Abstract
BACKGROUND
In October 2007, manufacturers voluntarily withdrew over-the-counter (OTC) infant cough and cold medications (CCMs) from the US market. A year later, manufacturers announced OTC CCM labeling would be revised to warn against OTC CCM use by children aged <4 years. We determined whether emergency department (ED) visits for CCM adverse drug events (ADEs) declined after these interventions.
METHODS
We used National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance data from 2004 to 2011 to estimate the number of ED visits for CCM ADEs before and after each intervention.
RESULTS
Among children aged <2 years, ED visits for CCM ADEs decreased from 4.1% of all ADE ED visits before the market withdrawal to 2.4% of all ADE visits afterward (difference in proportion: –1.7%, 95% confidence interval [CI]: –2.7% to –0.6%). Among children aged 2 to 3 years, ED visits for CCM ADEs decreased from 9.5% of all ADE ED visits before the labeling revision announcement to 6.5% of all ADE visits afterward (difference in proportion: –3.0%, 95% CI: –5.4% to –0.6%). Unsupervised ingestions accounted for 64.3% (95% CI: 51.1% to 77.5%) of CCM ADE ED visits involving children aged <2 years after the withdrawal and 88.8% (95% CI: 83.8% to 93.8%) of visits involving children aged 2 to 3 years after the labeling revision announcement.
CONCLUSIONS
After a voluntary market withdrawal and labeling revision, ED visits for CCM ADEs declined among children aged <2 years and 2 to 3 years relative to ADE ED visits for all drugs. Interventions addressing unsupervised ingestions are needed to reduce CCM ADEs.
Keywords: adverse events, drug safety, poisoning, medication errors, drug packaging, nasal decongestants, expectorants, antitussive agents, product withdrawals, nonprescription drugs
In October 2007, manufacturers voluntarily withdrew oral over-the-counter (OTC) infant cough and cold medications (CCMs) intended for use by children <2 years old amid considerable publicity.1–4 Studies had not demonstrated CCMs to be more effective than placebo in young children but had linked CCMs to significant numbers of emergency department (ED) visits and, in rare cases, infant deaths.5–8 The Food and Drug Administration (FDA) formally supported this withdrawal in January 2008.9 The next year, ED visits and calls to poison centers due to CCM adverse drug events (ADEs) involving young children declined.1,10
In October 2008, manufacturers of OTC CCMs announced, with FDA support, that OTC CCM labels would be revised to state that the medications should not be used in children <4 years old.11,12 Media attention and ongoing educational efforts by the FDA,13,14 professional organizations such as the American Academy of Pediatrics,15 and trade groups followed.16 Decreases in calls to poison centers for CCM ADEs involving young children were recorded in Texas the following year,17 in Texas and 4 other states through 2010,18 and nationally through 2010.19 However, national household surveys and surveys of caregivers of children treated in EDs indicate that many caregivers still give CCMs to young children.20–23
We used nationally representative data to assess whether the 2007 market withdrawal and the 2008 labeling revision, along with accompanying publicity and education efforts, were associated with persistent, national effects on ED visits for CCM ADEs among children.
METHODS
National estimates of ED visits for ADEs were based on data from the 58 general and 5 pediatric specialty hospitals that participate in the National Electronic Injury Surveillance System-Cooperative ADE Surveillance (NEISS–CADES) project, a nationally representative probability sample of hospitals with a minimum of 6 beds and a 24-hour ED in the United States and its territories. The NEISS-CADES project is a collaboration of the Centers for Disease Control and Prevention, the FDA, and the US Consumer Product Safety Commission and has been described in detail previously.24,25 In brief, trained abstractors review clinical diagnoses and supporting information in the medical records of each ED visit to identify ADEs diagnosed by treating clinicians. Abstractors report up to 2 medications implicated in each ADE, concomitant medications listed in the medical record, and narrative details of the event. ADE details, including manifestations and physician diagnoses, are classified using the Medical Dictionary for Regulatory Activities (MedDRA, version 9.1).
For this analysis, a case was defined as any ED visit from January 1, 2004, through December 31, 2011, by a patient <12 years of age for a problem that was attributed to use of a drug or a drug-specific adverse effect and that did not result in the patient’s death either in the ED or before arrival. Drugs included prescription and OTC medications, vaccines, and herbal remedies/dietary supplements. CCMs included orally administered prescription or OTC products containing decongestants, antitussive agents, and/or expectorants alone or in combination with each other and/or with analgesics or antihistamines. As part of an assessment of possible unintended increases in ADEs from non-CCM drugs after the market withdrawal and labeling revision announcement, nasal/ophthalmic decongestants, topical analgesics, herbal or alternative medicines, nonprescription analgesics, and antihistamines were considered to be drugs potentially substitutable for CCMs by caregivers seeking to treat respiratory symptoms. Cases were classified as “unsupervised ingestions” when children accessed medications without adult permission or oversight and “supervised administrations” when caregivers gave medications to children. ED visits for intentional self-harm, drug abuse, therapeutic failures, and drug withdrawal were excluded.
For children <2 years old, the 2007 market withdrawal’s targets, ED visits were considered to have occurred before the withdrawal if they happened before October 1, 2007, and to have occurred after the withdrawal if they occurred on or after October 1, 2007. ED visits involving children 2 to 11 years old were deemed to have occurred before the labeling revision announcement if they occurred before October 1, 2008, and to have occurred after the revision announcement if they occurred on or after October 1, 2008. We assessed ED visits involving children 2 to 11 years old before and after October 1, 2008, because previous studies found that the 2007 withdrawal of infant products had not significantly affected CCM ADEs among children $2 years old.1,10
Each NEISS-CADES case is assigned a sample weight based on the inverse probability of selection. The sample weights are modified for nonresponse rate and poststratified to adjust for the number of annual hospital ED visits. The national estimates of ED visits and their corresponding 95% confidence intervals (CIs) were calculated with the SURVEYMEANS procedure in SAS software, version 9.2 (SAS Institute, Cary, NC), to account for the sample weights and complex sample design. To obtain average annual estimates, NEISS-CADES estimates for each pre- and postintervention period were divided by the number of months in the period and then multiplied by 12. To compare ED visits for CCM ADEs in the pre- and postintervention periods, we calculated differences in the proportions of all estimated ADE ED visits that were due to CCMs and the corresponding 95% CIs. We compared differences in the proportions of ADEs that were due to CCMs to account for any overall increases or decreases in pediatric ADE ED visits. To compare the proportions of ED visits for CCM ADEs that had particular characteristics in the pre- and postintervention periods, we calculated differences in the proportions of ED visits for CCM ADEs with those characteristics and the corresponding 95% CIs. Estimated proportions for the respective periods were treated as statistically independent because the pre- and postintervention periods did not overlap. Estimates <1200 before annualization, based on <20 cases, or having a coefficient of variation >30% were considered to be statistically unreliable and are noted as such.24
To further evaluate changes in ED visits for CCM ADEs before and after the interventions, we used interrupted time series analysis.26 Trends in the quarterly (3-month) proportions of unweighted ADE ED cases that were due to CCMs at hospitals participating continuously in NEISS-CADES from 2004 through 2011 were assessed using log binomial regression models. The base models included variables reflecting the interventions’ immediate effects (ie, change in proportions immediately after the interventions) and slope parameters for changes over time before and after the intervention. We then used likelihood ratio statistics to assess the incremental statistical improvement from including the slope parameters for changes over time in the models. We conducted a sensitivity analysis assessing the effect of seasonal variation in CCM ADEs in which the period October 1 through March 31 was designated as likely having increased cough and cold ADE incidence.10 The interrupted time series analyses were performed using the SAS GENMOD procedure. A P value <.05 was considered statistically significant.
RESULTS
National Estimates
Between 2004 and 2011, an estimated 61 168 ED visits (95% CI: 47 139–75 196) occurred due to CCM ADEs involving children <12 years old. CCMs were the only medications implicated in an estimated 93.4% (95% CI: 91.6%–95.3%) of visits, and OTC CCMs accounted for more than three-fourths (76.6%) of estimated visits (Table 1). In an estimated 60.4% (95% CI: 54.3%–66.5%) of ED visits no ADE symptoms were documented. Most patients (89.7%, 95% CI: 84.5%–95.0%) did not require admission, observation, or transfer to another hospital. However, in an estimated 16.2% of ED visits, gastric decontamination was documented to have been given.
TABLE 1.
Numbers of Cases and National Estimates of Adverse Events From CCMs Among Children Aged <12 Years Treated in EDs According to Case Characteristics: United States, January 1, 2004 Through December 31, 2011
| Case Characteristics | Cases, n | National Estimate of ED Visits | ||
|---|---|---|---|---|
| n | % | 95% CI | ||
| Patient age, y | ||||
| <2 | 295 | 14 516 | 23.7 | 19.3–28.2 |
| 2–3 | 645 | 30 747 | 50.3 | 45.7–54.8 |
| 4–5 | 179 | 8766 | 14.3 | 11.6–17.1 |
| 6–11 | 151 | 7138 | 11.7 | 8.9–14.5 |
| Patient gender | ||||
| Female | 597 | 29 613 | 48.4 | 43.9–52.9 |
| Male | 673 | 31 555 | 51.6 | 47.1–56.1 |
| Medication marketing category | ||||
| OTC | 1008 | 46 875 | 76.6 | 72.1–81.2 |
| Prescription | 168 | 9622 | 15.7 | 11.2–20.3 |
| Unknown | 94 | 4671 | 7.6 | 5.2–10.1 |
| ED treatment and disposition | ||||
| Gastric decontamination | 224 | 9929 | 16.2 | 12.0–20.5 |
| Treated and released or left against medical advice | 1048 | 54 872 | 89.7 | 84.5–95.0 |
| Adverse event manifestation | ||||
| No symptoms documented | 742 | 36 973 | 60.4 | 54.3–66.5 |
| Allergic reaction | 236 | 13 011 | 21.3 | 16.2–26.4 |
| Neurologic | 195 | 7331 | 12.0 | 8.2–15.8 |
| Gastrointestinal | 79 | 2538 | 4.1 | 2.6–5.7 |
| Behavioral | 56 | 1948 | 3.2 | 1.6–4.8 |
| Total | 1270 | 61 168 | 100 | |
Case counts and estimates from National Electronic Injury Surveillance System–Cooperative Adverse Drug Event Surveillance, Centers for Disease Control and Prevention. CCMs include orally administered prescription or OTC products containing decongestants, antitussive agents, and/or expectorants alone or in combination with each other and/or with analgesics or antihistamines.
Comparing the period before the 2007 market withdrawal to the period after the withdrawal, there were no statistically significant differences in the proportions of ED visits for CCM ADEs by patient gender, medication marketing category, patient disposition, or the documentation of gastric decontamination among children <2 years old. Comparing the period before the 2008 labeling revision announcement to the period afterward, among children 2 to 3 years old, the proportion of ED visits for CCM ADEs in which gastric decontamination was documented declined from 26.0% to 14.1% (difference in proportions: –11.9%, 95% CI: –21.7% to –2.1%). Among children aged 2 to 3, 4 to 5, and 6 to 11 years, there were no statistically significant differences in the proportions of ED visits for CCM ADEs by patient gender, medication marketing category, or patient disposition before and after the labeling revision announcement.
Subsequent to the 2007 withdrawal of OTC infant CCM products, among children aged <2 years, the proportion of ED visits for all ADEs attributed to CCMs decreased by 41%, from 4.1% of all ADE visits to 2.4% (difference in proportions: –1.7%, 95% CI: –2.7% to –0.6%; Table 2). Considering only ED visits resulting from the supervised administration of medications, the proportion of ED visits that involved CCMs declined by 55%, from 3.3% to 1.5% (difference in proportions: –1.8%, 95% CI: –2.9% to –0.6%). Among ED visits for unsupervised ingestions of medications, the proportion of all ADE ED visits that involved CCMs did not decrease significantly. Unsupervised ingestions accounted for 64.3% (95% CI: 51.1%–77.5%) of ED visits for CCM ADEs involving children <2 years old after the withdrawal.
TABLE 2.
ED Visits for ADEs Among Children Aged <2 Years Treated Before and After OTC Infant CCM Market Withdrawal: United States, January 1, 2004–December 31, 2011
| Exposure Typea | Estimated Average Annual ED Visits Before Market Withdrawal |
Estimated Average Annual ED Visits After Market Withdrawal |
Difference in Proportions |
|||||
|---|---|---|---|---|---|---|---|---|
| No. of CCM RV | No. of All Medication RV |
Proportion of CCM-RV Among All Medication RV, % |
No. of CCM RV | No. of All Medication RV |
Proportion of CCM-RV Among All Medication RV, % |
% | 95% CI | |
| Total | 2138 | 52 543 | 4.1 | 1529 | 63 517 | 2.4 | −1.7 | −2.7 to –0.6 |
| Supervised administrations | 1031 | 31 318 | 3.3 | 546 | 36 513 | 1.5 | −1.8 | −2.9 to –0.6 |
| Unsupervised ingestions | 1107 | 21 226 | 5.2 | 984 | 27 005 | 3.6 | −1.6 | −3.4 to 0.3 |
Estimates from National Electronic Injury Surveillance System–Cooperative Adverse Drug Event Surveillance, Centers for Disease Control and Prevention. CCMs include orally administered prescription or OTC products containing decongestants, antitussive agents, and/or expectorants alone or in combination with each other and/or with analgesics or antihistamines. Market withdrawal of OTC CCMs labeled for infants was announced in October 2007. The period before withdrawal refers to the period beginning January 1, 2004, and ending September 30, 2007. The period after withdrawal refers to the period beginning October 1, 2007, and ending December 31, 2011. RV, related visits.
Exposure type was classified as supervised administrations when caregivers gave medications to children. Exposure type was classified as unsupervised ingestions when children accessed medications without adult permission or oversight.
Similarly, among children aged 2 to 3 years, the proportion of ADE ED visits attributed to CCMs decreased by 32% from 9.5% of all ADE visits to 6.5% (difference in proportions: –3.0%, 95% CI: –5.4% to –0.6%) after the 2008 OTC CCM labeling revision announcement (Table 3). Among ED visits resulting from the supervised administration of any medication, the proportion of ED visits that involved CCMs declined 54% from 5.4% to 2.5% (difference in proportions: –2.9%, 95% CI: –5.4% to –0.5%). Considering only ED visits for unsupervised ingestions of any medication, the proportion due to CCMs declined 24%, from 10.8% to 8.2% (difference in proportions: –2.6%, 95% CI: –5.3% to –0.01%). Unsupervised ingestions accounted for 88.8% (95% CI: 83.8% to 93.8%) of ED visits for CCM ADEs involving children aged 2 to 3 years after the revision announcement.
TABLE 3.
ED Visits for ADEs Among Children Aged 2 to 11 Years Treated Before and After OTC Infant CCM Labeling Revision Announcement: United States, January 1, 2004–December 31, 2011
| Age Group (y) and Exposure Typea |
Estimated Average Annual ED Visits Before Labeling Revision Announcement |
Estimated Average Annual ED Visits After Labeling Revision Announcement |
Difference in Proportions |
|||||
|---|---|---|---|---|---|---|---|---|
| No. of CCM RV | No. of All Medication RV |
Proportion of CCM-RV Among All Medication RV, % |
No. of CCM RV | No. of All Medication RV |
Proportion of CCM-RV Among All Medication RV, % |
% | 95% CI | |
| Children aged 2–3 | ||||||||
| Total | 4092 | 43 100 | 9.5 | 3480 | 53 447 | 6.5 | −3.0 | −5.4 to –0.6 |
| Supervised administrations | 577 | 10 618 | 5.4 | 390 | 15 634 | 2.5 | −2.9 | −5.4 to –0.5 |
| Unsupervised ingestions | 3515 | 32 482 | 10.8 | 3090 | 37 813 | 8.2 | −2.6 | −5.3 to -0.01 |
| Children aged 4–5 | ||||||||
| Total | 852 | 15 128 | 5.6 | 1453 | 22 338 | 6.5 | 0.9 | −1.7 to 3.4 |
| Supervised administrations | 485 | 10 354 | 4.7 | 762 | 16 059 | 4.7 | 0.1 | −2.8 to 2.9 |
| Unsupervised ingestions | 366 | 4774 | 7.7 | 691 | 6279 | 11.0 | 3.3 | −1.9 to 8.6 |
| Children aged 6–11 | ||||||||
| Total | 875 | 22 152 | 3.9 | 917 | 30 410 | 3.0 | −0.9 | −2.5 to 0.6 |
| Supervised administrations | 765 | 19 198 | 4.0 | 758 | 27 095 | 2.8 | −1.2 | −2.8 to 0.4 |
| Unsupervised ingestions | — | 2954 | — | — | 3315 | — | — | — |
Estimates from National Electronic Injury Surveillance System–Cooperative Adverse Drug Event Surveillance, Centers for Disease Control and Prevention. CCMs include orally administered prescription or OTC products containing decongestants, antitussive agents, and/or expectorants alone or in combination with each other and/or with analgesics or antihistamines. OTC CCM labeling revision was announced in October 2008. The period before withdrawal refers to the period beginning January 1, 2004, and ending September 30, 2008. The period after withdrawal refers to the period beginning October 1, 2008, and ending December 31, 2011. Estimates based on <20 cases are not shown. RV, related visits.
Exposure type was classified as supervised administrations when caregivers gave medications to children. Exposure type was classified as unsupervised ingestions when children accessed medications without adult permission or oversight.
In contrast to the younger age groups, the proportion of ADE ED visits attributed to CCMs did not change significantly after the CCM labeling revision announcement among children aged 4 to 5 or 6 to 11 years (Table 3). Unsupervised ingestions accounted for 47.6% (95% CI: 31.1%–64.0%) of ED visits for CCM ADEs involving children aged 4 to 5 years after the revision announcement. The estimates of ED visits for CCM unsupervised ingestions among children aged 6 to 11 years before and after the revision announcement were too small to be statistically stable.
After the 2007 market withdrawal, the proportion of supervised administration ADE ED visits attributable to potentially substitutable medications did not change significantly among children aged <2 years, increasing from 4.8% to 5.0% (difference in proportions: 0.2%, 95% CI: −1.7% to 2.2%). The proportion of supervised administration ADE ED visits attributable to potentially substitutable medications also did not change significantly among children aged 2 to 3 years (difference in proportions: −2.4%, 95% CI: −5.7% to 1.0%) after the 2008 labeling revision announcement.
Case-Based Interrupted Time Series
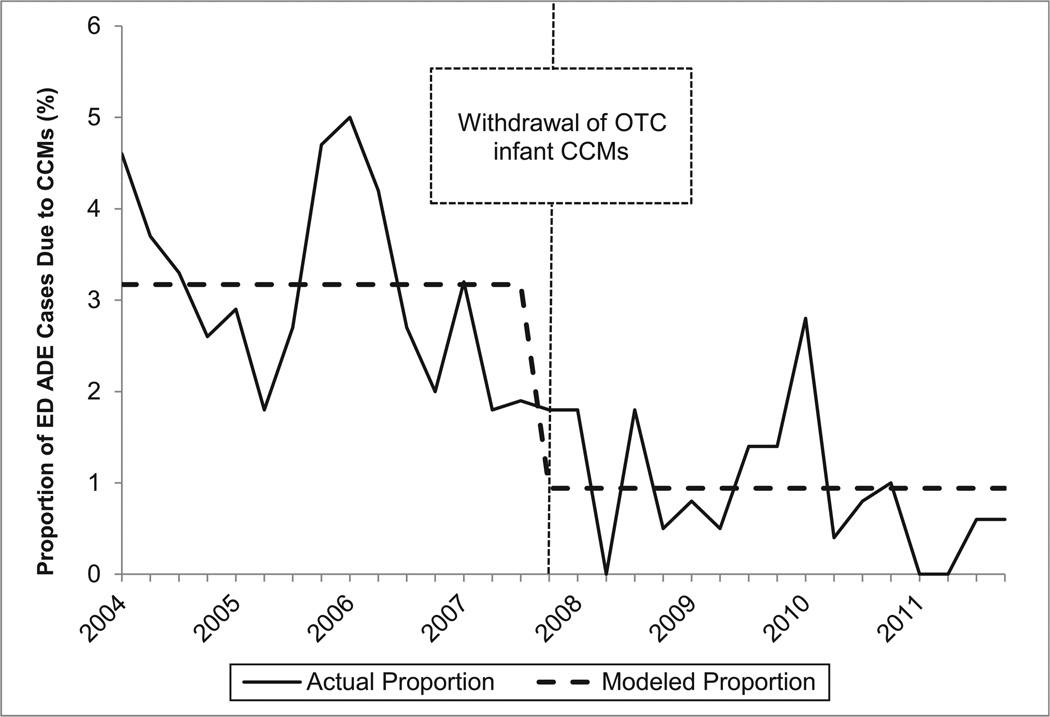
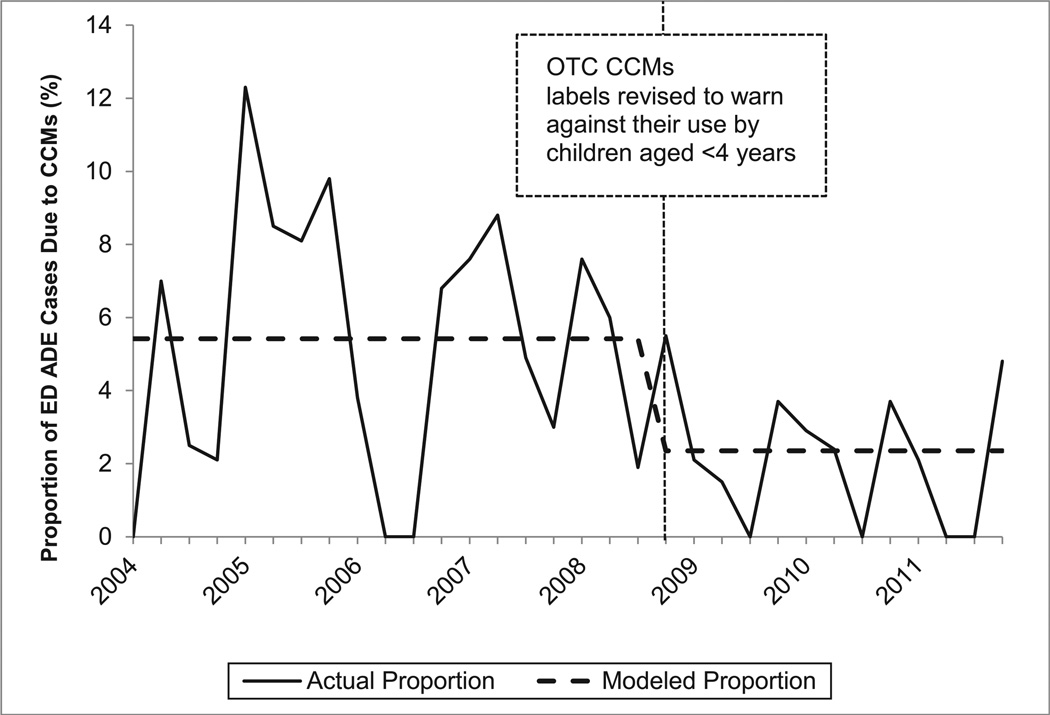
In the interrupted time series models, only the variables reflecting the immediate effects of the interventions were found to have statistically significant effects on the quarterly proportions of unweighted ADE ED cases involving children aged <2 or 2 to 3 years at NEISS-CADES hospitals that were due to CCMs. The slope parameters for changes over time before and after the interventions were not incrementally statistically significant and were therefore removed from the models. Among children aged <2 years, the proportion of unweighted supervised administration ADE ED cases that was due to CCMs declined 70.3% (95% CI: 55.0%–80.4%; Fig 1); and the proportion of unweighted unsupervised ingestion ED cases that was due to CCMs declined 36.2% (95% CI: 14.3%–52.5%). Among children aged 2 to 3 years, the proportion of unweighted supervised administration ADE ED cases that was due to CCMs declined 56.7% (95% CI: 31.1%–72.7%; Fig 2); and the proportion of unweighted unsupervised ingestion ED cases that was due to CCMs declined 31.2% (95% CI: 18.2%–42.2%). CCMs were found to account for significantly larger proportions of all unweighted ADE ED cases between October and March than between April and September in all models, but this seasonal variation did not significantly change the interventions’ estimated effects.
FIGURE 1.
Quarterly proportion of ED supervised administration ADE unweighted cases due to CCMs among children aged <2 years, NEISS-CADES continuously participating hospitals, 2004–2011. CCMs include oral prescription or OTC products containing decongestants, antitussive agents, and/or expectorants alone or in combination with each other and/or with analgesics or antihistamines. Modeled quarterly (3-month) proportions of ED ADE cases due to CCMs are based on interrupted time series binomial regression of immediate effects of the 2007 product withdrawal.
FIGURE 2.
Quarterly proportion of ED supervised administration ADEs unweighted cases due to CCMs among children aged 2 to 3 years, NEISS-CADES continuously participating hospitals, 2004–2011. CCMs include oral prescription or OTC products containing decongestants, antitussive agents, and/or expectorants alone or in combination with each other and/or with analgesics or antihistamines. Modeled quarterly (3-month) proportions of ED ADE cases due to CCMs are based on interrupted time series binomial regression of immediate effects of the 2008 labeling revision announcement.
DISCUSSION
After the 2007 OTC infant CCM withdrawal, the 2008 OTC CCM labeling revision announcement, and accompanying publicity and education efforts, ED visits for CCM ADEs declined for children aged <2 and 2 to 3 years relative to ADE visits due to other drugs. Case-based interrupted time series analyses suggest these declines were maintained over the 3 to 4 years that followed the interventions. After the market withdrawal and labeling revision announcement, unsupervised ingestions were implicated in nearly two-thirds of ED visits for CCM ADEs among children aged <2 years and nearly 90% among children aged 2 to 3 years.
The market withdrawal and labeling revision were directed primarily at preventing ADEs from the intentional administration of CCMs to children by caregivers. Although ED visits to treat ADEs from supervised administration of CCMs have not been eliminated, they have declined as a proportion of ED visits for ADEs from the supervised administration of all medications by 55% among children aged <2 years and 54% among children aged 2 to 3 years. However, in a recent survey many parents of children aged 0 to 3 years (25%–44%) reported that they had given their child a CCM the last time the child had upper respiratory tract infection symptoms.21 Efforts to educate parents and medical providers on CCMs’ risks for children aged <4 years by the FDA,14 CCM manufacturers,16 and professional organizations, such as the American Academy of Pediatrics’ contribution to the Choosing Wisely campaign,27 may help to reduce the number of ADEs from supervised administration of CCMs, but additional interventions are needed to further decrease CCM ADEs among children.
Reducing CCM ADEs among young children requires prevention of unsupervised ingestions. National estimates of ED visits for unsupervised ingestions of CCMs by children <2 years old did not decline after infant products were withdrawn, and unsupervised ingestions now account for most ED visits for CCM ADEs among children aged both <2 years and 2 to 3 years. Improved packaging designed to limit young children’s access to medications holds potential for reducing unsupervised ingestions.28 For example, adding flow restrictors to bottles can reduce young children’s ability to access liquid medications.29 Unsupervised ingestions could also be reduced through safer medication handling and storage practices as promoted by the American Association of Poison Control Centers,30 Safe Kids Worldwide,31 and the Up and Away and Out of Sight campaign.32 FDA efforts to remove from the market prescription CCMs that lack adequate evidence of safety and efficacy may also reduce CCM ADEs.33
Both the withdrawal of OTC infant CCMs and the labeling revision warning against use of OTC CCMs by children <4 years old could have caused unintended consequences. Each intervention had the potential to increase ADEs from other medications (eg, antihistamines or nonnarcotic analgesics) that could be substituted for OTC CCMs. In this analysis, neither intervention was associated with a significant increase in ED visits for ADEs from supervised administrations of drugs potentially substitutable for CCMs. In addition, calls to poison centers regarding prescription CCM therapeutic errors involving children <2 years old, which could have been substituted for OTC CCMs, declined through 2009.34
The fact that relative declines in ED visits for CCMADEs occurred after the market withdrawal and the labeling revision announcement does not prove causal relationships between the interventions and declines. Nevertheless, several pieces of evidence suggest that these interventions exerted important effects. First, in contrast to children aged <2 or 2 to 3 years, there were no significant relative declines in ED visits from CCM ADEs among children aged 4 to 5 or 6 to 11 years, suggesting that the declines among younger children resulted from the targeted interventions and not broader secular trends. Second, in the interrupted time series modeling of changes in CCM ADEs over time, all slope parameters that were independent of the interventions had nonsignificant effects. Finally, although limited to voluntary reports and covering different time periods, 5 studies based on poison center calls have found that CCM ADEs began declining only after the withdrawal of OTC infant CCMs.10,17–19,34
These findings are subject to several limitations. First, the data do not include ADE cases if the affected individual was not brought to the ED. However, in general, ED-based data may best capture outpatient ADE cases involving concern for serious harm. Second, NEISS-CADES data collection relieson ED physicians’ assessments and documentation and is therefore more likely to identify well-recognized ADEs than ADEs that are rare, previously unknown, or difficult to diagnose in EDs.24,25 Nevertheless, it is unlikely that lack of recognition caused the observed declines in CCM ADE ED visits because awareness of CCMs’ risks has probably increased since 2007. Third, the interrupted time series analysis reflects only changes in unweighted cases at sampled hospitals and without weighting should not necessarily be assumed to represent national trends. Fourth, the pre- and postintervention analyses’ results could have been affected by a seasonal imbalance. The months from January through September accounted for a slightly larger proportion of the period before the interventions than the period after the intervention, and the months from October through December accounted for a larger proportion of the periods after the interventions. However, the interrupted time series analysis suggests that the seasonal variations’ influence on the interventions’ effects were minor. In addition, the reduction in the estimated average annual number of ED visits for CCM ADEs after the interventions despite the 2009 H1N1 influenza pandemic during the postintervention period provides reassurance that the declines were not due to a lower average incidence of upper respiratory tract infections in the periods after the interventions.35,36 Finally, ED visit data cannot differentiate the effects of the market withdrawal and the labeling revision announcement from the effects of the media attention and public education efforts that accompanied them.
Although progress has been made in reducing ADE ED visits from supervised administrations of CCMs, more remains to be done to decrease all types of CCM ADEs among children. Addressing unsupervised ingestions has the greatest potential for further reductions in CCM ADEs. Public health surveillance will continue to be important for tracking the effects of efforts to reduce CCMADEs and for identifying future opportunities for reducing the burden of ADEs in the United States.
WHAT’S KNOWN ON THIS SUBJECT
In 2007, manufacturers voluntarily withdrew over-the-counter (OTC) infant cough and cold medications (CCMs) from the US market. A year later, manufacturers announced OTC CCM labeling would be revised to warn against OTC CCM use by children aged <4 years.
WHAT THIS STUDY ADDS
Among children aged <2 and 2 to 3 years, emergency department visits for CCM adverse events declined nationally after the withdrawal and labeling revision announcement relative to all adverse drug event visits. Unsupervised ingestions caused most CCM adverse events after each intervention.
ACKNOWLEDGMENTS
We thank Joel Friedman, Thomas Schroeder, Herman Burney, and the US Consumer Products Safety Commission analysts along with Ray Colucci and the National Electronic Injury Surveillance System coders for diligent data collection, Kelly Weidenbach and Katie Rose for data review and Maribeth Lovegrove for analytic assistance and insightful comments on the manuscript. Dr Nguyen would like to acknowledge previous training support from the Emory AIDS International Training and Research Program(grant NIH/FIC D43 TW01042).
FUNDING: No external funding.
ABBREVIATIONS
- ADE
adverse drug event
- CCM
cough and cold medication
- CI
confidence interval
- ED
emergency department
- FDA
Food and Drug Administration
- NEISS-CADES
National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance
- OTC
over-the-counter
Footnotes
Dr Hampton conceptualized and designed the study and drafted the initial manuscript; Dr Nguyen conceptualized and designed the study, carried out the initial analyses, and reviewed and revised the manuscript; Mr Edwards helped design the interrupted time series analysis and reviewed and revised the manuscript; Dr Budnitz conceptualized and designed the study and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Shehab N, Schaefer MK, Kegler SR, Budnitz DS. Adverse events from cough and cold medications after a market withdrawal of products labeled for infants. Pediatrics. 2010;126(6):1100–1107. doi: 10.1542/peds.2010-1839. [DOI] [PubMed] [Google Scholar]
- 2.Consumer Healthcare Products Association. Makers of OTC cough and cold medicines announce voluntary withdrawal of oral infant medicines. [Accessed June 7, 2013]; Available at: www.chpa-info.org/10_11_07_OralInfantMedicines.aspx. [Google Scholar]
- 3.Harris G. Manufacturers remove drugs for infant cold. New York Times. 2007 Oct 12; [Google Scholar]
- 4.Iwata E. Kids’ cough, cold medicines pulled. USA Today. 2007 Oct 14; [Google Scholar]
- 5.Gunn VL, Taha SH, Liebelt EL, Serwint JR. Toxicity of over-the-counter cough and cold medications. Pediatrics. 2001;108(3) doi: 10.1542/peds.108.3.e52. Available at: www.pediatrics.org/cgi/content/full/108/3/e52. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. Nonprescription Drug Advisory Committee Meeting—cold, cough, allergy, bronchodilator, antiasthmatic drug products for over-the-counter human use, October 18 and 19, 2007. [Accessed May 3, 2010]; Available at: www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4323b1-02-FDA.pdf.
- 7.Sharfstein JM, North M, Serwint JR. Over the counter but no longer under the radar —pediatric cough and cold medications. N Engl J Med. 2007;357(23):2321–2324. doi: 10.1056/NEJMp0707400. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Infant deaths associated with cough and cold medications—two states, 2005. MMWR Morb Mortal Wkly Rep. 2007;56(1):1–4. [PubMed] [Google Scholar]
- 9.Food and Drug Administration. News & Events—FDA releases recommendations regarding use of over-the-counter cough and cold products. Products should not be used in children under 2 years of age; evaluation continues in older populations. [Accessed April 16, 2013]; Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116839.htm.
- 10.Klein-Schwartz W, Sorkin JD, Doyon S. Impact of the voluntary withdrawal of over-the-counter cough and cold medications on pediatric ingestions reported to poison centers. Pharmacoepidemiol Drug Saf. 2010;19(8):819–824. doi: 10.1002/pds.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consumer Healthcare Product Association. Statement from CHPA on the voluntary label updates to oral OTC children’s cough and cold medicines. [Accessed June 7, 2013]; Available at: www.chpa-info.org/10_07_08_pedcc.aspx. [Google Scholar]
- 12.Food and Drug Administration. FDA statement following CHPA’s announcement on nonprescription over-the-counter cough and cold medicines in children. [Accessed June 7, 2013]; Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116964.htm.
- 13.Harris G. Child warning added to cold remedies. New York Times. 2008 Oct 7; [Google Scholar]
- 14.Food and Drug Administration. For Consumers —using over-the-counter cough and cold products in children. [Accessed June 27, 2013]; Available at: www.fda.gov/ForConsumers/ConsumerUpdates/ucm048515.htm.
- 15.American Academy of Pediatrics. Coughs and colds: medicines or home remedies? [Accessed May 7, 2013]; Available at: www.healthychildren.org/English/tips-tools/symptom-checker/Pages/Coughs-and-Colds-Medicines-or-Home-Remedies.aspx. [Google Scholar]
- 16.Consumer Healthcare Products Association. Children’s OTC cough and cold medicines. [Accessed June 27, 2013]; Available at: www.chpa-info.org/issues/Childrens_CC_Overview.aspx. [Google Scholar]
- 17.Forrester MB. Effect of cough and cold medication withdrawal and warning on ingestions by young children reported to Texas poison centers. Pediatr Emerg Care. 2012;28(6):510–513. doi: 10.1097/PEC.0b013e3182587b0c. [DOI] [PubMed] [Google Scholar]
- 18.Spiller HA, Beuhler MC, Ryan ML, Borys DJ, Aleguas A, Bosse GM. Evaluation of changes in poisoning in young children: 2000 to 2010. Pediatr Emerg Care. 2013;29(5):635–640. doi: 10.1097/PEC.0b013e31828e9d00. [DOI] [PubMed] [Google Scholar]
- 19.Mazer-Amirshahi M, Reid N, van den Anker J, Litovitz T. Effect of cough and cold medication restriction and label changes on pediatric ingestions reported to United States poison centers [published online ahead of print June 12, 2013] J Pediatr. doi: 10.1016/j.jpeds.2013.04.054. doi:10.1016/j.jpeds.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 20.C.S. Mott Children’s Hospital. National Poll on Children’s Health: despite warnings, parents continue to use cough and cold medicines for young kids. [Accessed May 7, 2013]; Available at: http://mottnpch.org/reports-surveys/despite-warnings-parents-continue-use-cough-cold-medicines-young-kids. [Google Scholar]
- 21.C.S. Mott Children’s Hospital. National Poll on Children’s Health: parents ignore warning labels, give cough and cold meds to young kids. [Accessed May 7, 2013]; Available at: http://mottnpch.org/reports-surveys/parents-ignore-warning-labels-give-cough-cold-meds-young-kids. [Google Scholar]
- 22.Varney SM, Bebarta VS, Pitotti RL, Vargas TE. Survey in the emergency department of parents’ understanding of cough and cold medication use in children younger than 2 years. Pediatr Emerg Care. 2012;28(9):883–885. doi: 10.1097/PEC.0b013e3182676518. [DOI] [PubMed] [Google Scholar]
- 23.Lazarus SG, Lanski SL, Smith AS, Simon HK. Cold preparation use in young children after FDA warnings: do concerns still exist? Clin Pediatr (Phila) 2013;52(6):534–539. doi: 10.1177/0009922813482761. [DOI] [PubMed] [Google Scholar]
- 24.Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296(15):1858–1866. doi: 10.1001/jama.296.15.1858. [DOI] [PubMed] [Google Scholar]
- 25.Jhung MA, Budnitz DS, Mendelsohn AB, Weidenbach KN, Nelson TD, Pollock DA. Evaluation and overview of the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance Project (NEISS-CADES) Med Care. 2007;45(10 suppl 2):S96–S102. doi: 10.1097/MLR.0b013e318041f737. [DOI] [PubMed] [Google Scholar]
- 26.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Pediatrics. Choosing Wisely campaign—AAP identifies lists of commonly used tests and treatments to question. [Accessed May 7, 2013]; Available at: www.aap.org/en-us/about-the-aap/aap-press-room/pages/Choosing-Wisely-Campaign.aspx. [Google Scholar]
- 28.Budnitz DS, Lovegrove MC. The last mile: taking the final steps in preventing pediatric pharmaceutical poisonings. J Pediatr. 2012;160(2):190–192. doi: 10.1016/j.jpeds.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovegrove MC, Hon S, Geller RG, et al. Efficacy of flow restrictors in limiting access of liquid medicines by young children. J Pediatr. 2013;163(4):1134–1139. e1. doi: 10.1016/j.jpeds.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Association of Poison Control Centers. Prevention. [Accessed June 18, 2013]; Available at: www.aapcc.org/prevention. [Google Scholar]
- 31.Safe Kids Worldwide. Medication safety. [Accessed June 18, 2013]; Available at: www.safekids.org/medicinesafety. [Google Scholar]
- 32.PROTECT Initiative. Up and away. [Accessed April 16, 2013]; Available at: www.upandaway.org. [Google Scholar]
- 33.Ostroff C, Lee CE, McMeekin J. Unapproved prescription cough, cold, and allergy drug products: recent US Food and Drug Administration regulatory action on unapproved cough, cold, and allergy medications. Chest. 2011;140(2):295–300. doi: 10.1378/chest.11-0981. [DOI] [PubMed] [Google Scholar]
- 34.Doyon S, Tra Y, Klein-Schwartz W. Decrease in therapeutic errors involving prescription cough and cold medications in young children. J Pediatr Pharmacol Ther. 2012;17(1):84–87. doi: 10.5863/1551-6776-17.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovegrove MC, Shehab N, Hales CM, Poneleit K, Crane E, Budnitz DS. Emergency department visits for antiviral adverse events during the 2009 H1N1 influenza pandemic. Public Health Rep. 2011;126(3):312–317. doi: 10.1177/003335491112600303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong GL, Brammer L, Finelli L. Timely assessment of the severity of the 2009 H1N1 influenza pandemic. Clin Infect Dis. 2011;52(suppl 1):S83–S89. doi: 10.1093/cid/ciq013. [DOI] [PubMed] [Google Scholar]