Abstract
Cell migration is central to normal physiology in embryogenesis, the inflammatory response and wound healing. In addition, the acquisition of a motile and invasive phenotype is an important step in the development of tumors and metastasis. Arf GTPase-activating proteins (GAPs) are nonredundant regulators of specialized membrane surfaces implicated in cell migration. Part of Arf GAP function is mediated by regulating the ADP ribosylation factor (Arf) family GTP-binding proteins. However, Arf GAPs can also function independently of their GAP enzymatic activity, in some cases working as Arf effectors. In this commentary, we discuss examples of Arf GAPs that function either as regulators of Arfs or independently of the GTPase activity to regulate membrane structures that mediate cell adhesion and movement.
Keywords: Arf GAP, Arf, effector, ADP-ribosylation factor, GTPase-activating protein, focal adhesions, podosomes, invadopodia, cell migration
Cell migration involves adhesive structures in which the cell membrane is integrated with the actin cytoskeleton.1 Cells acquire a spatial asymmetry to enable them to turn intracellular generated forces into a net cell body translocation. With the asymmetry, there is a clear distinction between the cell front and rear. Active membrane processes, including lamellipodia and filopodia, take place primarily around the cell front. Extension of both filopodia and lamellipodia is coupled with local actin polymerization, which generates protrusive force. In some cells, focal complexes form at the leading edge of lamellipodia and filopodia. Focal complexes are specialized surfaces of the plasma membrane that mediate attachment to the substratum, providing traction and allowing the cell edge to protrude. Focal complexes mature with cell migration to form another specialized surface in the plasma membrane, focal adhesions (FAs). FAs localize to the termini of stress fiber bundles and serve in longer-term anchorage at the rear of the cell.2 A contractile force is generated at the rear of the cell by the myosin motors to move the cell forward and cell-substratum (extracellular matrix) attachments are released to retract the cell rear. In some cells, podosomes are adhesive structures that mediate cell migration and sometimes invasion.
The structures involved in cell migration that are affected by Arf GAPs are FAs, podosomes and invadopodia. FAs contain multiple proteins, including integrins, which are transmembrane proteins.3 The extracellular part of integrins binds to the extracellular matrix. The cytoplasmic domains of integrins associate with multiple signaling proteins as well as proteins that are part of the actin cytoskeleton, thereby coordinating signaling events involved in cell migration and linking the extracellular matrix to the cytoskeleton. Cytoplasmic proteins critical to the function of FAs and that are often used as markers of FAs include vinculin, paxillin, and focal adhesion kinase. At least five distinct Arf GAPs have been found to associate with FAs, including GIT1, GIT2, ASAP1, ASAP3 and ARAP2.4
Podosomes and invadopodia are related structures induced by action of Src (for a review see ref. 5). They contain some proteins in common with FAs, but do have some differences that likely reflect different function and/or regulation. For example, podosomes contain ASAP1 but not ASAP3.6 Podosomes and invadopodia have not been examined for the presence of other Arf GAPs. Like FAs, podosomes and invadopodia mediate adhesion to extracellular surfaces. In addition, they are points of degradation of the extracellular matrix and may transfer tension along the extracellular matrix to enable the cell to move. Consistent with the function in motility, podosomes and invadopodia are dynamic structures, turning over in minutes. Podosomes are found in normal physiology of cells including smooth muscle cells, osteoclasts and macrophages and in Src-transformed fibroblasts. Invadopodia are observed in transformed cells, such as cells derived from breast cancers.
Two families of GTP-binding proteins within the Ras superfamily, Rho and Arf, are involved in both actin and membrane remodeling. RhoA regulates stress fibers (bundles of actin filaments that traverse the cell and are linked to the extracellular matrix through FAs) and the assembly of FAs.7 Rac1 regulates membrane ruffling and lamellipodia formation.8 Cdc42 regulates filopodia formation.9 Activation of Cdc42 has been shown to lead to the sequential activation of Rac1 and then RhoA in growth factor stimulated fibroblasts.
Arf proteins regulate membrane traffic and the actin cytoskeleton.10 There are 6 mammalian Arf proteins, divided into 3 classes based on their amino-acid sequence. Arf1, 2 and 3 are class I, Arf4 and Arf5 are class II and Arf6 is the single member of the class III group. Arf1 and Arf6 have been the most extensively studied. Most work has focused on Arf1 function in the Golgi apparatus and endocytic compartments although Arf1 has been found to affect paxillin recruitment to FAs and trafficking of epidermal growth factor receptor from the plasma membrane. Arf6 affects the endocytic pathway and the peripheral actin cytoskeleton.
The function of Rho and Arf family proteins depends on a cycle of binding and hydrolyzing GTP. However, Rho and Arf family proteins have slow intrinsic nucleotide exchange. Rho family proteins have slow intrinsic GTPase activity and Arf family proteins have no detectable intrinsic GTPase activity. The cycle of GTP binding and hydrolysis is driven by accessory proteins called guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). Rho family proteins are also regulated by guanine nucleotide dissociation inhibitors, which prevent spontaneous activation in the cytoplasm.
Arf GAPs are enzymes that catalyze the hydrolysis of GTP bound to Arf proteins, thereby converting Arf•GTP to Arf•GDP. Thirty-one genes in human encode proteins with Arf GAP domains (Figure 1). The Arf GAP family is divided into 10 subgroups based on domain structure and phylogenetic analysis.11 Six subgroups contain the Arf GAP domain at the N-terminus of the protein. Four groups contain a tandem of a PH, Arf GAP and Ankyrin repeat domains. The Arf GAP nomenclature is mostly based on the protein domain structure. For instance, the ASAP first identified, ASAP1, contains Arf GAP, SH3, Ank repeat and PH domains; ARAPs contain Arf GAP, Rho GAP, Ank repeat and PH domains; ACAPs contain Arf GAP, coiled-coil (later identified as BAR domain), Ank repeat and PH domains; and AGAPs contain Arf GAP, GTP-binding protein-like, Ank repeat and PH domains.
Figure 1. Domain structure of the Arf GAP family.
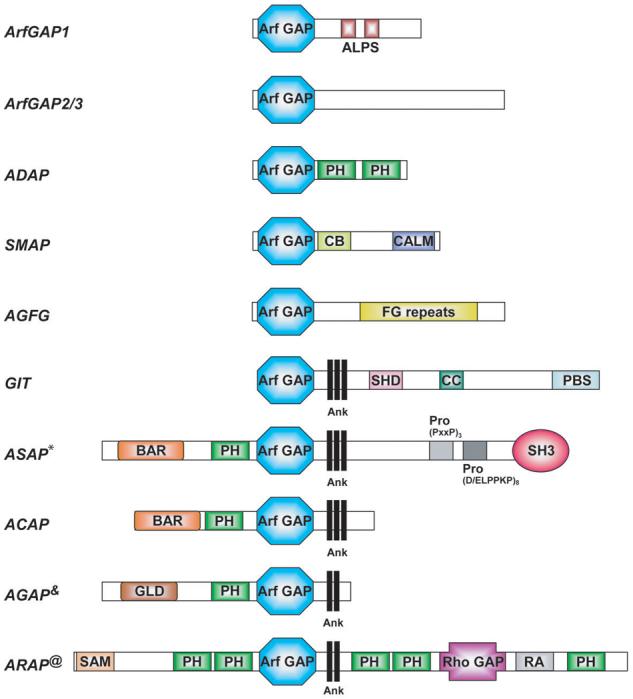
The schematic representation of the 10 groups of proteins containing the Arf GAP domain is not drawn to scale. Abbreviations used are: ALPS, ArfGAP1 Lipid-Packing Sensor domain; Ank, Ankyrin repeats; Arf GAP, Arf GTPase Activating domain; BAR, Bin/Amphiphysin/Rvs domain; CALM, CALM binding domain; CB, Clathrin Box; CC, Coiled-Coiled domain; FG repeats, multiple copies of the XXFG motif; GLD, GTP-binding protein-Like Domain; PBS, Paxillin Binding Site; PH, Pleckstrin Homology domain; Pro (PxxP)3, cluster of three Proline-rich (PxxP) motifs; Pro (D/ELPPKP)8, eigth tandem Prolin-rich (D/ELPPKP) motifs; RA, Ras Association motif; Rho GAP, Rho GTPase Activating domain; SAM, Sterile α-Motif; SH3, Src Homology 3 domain; SHD, Spa Homology Domain.
* ASAP2 and ASAP3 lack the Pro (D/ELPPKP)8 motifs. ASAP3 has no SH3 domain.
&AGAP2 has a splice variant with three N-terminal PxxP motifs, called PIKE-L.
@ARAP2 has an inactive Rho GAP domain.
The subcellular localization and function of a number of Arf GAPs have been identified. Arf GAP1, Arf GAP2 and Arf GAP3 are found in the Golgi apparatus where they control membrane traffic by regulating Arf1•GTP levels.12,13 Arf GAP1 has also been proposed to directly contribute to the formation of transport intermediates.14 SMAPs and AGAP1 and AGAP2 are associated with endosomes and regulate endocytic trafficking.14,15 ASAPs, ARAPs and Gits are associated with FAs. ASAPs, ARAPs and ACAPs are found in actin-rich membrane ruffles. ASAP1 is also found in invadopodia and podosomes.4 We propose that common to all Arf GAPs is that they laterally organize membranes, which maintain surfaces of specialized functions such as FAs and podosomes/invadopodia. Some Arf GAPs function primarily as Arf effectors with the turnover rate of the specialized membrane surface being determined by the catalytic rate of the GAP. Other Arf GAPs function as Arf regulators that integrate several signals.
ASAP1 is an example of an Arf GAP that may function as an Arf effector to regulate podosomes and invadopodia. ASAP1 is encoded by a gene on the short arm of chromosome 8. The gene is amplified in aggressive forms of uveal melanoma and cell migration rates correlate with ASAP1 expression levels in uveal melanoma16 and other cell types. ASAP1 function depends on cycling among four cellular locations, cytosol, FAs, lamellipodia and podosomes/invadopodia. ASAP1 is necessary for the formation of podosomes/invadopodia.17,18
The structural features of ASAP1 that are required to support podosome formation have been examined.17,18 ASAP1 contains, from the N-terminus, BAR, PH, Arf GAP, Ank repeat, proline rich and SH3 domains (Figure 2A).19 There are two major isoforms, ASAP1a and ASAP1b that differ in the proline rich domain. ASAP1a contains three SH3 binding motifs within the proline rich region including an atypical SH3 binding motif with 6 consecutive prolines. The atypical SH3 binding motif is absent in ASAP1b (Figure 2A). ASAP1 also has a highly conserved tyrosine between the Ank repeat and proline rich domains that is a site of phosphorylation by the oncogene Src.18
Figure 2. ASAP1 function in podosome and invadopodia formation.
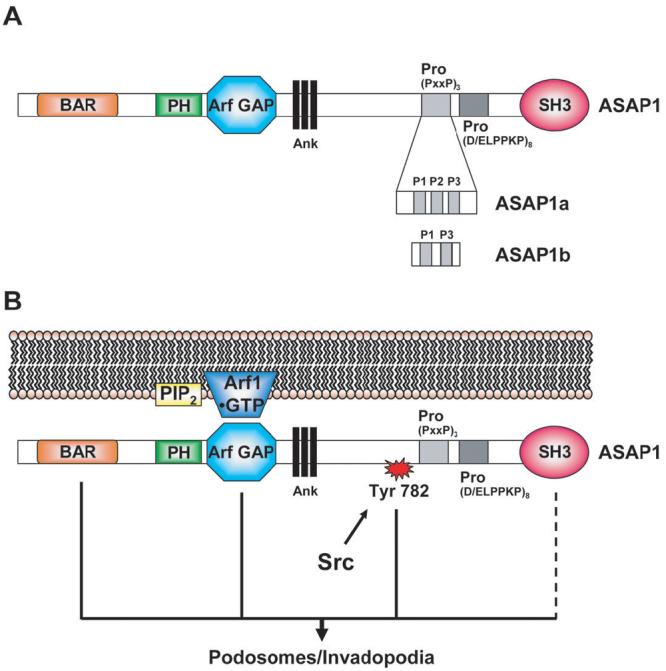
A. Domain structure of ASAP1 splice variants. ASAP1a contains three Proline-rich motifs, P1, P2 and P3. P1 and P3 contain a typical (PxxP) motif. P2 contains six Prolines. ASAP1b contains only P1 and P3. B. Model of ASAP1 functioning as an Arf effector to regulate podosome and invadopodia formation. ASAP1 integrates signals from Src, PIP2 and Arf•GTP. For abbreviations of the domain structure of ASAP1 see Figure 1.
Other abbreviations: PIP2, Phosphoinositides 4,5-biphosphate; Arf1, ADP-ribosylation factor 1.
The BAR domain is a bundle of 3 α-helices that homodimerizes to form a boomerang-shaped structure.20,21 BAR domains sense or induce membrane curvature.20 ASAP1 has been found to induce curvature dependent on its BAR domain.22 BAR domains are also protein binding sites.21 The BAR domain of ASAP1 binds to FIP3, a Rab11 and Arf6 binding proteins.23 Arf6-dependent targeting of ASAP1 is likely mediated by FIP3.23 Deletion of or introduction of point mutations into the BAR domain render ASAP1 inactive in supporting podosome formation. The relative role of membrane tubulation and protein binding in mediating the effect of the BAR domain on podosome formation has not been explored.
The SH3 domain of ASAP1 binds to focal adhesion kinase24 and pyk2.25 Either deletion of or introduction of point mutations into the SH3 domain abrogates the ability of ASAP1 to support podosome formation.18 The molecular basis for the function of the SH3 domain in podosome formation is not known. The proline rich domain binds to Src19 and CrkL.26 Whether it also binds to cortactin has not been resolved. Reports also conflict regarding the importance of the proline rich domain for podosomes/invadopodia formation.17,18
Three signals impinge on ASAP1 to drive podosome formation (Figure 2B). A conserved tyrosine between the Ank and proline rich motifs is phosphorylated by Src.18,25 Mutation of the tyrosine to phenylalanine results in a protein that functions as a dominant negative blocking podosome formation. ASAP1 with the tyrosine changed to glutamate can support podosome formation, but the mutant ASAP1 is not sufficient to drive podosome formation.18 Based on these results, phosphorylation of the conserved tyrosine is necessary but not sufficient to support podosome formation. Phosphatidylinositol 4,5-bisphosphate (PIP2) binds to the PH domain, which stimulates GAP activity in vitro.27 ASAP1 with mutations in the PH domain that abrogate binding, does not support podosome formation (Jian, Bharti and Randazzo, unpublished observations). Point mutations in the PH domain affect both the Km and the kcat for GAP activity. The effect of mutating the PH domain on the ability of ASAP1 to support podosome formation may be consequent to changes in binding Arf1•GTP; it is not likely the result of loss of GAP activity. ASAP1 with a point mutation in the GAP domain that prevents GAP activity but not Arf1•GTP binding is able to support podosome formation whereas a point mutant of ASAP1 that cannot bind Arf1•GTP does not (Jian, Bharti and Randazzo, unpublished observations).18 These data support the idea that ASAP1 integrates three signals, (i) PIP2, (ii) Src and (iii) Arf1•GTP. In response to the signals, ASAP1 functions as a scaffold and directly alters the lipid bilayer to create a domain within the plasma membrane that becomes a podosome. In this model, ASAP1 is functioning as an Arf effector and the GAP activity may regulate the turnover of podosomes.
ASAP3, another ASAP-type protein, is found in FAs.6 Reducing ASAP3 expression also reduces cell migration and invasion of mammary carcinoma cells through matrigel. Although ASAP3 does not affect the ability to form FAs, it does affect stress fiber formation and may affect focal adhesion maturation (Ha, Chen and Randazzo, unpublished observations).6 The molecular mechanisms underlying the effects of ASAP3 on the cytoskeleton are being examined including the possibility that, like ASAP1, ASAP3 integrates several signals and functions as an Arf effector.
ARAPs are examples of Arf GAP family proteins that function as Arf regulators. In common with ASAPs, they integrate a number of signaling pathways and affect the actin cytoskeleton. Three genes encode ARAPs in humans.11 Each of the ARAPs is comprised of a SAM, five PH, Arf GAP, Rho GAP, Ank repeat and Ras association domains. Two of the five PH domains have the consensus sequence for binding to the signaling lipid phosphoinositide 3,4,5-triphosphate (PIP3); however, when examined for ARAP1, PIP3 was not involved in membrane targeting (Campa, Balla and Randazzo, unpublished observations).
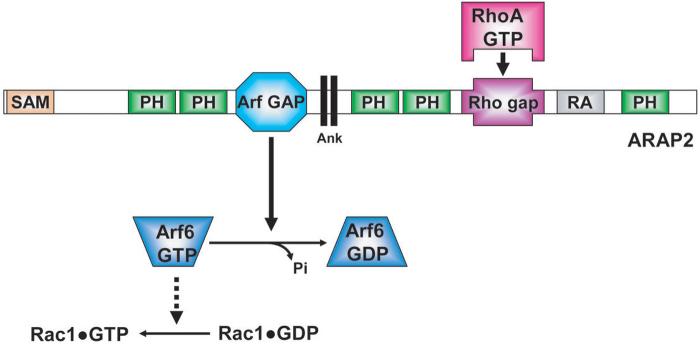
Examination of the role of ARAP2 in FA formation has provided information about the function of the GAP activity in the cellular function of an Arf GAP. ARAP2 selectively uses Arf6 as a substrate and, different from ARAP1 and ARAP3, has an inactive Rho GAP domain. The Rho GAP domain, however, retains the ability to selectively bind to RhoA•GTP. Also different from ARAP1 and ARAP3, ARAP2 associates with FAs. Cells with reduced expression of ARAP2, consequent to siRNA treatment, have fewer FAs and stress fibers and more focal complexes than control cells. The formation of FAs and stress fibers can be restored by expressing recombinant wild type ARAP2. A mutant of ARAP2 that lacks Arf GAP activity, while retaining the ability to bind to Arf6•GTP, cannot restore FA and stress fiber formation. Similarly, expression of a mutant of ARAP2 that is not able to bind RhoA•GTP cannot reverse the effect of reducing expression of endogenous ARAP2.28 These results support the idea that ARAP2 functions as an Arf GAP that is an effector of RhoA.
The model of ARAP2 functioning as a RhoA effector can explain the effects of ARAP2 on FAs (Figure 3). Arf6•GTP is involved in the formation of Rac1•GTP.29 Rac1•GTP drives lamellipodia and focal complex formation. The conversion of focal complexes to FAs is accompanied by an increase in RhoA•GTP and a decrease in Rac1•GTP. ARAP2 could function to mediate the reciprocal changes in RhoA and Rac1. RhoA•GTP formation leads to the activation of ARAP2. As a consequence of Arf6 GAP activity, Arf6•GTP is converted to Arf6•GDP. With reduced Arf6•GTP, Rac1•GTP concentration also decreases.
Figure 3. Model of ARAP2 as an Arf regulator that controls focal adhesion formation.
In this model, ARAP2 functions as a RhoA effector. The inactive Rho GAP domain of ARAP2 binds to RhoA•GTP, which contributes to activation of Arf6 GAP activity. ARAP2 hydrolyzes its substrate Arf6•GTP into Arf6•GDP. Subsequent to Arf6•GTP hydrolysis, Rac1•GTP concentration decreases. For abbreviations of the domain structure of ARAP2 see Figure 1.
The Arf GAP activity of other ARAPs may also be critical for cellular functions of the protein. Furthermore, the Rho GAP activity is slow for ARAP1 and ARAP3. It is possible that ARAP1 and ARAP3 can function as Rho effectors with an active Rho GAP domain analogously to ASAP1 functioning as an Arf effector. Further definition of the cellular function of ARAP1 and ARAP3 will provide opportunities to test this idea.
We have provided two examples of Arf GAPs that affect cell adhesion and migration. In one case, the Arf GAP appears to function as an Arf effector. In the other case, the Arf GAP functions as a regulator of Arf. The difference in function was discerned using Arf GAP mutants. If functioning as an Arf effector, an Arf GAP mutant that can bind Arf•GTP but not induce hydrolysis can reverse the effect of reduced endogenous Arf GAP, whereas a mutant that cannot bind Arf•GTP cannot replace endogenous Arf GAP. When working as an Arf regulator, a mutant that can bind Arf•GTP but not induce GTP hydrolysis cannot replace endogenous Arf GAP. Whether functioning as an effector or regulator, the rate of GAP activity determines the turnover rate of a specialized membrane surface maintained by Arf.
The Arf GAPs have specific sites of action within cells. Some contribute to malignancy, such as ASAP1, ASAP3, AGAP2 and SMAP1.30 The molecular basis of cellular function of each Arf GAPs is distinct. Here, we describe one Arf GAP that functions as an Arf effector and another that functions as an Arf regulator. Each class of Arf GAP has distinct sets of protein binding partners. Furthermore, catalytic mechanism differs among the GAPs. Because of these differences, Arf GAPs may be useful therapeutic targets for cancer therapy.
Acknowledgment
This work was supported by the Intramural Program at the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, USA.
Footnotes
The authors have no conflict of interest or financial disclosures to report.
References
- 1.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 2.Kaverina I, Krylyshkina O, Small JV. Regulation of substrate adhesion dynamics during cell motility. Int J Biochem Cell Biol. 2002;34:746–761. doi: 10.1016/s1357-2725(01)00171-6. [DOI] [PubMed] [Google Scholar]
- 3.Petit V, Thiery JP. Focal adhesions: structure and dynamics. Biol Cell. 2000;92:477–494. doi: 10.1016/s0248-4900(00)01101-1. [DOI] [PubMed] [Google Scholar]
- 4.Randazzo PA, Inoue H, Bharti S. Arf GAPs as regulators of the actin cytoskeleton. Biol Cell. 2007;99:583–600. doi: 10.1042/bc20070034. [DOI] [PubMed] [Google Scholar]
- 5.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 6.Ha VL, Bharti S, Inoue H, Vass WC, Campa F, Nie Z, de GA, Ward Y, Randazzo PA. ASAP3 is a focal adhesion-associated Arf GAP that functions in cell migration and invasion. J Biol Chem. 2008;283:14915–14926. doi: 10.1074/jbc.M709717200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 8.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 9.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 10.Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 11.Kahn RA, Bruford E, Inoue H, Logsdon JM, Nie Z, Premont RT, Randazzo PA, Satake M, Theibert AB, Zapp ML, Cassel D. Consensus nomenclature for the human ArfGAP domain-containing proteins. J Cell Biol. 2008 doi: 10.1083/jcb.200806041. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cukierman E, Huber I, Rotman M, Cassel D. The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science. 1995;270:1999–2002. doi: 10.1126/science.270.5244.1999. [DOI] [PubMed] [Google Scholar]
- 13.Frigerio G, Grimsey N, Dale M, Majoul I, Duden R. Two human ARFGAPs associated with COP-I-coated vesicles. Traffic. 2007;8:1644–1655. doi: 10.1111/j.1600-0854.2007.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nie Z, Randazzo PA. Arf GAPs and membrane traffic. J Cell Sci. 2006;119:1203–1211. doi: 10.1242/jcs.02924. [DOI] [PubMed] [Google Scholar]
- 15.Tanabe K, Kon S, Natsume W, Torii T, Watanabe T, Satake M. Involvement of a novel ADP-ribosylation factor GTPase-activating protein, SMAP, in membrane trafficking: implications in cancer cell biology. Cancer Sci. 2006;97:801–806. doi: 10.1111/j.1349-7006.2006.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehlers JP, Worley L, Onken MD, Harbour JW. DDEF1 is located in an amplified region of chromosome 8q and is overexpressed in uveal melanoma. Clin Cancer Res. 2005;11:3609–3613. doi: 10.1158/1078-0432.CCR-04-1941. [DOI] [PubMed] [Google Scholar]
- 17.Onodera Y, Hashimoto S, Hashimoto A, Morishige M, Mazaki Y, Yamada A, Ogawa E, Adachi M, Sakurai T, Manabe T, Wada H, Matsuura N, Sabe H. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 2005;24:963–973. doi: 10.1038/sj.emboj.7600588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bharti S, Inoue H, Bharti K, Hirsch DS, Nie Z, Yoon HY, Artym V, Yamada KM, Mueller SC, Barr VA, Randazzo PA. Src-dependent phosphorylation of ASAP1 regulates podosomes. Mol Cell Biol. 2007;27:8271–8283. doi: 10.1128/MCB.01781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown MT, Andrade J, Radhakrishna H, Donaldson JG, Cooper JA, Randazzo PA. ASAP1, a phospholipid-dependent arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol Cell Biol. 1998;18:7038–7051. doi: 10.1128/mcb.18.12.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 21.Habermann B. The BAR-domain family of proteins: a case of bending and binding? EMBO Rep. 2004;5:250–255. doi: 10.1038/sj.embor.7400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nie Z, Hirsch DS, Luo R, Jian X, Stauffer S, Cremesti A, Andrade J, Lebowitz J, Marino M, Ahvazi B, Hinshaw JE, Randazzo PA. A BAR domain in the N terminus of the Arf GAP ASAP1 affects membrane structure and trafficking of epidermal growth factor receptor. Curr Biol. 2006;16:130–139. doi: 10.1016/j.cub.2005.11.069. [DOI] [PubMed] [Google Scholar]
- 23.Inoue H, Ha VL, Prekeris R, Randazzo PA. Arf GAP ASAP1 Interacts with Rab11 Effector FIP3 and Regulates Pericentrosomal Localization of Transferrin Receptor-positive Recycling Endosome. Mol Biol Cell. 2008 doi: 10.1091/mbc.E08-03-0290. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Loijens JC, Martin KH, Karginov AV, Parsons JT. The association of ASAP1, an ADP ribosylation factor-GTPase activating protein, with focal adhesion kinase contributes to the process of focal adhesion assembly. Mol Biol Cell. 2002;13:2147–2156. doi: 10.1091/mbc.E02-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kruljac-Letunic A, Moelleken J, Kallin A, Wieland F, Blaukat A. The tyrosine kinase Pyk2 regulates Arf1 activity by phosphorylation and inhibition of the Arf-GTPase-activating protein ASAP1. J Biol Chem. 2003;278:29560–29570. doi: 10.1074/jbc.M302278200. [DOI] [PubMed] [Google Scholar]
- 26.Oda A, Wada I, Miura K, Okawa K, Kadoya T, Kato T, Nishihara H, Maeda M, Tanaka S, Nagashima K, Nishitani C, Matsuno K, Ishino M, Machesky LM, Fujita H, Randazzo P. CrkL directs ASAP1 to peripheral focal adhesions. J Biol Chem. 2003;278:6456–6460. doi: 10.1074/jbc.M210817200. [DOI] [PubMed] [Google Scholar]
- 27.Che MM, Boja ES, Yoon HY, Gruschus J, Jaffe H, Stauffer S, Schuck P, Fales HM, Randazzo PA. Regulation of ASAP1 by phospholipids is dependent on the interface between the PH and Arf GAP domains. Cell Signal. 2005;17:1276–1288. doi: 10.1016/j.cellsig.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Yoon HY, Miura K, Cuthbert EJ, Davis KK, Ahvazi B, Casanova JE, Randazzo PA. ARAP2 effects on the actin cytoskeleton are dependent on Arf6-specific GTPase-activating-protein activity and binding to RhoA-GTP. J Cell Sci. 2006;119:4650–4666. doi: 10.1242/jcs.03237. [DOI] [PubMed] [Google Scholar]
- 29.Radhakrishna H, Al-Awar O, Khachikian Z, Donaldson JG. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci. 1999;112(Pt 6):855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- 30.Ha VL, Luo R, Nie Z, Randazzo PA. Contribution of AZAP type Arf GAPs to cancer cell migration and invasion. Advances in Cancer Research. 2008 doi: 10.1016/S0065-230X(08)00401-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]