Abstract
Adiposity status and change are potential risk factors for Alzheimer’s disease (AD). The authors used data on 2,322 participants in the Baltimore Longitudinal Study of Aging to analyze the relation between AD incidence and adiposity in Cox proportional hazards models, with adjustment for sociodemographic factors and smoking status. Body mass index (BMI; weight (kg)/height (m)2) and waist circumference at specific ages were predicted by empirical Bayes estimators from mixed-effects regression models. After a median of 23.4 years of follow-up between 1958 and 2006, 187 participants developed AD. Among men, being underweight (BMI ≤ 18.5) at age 30, 40, or 45 years increased the likelihood of AD (hazard ratio (HR) = 5.76, 95% confidence interval (CI): 2.07, 16.00); among women, being obese (BMI ≥ 30) at age 30, 40, or 45 years and jointly centrally obese (waist circumference ≥ 80th percentile) at age 30, 35, or 50 years increased AD risk (HR = 6.57, 95% CI: 1.96, 22.02). Women who lost weight (BMI change <10th percentile) between ages 30 and 45 years were also at increased risk (HR = 2.02, 95% CI: 1.06, 3.85). Weight gain among men (BMI change >90th percentile) between ages 30 and 50 years increased AD risk (HR = 3.70, 95% CI: 1.43, 9.56). Future studies should identify age- and gender-specific optimal weights and weight-loss strategies for preventing AD and investigate potential mechanisms.
Keywords: adiposity, aging, Alzheimer disease, body mass index
Dementia affects 6–10% of persons aged 65 years or older, the majority of whom are diagnosed with Alzheimer’s disease (AD) (1). Recent research efforts have included AD as a potential outcome for a number of life-course cardiovascular and metabolic risk factors (2). One group of risk factors concerns adiposity, particularly body mass index (BMI; weight (kg)/height (m)2) and obesity (BMI ≥ 30), as well as weight change over time (3–19). Since the prevalence of obesity in industrialized countries has reached epidemic proportions—it is currently 30% among US adults—even a small detected effect of obesity on risk of incident AD could have great public health implications (20). Although prior work has suggested that obesity increases the risk of dementia, particularly incident AD (3–5), other investigators have not detected significant associations (6–8). In addition to BMI, recent research has focused on long-term vascular effects of central obesity using waist circumference as a primary measure (21). While one study did not find a significant effect of central obesity on AD (7), a more recent and larger study indicated that being in the upper quintile of waist circumference versus the lowest does more than double the risk of AD (22).
On the other hand, an increased risk of dementia was shown among older adults with a history of weight loss or underweight status based on cross-sectional and prospective cohort studies (9–19). Thus, a different picture emerges if exposure is measured in late life or long before subclinical dementia (23). Our recent review and meta-analysis of 10 prospective cohort studies (3–8, 24–27) suggested a U-shaped relation between BMI status and dementia, with both obesity and underweight increasing the risk of dementia; our analysis also suggested stronger associations in women than in men (28).
However, there is no consensus about the underlying pathogenesis linking dynamic BMI changes and AD, and there is no explanation for the previously observed gender differences in the association between weight status and AD. In particular, there is a paucity of research on the time-varying effects of weight status on AD incidence in later life. We aimed at investigating the effects of BMI/waist circumference status and their change through earlier stages of adulthood on the incidence of AD in later life using data from a prospective cohort study with a long median duration of follow-up (>20 years). Moreover, we tested gender differences in the associations based on BMI/waist circumference status and changes.
MATERIALS AND METHODS
Study population
Data from the Baltimore Longitudinal Study of Aging, a prospective cohort study of community-dwelling adults initiated by the National Institute on Aging in 1958, were used. The study has been described in detail elsewhere (29). Examinations were conducted at regular intervals (around 2 years) and included a battery of neuropsychological tests and neurologic, laboratory, and radiologic tests (30). All participants provided written informed consent.
The original study sample (sample 1 in Figure 1) consisted of 3,005 subjects (1,806 men and 1,199 women). The mean number of study visits between February 1958 and August 2006 was 7.98 (standard deviation, 6.90; range, 1–43). Subjects entered follow-up at age 30 years, were entered into the risk set at age 50 years, and exited follow-up at first failure, defined as being diagnosed with incident AD at or beyond age 50 years or being censored at the end of follow-up due to death or attrition. Subjects with non-AD dementia or mild cognitive impairment were included in the risk set, the group at risk of developing AD. Consequently, there were 187 incident AD cases or failures, for an incidence rate of 351 per 100,000 person-years (95% confidence interval (CI): 304, 406)—a rate comparable to that of other studies (<1% under the age of 70 years) (1). The median time at risk was 23.4 years (range, 0.01–66.01) (sample 2a in Figure 1). We further conducted a sensitivity analysis excluding visits made prior to 1986, to improve the accuracy of age of onset. Consequently, out of 1,979 subjects at risk, 165 incident AD cases were included in this analysis (sample 2b in Figure 1).
Figure 1.
Procedures used for inclusion and exclusion of Baltimore Longitudinal Study of Aging (BLSA) participants in analyses of the relation between adiposity and Alzheimer’s disease (AD) risk, 1958–2006. Sample 1 was used for prediction of body mass index/waist circumference with linear mixed models; samples 2a and 2b were used for fitting Cox proportional hazards models and constructing Kaplan-Meier survival curves. Solid line: subjects included in analysis; dashed line: subjects not included in analysis.
Outcome assessment
Subjects showing changes that indicated incident dementia were systematically studied. Diagnoses of dementia and dementia type were formulated during multidisciplinary evaluations based on prospectively collected evidence using National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria (31). Estimation of age at AD onset was based on informant reports and history of disease-free examinations.
Anthropometric measurements
Height and weight were measured with calibrated scales by trained technicians. At each study visit, BMI was calculated. Waist circumference was defined as the minimal abdominal perimeter located halfway between the rib cage and the pelvic crest and was measured using a flexible tape (30).
Linear mixed models were fitted to sample 1 (n = 3,005 subjects) to estimate predicted values of BMI and waist circumference at ages 30, 35, 40, 45, and 50 years, using age at visit as the time variable (the method is described in Web Appendix 1, presented on the Journal’s website (http://aje.oxfordjournals.org/)). Predicted BMI values at each target age were categorized on the basis of World Health Organization guidelines (32) as follows: <18.5 (underweight), 18.5–24.9 (normal), 25–29.9 (overweight), and ≥30 (obese). BMI change over a period of 5 years was also calculated and grouped into 3 categories (<10th percentile, 10th–90th percentile, and >90th percentile). Predicted values for waist circumference were categorized into quintiles, while change in waist circumference was grouped similarly to BMI change.
Covariates
Covariates considered in predictive models for BMI and waist circumference and in our main analyses included age at visit, gender, race/ethnicity (non-Hispanic white, non-Hispanic black, or other), years of education, smoking status (never, former, or current smoker), and year of birth. Other covariates were entered into our descriptive analyses exclusively, and these were reported hypertension, diabetes mellitus, cardiovascular disease (stroke, congestive heart failure, nonfatal myocardial infarction, or atrial fibrillation), and dyslipidemia.
Statistical analysis
All analyses were performed using STATA, version 10.0 (33). We first compared baseline characteristics according to number of visits and AD incidence status. Differences in continuous variables across visits and AD status categories were tested using Student’s t test, while differences between proportions were assessed using the chi-squared test. We used Cox proportional hazards to examine risk of AD associated with early to mid-adulthood adiposity status and change. The dependent measure was age at onset of AD or the last observed (censored) age of nondiagnosed subjects. All of our primary analyses were stratified by gender. Empirical Bayes predicted values for BMI and waist circumference were obtained from mixed-effects models (34), with age at visit being used as the time variable and with inclusion of fixed variables that might affect initial status and rate of change in BMI or waist circumference (see Web Appendix 1).
Our Cox proportional hazards models controlled for education, race/ethnicity, smoking status, and year of birth. The exposure was the adiposity status measure (BMI or waist circumference) at a specific age or 5-year change. High-risk groups for men and women were derived empirically and compared with the rest of the population within each gender in terms of risk for AD. To this end, Kaplan-Meier survival curves and log-rank tests (35) were used to compare the number of incident AD cases across risk groups.
RESULTS
Table 1 shows baseline characteristics, stratified by number of visits (1 visit vs. ≥2 visits; sample 1) and comparing subjects who were diagnosed with AD at follow-up with those who were not (sample 2a). Compared with subjects with only 1 baseline visit, those with 2 or more visits had a lower proportion female and a higher percentage of non-Hispanic whites, a higher mean education, a higher prevalence of hypertension, and a higher mean age at baseline. There were no differences in mean BMI, though obesity was slightly higher among persons with only 1 visit. On the other hand, incident AD cases (n = 187), as compared with subjects at risk with no AD at the end of follow-up, had a significantly lower mean BMI, were more likely to be former smokers but less likely to be current smokers at baseline, had a significantly higher proportion non-Hispanic white, and were more likely to have reported occurrence of cardiovascular disease. They were also significantly older at baseline (P < 0.05).
Table 1.
Baseline Characteristics (First Visit) of Subjects According to Number of Follow-Up Visits and Incident Alzheimer’s Disease Status, Baltimore Longitudinal Study of Aging, 1958–2006
| All Study Subjects
|
Subjects at Risk of Alzheimer’s Disease
|
||||
|---|---|---|---|---|---|
| ≥ 1 Visit (n = 3,005) | 1 Visit Only (n = 309) | ≥2 Visits (n = 2,696) | No Alzheimer’s Disease (n = 2,135) | Alzheimer’s Disease (n = 187) | |
| Gender, % women | 39.9 | 53.7 | 38.3*** | 36.4 | 38.0 (NS) |
| Race/ethnicity, % | |||||
| Non-Hispanic white | 80.7 | 64.2 | 82.1*** | 82.0 | 96.8*** |
| Non-Hispanic black | 16.2 | 29.7 | 14.5 | 15.2 | 3.2 |
| Other | 3.1 | 6.1 | 3.8 | 2.8 | 0.0 |
| Mean education, years | 16.6 (2.8)a | 15.7 (3.0) | 16.7 (2.7)*** | 16.6 (2.9) | 16.8 (2.7) (NS) |
| Smoking status, % | |||||
| Never smoker | 38.8 | 42.5 | 38.5 (NS) | 36.4 | 39.2*** |
| Former smoker | 38.0 | 34.6 | 38.2 | 40.5 | 48.4 |
| Current smoker | 23.2 | 22.9 | 23.3 | 23.1 | 12.4 |
| Type 2 diabetes mellitus, % | 2.5 | 2.9 | 2.4 (NS) | 3.2 | 2.1 (NS) |
| Hypertension, % | 32.7 | 26.8 | 33.4* | 38.3 | 41.4 (NS) |
| Cardiovascular disease, %b | 5.5 | 4.5 | 5.7 (NS) | 6.6 | 13.4*** |
| Dyslipidemia, % | 7.3 | 7.1 | 7.4 (NS) | 7.9 | 4.3† |
| Mean age at first visit, years | 52.1 (17.7) | 46.9 (18.5) | 52.8 (17.2)*** | 56.9 (15.4) | 68.6 (12.1)*** |
| ≤20 | 0.4 | 0.6 | 0.4*** | 0.0 | 0.0*** |
| 21–29 | 13.5 | 21.0 | 12.6 | 4.6 | 0.0 |
| 30–39 | 15.3 | 24.3 | 14.3 | 11.8 | 0.5 |
| 40–49 | 18.3 | 15.2 | 18.5 | 18.1 | 10.2 |
| 50–59 | 16.3 | 13.3 | 16.6 | 21.1 | 15.0 |
| 60–69 | 15.9 | 9.1 | 16.7 | 20.6 | 20.3 |
| 70–79 | 14.4 | 11.0 | 14.9 | 17.0 | 36.4 |
| ≥80 | 5.9 | 5.5 | 6.0 | 6.9 | 17.6 |
| Mean body mass indexc | 25.2 (3.9) | 25.4 (4.8) | 25.2 (3.8) (NS) | 25.4 (3.8) | 24.7 (3.3)** |
| Underweight (BMI ≤18.5) | 1.6 | 3.0 | 1.4* | 1.1 | 2.7* |
| Normal weight (BMI 18.6–24.9) | 52.2 | 50.3 | 52.4 | 49.3 | 56.1 |
| Overweight (BMI 25.0–29.9) | 35.7 | 33.4 | 36.0 | 38.4 | 35.3 |
| Obese (BMI ≥30) | 10.6 | 13.2 | 10.1 | 11.1 | 5.9 |
Abbreviation: NS, not significant.
P ≤ 0.10 (borderline-significant);
P < 0.05;
P < 0.01;
P < 0.001; NS: P > 0.10.
Numbers in parentheses, standard deviation.
Reporting any of the following conditions at the baseline visit: stroke, congestive heart failure, nonfatal myocardial infarction, or atrial fibrillation.
Weight (kg)/height (m)2.
Our results of fitting a taxonomy of multilevel/mixed models for change to the BMI and waist circumference data are presented in Web Appendix 2 (http://aje.oxfordjournals.org/). While model A did not include age as the time variable, model B suggests that overall the rate of change in BMI was positive and significant, with a fixed effect γ10 equal to 0.071 (standard error, 0.003) and a level-2 variance component of the rate of change equal to 0.020 ( ; standard error, 0.001). The same was observed in the case of waist circumference, in which the unconditional growth curve model indicated a rate of growth of 0.349 cm/year with a variance component of 0.100. For both outcomes (BMI and waist circumference), model E, which allowed for nonlinear growth by including a quadratic age term and allowed for secular trends by including year of birth, gave the best model fit. The correlation between year of birth and age at visit for the total Baltimore Longitudinal Study of Aging sample was −0.74.
Table 2 shows multivariate-adjusted hazard ratios for the development of AD by BMI status and BMI change over a period of 5 years. Among men, being underweight, as compared with normal weight, increased the risk of AD on average 4.16–11.89 times. Among women, the risk of AD was more than doubled for obese subjects as compared with those of normal weight, with the highest hazard ratio being observed at age 45 years (hazard ratio (HR) = 2.66, 95% CI: 0.91, 7.72). When we excluded study visits made prior to 1986 (i.e., using sample 2b; see Figure 1), the hazard ratio for obesity and AD among women was increased to 3.10 (95% CI: 1.06, 9.02) at age 45 years. Percentage weight loss among women in the range of −6.07% to −0.55% (<10th percentile of change) within 5 years between ages 30 and 45 years almost doubled the risk of AD in comparison with weight increases up to 5.33% (hazard ratios were 1.91 (P = 0.052), 1.93 (P = 0.045), and 2.00 (P = 0.036), respectively). Among men, in contrast, notable increases in BMI were associated with 4- to 5-fold increased risks of AD in comparison with normal weight fluctuations at all ages between 30 and 50 years (HR = 3.82–5.04; P < 0.05).
Table 2.
Risk of Incident Alzheimer’s Disease According to Body Mass Index Status and Change in Body Mass Index at Different Ages (Cox Proportional Hazards Models), Baltimore Longitudinal Study of Aging, 1958–2006a
| Range of Values, %b | BMIc |
|||||||
|---|---|---|---|---|---|---|---|---|
| Men
|
Range of Values, %b | Women
|
||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |||
| BMI statusd | ||||||||
| At age 30 years | ||||||||
| Underweight | 6.05*** | 2.14, 17.09 | 0.001 | 0.67 | 0.23, 1.89 | 0.447 | ||
| Normal | 1 | 1 | ||||||
| Overweight | 1.17 | 0.78, 1.75 | 0.438 | 1.17 | 0.59, 2.33 | 0.657 | ||
| Obese | 0.70 | 0.25, 1.96 | 0.499 | 2.44† | 0.85, 7.00 | 0.098 | ||
| At age 35 years | ||||||||
| Underweight | 4.16y | 0.99, 17.49 | 0.052 | 0.76 | 0.23, 2.50 | 0.656 | ||
| Normal | 1 | 1 | ||||||
| Overweight | 1.05 | 0.71, 1.55 | 0.818 | 1.04 | 0.51, 2.13 | 0.910 | ||
| Obese | 0.57 | 0.18, 1.87 | 0.357 | 2.33 | 0.81, 6.68 | 0.116 | ||
| At age 40 years | ||||||||
| Underweight | 11.89*** | 2.82, 50.15 | 0.001 | 1.34 | 0.41, 4.41 | 0.624 | ||
| Normal | 1 | 1 | ||||||
| Overweight | 1.07 | 0.72, 1.58 | 0.734 | 1.03 | 0.52, 2.05 | 0.928 | ||
| Obese | 0.41 | 0.10, 1.58 | 0.225 | 2.55† | 0.88, 7.37 | 0.083 | ||
| At age 45 years | ||||||||
| Underweight | 11.21*** | 2.65, 47.30 | 0.001 | 1.86 | 0.57, 6.12 | 0.303 | ||
| Normal | 1 | 1 | ||||||
| Overweight | 0.93 | 0.63, 1.36 | 0.701 | 1.24 | 0.66, 2.32 | 0.494 | ||
| Obese | 0.46 | 0.11, 1.93 | 0.290 | 2.66† | 0.91, 7.72 | 0.073 | ||
| At age 50 years | ||||||||
| Underweight | 2.96 | 0.68, 12.80 | 0.147 | |||||
| Normal | 1 | 1 | ||||||
| Overweight | 0.94 | 0.64, 1.38 | 0.753 | 1.11 | 0.61, 2.04 | 0.729 | ||
| Obese | 0.52 | 0.13, 2.17 | 0.374 | 2.43 | 0.84, 7.02 | 0.100 | ||
| Change in BMId | ||||||||
| Between ages 30 and 35 years | ||||||||
| <10th percentile | 33.84 to 30.55 | 1.14 | 0.70, 1.85 | 0.592 | −5.44 to −0.55 | 1.91† | 0.99, 3.65 | 0.052 |
| 10th–90th percentile | 30.54 to 5.33 | 1 | −0.54 to 5.33 | 1 | ||||
| >90th percentile | 5.37 to 27.34 | 4.58** | 1.75, 11.95 | 0.002 | 5.34 to 19.56 | 1.16 | 0.40, 3.37 | 0.784 |
| Between ages 35 and 40 years | ||||||||
| <10th percentile | −4.01 to −0.64 | 1.17 | 0.73, 1.86 | 0.519 | −5.74 to −0.64 | 1.93* | 1.01, 3.72 | 0.047 |
| 10th–90th percentile | −0.62 to 4.88 | 1 | 0.62 to 4.88 | 1 | ||||
| >90th percentile | 5.91 to 21.64 | 3.82** | 1.47, 9.93 | 0.006 | 4.92 to 16.40 | 1.32 | 0.50, 3.50 | 0.573 |
| Between ages 40 and 45 years | ||||||||
| <10th percentile | −4.21 to −0.73 | 1.03 | 0.64, 1.68 | 0.891 | −6.07 to −0.76 | 2.00* | 1.05, 3.83 | 0.036 |
| 10th–90th percentile | −0.73 to 5.11 | 1 | −0.71 to 5.11 | 1 | ||||
| >90th percentile | 5.12 to 17.93 | 5.04** | 1.76, 14.42 | 0.003 | 5.12 to 14.13 | 1.35 | 0.46, 3.91 | 0.582 |
| Between ages 45 and 50 years | ||||||||
| <10th percentile | −4.47 to −0.78 | 1.15 | 0.72, 1.83 | 0.548 | −6.45 to −0.78 | 1.67 | 0.87, 3.20 | 0.121 |
| 10th–90th percentile | −0.77 to 4.71 | 1 | −0.77 to 4.71 | 1 | ||||
| >90th percentile | 4.72 to 15.32 | 4.68** | 1.64, 13.37 | 0.004 | 4.72 to 12.41 | 1.24 | 0.43, 3.59 | 0.692 |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio.
P ≤ 0.10 (borderline-significant);
P < 0.05;
P < 0.01;
P < 0.001.
z scores were computed for change, and significant decrease and increase corresponded to the 10th and 90th percentiles (based on the whole Baltimore Longitudinal Study of Aging sample (n = 3,005)), respectively, of the standardized distribution of change. Numbers of subjects and failures were 1,343 and 110, respectively, for men and 671 and 64, respectively, for models with women only.
Cutpoints may vary or overlap because of rounding.
Weight (kg)/height (m)2.
BMI status was categorized as underweight (BMI <18.5), normal (BMI 18.5–24.9), overweight (BMI 25.0–29.9), or obese (BMI ≥30). BMI change was estimated by taking the difference between empirical Bayes predicted values for BMI at each age (using model E in Web Appendix 2) and then computing percent change from baseline. Models with BMI status and change as exposures controlled for education (years), ethnicity (Non-Hispanic black vs. other), and smoking status (former or current smoker vs. nonsmoker).
Table 3 shows multivariate-adjusted hazard ratios for development of AD by waist circumference quintile and change over a period of 5 years. Among men, compared with the lowest quintile, the third quintile was significantly protective against incident AD at age 30 years (HR = 0.55, 95% CI: 0.31, 0.99; P = 0.048). In contrast, women had more than 4-fold the risk of developing AD when belonging to the uppermost quintile at age 30 years (HR = 4.60, 95% CI: 1.28, 16.43). This effect of waist circumference among women was attenuated with age and was only borderline-significant at ages 35 and 50 years. Moreover, being in the fourth quintile as compared with the first significantly increased the risk of AD among women, particularly at ages 45 and 50 years. Notable longitudinal increases in waist circumference among women were protective against AD incidence as compared with normal fluctuations, particularly between the ages of 30 and 50 years, at all 5-year intervals (HRs = 0.49–0.50; P < 0.05). Most of those findings were similar when the analyses were repeated using sample 2b (Figure 1).
Table 3.
Risk of Incident Alzheimer’s Disease According to Central Obesity and Change in Waist Circumference at Different Ages (Cox Proportional Hazards Models), Baltimore Longitudinal Study of Aging, 1958–2006a
| Range of Valuesb | WC (cm)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Men
|
Range of Valuesb | Women
|
||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |||
| Quintile of WC statusc |
Range, cm
|
Range, cm
|
||||||
| At age 30 years | ||||||||
| Q1 (<20th percentile) | 60.6–77.4 | 1 | 45.4–61.2 | 1 | ||||
| Q2 | 77.4–81.0 | 0.69 | 0.42, 1.14 | 0.148 | 61.3–64.8 | 1.71† | 0.93, 3.12 | 0.083 |
| Q3 | 81.0–84.8 | 0.55* | 0.31, 0.99 | 0.048 | 64.8–70.3 | 1.38 | 0.64, 2.97 | 0.412 |
| Q4 | 84.8–89.9 | 1.26 | 0.72, 2.22 | 0.413 | 70.3–77.3 | 2.23 | 0.81, 6.18 | 0.112 |
| Q5 (>80th percentile) | 89.9–126.3 | 0.64 | 0.28, 1.46 | 0.294 | 77.4–112.9 | 4.60* | 1.28, 16.43 | 0.019 |
| At age 35 years | ||||||||
| Q1 (<20th percentile) | 64.4–79.2 | 1 | 50.1–63.3 | 1 | ||||
| Q2 | 79.2–82.8 | 0.84 | 0.52, 1.37 | 0.486 | 63.3–67.0 | 1.05 | 0.56, 1.95 | 0.885 |
| Q3 | 82.8–86.6 | 0.62 | 0.34, 1.12 | 0.115 | 67.0–72.8 | 1.22 | 0.61, 2.45 | 0.577 |
| Q4 | 86.6–91.8 | 1.16 | 0.65, 2.06 | 0.611 | 72.8–79.9 | 2.07 | 0.75, 5.71 | 0.159 |
| Q5 (>80th percentile) | 91.9–126.2 | 0.95 | 0.43, 2.08 | 0.898 | 80.1–114.4 | 3.17† | 0.91, 11.03 | 0.069 |
| At age 40 years | ||||||||
| Q1 (<20th percentile) | 67.2–80.9 | 1 | 53.7–65.3 | 1 | ||||
| Q2 | 80.9–84.6 | 0.73 | 0.45, 1.21 | 0.228 | 65.3–69.2 | 0.89 | 0.48, 1.65 | 0.706 |
| Q3 | 84.6–88.4 | 0.85 | 0.50, 1.45 | 0.556 | 69.2–75.2 | 0.91 | 0.44, 1.89 | 0.795 |
| Q4 | 88.4–93.6 | 0.99 | 0.56, 1.75 | 0.978 | 73.3–82.7 | 2.18 | 0.80, 5.97 | 0.127 |
| Q5 (>80th percentile) | 93.6–127.5 | 0.57 | 0.20, 1.62 | 0.294 | 82.8–115.8 | 2.52 | 0.73, 8.68 | 0.141 |
| At age 45 years | ||||||||
| Q1 (<20th percentile) | 68.5–82.7 | 1 | 55.8–67.2 | 1 | ||||
| Q2 | 82.7–86.5 | 0.92 | 0.56, 1.52 | 0.760 | 67.2–71.4 | 1.08 | 0.59, 1.97 | 0.808 |
| Q3 | 86.5–90.2 | 0.90 | 0.52, 1.55 | 0.702 | 71.4–77.8 | 0.76 | 0.34, 1.71 | 0.513 |
| Q4 | 90.2–95.6 | 1.07 | 0.60, 1.88 | 0.819 | 77.8–84.9 | 2.75* | 1.07, 7.06 | 0.035 |
| Q5 (>80th percentile) | 95.6–131.3 | 0.64 | 0.22, 1.82 | 0.400 | 85.0–117.3 | 2.45 | 0.71, 8.39 | 0.155 |
| At age 50 years | ||||||||
| Q1 (<20th percentile) | 69.8–84.1 | 1 | 57.9–69.2 | 1 | ||||
| Q2 | 84.2–88.3 | 0.85 | 0.52, 1.40 | 0.527 | 69.2–73.7 | 1.15 | 0.63, 2.11 | 0.647 |
| Q3 | 88.3–92.2 | 0.79 | 0.45, 1.36 | 0.396 | 73.8–80.1 | 0.88 | 0.41, 1.92 | 0.753 |
| Q4 | 92.2–97.7 | 1.00 | 0.57, 1.76 | 0.987 | 80.1–87.2 | 2.53† | 1.00, 6.44 | 0.051 |
| Q5 (>80th percentile) | 97.7–138.9 | 0.48 | 0.95, 1.07 | 0.235 | 87.2–118.8 | 3.04† | 0.87, 10.59 | 0.080 |
| Change in WCc |
Range, %
|
Range, %
|
||||||
| Between ages 30 and 35 years | ||||||||
| <10th percentile | −1.40 to 1.13 | 1.55 | 0.89; 2.69 | 0.118 | −2.98 to 1.10 | 1.37 | 0.41, 4.54 | 0.609 |
| 10th–90th percentile | 1.13 to 4.06 | 1 | 1.14 to 4.05 | 1 | ||||
| >90th percentile | 4.07 to 6.94 | 1.82 | 0.83, 4.01 | 0.133 | 4.08 to 16.20 | 0.50* | 0.26, 0.96 | 0.037 |
| Between ages 35 and 40 years | ||||||||
| <10th percentile | −1.40 to 1.13 | 1.56 | 0.90, 2.71 | 0.110 | −2.96 to 1.10 | 1.37 | 0.41, 4.54 | 0.609 |
| 10th–90th percentile | 1.13 to 3.92 | 1 | 1.15 to 3.92 | 1 | ||||
| >90th percentile | 3.93 to 6.51 | 1.83 | 0.83, 4.01 | 0.133 | 3.94 to 13.97 | 0.50* | 0.26, 0.96 | 0.037 |
| Between ages 40 and 45 years | ||||||||
| <10th percentile | −1.40 to 1.13 | 1.55 | 0.89, 2.69 | 0.118 | −3.03 to 1.11 | 1.37 | 0.41, 4.55 | 0.605 |
| 10th–90th percentile | 1.14 to 3.78 | 1 | 1.16 to 3.78 | 1 | ||||
| >90th percentile | 3.79 to 6.07 | 1.82 | 0.83, 4.01 | 0.133 | 3.79 to 12.28 | 0.49* | 0.26, 0.93 | 0.031 |
| Between ages 45 and 50 years | ||||||||
| <10th percentile | −1.41 to 1.13 | 1.60† | 0.92, 2.78 | 0.093 | −3.11 to 1.11 | 1.37 | 0.41, 4.55 | 0.605 |
| 10th–90th percentile | 1.13 to 3.67 | 1 | 1.17 to 3.67 | 1 | ||||
| >90th percentile | 3.70 to 5.78 | 1.83 | 0.84, 4.03 | 0.130 | 3.67 to 10.95 | 0.49* | 0.26, 0.94 | 0.031 |
Abbreviations: CI, confidence interval; HR, hazard ratio; Q, quintile; WC, waist circumference.
P≤ 0.10 (borderline-significant);
P < 0.05;
P < 0.01;
P < 0.001.
z scores were computed for change, and significant decrease and increase corresponded to the 10th and 90th percentiles (based on the whole Baltimore Longitudinal Study of Aging sample (n = 3,005)), respectively, of the standardized distribution of change. Numbers of subjects and failures were 1,343 and 110, respectively, for men and 669 and 64, respectively, for models with women only.
Cutpoints may vary or overlap because of rounding.
WC change was estimated by taking the difference between empirical Bayes predicted values for WC at each age (using model E in Web Appendix 2) and then computing percent change from baseline. Models with WC status and change as exposures controlled for education (years), ethnicity (non-Hispanic black vs. other), and smoking status (former or current smoker vs. nonsmoker).
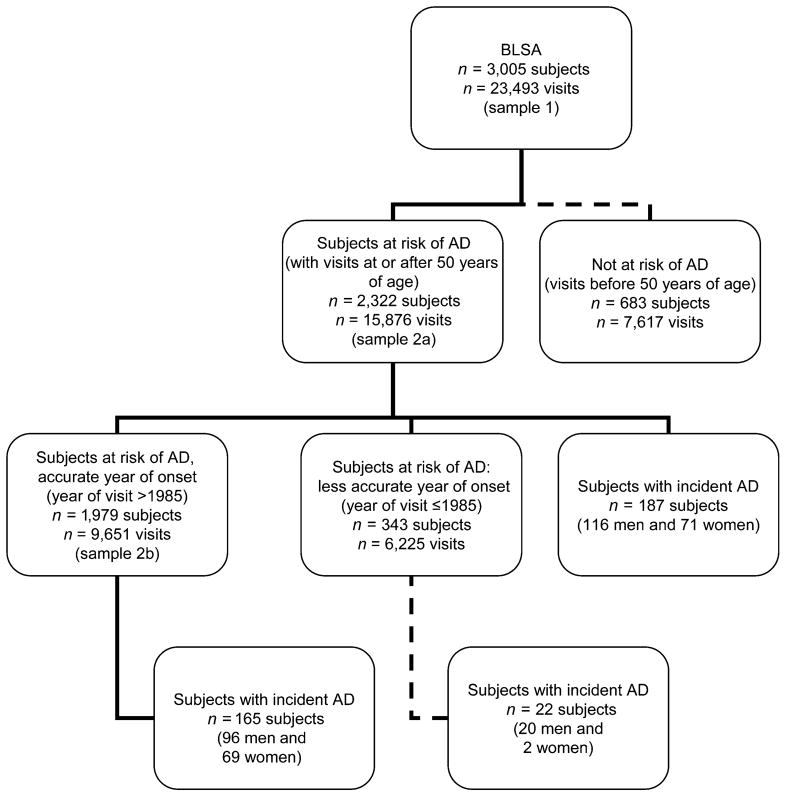
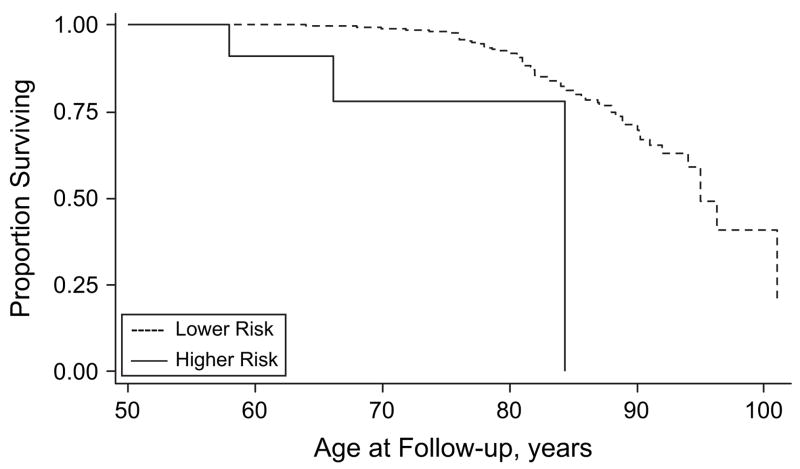
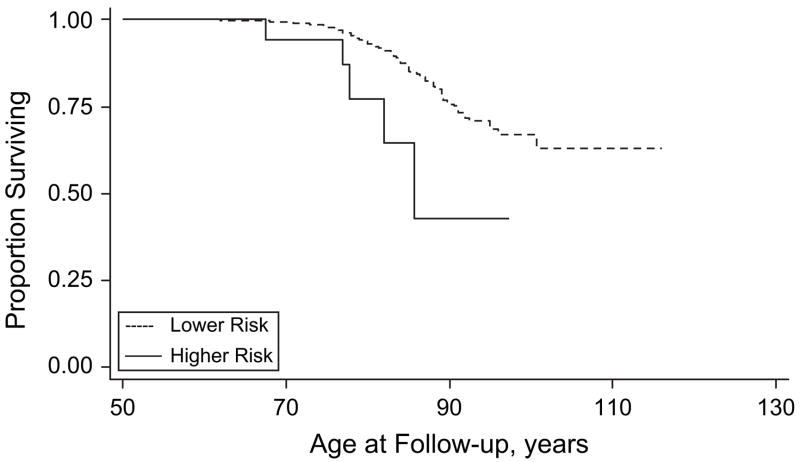
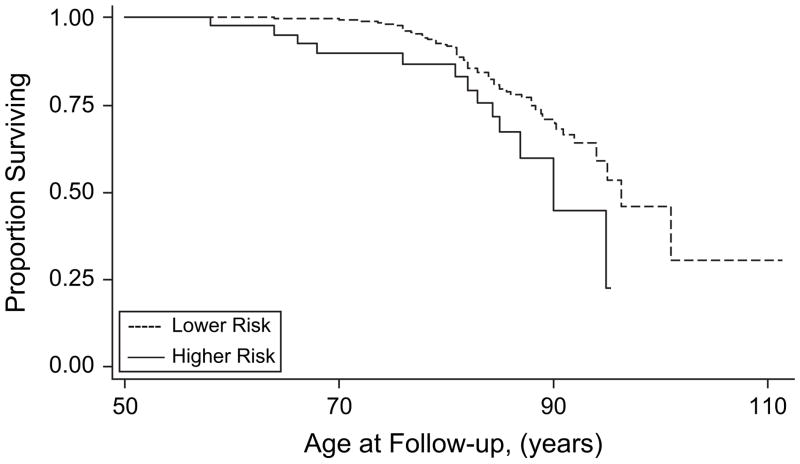
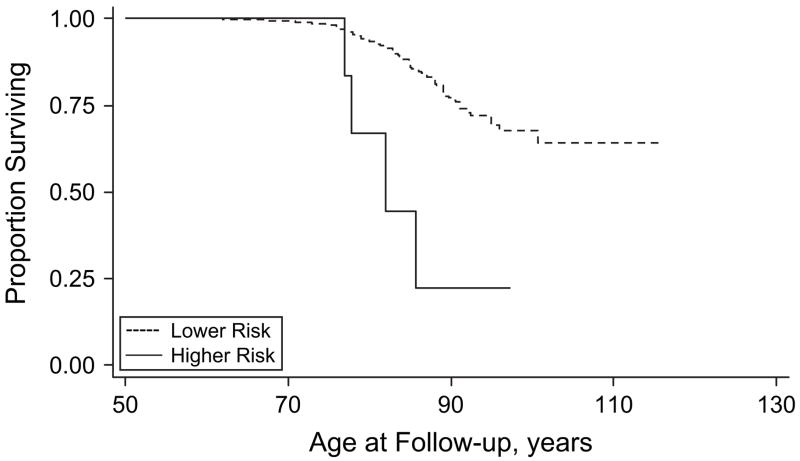
Figures 2–5 show results for Kaplan-Meier survival curves comparing AD incidence in empirically derived risk groups for each gender. Based on our above findings, women were at a somewhat increased risk of developing AD when they were obese at age 30, 40, or 45 years or had central obesity at age 30, 35, or 50 years. When women experienced all exposures simultaneously, however, the hazard ratio was considerably higher and the risk of AD was increased 6.57 times, on average (HR = 6.57, 95% CI: 1.96, 22.02; log-rank test: P = 0.0003) (Figure 2). Similarly, women experiencing appreciable weight loss during any 5-year period between ages 30 and 45 years had approximately double the risk of developing AD (HR = 2.02, 95% CI: 1.06, 3.85; log-rank test: P = 0.0266) (Figure 3). Among men, adiposity status defined by low BMI was a significant risk factor for AD, and being underweight at age 30, 40, or 45 years increased the risk more than 5 times (HR = 5.76, 95% CI: 2.07, 16.00; log-rank test: P = 0.001) (Figure 4). In addition, weight gain during any 5-year interval between ages 30 and 50 years increased the risk of AD more than 3-fold (HR = 3.70, 95% CI: 1.43, 9.56; log-rank test: P = 0.0166) (Figure 5).
Figure 2.
Kaplan-Meier survival curve for time to incident Alzheimer’s disease by adiposity risk status (elevated body mass index and waist circumference (obese at age 30, 40, or 45 years and in upper quintile of waist circumference at age 30, 35, or 50 years) vs. no elevation) among women, Baltimore Longitudinal Study of Aging, 1958–2006. The hazard ratio was 6.57 (95% confidence interval: 1.96, 22.02; P < 0.01; log-rank test: P =0.0003). Failure was defined as first diagnosis of incident Alzheimer’s disease at or after age 50 years. Hazard ratios were adjusted for education, race/ethnicity, smoking status, and year of birth.
Figure 5.
Kaplan-Meier survival curve for time to incident Alzheimer’s disease by adiposity risk status (increase in body mass index (change >90th percentile) during any 5-year interval between ages 30 and 50 years vs. no increase) among men, Baltimore Longitudinal Study of Aging, 1958–2006. The hazard ratio was 3.70 (95% confidence interval: 1.43, 9.56; * P < 0.01; log-rank test * P = 0.0166). Failure was defined as first diagnosis of incident Alzheimer’s disease at or after age 50 years. Hazard ratios were adjusted for education, race/ethnicity, smoking status, and year of birth.
Figure 3.
Kaplan-Meier survival curve for time to incident Alzheimer’s disease by adiposity risk status (significant decrease in body mass index (change <10th percentile) during any 5-year interval between ages 35 and 45 years vs. none) among women, Baltimore Longitudinal Study of Aging, 1958–2006. The hazard ratio was 2.02 (95% confidence interval: 1.06, 3.85; P < 0.05; log-rank test: * P = 0.0266). Failure was defined as first diagnosis of incident Alzheimer’s disease at or after age 50 years. Hazard ratios were adjusted for education, race/ethnicity, smoking status, and year of birth.
Figure 4.
Kaplan-Meier survival curve for time to incident Alzheimer’s disease by adiposity risk status (underweight (at age 30, 40, or 45 years vs. not underweight) among men, Baltimore Longitudinal Study of Aging, 1958–2006. The hazard ratio was 5.76 (95% confidence interval: 2.07, 16.00; P < 0.001; log-rank test: * P = 0.001). Failure was defined as first diagnosis of incident Alzheimer’s disease at or after age 50 years. Hazard ratios were adjusted for education, race/ethnicity, smoking status, and year of birth.
DISCUSSION
Of the 2,322 Baltimore Longitudinal Study of Aging participants at risk, 187 developed AD after a median of 23.4 years of follow-up. Both BMI and waist circumference increased with age at population-average annual rates of 0.071 units and 0.35 cm, respectively, with wide interindividual variability. Our analysis of the longitudinal data on the association of AD risk with BMI and waist circumference at baseline and their dynamic changes during follow-up showed marked gender differences. Among men, weight gain was associated with a more than 3-fold increased risk of AD at ages between 30 and 50 years for any 5-year interval (HR = 3.70, 95% CI: 1.43, 9.56); and men who were underweight at age 30, 40, or 45 years had an increased likelihood of developing AD (HR = 5.76, 95% CI: 2.07, 16.00). Among women, being obese (BMI ≥30) at age 30, 40, or 45 years and jointly centrally obese (waist circumference ≥80th percentile) at age 30, 35, or 50 years increased the risk of AD 6.6-fold (HR = 6.57, 95% CI: 1.96, 22.02). To our surprise, women who experienced appreciable weight loss between ages 30 and 45 years were found to have increased risk of AD (HR = 2.02, 95% CI: 1.06, 3.85); and a notable longitudinal increase in waist circumference, as compared with normal fluctuations, was found to be protective among women (HRs = 0.49–0.50), though not among men. Further research is needed to confirm our findings among women.
Our results regarding the association between obesity and AD are consistent with those of a number of previous studies (3, 5–7, 24). For instance, in the Cache County Study (n = 3,123; mean follow-up time = 3.2 years; 104 AD cases), Hayden et al. (24) showed that obesity increased the risk of AD in women (adjusted HR = 2.23, 95% CI: 1.09, 4.30) but not in men (adjusted HR = 1.48, 95% CI: 0.41, 4.18). In a Kaiser Permanente study, the largest study carried out to date (n = 10,136; follow-up time ≤36 years; 477 AD cases), Whitmer et al. (5) found hazard ratios of 2.60 (95% CI: 1.44, 4.69) among men and 3.38 (95% CI: 2.20, 5.19) among women, indicating a stronger effect among women. However, they did not find an association between underweight and risk of AD (5). Two other studies did not find statistically significant associations (6, 7), though they followed a similar trend.
Investigators have examined various other measures of adiposity, including waist circumference and subscapular and triceps skinfold thicknesses, and have obtained mixed findings (7, 22, 26, 27). In fact, while Yoshitake et al. (26) did not find an association between BMI and subscapular: triceps skinfold thickness ratio on the one hand and dementia or AD risk on the other for men and women separately, Luchsinger et al. (7) found that among both genders combined, having a waist circumference greater than 97 cm more than doubled the risk of vascular dementia (HR = 2.3, 95% CI: 1.0, 5.1) but not the risk of AD. Luchsinger et al. also found a positive association between vascular dementia and weight gain (7). More recently, among 6,583 subjects in the Kaiser Permanente cohort, Whitmer et al. (22) showed that in those with baseline central obesity (waist circumference measured between 1964 and 1973), the risk of developing dementia after an average of 36 years of follow-up was more than doubled when the uppermost quintile of waist circumference was compared with the lowest (HR = 2.72, 95% CI: 2.33, 3.33). Being defined as “obese” on the basis of both BMI and waist circumference concurrently increased the risk further (HR = 3.60, 95% CI: 2.85, 4.55) (22). These findings were similar to ours in terms of the joint effect of weight status and central obesity on the risk of AD, though our results were confined to women.
The fat-brain axis (36) and the hypothalamic-pituitary-adipose tissue axis (37) have been implicated in the biologic mechanisms that link adiposity to cognitive performance. In fact, compounds secreted by adipose tissue, such as leptin and adiponectin, have been shown to regulate energy expenditure and hyperphagic responses by interacting with the hypothalamus (38). In addition, direct administration of leptin to the hippocampus in mice was shown to improve memory processing and to shape the hypothalamus during the earliest stages (39). Recently, Fewlass et al. (40) found that leptin may contribute to amyloid beta deposition, while the results of another population-based study (41) suggested that a low serum leptin level is associated with cognitive decline even after adjustment for BMI. In addition, excess adiposity was shown to be linked with increased inflammation, particularly the release of cytokines such as interleukin-6 and C-reactive protein (42), which in turn was implicated in the cognitive decline process (43, 44).
Declining BMI may indicate pathologic processes that contribute to the subsequent development of AD (9). Some suggested mechanisms include increased energy expenditure, biologic disturbances, dysfunction in body weight regulation, mesial cortex temporal atrophy (16), and dysphagia (13). Studies also indicate that there may be complex relations between apolipoprotein E ε4, increased cerebrospinal fluid levels of cortisol, weight loss, and hippocampal atrophy, particularly among women, increasing the risk of AD (45, 46). In addition, in a study by Mayeux et al. (47), a lower BMI was associated with elevated plasma levels of amyloid β42, a possible risk factor for AD. As we showed in our recent review (28), at least 3 other prospective cohort studies indicated that weight loss or underweight status at the mild cognitive impairment stage or earlier was associated with increased incidence of AD (8, 48, 49). A general framework was recently proposed in which brain injury promoted by genetic and metabolic factors causes brain pathology and altered brain function, which in turn triggers changes in cognition, behavior, and appetite, promoting inadequate caloric intake, insufficient energy, impaired neuronal transport, and the stress response. These in turn may cause further increases in levels of free radicals, amyloid β, phosphorylated τ, cortisol, and cytokines and increase inflammation, which promotes further brain pathology and altered brain function (15).
On the other hand, it is well-established that obesity in general and central obesity in particular is only 1 component of an etiologic cluster known as the metabolic syndrome. Prior research showed positive associations of hypertension (50–52) and type 2 diabetes mellitus (53–55) with risk of dementia and cognitive decline. However, the influence of plasma lipid level (a third component of the metabolic syndrome) remains unclear. Cholesterol alters the degradation of the amyloid precursor protein, a major player in the pathogenesis of AD (56). Moreover, cerebrovascular disease that is associated with dyslipidemia may be related to the risk of AD (57). Conflicting results have also been noted in studies relating levels of total cholesterol (58, 59), high density lipoprotein cholesterol (60–62), and low density lipoprotein cholesterol (58) to AD. It was recently found that among 1,616 elders, the risk of developing cognitive impairment over a period of 4 years was increased significantly among those with the metabolic syndrome (63). In other recent studies linking the metabolic syndrome to incident or prevalent dementia, AD, and vascular dementia, researchers came to similar conclusions, though various measures of the metabolic syndrome were used (27, 64, 65), while Muller et al. (66) did not find an association between the metabolic syndrome and dementia risk. However, in the Kaiser Permanente study, adjustment for all other components of the metabolic syndrome did not attenuate the positive effect of mid-adulthood obesity (5), indicating that the effect of adiposity on the risk of AD may follow an independent pathway among both men and women.
Our study had several strengths. First, it was based on a large cohort of men and women who were followed for a relatively long period of time (median follow-up time: 23.4 years after age 50). Second, it was one of the few studies to examine the effects of central obesity (measured by waist circumference) and dynamic BMI and waist circumference changes during early to mid-adulthood on the incidence of AD. Finally, we used advanced statistical techniques, including mixed-effects regression models, to predict BMI and waist circumference in an efficient manner over time. However, our study also had limitations, including a lack of complete measurements for certain variables, including depressive symptoms and physical activity, at each age; this impeded our ability to adjust for these variables in a suitable manner, particularly when our exposure (BMI/waist circumference status and change) was age-dependent. In addition, the Baltimore Longitudinal Study of Aging was a sample of convenience; the cohort was not fixed, and recruitment and dropout were continuous throughout follow-up. We did not adjust for components of the metabolic syndrome, as we felt that they are potentially in the causal pathway and hence may act as mediators or effect modifiers. Finally, some of our results, particularly hazard ratios, were indicative of poor precision due to lack of statistical power. However, a sensitivity analysis that set the hazard ratio to be detected as the observed one and fixed the sample size of failures as well as the distribution of the main adiposity exposure (67) indicated that in fact power ranged between 0.80 and 1.00 for most of our analyses.
In conclusion, obesity, central obesity, and weight loss among women seem to play a role in the etiology of AD, while underweight and weight gain among men increase the risk. In future studies, investigators should address optimal age- and gender-specific healthy weight and weight loss strategies for prevention of AD. They should also suggest potential mechanisms for an effect of obesity or central obesity on AD, either through components of the metabolic syndrome or through other independent factors related to adiposity. Alternative pathways explaining the relation between weight loss and AD should also be investigated.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
The authors thank Drs. Susan Resnick and Larry Brant for internal review of the manuscript.
Abbreviations
- AD
Alzheimer’s disease
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
Footnotes
Conflict of interest: none declared.
References
- 1.Hendrie HC. Epidemiology of dementia and Alzheimer’s disease. Am J Geriatr Psychiatry. 1998;6(2 suppl 1):S3–S18. doi: 10.1097/00019442-199821001-00002. [DOI] [PubMed] [Google Scholar]
- 2.Gorospe EC, Dave JK. The risk of dementia with increased body mass index. Age Ageing. 2007;36(1):23–29. doi: 10.1093/ageing/afl123. [DOI] [PubMed] [Google Scholar]
- 3.Rosengren A, Skoog I, Gustafson D, et al. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165(3):321–326. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Whitmer RA, Gunderson EP, Barrett-Connor E, et al. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, et al. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4(2):103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 6.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10):1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 7.Luchsinger JA, Patel B, Tang MX, et al. Measures of adiposity and dementia risk in elderly persons. Arch Neurol. 2007;64(3):392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nourhashemi F, Deschamps V, Larrieu S, et al. Body mass index and incidence of dementia: The PAQUID Study. Neurology. 2003;60(1):117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 9.Buchman AS, Wilson RS, Bienias JL, et al. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65(6):892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 10.Buchman AS, Schneider JA, Wilson RS, et al. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology. 2006;67(11):1949–1954. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- 11.Barrett-Connor E, Edelstein S, Corey-Bloom J, et al. Weight loss precedes dementia in community-dwelling older adults. J Nutr Health Aging. 1998;2(2):113–114. [PubMed] [Google Scholar]
- 12.Barrett-Connor E, Edelstein SL, Corey-Bloom J, et al. Weight loss precedes dementia in community-dwelling older adults. J Am Geriatr Soc. 1996;44(10):1147–1152. doi: 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 13.Chouinard J, Lavigne E, Villeneuve C. Weight loss, dysphagia, and outcome in advanced dementia. Dysphagia. 1998;13(3):151–155. doi: 10.1007/PL00009565. [DOI] [PubMed] [Google Scholar]
- 14.Franklin CA, Karkeck J. Weight loss and senile dementia in an institutionalized elderly population. J Am Diet Assoc. 1989;89(6):790–792. [PubMed] [Google Scholar]
- 15.Grundman M. Weight loss in the elderly may be a sign of impending dementia. Arch Neurol. 2005;62(1):20–22. doi: 10.1001/archneur.62.1.20. [DOI] [PubMed] [Google Scholar]
- 16.Guyonnet S, Nourhashemi F, Reyes-Ortega G, et al. Weight loss in patients with Alzheimer-type dementia [in French] Rev Med Interne. 1997;18(10):776–785. doi: 10.1016/s0248-8663(97)89967-5. [DOI] [PubMed] [Google Scholar]
- 17.Knopman DS, Edland SD, Cha RH, et al. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69(8):739–746. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 18.Prasher VP, Metseagharun T, Haque S. Weight loss in adults with Down syndrome and with dementia in Alzheimer’s disease. Res Dev Disabil. 2004;25(1):1–7. doi: 10.1016/j.ridd.2003.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Wang SY. Weight loss and metabolic changes in dementia. J Nutr Health Aging. 2002;6(3):201–205. [PubMed] [Google Scholar]
- 20.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 21.Freiberg MS, Pencina MJ, D’Agostino RB, et al. BMI vs. waist circumference for identifying vascular risk. Obesity (Silver Spring) 2008;16(2):463–469. doi: 10.1038/oby.2007.75. [DOI] [PubMed] [Google Scholar]
- 22.Whitmer RA, Gustafson DR, Barrett-Connor E, et al. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008 Mar 26; doi: 10.1212/01.wnl.0000306313.89165.ef. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Whitmer RA. The epidemiology of adiposity and dementia. Curr Alzheimer Res. 2007;4(2):117–122. doi: 10.2174/156720507780362065. [DOI] [PubMed] [Google Scholar]
- 24.Hayden KM, Zandi PP, Lyketsos CG, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: The Cache County Study. Alzheimer Dis Assoc Disord. 2006;20(2):93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 25.Gustafson D, Rothenberg E, Blennow K, et al. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163(13):1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 26.Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: The Hisayama Study. Neurology. 1995;45(6):1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- 27.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia Aging Study. Arterioscler Thromb Vasc Biol. 2000;20(10):2255–2260. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 28.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008;9(3):204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawas C, Gray S, Brookmeyer R, et al. Age-specific incidence rates of Alzheimer’s disease: The Baltimore Longitudinal Study of Aging. Neurology. 2000;54(11):2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 30.Shock N, Greulich RC, Andres R, et al. Normal Human Aging: The Baltimore Longitudinal Study of Aging. Washington, DC: US GPO; 1984. [Google Scholar]
- 31.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Report of a WHO Consultation. Geneva, Switzerland: World Health Organization; 1997. Obesity: Preventing and Managing the Global Epidemic. (Publication no. WHO/NUT/NCD/98.1) [PubMed] [Google Scholar]
- 33.Stata Corporation. Statistics/Data Analysis: Release 10.0. College Station, TX: Stata Corporation; 2007. [Google Scholar]
- 34.Singer JD, Willet JB, editors. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. 1. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 35.Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982;1(2):121–129. doi: 10.1002/sim.4780010204. [DOI] [PubMed] [Google Scholar]
- 36.Elmquist JK, Flier JS. Neuroscience. The fat-brain axis enters a new dimension. Science. 2004;304(5667):63–64. doi: 10.1126/science.1096746. [DOI] [PubMed] [Google Scholar]
- 37.Schaffler A, Binart N, Scholmerich J, et al. Hypothesis paper. Brain talks with fat—evidence for a hypothalamic-pituitary-adipose axis? Neuropeptides. 2005;39(4):363–367. doi: 10.1016/j.npep.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Speakman JR. Obesity: the integrated roles of environment and genetics. J Nutr. 2004;134(8 suppl):2090S–2105S. doi: 10.1093/jn/134.8.2090S. [DOI] [PubMed] [Google Scholar]
- 39.Harvey J, Shanley LJ, O’Malley D, et al. Leptin: a potential cognitive enhancer? Biochem Soc Trans. 2005;33(pt 5):1029–1032. doi: 10.1042/BST20051029. [DOI] [PubMed] [Google Scholar]
- 40.Fewlass DC, Noboa K, Pi-Sunyer FX, et al. Obesity-related leptin regulates Alzheimer’s Aβ. FASEB J. 2004;18(15):1870–1878. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- 41.Holden KF, Lindquist K, Tylavsky FA, et al. Serum leptin level and cognition in the elderly: findings from the Health ABC Study. Neurobiol Aging. 2008 Mar 19; doi: 10.1016/j.neurobiolaging.2007.11.024. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83(2):461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 43.Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 44.Yucesoy B, Peila R, White LR, et al. Association of interleukin-1 gene polymorphisms with dementia in a community-based sample: The Honolulu-Asia Aging Study. Neurobiol Aging. 2006;27(2):211–217. doi: 10.1016/j.neurobiolaging.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Peskind ER, Wilkinson CW, Petrie EC, et al. Increased CSF cortisol in AD is a function of APOE genotype. Neurology. 2001;56(8):1094–1098. doi: 10.1212/wnl.56.8.1094. [DOI] [PubMed] [Google Scholar]
- 46.Vanhanen M, Kivipelto M, Koivisto K, et al. APOE-ε4 is associated with weight loss in women with AD: a population-based study. Neurology. 2001;56(5):655–659. doi: 10.1212/wnl.56.5.655. [DOI] [PubMed] [Google Scholar]
- 47.Mayeux R, Honig LS, Tang MX, et al. Plasma Aβ40 and Aβ42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61(9):1185–1190. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- 48.Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia: The Honolulu-Asia Aging Study. Arch Neurol. 2005;62(1):55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 49.Borenstein Graves A, Mortimer JA, Bowen JD, et al. Head circumference and incident Alzheimer’s disease: modification by apolipoprotein E. Neurology. 2001;57(8):1453–1460. doi: 10.1212/wnl.57.8.1453. [DOI] [PubMed] [Google Scholar]
- 50.El-Atat F, Aneja A, McFarlane S, et al. Obesity and hypertension. Endocrinol Metab Clin North Am. 2003;32(4):823–854. doi: 10.1016/s0889-8529(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 51.Hall JE, Jones DW, Kuo JJ, et al. Impact of the obesity epidemic on hypertension and renal disease. Curr Hypertens Rep. 2003;5(5):386–392. doi: 10.1007/s11906-003-0084-z. [DOI] [PubMed] [Google Scholar]
- 52.Najman DM, Kapoor P, Serrano A, et al. Hypertension and obesity [letter] Arch Intern Med. 2003;163(9):1114–1115. doi: 10.1001/archinte.163.9.1114-b. [DOI] [PubMed] [Google Scholar]
- 53.Arvanitakis Z, Wilson RS, Bienias JL, et al. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61(5):661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 54.Yaffe K, Blackwell T, Kanaya AM, et al. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63(4):658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 55.Hassing LB, Hofer SM, Nilsson SE, et al. Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: evidence from a longitudinal study. Age Ageing. 2004;33(4):355–361. doi: 10.1093/ageing/afh100. [DOI] [PubMed] [Google Scholar]
- 56.Burns M, Duff K. Cholesterol in Alzheimer’s disease and tauopathy. Ann N Y Acad Sci. 2002;977:367–375. doi: 10.1111/j.1749-6632.2002.tb04839.x. [DOI] [PubMed] [Google Scholar]
- 57.Geula C, Farlow M, Cummings J, et al. Alzheimer’s disease: translating neurochemical insights into chemical benefits. J Clin Psychiatry. 2000;61(10):791–802. doi: 10.4088/jcp.v61n1012. [DOI] [PubMed] [Google Scholar]
- 58.Scacchi R, De Bernardini L, Mantuano E, et al. DNA polymorphisms of apolipoprotein B and angiotensin I-converting enzyme genes and relationships with lipid levels in Italian patients with vascular dementia or Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9(4):186–190. doi: 10.1159/000017045. [DOI] [PubMed] [Google Scholar]
- 59.Lesser G, Kandiah K, Libow LS, et al. Elevated serum total and LDL cholesterol in very old patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2001;12(2):138–145. doi: 10.1159/000051248. [DOI] [PubMed] [Google Scholar]
- 60.Kuriyama M, Hokezu Y, Togo S, et al. Serum lipids, lipoproteins and apolipoproteins in patients with senile dementia [in Japanese] Nippon Ronen Igakkai Zasshi. 1992;29(7–8):559–564. doi: 10.3143/geriatrics.29.559. [DOI] [PubMed] [Google Scholar]
- 61.Anttila T, Helkala EL, Viitanen M, et al. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ. 2004;329(7465):539. doi: 10.1136/bmj.38181.418958.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michikawa M. Cholesterol paradox: is high total or low HDL cholesterol level a risk for Alzheimer’s disease? J Neurosci Res. 2003;72(2):141–146. doi: 10.1002/jnr.10585. [DOI] [PubMed] [Google Scholar]
- 63.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292(18):2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 64.Martins IJ, Hone E, Foster JK, et al. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol Psychiatry. 2006;11(8):721–736. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- 65.Vanhanen M, Koivisto K, Moilanen L, et al. Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology. 2006;67(5):843–847. doi: 10.1212/01.wnl.0000234037.91185.99. [DOI] [PubMed] [Google Scholar]
- 66.Muller M, Tang MX, Schupf N, et al. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dement Geriatr Cogn Disord. 2007;24(3):185–192. doi: 10.1159/000105927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials. 1981;2(2):93–113. doi: 10.1016/0197-2456(81)90001-5. [DOI] [PubMed] [Google Scholar]