Abstract
Variation in the expression level and activity of genes involved in drug disposition and action (“pharmacogenes”) can affect drug response and toxicity, especially when in tissues of pharmacological importance. Previous studies have relied primarily on microarrays to understand gene expression differences, or have focused on a single tissue or small number of samples. The goal of this study was to use RNA-seq to determine the expression levels and alternative splicing of 389 PGRN pharmacogenes across four tissues (liver, kidney, heart and adipose) and lymphoblastoid cell lines (LCLs), which are used widely in pharmacogenomics studies. Analysis of RNA-seq data from 139 different individuals across the 5 tissues (20–45 individuals per tissue type) revealed substantial variation in both expression levels and splicing across samples and tissue types. This in-depth exploration also revealed 183 splicing events in pharmacogenes that were previously not annotated. Overall, this study serves as a rich resource for the research community to inform biomarker and drug discovery and use.
Keywords: RNA-seq, Pharmacogenes, Splicing, Biomarkers, JuncBASE, Pharmacogenomics of Research Network
Introduction
Variation in the expression levels and splicing of drug metabolizing enzymes, transporters, and targets, such as receptors and ion channels, has been associated with inter-individual differences in optimal drug dose, drug efficacy, and adverse drug events 1, 2. Thus, a comprehensive study of variation in the transcriptome profiles of pharmacologically relevant tissues promises to yield important insights into the molecular basis of variation in drug response. Technological advances in quantifying the transcriptome and the rapid development of high-throughput screening methodologies have led to the identification and characterization of many biomarkers of drug response 3, 4. These innovations have transformed the way we design and analyze pharmacogenomics studies and are increasingly informing development of approaches to clinical practice.
Transcriptome sequencing, or RNA-Seq, is facilitating analyses at the transcript level with an unprecedented resolution. As the technology has developed, longer reads and higher throughput have allowed for detailed evaluation of whole transcriptomes across many samples 5. Analytical approaches have emerged, including Cufflinks 6 and DESeq 7 for gene expression analysis and DEXSeq 8, MISO 9, and JuncBASE 10 for splicing analysis. However, the use of next-generation sequencing technology for pharmacogenomics research has been limited 4, 11. Although community-wide efforts such as GTEx 12 are facilitating studies of expression quantitative trait loci (eQTLs), there has not been an application of RNA-Seq to large sample sets across diverse human tissues with a focus on genes involved in drug disposition and tissues of greater pharmacological relevance and action.
In pharmacogenomics, polymorphisms that affect expression levels or result in alternative splicing of drug metabolizing enzymes are known to have large effects on drug disposition and response. For example, UGT1A1*28 (rs8175347), with seven thymine-adenine 13 repeats in the promoter region, leads to reduced transcription rates of this enzyme and profound toxicity in patients receiving the topoisomerase inhibitor, irinotecan 14, 15. Likewise, alternative splicing of CYP2D6 occurs frequently in human populations and is responsible for reduced activity of the enzyme 16. Given these large and clinically important effects in drug metabolizing enzymes, a systematic study of the transcriptome with a focus on pharmacogenes is clearly needed. While several research groups have performed transcriptome profiling and alternative splicing event analyses in human cell lines and tissues 17–19, these studies are limited to single-tissue types or use pooled samples. Thus, information about inter-individual variation in gene expression and splicing from a given tissue type or inter-tissue variation is limited, despite the value of such studies in identifying biomarkers for differential drug response or toxicity.
Given these limitations, the NIH-supported Pharmacogenomics Research Network (PGRN) initiated a transcriptome sequencing project to catalog variation in gene expression and splicing across individuals in tissues and genes of pharmacologic importance. Tissues studied include liver, a key organ for drug metabolism 20, 21, kidney, the site of excretion for many drugs 22, as well as heart and adipose tissue, where pharmacogenes can affect local drug distribution and action 23. Lymphoblastoid cell lines were also included, as they have been widely used as a cell-based model for a variety of pharmacogenomics studies 24–26. In this article, we characterized the variability in the expression and splicing of 389 PGRN pharmacogenes across individuals and between four human tissue types and lymphoblastoid cell lines, and identified novel alternative splicing events in these samples. Further, we provide this information for community use, in the form of expression and splicing profiles for 139 individuals. This resource will be valuable for future pharmacogenomics studies as both a discovery and validation platform.
Materials and Methods
Selection of Pharmacogenes
Protein coding genes were defined as those with a start codon in the Gencode v12 13 annotation. A subset of these was defined as “PGRN pharmacogenes.” Our list of 389 pharmacogenes was compiled from PharmGKB27, a curated knowledgebase about the impact of genetic variation on drug response, PharmaADME 28, the U.S. Food and Drug Administration (FDA) Pharmacogenomics Biomarkers 29 and the literature 24, 30–33. Genes that are annotated in at least two of these resources or publications were selected as PGRN pharmacogenes. These include 160 enzymes, 84 transporters, 15 ion-channels, 27 receptors, 24 nuclear receptors and other transcription factors, as well as 22 other genes, including G-protein coupled receptors that are drug targets and play an important role in drug disposition, response, or toxicity (Supplemental Table S1).
Tissues Collection, RNA Isolation, and Preparation of RNA-sequencing Library
Tissue from 24 liver, 20 kidney (cortex), 25 heart (left ventricle), 25 adipose (subcutaneous) samples, and 45 lymphoblastoid cell lines (LCLs) were obtained from PGRN research groups: the Pharmacogenomics of Anticancer Agents Research in Children (PAAR4Kids) provided liver tissues, Pharmacogenomics of Membrane Transporters (PMT) provided kidney samples, Pharmacogenomics and Risk of Cardiovascular Disease (PARC) provided adipose tissue and lymphoblastoid cell lines, and Pharmacogenomics of Arrhythmia Therapy (PAT) provided heart tissue. Demographic information on the samples is described in Supplementary Table S2.
Total RNA was extracted for each sample, selected for mRNA by poly-A selection, and then fragmented to a mean length of ~120–180 base pairs. Strand-specific cDNA libraries were prepared and sequenced on an Illumina HiSeq 2000 at depths of 45–171 million paired end 100bp reads per sample.
Alignment and Transcriptome Analysis
Raw reads were mapped to the human genome sequence (hg19) 34 using Tophat v2.0.6 35 and PCR duplicates were removed. Some samples had a low percentage of unique reads likely due to limited starting material. Transcript structure assembly was performed with Cufflinks (v.2.0.2) 6 on each sample for each tissue type. To control for differing sequencing depths between tissue types, and the variable number of samples analyzed for each tissue type, gene expression analysis was performed on a subset of the data: 20 million reads per sample and 18 samples per tissue type. Gene expression values (in Fragments per Kilobase of Exon Mapped, FPKM) were calculated by summing per-isoform FPKM values generated by Cuffdiff (v2.2.1) 6 for each sample or by tissue type. Throughout, gene estimates are used unless isoforms are specifically mentioned.
To discover novel splice events and analyze differential splicing, the subsampled reads were run through the JuncBASE v0.6 10 pipeline. JuncBASE uses junction reads from an RNA-Seq experiment to calculate inclusion and exclusion of individual splicing events. These are measured as percent spliced in (PSI). Such measures are generally more reliable than isoform reconstruction as they require less inference.
Validation
To validate selected splice events that were not found in the gene annotations, we created primers specific to the novel event and looked for amplification by PCR using pooled liver cDNA. (Supplemental Figure S1). To validate the PSI estimates derived from RNA-seq, PSI values for two common and previously annotated splice variants in HMGCR13(−) 13 and LDLR4(−) 13, were quantified by qPCR in LCLs (n=39) from the same RNA that was used to prepare the RNA-seq libraries. The PSI values for these two events in LCLs calculated by qPCR and RNA-seq were positively correlated with R2 values of 0.43 and 0.5 (Supplemental Figure S2).
To validate the patterns of pharmacogene expression and splicing identified in this study, we analyzed data from the Genotype Tissue Expression (GTEx) Project (v4)36. Expression (RPKM, mapped reads per kilobase per million mapped reads) values per individual, per gene were downloaded from the GTEx portal (http://gtexportal.org) to study variability in gene expression and patterns of expression across tissues. Aligned reads were downloaded from SRA/dbGaP and run through the JuncBASE pipeline in the same way as was done for the PGRN data to compare differential splicing patterns between the two datasets and novel junctions identified in the PGRN dataset.
Further details regarding all methodology are included in the supplemental methods.
Results
The Pharmacogenomics Research Network RNA-Seq Project
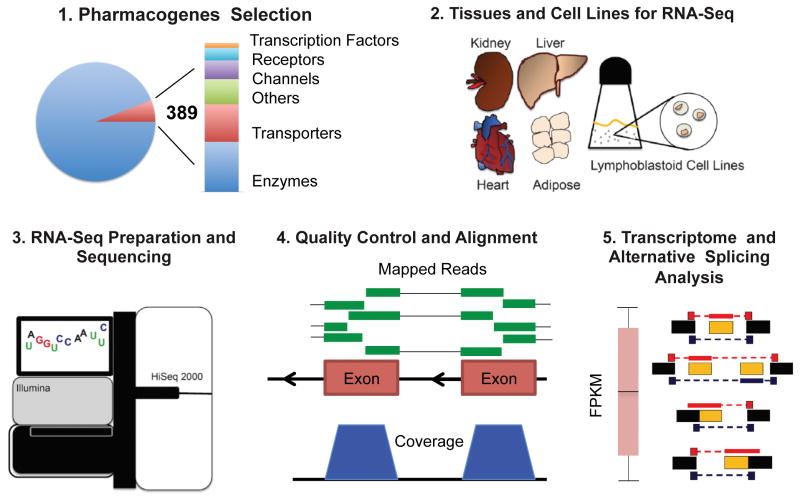
The Pharmacogenomics Research Network (PGRN) RNA-Seq project was designed to provide in-depth investigation of the transcriptomes of pharmacologically relevant human tissues with a focus on genes of particular interest to the pharmacogenomics community (Figure 1). In order to study inter-individual variability in expression and splicing of PGRN pharmacogenes (Supplemental Table S1), we generated transcriptome data from 24 liver, 20 kidney, 25 heart, and 25 adipose samples and 45 LCLs (Supplemental Table S2). For each sample, reads were mapped to the human genome 35, resulting in 10 to 97 million mapped reads per sample. To control for this substantial difference in sequencing depth and sample number between tissues, 18 samples for each tissue were selected and subsampled down to 20 million reads/sample for further expression and splicing analyses, resulting in a total of 90 samples. Gene expression and splicing results are available for download (http://pharmacogenetics.ucsf.edu/expression/rnaseqdata.html, GSE70503 http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=kvsleaoqfnittwr&acc=GSE70503), and reads are deposited in the Sequence Read Archive (SRP060355). The expression profiles of all pharmacogenes across tissues and individuals is included in Supplemental Figure S3. A brief overview of alternative splicing and gene expression of all protein coding genes can be found in the supplemental results.
Figure 1.
Overview of the Pharmacogenomics Research Network RNA-Seq project. 1. 389 “PGRN pharmacogenes” were selected representing genes that play a key role in drug disposition. 2. RNA from multiple samples for human liver, heart, kidney, adipose tissue, and lymphoblastoid cell lines was collected. 3. cDNA libraries were prepared from these samples and sequenced using an Illumina HiSeq 2000. 4. Rigorous pre- and post- alignment quality control procedures were applied to the data. 5. Gene expression was quantified and splicing events identified for the PGRN pharmacogenes across samples and tissue types. This information is provided as a resource to the pharmacogenomics community.
Analysis of PGRN pharmacogene gene expression
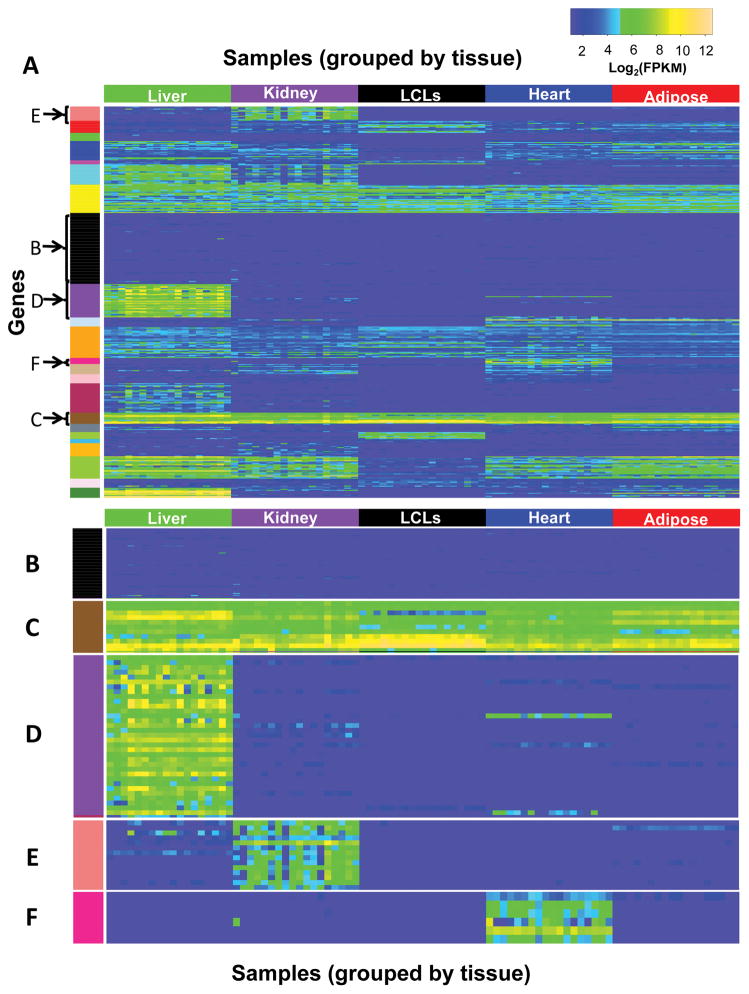
We found that 161 (of 389) of our PGRN pharmacogenes were expressed at FPKM ≥ 1 in at least one sample across all five tissue types in our data set and 87 pharmacogenes were expressed at FPKM ≥1 in all samples of all five tissue types (Supplemental Table S3A). As a group, PGRN pharmacogenes were significantly enriched for variable gene expression between individuals, and were among the top ten most variably expressed gene sets (classified by gene ontology biological process 37) in the physiological tissues (Supplemental Table S4). We also observed subsets of pharmacogenes that showed similar patterns of expression across the different tissues (k-means clustering of gene expression of 389 pharmacogenes, Figure 2A). For example, some pharmacogenes were expressed consistently at low levels across all tissues and samples (e.g. ABCC12 and ESR2, Figure 2B). In contrast, 11 pharmacogenes were very highly expressed, though to different levels, across all tissues and LCLs (Figure 2C); these include genes involved in mitochondrial structure or function (ADH5, ALDH2, CYB5R3, SOD2) and glutathione transferase activity (GSTK1, GSTO1, GSTP1) 37.
Figure 2.
(A) Heatmap of the 389 PGRN pharmacogenes’ expression (FPKM) across 90 samples. Samples are arranged horizontally, grouped by tissue. Pharmacogenes are arranged vertically, grouped by clusters identified by K-means clustering; clusters are indicated by colors along the left side of the heatmap. Selected clusters show (B) genes expressed at low levels across all samples (ABCB5, ABCC12, ABCC8, ADH7, ADRB3, ALDH3A1, BDNF, CACNA1S, CFTR, CHRM3, CHST13, CHST4, CHST5, CHST6, CHST8, CRHR1, CYP11B1, CYP11B2, CYP26A1, CYP26C1, CYP2A13, CYP2F1, CYP2S1, CYP4F8, CYP4Z1, CYP7A1, DRD1, DRD2, DRD3, DRD4, DRD5, ESR2, FMO6P, GNB3, GRM3, GSTA3, GSTA5, GSTT2, HTR1A, HTR2A, IL28B, KCNE2, MMP3, OPRM1, P2RY1, PNMT, PRSS53, RYR1, SCN3B, SLC10A2, SLC22A13, SLC22A14, SLC22A16, SLC22A4, SLC28A2, SLC28A3, SLC6A3, SLC6A4, SLCO1A2, SLCO6A1, SULT1A3, SULT4A1, TPH1, TPH2, TPSG1, UGT1A10, UGT1A5, UGT1A8, UGT2B11, UGT2B28) (C) genes highly expressed across all samples (ADD1, ADH5, ALDH2, CYB5A, CYB5R3, GSTK1, GSTO1, GSTP1, HLA-B, RPL13, SOD2) or genes expressed at higher levels in (D) liver (ABCB4, ABCC2, ADH1A, ADH4, APOA4, APOB, CYP2A6, CYP2B6, CYP2C18, CYP2C8, CYP2C9, CYP2D6, CYP2J2, CYP3A4, CYP3A5, CYP4F11, CYP8B1, F2, F5, MAT1A, NAT2, PON1, PON3, SERPINA7, SLC22A1, SLCO1B1, SLCO1B3, SULT2A1, UGT1A1, UGT1A4, UGT2B10, UGT2B15, UGT2B4), (E) kidney (ABP1, FMO1, GSTA2, GSTO2, HSD11B2, SLC13A1, SLC13A3, SLC22A11, SLC22A12, SLC22A2, SLC22A6, SLC22A8, SULT1C2, UGT8), or (F) heart (ADRB1, CACNA1C, KCNH2, NPPB, RYR2, SCN5A). Gene names are listed in order from top to bottom in each cluster in the figure. Plot drawn using R package gplots. 74
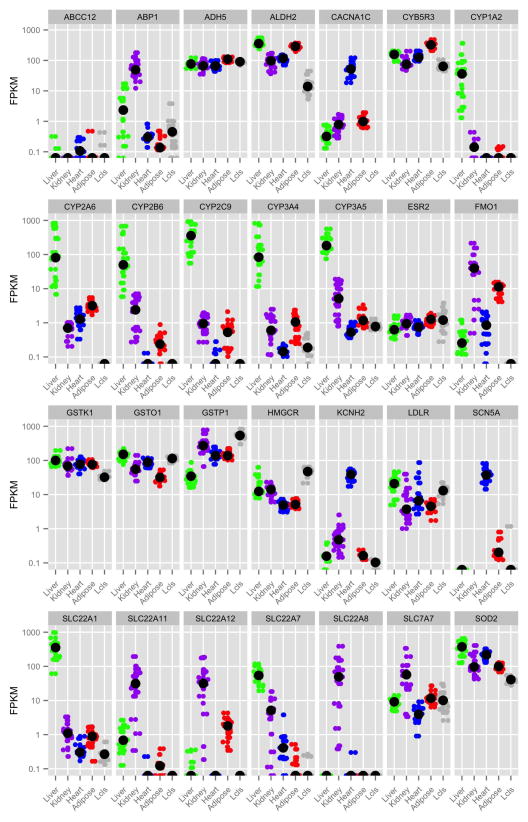
Not surprisingly, PGRN pharmacogenes are generally more highly expressed in liver compared to the other tissues (Supplemental Figure S4). Many genes coding for xenobiotic metabolizing enzymes and transporters were highly and specifically expressed in the liver, an organ important for drug metabolism (Figure 2D, Supplemental Table S5A). Pharmacogenes expressed at highest abundance in the kidney, the major organ for secretion and reabsorption, include a number of solute carrier transporters (SLC genes) (Figure 2E), which play important roles in drug secretion or reabsorption 38, as well as enzymes such as ABP1 and FMO1. In addition, pharmacogene expression levels in the liver and kidney varied greatly among individuals. For example, the expression levels of a number of CYPs in the liver and SLC transporters in the kidney varied by over 100-fold and 1000-fold respectively (Figure 3).
Figure 3.
Gene expression (FPKM) by sample across each tissue type and LCLs for selected cytochrome P450 (CYP) enzymes, solute carrier family (SLC) transporters, and other pharmacogenes discussed in this article from subsampled data (18 samples per tissue type, 20 million reads per sample). The black dot indicates median FPKM per gene and tissue type. See Supplemental Figure 3 for plots for all pharmacogenes. Plots drawn using R package ggplot2.75
The list of PGRN pharmacogenes included 119 (out of 389) genes that are currently drug targets or are under clinical development as potential targets for various diseases 39. These drug target genes may be expressed abundantly in tissues not primarily involved in drug disposition. For example, a small number of pharmacogenes were highly expressed solely in the heart (Figure 2F). These genes are all involved with cardiac contractility and include, for example, ion channels involved in cardiac conductance (SCN5A, CACNA1C, KCNH2) that are targeted by many drugs 40–42. Most pharmacogenes expressed (FPKM ≥ 1) in adipose tissue were expressed in other tissues as well (Figure 2A). The strongest correlation of pharmacogene expression profiles among tissues were detected between adipose and heart (r=0.83), as is true for all protein-coding genes expression between adipose and heart (r=0.90) (Supplemental Table S6).
Compared to the four physiological tissues, LCLs showed lower overall expression levels of pharmacogenes: proportionally fewer pharmacogenes were expressed in at least one LCL sample or expressed in all LCL samples compared to all protein-coding genes (chi-square test: 48% vs. 64%, p<0.0001 and 30% vs. 48%, p<0.0001 in at least one sample or all samples respectively, Supplementary Table 3). Pharmacogenes expressed at lower levels in LCLs than in the tissues assayed include genes important for drug disposition - for example, genes coding for enzymes (cytochrome P450s, UGTs, SULTs), SLC transporters, ion channels and receptors (Supplemental Table S5B).
Analysis of PGRN pharmacogene splicing
We found that 278 of the 389 pharmacogenes (72%) showed clear evidence of being alternatively spliced (≥ 2 isoforms) in our data set. Receptor and channel genes are the least alternatively spliced (<50%, Supplementary Table S7), although, likely due to the small numbers of genes, only receptors are significantly depleted (Bonferroni-corrected p<0.05, hypergeometric test). Another 66 pharmacogenes had inconclusive evidence of being alternatively spliced either because the alternative splice event is very rare, or because of low gene expression. The other 45 pharmacogenes are substantially expressed (FPKM>10) in at least one sample but have no evidence of alternative splice events in this dataset.
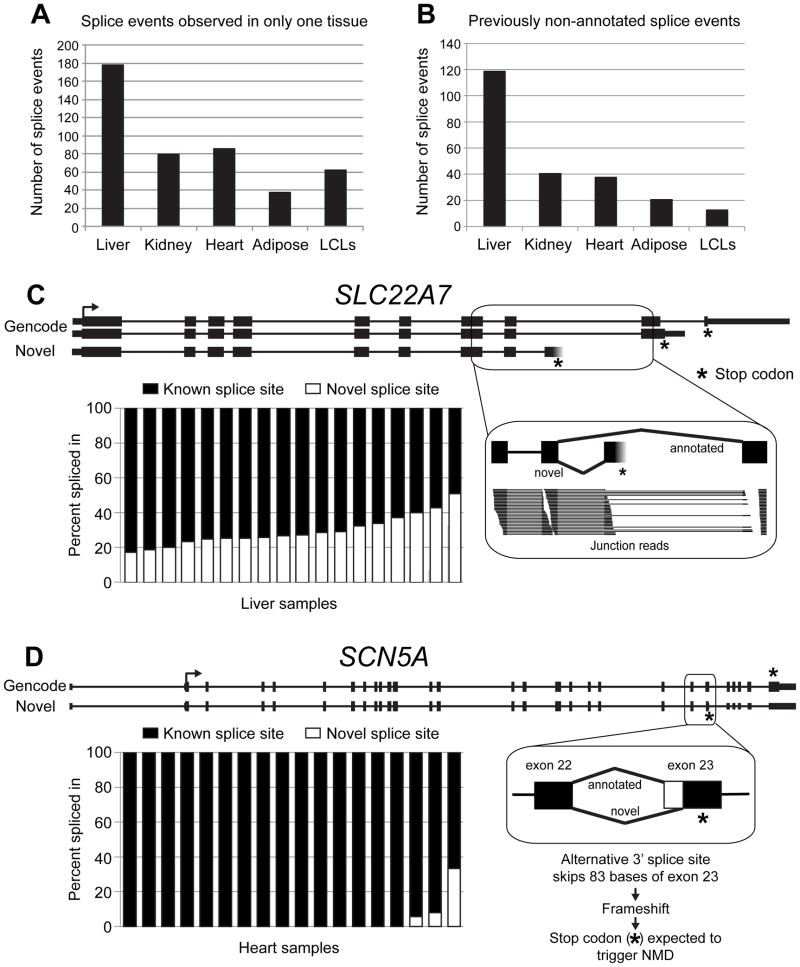
Differential alternative splicing between pairs of tissues was evident for 30–70% of PGRN pharmacogenes tested (Wilcoxon test, FDR<0.05) (Supplementary Table S8), with LCLs showing the greatest differences in splicing events compared with the other tissues. We also found dozens of inferred splice events that were only observed in one of our five tissue types, often because the gene was not expressed in other tissues but also possibly because only alternative splice events were used in those tissue types (Figure 4A). When we control for gene expression differences between tissues by requiring the potentially alternatively spliced region to have high total read coverage in a number of samples for the four other tissues, we see only a very small fraction of genes (0–5%) have tissue-specific splice events (Supplementary Table S9).
Figure 4.
(A) Splice events in PGRN pharmacogenes with PSI (percent spliced in) ≥5 and coverage ≥1 reads/100bp in at least one sample of one tissue and no coverage in any of the four other tissues. (B) Splice events in pharmacogenes not present in current gene annotations with coverage ≥5 reads/100bp in at least one sample. These splice events were identified in 68, 31, 18, 16, and 10 pharmacogenes in liver, kidney, heart, adipose tissue and LCLs respectively. (C) An alternative last exon in SLC22A7, not previously annotated, was observed in liver samples and would alter the C-terminal end of the protein. Chart: fraction of transcripts from SLC22A7 that contain the novel (white) or known (black) splice event in each liver sample. Inset: reads crossing the alternative junctions in a liver sample. (D) A novel alternative 3′ splice site in SCN5A was identified that results in an 83-base deletion of the coding sequence of SCN5A, creating a premature stop codon expected to trigger nonsense-mediated mRNA decay. Chart: fraction of transcripts from SCN5A that contain the novel (white) or known (black) splice event in each heart sample.
Notably, a total of 183 alternative splicing events (in 102 out of 389 genes) included splice junctions not previously annotated, but which were present with a robust coverage of at least 5 reads/100bp in at least one sample (Figure 4B). The greatest number of previously non-annotated pharmacogene splicing events was observed in the liver samples, likely because many of those genes are very highly expressed in that tissue, making it easier to observe these often low expressed events. One of the novel splicing events observed in liver was an alternative last exon of SLC22A7, a gene that encodes a transporter of endogenous compounds and prescription drugs (Figure 4C). This newly found alternative event was validated by PCR (Supplemental Figure S1), is predicted to produce a protein with a truncated C-terminus, and was substantially and variably expressed in the liver samples. A novel splicing event observed in heart was in SCN5A, a gene encoding a sodium channel important in maintaining normal cardiac rhythm (Figure 4D). Observed in three heart samples, this novel alternative 3′ splice site in exon 23 excludes 83 bases and generates a downstream premature termination codon that is expected to cause the transcript to be degraded by the nonsense-mediated mRNA decay pathway.
Discussion
Over the last several years, there have been many studies using RNA-Seq to quantify gene expression and to identify novel alternative splicing events in many tissue and cell types 43–48. Here, we applied this approach to characterize the expression of 389 genes of pharmacologic importance (genes involved in drug disposition, response or toxicity) in multiple human tissue types and LCLs. Unlike many other transcriptome profiling studies using RNA-Seq, this report presents findings for multiple samples across tissues, allowing the capture of inter-individual variation in expression levels in addition to comparison of expression and splicing across different tissues. Further, results in multiple subjects act as biological replicates for a given tissue type, allowing for a more accurate representation of tissue-specific splicing and expression. By incorporating inter-individual variation in our study of several human tissues, our data represent an important addition to our understanding of human transcriptomics. This dataset is available at http://pharmacogenetics.ucsf.edu/expression/rnaseqdata.html.
In comparing global analyses of protein-coding and pharmacogene expression, we observed several interesting patterns. Prominently, the majority of PGRN pharmacogenes were expressed at lower levels in LCLs compared to the four physiological tissues studied, in contrast to expression levels across all protein coding genes. As an actively and aggressively proliferating cell type, gene expression in LCLs is tuned to growth, and thus relative expression of genes involved in other cellular processes may be suppressed. Further, it is possible that peripheral B-lymphocytes, the primary cells from which LCLs are derived, also show significantly different patterns of expression from the other four physiological tissues included in this study. These results suggest that consideration of the phenotype and gene of interest is important when using LCLs as a proxy for other tissues in pharmacogenetic studies, as well as when using tissues as proxies for each other. Overall, more pharmacogenes were expressed at higher levels in the liver compared to other tissues. While this result is not unexpected given the importance of the liver in drug metabolism and transport and the bias towards liver-specific genes in the field of pharmacogenomics, it also demonstrates the importance of conducting studies in samples of the relevant tissue type where possible. We also observed high correlation in gene expression values between adipose and heart tissues among both protein coding genes and the subset of PGRN pharmacogenes. This result is consistent with the finding that adipose derived stem cells have been shown to spontaneously differentiate into cardiomyocytes and that both adipose and cardiac tissues derive from the mesoderm 49, 50.
We also observed interesting patterns of alternative splicing in this study, including the discovery of splicing events not previously annotated and significant differential splicing between LCLs and other tissue types. Since splicing detection is dependent on sequencing coverage and the number of samples analyzed, we investigated the effects of subsampling down the number of reads and samples to make them equivalent between tissues (Supplementary Results, Supplementary Figure S6). Using only 18 samples per tissue, we were still able to detect 95% of splice events we would be able to observe with all samples in our data set. Subsampling the reads limits the detection of rare splicing events which particularly affects novel splice events, as they generally have low PSI values and, thus, low read coverage (Supplementary Figure S7), or occur in only a small number of samples. As rare splice events may represent physiologically relevant alternative splicing, the splicing results from using all of our data (all reads, all samples) are also available to download.
Among the splicing events identified, we observe both previously characterized as well as novel alternative splicing events. For example, an alternative 3′ splice site in the drug target SCN5A generates a premature termination codon predicted to trigger the nonsense-mediated mRNA decay pathway (NMD). SCN5A encodes the main cardiac voltage-gated sodium channel important in maintaining normal cardiac conduction. A number of drugs target sodium channels, including antiarrhythmics and non-antiarrhythmic sodium channel blockers. Changes in structure, activity, and expression of drug targets, such as that encoded by SCN5A, can alter the efficacy of drugs designed to target these proteins 51, 52. This event may be indicative of a novel role for alternative splicing coupled with NMD in the regulation of this gene 53. Additionally, a novel truncated isoform of the transporter SLC22A7 was identified. The gene SLC22A7 is expressed in both kidney and liver and is important for transport of endogenous compounds 54 and a number of prescription drugs 55–57.
We also observed substantial variability in gene expression, particularly among drug transporters and drug metabolizing enzymes. In the liver, several cytochrome P450 (CYP) enzymes showed significant variability in expression levels between individuals; such variability can drive differences in drug metabolism across individuals, leading to variation in drug efficacy and susceptibility to toxicity 58. One example includes CYP3A4, which is responsible for activation and deactivation of a number of drugs by oxidation in the liver. Induction of CYP3A4 by concomitant medications or dietary supplements is well-established, and is considered a major source of variation in drug response 59. The enormous inter-individual variation in the expression levels of CYP3A4 we observe in the liver samples may be due to differences in diet, including dietary supplements, or medications among the individuals, in addition to genetic variation. Like drug metabolism, renal elimination of drugs is also variable across individuals in part due to variation in renal secretion and reabsorption; this variation can be driven by differences in expression levels of renal transporters across individuals. We observed profound differences in the expression levels of renal secretory and reabsorptive transporters, particularly the solute carrier transporters (SLCs). For example, expression of the uric acid transporter SLC22A12 varied almost 1000-fold between individuals in the kidney (Figure 3). As a target for drugs that treat hyperuricemia 60, the expression level of SLC22A12 could be an important determinant of drug response.
As is true of any study using human organs, while only healthy tissues were used for mRNA extraction, the patients themselves may have had a disease affecting other organs or may have been taking medications. In particular for the study of pharmacogenes, the variability in xenobiotic exposure is a concern, as such exposure is known to alter pharmacogene expression 61, 62 and splicing 63–67 profiles. The fact that the variability in splicing and expression both within and between tissues was similar to that identified in an alternate dataset with different samples suggests that the patterns of splicing and expression detected are not driven by a single overrepresented disease, phenotype, or environmental exposure in our dataset; however, in both datasets the variability detected may be driven in part by differences in health status or exposures between individuals. Patient age and sex can also explain a portion of variability in gene expression 68, 69; for example, a few pharmacogenes appeared to show higher expression levels in samples from pediatric patients. Given the small sample sizes and skewed sex and age distributions in some of the tissue types, this study was not optimal for investigating variation due to these two factors. Finally, other potential sources of variability in our dataset include subtle differences in cellular composition of the tissue samples or sample collection protocols.
Pharmacogenomic studies have largely focused on the effects of genetic polymorphisms in pharmacogenes on drug response and drug toxicity 70, 71. Our data suggest that genes involved in drug disposition and toxicity can be variably spliced and expressed among individuals and across tissues. Further, given that splicing can affect expression, localization, and function of genes 72, 73, our results suggest that splicing may be a relatively unexplored source of variability in drug response, toxicity, and efficacy. Transcriptome profiling (including both expression and splicing) of pharmacogenes may be a valuable tool for identification of mechanisms and possible prediction of this variability. As the first in depth analysis of transcript structure and expression of genes that play a key role in drug disposition, this PGRN RNAseq resource will be valuable for biomarker and drug target discovery and validation.
Supplementary Material
Acknowledgments
This study is supported in part by the NIH Pharmacogenomics Research Network (PGRN) RNA Sequencing Project and grants U01GM61390, HL65962, U19HL069757, R01GM094418 and U01GM092666. AC was supported by U01GM61390 and GM007175. CEF was supported by the Department of Defense (DoD) through the National Defense Science & Engineering Graduate Fellowship (NDSEG) Program. SEB received funding from Tata Consultancy Services.
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
Supplementary information is available at The Pharmacogenomics Journal’s website.
References
Uncategorized References
- 1.Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. The New England journal of medicine. 2011;364(12):1144–1153. doi: 10.1056/NEJMra1010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans WE, McLeod HL. Pharmacogenomics--drug disposition, drug targets, and side effects. The New England journal of medicine. 2003;348(6):538–549. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed S, Syed BA. Commercial prospects for genomic sequencing technologies. Nature reviews Drug discovery. 2013;12(5):341–342. doi: 10.1038/nrd4006. [DOI] [PubMed] [Google Scholar]
- 4.Smith RP, Lam ET, Markova S, Yee SW, Ahituv N. Pharmacogene regulatory elements: from discovery to applications. Genome medicine. 2012;4(5):45. doi: 10.1186/gm344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dijk EL, Auger H, Jaszczyszyn Y, Thermes C. Ten years of next-generation sequencing technology. Trends in genetics : TIG. 2014;30(9):418–426. doi: 10.1016/j.tig.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature protocols. 2012;7(3):562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anders S, Huber W. Differential expression analysis for sequence count data. Genome biology. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anders S, Reyes A, Huber W. Detecting differential usage of exons from RNA-seq data. Genome research. 2012;22(10):2008–2017. doi: 10.1101/gr.133744.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz Y, Wang ET, Airoldi EM, Burge CB. Analysis and design of RNA sequencing experiments for identifying isoform regulation. Nature methods. 2010;7(12):1009–1015. doi: 10.1038/nmeth.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks AN, Yang L, Duff MO, Hansen KD, Park JW, Dudoit S, et al. Conservation of an RNA regulatory map between Drosophila and mammals. Genome research. 2011;21(2):193–202. doi: 10.1101/gr.108662.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy JJ, McLeod HL, Ginsburg GS. Genomic medicine: a decade of successes, challenges, and opportunities. Science translational medicine. 2013;5(189):189sr184. doi: 10.1126/scitranslmed.3005785. [DOI] [PubMed] [Google Scholar]
- 12.The Genotype-Tissue Expression (GTEx) project. Nature genetics. 2013;45(6):580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome research. 2012;22(9):1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyer L, Das S, Janisch L, Wen M, Ramirez J, Karrison T, et al. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. The pharmacogenomics journal. 2002;2(1):43–47. doi: 10.1038/sj.tpj.6500072. [DOI] [PubMed] [Google Scholar]
- 15.Tukey RH, Strassburg CP, Mackenzie PI. Pharmacogenomics of human UDP-glucuronosyltransferases and irinotecan toxicity. Molecular pharmacology. 2002;62(3):446–450. doi: 10.1124/mol.62.3.446. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Poi MJ, Sun X, Gaedigk A, Leeder JS, Sadee W. Common CYP2D6 polymorphisms affecting alternative splicing and transcription: long-range haplotypes with two regulatory variants modulate CYP2D6 activity. Human molecular genetics. 2014;23(1):268–278. doi: 10.1093/hmg/ddt417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, Zhao K, Jiang P, Lu ZX, Wang J, Murray JC, et al. Transcriptome landscape of the human placenta. BMC genomics. 2012;13:115. doi: 10.1186/1471-2164-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farkas MH, Grant GR, White JA, Sousa ME, Consugar MB, Pierce EA. Transcriptome analyses of the human retina identify unprecedented transcript diversity and 3. 5 Mb of novel transcribed sequence via significant alternative splicing and novel genes. BMC genomics. 2013;14(1):486. doi: 10.1186/1471-2164-14-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature methods. 2008;5(7):621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 20.Barton HA, Lai Y, Goosen TC, Jones HM, El-Kattan AF, Gosset JR, et al. Model-based approaches to predict drug-drug interactions associated with hepatic uptake transporters: preclinical, clinical and beyond. Expert opinion on drug metabolism & toxicology. 2013;9(4):459–472. doi: 10.1517/17425255.2013.759210. [DOI] [PubMed] [Google Scholar]
- 21.Gandhi A, Moorthy B, Ghose R. Drug disposition in pathophysiological conditions. Current drug metabolism. 2012;13(9):1327–1344. doi: 10.2174/138920012803341302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masereeuw R, Russel FG. Therapeutic implications of renal anionic drug transporters. Pharmacology & therapeutics. 2010;126(2):200–216. doi: 10.1016/j.pharmthera.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Lai Y, Varma M, Feng B, Stephens JC, Kimoto E, El-Kattan A, et al. Impact of drug transporter pharmacogenomics on pharmacokinetic and pharmacodynamic variability - considerations for drug development. Expert opinion on drug metabolism & toxicology. 2012;8(6):723–743. doi: 10.1517/17425255.2012.678048. [DOI] [PubMed] [Google Scholar]
- 24.Huang RS, Duan S, Kistner EO, Zhang W, Bleibel WK, Cox NJ, et al. Identification of genetic variants and gene expression relationships associated with pharmacogenes in humans. Pharmacogenetics and genomics. 2008;18(6):545–549. doi: 10.1097/FPC.0b013e3282fe1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangravite LM, Engelhardt BE, Medina MW, Smith JD, Brown CD, Chasman DI, et al. A statin-dependent QTL for GATM expression is associated with statin-induced myopathy. Nature. 2013 doi: 10.1038/nature12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheeler HE, Dolan ME. Lymphoblastoid cell lines in pharmacogenomic discovery and clinical translation. Pharmacogenomics. 2012;13(1):55–70. doi: 10.2217/pgs.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, et al. Pharmacogenomics knowledge for personalized medicine. Clinical pharmacology and therapeutics. 2012;92(4):414–417. doi: 10.1038/clpt.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montreal Heart Institute Pharmacogenomics Center. PharmaADME. 2013. [Google Scholar]
- 29.U.S. Food and Drug Admnistration. Table of Pharmacogenomic Biomarkers in Drug Labels. 2013. [Google Scholar]
- 30.Rukov JL, Wilentzik R, Jaffe I, Vinther J, Shomron N. Pharmaco-miR: linking microRNAs and drug effects. Briefings in bioinformatics. 2013 doi: 10.1093/bib/bbs082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivanov M, Kals M, Kacevska M, Metspalu A, Ingelman-Sundberg M, Milani L. In-solution hybrid capture of bisulfite-converted DNA for targeted bisulfite sequencing of 174 ADME genes. Nucleic acids research. 2013;41(6):e72. doi: 10.1093/nar/gks1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gamazon ER, Skol AD, Perera MA. The limits of genome-wide methods for pharmacogenomic testing. Pharmacogenetics and genomics. 2012;22(4):261–272. doi: 10.1097/FPC.0b013e328350ca5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sissung TM, English BC, Venzon D, Figg WD, Deeken JF. Clinical pharmacology and pharmacogenetics in a genomics era: the DMET platform. Pharmacogenomics. 2010;11(1):89–103. doi: 10.2217/pgs.09.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karolchik D, Barber GP, Casper J, Clawson H, Cline MS, Diekhans M, et al. The UCSC Genome Browser database: 2014 update. Nucleic acids research. 2014;42(Database issue):D764–770. doi: 10.1093/nar/gkt1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Consortium GT. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nature genetics. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. Renal transporters in drug development. Annual review of pharmacology and toxicology. 2013;53:503–529. doi: 10.1146/annurev-pharmtox-011112-140317. [DOI] [PubMed] [Google Scholar]
- 39.Rask-Andersen M, Masuram S, Schioth HB. The druggable genome: Evaluation of drug targets in clinical trials suggests major shifts in molecular class and indication. Annual review of pharmacology and toxicology. 2014;54:9–26. doi: 10.1146/annurev-pharmtox-011613-135943. [DOI] [PubMed] [Google Scholar]
- 40.Abernethy DR, Schwartz JB. Calcium-antagonist drugs. The New England journal of medicine. 1999;341(19):1447–1457. doi: 10.1056/NEJM199911043411907. [DOI] [PubMed] [Google Scholar]
- 41.George AL., Jr Recent genetic discoveries implicating ion channels in human cardiovascular diseases. Current opinion in pharmacology. 2014;15:47–52. doi: 10.1016/j.coph.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oshiro C, Thorn CF, Roden DM, Klein TE, Altman RB. KCNH2 pharmacogenomics summary. Pharmacogenetics and genomics. 2010;20(12):775–777. doi: 10.1097/FPC.0b013e3283349e9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nature genetics. 2008;40(12):1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 44.Sultan M, Schulz MH, Richard H, Magen A, Klingenhoff A, Scherf M, et al. A global view of gene activity and alternative splicing by deep sequencing of the human transcriptome. Science. 2008;321(5891):956–960. doi: 10.1126/science.1160342. [DOI] [PubMed] [Google Scholar]
- 45.Li M, Jia C, Kazmierkiewicz KL, Bowman AS, Tian L, Liu Y, et al. Comprehensive analysis of gene expression in human retina and supporting tissues. Human molecular genetics. 2014;23(15):4001–4014. doi: 10.1093/hmg/ddu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webb A, Papp AC, Sanford JC, Huang K, Parvin JD, Sadee W. Expression of mRNA transcripts encoding membrane transporters detected with whole transcriptome sequencing of human brain and liver. Pharmacogenetics and genomics. 2013;23(5):269–278. doi: 10.1097/FPC.0b013e32835ff536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Molecular & cellular proteomics : MCP. 2014;13(2):397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gremel G, Wanders A, Cedernaes J, Fagerberg L, Hallstrom B, Edlund K, et al. The human gastrointestinal tract-specific transcriptome and proteome as defined by RNA sequencing and antibody-based profiling. Journal of gastroenterology. 2014 doi: 10.1007/s00535-014-0958-7. [DOI] [PubMed] [Google Scholar]
- 49.Choi YS, Dusting GJ, Stubbs S, Arunothayaraj S, Han XL, Collas P, et al. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. Journal of cellular and molecular medicine. 2010;14(4):878–889. doi: 10.1111/j.1582-4934.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Planat-Benard V, Menard C, Andre M, Puceat M, Perez A, Garcia-Verdugo JM, et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circulation research. 2004;94(2):223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 51.Makita N, Horie M, Nakamura T, Ai T, Sasaki K, Yokoi H, et al. Drug-induced long-QT syndrome associated with a subclinical SCN5A mutation. Circulation. 2002;106(10):1269–1274. doi: 10.1161/01.cir.0000027139.42087.b6. [DOI] [PubMed] [Google Scholar]
- 52.Shuraih M, Ai T, Vatta M, Sohma Y, Merkle EM, Taylor E, et al. A common SCN5A variant alters the responsiveness of human sodium channels to class I antiarrhythmic agents. Journal of cardiovascular electrophysiology. 2007;18(4):434–440. doi: 10.1111/j.1540-8167.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 53.Lewis BP, Green RE, Brenner SE. Evidence for the widespread coupling of alternative splicing and nonsense-mediated mRNA decay in humans. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):189–192. doi: 10.1073/pnas.0136770100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cropp CD, Komori T, Shima JE, Urban TJ, Yee SW, More SS, et al. Organic anion transporter 2 (SLC22A7) is a facilitative transporter of cGMP. Molecular pharmacology. 2008;73(4):1151–1158. doi: 10.1124/mol.107.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dahlin A, Geier E, Stocker SL, Cropp CD, Grigorenko E, Bloomer M, et al. Gene expression profiling of transporters in the solute carrier and ATP-binding cassette superfamilies in human eye substructures. Molecular pharmaceutics. 2013;10(2):650–663. doi: 10.1021/mp300429e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobayashi Y, Sakai R, Ohshiro N, Ohbayashi M, Kohyama N, Yamamoto T. Possible involvement of organic anion transporter 2 on the interaction of theophylline with erythromycin in the human liver. Drug metabolism and disposition: the biological fate of chemicals. 2005;33(5):619–622. doi: 10.1124/dmd.104.003301. [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi Y, Ohshiro N, Sakai R, Ohbayashi M, Kohyama N, Yamamoto T. Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]) The Journal of pharmacy and pharmacology. 2005;57(5):573–578. doi: 10.1211/0022357055966. [DOI] [PubMed] [Google Scholar]
- 58.Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacology & therapeutics. 2013;138(1):103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 59.U.S. Department of Health and Human Services FaDAF, (CDER) CfDEaR. Guidance for Industry: Drug Interaction Studies — Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. 2012. [Google Scholar]
- 60.Wempe MF, Lightner JW, Miller B, Iwen TJ, Rice PJ, Wakui S, et al. Potent human uric acid transporter 1 inhibitors: in vitro and in vivo metabolism and pharmacokinetic studies. Drug design, development and therapy. 2012;6:323–339. doi: 10.2147/DDDT.S35805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313(5795):1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 62.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nature reviews Cancer. 2006;6(10):813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 63.Wernicke C, Hellmann J, Finckh U, Rommelspacher H. Chronic ethanol exposure changes dopamine D2 receptor splicing during retinoic acid-induced differentiation of human SH-SY5Y cells. Pharmacological reports : PR. 2010;62(4):649–663. doi: 10.1016/s1734-1140(10)70322-4. [DOI] [PubMed] [Google Scholar]
- 64.Medina MW, Gao F, Naidoo D, Rudel LL, Temel RE, McDaniel AL, et al. Coordinately regulated alternative splicing of genes involved in cholesterol biosynthesis and uptake. PloS one. 2011;6(4):e19420. doi: 10.1371/journal.pone.0019420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solier S, Barb J, Zeeberg BR, Varma S, Ryan MC, Kohn KW, et al. Genome-wide analysis of novel splice variants induced by topoisomerase I poisoning shows preferential occurrence in genes encoding splicing factors. Cancer research. 2010;70(20):8055–8065. doi: 10.1158/0008-5472.CAN-10-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stormo C, Kringen MK, Lyle R, Olstad OK, Sachse D, Berg JP, et al. RNA-Sequencing Analysis of HepG2 Cells Treated with Atorvastatin. PloS one. 2014;9(8):e105836. doi: 10.1371/journal.pone.0105836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vivarelli S, Lenzken SC, Ruepp MD, Ranzini F, Maffioletti A, Alvarez R, et al. Paraquat modulates alternative pre-mRNA splicing by modifying the intracellular distribution of SRPK2. PloS one. 2013;8(4):e61980. doi: 10.1371/journal.pone.0061980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whitney AR, Diehn M, Popper SJ, Alizadeh AA, Boldrick JC, Relman DA, et al. Individuality and variation in gene expression patterns in human blood. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):1896–1901. doi: 10.1073/pnas.252784499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schadt EE, Molony C, Chudin E, Hao K, Yang X, Lum PY, et al. Mapping the genetic architecture of gene expression in human liver. PLoS biology. 2008;6(5):e107. doi: 10.1371/journal.pbio.0060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daly AK. Using genome-wide association studies to identify genes important in serious adverse drug reactions. Annual review of pharmacology and toxicology. 2012;52:21–35. doi: 10.1146/annurev-pharmtox-010611-134743. [DOI] [PubMed] [Google Scholar]
- 71.Daly AK. Pharmacogenomics of adverse drug reactions. Genome medicine. 2013;5(1):5. doi: 10.1186/gm409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barrie ES, Smith RM, Sanford JC, Sadee W. mRNA transcript diversity creates new opportunities for pharmacological intervention. Molecular pharmacology. 2012;81(5):620–630. doi: 10.1124/mol.111.076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pal S, Gupta R, Kim H, Wickramasinghe P, Baubet V, Showe LC, et al. Alternative transcription exceeds alternative splicing in generating the transcriptome diversity of cerebellar development. Genome research. 2011;21(8):1260–1272. doi: 10.1101/gr.120535.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Warnes GR. gplots: Various R Programming Tools for Plotting Data. 2015. [Google Scholar]
- 75.Wickham H. ggplot2: elegant graphics for data analysis. Springer; New York: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.