SUMMARY
The role that animals play in the transmission of Schistosoma japonicum to humans in the Philippines remains uncertain and prior studies have not included several species, adjustment for misclassification error and clustering, or used a cohort design. A cohort study of 2468 people providing stool samples at 12 months following praziquantel treatment in 50 villages of Western Samar, the Philippines, was conducted. Stool samples from dogs, cats, rats, and water buffaloes were collected at baseline (2003–2004) and follow-up (2005). Latent-class hierarchical Bayesian log-binomial models adjusting for misclassification errors in diagnostic tests were used. The village-level baseline and follow-up prevalences of cat, dog, and rat S. japonicum infection were associated with the 12-month cumulative incidence of human S. japonicum infection, with similar magnitude and precision of effect, but correlation between infection levels made it difficult to divide their respective effects. The cumulative incidence ratios associated with a 1% increase in the prevalence of infection in dogs at baseline and in rats at follow-up were 1·04 [95% Bayesian credible interval (BCI) 1·02–1·07] and 1·02 (95% BCI 1·01–1·04), respectively, when both species were entered in the model. Dogs appear to play a role in human schistosomiasis infection while rats could be used as schistosomiasis sentinels.
Keywords: Bayesian hierarchical model, cohort, misclassification error, Schistosoma japonicum, zoonosis
INTRODUCTION
Schistosomiasis, a chronic parasitic infection caused by trematode worms, is believed to be endemic in 76 countries and territories [1, 2]. Schistosoma haematobium, S. japonicum, and S. mansoni are estimated to account for 90% of the estimated 207 million schistosomiasis infections worldwide [1, 3]. S. japonicum occurs mainly in China, the Philippines, and parts of Indonesia, and, unlike other forms of schistosomes, S. japonicum can naturally infect humans and at least 46 other mammalian species [4, 5]. Schistosomiasis japonica symptoms include anaemia, chronic pain, and decreased nutritional status [6–8].
In the Philippines, it is believed that 12 million people are at risk for infection, with 2·5 million directly exposed to S. japonicum through water contact activities [3, 9]. In 2000, the Philippines’ Department of Health adopted a mass drug administration (MDA) with praziquantel as their control strategy. However, implementing MDA has been challenging due to ubiquitous water contact, heterogeneous acceptance of MDA and the presence of other mammalian reservoirs [9–12]. All mammalian hosts contribute to transmission by shedding S. japonicum eggs into or near water bodies, thereby increasing the risk of infection in all hosts [5, 13]. In China, the results from a clustered randomized trial conducted in four pairs of villages suggest that biannual MDA of bovines with praziquantel improves the effectiveness of human annual MDA with praziquantel, thus supporting the hypothesis that bovines act as a reservoir, although the sample size was small (eight villages) [14]. However, bovines may not be the only animals playing a role in transmission. In Anhui Province, China, a 5-year intervention study evaluated the effectiveness of an integrated control strategy including the elimination of bovines from the study area, annual treatment of all residents aged 5–65 years that tested positive with S. japonicum, improvement of sanitation facilities, health education to limit water contact with snail-inhabited sites and changing agricultural methods to reduce contact with snail habitats [15]. Human infection decreased during the initial 3 years, but remained stable for the remaining 2 years, suggesting that other reservoirs contributed to the transmission [15], as was suggested from a cross-sectional study conducted in the hilly areas of Anhui Province [16].
In the Philippines, the role played by different mammalian hosts remains uncertain. In Western Samar, a cross-sectional association between the village-level intensity of cat and dog S. japonicum infection and the prevalence of human infection has been reported [17]. Genetic analyses using these samples showed a high frequency of parasite gene-flow across species, but particularly between dogs and humans [18]. However, a transmission dynamics model using the same data found humans to be the most important source of human infections, with rats possibly playing a role [19]. The previous studies conducted in Samar were cross-sectional and could not be used to assess causal relationships between non-human and human S. japonicum infections. In addition, the cross-sectional study by McGarvey et al. [17] used a cumulative-logit model for a relatively common outcome which may result in the prevalence odds ratio estimates overestimating the prevalence proportion ratio estimates [20]. Such overestimation can lead to selecting control strategies based on the impression that some risk factors have a higher magnitude of effect, as suggested by an odds ratio, compared to the effect that would have been measured with a prevalence ratio. Finally, the lack of adjustment for misclassification error in animal infection could have biased the measures of association.
The purpose of this study is to estimate the magnitude of the effect of village-level prevalence of animal (dogs, cats, rats, water buffaloes) infection with S. japonicum on the 12-month cumulative incidence of infection with S. japonicum in humans in Western Samar Province, the Philippines.
MATERIALS AND METHODS
Study design
This cohort study was conducted in 50 villages of Western Samar Province, Eastern Visayas region, the Philippines. At baseline (August 2003–April 2004), participants were asked to provide three stool samples and answer a socio-demographic questionnaire to measure potential confounders. From 4 to 8 months after baseline, all villages were offered praziquantel MDA. All villages were visited 11–15 months after MDA (February–December 2005) and participants were asked to provide three stool samples. Stool samples from dogs, cats, rats, pigs, and water buffaloes were collected at baseline and follow-up.
Sampling strategy and study population
Seventy-five of 134 endemic villages met the eligibility criteria, namely safe and relatively easy access, at least 35 households, and not located on the seashore. Of these, 50 were sampled to represent the most ‘rain-fed’ (n = 25) and the most ‘man-made’ (n = 25) irrigation schemes [17, 21].
In each village, 35 eligible households were randomly selected. Each eligible household had at least five members with at least one working full time in a rain-fed farm in ‘rain-fed’ villages and at least 50% of the time in a man-made irrigated farm in ‘irrigated’ villages. In each household, at most six individuals including at least one full-time rice farmer were randomly selected and invited to participate.
MDA
From December 2003 to December 2004, all residents were offered two equal split doses praziquantel treatment (60 mg/kg) following community preparation activities. The village-level participation proportion in MDA varied from 16% to 81% [11]. Participants that tested positive for schistosomiasis were especially encouraged to receive treatment.
Follow-up stool collection in humans
Participants were asked to provide one stool sample per day for three consecutive days. Two slides were prepared from each stool sample 2–3 h after collection. Slides were placed in a cooler at the end of each day and transferred to a refrigerator at the end of each week. The Kato-Katz technique was used to count the number of S. japonicum eggs/g stool [22].
Baseline and follow-up animal stool samples and analysis
At baseline and follow-up, the census of households that owned pigs, dogs and cats was used to randomly sample 35 animals of each species, one in each household, except in the first 10 villages where all animals were sampled. Rats were trapped in 30 cages placed at different locations that changed every day with the aim of trapping 35 rats in each village.
Pigs, water buffaloes and adult dogs had stool samples collected intra-rectally on two or three consecutive days. Cats, puppies and rats were placed in a cage and faeces collected for 2–3 consecutive days. The stool samples were analysed using the Danish Bilharziasis Laboratory (DBL) method [23].
Human and animal ethical review
The human participant research was approved by the institutional review board of Brown University and of the Research Institute for Tropical Medicine. The animal protocol was approved by the Brown University Institutional Animal Care and Use Committee, the Research Institute for Tropical Medicine’s Animal Protection Committee, and the DBL Institute for Health Research and Development.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.
Statistical analysis
Definition of the population at risk
Only participants answering the baseline questionnaire, receiving both doses of praziquantel and providing a stool sample at follow-up were considered at risk of infection. We assumed 100% efficacy of praziquantel for the treatment of schistosomiasis japonica, based on recent evidence from China reporting efficacies of over 99·8% [24, 25].
Adjustment for misclassification error in S. japonicum infection
Both the Kato-Katz and the DBL tests have imperfect diagnostic accuracies for S. japonicum, and no gold standards exist. In addition, the tests’ sensitivities and specificities change with the number of stools examined [26–28]. Since we had 1–3 stool samples obtained on consecutive days for each animal and human participant, we used a Bayesian latent-class model adapted from Joseph et al. to adjust for the imperfect and changing sensitivities and specificities of the tests in all species [29]. The prior values (s.d.) for the sensitivity and specificity of the Kato- Katz test for human S. japonicum diagnosis were set at 54·1% (10·1%) and 94·7% (4·0%), respectively [17]. Prior values for the diagnosis of animal S. japonicum were taken from a pilot study conducted in the same area, with sensitivity and specificity values (s.d.) of 75·0% (3·9%) and 96·9% (0·8%) for dogs, 66·3% (7·5%) and 97·2% (0·9%) for cats, 76·8% (6·9%) and 92·9% (2·6%) for rats, and 78·0% (15·0%) and 98·7% (1·0%) for water buffaloes, respectively [28]. Models with uniform priors between 0 and 1 for both sensitivity and specificity were also conducted for water buffaloes which showed very poor sensitivity values.
Description of the association between cat, dog and rat prevalence of infection
The village-level prevalence proportions of infection in animals at baseline, adjusted for misclassification error, were modelled in a Bayesian linear regression to estimate the regression coefficient.
Modelling the effect of animal infection on human 12-month cumulative incidence of infection
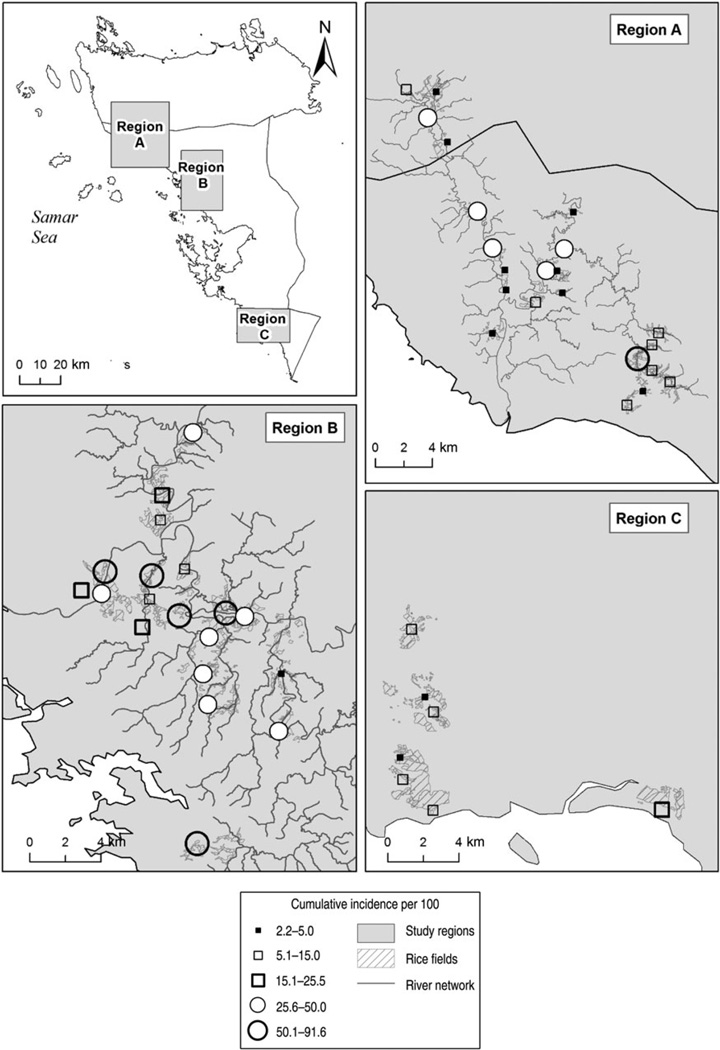
We used a hierarchical log-binomial model [30] with the latent variable for human cumulative incidence of infection as the outcome. The exposure of interest, the prevalence of infection at baseline or follow-up or their difference, adjusted for misclassification error, in dogs, cats, water buffaloes or rats, were modelled as fixed effects at the village level. Confounding variables included human age, sex, their interaction, and occupation as fixed effects at the individual level. The intercept was a random effect at the village level. A region effect was included at the village level, based on baseline results that these regions (Fig. 1) had an important impact on human prevalence [21]. The effect of MDA, irrigation status of the village, and number of days between the baseline and follow-up visits or between treatment and follow-up visit did not have an impact on the cumulative incidence of human infection and are not discussed further.
Fig. 1.
Twelve-month cumulative incidence (adjusted for age, sex, occupation and misclassification error) of human infection with Schistosoma japonicum in people having received praziquantel treatment in 50 villages of Samar province, the Philippines, 2005.
A number of models were fitted, exploring the effect of the difference between the baseline and follow-up prevalences of animal infection, or including the baseline prevalence of infection in several species simultaneously.
WinBugs software (version 1.4·3, MRC Biostatistics Unit, Cambridge, UK) was used to implement the Gibbs sampler algorithm. Even in routine regression models, whether frequentist or Bayesian, parameter constraints such as those required in the estimation of incidence ratios, require special numerical methods [30], and the computational challenges increase when there is hierarchy in the data. Bayesian modelling is naturally hierarchical and it can be done via Gibbs sampling as implemented in WinBugs. Posterior medians of random samples derived from marginal posterior densities were used as point estimates, reported with 95% Bayesian credible intervals (BCI). The regression coefficients were exponentiated to obtain cumulative incidence ratios (CIR). Each model was run with two chains with at least 20 000 iterations. The programs written in WinBugs are available upon request from the authors.
RESULTS
Of the 6918 individuals who agreed to participate in the study and completed a baseline questionnaire, 2394 (34·6%) did not provide a stool sample at follow-up, and 2056 (29·7%) did not receive praziquantel during MDA, resulting in 2468 (35·7%) participants ‘at-risk’ for this analysis. Table 1 compares the proportion of at-risk and not-at-risk participants according to age and gender, and the baseline parasitological examination results.
Table 1.
Characteristics of the individuals who were included in the analysis (‘at-risk’ group) and the individuals who provided stool sample at baseline but were not included in the analysis (‘not-at-risk’ group) with the estimated ratio of participation for each characteristic*
| Characteristic | At-risk group n (%) | Not-at-risk group n (%) | Proportion ratio (95% BCI) |
|---|---|---|---|
| N | 2468 (35·7) | 4450 (64·3) | |
| Males, age (years) | |||
| <10 | 364 (33·9) | 710 (66·1) | 0·69 (0·62–0·78) |
| 11–15 | 267 (44·3) | 336 (55·7) | 0·69 (0·62–0·78) |
| 16–40 | 440 (32·7) | 905 (67·3) | 0·67 (0·60–0·75) |
| >40 | 328 (48·8) | 344 (51·2) | Reference |
| Females, age (years) | |||
| <10 | 359 (32·6) | 742 (67·4) | 0·66 (0·59–0·75) |
| 11–15 | 178 (37·2) | 301 (62·8) | 0·76 (0·66–0·87) |
| 16–40 | 237 (22·7) | 805 (77·3) | 0·46 (0·40–0·53) |
| >40 | 295 (49·1) | 306 (50·9) | Reference |
| S. japonicum at baseline (based on results of the Kato-Katz test) | |||
| Yes | 537 (60·8) | 347 (39·3) | 1·64 (1·54–1·75) |
| No | 1753 (37·0) | 2987 (63·0) | Reference |
| Unknown | 178 (13·8) | 1116 (86·2) | 0·37 (0·32–0·43) |
BCI, Bayesian credible interval.
One participant did not provide age and gender information.
The proportion accepting praziquantel MDA was much higher in those with a positive schistosomiasis test at baseline [11]. This was expected since these individuals were particularly encouraged to receive treatment during the mass treatment period. However, having been infected with S. japonicum at baseline did not have an impact on the probability of providing a stool sample at follow-up for those people who did receive treatment (76% in those positive and negative at baseline). The proportion taking praziquantel was lower in people without a baseline stool sample (48%). Some of those individuals may have left the village at the time of the follow-up visit. The proportion of participants ‘at risk’ was lowest in women aged 16–40 years, since praziquantel was not offered to pregnant and lactating women. Men in that age group also showed lower participation, as did children aged <10 years since praziquantel was only offered to those aged ≥5 years.
Figure 1 depicts the 12-month cumulative incidence of human infection according to the three regions sampled. This map clearly shows that the cumulative incidence was highest in region B, followed by region A, and region C.
Dog, cat, and rat prevalence of infection at baseline (Table 2) and follow-up (Table 3) were all associated with the cumulative incidence of human infection in models including only one species.
Table 2.
Adjusted* cumulative incidence ratio estimates of human infection with Schistosoma japonicum from a one unit increase (1%) in the baseline prevalence proportion of dog, cat, water buffalo and rat S. japonicum infection using a Bayesian hierarchical model adjusting for misclassification error
| Cumulative incidence ratio estimate (95% BCI) | ||||
|---|---|---|---|---|
| Animals species included in the model | Dogs | Rats | Cats | Water buffaloes |
| Dogs only | 1·04 (1·02–1·07) | |||
| Rats only | 1·02 (1·00–1·04) | |||
| Cats only | 1·17 (1·01–1·39) | |||
| Water buffaloes only | 1·02 (0·98–1·05) | |||
| Dogs and rats | 1·04 (1·02–1·07) | 1·01 (1·00–1·03) | ||
| Dogs and cats | 1·05 (1·02–1·09) | 0·95 (0·77–1·15) | ||
| Dogs and water buffaloes | 1·05 (1·02–1·07) | 1·01 (0·98–1·04) | ||
BCI, Bayesian credible interval.
All models are adjusted for age, gender, occupation at the individual level and region at the village level and include village-level random-effect intercepts.
Table 3.
Adjusted* cumulative incidence ratio estimates of human infection with Schistosoma japonicum from a one unit increase (1%) in the follow-up prevalence proportion of dog, cat, water buffalo and rat S. japonicum infection using a Bayesian hierarchical model adjusting for misclassification error
| Cumulative incidence ratio estimate (95% BCI) | ||||
|---|---|---|---|---|
| Animals species included in the model | Dogs | Rats | Cats | Water buffaloes |
| Dogs only | 1·04 (1·02–1·06) | |||
| Rats only | 1·04 (1·02–1·06) | |||
| Cats only | 1·24 (1·01–1·46) | |||
| Water buffaloes only | 1·03 (0·96–1·06) | |||
| Dogs and rats | 1·02 (1·00–1·05) | 1·02 (1·00–1·05) | ||
| Dogs and cats | 1·03 (1·01–1·06) | 1·16 (0·87–1·35) | ||
| Dogs and water buffaloes | 1·04 (1·02–1·06) | 1·03 (0·97–1·05) | ||
BCI, Bayesian credible interval.
All models are adjusted for age, gender, occupation at the individual level and region at the village level and include village-level random-effect intercepts.
When the baseline or follow-up dog and cat prevalence proportions were included in the same model, the width of the 95% BCI increased considerably for the effect of cat infection. This can be explained by the high correlation between dog and cat infections (Table 4) and the wide 95% BCI around the sensitivity estimate of the DBL for the diagnosis of cat infections (Table 5).
Table 4.
Linear regression coefficient among animal infection at baseline
| Animals | Baseline, coefficient (95% BCI) |
|---|---|
| Dogs and cats | 3·73 (2·16 to 5·6) |
| Dogs and water buffaloes | 0·05 (−0·63 to 0·60) |
| Dogs and rats | 0·30 (0·01 to 0·61) |
| Cats and water buffaloes | −1·61 (−7·5 to 0·07) |
| Cats and rats | 0·12 (−0·46 to 0·58) |
| Rats and water buffaloes | 0·09 (−1·01 to 1·07) |
BCI, Bayesian credible interval.
Table 5.
Posterior sensitivity and specificity estimates the Danish Bilharziasis Laboratory test for the detection of animal infection using one stool sample
| Baseline (95% BCI) | Follow-up (95% BCI) | |||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| Dogs | 66·7 (60·7–72·5) | 98·3 (97·4–99·0) | 74·6 (68·2–80·4) | 98·4 (97·6–99·1) |
| Rats | 54·9 (49·1–60·9) | 98·4 (97·1–99·3) | 64·9 (55·5–73·3) | 99·1 (98·3–99·6) |
| Cats | 42·5 (31·6–55·7) | 99·5 (99·2–99·8) | 51·5 (35·1–69·1) | 99·1 (98·4–99·6) |
| Water buffaloes | 5·1 (3·3–7·9) | 99·9 (99·4–100·0) | 3·3 (1·7–5·5) | 99·6 (98·7–100·0) |
BCI, Bayesian credible interval.
The models including the baseline or follow-up dog and rat prevalence proportions suggested that infection in both species is related to the cumulative incidence of human infection (Tables 2 and 3). The magnitudes of the association were reduced and the width of the 95% BCI increased in a model using the follow-up dog and rat prevalence proportions (Table 3) compared to a model using the baseline values. However, the magnitude of the effect remained quite strong and the 95% BCI stable in a model including baseline dog infection (CIR 1·03, 95% BCI 1·01–1·06) and follow-up rat infection (CIR 1·02, 95% BCI 1·01–1·03) prevalences (Table 6). The magnitude and the 95% BCI of the association between the difference in the prevalence of infection at baseline and follow-up in dogs, and rats were inconclusive, as were the CIR and 95% BCI for the effect of water buffalo infection at baseline (Table 2) and follow-up (Table 3). It is noteworthy that the sensitivity of the DBL to detect water buffalo infection was very poor (Table 5).
Table 6.
Cumulative incidence ratio estimates of all variables included in the final Bayesian hierarchical log-binomial model of human infection with Schistosoma japonicum
| Variable | Category | Reference | RR (95% BCI) |
|---|---|---|---|
| Dog prevalence of infection at baseline | Each percent increase in prevalence | 1·03 (1·01–1·06) | |
| Rat prevalence of infection at follow-up | Each percent increase in prevalence | 1·02 (1·01–1·04) | |
| Age effect, boys | 10–15 yr | <10 yr | 1·89 (1·21–3·00) |
| 16–40 yr | 1·41 (1·01–2·05) | ||
| >40 yr | 0·74 (0·44–1·19) | ||
| Age effect, girls | 10–15 yr | <10 yr | 2·62 (1·35–5·65) |
| 16–40 yr | 0·81 (0·31–1·59) | ||
| >40 yr | 0·67 (0·31–1·21) | ||
| Gender effect for age <10 yr | Male | Female | 1·43 (0·92–2·30) |
| Gender effect for age 10–16 yr | Male | 1·03 (0·53–1·87) | |
| Gender effect for age 17–40 yr | Male | 2·51 (1·39–6·16) | |
| Gender effect for age >40 yr | Male | 1·59 (0·87–3·51) | |
| Rice farming | Some of the time | All the time | 0·99 (0·51–1·67) |
| Other type of farming | 0·77 (0·10–2·03) | ||
| No farming | 0·69 (0·35–1·22) | ||
| Pupil/student | 0·54 (0·30–0·90) | ||
| Region effect | B | A | 6·20 (2·47–18·20) |
| C | 2·58 (0·66–10·2) |
RR, Risk ratio; BCI, Bayesian credible interval.
In addition to the association of animal infection prevalence, the region of residence influenced the 12-month cumulative incidence of infection, especially for people living in region B (Table 6). This association was independent from that of animal infection, as the inclusion of any village-level animal prevalence only minimally influenced the effect of region. Age was found to modify the impact of gender, and pupils or students were at lower risk of infection than those working on rice farms.
DISCUSSION
Our findings suggest that dog, rat and cat village-level S. japonicum infection prevalence proportions measured at baseline or follow-up were associated with the 12-month cumulative incidence of human infection after treatment with praziquantel in 50 villages of Samar. However, the poor precision of the estimated sensitivity of the test to detect cat infections combined with high correlation between the infection prevalence proportions between dogs and cats made it difficult to separate out these two associations.
This study confirms our prior cross-sectional and schistosome genotyping findings that S. japonicum infection in dogs is associated with S. japonicum infection in humans [17, 18]. The magnitude of the effect was similar in the cross-sectional model although it measured the odds ratio for the unadjusted unit increase in 1 egg/g in dog infection at the village level, which could have been an overestimate of the effect. The magnitude of the effect of dog infection on human infection is very similar in the current study, but more precise, as would be expected from a cumulative incidence measure [20], and indicates the increase in human risk per 1% increase in the adjusted prevalence of dog infection. We believe that estimate to be accurate and also easier to interpret for decision makers. The genotyping study found minimal genetic differences in schistosomes from human and dog samples thereby suggesting the possibility of high transmission between dogs and humans [18]. The cross-sectional data had found some associations between the village-level average number of dog and cat S. japonicum eggs/g (not adjusted for measurement error) with human infection [17]. Our findings support that of a longitudinal study conducted in marshland and hilly villages in China where a high transmission index in dogs in the absence of bovines was found [16]. However, our findings cannot determine if this association is due to dogs and humans sharing the same strains of S. japonicum and being exposed at the same sites or if dogs are the sources of human infection. Nevertheless, the cohort nature of our study and a more conclusive effect of baseline village-level prevalence of dog infection, instead of the less conclusive effect of follow-up prevalence or change in prevalence of dog infection, suggest that there may be a delay between the environmental contamination with dog faeces and the resulting infection in humans. First, biologically, there would need to be a delay of at least 2–3 months between a dog infection and the detection of human infection due to the complex life-cycle of schistosomiasis [31]. Second, our data from Samar show that the 12-month cumulative incidence of human schistosomiasis was almost as high as the prevalence estimates observed at baseline [17]. This is in contrast to the results of a transmission dynamics model using data from Bohol in the Philippines in the 1980s where the prevalence of infection 12 months following mass drug treatment with a coverage of 50% was about half of that estimated at baseline [13]. Hence, although we cannot be certain if the association between village-level prevalence of dog infection at baseline and human cumulative incidence of infection is due to dogs and humans sharing the same strains of S. japonicum and being exposed at the same sites or if dogs are the sources of human infection, our data and the literature tend to favour the latter hypothesis. The less conclusive association found with baseline village-level prevalence of infection in rats, but more conclusive association with the follow-up prevalence of infection, support our previous finding from a transmission dynamics model that rat infection may be weakly associated with human infection [19]. It may suggest that rats are good indicators of the presence of the snail vectors, but may not share with humans the same strain of S. japonicum.
Our findings demonstrate that socio-demographic and geographical factors are risk factors for human S. japonicum infection. Age, gender, occupation, and region showed strong magnitude of effect on the 12-month cumulative incidence of infection. This supports the prior transmission dynamics model and the genetic analysis suggesting that the most important source of human infection with schistosomiasis remains the human host [18, 19]. The very strong effect of the region of residence will require more investigation to determine what environmental factors make some populations more at risk. The irrigation status of the village did not impact human infection.
Several strengths of this study are noteworthy. This is the first cohort study to report the cumulative incidence of human schistosomiasis in such a large number of villages and residents. The reported cumulative incidences are adjusted for misclassification error due to the poor accuracy of the Kato-Katz test and the fact that participants provided different numbers of faecal samples. It is unique in its estimation of the strength of the effect of village-level prevalences of infection in four mammalian species (cats, dogs, water buffaloes, rats) and the 12-month cumulative incidence of S. japonicum infection in humans, while adjusting for misclassification error in the diagnosis of infections in humans and other mammalian hosts, and taking the clustering of infection into account. This study used a log-binomial model to estimate CIRs, which are more appropriate measures of association than odds ratios for cohort studies and for outcomes that are common [20]. Therefore, we believe our estimated measures of association to have little bias.
This study has some limitations. First, treatment with praziquantel was assumed to be 100% effective based on previous studies [24, 25], so it is likely that those who did receive treatment, which was observed, were successfully treated. Second, relatively fewer women aged 17–40 years and individuals not providing a baseline stool sample were included in the ‘at risk’ population. Women in that age group were found to be less intensely infected than their male counterparts at baseline [21]. Moreover, MDA participation in those who had not provided a baseline stool sample was lower, most likely due to their absence from the village. Hence, the lower proportion of participants in these two populations will minimally bias our findings given their limited role in the transmission cycle of schistosomiasis, either by not working on the rice farm or being absent from the village. Third, recent studies of water buffalo S. japonicum infection in Leyte and Samar, using more sensitive molecular and coprological techniques [32, 33], showed much higher prevalence of infection in water buffaloes in results unadjusted for misclassification error. Our unadjusted results show a very low prevalence of infection in water buffaloes. However, our adjusted median prevalence results using unrestricted sensitivity and specificity prior values are consistent with previous findings [32, 33]. On the other hand, the very small number of animals found positive for S. japonicum limited our ability to obtain precise estimates. The studies in China which had suggested an association between infection in water buffaloes and humans had used the miracidia hatching technique [14, 16], which was found to be even less sensitive than the DBL method when compared to PCR [32]. Hence, if water buffaloes do play a role in the transmission of infection to humans in Samar, it may be to a lesser extent than that reported in China.
CONCLUSIONS
Dogs, cats and rats were found to play a role in schistosomiasis transmission, with dogs and rats showing more consistent effects. Therefore, schistosomiasis elimination efforts should not exclude the possibility of targeting infection in dogs, and possibly cats, as well as in humans, as this approach could improve the effectiveness of MDA programmes in this area of the Philippines. Rats could be considered as a good sentinel to monitor infection levels in the environment. Further genetic and aetiological research is needed to understand the role of other animals in transmission to humans, as well as the environmental factors associated with the effect of the region of residence. Employing a One Health approach which would involve experts in environmental sciences, veterinary and human medicine may contribute to the elimination of schistosomiasis in human communities [34].
ACKNOWLEDGEMENTS
This project was funded by the National Institutes of Health/National Science Foundation Ecology of Infectious Diseases programme (R01 TW01582).
Footnotes
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Nelson KE, Williams CM. Infectious Disease Epidemiology: Theory and Practice. 2nd edn. Sudbury, MA: Jones and Bartlett Publishers; 2007. p. xvi.p. 1207. [Google Scholar]
- 2.Lustigman S, et al. A research agenda for helminth diseases of humans: the problem of helminthiases. PLoS Neglected Tropical Diseases. 2012;6:e1582. doi: 10.1371/journal.pntd.0001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou XN, et al. Schistosomiasis japonica control and research needs. Advances in Parasitology. 2010;72:145–178. doi: 10.1016/S0065-308X(10)72006-6. [DOI] [PubMed] [Google Scholar]
- 4.He YX, Salafsky B, Ramaswamy K. Host–parasite relationships of Schistosoma japonicum in mammalian hosts. Trends in Parasitology. 2001;17:320–324. doi: 10.1016/s1471-4922(01)01904-3. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez TJ, Jr, et al. Prevalence of Schistosoma japonicum infection among animals in fifty villages of Samar province, the Philippines. Vector Borne and Zoonotic Diseases. 2007;7:147–155. doi: 10.1089/vbz.2006.0565. [DOI] [PubMed] [Google Scholar]
- 6.McGarvey ST, et al. Schistosomiasis japonica and childhood nutritional status in northeastern Leyte, the Philippines: a randomized trial of praziquantel versus placebo. American Journal of Tropical Medicine and Hygiene. 1996;54:498–502. doi: 10.4269/ajtmh.1996.54.498. [DOI] [PubMed] [Google Scholar]
- 7.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 8.Friedman JF, et al. Relationship between Schistosoma japonicum and nutritional status among children and young adults in Leyte, The Philippines. American Journal of Tropical Medicine and Hygiene. 2005;72:527–533. [PubMed] [Google Scholar]
- 9.Leonardo LR, et al. Difficulties and strategies in the control of schistosomiasis in the Philippines. Acta Tropica. 2002;82:295–299. doi: 10.1016/s0001-706x(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 10.Li YS, et al. Epidemiology of Schistosoma japonicum in China: morbidity and strategies for control in the Dongting Lake region. International journal for Parasitology. 2000;30:273–281. doi: 10.1016/s0020-7519(99)00201-5. [DOI] [PubMed] [Google Scholar]
- 11.Tallo VL, et al. Is mass treatment the appropriate schistosomiasis elimination strategy? Bulletin of the World Health Organization. 2008;86:765–771. doi: 10.2471/BLT.07.047563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonardo L, et al. A national baseline prevalence survey of schistosomiasis in the Philippines using stratified two-step systematic cluster sampling design. Journal of Tropical Medicine. 2012;2012 doi: 10.1155/2012/936128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishikawa H, Ohmae H. Modeling the dynamics and control of transmission of Schistosoma japonicum and S. mekongi in Southeast Asia. Korean Journal of Parasitology. 2009;47:1–5. doi: 10.3347/kjp.2009.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray DJ, et al. A cluster-randomised intervention trial against Schistosoma japonicum in the Peoples’ Republic of China: bovine and human transmission. PLoS ONE. 2009:4. doi: 10.1371/journal.pone.0005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou YB, et al. An integrated strategy for transmission control of Schistosoma japonicum in a marshland area of China: findings from a five-year longitudinal survey and mathematical modeling. American Journal of Tropical Medicine and Hygiene. 2011;85:83–88. doi: 10.4269/ajtmh.2011.10-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu DB, et al. Contrasting reservoirs for Schistosoma japonicum between marshland and hilly regions in Anhui, China – a two-year longitudinal parasitological survey. Parasitology. 2010;137:99–110. doi: 10.1017/S003118200999103X. [DOI] [PubMed] [Google Scholar]
- 17.McGarvey ST, et al. Cross-sectional associations between intensity of animal and human infection with Schistosoma japonicum in Western Samar province, Philippines. Bulletin of the World Health Organization. 2006;84:446–452. doi: 10.2471/blt.05.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudge JW, et al. Population genetics of Schistosoma japonicum within the Philippines suggest high levels of transmission between humans and dogs. PLoS Neglected Tropical Diseases. 2008;2 doi: 10.1371/journal.pntd.0000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riley S, et al. Multi-host transmission dynamics of Schistosoma japonicum in Samar Province, the Philippines. PLoS Medicine. 2008;5:70–78. doi: 10.1371/journal.pmed.0050018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNutt LA, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. American Journal of Epidemiology. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 21.Tarafder MR, et al. A cross-sectional study of the prevalence of intensity of infection with Schistosoma japonicum in 50 irrigated and rain-fed villages in Samar Province, the Philippines. BMC Public Health. 2006;6:61. doi: 10.1186/1471-2458-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters PA, et al. Quick Kato smear for field quantification of Schistosoma mansoni eggs. American Journal of Tropical Medicine and Hygiene. 1980;29:217–219. doi: 10.4269/ajtmh.1980.29.217. [DOI] [PubMed] [Google Scholar]
- 23.Willingham AL, Johansen MV, Barnes EH. A new technic for counting Schistosoma japonicum eggs in pig feces. Southeast Asian Journal of Tropical Medicine and Public Health. 1998;29:128–130. [PubMed] [Google Scholar]
- 24.Seto EY, et al. Human schistosomiasis resistance to praziquantel in China: should we be worried? American Journal of Tropical Medicine and Hygiene. 2011;85:74–82. doi: 10.4269/ajtmh.2011.10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, et al. The sensitivity of Schistosoma japonicum to praziquantel: a field evaluation in areas with low endemicity of China. American Journal of Tropical Medicine and Hygiene. 2012;86:834–836. doi: 10.4269/ajtmh.2012.11-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devlas SJ, Gryseels B. Underestimation of Schistosoma mansoni prevalences. ParasitologyToday. 1992;8:274–277. doi: 10.1016/0169-4758(92)90144-q. [DOI] [PubMed] [Google Scholar]
- 27.Engels D, Sinzinkayo E, Gryseels B. Day-to-day egg count fluctuation in Schistosoma mansoni infection and its operational implications. American Journal of Tropical Medicine and Hygiene. 1996;54:319–324. doi: 10.4269/ajtmh.1996.54.319. [DOI] [PubMed] [Google Scholar]
- 28.Carabin H, et al. Estimating sensitivity and specificity of a faecal examination method for Schistosoma japonicum infection in cats, dogs, water buffaloes, pigs, and rats in Western Samar and Sorsogon Provinces, The Philippines. International Journal for Parasitology. 2005;35:1517–1524. doi: 10.1016/j.ijpara.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Joseph L, Gyorkos TW, Coupal L. Bayesian estimation of disease prevalence and the parameters of diagnostic tests in the absence of a gold standard. American Journal of Epidemiology. 1995;141:263–272. doi: 10.1093/oxfordjournals.aje.a117428. [DOI] [PubMed] [Google Scholar]
- 30.De Andrade BB, Carabin H. On the estimation of relative risks via log binomial regression. Revista Brasileira de Biometria. 2011;29:1–15. [Google Scholar]
- 31.Gryseels B, et al. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 32.Wu HW, et al. High prevalence of Schistosoma japonicum infection in water buffaloes in the Philippines assessed by real-time polymerase chain reaction. American Journal of Tropical Medicine and Hygiene. 2010;82:646–652. doi: 10.4269/ajtmh.2010.09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon CA, et al. High prevalence of Schistosoma japonicum infection in Carabao from Samar Province, the Philippines: implications for transmission and control. PLoS Neglected Tropical Diseases. 2012;6:e1778. doi: 10.1371/journal.pntd.0001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.One Health Initiative. [Accessed 16 June 2014]; http://www.onehealthinitiative.com/about.php. [Google Scholar]