Abstract
Objective
Obesity is associated with alterations in thyroid hormone (TH) levels in obese, pregnant individuals. The maintenance of TH levels throughout gestation is important for proper fetal development. The aim of this study was to measure levels of fT3, fT4 and TSH in maternal and matched cord blood serum from normal weight, overweight and obese gravidae to determine alterations in maternal and neonatal TH levels by virtue of maternal obesity.
Design, Setting, Subjects, Outcome Measures
ELISA was utilized to measure fT3, fT4 and TSH levels from banked, matched maternal and neonatal (cord blood) serum (N=205 matched pairs). Data was stratified according to pre-pregnancy or first trimester BMI.
Results
Both maternal and neonatal fT3 levels consistently increased with increasing maternal obesity, and maternal and neonatal fT3 were significantly correlated (r =0.422, p<0.001). Maternal and neonatal fT3 were also significantly associated with birthweight (β=0.155, p=0.027 and β=0.171, p=0.018 respectively). Both the maternal and neonatal fT3 to fT4 ratio significantly increased with increasing maternal obesity. We further found that excess gestational weight gain was associated with a decrease in maternal fT4 compared with gravidae who had insufficient gestational weight gain (0.86±0.17 vs. 0.95±0.22, p<0.01).
Conclusion
Maternal obesity is not only associated with maternal alterations in TH, but with accompanying neonatal changes. Because both maternal obesity and alterations in TH levels are associated with childhood obesity, based on these findings and our prior analyses in a non-human primate model we propose that changes in fT3 levels in the offspring of obese mothers may be a potential molecular mediator of fetal overgrowth and childhood obesity.
Key terms: thyroid hormone, obesity, pregnancy, gestational weight gain
INTRODUCTION
Maintenance of proper thyroid hormone (TH) levels throughout pregnancy is essential for fetal growth and development. Undiagnosed hypothyroidism in pregnant women has been associated with alterations in neuropsychological development of the offspring (1–3). Both maternal and fetal TH levels have been implicated in regulating fetal growth (4–6). Recent data from the FaSTER trial revealed that lower median birthweights occur in women in the highest free T4 (fT4) quintile, but that this is not associated with adverse pregnancy outcomes (7). However, how alterations in maternal and fetal TH levels may affect child development is an important area of study. Data from the Generation R study reveals that maternal thyroid levels during pregnancy may influence childhood adiposity and potentially cardiovascular development (8). Lower maternal TSH levels and higher maternal fT4 levels were associated with lower childhood BMI.
Alterations in TH levels have also been implicated in the etiology of obesity, although their role remains poorly understood. THs help to regulate appetite, temperature, and the availability of energy substrates (9). THs also increase the basal metabolic rate (10). It has been shown that there is an increased prevalence of thyroid function abnormalities in obese individuals, and that BMI is positively correlated with fT3 and TSH levels (11–13). The effects of thyroid hormone treatments on both hypothyroid and hyperthyroid individuals have been long studied, however, the data is not consistent data regarding weight gain and loss based on treatment (14). Similarly, data is not consistent showing thyroid hormone changes after weight gain or loss (14).
While obesity is associated with adverse health outcomes for the individual, obesity during pregnancy bears unique maternal and fetal risks. The incidence of obesity among pregnant women is estimated between 18.5% and 38.3% (15). These women are more susceptible to preeclampsia and gestational diabetes (15, 16). They are also more susceptible to delivery complications including an increased risk of cesarean section, slower labor progression rate, shoulder dystocia and endometritis (15, 16). Newborns of obese and overweight women are more likely to be large for gestational age (LGA), having a higher birthweight (17).
According to the developmental origins of health and disease (DOHaD), adverse experiences in utero can predispose an individual to the adult onset of metabolic disease (18, 19). The in utero milieu may account for upwards of 60% of the variation in infant birthweight (20). LGA infants are more likely to develop metabolic syndrome in childhood, whose symptoms include obesity, glucose intolerance, hyperlipidemia and insulin resistance (21). Individuals born LGA are also at a greater risk of developing cardiovascular and metabolic problems in adulthood (22, 23). A high prevalence of fetal macrosomia occurs among overweight and obese women without insulin resistance or diabetes (24, 25). Determining the molecular mechanisms driving these associations between fetal overgrowth and maternal obesity is essential to preventing obesity in the next generation.
We have previously reported in a non-human primate model of maternal obesity that a maternal high fat diet is associated with alterations in the fetal thyroid levels at the beginning of the third trimester (26). Specifically, we found that fetal fT4 is decreased in association with epigenomic-driven alterations in expression of genes involved in thyroid hormone synthesis. We hypothesized that THs are potential molecular mediators of overgrowth observed in newborns from obese gravidae. In this study we aimed to investigate changes in maternal and neonatal (cord blood) thyroid hormone levels with increasing maternal obesity, as well as the associations between maternal and fetal thyroid hormone levels and birthweight.
MATERIALS AND METHODS
Study population
Subject data and serum samples were obtained following full and informed subject consent under the PeriBank protocol IRB H-26364 at Baylor College of Medicine. Additional IRB approval for this research was obtained under H-30688. Subjects were recruited by trained PeriBank study personnel at the time of admission to labor and delivery. Recruitment occurred between 2011 and 2014. After consent was obtained, over 4,700 variables of clinical information (clinical metadata) were directly extracted from the electronic medical record and accompanying prenatal records alongside directed subject questioning. Neonatal (cord) blood was collected immediately following delivery. Maternal blood was collected within 8 hours following delivery. Iodine skin cleansers were not used during delivery as this would be a confounder of this study. Samples were stored at −80°C until use. Exclusion criteria were multiple gestations, preterm delivery, and subjects with known thyroid disease or taking thyroid medication. A total of 205 subjects were enrolled and categorized into one of five groups according to pre-pregnancy or first trimester BMI: Normal Weight (BMI 18.5–24.9, N=46); Overweight (BMI 25.0–29.9, N=48); Obese 1 (30.0–34.9, N=49); Obese 2 (35.0–39.9, N=36); and Obese 3 (BMI ≥ 40.0, N=20).
Thyroid parameters
Matched maternal and neonatal (cord) serum samples were utilized to measure fT3, fT4 and TSH levels using commercially available ELISA kits (Alpco, Salem, NH). For fT4, the reference range of the ELISA is 0–8ng/dL; for fT3 the range is 0–19pg/mL; and for TSH 0.1–10μIU/mL. All samples were done in duplicate. A standard curve for each plate was created using a 4 Parameter Logistic nonlinear regression model in R, which was utilized to determine the concentration of each sample based on absorbance.
fT3 to fT4 ratio
Both fT3 and fT4 were converted to pmol/L before calculating the ratio (27). Kruskall-Wallis One Way ANOVA on Ranks was utilized to determine significant changes between groups. Pearson Product Moment Correlation was calculated to determine the correlation between maternal and cord fT3:fT4 ratios.
Gestational Weight Gain Calculation
To assess the extent of weight gain, patients were stratified according to three categories (insufficient weight gain, normal weight and excessive weight gain) based on pre-pregnancy or first trimester weight and weight at delivery. This was achieved by applying the 2009 IOM (Institute of Medicine) guidelines for weight gain during pregnancy (28). According to these guidelines, normal weight pregnant females should gain 0.8–1 lbs/wk during the 2nd and 3rd trimester, overweight females 0.5–0.7 lbs/wk and obese females (all classes of obesity) 0.4–0.6 lbs/wk. Whenever the predetermined weight gain was not achieved or exceeded, the patient was categorized as having insufficient or excessive weight gain, otherwise as exhibiting normal weight gain.
Statistical Analysis
Information on BMI, maternal age, birthweight and infant gender for all pregnancies was available through the PeriBank database. Gestational weight gain for each patient was calculated on the basis of pre-pregnancy weight and mother’s weight at delivery using the formula described above. Further statistical tests were performed using SPSS software (version 22, IBM). In order to characterize the demographic and clinical variables of our subjects, descriptive statistics were utilized (frequency, median and interquartile range). Komogorov-Smirnov tests revealed a non-normal distribution of our measured fT3, fT4 and TSH values. Accordingly, differences in maternal and neonatal thyroid hormone levels across all BMI groups were tested using Kruskal-Wallis tests. Post-hoc Mann-Whitney U tests were applied in order to assess differences between thyroid hormone levels of pregnant females with a normal BMI versus those of females with BMI categories Overweight, Obese 1, Obese 2 and Obese 3. In addition, the associations between thyroid hormone levels and BMI were assessed with multivariate linear regression analysis utilizing log transformed variables, correcting for age of the mother, infant gender and birthweight. Correlation of maternal and neonatal thyroid hormone levels with the infant’s birthweight was achieved by application of Spearman’s Rank correlation test. Differences in thyroid hormone levels between the three individual gestational weight gain groups were analyzed using Kruskal-Wallis tests. Within our statistical analysis, p < 0.05 was accepted as significant.
RESULTS
Characteristics of the study population
Subjects were grouped into 5 IOM obesity categories based on documented pre-pregnancy or first trimester BMI (Table 1). Maternal age was significantly different in the Overweight and Obese 1 groups compared to Normal weight subject cohorts. Consistent with prior observations by others, infant birthweight was significantly higher in the morbidly obese group compared with both Normal weight and Overweight. There was no significant difference in gestational age and weight gain. BMI significantly differed between groups by virtue of the study design.
Table 1.
Characteristics of study population
| Maternal Age (years) | Infant Birthweight (grams) | Gestational Age (weeks) | Weight gain (lbs) | BMI (kg/m2) | |
|---|---|---|---|---|---|
| Normal (N=46) | 26.3 ± 0.83 | 3223.2 ± 69.7 | 38.3 ± 0.9 | 25.9 ± 1.4 | 21.7 ± 0.3 |
| Overweight (N=48) | 30.1 ± 0.94* | 3243.8 ± 71.7 | 38.6 ± 0.2 | 23.8 ± 1.7 | 27.0 ± 0.2 |
| Obese 1 (N=49) | 31.9 ± 0.90* | 3414.9 ± 87.8 | 38.8 ± 0.3 | 21.8 ± 2.1 | 31.9 ± 0.2 |
| Obese 2 (N=36) | 29.5 ± 1.01 | 3506.1 ± 65.8 | 37.8 ± 1.1 | 18.1 ± 2.7 | 37.4 ± 0.2 |
| Obese 3 (N=20) | 29.2 ± 1.46 | 3647.7 ± 139.8*# | 38.9 ± 0.4 | 21.3 ± 4.2 | 44.8 ± 0.9 |
significant difference from normal weight group (p<0.05)
significant difference from overweight group, (p<0.05)
+/− = standard deviation
Maternal and neonatal (cord blood) serum fT3 and fT4 are altered with increasing maternal obesity
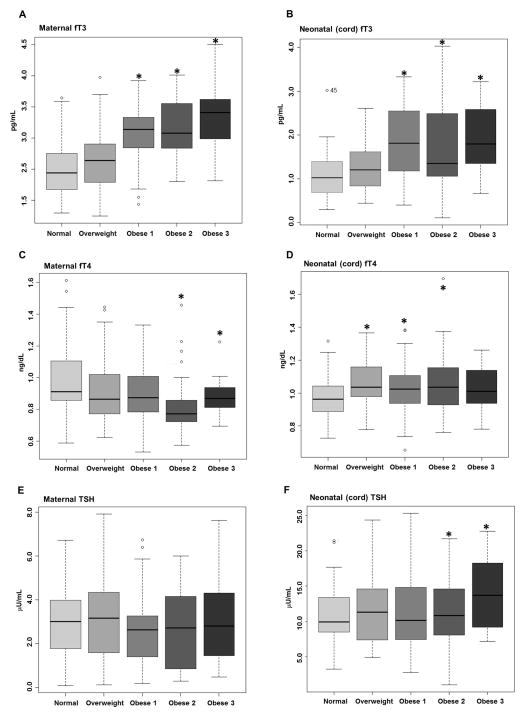
Maternal and neonatal fT3, fT4 and TSH levels were measured using ELISA. Thyroid function data was stratified into 5 categories based on pre-pregnancy or first trimester BMI. With increasing maternal obesity both maternal (Figure 1A) and neonatal (Figure 1B) fT3 increased compared to normal weight. With increasing maternal obesity, maternal fT4 decreased in the Obese 2 and 3 groups compared to Normal Weight (Figure 1C). However, neonatal fT4 increased with increasing maternal obesity in the Overweight, Obese 1 and Obese 2 categories compared with Normal Weight (Figure 1D). Maternal TSH levels were unchanged (Figure 1 E), while neonatal TSH levels were slightly increased in the Obese 2 and 3 groups compared with Normal weight (Figure 1 F).
Figure 1. Maternal and neonatal fT3 levels increase with increasing maternal obesity.
Matched maternal and neonatal (cord) serum levels of THs were measured using ELISA. Maternal (A) and neonatal (cord) (B) fT3 increased with increasing maternal obesity. Maternal fT4 (C) decreased only in the most obese subjects, while cord fT4 (D) is increased in offspring from Overweight, Obese 1 and Obese 2 subjects. While no changes in maternal TSH (E) were observed, neonatal TSH (F) slightly increases in the offspring from Obese 2 and 3 subjects.
Maternal and neonatal fT3 are positively correlated
Pearson Product Moment Correlation Coefficients were calculated to determine if maternal and neonatal thyroid parameters showed a significant correlation. Correlation coefficients were calculated for fT3, fT4, TSH and the fT3 to fT4 ratio. Maternal and neonatal fT3 levels showed a significant positive correlation (r=0.422, p<0.001, Table 2). No other parameters tested revealed a significant correlation (Table 2).
Table 2.
Maternal and neonatal (cord blood) serum fT3 are significantly correlated
| Correlation Coefficient | P-value | |
|---|---|---|
| fT3 | 0.422 | p<0.001 |
| fT4 | −0.0696 | p=0.322 |
| TSH | 0.0144 | p=0.864 |
| fT3 to fT4 ratio | 0.0949 | p=0.192 |
Maternal and neonatal fT3 to fT4 ratios are altered in association with increasing maternal obesity
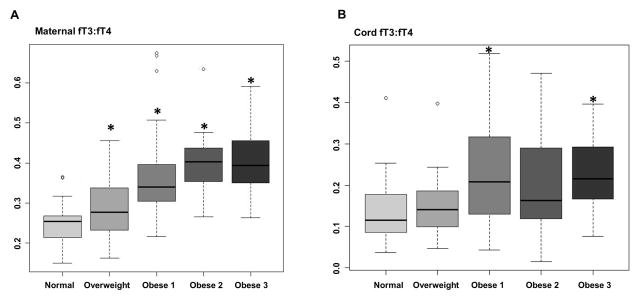
Maternal and neonatal fT3 and fT4 levels were converted to pmol/L and the ratio of fT3 to fT4 was calculated. The maternal fT3 to fT4 ratio increased with increasing maternal obesity (Figure 2A) compared with Normal weight cohorts. The neonatal fT3 to fT4 ratio only significantly differed between the Normal weight group and Obese 1 and 3 (Figure 2B).
Figure 2. Maternal and neonatal fT3 to fT4 ratios are altered in association with increasing maternal obesity.
Maternal fT3 to fT4 ratio increased with increasing maternal obesity (A) compared with Normal weight cohorts, while the neonatal fT3 to fT4 ratio only significantly differed between the Normal weight group in the Obese 1 and 3 groups (B).
Excess gestational weight gain is associated with significantly reduced maternal fT4
The potential influence of maternal weight gain (as a surrogate for caloric density or caloric load) during pregnancy on thyroid hormone levels was investigated, revealing that among all BMI categories, females with excessive weight gain exhibited significantly lower maternal fT4 levels (p=0.013) when compared to those with insufficient weight gain (Table 3).
Table 3.
Maternal fT4 decreases with excess gestational weight gain
| Insufficient | Sufficient | Excess | ||||
|---|---|---|---|---|---|---|
| Maternal | Cord | Maternal | Cord | Maternal | Cord | |
| fT3 (pg/mL) | 2.38± 0.51 | 1.44±0.82 | 2.32± 0.51 | 1.43± 0.76 | 2.45± 0.62 | 1.72± 1.04 |
| fT4 (ng/dL) | 0.95± 0.22 | 1.00± 0.15 | 0.93± 0.24 | 1.04± 0.15 | 0.86± 0.17* | 1.04± 0.14 |
| TSH (μU/mL) | 3.40± 2.34 | 12.07± 8.18 | 3.24± 2.25 | 15.59± 9.85 | 3.36± 2.54 | 14.48± 9.88 |
Significantly different compared to insufficient weight gain, p<0.05.
Maternal and neonatal fT3 and fT3:fT4 correlate with birthweight
Table 4 shows correlations between maternal and neonatal thyroid hormone levels and infant birthweight. Statistical analysis revealed that both maternal and neonatal fT3 levels and fT3 to fT4 ratios were associated with the infant’s birthweight (Table 4). We did not find any significant correlations between maternal and neonatal fT4 or TSH levels and birthweight (Table 4).
Table 4.
Maternal and fetal thyroid hormone levels correlate with birthweight
| Maternal | Neonatal (Cord blood) | |||
|---|---|---|---|---|
| β-Coefficient | Significance | β-Coefficient | Significance | |
| fT4 | −0.12 | p=0.086 | −0.085 | p=0.227 |
| fT3 | 0.155 | p=0.027 | 0.171 | p=0.018 |
| fT3 to fT4 ratio | 0.212 | p=0.002 | 0.200 | p=0.006 |
| TSH | 0.115 | p=0.136 | −0.077 | p=0.318 |
DISCUSSION
The association between altered THs, especially TSH and T3, with obesity has been well studied (9, 14). Whether these alterations are consequence or cause remains controversial. However, it is well documented that maintenance of proper TH levels during pregnancy is of great importance for fetal development (12, 21, 25). According to the DOHaD hypothesis, a metabolically perturbed in utero environment may predispose the offspring to later in life metabolic disorders (18, 29). Infants of obese mothers are more likely to be born LGA and to develop obesity in childhood (30). In this study, we sought to investigate THs as potential molecular mediators of fetal overgrowth and childhood obesity. While our study limitations include a lack of information on thyroxine binding globulin (TBG) or thyroid peroxidase (TPO) antibody levels or urinary iodine concentrations, our study has the significant strength of measuring fT3, fT4 and TSH in matched maternal and fetal serum.
It has previously been shown in non-pregnant individuals that there is a positive association between fT3 levels and body weight (11, 12). Our data supports a similar association in pregnant women at term, as fT3 levels rise with increasing maternal obesity. Of importance, we find a similar increase in fT3 in the neonate, with higher neonatal (cord blood) fT3 levels from mothers who are obese (Figure 1B), and we show that maternal and neonatal fT3 levels are significantly correlated (Table 3). We also report a significant association between maternal and neonatal fT3 with birthweight (Table 4). While the consequences of an elevated fT3 in the neonate are unknown, elevated fT3 levels have been reported in association with childhood obesity (31). It is well established that infants of mothers who are obese are more likely to be born LGA (30). How both maternal and fetal TH levels in association with maternal obesity contribute to fetal overgrowth is an important area of study.
The conversion of fT4 to fT3 occurs with deiodinase (DIO gene product) activity, which regulates local availability of fT3 (32). The concerted activity of the deiodinases can be inferred by the fT3 to fT4 ratio (33). It has been reported that both lower fT4 and a higher fT3 to fT4 ratio are associated with a less favorable metabolic outcome in healthy pregnant women as assessed by pre and post-load glucose levels, glycosylated hemoglobin, fasting insulin, HOMA-IR and triglycerides (34). Consistent with this data, we have found both decreased maternal fT4 (Figure 1C) and an increase in the maternal fT3 to fT4 ratio (Figure 2A) with increasing maternal obesity, as well as a decrease in maternal fT4 with excess gestational weight gain (Table 2). In the fetus, our data also reveal an increase in the fT3 to fT4 ratio (in the obese 1 and 3 groups, Figure 2B). However, the neonatal fT4 levels are not correlated with the maternal levels, and in fact, increase compared to normal weight in the overweight, obese 1 and 2 groups. The discrepant results between the fT3 to fT4 ratios and fT4 levels may be accounted for by the fact that the placenta has potent deiodinase activities (35, 36); investigation into potential alterations of deiodinase activity with increasing maternal obesity is warranted. These findings in humans are consistent with our prior detailed observations in our non-human primate model, and collectively support the notion that there are maternal, fetal, and likely placental modulators of circulating thyroid hormone levels during gestation (17,24).
In sum, what is of notable interest in the current study is the lack of correlation between neonatal fT4 and TSH, while fT3 is tightly and significantly correlated between maternal and fetal serum levels. While THs are essential for fetal development throughout gestation, the fetus has a fully functioning thyroid gland by the midpoint of gestation (16–20 weeks; 35). By term, it is believed that fetal TH levels are being maintained by the fetus’s own hypothalamic- pituitary- thyroid axis (37). Therefore, the extent to which maternal TH levels influence fetal levels remains to be determined. Extensive mechanistic studies of placental transport with maternal obesity are essential to determine how the maternal TH levels interact with and determine fetal levels, and to understand what long term consequences of alterations of TH levels experienced in utero may be.
Acknowledgments
We would like to acknowledge members of the Aagaard and Hawkins labs for constructive feedback. We would also like to acknowledge the PeriBank team for aiding with sample acquisition and data collection. This work was funded by NIH/NICHD K99-HD075858-02 to MAS and by the DFG (German Research Foundation, SA 2795/2-1) to MKK.
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 2.Henrichs J, Ghassabian A, Peeters RP, et al. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin Endocrinol (Oxf) 2013;79:152–162. doi: 10.1111/cen.12227. [DOI] [PubMed] [Google Scholar]
- 3.de Escobar GM, Ares S, Berbel P, et al. The changing role of maternal thyroid hormone in fetal brain development. Semin Perinatol. 2008;32:380–386. doi: 10.1053/j.semperi.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Millar LK, Wing DA, Leung AS, et al. Low birth weight and preeclampsia in pregnancies complicated by hyperthyroidism. Obstet Gynecol. 1994;84:946–949. [PubMed] [Google Scholar]
- 5.Shields BM, Knight BA, Hill A, et al. Fetal thyroid hormone level at birth is associated with fetal growth. J Clin Endocrinol Metab. 2011;96:E934–938. doi: 10.1210/jc.2010-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaidya B, Campbell V, Tripp JH, et al. Premature birth and low birth weight associated with nonautoimmune hyperthyroidism due to an activating thyrotropin receptor gene mutation. Clin Endocrinol (Oxf) 2004;60:711–718. doi: 10.1111/j.1365-2265.2004.02040.x. [DOI] [PubMed] [Google Scholar]
- 7.Haddow JE, Craig WY, Neveux LM, et al. Implications of High Free Thyroxine (FT4) concentrations in euthyroid pregnancies: the FaSTER trial. J Clin Endocrinol Metab. 2014;99:2038–2044. doi: 10.1210/jc.2014-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godoy GA, Korevaar TI, Peeters RP, et al. Maternal thyroid hormones during pregnancy, childhood adiposity and cardiovascular risk factors: the Generation R Study. Clin Endocrinol (Oxf) 2014;81:117–125. doi: 10.1111/cen.12399. [DOI] [PubMed] [Google Scholar]
- 9.Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010;316:165–171. doi: 10.1016/j.mce.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18:141–144. doi: 10.1089/thy.2007.0266. [DOI] [PubMed] [Google Scholar]
- 11.Bray GA, Fisher DA, Chopra IJ. Relation of thyroid hormones to body-weight. Lancet. 1976;1:1206–1208. doi: 10.1016/s0140-6736(76)92158-9. [DOI] [PubMed] [Google Scholar]
- 12.Michalaki MA, Vagenakis AG, Leonardou AS, et al. Thyroid function in humans with morbid obesity. Thyroid. 2006;16:73–78. doi: 10.1089/thy.2006.16.73. [DOI] [PubMed] [Google Scholar]
- 13.Kitahara CM, Platz EA, Ladenson PW, et al. Body fatness and markers of thyroid function among U.S. men and women. PLoS One. 2012;7:e34979. doi: 10.1371/journal.pone.0034979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce EN. Thyroid hormone and obesity. Curr Opin Endocrinol Diabetes Obes. 2012;19:408–413. doi: 10.1097/MED.0b013e328355cd6c. [DOI] [PubMed] [Google Scholar]
- 15.Yogev Y, Catalano PM. Pregnancy and obesity. Obstet Gynecol Clin North Am. 2009;36:285–300. viii. doi: 10.1016/j.ogc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Aviram A, Hod M, Yogev Y. Maternal obesity: implications for pregnancy outcome and long-term risks-a link to maternal nutrition. Int J Gynaecol Obstet. 2011;115(Suppl 1):S6–10. doi: 10.1016/S0020-7292(11)60004-0. [DOI] [PubMed] [Google Scholar]
- 17.Walsh JM, McAuliffe FM. Prediction and prevention of the macrosomic fetus. Eur J Obstet Gynecol Reprod Biol. 2012;162:125–130. doi: 10.1016/j.ejogrb.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Hanson M, Godfrey KM, Lillycrop KA, et al. Developmental plasticity and developmental origins of non-communicable disease: theoretical considerations and epigenetic mechanisms. Prog Biophys Mol Biol. 2011;106:272–280. doi: 10.1016/j.pbiomolbio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Suter MA, Aagaard-Tillery KM. Environmental influences on epigenetic profiles. Semin Reprod Med. 2009;27:380–390. doi: 10.1055/s-0029-1237426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holt RI. Fetal programming of the growth hormone-insulin-like growth factor axis. Trends Endocrinol Metab. 2002;13:392–397. doi: 10.1016/s1043-2760(02)00697-5. [DOI] [PubMed] [Google Scholar]
- 21.Boney CM, Verma A, Tucker R, et al. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 22.Hermann GM, Dallas LM, Haskell SE, et al. Neonatal macrosomia is an independent risk factor for adult metabolic syndrome. Neonatology. 2010;98:238–244. doi: 10.1159/000285629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ornoy A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol. 2011;32:205–212. doi: 10.1016/j.reprotox.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Owens LA, O’Sullivan EP, Kirwan B, et al. ATLANTIC DIP: the impact of obesity on pregnancy outcome in glucose-tolerant women. Diabetes Care. 2010;33:577–579. doi: 10.2337/dc09-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen DM, Damm P, Sorensen B, et al. Pregnancy outcome and prepregnancy body mass index in 2459 glucose-tolerant Danish women. Am J Obstet Gynecol. 2003;189:239–244. doi: 10.1067/mob.2003.441. [DOI] [PubMed] [Google Scholar]
- 26.Suter MA, Sangi-Haghpeykar H, Showalter L, et al. Maternal high-fat diet modulates the fetal thyroid axis and thyroid gene expression in a nonhuman primate model. Mol Endocrinol. 2012;26:2071–2080. doi: 10.1210/me.2012-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonklaas J, Kahric-Janicic N, Soldin OP, et al. Correlations of free thyroid hormones measured by tandem mass spectrometry and immunoassay with thyroid-stimulating hormone across 4 patient populations. Clin Chem. 2009;55:1380–1388. doi: 10.1373/clinchem.2008.118752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson J, Clifton RG, Roberts JM, et al. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine guidelines. Obstet Gynecol. 2013;121:969–975. doi: 10.1097/AOG.0b013e31828aea03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armitage JA, Poston L, Taylor PD. Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res. 2008;36:73–84. doi: 10.1159/000115355. [DOI] [PubMed] [Google Scholar]
- 30.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. Bjog. 2006;113:1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 31.Bas VN, Aycan Z, Agladioglu SY, et al. Prevalence of hyperthyrotropinemia in obese children before and after weight loss. Eat Weight Disord. 2013;18:87–90. doi: 10.1007/s40519-013-0008-0. [DOI] [PubMed] [Google Scholar]
- 32.Kohrle J. Thyroid hormone transporters in health and disease: advances in thyroid hormone deiodination. Best Pract Res Clin Endocrinol Metab. 2007;21:173–191. doi: 10.1016/j.beem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Nicoloff JT, Lum SM, Spencer CA, et al. Peripheral autoregulation of thyroxine to triiodothyronine conversion in man. Horm Metab Res Suppl. 1984;14:74–79. [PubMed] [Google Scholar]
- 34.Bassols J, Prats-Puig A, Soriano-Rodriguez P, et al. Lower free thyroxin associates with a less favorable metabolic phenotype in healthy pregnant women. J Clin Endocrinol Metab. 2011;96:3717–3723. doi: 10.1210/jc.2011-1784. [DOI] [PubMed] [Google Scholar]
- 35.Kurlak LO, Mistry HD, Kaptein E, et al. Thyroid hormones and their placental deiodination in normal and pre-eclamptic pregnancy. Placenta. 2013;34:395–400. doi: 10.1016/j.placenta.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 36.McKinnon B, Li H, Richard K, et al. Synthesis of thyroid hormone binding proteins transthyretin and albumin by human trophoblast. J Clin Endocrinol Metab. 2005;90:6714–6720. doi: 10.1210/jc.2005-0696. [DOI] [PubMed] [Google Scholar]
- 37.Patel J, Landers K, Li H, et al. Delivery of maternal thyroid hormones to the fetus. Trends Endocrinol Metab. 2011;22:164–170. doi: 10.1016/j.tem.2011.02.002. [DOI] [PubMed] [Google Scholar]