSIONS
The described mechanisms of oncolysis mediated by paramyxoviruses indicate that these viruses represent exceptionally attractive instruments for cancer therapy. The genomes of paramyxoviruses are comparatively stable. The viruses induce efficient syncytium-mediated lysis of cancer cells and they elicit strong immunomodulatory effects, which dramatically enforce immune surveillance and anticancer resistance. The use of nonhuman paramyxoviruses, such as NDV, or SeV, or attenuated vaccine strains of MV, or Mumps viruses hold great advantage, as these virus strains are not associated with any serious pathology in humans.
Fast screening tests of biopsy materials are needed because variable malignancies might support a particular virus representative reproduction to a different level. Such tests should reveal which virus kills a particular malignancy in the most efficient way. In other words, an experimentally verified matching of a particular malignancy with a particular virus is highly desirable. In case several different virus representatives (perhaps even from a few different viral families) would be found to be efficient for one type of malignancy, they all might be used for virotherapy in a sequential or parallel way. Because of the potential for fast immune-mediated clearance of a particular virus from a patient, the sequential therapy may ameliorate treatment efficiency decline due to such clearance.
ACKNOWLEDGMENTS
We thank Vyacheslav M. Senin for inspiring ideas, John F. Atkins, Dmitriy Zamarin and Anna Senina for critical reading of the manuscript. We are grateful to Brigit Sullivan, ORS, NIH Library Writing Center, for editing assistance. The work was supported by a grant 11-04-00410 from the Russian Foundation for Basic Research and by a grant from Russian Scientific Fund 14-15-01073 (P.M.C.). This research was supported in part by the Intramural Research Program of the National Library of Medicine, NIH.
Footnotes
CONFLICT OF INTEREST
Olga V. Matveeva is a co-author of a patent entitled “Method for cancer immunotherapy and pharmaceutical compositions based on oncolytic non-pathogenic Sendai virus.”
REFERENCES
- 1.Lamb RA, Parks GD. Paramyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th edn. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1449–1496. [Google Scholar]
- 2.Villar E, Barroso IM. Role of sialic acid-containing molecules in paramyxovirus entry into the host cell: a minireview. Glycoconj J. 2006;23:5–17. doi: 10.1007/s10719-006-5433-0. [DOI] [PubMed] [Google Scholar]
- 3.Cantín C, Holguera J, Ferreira L, Villar E, Muñoz-Barroso I. Newcastle disease virus may enter cells by caveolae-mediated endocytosis. J Gen Virol. 2007;88(Pt 2):559–569. doi: 10.1099/vir.0.82150-0. [DOI] [PubMed] [Google Scholar]
- 4.Higuchi H, Bronk SF, Bateman A, Harrington K, Vile RG, Gores GJ. Viral fusogenic membrane glycoprotein expression causes syncytia formation with bioenergetic cell death: implications for gene therapy. Cancer Res. 2000;60:6396–6402. [PubMed] [Google Scholar]
- 5.Bossart KN, Fusco DL, Broder CC. Paramyxovirus entry. Adv Exp Med Biol. 2013;790:95–127. doi: 10.1007/978-1-4614-7651-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matrosovich M, Herrler G, Klenk HD. Sialic acid receptors of viruses. Top Curr Chem. 2013 doi: 10.1007/128_2013_466. (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Büll C, Stoel MA, den Brok MH, Adema GJ. Sialic acids sweeten a tumor’s life. Cancer Res. 2014;74:3199–3204. doi: 10.1158/0008-5472.CAN-14-0728. [DOI] [PubMed] [Google Scholar]
- 8.Büll C, den Brok MH, Adema GJ. Sweet escape: sialic acids in tumor immune evasion. Biochim Biophys Acta. 2014;1846:238–246. doi: 10.1016/j.bbcan.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi Y, Miyamoto Y, Inoue T, Kaneda Y. Efficient eradication of hormone-resistant human prostate cancers by inactivated Sendai virus particle. Int J Cancer. 2009;124:2478–2487. doi: 10.1002/ijc.24234. [DOI] [PubMed] [Google Scholar]
- 10.Myers R, Greiner S, Harvey M, Soeffker D, Frenzke M, Abraham K, et al. Oncolytic activities of approved mumps and measles vaccines for therapy of ovarian cancer. Cancer Gene Ther. 2005;12:593–599. doi: 10.1038/sj.cgt.7700823. [DOI] [PubMed] [Google Scholar]
- 11.Anderson BD, Nakamura T, Russell SJ, Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 12.Noyce RS, Bondre DG, Ha MN, Lin LT, Sisson G, Tsao MS, et al. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011;7:e1002240. doi: 10.1371/journal.ppat.1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takano A, Ishikawa N, Nishino R, Masuda K, Yasui W, Inai K, et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009;69:6694–6703. doi: 10.1158/0008-5472.CAN-09-0016. [DOI] [PubMed] [Google Scholar]
- 14.Derycke MS, Pambuccian SE, Gilks CB, Kalloger SE, Ghidouche A, Lopez M, et al. Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker. Am J Clin Pathol. 2010;134:835–845. doi: 10.1309/AJCPGXK0FR4MHIHB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabre-Lafay S, Monville F, Garrido-Urbani S, Berruyer-Pouyet C, Ginestier C, Reymond N, et al. Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer. 2007;7:73. doi: 10.1186/1471-2407-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cattaneo R. Paramyxovirus entry and targeted vectors for cancer therapy. PLoS Pathog. 2010;6:e1000973. doi: 10.1371/journal.ppat.1000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 19.Nagini S. RECKing MMP: relevance of reversion-inducing cysteine-rich protein with kazal motifs as a prognostic marker and therapeutic target for cancer (a review) Anticancer Agents Med Chem. 2012;12:718–725. doi: 10.2174/187152012802650237. [DOI] [PubMed] [Google Scholar]
- 20.Hadler-Olsen E, Winberg JO, Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol. 2013;34:2041–2051. doi: 10.1007/s13277-013-0842-8. [DOI] [PubMed] [Google Scholar]
- 21.Kinoh H, Inoue M, Washizawa K, Yamamoto T, Fujikawa S, Tokusumi Y, et al. Generation of a recombinant Sendai virus that is selectively activated and lyses human tumor cells expressing matrix metalloproteinases. Gene Ther. 2004;11:1137–1145. doi: 10.1038/sj.gt.3302272. [DOI] [PubMed] [Google Scholar]
- 22.Springfeld C, von Messling V, Frenzke M, Ungerechts G, Buchholz CJ, Cattaneo R. Oncolytic efficacy and enhanced safety of measles virus activated by tumor-secreted matrix metalloproteinases. Cancer Res. 2006;66:7694–7700. doi: 10.1158/0008-5472.CAN-06-0538. [DOI] [PubMed] [Google Scholar]
- 23.Mühlebach MD, Schaser T, Zimmermann M, Armeanu S, Hanschmann KM, Cattaneo R, et al. Liver cancer protease activity profiles support therapeutic options with matrix metalloproteinase-activatable oncolytic measles virus. Cancer Res. 2010;70:7620–7629. doi: 10.1158/0008-5472.CAN-09-4650. [DOI] [PubMed] [Google Scholar]
- 24.Haus O. The genes of interferons and interferon-related factors: localization and relationships with chromosome aberrations in cancer. Arch Immunol Ther Exp (Warsz) 2000;48:95–100. [PubMed] [Google Scholar]
- 25.Mansour M, Palese P, Zamarin D. Oncolytic specificity of Newcastle disease virus is mediated by selectivity for apoptosis-resistant cells. J Virol. 2011;85:6015–6023. doi: 10.1128/JVI.01537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galanis E. Therapeutic potential of oncolytic measles virus: promises and challenges. Clin Pharmacol Ther. 2010;88:620–625. doi: 10.1038/clpt.2010.211. [DOI] [PubMed] [Google Scholar]
- 27.Bateman AR, Harrington KJ, Kottke T, Ahmed A, Melcher AA, Gough MJ, et al. Viral fusogenic membrane glycoproteins kill solid tumor cells by nonapoptotic mechanisms that promote cross presentation of tumor antigens by dendritic cells. Cancer Res. 2002;62:6566–6578. [PubMed] [Google Scholar]
- 28.Lin EH, Salon C, Brambilla E, Lavillette D, Szecsi J, Cosset FL, et al. Fusogenic membrane glycoproteins induce syncytia formation and death in vitro and in vivo: a potential therapy agent for lung cancer. Cancer Gene Ther. 2010;17:256–265. doi: 10.1038/cgt.2009.74. [DOI] [PubMed] [Google Scholar]
- 29.Ebert O, Shinozaki K, Kournioti C, Park MS, García-Sastre A, Woo SL. Syncytia induction enhances the oncolytic potential of vesicular stomatitis virus in virotherapy for cancer. Cancer Res. 2004;64:3265–3270. doi: 10.1158/0008-5472.can-03-3753. [DOI] [PubMed] [Google Scholar]
- 30.Nakamori M, Fu X, Meng F, Jin A, Tao L, Bast RC, Jr, et al. Effective therapy of metastatic ovarian cancer with an oncolytic herpes simplex virus incorporating two membrane fusion mechanisms. Clin Cancer Res. 2003;9:2727–2733. [PubMed] [Google Scholar]
- 31.Altomonte J, Marozin S, Schmid RM, Ebert O. Engineered newcastle disease virus as an improved oncolytic agent against hepatocellular carcinoma. Mol Ther. 2010;18:275–284. doi: 10.1038/mt.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gainey MD, Manuse MJ, Parks GD. A hyperfusogenic F protein enhances the oncolytic potency of a paramyxovirus simian virus 5 P/V mutant without compromising sensitivity to type I interferon. J Virol. 2008;82:9369–9380. doi: 10.1128/JVI.01054-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bateman A, Bullough F, Murphy S, Emiliusen L, Lavillette D, Cosset FL, et al. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res. 2000;60:1492–1497. [PubMed] [Google Scholar]
- 34.Galanis E, Bateman A, Johnson K, Diaz RM, James CD, Vile R, et al. Use of viral fusogenic membrane glycoproteins as novel therapeutic transgenes in gliomas. Hum Gene Ther. 2001;12:811–821. doi: 10.1089/104303401750148766. [DOI] [PubMed] [Google Scholar]
- 35.Allen C, McDonald C, Giannini C, Peng KW, Rosales G, Russell SJ, et al. Adenoviral vectors expressing fusogenic membrane glycoproteins activated via matrix metalloproteinase cleavable linkers have significant antitumor potential in the gene therapy of gliomas. J Gene Med. 2004;6:1216–1227. doi: 10.1002/jgm.616. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann D, Bayer W, Wildner O. In situ tumor vaccination with adenovirus vectors encoding measles virus fusogenic membrane proteins and cytokines. World J Gastroenterol. 2007;13:3063–3070. doi: 10.3748/wjg.v13.i22.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmann D, Bayer W, Wildner O. Local and distant immune-mediated control of colon cancer growth with fusogenic membrane glycoproteins in combination with viral oncolysis. Hum Gene Ther. 2007;18:435–450. doi: 10.1089/hum.2006.185. [DOI] [PubMed] [Google Scholar]
- 38.Washburn B, Schirrmacher V. Human tumor cell infection by Newcastle Disease Virus leads to upregulation of HLA and cell adhesion molecules and to induction of interferons, chemokines and finally apoptosis. Int J Oncol. 2002;21:85–93. doi: 10.3892/ijo.21.1.85. [DOI] [PubMed] [Google Scholar]
- 39.Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6:226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 42.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 43.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo ZS, Liu Z, Bartlett DL. Oncolytic immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front Oncol. 2014;4:74. doi: 10.3389/fonc.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012;22:275–281. doi: 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y, et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer. 2013;12:103. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoue H, Tani K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ. 2014;21:39–49. doi: 10.1038/cdd.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svet-Moldavsky GJ, Hamburg VP. Quantitative relationships in viral oncolysis and the possibility of artificial heterogenization of tumours. Nature. 1964;202:303–304. doi: 10.1038/202303b0. [DOI] [PubMed] [Google Scholar]
- 49.Kuzumaki N, Moriuchi T, Kodama T, Kobayashi H. Xenogenization of rat erythroid cells by lymphatic leukemia virus: its role in induction of autoimmune hemolytic anemia. J Immunol. 1976;117:1250–1255. [PubMed] [Google Scholar]
- 50.Donnelly OG, Errington-Mais F, Steele L, Hadac E, Jennings V, Scott K, et al. Measles virus causes immunogenic cell death in human melanoma. Gene Ther. 2013;20:7–15. doi: 10.1038/gt.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gauvrit A, Brandler S, Sapede-Peroz C, Boisgerault N, Tangy F, Gregoire M. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 2008;68:4882–4892. doi: 10.1158/0008-5472.CAN-07-6265. [DOI] [PubMed] [Google Scholar]
- 52.Guillerme JB, Marc G, Tangy F, Fonteneau JF. Antitumor virotherapy by attenuated measles virus. Biology. 2013;2:587–602. doi: 10.3390/biology2020587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siddiqui MA, Malathi K. RNase L induces autophagy via c-Jun N-terminal kinase and double-stranded RNA-dependent protein kinase signaling pathways. J Biol Chem. 2012;287:43651–43664. doi: 10.1074/jbc.M112.399964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 55.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richetta C, Grégoire IP, Verlhac P, Azocar O, Baguet J, Flacher M, et al. Sustained autophagy contributes to measles virus infectivity. PLoS Pathog. 2013;9:e1003599. doi: 10.1371/journal.ppat.1003599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng C, Qiu X, Jin S, Yu S, Chen H, Ding C. Whole genome sequencing and biological characterization of Duck/JS/10, a new lentogenic class I Newcastle disease virus. Arch Virol. 2012;157:869–880. doi: 10.1007/s00705-012-1248-4. [DOI] [PubMed] [Google Scholar]
- 58.Delpeut S, Rudd PA, Labonte P, von Messling V. Membrane fusion-mediated autophagy induction enhances morbillivirus cell-to-cell spread. J Virol. 2012;86:8527–8535. doi: 10.1128/JVI.00807-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joubert PE, Meiffren G, Grégoire IP, Pontini G, Richetta C, Flacher M, et al. Autophagy induction by the pathogen receptor CD46. Cell Host Microbe. 2009;6:354–366. doi: 10.1016/j.chom.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 60.Park MS, Shaw ML, Muñoz-Jordan J, Cros JF, Nakaya T, Bouvier N, et al. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J Virol. 2003;77:1501–1511. doi: 10.1128/JVI.77.2.1501-1511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gotoh B, Komatsu T, Takeuchi K, Yokoo J. Paramyxovirus accessory proteins as interferon antagonists. Microbiol Immunol. 2001;45:787–800. doi: 10.1111/j.1348-0421.2001.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 62.Childs K, Randall R, Goodbourn S. Paramyxovirus V proteins interact with the RNA Helicase LGP2 to inhibit RIG-I-dependent interferon induction. J Virol. 2012;86:3411–3421. doi: 10.1128/JVI.06405-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takeuchi K, Kadota SI, Takeda M, Miyajima N, Nagata K. Measles virus V protein blocks interferon (IFN)-alpha/beta but not IFN-gamma signaling by inhibiting STAT1 and STAT2 phosphorylation. FEBS Lett. 2003;545:177–182. doi: 10.1016/s0014-5793(03)00528-3. [DOI] [PubMed] [Google Scholar]
- 64.Takayama I, Sato H, Watanabe A, Omi-Furutani M, Sugai A, Kanki K, et al. The nucleocapsid protein of measles virus blocks host interferon response. Virology. 2012;424:45–55. doi: 10.1016/j.virol.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Shaffer JA, Bellini WJ, Rota PA. The C protein of measles virus inhibits the type I interferon response. Virology. 2003;315:389–397. doi: 10.1016/s0042-6822(03)00537-3. [DOI] [PubMed] [Google Scholar]
- 66.Zimmermann M, Armeanu-Ebinger S, Bossow S, Lampe J, Smirnow I, Schenk A, et al. Attenuated and protease-profile modified sendai virus vectors as a new tool for virotherapy of solid tumors. PLoS ONE. 2014;9:e90508. doi: 10.1371/journal.pone.0090508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cantell K, Hirvonen S, Kauppinen HL, Myllylä G. Production of interferon in human leukocytes from normal donors with the use of Sendai virus. Meth Enzymol. 1981;78(Pt A):29–38. doi: 10.1016/0076-6879(81)78094-7. [DOI] [PubMed] [Google Scholar]
- 68.Hua J, Liao MJ, Rashidbaigi A. Cytokines induced by Sendai virus in human peripheral blood leukocytes. J Leukoc Biol. 1996;60:125–128. doi: 10.1002/jlb.60.1.125. [DOI] [PubMed] [Google Scholar]
- 69.Nyman TA, Tölö H, Parkkinen J, Kalkkinen N. Identification of nine interferon-alpha subtypes produced by Sendai virus-induced human peripheral blood leucocytes. Biochem J. 1998;329(Pt 2):295–302. doi: 10.1042/bj3290295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costas MA, Mella D, Criscuolo M, Díaz A, Finkielman S, Nahmod VE, et al. Superinduction of mitogen-stimulated interferon-gamma production and other lymphokines by Sendai virus. J Interferon Res. 1993;13:407–412. doi: 10.1089/jir.1993.13.407. [DOI] [PubMed] [Google Scholar]
- 71.Zeng J, Fournier P, Schirrmacher V. Induction of interferon-alpha and tumor necrosis factor-related apoptosis-inducing ligand in human blood mononuclear cells by hemagglutinin-neuraminidase but not F protein of Newcastle disease virus. Virology. 2002;297:19–30. doi: 10.1006/viro.2002.1413. [DOI] [PubMed] [Google Scholar]
- 72.Fournier P, Zeng J, Schirrmacher V. Two ways to induce innate immune responses in human PBMCs: paracrine stimulation of IFN-alpha responses by viral protein or dsRNA. Int J Oncol. 2003;23:673–680. [PubMed] [Google Scholar]
- 73.Schirrmacher V, Fournier P. Newcastle disease virus: a promising vector for viral therapy, immune therapy, and gene therapy of cancer. Methods Mol Biol. 2009;542:565–605. doi: 10.1007/978-1-59745-561-9_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang BX, Rahbar R, Fish EN. Interferon: current status and future prospects in cancer therapy. J Interferon Cytokine Res. 2011;31:545–552. doi: 10.1089/jir.2010.0158. [DOI] [PubMed] [Google Scholar]
- 75.Williams RF, Sims TL, Tracey L, Myers AL, Ng CY, Poppleton H, et al. Maturation of tumor vasculature by interferon-beta disrupts the vascular niche of glioma stem cells. Anticancer Res. 2010;30:3301–3308. [PubMed] [Google Scholar]
- 76.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, et al. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zaidi MR, Merlino G. The two faces of interferon-γ in cancer. Clin Cancer Res. 2011;17:6118–6124. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bekisz J, Sato Y, Johnson C, Husain SR, Puri RK, Zoon KC. Immunomodulatory effects of interferons in malignancies. J Interferon Cytokine Res. 2013;33:154–161. doi: 10.1089/jir.2012.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seliger B, Ruiz-Cabello F, Garrido F. IFN inducibility of major histocompatibility antigens in tumors. Adv Cancer Res. 2008;101:249–276. doi: 10.1016/S0065-230X(08)00407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 81.Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 82.Saga K, Tamai K, Yamazaki T, Kaneda Y. Systemic administration of a novel immune-stimulatory pseudovirion suppresses lung metastatic melanoma by regionally enhancing IFN-γ production. Clin Cancer Res. 2013;19:668–679. doi: 10.1158/1078-0432.CCR-12-1947. [DOI] [PubMed] [Google Scholar]
- 83.Kurooka M, Kaneda Y. Inactivated Sendai virus particles eradicate tumors by inducing immune responses through blocking regulatory T cells. Cancer Res. 2007;67:227–236. doi: 10.1158/0008-5472.CAN-06-1615. [DOI] [PubMed] [Google Scholar]
- 84.Suzuki H, Kurooka M, Hiroaki Y, Fujiyoshi Y, Kaneda Y. Sendai virus F glycoprotein induces IL-6 production in dendritic cells in a fusion-independent manner. FEBS Lett. 2008;582:1325–1329. doi: 10.1016/j.febslet.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 85.Fujihara A, Kurooka M, Miki T, Kaneda Y. Intratumoral injection of inactivated Sendai virus particles elicits strong antitumor activity by enhancing local CXCL10 expression and systemic NK cell activation. Cancer Immunol Immunother. 2008;57:73–84. doi: 10.1007/s00262-007-0351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 87.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 88.Lorence RM, Rood PA, Kelley KW. Newcastle disease virus as an antineoplastic agent: induction of tumor necrosis factor-alpha and augmentation of its cytotoxicity. J Natl Cancer Inst. 1988;80:1305–1312. doi: 10.1093/jnci/80.16.1305. [DOI] [PubMed] [Google Scholar]
- 89.Zorn U, Dallmann I, Grosse J, Kirchner H, Poliwoda H, Atzpodien J. Induction of cytokines and cytotoxicity against tumor cells by Newcastle disease virus. Cancer Biother. 1994;9:225–235. doi: 10.1089/cbr.1994.9.225. [DOI] [PubMed] [Google Scholar]
- 90.Washburn B, Weigand MA, Grosse-Wilde A, Janke M, Stahl H, Rieser E, et al. TNF-related apoptosis-inducing ligand mediates tumoricidal activity of human monocytes stimulated by Newcastle disease virus. J Immunol. 2003;170:1814–1821. doi: 10.4049/jimmunol.170.4.1814. [DOI] [PubMed] [Google Scholar]
- 91.Schirrmacher V, Bai L, Umansky V, Yu L, Xing Y, Qian Z. Newcastle disease virus activates macrophages for anti-tumor activity. Int J Oncol. 2000;16:363–373. [PubMed] [Google Scholar]
- 92.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jarahian M, Watzl C, Fournier P, Arnold A, Djandji D, Zahedi S, et al. Activation of natural killer cells by newcastle disease virus hemagglutinin-neuraminidase. J Virol. 2009;83:8108–8121. doi: 10.1128/JVI.00211-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arnon TI, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31:2680–2689. doi: 10.1002/1521-4141(200109)31:9<2680::aid-immu2680>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 95.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 96.Arnon TI, Achdout H, Lieberman N, Gazit R, Gonen-Gross T, Katz G, et al. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood. 2004;103:664–672. doi: 10.1182/blood-2003-05-1716. [DOI] [PubMed] [Google Scholar]
- 97.Chinnery F, King CA, Elliott T, Bateman AR, James E. Viral antigen mediated NKp46 activation of NK cells results in tumor rejection via NK-DC crosstalk. Oncoimmunology. 2012;1:874–883. doi: 10.4161/onci.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harada Y, Yonemitsu Y. Dramatic improvement of DC-based immunotherapy against various malignancies. Front Biosci (Landmark Ed) 2011;16:2233–2242. doi: 10.2741/3850. [DOI] [PubMed] [Google Scholar]
- 99.Okano S, Yonemitsu Y, Shirabe K, Kakeji Y, Maehara Y, Harada M, et al. Provision of continuous maturation signaling to dendritic cells by RIG-I-stimulating cytosolic RNA synthesis of Sendai virus. J Immunol. 2011;186:1828–1839. doi: 10.4049/jimmunol.0901641. [DOI] [PubMed] [Google Scholar]
- 100.Sugiyama M, Kakeji Y, Tsujitani S, Harada Y, Onimaru M, Yoshida K, et al. Antagonism of VEGF by genetically engineered dendritic cells is essential to induce antitumor immunity against malignant ascites. Mol Cancer Ther. 2011;10:540–549. doi: 10.1158/1535-7163.MCT-10-0479. [DOI] [PubMed] [Google Scholar]
- 101.Yoneyama Y, Ueda Y, Akutsu Y, Matsunaga A, Shimada H, Kato T, et al. Development of immunostimulatory virotherapy using non-transmissible Sendai virus-activated dendritic cells. Biochem Biophys Res Commun. 2007;355:129–135. doi: 10.1016/j.bbrc.2007.01.132. [DOI] [PubMed] [Google Scholar]
- 102.Yonemitsu Y, Ueda Y, Kinoh H, Hasegawa M. Immunostimulatory virotherapy using recombinant Sendai virus as a new cancer therapeutic regimen. Front Biosci. 2008;13:1892–1898. doi: 10.2741/2809. [DOI] [PubMed] [Google Scholar]
- 103.Komaru A, Ueda Y, Furuya A, Tanaka S, Yoshida K, Kato T, et al. Sustained and NK/CD4+ T cell-dependent efficient prevention of lung metastasis induced by dendritic cells harboring recombinant Sendai virus. J Immunol. 2009;183:4211–4219. doi: 10.4049/jimmunol.0803845. [DOI] [PubMed] [Google Scholar]
- 104.Kato T, Ueda Y, Kinoh H, Yoneyama Y, Matsunaga A, Komaru A, et al. RIG-I helicase-independent pathway in sendai virus-activated dendritic cells is critical for preventing lung metastasis of AT6.3 prostate cancer. Neoplasia. 2010;12:906–914. doi: 10.1593/neo.10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schirrmacher V, Haas C, Bonifer R, Ertel C. Virus potentiation of tumor vaccine T-cell stimulatory capacity requires cell surface binding but not infection. Clin Cancer Res. 1997;3:1135–1148. [PubMed] [Google Scholar]
- 106.Pearlstein E, Salk PL, Yogeeswaran G, Karpatkin S. Correlation between spontaneous metastatic potential, platelet-aggregating activity of cell surface extracts, and cell surface sialylation in 10 metastatic-variant derivatives of a rat renal sarcoma cell line. Proc Natl Acad Sci USA. 1980;77:4336–4339. doi: 10.1073/pnas.77.7.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yogeeswaran G, Salk PL. Metastatic potential is positively correlated with cell surface sialylation of cultured murine tumor cell lines. Science. 1981;212:1514–1516. doi: 10.1126/science.7233237. [DOI] [PubMed] [Google Scholar]
- 108.Narayanan S. Sialic acid as a tumor marker. Ann Clin Lab Sci. 1994;24:376–384. [PubMed] [Google Scholar]
- 109.Komminoth P, Roth J, Saremaslani P, Matias-Guiu X, Wolfe HJ, Heitz PU. Polysialic acid of the neural cell adhesion molecule in the human thyroid: a marker for medullary thyroid carcinoma and primary C-cell hyperplasia. An immunohistochemical study on 79 thyroid lesions. Am J Surg Pathol. 1994;18:399–411. doi: 10.1097/00000478-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 110.Sata T, Roth J, Zuber C, Stamm B, Heitz PU. Expression of alpha 2,6-linked sialic acid residues in neoplastic but not in normal human colonic mucosa. A lectin-gold cytochemical study with Sambucus nigra and Maackia amurensis lectins. Am J Pathol. 1991;139:1435–1448. [PMC free article] [PubMed] [Google Scholar]
- 111.Pousset D, Piller V, Bureaud N, Monsigny M, Piller F. Increased alpha2,6 sialylation of N-glycans in a transgenic mouse model of hepatocellular carcinoma. Cancer Res. 1997;57:4249–4256. [PubMed] [Google Scholar]
- 112.Fernández-Rodríguez J, Feijoo-Carnero C, Merino-Trigo A, Páez de la Cadena M, Rodríguez-Berrocal FJ, de Carlos A, et al. Immunohistochemical analysis of sialic acid and fucose composition in human colorectal adenocarcinoma. Tumour Biol. 2000;21:153–164. doi: 10.1159/000030122. [DOI] [PubMed] [Google Scholar]
- 113.Lu DY, Lu TR, Wu HY. Development of antimetastatic drugs by targeting tumor sialic acids. Sci Pharm. 2012;80:497–508. doi: 10.3797/scipharm.1205-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vierbuchen M, Schröder S, Larena A, Uhlenbruck G, Fischer R. Native and sialic acid masked Lewis(a) antigen reactivity in medullary thyroid carcinoma. Distinct tumour-associated and prognostic relevant antigens. Virchows Arch. 1994;424:205–211. doi: 10.1007/BF00193501. [DOI] [PubMed] [Google Scholar]
- 115.Cohen M, Elkabets M, Perlmutter M, Porgador A, Voronov E, Apte RN, et al. Sialylation of 3-methylcholanthrene-induced fibrosarcoma determines antitumor immune responses during immunoediting. J Immunol. 2010;185:5869–5878. doi: 10.4049/jimmunol.1001635. [DOI] [PubMed] [Google Scholar]
- 116.Kingsbury DW. The Paramyxoviruses. New York: Plenum Press; 1991. [Google Scholar]
- 117.Enders G. Paramyxoviruses. Galveston: University of Texas Medical Branch at Galveston; 1996. [PubMed] [Google Scholar]
- 118.Powell LD, Whiteheart SW, Hart GW. Cell surface sialic acid influences tumor cell recognition in the mixed lymphocyte reaction. J Immunol. 1987;139:262–270. [PubMed] [Google Scholar]
- 119.Miest TS, Cattaneo R. New viruses for cancer therapy: meeting clinical needs. Nat Rev Microbiol. 2014;12:23–34. doi: 10.1038/nrmicro3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pond AR, Manuelidis EE. Oncolytic effect of poliomyelitis virus on human epidermoid carcinoma (hela tumor) heterologously transplanted to guinea pigs. Am J Pathol. 1964;45:233–249. [PMC free article] [PubMed] [Google Scholar]
- 121.Tsypkin LB, Voroshilova MK, Goryunova AG, Lavrova IK, Koroleva GA. The morphology of tumors of the human gastrointestinal tract in short-term organ culture and the reaction of these tumors to infection with poliovirus. Cancer. 1976;38:1796–1806. doi: 10.1002/1097-0142(197610)38:4<1796::aid-cncr2820380457>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 122.Toyoda H, Wimmer E, Cello J. Oncolytic poliovirus therapy and immunization with poliovirus-infected cell lysate induces potent antitumor immunity against neuroblastoma in vivo. Int J Oncol. 2011;38:81–87. [PubMed] [Google Scholar]
- 123.Chumakov PM, Morozova VV, Babkin IV, Baikov IK, Netesov SV, Tikunova NV. [Oncolytic enteroviruses] Mol Biol (Mosk) 2012;46:712–725. [PubMed] [Google Scholar]
- 124.Twigger K, Roulstone V, Kyula J, Karapanagiotou EM, Syrigos KN, Morgan R, et al. Reovirus exerts potent oncolytic effects in head and neck cancer cell lines that are independent of signalling in the EGFR pathway. BMC Cancer. 2012;12:368. doi: 10.1186/1471-2407-12-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rommelaere J, Geletneky K, Angelova AL, Daeffler L, Dinsart C, Kiprianova I, et al. Oncolytic parvoviruses as cancer therapeutics. Cytokine Growth Factor Rev. 2010;21:185–195. doi: 10.1016/j.cytogfr.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 126.Kirn DH, McCormick F. Replicating viruses as selective cancer therapeutics. Mol Med Today. 1996;2:519–527. doi: 10.1016/s1357-4310(97)81456-6. [DOI] [PubMed] [Google Scholar]
- 127.Sviatchenko VA, Tarasova MV, Netesov SV, Chumakov PM. [Oncolytic adenoviruses in anti-cancer therapy: current status and perspectives] Mol Biol (Mosk) 2012;46:556–569. [PubMed] [Google Scholar]
- 128.Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 129.Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17:516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- 130.Nakamura T, Russell SJ. Oncolytic measles viruses for cancer therapy. Expert Opin Biol Ther. 2004;4:1685–1692. doi: 10.1517/14712598.4.10.1685. [DOI] [PubMed] [Google Scholar]
- 131.Kirn D, Caroline B. Oncolytic vaccinia virus cancer therapy. 20100303714 A1 and 8747837 B2. US patents. 2008
- 132.Kochneva GV, Sivolobova GF, Yudina KV, Babkin IV, Chumakov PM, Netesov SV. Oncolytic poxviruses. Mol Genet Microbiol Virol. 2012;27:8–15. [PubMed] [Google Scholar]
- 133.Bian H, Fournier P, Moormann R, Peeters B, Schirrmacher V. Selective gene transfer to tumor cells by recombinant Newcastle Disease Virus via a bispecific fusion protein. Int J Oncol. 2005;26:431–439. [PubMed] [Google Scholar]
- 134.Msaouel P, Iankov ID, Allen C, Russell SJ, Galanis E. Oncolytic measles virus retargeting by ligand display. Methods Mol Biol. 2012;797:141–162. doi: 10.1007/978-1-61779-340-0_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nussbaum O, Broder CC, Moss B, Stern LB, Rozenblatt S, Berger EA. Functional and structural interactions between measles virus hemagglutinin and CD46. J Virol. 1995;69:3341–3349. doi: 10.1128/jvi.69.6.3341-3349.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Allen C, Vongpunsawad S, Nakamura T, James CD, Schroeder M, Cattaneo R, et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006;66:11840–11850. doi: 10.1158/0008-5472.CAN-06-1200. [DOI] [PubMed] [Google Scholar]
- 137.Nakamura T, Peng KW, Harvey M, Greiner S, Lorimer IA, James CD, et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat Biotechnol. 2005;23:209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- 138.Paraskevakou G, Allen C, Nakamura T, Zollman P, James CD, Peng KW, et al. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol Ther. 2007;15:677–686. doi: 10.1038/sj.mt.6300105. [DOI] [PubMed] [Google Scholar]
- 139.Hasegawa K, Hu C, Nakamura T, Marks JD, Russell SJ, Peng KW. Affinity thresholds for membrane fusion triggering by viral glycoproteins. J Virol. 2007;81:13149–13157. doi: 10.1128/JVI.01415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hasegawa K, Nakamura T, Harvey M, Ikeda Y, Oberg A, Figini M, et al. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6170–6178. doi: 10.1158/1078-0432.CCR-06-0992. [DOI] [PubMed] [Google Scholar]
- 141.Ungerechts G, Springfeld C, Frenzke ME, Lampe J, Johnston PB, Parker WB, et al. Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine. Cancer Res. 2007;67:10939–10947. doi: 10.1158/0008-5472.CAN-07-1252. [DOI] [PubMed] [Google Scholar]
- 142.Hallak LK, Merchan JR, Storgard CM, Loftus JC, Russell SJ. Targeted measles virus vector displaying echistatin infects endothelial cells via alpha(v)beta3 and leads to tumor regression. Cancer Res. 2005;65:5292–5300. doi: 10.1158/0008-5472.CAN-04-2879. [DOI] [PubMed] [Google Scholar]
- 143.Allen C, Paraskevakou G, Iankov I, Giannini C, Schroeder M, Sarkaria J, et al. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol Ther. 2008;16:1556–1564. doi: 10.1038/mt.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Peeters BP, de Leeuw OS, Koch G, Gielkens AL. Rescue of Newcastle disease virus from cloned cDNA: evidence that cleavability of the fusion protein is a major determinant for virulence. J Virol. 1999;73:5001–5009. doi: 10.1128/jvi.73.6.5001-5009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dortmans JC, Koch G, Rottier PJ, Peeters BP. Virulence of Newcastle disease virus: what is known so far? Vet Res. 2011;42:122. doi: 10.1186/1297-9716-42-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zamarin D, Palese P. Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Future Microbiol. 2012;7:347–367. doi: 10.2217/fmb.12.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vigil A, Park MS, Martinez O, Chua MA, Xiao S, Cros JF, et al. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 2007;67:8285–8292. doi: 10.1158/0008-5472.CAN-07-1025. [DOI] [PubMed] [Google Scholar]
- 148.Zamarin D, Martínez-Sobrido L, Kelly K, Mansour M, Sheng G, Vigil A, et al. Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses. Mol Ther. 2009;17:697–706. doi: 10.1038/mt.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zamarin D, Vigil A, Kelly K, García-Sastre A, Fong Y. Genetically engineered Newcastle disease virus for malignant melanoma therapy. Gene Ther. 2009;16:796–804. doi: 10.1038/gt.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Song KY, Wong J, Gonzalez L, Sheng G, Zamarin D, Fong Y. Antitumor efficacy of viral therapy using genetically engineered Newcastle disease virus [NDV(F3aa)-GFP] for peritoneally disseminated gastric cancer. J Mol Med. 2010;88:589–596. doi: 10.1007/s00109-010-0605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Silberhumer GR, Brader P, Wong J, Serganova IS, Gönen M, Gonzalez SJ, et al. Genetically engineered oncolytic Newcastle disease virus effectively induces sustained remission of malignant pleural mesothelioma. Mol Cancer Ther. 2010;9:2761–2769. doi: 10.1158/1535-7163.MCT-10-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li P, Chen CH, Li S, Givi B, Yu Z, Zamarin D, et al. Therapeutic effects of a fusogenic newcastle disease virus in treating head and neck cancer. Head Neck. 2011;33:1394–1399. doi: 10.1002/hed.21609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Elankumaran S, Rockemann D, Samal SK. Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death. J Virol. 2006;80:7522–7534. doi: 10.1128/JVI.00241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kinoh H, Inoue M. New cancer therapy using genetically-engineered oncolytic Sendai virus vector. Front Biosci. 2008;13:2327–2334. doi: 10.2741/2847. [DOI] [PubMed] [Google Scholar]
- 155.Kinoh H, Inoue M, Komaru A, Ueda Y, Hasegawa M, Yonemitsu Y. Generation of optimized and urokinase-targeted oncolytic Sendai virus vectors applicable for various human malignancies. Gene Ther. 2009;16:392–403. doi: 10.1038/gt.2008.167. [DOI] [PubMed] [Google Scholar]
- 156.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. IMPACTStudy Investigators Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 157.Surowiak P, Materna V, Maciejczyk A, Kaplenko I, Spaczynski M, Dietel M, et al. CD46 expression is indicative of shorter revival-free survival for ovarian cancer patients. Anticancer Res. 2006;26(6C):4943–4948. [PubMed] [Google Scholar]
- 158.Maciejczyk A, Szelachowska J, Szynglarewicz B, Szulc R, Szulc A, Wysocka T, et al. CD46 Expression is an unfavorable prognostic factor in breast cancer cases. Appl Immunohistochem Mol Morphol. 2011;19:540–546. doi: 10.1097/PAI.0b013e31821a0be9. [DOI] [PubMed] [Google Scholar]
- 159.Noyce RS, Richardson CD. Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol. 2012;19:19. doi: 10.1016/j.tim.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 160.Fabre-Lafay S, Garrido-Urbani S, Reymond N, Gonçalves A, Dubreuil P, Lopez M. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-alpha- converting enzyme (TACE)/ADAM-17. J Biol Chem. 2005;280:19543–19550. doi: 10.1074/jbc.M410943200. [DOI] [PubMed] [Google Scholar]