Abstract
Objective
To assess whether markers of acculturation (birthplace, number of U.S. generations) and socioeconomic status (SES) are associated with carotid artery plaque, internal carotid intima-media thickness (IMT), and albuminuria, in four racial/ethnic groups.
Methods
Using Multi-Ethnic Study of Atherosclerosis data (n = 6,716; age: 45-84) and race-specific binomial regression models, we computed prevalence ratios, adjusted for demographics and traditional cardiovascular risk factors.
Results
The adjusted U.S. to foreign-born prevalence ratio (99% CI) for carotid plaque was 1.20 (0.97, 1.39) in Whites, 1.91 (0.94, 2.94) in Chinese, 1.62 (1.28, 2.06) in Blacks, and 1.23 (1.15, 1.31) in Hispanics. Greater carotid plaque prevalence was also found among Whites, Blacks, and Hispanics with more generations of US residence (p<0.001). Lower educational attainment and/or income were associated with greater carotid plaque prevalence in Whites and Blacks. Similar associations were observed with IMT. There was also some evidence of an inverse association between albuminuria and SES, in Whites and Hispanics.
Conclusions
Greater U.S. acculturation and lower SES were associated with a higher prevalence of carotid plaque and IMT, while little association was found with albuminuria.
Introduction
Originating with the Ni-Hon-San study(1, 2), research has associated increased acculturation to Western lifestyles with more adverse cardiovascular disease (CVD) risk factor profiles and with increased CVD morbidity and mortality. Specifically, greater Western acculturation has frequently been linked to increased BMI(3-5), waist circumference and abdominal obesity(6, 7), hypertension(7-9), type II diabetes(10, 11), and CVD morbidity and mortality(1, 12, 13). However, little research has explored associations between acculturation and subclinical CVD(14, 15).
Abundant research also exists linking low SES to increased levels of CVD risk factors, morbidity, and mortality(14, 16-18). In general, SES has been found to be inversely related to subclinical measures of CVD, including coronary artery calcification (CAC)(14, 19-22), carotid artery plaque or intima-media thickness (IMT)(20, 23-26), and albuminuria(27), while relations with peripheral artery disease have been inconsistent(28-30). The extent to which these associations vary by race/ethnicity has been examined infrequently. There is, however, some evidence that the relationship between SES and disease may differ across racial/ethnic groups(14, 31, 32). Specifically, in the Multi-Ethnic Study of Atherosclerosis (MESA) a higher prevalence of CAC was found among whites with low education as compared to those with higher education, while the reverse was true for Hispanics(14).
The aim of the present study was to assess whether acculturation and SES are associated with other measures of subclinical disease, specifically with carotid plaque and albuminuria. The relations of acculturation and SES to CAC have been described previously in MESA(14). Although CAC, carotid plaque, and albuminuria are all subclinical measures of CVD and are related to adverse clinical outcomes, these measures represent different aspects of the disease process, and have relatively weak intercorrelations(33). Thus, they may be differentially related to our exposures of interest. The investigation of these patterns is important from a public health perspective, and may also yield clues regarding the etiology of atherosclerosis. Based on prior work(14) we hypothesized that increased Western acculturation, as assessed by place of birth, migration history, and duration of U.S. residence, would be associated with increased carotid plaque, IMT, and albuminuria. Additionally, we expected there to be an interaction between race/ethnicity and SES with respect to their associations with subclinical disease. Specifically, we expected Caucasians and Blacks at lower SES to have more adverse subclinical CVD profiles than those at higher SES, while for Hispanics and Chinese we expected the reverse to be true.
Methods
Study Population
MESA is a prospective epidemiological cohort study initiated in July 2000, with the aim of exploring the prevalence, correlates, and progression of subclinical and clinical CVD(34). A specific objective of MESA is to assess racial/ethnic, age, and sex differences in subclinical and clinical CVD. Local institutional review committees approved the MESA protocol, and all subjects gave informed consent. 6,814 men and women between the ages of 45 and 84, all of whom were free of reported clinical CVD at baseline, were recruited in 6 U.S. field centers.
Individuals were classified as Hispanic, non-Hispanic black, non-Hispanic white, or non-Hispanic Chinese based on their answers to race and ethnicity questions modeled on the Year 2000 Census. White participants were recruited from all study sites, while Blacks were recruited from Forysth County, NC; Chicago, IL; New York, NY; Baltimore, MD and Los Angeles, CA; Hispanics from St. Paul, MN; New York, NY; and Los Angeles, CA; and Chinese from Chicago, IL and Los Angeles, CA.
Acculturation and SES Definitions
Questionnaires administered as part of the baseline visit were used to obtain information on acculturation and SES. Country of birth, migration history, and time in the U.S. (for those not born in the U.S.) were used as proxy measures of acculturation. For migration history, or number of generations in the U.S., participants not born in the U.S. were considered generation zero, while those with ≥ 1 parent not born in the U.S. were considered first generation, those with both parents born in the U.S. but ≥ 2 grandparents not born in the U.S. were classified as second generation, and those with both parents born in the U.S. and ≥3 grandparents born in the U.S. were categorized as third generation(14). Country of birth was collapsed into region of birth, and was used in analyses of the Black and Hispanic subgroups. Blacks were categorized as being from Central America or the Caribbean, Africa, or the U.S., whereas Hispanics were classified as being from Mexico, Central America or the Caribbean, South America, or the U.S.
Education and income levels were used as indicators of SES. Participants were asked to select their highest level of schooling completed from eight categories, and their gross family income from thirteen categories(14). A priori, the education and income levels were collapsed into the following smaller categories for use as indicator variables in multivariate analyses: education – grade 8 or less, grade 9-11, completed high school or GED, technical school or associate degree or some college, bachelor's degree, graduate or professional school; income – less than $12,000, $12,000 to $24,999, $25,000 to $34,999, $35,000 to $49,999, $50,000 to $74,999, $75,000 to $99,999, $100,000 or more.
Subclinical CVD Assessment
Images of bilateral common carotid and internal carotid arteries were obtained via high-resolution B-mode ultrasonography using a Logiq 700 ultrasound machine (GE Medical Systems, Waukesha, Wisconsin). Images of the near and far walls were obtained, as in a previous study(35). Central reading of the intima-media thickness took place at Tufts-New England Medical Center (Boston, MA). The maximum thicknesses of the common and internal carotid arteries were used in the analyses. Additionally, a dichotomous variable indicated the presence of atherosclerotic plaque (any stenosis in either the right or left internal or common carotid artery).
Urine albumin and creatinine concentrations were assayed in a single untimed urine sample at the Fletcher Allen Health Care Clinical Chemistry Laboratory (Burlington, VT). Urine albumin was measured by the Array 360 CE Protein Analyzer (Beckman Instruments, Inc., Drea, CA), while serum creatinine was measured by rate reflectance spectrophotometry using thin film adaptation of the creatine amidinohydrolase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY). To estimate the albumin excretion rate (A/kC), sex-standardized urine albumin (μg/mL) to creatinine (mg/mL) ratios were calculated after multiplying men's urine creatinine concentrations by k = 25/17, based on the higher rate of creatinine excretion typical of men compared to women(36, 37). The sex-standardized urine albumin to creatinine ratio is represented both linearly and dichotomously, with participants having values ≥ 25 mg/g defined as having albuminuria(36).
Additional Covariates
Sex, age, current cigarette smoking status (current, former, never), and use of statins, antihypertensives, and diabetes medications were self-reported. Body mass index (BMI) was calculated as weight over height squared (kg)/(m2). Resting blood pressure was measured three times in the seated position using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, Florida). The average of the last two measurements was used in analyses. Participants were asked to fast for at least 8 hours prior to their baseline visit. Serum glucose was measured by rate reflectance spectrophotometry using thin film adaptation of the glucose oxidase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY). HDL cholesterol was assessed in EDTA plasma using the cholesterol oxidase method (Roche Diagnostics) after precipitation of non-HDL-cholesterol with magnesium/dextran, and LDL cholesterol was calculated in plasma specimens having a triglyceride value <400 mg/dL using the Friedewald formula. Serum assays were preformed at the Collaborative Studies Clinical Laboratory at Fairview-University Medical Center (Minneapolis, MN).
At baseline, diet was assessed using a staff-assisted self-administered 127-item food frequency questionnaire (FFQ) and dietary supplement form, which has been described elsewhere(38). Servings per day of the following food groups were calculated: fruit, vegetables, whole grains, refined grains, dairy, fish and poultry, and meat. Physical activity was assessed using a detailed, semi-quantitative questionnaire adapted from the Cross-Cultural Activity Participation Study (personal communication, Barbara Ainsworth, San Diego State University). Leisure physical activity was computed as the sum of MET(39)-min/wk of walking, conditioning, sports, and dance, while a sedentary lifestyle score was the sum MET-min/wk of sitting or reclining, reading, knitting, sewing, driving a car, or watching television.
Statistical Analysis
All analyses were stratified by race/ethnicity, and were performed with SAS (version 9.1; Cary, NC: SAS Institute Inc.). Given the large number of comparisons being conducted in this analysis, p < 0.01 was required for statistical significance, but exact p-values are reported. Means and frequencies of unadjusted demographics, acculturation measures, socioeconomic indicators, and measures of subclinical CVD were computed. Age- and sex-adjusted demographics and traditional CVD risk factors were also computed, stratified by place of birth (U.S. or other).
For our analyses of plaque and albuminuria, race/ethnicity-specific binomial regression models with a logit link (PROC GLIMMIX) were used, and predicted logits were back-transformed to give adjusted median prevalences of plaque and albuminuria, and to assess differences between prevalences. Mean differences in ln(internal carotid IMT) and ln(urine albumin excretion) were assessed via multiple linear regression (PROC GLM). For primary exposures with more than two levels (i.e. education, income, generations of U.S. residence), adjusted prevalences and means were computed with the exposures entered as indicator variables, while the p-value for the linear trend across the groups was computed with ordinal categories entered as continuous variables. The base model (model 1) adjusted for sex, age (years), and relevant combinations of country of birth (U.S. or other), education (6-level indicator variable), and income (7-level indicator variable). Our second model aimed at determining whether associations observed were independent of traditional CVD risk factors. This model controlled for model 1 covariates, and additionally adjusted for major CVD risk factors including smoking status (current, former, never), body mass index (BMI) (continuous), LDL cholesterol (continuous), HDL cholesterol (continuous), statin use (Y/N), systolic blood pressure (continuous), hypertension medication use (Y/N), and diabetes status by American Diabetes Association 2003 criteria (Y/N). Overall, differences between the base model (model 1) and the model adjusted for major CVD risk factors (model 2) were minimal. Results from the base model are discussed, and instances where adjustment for major CVD risk factors substantially impacted estimates are noted. A third model, which further adjusted for diet (fruit, vegetables, refined grains, whole grains, dairy, and meat) and physical activity (leisure physical activity, sedentary lifestyle score), was also explored. However, as these additional adjustments rarely influenced our results we chose not to present these data.
Results
The sample included 2,624 Whites, 803 Chinese, 1,895 Blacks, and 1,492 Hispanics aged 45-84 years. Race/ethnicity-stratified acculturation measures, socioeconomic indicators, and subclinical measures are shown in Table 1. Chinese participants were most likely to have been born in another country (96.3%), followed by Hispanics (68.8%), Blacks (9.0%), and Whites (6.6%). On average, Whites born elsewhere had lived in the U.S. for a longer duration (mean years ± SD) (46 ± 19) than Blacks (38 ± 18), Hispanics (30 ± 15), and Chinese (19 ± 12), respectively. Of Blacks born outside the U.S., 66% originated in Central America or the Caribbean, while 27% were born in Africa. Among Hispanics born in another country, 36% were from Mexico, 34% were from Central America or the Caribbean, and 11% were from South America.
Table 1.
Measures of acculturation, socioeconomic indicators, and subclinical CVD measures by race/ethnicity in 6,814 participants, MESA 2000-2002*
| Whites | Chinese | Blacks | Hispanics | |
|---|---|---|---|---|
| N | 2624 | 803 | 1895 | 1492 |
| Mean age, y (SD) | 62.6 (10.2) | 62.3 (10.3) | 62.1 (10.0) | 61.3 (10.3) |
| Male, % | 1261 (48.1) | 390 (48.6) | 844 (44.5) | 718 (48.1) |
| Place of birth†, % | ||||
| United States | 2445 (93.4) | 30 (3.7) | 1712 (91.0) | 465 (31.2) |
| Other country | 172 (6.6) | 772 (96.3) | 169 (9.0) | 1027 (68.8) |
| Generations in the U.S.§†, % | ||||
| 0 | 172 (6.6) | 772 (96.3) | 169 (9.0) | 1027 (68.8) |
| 1 | 455 (17.4) | 28 (3.5) | 36 (1.9) | 301 (20.2) |
| 2 | 656 (25.0) | 2 (0.2) | 23 (1.2) | 111 (7.4) |
| 3 | 1334 (51.0) | 0 (0.0) | 1653 (87.9) | 53 (3.6) |
| Income†, % | ||||
| < $20,000 | 277 (10.9) | 332 (41.7) | 378 (21.8) | 573 (39.3) |
| $20,000-$49,999 | 819 (32.1) | 238 (29.9) | 710 (41.0) | 625 (42.9) |
| ≥ $50,000 | 1457 (57.0) | 227 (28.4) | 646 (37.2) | 259 (17.8) |
| Education†, % | ||||
| < High school degree | 129 (4.9) | 199 (24.8) | 230 (12.2) | 667 (44.7) |
| High school degree or some college | 1189 (45.5) | 291 (36.3) | 1015 (54.0) | 678 (45.4) |
| College degree | 1298 (49.6) | 312 (38.9) | 636 (33.8) | 147 (9.9) |
| Carotid plaque, % | 1197 (46) | 208 (26) | 810 (44) | 574 (39) |
| Common carotid IMTψ (mm) | 0.86 | 0.82 | 0.90 | 0.85 |
| Internal carotid IMTψ (mm) | 1.06 | 0.83 | 1.03 | 0.97 |
| Percent with A/kC > 25 | 248 (9.5) | 134 (16.7) | 299 (15.8) | 234 (15.7) |
| Urine albumin excretionψ (mg/g) | 6.95 | 10.29 | 8.71 | 10.01 |
For racial/ethnic differences, p<0.0001 for all variables except age (p=0.002), and % male (p=0.06).
Due to missing values in some instances the sum of the categories is less than the race-specific total.
Geometric mean
Generation 0 means participant was not born in the U.S.; first generation, 1 or both parents were not born in the U.S.; second generation, both parents were born in the U.S. but ≥ 2 grandparents were not born in the U.S.; and third generation, both parents were born in the U.S. and ≥3 grandparents were born in the U.S..
Chinese and Hispanics not born in the U.S. generally had lower levels of income and formal education than those born in the U.S. (Table 2). BMI was significantly higher in US-born Chinese and Hispanics, as compared to their non-US-born counterparts. Blacks born outside the U.S. were less likely to smoke than those born in the U.S.
Table 2.
Socioeconomic characteristics and cardiovascular risk factors of U.S.and Non-US-Born participants by race/ethnicity, MESA 2000-2002*
| White | Chinese | Black | Hispanic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birthplace | US | Other | p-value¥ | US | Other | p-value¥ | US | Other | p-value¥ | US | Other | p-value¥ |
| N | 2,445 | 172 | 30 | 772 | 1,712 | 169 | 465 | 1,029 | ||||
| Male, % | 48.3 | 43.5 | 0.23 | 56.7 | 48.2 | 0.36 | 44.6 | 45.7 | 0.77 | 52.9 | 45.9 | 0.01 |
| Mean age, y | 62.5 | 63.8 | 0.12 | 61.1 | 62.4 | 0.52 | 62.5 | 58.4 | <0.0001 | 61.6 | 61.1 | 0.38 |
| Education† | 0.08 | 0.005 | 0.09 | <0.0001 | ||||||||
| < High school degree | 4.8 | 6.4 | 3.3 | 25.7 | 11.9 | 15.4 | 20.0 | 55.9 | ||||
| High school degree or some college | 46.0 | 37.4 | 33.3 | 36.4 | 53.6 | 58.0 | 67.7 | 35.4 | ||||
| College degree | 49.2 | 56.1 | 63.3 | 38.0 | 34.5 | 26.6 | 12.3 | 8.8 | ||||
| Income, %† | 0.96 | <0.0001 | 0.87 | <0.0001 | ||||||||
| <$20,000 | 10.8 | 11.2 | 3.5 | 43.1 | 21.6 | 23.5 | 25.5 | 45.7 | ||||
| $20,000-$49,999 | 32.1 | 31.2 | 34.5 | 29.7 | 41.0 | 40.1 | 45.8 | 41.6 | ||||
| ≥$50,000 | 57.0 | 57.7 | 62.1 | 27.2 | 37.3 | 36.4 | 28.8 | 12.7 | ||||
| Smoked w/in past 30 days, % | 11.3 | 14.5 | 0.21 | 5.9 | 5.6 | 0.95 | 19.3 | 4.8 | <0.0001 | 14.6 | 13.1 | 0.42 |
| Diabetes, % | 4.3 | 5.2 | 0.58 | 7.1 | 10.4 | 0.56 | 14.2 | 5.5 | 0.67 | 16.5 | 13.5 | 0.12 |
| Mean BMI, kg/m2 | 27.8 | 27.2 | 0.14 | 25.7 | 23.9 | 0.004 | 30.2 | 29.4 | 0.05 | 30.6 | 28.9 | <0.0001 |
| Statin use, % | 16.6 | 16.5 | 0.96 | 24.5 | 12.4 | 0.05 | 15.8 | 12.2 | 0.23 | 14.1 | 11.2 | 0.11 |
| Mean LDL cholesterol, mg/dL | 117 | 119 | 0.36 | 108 | 115 | 0.16 | 116 | 119 | 0.38 | 117 | 121 | 0.05 |
| Mean HDL cholesterol, mg/dL | 52.2 | 53.2 | 0.34 | 53.8 | 49.4 | 0.05 | 52.3 | 53.3 | 0.35 | 47.8 | 47.6 | 0.80 |
| Hypertension medication use, % | 32.9 | 35.4 | 0.49 | 18.4 | 29.2 | 0.18 | 50.4 | 47.7 | 0.48 | 32.2 | 32.5 | 0.91 |
| Mean systolic blood pressure, mmHg | 124 | 120 | 0.02 | 125 | 125 | 0.82 | 131 | 129 | 0.08 | 126 | 127 | 0.17 |
Means and proportions of cardiovascular risk factors are adjusted for age and sex.
Probability value for difference between U.S. and foreign born individuals.
Unadjusted values.
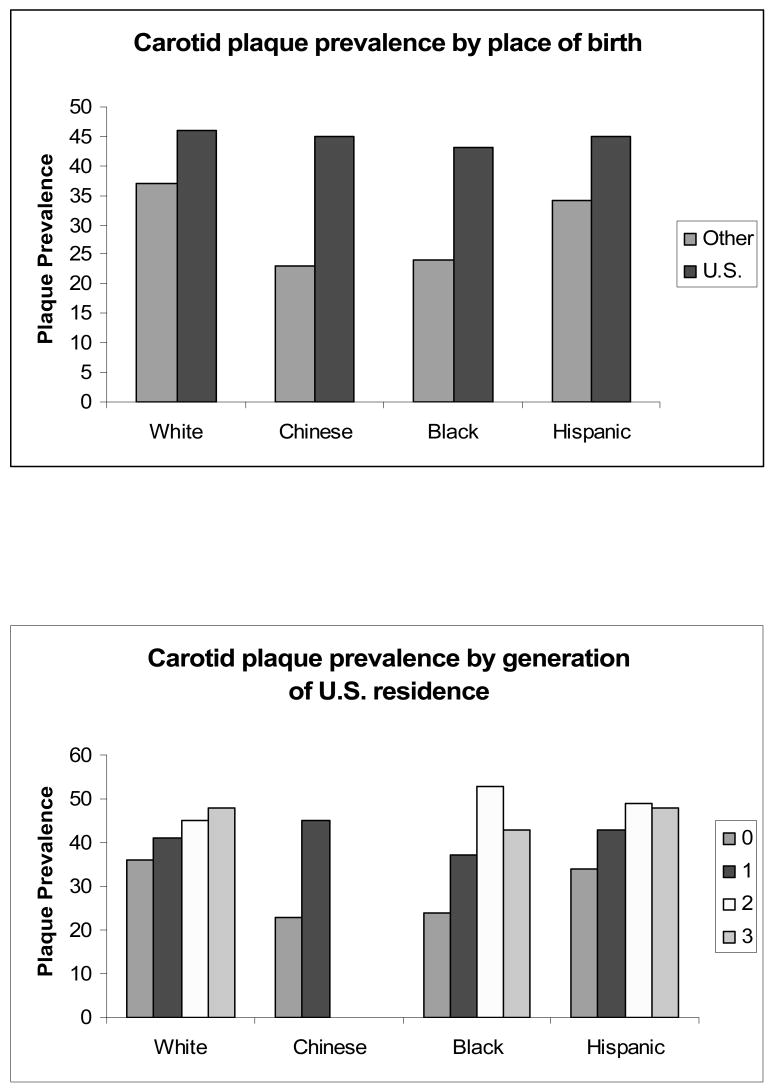
Foreign-born Blacks and Hispanics had a significantly lower prevalence of carotid plaque, as compared to their U.S.-born counterparts (Table 3, Figure 1). A similar trend was observed for Whites and Chinese, though these contrasts did not reach statistical significance. After model 1 adjustments, the U.S. to foreign-born carotid plaque prevalence ratio (99% CI) was 1.20 (0.97, 1.39) in Whites, 1.91 (0.94, 2.94) in Chinese, 1.62 (1.28, 2.06) in Blacks, and 1.23 (1.15, 1.31) in Hispanics. Although plaque prevalence increased with the number of generations the participants' family had lived in the U.S. in all racial/ethnic groups, it did not reach the chosen significance level in Chinese (p < 0.001 for all but Chinese). Among Hispanics born outside the U.S. each additional decade of U.S. residence was associated with a 4.4% greater prevalence of plaque, following model 1 adjustments (p = 0.001). Decades of U.S. residence was not significantly associated with plaque among other racial/ethnic groups (data not shown).
Table 3.
Race/ethnicity stratified prevalences of carotid plaque and mean maximum internal carotid IMT by acculturative and socioeconomic characteristics, MESA 2000-2002.
| Prevalence of Carotid Plaque (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| White | Chinese | Black | Hispanic | |||||
| N | 2,624 | 803 | 1,895 | 1,492 | ||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Place of birth | ||||||||
| Other country | 37 | 35 | 23 | 22 | 24 | 25 | 34 | 34 |
| United States | 46 | 45 | 45 | 44 | 43 | 42 | 45 | 44 |
| p-value | 0.03 | 0.02 | 0.02 | 0.02 | <0.0001 | 0.0003 | 0.001 | 0.003 |
| Generations in U.S.§ | ||||||||
| 0 | 36 | 35 | 23 | 22 | 24 | 25 | 34 | 34 |
| 1 | 41 | 41 | 45 | 44 | 37 | 36 | 43 | 42 |
| 2 | 45 | 45 | -- | -- | 53 | 53 | 49 | 48 |
| 3 | 48 | 47 | -- | -- | 43 | 42 | 48 | 48 |
| p-value† | 0.0007 | 0.003 | 0.02 | 0.02 | <0.0001 | 0.0005 | 0.001 | 0.002 |
| Education | ||||||||
| < High school degree | 51 | 46 | 24 | 23 | 44 | 44 | 40 | 38 |
| High school degree or some college | 52 | 50 | 23 | 22 | 40 | 40 | 37 | 37 |
| College degree | 39 | 40 | 23 | 22 | 40 | 41 | 29 | 30 |
| p-value† | <0.0001 | 0.0009 | 0.74 | 0.80 | 0.53 | 0.73 | 0.08 | 0.20 |
| Income | ||||||||
| <$20,000 | 48 | 45 | 23 | 23 | 48 | 46 | 39 | 38 |
| $20,000-$49,999 | 49 | 48 | 29 | 28 | 42 | 41 | 37 | 36 |
| ≥$50,000 | 42 | 42 | 19 | 17 | 35 | 37 | 35 | 37 |
| p-value† | 0.02 | 0.10 | 0.53 | 0.35 | 0.002 | 0.02 | 0.41 | 0.12 |
|
| ||||||||
| Mean Maximum Internal Carotid IMT* (mm) | ||||||||
|
| ||||||||
| Place of birth | ||||||||
| Other country | 1.01 | 1.01 | 0.82 | 0.82 | 0.88 | 0.91 | 0.94 | 0.95 |
| United States | 1.05 | 1.05 | 1.02 | 1.04 | 1.03 | 1.03 | 1.04 | 1.03 |
| p-value | 0.27 | 0.25 | 0.007 | 0.004 | 0.0002 | 0.003 | 0.0004 | 0.003 |
| Generations in U.S.§ | ||||||||
| 0 | 1.01 | 1.01 | 0.82 | 0.82 | 0.88 | 0.91 | 0.94 | 0.95 |
| 1 | 1.02 | 1.03 | 1.02 | 1.04 | 0.94 | 0.94 | 1.01 | 1.00 |
| 2 | 1.05 | 1.05 | -- | -- | 1.07 | 1.07 | 1.03 | 1.02 |
| 3 | 1.07 | 1.06 | -- | -- | 1.03 | 1.03 | 1.21 | 1.21 |
| p-value† | 0.05 | 0.14 | 0.007 | 0.004 | 0.0002 | 0.002 | <0.0001 | 0.0001 |
| Education | ||||||||
| < High school degree | 1.16 | 1.12 | 0.86 | 0.86 | 1.10 | 1.10 | 0.98 | 0.97 |
| High school degree or some college | 1.11 | 1.09 | 0.81 | 0.81 | 1.00 | 1.00 | 0.98 | 0.98 |
| College degree | 0.99 | 1.00 | 0.83 | 0.83 | 1.00 | 1.01 | 0.92 | 0.93 |
| p-value† | <0.0001 | <0.0001 | 0.40 | 0.42 | 0.11 | 0.15 | 0.40 | 0.71 |
| Income | ||||||||
| <$20,000 | 1.08 | 1.05 | 0.83 | 0.84 | 1.06 | 1.05 | 0.98 | 0.98 |
| $20,000-$49,999 | 1.08 | 1.07 | 0.86 | 0.85 | 1.02 | 1.02 | 0.97 | 0.96 |
| ≥$50,000 | 1.03 | 1.04 | 0.80 | 0.80 | 0.98 | 0.99 | 0.94 | 0.97 |
| p-value† | 0.05 | 0.29 | 0.56 | 0.34 | 0.04 | 0.16 | 0.32 | 0.62 |
p-value for linear trend across groups
Generation 0 means participant was not born in the U.S.; first generation, 1 or both parents were not born in the U.S.; second generation, both parents were born in the U.S. but ≥ 2 grandparents were not born in the U.S.; and third generation, both parents were born in the U.S. and ≥3 grandparents were born in the U.S.
Geometric means reported
Non-estimable due to small sample size
Model 1: Each independent variable (place of birth, generations in U.S., education, and income) was considered one at a time, and adjustments were made for age and sex. Model 1 for place of birth and generations in U.S. each additionally included education (6 levels) and income (7 levels). Model 1 for income (3 levels) also included place of birth and education (6 levels), while Model 1 for education (3 levels) additionally included place of birth and income (7 levels).
Model 2: Adjusted for model 1 + LDL cholesterol, HDL cholesterol, statin use, BMI, systolic blood pressure, hypertension medication use, smoking status (current, former, never), diabetes status.
Figure 1.
Race/ethnicity stratified prevalences of carotid plaque by place of birth and generations of U.S. residence, MESA 2000-2002.
After model 1 adjustments, examination of region of birth revealed that Blacks born in Africa had a lower prevalence of plaque (11.4%) than those born in the Caribbean and Central America (27.8%), who in turn had a lower prevalence than Blacks born in the U.S. (42.8%) (p < 0.0001 overall). Among Hispanics, those born in South America had the lowest prevalence of plaque (26.2%), followed by those born in the Caribbean and Central America (32.6%), Mexico (36.3%), and the U.S. (45.1%), respectively (p = 0.0008 overall). Among Chinese, Blacks, and Hispanics, greater Western acculturation, as assessed by both place of birth and generation of U.S. residence, was associated with greater mean internal carotid IMT, while no associations were observed among Whites.
Greater educational attainment was associated with a lower prevalence of carotid plaque and with lower mean internal carotid IMT among Whites, but not among other racial/ethnic groups. In Blacks, a higher prevalence of carotid plaque was observed among those with lower income, though this association was attenuated with further adjustments. No associations were observed between mean internal carotid IMT and income.
As shown in Supplemental (Online) Table 1, place of birth and the number of generations of U.S. residence were not significantly associated with the prevalence of albuminuria or with mean urine albumin excretion in any racial/ethnic group. Among Whites, educational attainment was inversely associated with the prevalence of albuminuria in models 1 and 2. Mean urinary albumin excretion was also lower among Whites with greater education in model 1, but was not associated after model 2 adjustments. In Hispanics, there was some evidence that greater income was associated with a lower prevalence of albuminuria and lower urine albumin excretion, while no relations were observed in other racial/ethnic groups.
Discussion
Our main and novel finding was that among Blacks and Hispanics, being born in the U.S. and more generations of U.S. residence were strongly associated with a greater prevalence of carotid plaque. Similar trends were observed among Whites and Chinese, though at times they did not reach statistical significance. Moreover, among Hispanic immigrants, the duration of U.S. residence was positively associated with plaque prevalence. Greater Western acculturation was also associated with higher mean maximum internal carotid IMT in Chinese, Blacks, and Hispanics. These findings are consistent with previous research which has reported positive associations between Westernization and carotid IMT in Chinese(15), and in MESA, between acculturation and CAC(14).
Within-race analyses according to region of birth also support the notion that greater acculturation is associated with a greater prevalence of carotid plaque. In our data, Blacks born in Africa had a lower prevalence of plaque (11.4%) than those born in the Caribbean and Central America (27.8%), who in turn had a lower prevalence than Blacks born in the U.S. (42.8%). Among Hispanics, those born in South America had the lowest prevalence of plaque (26.2%), followed by those born in the Caribbean and Central America (32.6%), Mexico (36.3%), and the U.S. (45.1%), respectively.
Furthermore, there was some evidence of an inverse association between SES and carotid plaque. In Whites, educational attainment was inversely related to carotid plaque prevalence and mean internal carotid IMT, while in Blacks an inverse association was observed between household income and carotid plaque prevalence. Inverse associations between carotid plaque and SES have been reported previously in other populations(20, 23-26).
In a recent meta-analysis, the relative risk (95% CI) per 0.10 mm common carotid IMT difference was 1.15 (1.12, 1.17) for myocardial infarction and 1.18 (1.16, 1.21) for stroke(40). Thus the IMT differences we observed in relation to acculturation and SES, frequently on the magnitude of 0.05 mm, are potentially clinically relevant. Current levels of CVD risk factors (sex, age, LDL cholesterol, HDL cholesterol, statin use, BMI, systolic blood pressure, hypertension medication use, smoking status, diabetes status) did not appear to account for the patterns of acculturation and SES we observed with carotid plaque. Though surprising, several possible explanations exist. First, error in the measurement of risk factors may have contributed. Second, as has been suggested previously(41-43), early risk factors levels and cumulative environments, which we did not measure, are probably important in the development of atherosclerotic carotid plaque. Finally, it is possible that other unidentified factors related to U.S. acculturation and SES explain their associations with carotid plaque.
Within racial/ethnic groups, prevalent albuminuria was not associated with acculturation, and little variation was observed with level of SES. Specifically, the prevalence of albuminuria was inversely associated with education in Whites, and with income in Hispanics, while no other associations were observed. These findings are concordant with data from the Third National Health and Nutrition Examination Survey, which found a greater of prevalence of albuminuria among participants living in poverty as compared to those above the poverty level(27).
The extent of differences in the relation between carotid plaque and measures of acculturation and SES, as compared to those observed with albuminuria, is quite striking. However, these subclinical measures are heterogeneous and are relatively weakly correlated(33), and each assesses a different aspect of subclinical CVD. Carotid IMT is an intermediate phenotype for early atherosclerosis in the large arteries(40), whereas albuminuria is an indicator of microvascular disease(44). Thus, the fact that different associations were observed between our exposures and carotid plaque and albuminuria is not entirely unexpected.
A major limitation of the present analysis is that, although the sample size for the different racial/ethnic groups may be large as compared to other studies, stratification of acculturation and SES within racial/ethnic groups sometimes resulted in small numbers. Consequently, the study may have been underpowered to find weak but real associations in some instances. Furthermore, within races/ethnicities acculturation and SES are considerably associated with age, and despite age-adjustment, residual confounding by age may have remained.
An additional limitation of this study was its cross-sectional design, hampering our ability to infer that either U.S. acculturation or SES was causally related to measures of subclinical CVD. Further, acculturation, and similarly SES, are complex constructs which are difficult to accurately measure. As demonstrated by the Ni-Hon-San study, assessing migration status or duration of residence may not be sufficient to fully capture acculturative processes(2). Unfortunately, a validated acculturation scale was not administered in MESA. Yet, the measures we operationalized – place of birth and generations of U.S. residence – have been utilized widely in other studies of acculturation, and are thought to provide valuable information. Similarly, education and income are among the most commonly employed indicators of SES. Although there is no single best indicator of SES, as each emphasizes a particular aspect of social stratification, most indicators of SES are correlated(45). Additionally, it has been suggested by some that indicators of SES may have different meanings in different racial/ethnic groups(46, 47). As a final limitation, the measures of SES we examined were assessed only once in middle or late adulthood. Adult measures are only limited proxies for socioeconomic trajectories over the life course, that are also clearly relevant to the development of atherosclerosis(14).
In summary, across all racial/ethnic groups the prevalence of carotid plaque and mean IMT were substantially higher among those with greater U.S. acculturation and lower SES, even after adjustment for traditional CVD risk factors. The relations with albuminuria were less convincing, though there was some evidence that SES was inversely associated with albuminuria prevalence. An implication of this analysis is that, as greater acculturation was associated with increased carotid plaque, efforts should be considered to encourage maintenance of healthful habits among recent immigrants. This recommendation is particularly salient, given that the proportion of immigrants living in the U.S. has increased dramatically in recent decades, with immigrants comprising an estimated 10.4% of the nation's population in 2000(48). These data also highlight the known association between low SES and CVD health disparities within the U.S.
Supplementary Material
Acknowledgments
This research was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. Pamela Lutsey was supported as a predoctoral fellow on the training grant T32 HL07779. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Reprint Requests: None requested.
Author Contributions: PLL and ARF designed the study and led in writing the manuscript. PLL and DRJ conducted the analyses. All authors helped to conceptualize ideas, interpret findings, and review drafts of the manuscript.
Statement of Institutional Review: Local institutional review committees approved the MESA protocol, and all subjects gave informed consent.
References
- 1.Robertson TL, Kato H, Rhoads GG, Kagan A, Marmot M, Syme SL, et al. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California. Incidence of myocardial infarction and death from coronary heart disease. American Journal of Cardiology. 1977;39(2):239–243. doi: 10.1016/s0002-9149(77)80197-5. [DOI] [PubMed] [Google Scholar]
- 2.Marmot MG, Syme SL. Acculturation and coronary heart disease in Japanese-Americans. Am J Epidemiol. 1976;104(3):225–47. doi: 10.1093/oxfordjournals.aje.a112296. [DOI] [PubMed] [Google Scholar]
- 3.Gordon-Larsen P, Harris KM, Ward DS, Popkin BM. National Longitudinal Study of Adolescent H. Acculturation and overweight-related behaviors among Hispanic immigrants to the US: the National Longitudinal Study of Adolescent Health. Social Science & Medicine. 2003;57(11):2023–2034. doi: 10.1016/s0277-9536(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 4.Popkin BM, Udry JR. Adolescent obesity increases significantly in second and third generation U.S. immigrants: the National Longitudinal Study of Adolescent Health. Journal of Nutrition. 1998;128(4):701–706. doi: 10.1093/jn/128.4.701. [DOI] [PubMed] [Google Scholar]
- 5.Lauderdale DS, Rathouz PJ. Body mass index in a US national sample of Asian Americans: effects of nativity, years since immigration and socioeconomic status. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2000;24(9):1188–1194. doi: 10.1038/sj.ijo.0801365. [DOI] [PubMed] [Google Scholar]; Int J Obes Relat Metab Disord. 2002 Nov;26(11):1521. erratum appears. [Google Scholar]
- 6.Sundquist J, Winkleby M. Country of birth, acculturation status and abdominal obesity in a national sample of Mexican-American women and men. International Journal of Epidemiology. 2000;29(3):470–477. [PubMed] [Google Scholar]
- 7.Kaufman JS, Durazo-Arvizu RA, Rotimi CN, McGee DL, Cooper RS. Obesity and hypertension prevalence in populations of African origin. The Investigators of the International Collaborative Study on Hypertension in Blacks. Epidemiology. 1996;7(4):398–405. doi: 10.1097/00001648-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Goslar PW, Macera CA, Castellanos LG, Hussey JR, Sy FS, Sharpe PA. Blood pressure in Hispanic women: the role of diet, acculturation, and physical activity. Ethnicity & Disease. 1997;7(2):106–113. [PubMed] [Google Scholar]
- 9.Espino DV, Maldonado D. Hypertension and acculturation in elderly Mexican Americans: results from 1982-84 Hispanic HANES. Journals of Gerontology. 1990;45(6):M209–M214. doi: 10.1093/geronj/45.6.m209. [DOI] [PubMed] [Google Scholar]
- 10.West SK, Munoz B, Klein R, Broman AT, Sanchez R, Rodriguez J, et al. Risk factors for Type II diabetes and diabetic retinopathy in a mexican-american population: Proyecto VER. American Journal of Ophthalmology. 2002;134(3):390–398. doi: 10.1016/s0002-9394(02)01595-7. [DOI] [PubMed] [Google Scholar]
- 11.Cooper RS, Rotimi CN, Kaufman JS, Owoaje EE, Fraser H, Forrester T, et al. Prevalence of NIDDM among populations of the African diaspora. Diabetes Care. 1997;20(3):343–8. doi: 10.2337/diacare.20.3.343. [DOI] [PubMed] [Google Scholar]
- 12.Sundquist J, Winkleby MA. Cardiovascular risk factors in Mexican American adults: a transcultural analysis of NHANES III, 1988-1994. American Journal of Public Health. 1999;89(5):723–730. doi: 10.2105/ajph.89.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang J, Madhavan S, Alderman MH. The association between birthplace and mortality from cardiovascular causes among black and white residents of New York City. New England Journal of Medicine. 1996;335(21):1545–1551. doi: 10.1056/NEJM199611213352101. see comment. [DOI] [PubMed] [Google Scholar]
- 14.Diez Roux AV, Detrano R, Jackson S, Jacobs DR, Jr, Schreiner PJ, Shea S, et al. Acculturation and socioeconomic position as predictors of coronary calcification in a multiethnic sample. Circulation. 2005;112(11):1557–1565. doi: 10.1161/CIRCULATIONAHA.104.530147. [DOI] [PubMed] [Google Scholar]
- 15.Woo KS, Chook P, Raitakari OT, McQuillan B, Feng JZ, Celermajer DS. Westernization of Chinese adults and increased subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19(10):2487–93. doi: 10.1161/01.atv.19.10.2487. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88(4 Pt 1):1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 17.Smith GD, Hart C, Watt G, Hole D, Hawthorne V. Individual social class, area-based deprivation, cardiovascular disease risk factors, and mortality: the Renfrew and Paisley Study. Journal of Epidemiology & Community Health. 1998;52(6):399–405. doi: 10.1136/jech.52.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyroler HA. The influence of socioeconomic factors on cardiovascular disease risk factor development. Preventive medicine. 1999;29(6 Pt 2):S36–40. doi: 10.1006/pmed.1998.0441. [DOI] [PubMed] [Google Scholar]
- 19.Colhoun HM, Rubens MB, Underwood SR, Fuller JH. Cross sectional study of differences in coronary artery calcification by socioeconomic status. BMJ. 2000;321(7271):1262–1263. doi: 10.1136/bmj.321.7271.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch J, Kaplan GA, Salonen R, Salonen JT. Socioeconomic status and progression of carotid atherosclerosis. Prospective evidence from the Kuopio Ischemic Heart Disease Risk Factor Study. Arteriosclerosis, Thrombosis & Vascular Biology. 1997;17(3):513–519. doi: 10.1161/01.atv.17.3.513. [DOI] [PubMed] [Google Scholar]
- 21.Yan LL, Liu K, Daviglus ML, Colangelo LA, Kiefe CI, Sidney S, et al. Education, 15-year risk factor progression, and coronary artery calcium in young adulthood and early middle age: the Coronary Artery Risk Development in Young Adults study. JAMA. 2006;295(15):1793–1800. doi: 10.1001/jama.295.15.1793. [DOI] [PubMed] [Google Scholar]
- 22.Gallo LC, Matthews KA, Kuller LH, Sutton-Tyrrell K, Edmundowicz D. Educational attainment and coronary and aortic calcification in postmenopausal women. Psychosomatic medicine. 2001;63(6):925–935. doi: 10.1097/00006842-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Ranjit N, Diez-Roux AV, Chambless L, Jacobs DR, Jr, Nieto FJ, Szklo M. Socioeconomic differences in progression of carotid intima-media thickness in the Atherosclerosis Risk in Communities study. Arteriosclerosis, Thrombosis & Vascular Biology. 2006;26(2):411–416. doi: 10.1161/01.ATV.0000198245.16342.3d. [DOI] [PubMed] [Google Scholar]
- 24.Lynch J, Kaplan GA, Salonen R, Cohen RD, Salonen JT. Socioeconomic status and carotid atherosclerosis. Circulation. 1995;92(7):1786–1792. doi: 10.1161/01.cir.92.7.1786. [DOI] [PubMed] [Google Scholar]
- 25.Rosvall M, Ostergren PO, Hedblad B, Isacsson SO, Janzon L, Berglund G. Socioeconomic differences in the progression of carotid atherosclerosis in middle-aged men and women with subclinical atherosclerosis in Sweden. Social science & medicine. 2006;62(7):1785–1798. doi: 10.1016/j.socscimed.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 26.Rosvall M, Ostergren PO, Hedblad B, Isacsson SO, Janzon L, Berglund G. Occupational status, educational level, and the prevalence of carotid atherosclerosis in a general population sample of middle-aged Swedish men and women: results from the Malmo Diet and Cancer Study. American Journal of Epidemiology. 2000;152(4):334–346. doi: 10.1093/aje/152.4.334. [DOI] [PubMed] [Google Scholar]
- 27.Martins D, Tareen N, Zadshir A, Pan D, Vargas R, Nissenson A, et al. The association of poverty with the prevalence of albuminuria: data from the Third National Health and Nutrition Examination Survey (NHANES III) American Journal of Kidney Diseases. 2006;47(6):965–971. doi: 10.1053/j.ajkd.2006.02.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coppin AK, Ferrucci L, Lauretani F, Phillips C, Chang M, Bandinelli S, et al. Low socioeconomic status and disability in old age: evidence from the InChianti study for the mediating role of physiological impairments. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2006;61(1):86–91. doi: 10.1093/gerona/61.1.86. [DOI] [PubMed] [Google Scholar]
- 29.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. International journal of epidemiology. 1991;20(2):384–392. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- 30.Woo J, Lynn H, Wong SY, Hong A, Tang YN, Lau WY, et al. Correlates for a low ankle-brachial index in elderly Chinese. Atherosclerosis. 2006;186(2):360–6. doi: 10.1016/j.atherosclerosis.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 31.Diez-Roux AV, Nieto FJ, Muntaner C, Tyroler HA, Comstock GW, Shahar E, et al. Neighborhood environments and coronary heart disease: a multilevel analysis. American Journal of Epidemiology. 1997;146(1):48–63. doi: 10.1093/oxfordjournals.aje.a009191. [DOI] [PubMed] [Google Scholar]
- 32.Diez-Roux AV, Nieto FJ, Tyroler HA, Crum LD, Szklo M. Social inequalities and atherosclerosis. The atherosclerosis risk in communities study. American Journal of Epidemiology. 1995;141(10):960–972. doi: 10.1093/oxfordjournals.aje.a117363. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs DRJ, Crow RS. Subclinical Cardiovascular Disease Markers Applicable to Studies of Oral Health: Multiethnic Study of Atherosclerosis. Ann NY Acad Sci. 2007;1098(1):269–287. doi: 10.1196/annals.1384.029. [DOI] [PubMed] [Google Scholar]
- 34.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. American Journal of Epidemiology. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 35.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. New England Journal of Medicine. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103. see comment. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs DR, Jr, Murtaugh MA, Steffes M, Yu X, Roseman J, Goetz FC. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens: the Coronary Artery Risk Development in Young Adults Study. American Journal of Epidemiology. 2002;155(12):1114–1119. doi: 10.1093/aje/155.12.1114. [DOI] [PubMed] [Google Scholar]
- 37.Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. Journal of the American Society of Nephrology. 1996;7(6):930–937. doi: 10.1681/ASN.V76930. [DOI] [PubMed] [Google Scholar]
- 38.Lutsey PJ, D R, Jr, Kori S, Mayer-Davis E, Shea S, Steffen LM, Szklo M, Tracy R. Whole grain intake and its cross-sectional association with obesity, insulin resistance, inflammation, diabetes and subclinical cardiovascular disease: The MESA Study. BJN. 2007 doi: 10.1017/S0007114507700715. In Press. [DOI] [PubMed] [Google Scholar]
- 39.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 40.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 41.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, et al. Childhood Cardiovascular Risk Factors and Carotid Vascular Changes in Adulthood: The Bogalusa Heart Study. JAMA. 2003;290(17):2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 42.Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, et al. Cardiovascular Risk Factors in Childhood and Carotid Artery Intima-Media Thickness in Adulthood: The Cardiovascular Risk in Young Finns Study. JAMA. 2003;290(17):2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 43.Carson AP, Rose KM, Catellier DJ, Kaufman JS, Wyatt SB, Diez-Roux AV, et al. Cumulative Socioeconomic Status Across the Life Course and Subclinical Atherosclerosis. Annals of Epidemiology. doi: 10.1016/j.annepidem.2006.07.009. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 44.Sarafidis PA, Bakris GL. Microalbuminuria and chronic kidney disease as risk factors for cardiovascular disease. Nephrol Dial Transplant. 2006;21(9):2366–2374. doi: 10.1093/ndt/gfl309. [DOI] [PubMed] [Google Scholar]
- 45.Oakes JK, J S. Methods in Social Epidemiology. San Francisco: Jossey-Bass; 2006. [Google Scholar]
- 46.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, et al. Socioeconomic Status in Health Research: One Size Does Not Fit All. JAMA. 2005;294(22):2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 47.Kaufman JS, Cooper RS, McGee DL. Socioeconomic status and health in blacks and whites: the problem of residual confounding and the resiliency of race. Epidemiology. 1997;8(6):621–8. [PubMed] [Google Scholar]
- 48.Schmidley A. Profile of the foreign-born population in the United States: 2000. Washington, DC: Census Bureau; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.