Abstract
Introduction
Campylobacteriosis is one of the leading causes of gastroenteritis worldwide. This study describes the epidemiology of laboratory-confirmed Campylobacter diarrheal infections in two facility-based surveillance sites in Guatemala.
Methods
Clinical, epidemiologic, and laboratory data were collected on patients presenting with acute diarrhea from select healthcare facilities in the departments of Santa Rosa and Quetzaltenango, Guatemala, from January 2008 through August 2012. Stool specimens were cultured for Campylobacter and antimicrobial susceptibility testing was performed on a subset of isolates. Multidrug resistance (MDR) was defined as resistance to ≥3 antimicrobial classes.
Results
Campylobacter was isolated from 306 (6.0%) of 5137 stool specimens collected. For children <5 years of age, annual incidence was as high as 1288.8 per 100,000 children in Santa Rosa and 185.5 per 100,000 children in Quetzaltenango. Among 224 ambulatory care patients with Campylobacter, 169 (75.5%) received metronidazole or trimethoprim-sulfamethoxazole, and 152 (66.7%) received or were prescribed oral rehydration therapy. Antimicrobial susceptibilities were tested in 96 isolates; 57 (59.4%) were resistant to ciprofloxacin and 12 (12.5%) were MDR.
Conclusion
Campylobacter was a major cause of diarrhea in children in two departments in Guatemala; antimicrobial resistance was high, and treatment regimens in the ambulatory setting which included metronidazole and trimethoprim-sulfamethoxazole and lacked oral rehydration were sub-optimal.
Keywords: Campylobacter, Guatemala, Epidemiology, Antimicrobial resistance
1. Introduction
Campylobacteriosis, usually acquired by the consumption and handling of poultry – is one of the leading causes of gastroenteritis worldwide [1]. The illness is characterized by diarrhea, abdominal cramps, and fever [2]. Although mortality is rare, significant post-infectious sequelae such as Guillain–Barré syndrome, irritable bowel syndrome, and reactive arthritis do occur [3–6]. The utilization of fluoroquinolones in feed animals to treat illness and promote growth has contributed to increasingly quinolone-resistant Campylobacter strains [7,8], complicating the treatment for patients with severe disease and immunocompromised states, especially children [1].
In Guatemala, diarrhea is the second most common cause of morbidity and mortality in children <5 years of age [9]. Small, community-based studies in Guatemala suggest that Campylobacter is a common cause of diarrhea in children [10,11]. However, no estimates have been generated for the incidence of campylobacteriosis in Guatemala or for the degree of antimicrobial resistance. Identification of Campylobacter requires specific culturing techniques with micro-aerobic environments [12], and few laboratories in Guatemala routinely culture for this pathogen.
In this report, cases are characterized and the incidence of laboratory-confirmed campylobacteriosis is estimated from a facility-based surveillance system in Guatemala from 2008 through 2012.
2. Methods
2.1. Study sites
In July 2007, the U.S. Centers for Disease Control and Prevention’s (CDC) International Emerging Infections Program (IEIP) in Guatemala, in collaboration with the Ministry of Public Health and Welfare (MSPAS) and the Universidad del Valle de Guatemala (UVG), initiated a facility-based surveillance system in the department of Santa Rosa. Additional sites in the department of Quetzaltenango were added in February of 2009. The surveillance system captures patients of all ages in both ambulatory and hospital settings, and diarrhea is one of the syndromes under surveillance.
Santa Rosa, with a population of 346,590 persons, is one of 22 administrative departments in Guatemala, and it is located in the semi-tropical southern part of the country. Ethnically, the population is 15% Amerindian indigenous [13]. Quetzaltenango, with a population of 789,358 persons, is in the western highlands and has a population that is 62% Amerindian indigenous. Government health facilities include hospitals, health centers staffed by a physician and nurses, and health posts staffed by nurses. In both surveillance sites, the facility-based system includes a hospital and an ambulatory component. In Santa Rosa, the surveillance system includes the regional hospital in Cuilapa, the municipal capital of Santa Rosa, as well as the health center and five health posts of the municipality of Nueva Santa Rosa, located 30 km north of Cuilapa. In Quetzaltenango, surveillance includes the regional hospital in the capital of Quetzaltenango, as well as the three health centers and one health post in the municipalities around the capital. In this analysis, the health centers and health posts were collectively considered ambulatory care facilities, and cases captured in these facilities were used for ambulatory surveillance. Data were included from Santa Rosa between January 2008 and August 2012, and from Quetzaltenango between February 2009 and August 2012.
2.2. Case detection and data collection
A case of diarrhea was defined as≥3 loose or liquid stools in a 24-h period with onset within the seven days preceding presentation to any participating facility by a patient residing in a municipality covered by the surveillance system. To avoid enrolling patients with chronic diarrhea, subjects were excluded if they had any signs or symptoms of diarrhea within the seven days prior to the onset of the current illness. Surveillance nurses screened patients by reviewing log book entries and assessing chief complaints for diarrhea-related visits and admissions. These patients were interviewed, and if found to meet the case definition, detailed clinical, epidemiologic, demographic, and socioeconomic data were obtained through structured patient interviews and medical chart abstractions [14]. In the ambulatory setting, facilities were staffed with surveillance nurses during all working hours and all patients presenting with diarrhea were screened for eligibility. In the hospital setting, except for holidays, surveillance nurses were on-duty seven days per week from 8:00 AM to 5:00 PM. Only patients who were admitted to the hospital were screened for eligibility. This insured that only severe cases of diarrhea were enrolled in hospital surveillance, and it also was the only feasible approach since surveillance nurses were not able to collect all the necessary laboratory and epidemiologic data from patients in the emergency department (ED) before they were discharged.
2.3. Laboratory methods
A stool specimen was requested of all consenting patients. For children <5 years of age who were unable to produce a specimen, a rectal swab was collected and placed in Cary-Blair transport media. Stool samples from the ambulatory facilities were stored in Cary-Blair transport media in an insulated cooler at 4 °C, and transported within 24 h to one of the two regional hospitals for initial processing and testing. Samples were streaked by direct plating onto Campylobacter selective agar base, Karmali (Oxoid, Basingstoke, UK) and incubated at 42 °C for 48 h under microaerophilic conditions provided by the CampyGen™ Generating System (Oxoid, Basingstoke, UK). Small, gray, moist, and flat spreading colonies were considered suspicious of Campylobacter, and were placed on a stained slide for microscopic examination. On visual examination, colonies with “gull-winged,” spiral, or “S”-shaped structures were considered microscopically suspect colonies, and were subsequently plated on Mueller Hinton (Oxoid, Basingstoke, UK) or blood agar and tested with oxidase and catalase and the Dryspot Campylobacter test kit (Oxoid, Basingstoke, UK) to confirm Campylobacter. The hippurate test was used to identify C. jejuni [15]. A specimen was considered negative for Campylobacter if no suspicious colonies grew after 72 h of incubation. Isolates were sent to UVG laboratories for Campylobacter and C. jejuni confirmation and antimicrobial susceptibility testing. Due to limited supplies, antimicrobial susceptibility testing was performed on all cultures done from 2010 to 2011 that grew Campylobacter, using minimum inhibitory concentrations (MIC) via Etest® (Biomérieux, Marcy l’Etoile, France) for the following antimicrobial agents: nalidixic acid (NA), chloramphenicol, ciprofloxacin, erythromycin, and tetracycline [16]. Multidrug-resistance (MDR) was defined as resistance to at least one antimicrobial in at least three of the following antimicrobial groups: macrolides, quinolones, phenicol, and tetracycline [17,18].
2.4. Data analysis
The number of laboratory-confirmed Campylobacter infections was examined by quarter and stratified by ambulatory and hospital settings separately for Santa Rosa and Quetzaltenango. The total number of diarrhea cases that were captured by the surveillance system was also displayed per quarter. Seasonality of Campylobacter infections was assessed visually using time series graphs.
Annual crude rates for Campylobacter were calculated. Population denominators were obtained for the catchment area of the surveillance facilities by healthcare setting for all ages and for children <5 years, from 2000 through 2010 municipality data from Guatemala’s National Institute of Statistics (INE) [13]. Population estimates for the catchment areas in 2011 and 2012 were generated by calculating the average change in municipality population by age from 2009 to 2010, then assuming that same change for 2011 and 2012.
Demographic and clinical characteristics of case–patients treated at ambulatory and hospital settings were compared using chi square tests. Fisher’s exact tests were used when cell sizes had counts of five or less. Demographic, geographic, healthcare setting, and clinical differences were explored between patients who had antimicrobial susceptibility data versus those who did not using chi square tests. The proportion resistant was calculated for each of the five tested antimicrobials for all Campylobacter and the subset of C. jejuni. In addition, the difference was tested in the proportion of isolates that were MDR from ambulatory versus hospital settings using Fisher’s exact test.
2.5. Ethics
The surveillance protocol was approved by the institutional review boards of the CDC and the UVG, and approved by the Guatemalan MSPAS. Verbal consent was requested of patients in order to screen them for eligibility. Written, informed consent was obtained from eligible patients who were willing to participate. For patients <18 years of age, parents or guardians were asked to provide written, informed consent for the participation of the patient. In addition, children aged 7 through 17 were asked for written, informed assent.
3. Results
During the five-year analysis period in Santa Rosa, 4327 patients met the case definition for diarrhea and all but one consented to participate; 246 (6.3%) of 3929 (90.8%) stool specimens collected yielded cultures positive for Campylobacter. During the three-year analysis period in Quetzaltenango, 1336 patients met the case definition for diarrhea and all but one consented to participate; 60 (5.0%) of 1208 (90.4%) stool specimens collected yielded cultures positive for Campylobacter. No seasonal pattern of disease was evident by visual inspection of time series graphs. In the hospital setting in Santa Rosa during 2008 through 2012, the median crude annual incidence of Campylobacter infections was 2.4 per 100,000 persons (range, 0–6.6) for all ages, and 16.1 per 100,000 persons (range, 0–45.0) for children <5 years old (Table 1). Median incidence in the ambulatory setting in Santa Rosa was 149.5 per 100,000 persons (range, 92.5–215.8) for all ages, and 910.7 per 100,000 persons (range, 594.5–1288.8) for children <5 years old. In the ambulatory setting in Quetzaltenango during 2010 through 2012, the median crude annual rate was 9.4 per 100,000 persons (range, 8.5–27.3) for all ages, and 55.2 per 100,000 persons (range, 48.3–185.5) for children <5 years old. Of the 306 Campylobacter cases, 235 (76.8%) were C. jejuni (Table 2).
Table 1.
Number and crude rates of laboratory-confirmed Campylobacter cases captured at Santa Rosa and Quetzaltenangoa surveillance sites by healthcare setting, January 1, 2008 – August 31, 2012.
| Santa Rosa | Quetzaltenango | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Hospital | Ambulatory | Hospital | Ambulatory | |||||
|
|
|
|||||||
| Number of cases | Crude rate per 100,000 persons | Number of cases | Crude rate per 100,000 persons | Number of cases | Crude rate per 100,000 persons | Number of cases | Crude rate per 100,000 persons | |
| 2008 | ||||||||
| <5 years | 3 | 8.1 | 27 | 594.5 | n/a | n/a | n/a | n/a |
| All | 3 | 1.2 | 28 | 92.5 | ||||
| 2009 | ||||||||
| <5 years | 6 | 16.1 | 34 | 745.5 | n/a | n/a | n/a | n/a |
| All | 6 | 2.4 | 39 | 127.3 | ||||
| 2010 | ||||||||
| <5 years | 17 | 45.0 | 59 | 1288.8 | 3 | 5.4 | 6 | 48.3 |
| All | 17 | 6.6 | 67 | 215.8 | 3 | 0.8 | 7 | 8.5 |
| 2011 | ||||||||
| <5 years | 0 | 0 | 46 | 1001.1 | 3 | 5.3 | 7 | 55.2 |
| All | 0 | 0 | 47 | 149.5 | 4 | 1.1 | 8 | 9.4 |
| 2012b | ||||||||
| <5 years | 4 | 25.0 | 28 | 910.7 | 5 | 13.2 | 16 | 185.5 |
| All | 4 | 4.1 | 32 | 150.8 | 5 | 1.9 | 16 | 27.3 |
Surveillance in Quetzaltenango began in 2009.
Rates account for partial year through August 31.
Table 2.
Characteristics of 306 laboratory-confirmed Campylobacter cases captured at Santa Rosa and Quetzaltenango surveillance sites, stratified by healthcare setting, January 1, 2008 to August 31, 2012.
| Total | Ambulatory | Hospital | p value | |
|---|---|---|---|---|
|
| ||||
|
N = 306 n (%) |
N = 257 n (%) |
N = 49 n (%) |
||
| Demographics | ||||
| Age in years | 0.15 | |||
| <1 | 127 (41.5) | 101 (39.3) | 26 (53.1) | a |
| 1–4 | 155 (50.7) | 135 (52.5) | 20 (40.8) | |
| 5–18 | 11 (3.6) | 11 (4.3) | 0 | |
| 19–50 | 6 (2.0) | 5 (2.0) | 1 (2.0) | |
| >50 | 7 (2.3) | 5 (2.0) | 2 (4.1) | |
| Male sex | 171 (55.9) | 148 (57.6) | 23 (46.9) | 0.17 |
| History | ||||
| Fever b | 144 (53.3) | 114 (51.4) | 30 (62.5) | 0.16 |
| Bloody diarrhea | 27 (8.8) | 25 (9.7) | 2 (4.1) | 0.28 a |
| Abdominal pain/cramping b | 145 (51.6) | 136 (56.0) | 9 (23.7) | 0.0002 |
| Clinical | ||||
| Measured fever ≥ 38° C b | 58 (21.3) | 38 (17.0) | 20 (40.8) | 0.0002 |
| Sunken eyes | 104 (34.0) | 86 (33.5) | 18 (36.7) | 0.66 |
| Oral mucosa | <.0001 | |||
| Somewhat dry | 133 (43.5) | 99 (38.5) | 34 (69.4) | |
| Very dry | 19 (6.2) | 13 (5.1) | 6 (12.2) | |
| Prolonged capillary refill b | 13 (4.5) | 11 (4.5) | 2 (4.2) | 0.92 |
| Decreased skin turgor b | 5 (1.7) | 5 (2.0) | 0 | 1.00a |
| Laboratory speciation | 0.82 | |||
| Campylobacter jejuni | 235 (76.8) | 198 (77.0) | 37 (75.5) | |
| Campylobacter sp. – unknown | 71 (23.2) | 59 (23.0) | 12 (24.5) | |
| Treatment | ||||
| Admitted to intensive care unit b,c | 4 (8.5) | |||
| Received intravenous fluids b,c | 45 (94.0) | |||
| Received or prescribed oral rehydration solution b,d | 152 (66.7) | |||
| Received or prescribed medication b,d | 214 (93.9) | |||
| Azithromycin b | 2 (0.9) | 1 (2.7) | ||
| Chloramphenicol | 0 | 0 | ||
| Clindamycinb | 0 | 0 | ||
| Ciprofloxacin b | 3 (1.3) | 0 | ||
| Doxycycline b | 0 | 0 | ||
| Erythromycin b | 6 (3.2) | 1 (2.7) | ||
| Levofloxacin**b | 0 | 0 | ||
| Metronidazole b | 56 (25.0) | 1 (2.7) | ||
| Trimethoprim sulfamethoxazole b | 113 (50.5) | 0 | ||
Fisher’s exact test.
Proportions based on non-missing data.
Data only available in hospital setting.
Data only available in ambulatory setting.
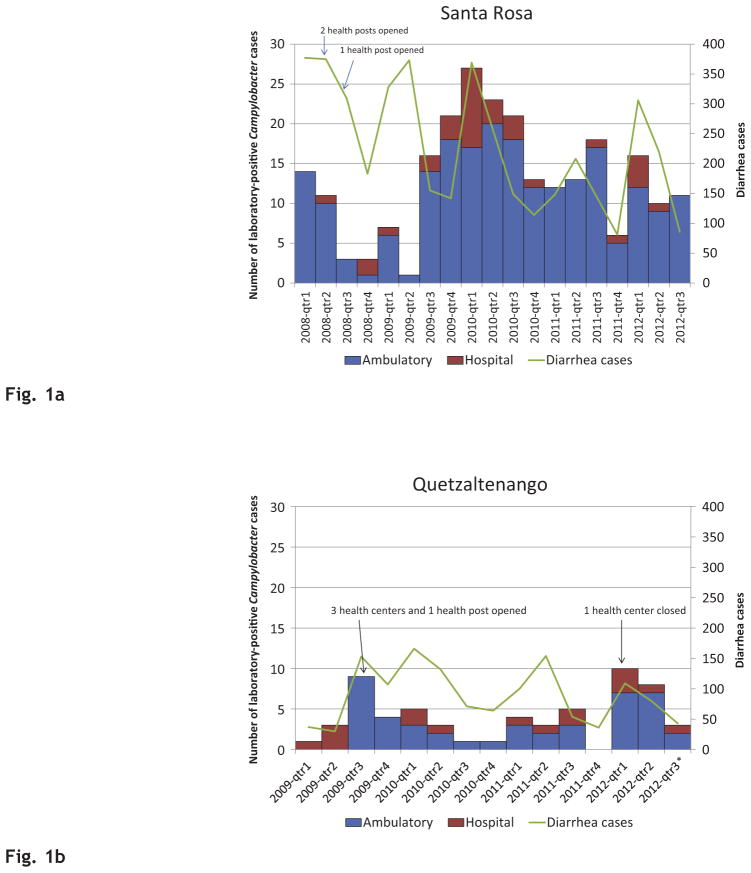
During the study period, the number of laboratory-confirmed Campylobacter infections and diarrhea cases captured by the surveillance system per quarter varied, especially for Santa Rosa (Fig. 1a and b). In Santa Rosa, the median proportion of cases with a confirmed Campylobacter infection per stool culture performed per quarter was 8.0% (range, 0.4–17.1%) in the ambulatory setting and 4.1% (range, 0–11.1%) in the hospital setting. In Quetzaltenango, the median proportion was 3.9% (range, 0–13.5%) in the ambulatory setting and 5.0% (range, 0–15.8%) in the hospital setting. The proportion of cases presenting to ambulatory versus hospital settings was higher for both Santa Rosa (86.6%) and Quetzaltenango (73.3%).
Fig. 1.
Fig. 1a. Number of laboratory-confirmed Campylobacter cases and diarrhea cases per quarter-year by patient-care setting, Santa Rosa, Guatemala 2008–2012.
Fig. 1b. Number of laboratory-confirmed Campylobacter cases and diarrhea cases per quarter-year by patient-care setting, Quetzaltenango, Guatemala 2009–2012.
Over 40% of the Campylobacter infections occurred in children <1 year of age, over 90% in children <5 years (<50% of patients screened were <5 years of age) (Table 2). More patients presenting to the ambulatory versus hospital settings stated that they had abdominal pain or cramping (p = 0.0002). Over 40% of hospitalized versus 17.0% of ambulatory case–patients had an axillary temperature of ≥38 °C (p = 0.0002). A higher proportion of hospitalized case–patients showed signs of dehydration with somewhat or very dry oral mucosa (p < 0.0001). Overall, 27 (8.8%) of the case–patients reported bloody diarrhea.
Among hospitalized case–patients, 45 (94.0%) received intravenous fluids; 4 (8.5%) were admitted to the intensive care unit, all were ≤6 months of age (Table 2). In the ambulatory setting, 152 patients (66.7%) received or were prescribed oral rehydration solution. Of the 76 patients who did not receive oral rehydration solution in the ambulatory setting, 64 (84.2%) were <5 years of age and 22 (34.4%) of them had sunken eyes, very dry mucosa, delayed capillary refill, or decreased skin turgor (data not shown). In the ambulatory setting, most patients (75.5%) received or were prescribed empiric therapy with metronidazole or trimethoprim-sulfamethoxazole. No patients died.
Antimicrobial susceptibility testing was performed on 96 (31.4%) of the 306 laboratory-confirmed Campylobacter case isolates; there were no demographic, geographic, healthcare setting, or clinical differences between patients with and patients without antimicrobial susceptibility data. Most specimens were C. jejuni (67.7%). Chloramphenicol had the lowest resistance rates while the quinolones including nalidixic acid and ciprofloxacin had the highest (Table 3). A total of 83 (86.4%) isolates were resistant to at least one antimicrobial class and 12 (12.5%) were MDR (Table 4). There was no difference in the proportion of isolates that were MDR in ambulatory versus hospital settings (p = 0.44); 9 (75.0%) of the 12 macrolide-resistant strains were MDR (data not shown).
Table 3.
Number and percentage of Campylobacter isolates resistant to antimicrobial agents, Santa Rosa and Quetzaltenango, Guatemala 2010–2011.
| Antimicrobial class | Antimicrobial | All Campylobacter N = 96 n (%) |
Campylobacter jejuni N = 65 n (%) |
|---|---|---|---|
| Macrolide | Erythromycin | 12 (12.5) | 8 (12.3) |
| Quinolones | Ciprofloxacin | 57 (59.4) | 42 (64.6) |
| Nalidixic acid | 62 (66.7) | 43 (68.3) | |
| Phenicol | Chloramphenicol | 11 (11.5) | 7 (10.8) |
| Tetracycline | Tetracycline | 59 (61.5) | 41 (63.1) |
Table 4.
Resistance patterns of Campylobacter isolates, Santa Rosa and Quetzaltenango, Guatemala 2010–2011.
| Antimicrobial | All Campylobacter N = 96 n (%) |
Campylobacter jejuni N = 65 n (%) |
|---|---|---|
| No resistance | 13 (13.5) | 10 (15.4) |
| Resistance to one antimicrobial class | 32 (33.3) | 18 (27.7) |
| Resistance to two antimicrobial classes | 39 (40.6) | 29 (44.6) |
| Resistance to three antimicrobial classes | 10 (10.4) | 7 (10.8) |
| Resistance to four antimicrobial classes | 2 (2.1) | 1 (1.5) |
4. Discussion
This study is the first large study to describe laboratory-confirmed Campylobacter infections in Guatemala. In the ambulatory setting in Santa Rosa in 2010, crude annual incidence rates were as high as 215.8 per 100,000 for all ages, and 1288.8 per 100,000 in children <5 years old. Campylobacter primarily affected children <5 years of age, and most patients presented to ambulatory care centers for treatment. Among isolates tested, the proportion that was MDR was 12.5%.
In 2009 and 2010 the incidence of Campylobacter infections in the United States reported by FoodNet, which provides population-based estimates of laboratory-confirmed infections commonly transmitted through food from 10 sites, was 13.0 and 13.6 per 100,000 persons, respectively, for all ages [19,20]. In the ambulatory setting in Santa Rosa, crude incidence rates for all ages in 2009 and 2010 were 10-fold higher than corresponding rates in the United States in 2009 and 16-fold higher for 2010. For the United States in 2010, FoodNet reported the incidence among children <5 years old of 24.4 per 100,000 persons, while in Santa Rosa during that year, this study found an incidence of 1288.8 – over 50-fold greater [19]. The reported incidence rates in other parts of the world varied, including 400 per 100,000 persons in New Zealand (2003) [21], >120 per 100,000 in Australia (2005) [22], 44.1 per 100,000 in Europe (2008) [23], and 30 per 100,000 in Canada (2004) [24]. Rates from Quetzaltenango, Guatemala were lower than Santa Rosa. It is important to note that differences in the surveillance methodology may impact incidence rates.
Quinolone-resistant Campylobacter strains have been rising worldwide in the past two decades [1,6]. The proportion of Campylobacter isolates resistant to ciprofloxacin in the U.S. National Antimicrobial Resistance Monitoring System (NARMS) in 2010 was 22.4% [17]. In Finland (2003–2005), 45% of Campylobacter strains were resistant to ciprofloxacin [18]. In SENTRY, a worldwide laboratory surveillance network, 42.6% of Campylobacter strains from 10 medical centers in Latin America and 20 in Europe were resistant to ciprofloxacin in 2003 [25]. The data from this study, from April 2010 through December 2011, show an even higher proportion of ciprofloxacin-resistance of 59.4%. In addition, whereas 2.1% of isolates reported in NARMS in 2010 were MDR, this study found 12.5% of isolates from the Guatemalan surveillance system to be MDR. Macrolide resistance prevalence in the U.S. has been low (≤3%) and steady over the past ten years. In Guatemala, resistance prevalence was fourfold (12.5%) higher compared with NARMS. Similar to Finnish data [18], strains that were macrolide-resistant tended to be MDR.
Although most Campylobacter infections are self-limited and do not require antimicrobial therapy, patients with severe infections, and those who are immunocompromised or pregnant may require antimicrobial treatment. This study’s data suggest that resistant strains of Campylobacter are prevalent, making antimicrobial agent selection difficult. In the sentinel sites in Guatemala, >75% of patients in the ambulatory setting received metronidazole or trimethoprim-sulfamethoxazole, neither of which is effective against Campylobacter . At the same time, one-third of patients in the outpatient setting was not prescribed or did not receive oral rehydration solution. Of those, 84.2% were children <5 years of age. One-third of those patients <5 years of age who had not received oral rehydration solution had at least one sign of dehydration on physical exam. These findings highlight the need to reemphasize the adequate training and performance monitoring of proper oral rehydration solution administration, especially among young vulnerable populations.
Similar to the geographic variation in campylobacteriosis in FoodNet sites in the U.S. [26], this study also found large differences in the rates of Campylobacter infections and the number of patients presenting with diarrhea in Santa Rosa and Quetzaltenango. The warm climate of Santa Rosa likely contributes to the higher rate of diarrheal disease in the department [27]. Differences in healthcare seeking behaviors and hospital admitting practices may also partially explain the difference. In the United States, campylobacteriosis is a seasonal disease that peaks in the summer months [26]. In Guatemala, a definitive seasonal pattern was not observed, perhaps because temperatures remain relatively stable throughout the year.
The findings of this study are subject to a number of limitations. Although the surveillance platform includes most of the government facilities in the populations studied, not all patients seek care in these facilities and some do not seek care at all. Therefore, the crude incidence rates are an underestimate of the true incidence of Campylobacter disease. In addition, surveillance in the hospital setting only includes patients who are admitted. Thus, patients seen in the ED and then discharged or transferred will not be included in this data, again leading to underestimates in the rate calculations. The presence of Campylobacter in healthy controls was not tested to determine what proportion of the cases identified in this surveillance system may have been asymptomatic carriers of Campylobacter whose disease was caused by another enteric pathogen. Finally, these data are from the departments of Santa Rosa and Quetzaltenango and therefore may not be generalizable to all of Guatemala. These data, however, are the most comprehensive description of campylobacteriosis in Guatemala and Central America in the healthcare setting.
5. Conclusion
Campylobacter is a major cause of diarrhea in children <5 years of age in Guatemala with disease rates in the ambulatory setting significantly higher than those of the United States. Consistent with the epidemiology of Campylobacter elsewhere, disease predominantly affected younger age groups. Similar to FoodNet data from the United States, rates varied substantially between sites, but unlike FoodNet, no seasonal pattern was apparent. Although treatment is normally supportive, one-third of patients in the outpatient setting was not prescribed or given oral rehydration therapy and >75% were given an antimicrobial ineffective against Campylobacter. Quinolone-resistant and MDR Campylobacter proportions were higher in Guatemala than in other countries, potentially complicating the treatment for immunocompromised and pregnant patients or those with severe disease. This study illustrates the capacity of a sentinel healthcare facility-based surveillance system to provide working estimates of the incidence of an acute infectious disease. The next steps in addressing the burden of campylobacteriosis in Guatemala include the utilization of this platform to identify risk factors for Campylobacter infection through focused case–control studies; test interventions based on results of risk-factor studies, aimed at reducing the burden of disease; identify optimal treatment regimens for diarrhea in these populations; and establish the further burden of post-infectious sequelae of campylobacteriosis and the economic cost of the illness.
Acknowledgments
We would like to thank the Guatemala National Center for Epidemiology, the Health Areas of Santa Rosa and Quetzaltenango, and the surveillance hospitals, health centers, and health posts for their cooperation. We are grateful to Lesbia Arevalo, Liliana de Alvarez, Aleida Roldán and César Racancoj for their laboratory support and Gerard Lopez, Fredy Muñoz and their team of programmers for the development of the Questionnaire Mobile program for data entry on PDAs. We also thank Chris Bernart, Fabiola Moscoso, and our surveillance nurses and field staff for their administrative and technical support. We are very thankful for the active participation of the residents of Santa Rosa and Quetzaltenango. Financial support: This publication was supported by Cooperative Agreement Number UO1 GH000028-02 from the U.S. Centers for Disease Control and Prevention (CDC). The CDC participated in all aspects of study design, data collection, data analysis, and manuscript preparation.
Footnotes
Conflict of interest
None declared.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Allos BM. Campylobacter jejuni Infections: update on emerging issues and trends. Clin Infect Dis. 2001;32(8):1201–6. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- 2.Blaser MJ. Epidemiologic and clinical features of Campylobacter jejuni infections. J Infect Dis. 1997;176(Suppl 2):S103–5. doi: 10.1086/513780. [DOI] [PubMed] [Google Scholar]
- 3.Allos BM. Association between Campylobacter infection and Guillain-Barré syndrome. J Infect Dis. 1997;176(Suppl 2):S125–8. doi: 10.1086/513783. [DOI] [PubMed] [Google Scholar]
- 4.Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome–a meta-analysis. Am J Gastroenterol. 2006;101(8):1894–9. doi: 10.1111/j.1572-0241.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 5.DuPont AW. Postinfectious irritable bowel syndrome. Clin Infect Dis. 2008;46(4):594–9. doi: 10.1086/526774. [DOI] [PubMed] [Google Scholar]
- 6.Kirkpatrick BD, Tribble DR. Update on human Campylobacter jejuni infections. Curr Opin Gastroenterol. 2011;27(1):1–7. doi: 10.1097/MOG.0b013e3283413763. [DOI] [PubMed] [Google Scholar]
- 7.Smith JL, Fratamico PM. Fluoroquinolone resistance in Campylobacter. J Food Prot. 2010;73(6):1141–52. doi: 10.4315/0362-028x-73.6.1141. [DOI] [PubMed] [Google Scholar]
- 8.Payot S, Bolla JM, Corcoran D, Fanning S, Mégraud F, Zhang Q. Mechanisms of fluoroquinolone and macrolide resistance in Campylobacter spp. Microbes Infect. 2006;8(7):1967–71. doi: 10.1016/j.micinf.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Pan American Health Organization (PAHO) [Accessed on June 15, 2013]; Available at: < http://www1.paho.org/english/dd/ais/cp_320.htm>.
- 10.Jensen LA, Marlin JW, Dyck DD, Laubach HE. Prevalence of multi-gastrointestinal infections with helminth, protozoan and Campylobacter spp. in Guatemalan children. J Infect Dev Ctries. 2009;3(3):229–34. doi: 10.3855/jidc.41. [DOI] [PubMed] [Google Scholar]
- 11.Cruz JR, Cano F, Bartlett AV, Méndez H. Infection, diarrhea, and dysentery caused by Shigella species and Campylobacter jejuni among Guatemalan rural children. Pediatr Infect Dis J. 1994;13(3):216–23. doi: 10.1097/00006454-199403000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Hurd S, Patrick M, Hatch J, Clogher P, Wymore K, Cronquist AB, et al. Clinical Laboratory Practices for the isolation and identification of campylobacter in foodborne diseases active surveillance network (FoodNet) Sites: baseline information for understanding changes in surveillance data. Clin Infect Dis. 2012;54(Suppl 5):S440–5. doi: 10.1093/cid/cis245. [DOI] [PubMed] [Google Scholar]
- 13.Instituto Nacional de Estadistica. [accessed on June 20.06.12]; Available at: < http://www.ine.gob.gt/np/poblacion/index.htm>.
- 14.Reyes L, Arvelo W, Estevez A, Gray J, Moir JC, Gordillo B, et al. Population-based surveillance for 2009 pandemic influenza A (H1N1) virus in Guatemala, 2009. Influenza Other Respi Viruses. 2010;4(3):129–40. doi: 10.1111/j.1750-2659.2010.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilligan PH, Whittier S. Reagents. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of clinical microbiology. Washington, D.C: ASM Press; 1999. pp. 526–38. [Google Scholar]
- 16.Clinical and Laboratory Standards Institute (CLSI) CLSI-document M45–A2. Wayne, PA: 2010. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; Approved guideline-Second edition. [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS): Human Isolates Final Report. Atlanta. Georgia: U.S. Department of Health and Human Services, CDC; 2010. 2012. [Google Scholar]
- 18.Lehtopolku M, Nakari UM, Kotilainen P, Huovinen P, Siitonen A, Hakanen AJ. Antimicrobial susceptibilities of multidrug-resistant Campylobacter jejuni and C. coli strains: in vitro activities of 20 antimicrobial agents. Antimicrob Agents Chemother. 2010;54(3):1232–6. doi: 10.1128/AAC.00898-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food - 10 states 2009. MMWR Morb Mortal Wkly Rep. 2010;59(14):418–22. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Vital signs: incidence and trends of infection with pathogens transmitted commonly through food–foodborne diseases active surveillance network, 10 U.S sites, 1996–2010. MMWR Morb Mortal Wkly Rep. 2011;60(22):749–55. [PubMed] [Google Scholar]
- 21.Baker MG, Sneyd E, Wilson NA. Is the major increase in notified campylobacteriosis in New Zealand real? Epidemiol Infect. 2007;135(1):163–70. doi: 10.1017/S0950268806006583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.OzFoodNet Working Group. Burden and causes of foodborne disease in Australia: annual report of the OzFoodNet network, 2005. Commun Dis Intell. 2006;30:278–300. [PubMed] [Google Scholar]
- 23.European Centre for Disease Prevention and Control. [accessed on 25.09.12];Annual epidemiological report on communicable disease in Europe. 2010 Available at: < http://ecdc.europa.eu/en/publications/Publications/1011_SUR_Annual_Epidemiological_Report_on_Communicable_Diseases_in_Europe.pdf>. [PubMed]
- 24.Galanis E. Campylobacter and bacterial gastroenteritis. CMAJ. 2007;177(6):570–1. doi: 10.1503/cmaj.070660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streit JM, Jones RN, Toleman MA, Stratchounski LS, Fritsche TR. Prevalence and antimicrobial susceptibility patterns among gastroenteritis-causing pathogens recovered in Europe and Latin America and Salmonella isolates recovered from bloodstream infections in North America and Latin America: report from the SENTRY Antimicrobial Surveillance Program (2003) Int J Antimicrob Agents. 2006;27(5):367–75. doi: 10.1016/j.ijantimicag.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Ailes E, Demma L, Hurd S, Hatch J, Jones TF, Vugia D, et al. Continued decline in the incidence of Campylobacter infections, FoodNet 1996–2006. Foodborne Pathog Dis. 2008;5(3):329–37. doi: 10.1089/fpd.2008.0090. [DOI] [PubMed] [Google Scholar]
- 27.Lake IR, Gillespie IA, Bentham G, Nichols GL, Lane C, Adak GK, et al. A re-evaluation of the impact of temperature and climate change on foodborne illness. Epidemiol Infect. 2009;137(11):1538–47. doi: 10.1017/S0950268809002477. [DOI] [PubMed] [Google Scholar]