Abstract
Rotavirus vaccine was introduced in El Salvador in 2006 and is recommended to be given concomitantly with DTP–HepB–Haemophilus influenzae type b (pentavalent) vaccine at ages 2 months (upper age limit 15 weeks) and 4 months (upper age limit 8 months) of age. However, rotavirus vaccination coverage continues to lag behind that of pentavalent vaccine, even in years when national rotavirus vaccine stockouts have not occurred. We analyzed factors associated with receipt of oral rotavirus vaccine among children who received at least 2 doses of pentavalent vaccine in a stratified cluster survey of children aged 24–59 months conducted in El Salvador in 2011. Vaccine doses included were documented on vaccination cards (94.4%) or in health facility records (5.6%). Logistic regression and survival analysis were used to assess factors associated with vaccination status and age at vaccination. Receipt of pentavalent vaccine by age 15 weeks was associated with rotavirus vaccination (OR: 5.1; 95% CI 2.7, 9.4), and receipt of the second pentavalent dose by age 32 weeks was associated with receipt of two rotavirus vaccine doses (OR: 5.0; 95% CI 2.1–12.3). Timely coverage with the first pentavalent vaccine dose was 88.2% in the 2007 cohort and 91.1% in the 2008 cohort (p = 0.04). Children born in 2009, when a four-month national rotavirus vaccine stock-out occurred, had an older median age of receipt of rotavirus vaccine and were less likely to receive rotavirus on the same date as the same dose of pentavalent vaccine than children born in 2007 and 2008. Upper age limit recommendations for rotavirus vaccine administration contributed to suboptimal vaccination coverage. Survey data suggest that late rotavirus vaccination and co-administration with later doses of pentavalent vaccine among children born in 2009 helped increase rotavirus vaccine coverage following shortages.
Keywords: Rotavirus vaccine, El Salvador, Vaccination timeliness, Routine vaccination, Vaccination coverage
1. Introduction
Diarrhea due to rotavirus is one of the leading causes of death in children under 5 years of age internationally [1]. Since 2006, second generation live orally administered rotavirus vaccines have been recommended as a two-dose monovalent rotavirus vaccine (RV1; Rotarix, GlaxoSmithKline Biologicals) or three-dose pentavalent rotavirus vaccine (RV5; RotaTeq, Merck & Co., Inc.) regimen by the World Health Organization (WHO) [1]. The El Salvador Expanded Programme on Immunization (EPI) introduced a 2-dose oral rotavirus vaccination series in October 2006 entirely with government funds, as a low-middle income but non-Gavi eligible country, and recommended administration at 2 and 4 months of age, concurrently with injected diphtheria–tetanus–pertussis–hepatitis B–Haemophilus influenzae type b (pentavalent) vaccine and live oral poliovirus vaccine (OPV) [2,3]. Studies have shown a positive impact of rotavirus vaccine in El Salvador: a 2010 vaccine effectiveness study demonstrated a four-fold reduction (OR: 0.24) in hospitalizations for rotavirus infection among children who received two doses of vaccine [4]; and a 2011 study found an overall reduction in rotavirus diarrhea hospitalizations by age group in children under five years of age, with the most significant benefits in birth cohorts that had been eligible for vaccination [5].
When second generation rotavirus vaccines were introduced, the WHO Strategic Advisory Group of Experts (SAGE) recommended upper age limits of 15 weeks of age for the first dose and 8 months of age for completion of the two- or three-dose series [1,6-8]. In 2012, WHO updated its recommendations supporting co-administering rotavirus vaccine with diphtheria–tetanus–pertussis (DTP)-containing vaccine regardless of the child’s age [1]; the same year, the Technical Advisory Group on Vaccine-preventable Disease (TAG) of the Pan American Health Organization (PAHO) recommended that countries of the Americas work to improve adherence to the national routine vaccination schedule to ensure timely vaccination, with a consideration of possible benefits of late rotavirus vaccination under some circumstances [9]. Before these modified recommendations, rotavirus vaccines were the only vaccines in the routine infant vaccination schedule with upper age limits for administration [1,7]. The upper age limit recommendations were informed by experiences with the first licensed rotavirus vaccine, which was withdrawn in 1999 because of an increased risk of intussusception, a potentially fatal bowel obstruction caused by telescoping of one part of the intestine into an adjacent segment, especially among older infants [6,10,11]. Based on large safety and efficacy trials and observational studies[1,6-8,12,13], the risk of intussusception following receipt of second generation rotavirus vaccines was shown to be greatly reduced compared to the earlier vaccine, although continued monitoring of this risk is still warranted.
Rotavirus vaccine is highly effective in reducing diarrheal disease hospitalizations [4,5]. However, coverage with rotavirus vaccine is often lower than that of co-administrated vaccines[2,3,14,15]. De Oliveira et al. [3] reported lower coverage with rotavirus vaccine than pentavalent vaccine in El Salvador in 2007, 2008, and 2009. The authors hypothesized that the upper age limits for administration resulted in coverage discrepancies between rotavirus and pentavalent vaccines. There have been no studies investigating the impact of the upper age limits on rotavirus vaccine coverage using data from individual children in low or middle income settings in the Americas.
A national cross-sectional survey of vaccination coverage among children aged 24–59 months was completed in El Salvador in 2011. The primary analysis by Suarez Castaneda et al. [2] showed rotavirus vaccination coverage, estimated at 93.7% for the first dose and 86.3% for the second, to be lower than coverage with the corresponding doses of pentavalent vaccine, estimated at 99.9% for both doses. Additionally, El Salvador experienced a nationwide shortage of rotavirus vaccine between July and October of 2009 [2]. Year of birth was a predictor of rotavirus vaccination timeliness and the primary analysis of that survey concluded that further investigation of the reasons for lower rotavirus coverage was needed [2].
We used the dataset from the 2011 vaccination coverage survey to investigate birth cohort-specific timeliness of rotavirus and pentavalent vaccines, differences in timeliness between doses and vaccines, and co-administration patterns to further understand upper age limits and vaccine shortages as factors in lower rotavirus vaccine coverage in El Salvador.
2. Methods
2.1. Study design
The methods of the study design have been described by Suarez-Castaneda et al. [2]. Briefly, this was a multi-stage stratified cluster survey of all five regions of El Salvador, conducted from 1 November to 2 December 2011. Thirty clusters were sampled via probability proportional to size from each of the 5 regions. Seventeen households within each locality were selected (details described in [2]), and one eligible child was randomly selected in each household, yielding a sample size of 2550 2- to 4-year-old children born between 4 November 2006 and 12 December 2009. Caregivers were interviewed about their child’s vaccination status and their attitudes toward vaccination. Vaccination dates were obtained from children’s vaccination cards at home (94.4%) or at health facilities if the card was unavailable (5.6%). The survey based coverage estimates on the 2006 national vaccination schedule for children less than two years of age. Only two children had no written record of vaccination and were excluded; both had received vaccines according to parental report. For each missing dose of vaccine, the parent or guardian was asked to recall the reason it was not administered. Parents or guardians were also surveyed about family and community characteristics, such as parental education level and marital status, number of people in the household, levels of community violence (e.g., gang activity), and accessibility of vaccination clinics. These self-reported factors were recorded for each child.
2.2. Analytic methods
The current analysis is limited to the sample of children born in 2007–2009 with at least 2 documented doses of pentavalent vaccine (N = 2492); children born in 2006 (n = 55) and children who had not received at least 2 doses of pentavalent vaccine (n = 3) were excluded. To reflect national policy and facilitate comparisons between the doses, schedule adherence for both vaccines was categorized using the recommended upper age limits for rotavirus vaccine of 104 days for the first dose and 223 days for the final dose of the series. Percentages and (Wald) confidence intervals were calculated accounting for the survey design and the weights provide by the original authors using SAS v9.3 (Cary, NC). These are reported for defined sub-populations overall, and by birth year. The weighted median ages of administration of rotavirus and pentavalent vaccines are presented with absolute ranges. Logistic regression models, also accounting for survey design and weights, were developed for rotavirus vaccination status predicted by the timing of the corresponding dose of pentavalent vaccine, that is administered before or after the upper age limit for rotavirus vaccination, and year of birth; categorical pentavalent timeliness (doses administered within 30 days of the recommended age) was predicted by year of birth. Confounding was assessed using the backwards change in estimate approach [16].
In the time-to-event analysis, children were considered eligible for each dose of vaccine from birth. Children without a written record of the vaccine of interest were censored at their age at the time of the survey. For the second dose of vaccine, children were considered vaccinated if they had a written record for the first and second doses. The results are presented in graphs plotting one minus the proportion of unvaccinated children by age in months. These images were generated using R (3.0) survey method survival analysis package to account for the sample weights and survey design.
The survey was reviewed by the national and PAHO ethical committees and considered non-research. This secondary analysis was approved by Emory University’s Institutional Review Board and the Centers for Disease Control and Prevention.
3. Results
Table 1 describes the characteristics of the surveyed children and their households. Of 2495 children included in El Salvador’s 2011 national vaccination coverage survey born during 2007–2009, 2492 (99.8%) had received at least two doses of pentavalent vaccine. Among these, 2338 (93.8%) of 2492 had documentation of receipt of the first dose of oral rotavirus vaccine and 2162 (86.3%) had completed the two-dose rotavirus vaccination series. Median age at receipt of first dose of pentavalent vaccine (penta1) was 62.4 days (range: 0–1234 days) and median age at receipt of the second dose (penta2) was 125.3 days (range: 58–1398 days), close to the recommended ages of 2 and 4 months, respectively. Similarly, oral rotavirus vaccine doses were received at a median age of 63.7 days (range: 0–1183 days) for the first dose (rota1) and 126.8 days (Range: 58–1463 days) for the second dose (rota2). Among 2338 children who received the first dose of rotavirus vaccine, 1814 (77.2%) received rota1 and penta1 on the same date, and 453 (19.9%) received rota1 a median of 55.2 days after penta1 (Table 2). Among 2162 children who received the second dose of rotavirus vaccine, 1613 (74.8%) received rota2 and penta2 on the same date, while 429 (19.9%) received rota2 a median of 40.9 days after penta2. Among children who received rota1 on a different date than penta1 and penta2, 99.1% received OPV1 on the same day as penta1; among children who received rota2 on a different date than penta2 and penta3, 96.8% received OPV2 on the same day as penta2.
Table 1.
Selected characteristics of surveyed children born 2007-2009 with at least 2 documented doses of pentavalent vaccine, their families and communities. El Salvador, 2011.
| Year of birth |
|||||||
|---|---|---|---|---|---|---|---|
| 2007 (N=806) |
2008 (N=877) |
2009 (N=809) |
|||||
| n | % | n | % | n | % | ||
| Gender | Female | 387 | 48.0 | 428 | 48.8 | 383 | 47.3 |
| Parental marital status | Partnered/married | 652 | 80.9 | 702 | 80.1 | 650 | 80.4 |
| Divorced/separated | 22 | 2.7 | 24 | 2.7 | 17 | 2.1 | |
| Single | 124 | 15.4 | 143 | 16.3 | 135 | 16.7 | |
| Widowed | 8 | 1.0 | 8 | 0.9 | 7 | 0.9 | |
| Parental education level | Less than 7th grade | 442 | 54.8 | 482 | 55.0 | 481 | 59.5 |
| 7th grade or higher | 364 | 45.2 | 395 | 45.0 | 328 | 40.5 | |
| Parental employment status | Not employed | 572 | 71.1 | 602 | 68.6 | 564 | 69.7 |
| Outside the home | 234 | 29.0 | 275 | 31.4 | 245 | 30.3 | |
| Number of people in the household | 2–5 | 503 | 62.4 | 564 | 64.3 | 503 | 62.2 |
| 6 or more | 303 | 37.6 | 313 | 35.7 | 306 | 37.8 | |
| Primary mode of transportation | Foot | 439 | 54.5 | 462 | 52.7 | 391 | 48.3 |
| Bus | 243 | 30.2 | 275 | 31.4 | 299 | 37.0 | |
| Personal vehicle | 53 | 6.6 | 64 | 7.3 | 61 | 7.5 | |
| Other | 71 | 8.8 | 76 | 8.7 | 58 | 7.2 | |
| Area of residence | Urban area | 363 | 45.0 | 443 | 50.5 | 361 | 44.6 |
| Presence of organized crime | Yes | 131 | 16.3 | 143 | 16.3 | 142 | 17.6 |
| Region | Central | 154 | 19.1 | 184 | 21.0 | 162 | 20.0 |
| Metropolitan | 152 | 18.9 | 179 | 20.4 | 158 | 19.5 | |
| Occidental | 169 | 21.0 | 175 | 20.0 | 155 | 19.2 | |
| Oriental | 161 | 20.0 | 181 | 20.6 | 160 | 19.8 | |
| Paracentral | 170 | 21.1 | 158 | 18.0 | 174 | 21.5 | |
Table 2.
Concurrent administration of rotavirus and pentavalenta vaccines among children born in 2007-2009. El Salvador, 2011.
| Year of birth |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total |
2007 (N=806) |
2008 (N=877) |
2009 (N = 809) |
||||||||||
| n | % | CI | n | % | CI | n | % | CI | n | % | CI | ||
| Rotavirus dose 1 (N = 2338) |
With penta1b | 1814 | 77.2 | (75.0, 79.4) | 592 | 80.6 | (77.5, 83.7) | 701 | 84.4 | (81.8,87.0) | 521 | 66.4 | (62.2, 70.7) |
| With penta2c | 166 | 7.4 | (6.1, 8.6) | 50 | 7.2 | (5.1,9.2) | 34 | 4.1 | (2.6, 5.7) | 83 | 11.0 | (8.5, 13.5) | |
| In a separate visit | 358 | 15.4 | (13.7, 17.2) | 90 | 12.3 | (9.5,15.1) | 97 | 11.4 | (9.2,13.7) | 171 | 22.6 | (19.0, 26.1) | |
| Rotavirus dose 2 (N = 2162) |
With penta2 | 1613 | 74.8 | (72.8, 76.9) | 542 | 77.9 | (74.6, 81.3) | 610 | 80.9 | (77.5, 84.2) | 461 | 66.5 | (61.6, 69.4) |
| With penta3d | 95 | 4.8 | (3.7, 5.9) | 20 | 2.9 | (1.6, 4.3) | 19 | 2.4 | (1.3,3.6) | 56 | 9.1 | (6.2, 12.0) | |
| In a separate visit | 454 | 20.4 | (18.6, 22.2) | 138 | 19.1 | (16.0, 22.3) | 125 | 16.7 | (13.6,19.8) | 191 | 25.4 | (22.0, 28.9) | |
Diphtheria–tetanus–pertussis–Hepatitis B–Haemophilus influenzae type b vaccine.
Penta1 is the first dose of pentavalent vaccine.
Penta2 is the second dose of pentavalent vaccine.
Penta2 is the second dose of pentavalent vaccine.
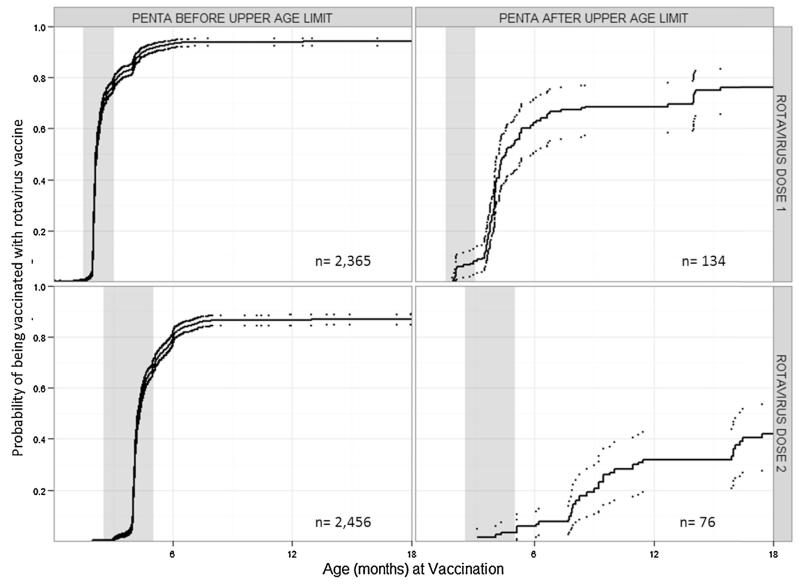
Delayed receipt of pentavalent vaccine was strongly associated with non-receipt of rotavirus vaccine or incomplete rotavirus vaccination. Among 154 children with zero doses of rotavirus vaccine despite having received two doses of pentavalent vaccine, 29 (19.7%) had received the first pentavalent vaccine after 15 weeks of age. Among 176 children with only one dose of rotavirus vaccine, 12 (7.3%) had received penta2 after 32 weeks of age. After adjusting for year of birth and maternal education, odds of receipt of oral rotavirus vaccine were lower among children who received penta1 after 15 weeks of age compared with those who received penta1 before 15 weeks of age (OR: 0.2; 95% CI 0.1, 0.4) (Table 3). Among children who received rota1, odds of receipt of rota2 were lower among those who received penta2 after 32 weeks of age compared with those who received the vaccine before 32 weeks (OR: 0.1; 95% CI 0.1, 0.2). Urban residence, maternal employment status, and number of residents in the household were not found to be confounders. Uptake of rotavirus vaccine in children who received pentavalent vaccine before and after the upper age limits of administration are visualized using cumulative incidence curves (Fig. 1).
Table 3.
Odds ratios of receiving rotavirus vaccine by pentavalent timing and birth cohort among children born 2007–2009 who received a dose of pentavalent vaccine. El Salvador, 2011.
| Rotavirus dose 1 |
Rotavirus dose 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Administered |
Crude | Adjusteda | Administered |
Crude | Adjustedb | |||
| n | %(CI) | n | %(CI) | |||||
| Pentavalent before age limit |
2236 | 94.7 (93.3, 96.1) | 1.0 (reference) | 1.0 (reference) | 2129 | 87.7 (85.7, 89.7) | 1.0 (reference) | 1.0 (reference) |
| Pentavalent after age limit |
102 | 78.3 (69.3, 87.4) | 0.2 (0.1,0.4) | 0.2 (0.1,0.4) | 85 | 64.1 (54.0, 74.1) | 0.1 (0.1, 0.2) | 0.1 (0.1, 0.2) |
| Born in 2007 | 732 | 90.6 (88.2,93.1) | 1.0 (reference) | 1.0 (reference) | 700 | 86.4 (83.5, 89.4) | 1.0 (reference) | 1.0 (reference) |
| Born in 2008 | 832 | 94.8 (92.9, 97.8) | 1.9 (1.3, 2.8) | 1.9 (1.2, 2.6) | 754 | 85.2 (82.0, 88.4) | 0.9 (0.7, 1.2) | 0.8 (0.6, 1.1) |
| Born in 2009 | 774 | 95.7 (94.1, 97.3) | 2.3 (1.6, 3.4) | 2.3 (1.5, 3.3) | 708 | 87.4 (84.5, 90.2) | 1.1 (0.8, 1.4) | 1.0 (0.7, 1.3) |
| Less than 7th grade education |
1323 | 93.9 (92.3, 95.5) | 0.9 (0.7, 1.3) | 1.1 (0.7, 1.4) | 933 | 85.0 (81.9, 88.1) | 1.2 (0.9, 1.6) | 1.1 (0.9, 1.5) |
| 7th grade education or higher |
1015 | 93.5 (91.6, 95.6) | 1.0 (reference) | 1.0 (reference) | 1229 | 87.3 (84.9, 89.6) | 1.0 (reference) | 1.0 (reference) |
Adjusted for pentavalent dose 1 categorical timeliness, parental education and birth cohort.
Adjusted for pentavalent dose 2 categorical timeliness, parental education and birth cohort.
Fig. 1.
Cumulative incidence curves showing the probability of rotavirus vaccination for children who received pentavalent vaccine before and after the upper age limit of rotavirus vaccine administration, El Salvador, 2011.
The probability of rotavirus vaccination is shown with solid lines and confidence intervals are shown as dotted lines. The top row shows the probability of vaccination with the first dose of rotavirus vaccine, among children who received the first dose of pentavalent vaccine before the first dose rotavirus vaccine upper age limit (15 weeks of age) in the left column and after the upper age limit in the right column. The bottom row shows the probability of vaccination with the second dose of rotavirus vaccine, among children who received the second dose of pentavalent vaccine before the series rotavirus vaccine upper age limit (8 months of age) in the left column and after the upper age limit in the right column. The gray boxes highlight the minimum acceptable age until 30 days after the recommended age for the rotavirus dose.
In the first two birth cohorts to receive oral rotavirus vaccine before national shortages in 2009, we observed significant improvement in timeliness of pentavalent vaccination among children included in the survey. The percentage of children who had received a valid dose of penta1 by 3 months (90 days) of age increased from 88.2% in the 2007 cohort to 91.1% in the 2008 cohort (p = 0.04), although there was little difference in the median age of penta1: 62.5 compared with 62.3 days. Controlling for maternal education, odds of penta1 receipt by 3 months of age were 1.6-fold higher among children born in 2008 compared with those born in 2007 (OR: 1.6; 95% CI 1.1, 2.3). The percentage of children who had received penta2 by 5 months of age was 84.6% in the 2007 cohort compared to 87.4% among those born in 2008 (p = 0.10); odds of timely vaccination was not statistically different (OR: 1.1; 95% CI: 0.8, 1.6) after controlling for maternal education, penta1 timeliness, urban residence, maternal employment status, and number of residents in the household.
Despite nationwide shortages of rotavirus vaccine from July to October, 2009, 95.7% (95% CI: 94.1, 97.3) of children born in 2009 received rota1 and 87.4% (95% CI: 84.5, 90.2) received rota2, similar to percentages of children born in 2007 (90.6% and 86.4%) and 2008 (94.8% and 85.2%, respectively). However, the median age of receipt of penta2 was 124.7 days in 2008 compared with 125.9 in 2009, and 22.1% of children born in 2009 received rota1 after 15 weeks of age compared with 9.0% in 2008. Compared with children in the 2007 and 2008 birth cohorts, children born in 2009 were more likely to have received rota2 with the third dose of pentavalent vaccine (penta3) or on separate dates from penta2 and penta3 (2007/2008: 20.6%, 2009: 34.5%; p: 0.001) (Table 2). The primary reason parents gave for their children not receiving rotavirus vaccine was that there was no rotavirus vaccine at the time of their visit, with 63.2, 72.1, and 89.4% of parents citing this reason in the 2007, 2008, and 2009 birth cohort, respectively.
The number of surveyed children who received rota1 in November 2009, the month following the nationwide shortage was 134, higher than the number of children aged 2–3 months (n = 80) and exceeding the number of rota1 doses received in any other month children in the study were vaccinated with the first dose of rotavirus vaccine. The increase in rota1 doses received in November 2009 was followed by an increased number of rota2 doses received in January 2010.
4. Discussion
This analysis suggests that shortages of vaccine and missed opportunities led to suboptimal rotavirus vaccine coverage during the first three years following rotavirus vaccine introduction in El Salvador’s national immunization program, though first dose rotavirus vaccine coverage was improving by birth cohort. High coverage and timeliness of other routine vaccinations indicate the overall strength of the program at the time of rotavirus vaccine introduction; adherence to upper age limits during the first two years also indicates well-trained vaccination staff. This analysis also showed an increased proportion of infants receiving both doses of rotavirus vaccine after the recommended ages in the 2009 birth cohort, suggesting efforts to provide rotavirus vaccine to infants eligible for vaccination during vaccine shortages. When forecasting vaccination coverage after new vaccine introduction, it is often assumed that a newly introduced vaccine will quickly achieve the same coverage level as established vaccines recommended at the same ages [3] and previous publications have hypothesized that the recommended upper age limits for rotavirus vaccine are related to lower coverage [1,6,14]. This analysis showed that age-specific recommendations contributed to lower coverage, though to a lesser extent than missed opportunities and vaccine shortages. Other variables associated with delayed pentavalent or rotavirus vaccination included child’s year of birth and gender, vaccination in the private sector, and mother’s education and marital status [2]. Our findings support the revised recommendations from WHO and PAHO advisory bodies to consider the benefits and risks of rotavirus vaccination among older infants, while still working to improve schedule adherence.
This analysis also showed that the timeliness of the first dose of pentavalent vaccine increased significantly as the rotavirus vaccine program matured during the first two years after introduction, before national vaccine shortages. Previous studies found an association between rotavirus vaccine introduction and improved timeliness of other vaccines [15,17] and that new vaccine introduction can strengthen service delivery in existing routine vaccination programs [18]. As this survey did not include cohorts born before and after rotavirus vaccine introduction, we were unable to assess improved timeliness in administration for other routine infant vaccines as observed in Australia [17] and Paraguay (unpublished data 2011).
Our results also highlight challenges of new vaccine introduction, including the implications of shortages on vaccination coverage and timely administration [3,19-21]. The results of this survey show that the immunization program in El Salvador was flexible in its handling of the national rotavirus vaccine shortages, resulting in a minimal reduction in rotavirus vaccination coverage in 2009 but delayed administration. This was evidenced through adaptability in co-administration and diligent follow-up of children who had not received rotavirus vaccine, or who were partially vaccinated, in the months following the national stock-out. Other subnational shortages likely played a role in lower and less timely coverage with rotavirus vaccine during the study period, as suggested by the reasons parents provided for not having received rotavirus vaccine even before 2009.
This study had several limitations. Because the survey did not include cohorts before the rotavirus vaccine was introduced, we were unable to draw conclusions about the association between rotavirus vaccine introduction and the timeliness of routine infant vaccinations. The 2009 national vaccine shortage also limited our ability to look at improvements in timeliness across birth cohorts. Although the unavailability of the vaccine was identified as a primary reason for non-vaccination [2], we were unable to verify information about local vaccination stock or consider provider attitudes toward vaccinations and contraindications to vaccination for individual children.
This study also has several strengths. The analysis included three birth cohorts of children eligible for rotavirus and pentavalent vaccines with complete documentation of the dates of administration. As receiving pentavalent vaccine was nearly universal, it is clear that there is access to immunization services in this strong program. In addition, because the vaccination and community and family factor data were individually linked, we were able to assess associations based on individual information, rather than ecological and aggregated data. Finally, the overall sample size was sufficiently large to allow us to produce estimates by birth cohort.
Our findings add to the limited literature about the use and coverage of rotavirus vaccine, with a restricted period for valid administration and its impact on timing and coverage. The experience with rotavirus vaccine introduction in El Salvador is unique, but it can provide potentially helpful information for other country programs considering introducing this and other new vaccines, as well as promoting the use of existing survey data to answer specific questions regarding newer vaccines. The findings of this study also add to the growing number of analyses looking at vaccination timeliness and adherence to recommended ages for administration in low and middle income countries [2,22,23] and follows PAHO’s new guidance tool for secondary survey analyses created in collaboration with the US Centers for Disease Control and Prevention [24]. El Salvador and other countries that have or will soon introduce new vaccines should continue to carefully monitor availability of vaccine, vaccination coverage, and timeliness and simultaneity of vaccine administration. Vaccination programs should encourage administration of all recommended vaccines during vaccination visits to avoid missed opportunities and rapidly accelerate coverage of new vaccines.
Acknowledgements
We would like to acknowledge Elner Osmin Crespin-Elias, Oscar A. Rivera Pleitez, Maria Isalbel Quintanilla de Campos of Francisco Gavidia University for their contributions to study design and data collection, Kathleen Wannemuehler for her statistical support, and Umesh Parashar and Jacqueline Gindler for invalueable help in editing.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
Conflict of interest: None.
References
- [1].World Health Organization (WHO) Weekly Epidemiological Record 87; Meeting of the Strategic Advisory Group of Experts on immunization, April 2012 – conclusions and recommendations; May 25, 2012; pp. 201–16. [PubMed] [Google Scholar]
- [2].Suarez-Castaneda E, Pezzoli L, Elas M, Baltrons R, Crespin-Elias EO, Pleitez OA, et al. Routine childhood vaccination programme coverage, El Salvador, 2011 – in search of timeliness. Vaccine. 2014 Jan 16;32(4):437–44. doi: 10.1016/j.vaccine.2013.11.072. [DOI] [PubMed] [Google Scholar]
- [3].de Oliveira LH, Danovaro-Holliday MC, Sanwogou NJ, Ruiz-Matus C, Tambini G, Andrus JK. Progress in the Introduction of the rotavirus vaccine in Latin America and the Caribbean: four years of accumulated experience. Pediatr Infect Dis J. 2011 Jan;30(1 Suppl.):S61–6. doi: 10.1097/INF.0b013e3181fefdd6. [DOI] [PubMed] [Google Scholar]
- [4].de Palma O, Cruz L, Ramos H, de Baires A, Villatoro N, Pastor D, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case control study. BMJ. 2010;340:c2825. doi: 10.1136/bmj.c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yen C, Armero Guardado JA, Alberto P, Rodriguez Araujo DS, Mena C, Cuellar E, et al. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J. 2011 Jan;30(1 Suppl.):S6–10. doi: 10.1097/INF.0b013e3181fefa05. [DOI] [PubMed] [Google Scholar]
- [6].Patel MM, Haber P, Baggs J, Zuber P, Bines JE, Parashar UD. Intussusception and rotavirus vaccination: a review of the available evidence. Expert Rev Vaccines. 2009 Nov;8(11):1555–64. doi: 10.1586/erv.09.106. [DOI] [PubMed] [Google Scholar]
- [7].Cortese MM, Parashar UD. Control centers for disease and prevention. prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2009 Feb 6;58(RR–2):1–25. [PubMed] [Google Scholar]
- [8].World Health Organization (WHO) Meeting of the Strategic Advisory Group of Experts on immunization, April 2012 – conclusions and recommendations; August 10, 2007; pp. 285–96. Weekly epidemiological record 82. [Google Scholar]
- [9].Pan American Health Organization (PAHO) Paving the way for immunization; Meeting of the Technical Advisory Group on Vaccine-Preventable Diseases (TAG); Washington DC, USA. October 2012; http://www.who.int/immunization/sage/meetings/2012/november/9_FINAL_TAG_Meeting_2012_report.pdf. [Google Scholar]
- [10].Centers for Disease, Control, and Prevention Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999 Nov 5;48(43):1007. [PubMed] [Google Scholar]
- [11].Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001 Feb 22;344(8):564–72. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- [12].Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, et al. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011;29(16):3061–6. doi: 10.1016/j.vaccine.2011.01.088. [DOI] [PubMed] [Google Scholar]
- [13].Patel MM, López-Collada VR, Bulhões MM, De Oliveira LH, Márquez AB, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364(24):2283–92. doi: 10.1056/NEJMoa1012952. [DOI] [PubMed] [Google Scholar]
- [14].Flannery B, Samad S, de Moraes JC, Tate JE, Danovaro-Holliday MC, de Oliveira LH, et al. Uptake of oral rotavirus vaccine and timeliness of routine immunization in Brazil’s National Immunization Program. Vaccine. 2013 Mar 1;31(11):1523–8. doi: 10.1016/j.vaccine.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hull BP, Menzies R, Macartney K, McIntyre PB. Impact of the introduction of rotavirus vaccine on the timeliness of other scheduled vaccines: the Australian experience. Vaccine. 2013 Apr 8;31(15):1964–9. doi: 10.1016/j.vaccine.2013.02.007. [DOI] [PubMed] [Google Scholar]
- [16].Kleinbaum DG, Klein M, Rihl Pryor E. Statistics in the health sciences. 3rd ed. Springer; New York: 2010. Logistic regression: a self learning text. [Google Scholar]
- [17].Bissinger W. Vaccination with 3-dose paediatric rotavirus vaccine (Rotateq(R)): impact on the timeliness of uptake of the primary course of DTPa vaccine. Vaccine. 2012 Jul 27;30(35):5293–7. doi: 10.1016/j.vaccine.2012.04.071. [DOI] [PubMed] [Google Scholar]
- [18].Hyde TB, Dentz H, Wang SA, Burchett HE, Mounier-Jack S, Mantel CF. The impact of new vaccine introduction on immunization and health systems: a review of the published literature. Vaccine. 2012 Oct 5;30(45):6347–58. doi: 10.1016/j.vaccine.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Groom AV, Cheek JE, Bryan RT. Effect of a national vaccine shortage on vaccine coverage for American Indian/Alaska Native children. Am J Public Health. 2006;96(4):697. doi: 10.2105/AJPH.2004.053413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Santibanez TA, Santoli JM, Barker LE. Differential effects of the DTaP and MMR vaccine shortages on timeliness of childhood vaccination coverage. Am J Public Health. 2006;96(4):691. doi: 10.2105/AJPH.2004.053306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Smith PJ, Nuorti JP, Singleton JA, Zhao Z, Wolter KM. Effect of vaccine shortages on timeliness of pneumococcal conjugate vaccination: results from the 2001–2005 National Immunization Survey. Pediatrics. 2007;120(5):e1165–73. doi: 10.1542/peds.2007-0037. [DOI] [PubMed] [Google Scholar]
- [22].Akmatov MK, Mikolajczyk RT. Timeliness of childhood vaccinations in 31 low and middle-income countries. J Epidemiol Commun Health. 2012;66(7):e14e14. doi: 10.1136/jech.2010.124651. [DOI] [PubMed] [Google Scholar]
- [23].Fadnes LT, Jackson D, Engebretsen IMS, Zembe W, Sanders D, Sommerfelt H, et al. Vaccination coverage and timeliness in three South African areas: a prospective study. BMC Public Health. 2011;11(1):404. doi: 10.1186/1471-2458-11-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pan American Health Organization (PAHO) Módulo 6: Análisis de datos de encuestas y registros nominales. 2015 http://www.paho.org/immunization/toolkit/resources/reporting-monitoring/es/Modulo6-analisis-datos-encuestas-y-registros-nominales.pdf?ua=1.