Abstract
Cell surface carbohydrates are important for cell migration and invasion of prostate cancer (PCa). Accordingly, the I-branching N-acetylglucosaminyltransferase (GCNT2) converts linear i-antigen to I-branching glycan, and its expression is associated with breast cancer progression. In the present study, we identified relationships between GCNT2 expression and clinicopathological parameters in patients with PCa. Paraffin-embedded PCa specimens were immunohistochemically tested for GCNT2 expression, and the roles of GCNT2 in PCa progression were investigated using cell lines with high GCNT2 expression and low GCNT2 expression. GCNT2-positive cells were significantly lesser in organ-confined disease than in that with extra-capsular extensions, and GCNT2-negative tumors were associated with significantly better prostate-specific antigen (PSA)-free survival compared with GCNT2-positive tumors. Subsequent functional studies revealed that knockdown of GCNT2 expression in PCa cell lines significantly inhibited cell migration and invasion. GCNT2 regulated the expression of cell surface I-antigen on the O-glycan and glycolipid. Moreover, I-antigen-bearing glycolipids were subject to α5β1 integrin–fibronectin mediated protein kinase B (AKT) phosphorylation. In conclusion, GCNT2 expression is closely associated with invasive potential of PCa.
Keywords: Prostate cancer, I-branching N-acetylglucosaminyltransferase, I-antigen, cell migration, glycolipid
Introduction
Cell surface carbohydrates reportedly play significant roles in glycoprotein function and in tumor cell proliferation and invasion.(1-3) It is also widely accepted that invasion and metastasis of tumor cells from primary lesion correlates with poor prognosis in several epithelial cancers, including in prostate cancer (PCa).(4)
PCa is the most common malignancy in men and the second leading cause of cancer-related death in the United States and Europe.(5, 6) Its incidence is rapidly increasing in the Asia–Pacific region,(7) and the associated clinical issues have attracted global research interest. Whereas primary prostate tumors are moderately sensitive to androgens, metastatic prostate cancers acquire adaptive hormone independency after androgen deprivation therapy.(8) Although androgen-independent prostate cancers exhibited changes in cell surface carbohydrate structures,(9) the mechanisms related to aberrant glycan with prostate cancer metastasis are yet unclear.
I-branching N-acetylglucosaminyltransferase (GCNT2) (10-12) synthesizes I-branched polylactosamine chains (I-antigen) by catalyzing the transfer of N-acetylglucosamine (GlcNAc) from uridine diphosphate (UDP)-GlcNAc with a galactose β1-6 linkage of linear lactosamine chains (i-antigens; Fig. 1A). The presentation of these i/I antigens changes dramatically during human development.(13) The i–I antigen conversion reportedly increases the presentation of polylactosamine chains and their functional terminal structures sialyl Lewis X and sialyl Lewis A.(14) In addition, branched polylactosamine chains have increased affinity for specific lectins.(15) In cancer cells, mannosyl (alpha-1,6-)-glycoprotein beta-1,6-N-acetyl-glucosaminyltransferase (MGAT5)-mediated polylactosamine chain branching resulted in increased PCa cell invasion.(16) Moreover, GCNT1-mediated core2 branching of O-glycan increased testicular tumor invasion and PCa aggressiveness.(17, 18) However, few previous studies have investigated the relationship between GCNT2 and PCa invasiveness and metastasis, despite the potential for formation of branching glycans. Recently, the expression of GCNT2 was closely associated with the malignant potential of breast cancers.(19) Thus, we investigated associations between GCNT2 expression and clinical features in patients with PCa.
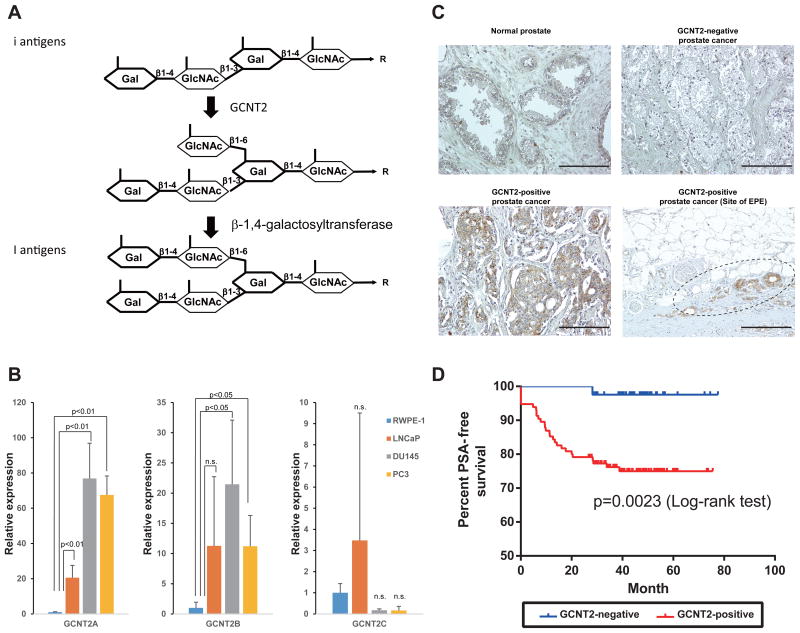
Figure 1.
I-branching N-acetylglucosaminyltransferase (GCNT2) expression correlates with prostate cancer (PCa) progression. (A) Biosynthetic pathways for I-antigen. (B) Expression level of GCNT2 was determined using quantitative polymerase chain reaction analysis. (C) PCa specimens were stained with an anti-GCNT2 antibody followed by staining with a horseradish peroxidase-conjugated secondary antibody. Counterstaining was performed using hematoxylin and eosin. GCNT2-positive cancer cells are indicated in brown. (D) Prostate-specific antigen-free survival periods were compared between GCNT2-positive and GCNT2-negative specimens, and survival analysis was conducted using Kaplan–Meier curves. Scale bars: 200 μm.
In this study, we demonstrated that GCNT2 expression in PCa specimens from radical prostatectomy procedures was associated with PCa aggressiveness. In addition, knockdown of GCNT2 expression in PCa cells significantly decreased cell migration and invasion. We also demonstrated that GCNT2-expressing PCa cells presented I-antigen carrying O-glycans and glycolipids on the cell surface. Moreover, I-antigen-expressing PCa cells had increased α5β1 integrin-mediated protein kinase B (AKT) phosphorylation and migration. Collectively, the present data indicate important the roles of GCNT2 in the progression and invasion of PCa cells.
Materials and methods
Materials
The O-glycosylation inhibitor benzyl-α-GalNAc (BAG) was purchased from Sigma–Aldrich (St Louis, MO, USA). The N-glycosylation inhibitor tunicamycin (TM) was purchased from Wako Pure Chemical Company (Osaka, Japan). The glucosylceramide synthetase inhibitor DL-threo-1-phenyl-2-palmitoylamino-3-morpholino-1-propanol hydrochloride (PPMP) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibodies used in this study were listed in Doc. S1.
Cells
The human PCa cell line LNCaP, DU145 and PC3 were purchased from the American Type Culture Collection (Rockville, MD, USA). Cells were maintained in RPMI1640 medium (Wako, Osaka, Japan) containing 100-U/mL penicillin and 100 μg/mL streptomycin with 10% FBS (Hyclone, UT, USA). Immortalized RWPE-1 prostate epithelial cells were also purchased from the American Type Culture Collection. RWPE-1 was maintained in keratinocyte serum-free medium (Life technologies, Carlsbad, CA, USA). DU145-derived GCNT2 knockdown cell lines were established by transfection of 4 different GCNT2siRNA vectors (Doc.S1). All cells were analyzed by STR analysis (BEX co., Ltd., Tokyo, Japan).
Immunohistochemical analysis of PCa specimens
Between 2005 and 2011, 156 PCa patients were treated with radical prostatectomy at the Department of Urology, Hirosaki University Graduate School of Medicine, Hirosaki, Japan. Staging and grading of tumors and patient follow-up were previously described.(20) In brief, Postoperative PSA levels were considered to be increased (PSA recurrence) if they were ≥ 0.2 ng/mL during two consecutive visits in a 1-month interval. Time zero was defined as the day of surgery. Patients with constantly detectable PSA levels (< 0.001 ng/mL) after surgery were recorded as recurrences at time zero. Follow-up intervals were calculated from the date of surgery to the last recorded follow-up (median, 47.9 months; range, 26.7-79.6 months).(20) All tumor specimens after radical prostatectomy from these patients were formalin-fixed and embedded in paraffin. Deparaffinized specimens were incubated with the rabbit anti-human GCNT2 polyclonal Ab (1:200, HPA026776; Sigma) followed by incubation with HRP-conjugated goat anti-mouse/rabbit IgG antibody (Dako, Tokyo, Japan). Based on the staining status of Golgi apparatus, specimens with 10% or more positive cancer cells were judged as GCNT2 positive. Informed consent was obtained from all patients prior to participation in this study. The ethics committee of Hirosaki University approved the study protocol, and the study was performed in accordance with the ethical standards of the Declaration of Helsinki.
Determination of GCNT2 expression in prostate cancer cell lines using quantitative real-time PCR
Total RNA was isolated using ISOGEN II reagent, and then qPCR analyses were performed using GeneAce SYBR® qPCR Mix α No ROX (Nippon gene, Tokyo, Japan) according to the manufacturer's instructions. Primer sets are listed in Table S1. Gene expression levels were normalized to that of human glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Cell proliferation, migration and invasion assays
In vitro cell proliferation assays were performed as described previously.(17) In vitro cell migration and invasion assays were performed using Boyden chambers as described previously.(18) The numbers of cells on the lower side were counted in triplicate using a Hybrid Cell Counting System (Keyence, Osaka, Japan).
Flow cytometry
Cells (1 × 105) were incubated with or without primary antibody in 100 μL of 1% BSA–PBS for 30 min on ice followed by the incubation with fluorescence-conjugated isotype-specific secondary antibodies.
Immunocytochemical analysis of I-antigen presentation on PCa cells
Cells were cultured to 80% confluence on glass slides and were treated with 0.6% DMSO, 2 mM BAG, 0.2 μg/mL TM, and 20 μg/mL PPMP on glass slides for 48 h. Immunocytochemical analysis was performed as described previously.(21)
Fibronectin adhesion and spreading assays
Adhesion assays and spreading assays were performed using fibronectin-coated dishes as previously described.(22)
Immunoprecipitation
α5β1 Integrin heterodimer complex levels were determined in lysates from DU145-derived cells. Cells were incubated with 2 μg/mL rabbit monoclonal anti-α5 integrin antibody (EPR7854, Abcam, Cambridge, MA, USA) or mouse monoclonal anti-β1 integrin antibody (P5D2, Abcam) and were then incubated with protein G Dynabeads (Life Technologies). Immune complexes were eluted from Dynabeads using 3× Laemmli SDS-PAGE sample buffer.
Western blotting
Total cell lysates were prepared using 1% Igepal CA-630 (Sigma, St. Louis, MO, USA) containing protease inhibitor cocktail (Roche, Basel Schweiz). Briefly, samples were separated using 4%–15% SDS-PAGE gradient gels (Bio-Rad, Hercules, CA, USA) and were then transferred onto PVDF membranes. Western blotting analysis was performed using specific primary antibodies and horseradish peroxidase-conjugated secondary antibodies. After incubation with secondary antibodies, all samples were enzymatically visualized using Novex® ECL Chemiluminescent Substrate Reagent Kits (Life Technologies) and a ChemiDoc™ XRS+ System (Bio-Rad).
Focal adhesion kinase and protein kinase B stimulation on fibronectin
DU145-derived cell lines were cultured in the absence of serum for 48 h and were then detached using an enzyme-free cell dissociation solution (Millipore). Subsequently, 1 × 105 cells were seeded on 20 μg/mL fibronectin coated 6-well plates. After incubation for 5 min, 10 min, and 20 min, cells were washed once in PBS and were lysed using 1% Igepal CA-630 solution containing protease inhibitor cocktail and PhosStop (Roche).
Inhibition assays
Cells were pretreated with 20 μg/mL anti-α5 integrin antibody (NKI-SAM-1), 10 μg/mL of anti-β1 integrin antibody (P5D2), or 20 μg/mL of corresponding control isotype antibodies at on ice for 30 min and migration and fibronectin stimulation assays were performed.
Cells were treated with the AKT inhibitor VIII (10 μM; Cayman Chemical Company, Ann Arbor, MI, USA) or with DMSO, and migration assays were performed. In separate experiments, cells were cultured with the BAG (2 mM), PPMP (20 μg/mL), or DMSO for 48 h and were then subjected to migration and fibronectin stimulation assays. In RGD peptide blocking assay, cells were pretreated with 100, 200, 400, 800 μM of RGD peptide (sc-201176; Santa Cruz) or vehicle control at on ice for 30 min and fibronectin stimulation assays were performed.
Statistical analysis
Associations of GCNT2 status with clinical and histopathological parameters were analyzed using chi-squared tests. PSA-free survival was evaluated using Kaplan–Meier curves, and differences between groups were assessed using the log-rank test. All statistical analyses were performed using SPSS 21.0 software (SPSS, Chicago, IL, USA). Multivariate analysis of in this study used Cox proportional hazards regression analysis to test the association of GCNT2 status with other clinical and pathological parameters, including patients age, initial PSA, clinical stage, biopsy gleason score (GS), post-operation GS, pathological stage, margin status and perineural invasion for the prediction of PSA recurrence.
Results
GCNT2 expression in PCa positively correlates with cancer invasion and PSA recurrence
To confirm that GCNT2 expression correlates with PCa aggressiveness, expression levels of three isoforms of GCNT2 were determined in PCa cell lines using qPCR. A transcript variant (isoform A) of GCNT2 was the major isoform expressed in PCa cell lines. Whereas high expression of GCNT2 was observed in the highly invasive PCa cell lines DU145 and PC3, low-level expression of GCNT2 was observed in the poorly invasive LNCaP cell line (Fig. 1B). This result suggested that the high expression of GCNT2 correlates with invasive characteristics in PCa cell lines. To evaluate the role of GCNT2 in PCa aggressiveness, PCa specimens were immunohistochemically analyzed using a rabbit anti-GCNT2 polyclonal antibody. In these experiments, GCNT2 expression was detected in a partially healthy prostate gland and was highly expressed in some PCa cells (Fig. 1C). No significant differences in clinical parameters were observed between GCNT2-postive and GCNT2-negative PCa specimens from 156 patients (Table S2). However, >80% of tumor specimens had extraprostatic extensions (pT3 and pT4) that expressed GCNT2 in accordance with pathological parameters (Table S3), and GCNT2-positive patients were at significantly higher risk of PSA recurrence after radical prostatectomy (Fig. 1D). Moreover, nodal metastatic PCa cells also expressed GCNT2 (Figure S1). According to multivariate analyses, PSA levels, margin status, and GCNT2 expression in tumors were independent risk factors for PSA recurrence (Table 1). These results indicate that GCNT2 expression correlates with PCa invasion and progression.
Table 1. Cox proportional-hazards model for predicting prostate specific antigen-free survival Multivariate analysis.
| p value | Exp (B) | 95.0% CI | ||
|---|---|---|---|---|
| min | max | |||
| Age | 0.759 | 1.012 | 0.940 | 1.089 |
| iPSA† | 0.022 | 1.065 | 1.009 | 1.123 |
| cT2≤ | 0.920 | 1.043 | 0.458 | 2.374 |
| biopsy GS‡ | 0.076 | 2.622 | 0.903 | 7.611 |
| post-Ope GS‡ | 0.701 | 0.819 | 0.295 | 2.276 |
| pT3≤§ | 0.446 | 1.534 | 0.510 | 4.612 |
| Margin status¶ | 0.027 | 0.134 | 0.015 | 0.253 |
| perineural invasion | 0.822 | 1.121 | 0.413 | 3.041 |
| GCNT2 status | 0.032 | 9.021 | 1.203 | 67.630 |
pre-treatment with prostate specific antigen,
Gleason score,
extra-capsular extension,
cancer presence at the resected margin, GCNT2; I-branching N-acetylglucosaminyltransferase
GCNT2 regulates PCa cell migration and invasion
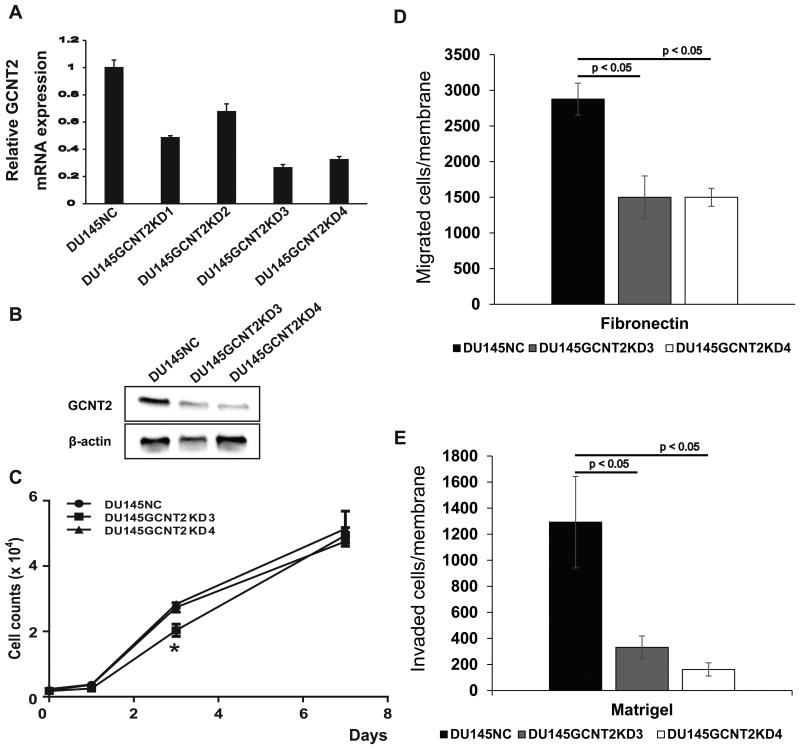
To investigate the role of GCNT2 expression in PCa cells, we established GCNT2 knockdown DU145 cell lines. In subsequently qPCR analyses and western blotting, clone 3 and clone 4 showed >70% inhibition of GCNT2 expression (Fig.2A and 2B). Although GCNT2 knockdown significantly inhibited cell proliferation at day three in clone 3 (DU145GCNT2KD3), total cell numbers at day seven did not differ between siControl (DU145NC), DU145GCNT2KD3, and clone 4 (DU145GCNT2KD4) cells (Fig. 2C). These results suggest that GCNT2 expression is not critical for cell proliferation in vitro. In subsequent experiments, the effects of GCNT2 expression were examined using migration and invasion assays in DU145NC, GCNT2KD3, and GCNT2KD4 cells using a Transwell system. In comparisons with DU145NC cells, migration and invasion was strongly inhibited in GCNT2 knockdown cell lines (Figs. 2D and 2E). In further experiments, GCNT2 expression was transiently inhibited using siRNA transfection in PC3 cells and resulted in decreased invasion potential (Figure S2A). Moreover, wound healing assays showed significantly decreased surface coverage rates in GCNT2 knockdown cell lines compared with that in DU145NC cells (Figure S2B). In a previous study, high expression of GCNT2 was associated with the epithelial-mesenchymal transition (EMT) and accelerated cell invasion in breast cancers.(19) In agreement, comparisons of the present DU145NC and DU145GCNT2KD4 cell lines with PC3 cells revealed similar patterns of EMT marker expression (Figure S2C), suggesting that GCNT2 regulates migration and invasion without stimulating the EMT in PCa cells.
Figure 2.
I-branching N-acetylglucosaminyltransferase (GCNT2) expression controls prostate cancer cell invasion. (A) Messenger RNA expression of GCNT2 was determined in DU145 cells using qPCR. GCNT2 expression levels were normalized to those of human glyceraldehyde-3-phosphate dehydrogenase. Clone 3 and Clone 4 (DU145GCNT2KD3 and DU145GCNT2KD4) showed >70% inhibition of GCNT2 expression. (B) Protein expression of GCNT2 was determined in DU145-derived cells using western blotting. The GCNT2 expression level was lower in DU145GCNT2KD3 and 4 than that in DU145NC. (C) In vitro cell proliferation was similar in DU145GCNT2KD3 and DU145GCNT2KD4 cells at day seven, and strongly reduced cell migration (D) and invasion (E) was observed in Transwell assays. Assays were conducted in triplicate; *, p < 0.05
GCNT2 catalyzes the formation of I-antigens on O-glycosylated proteins and glycolipids of PCa cell membranes
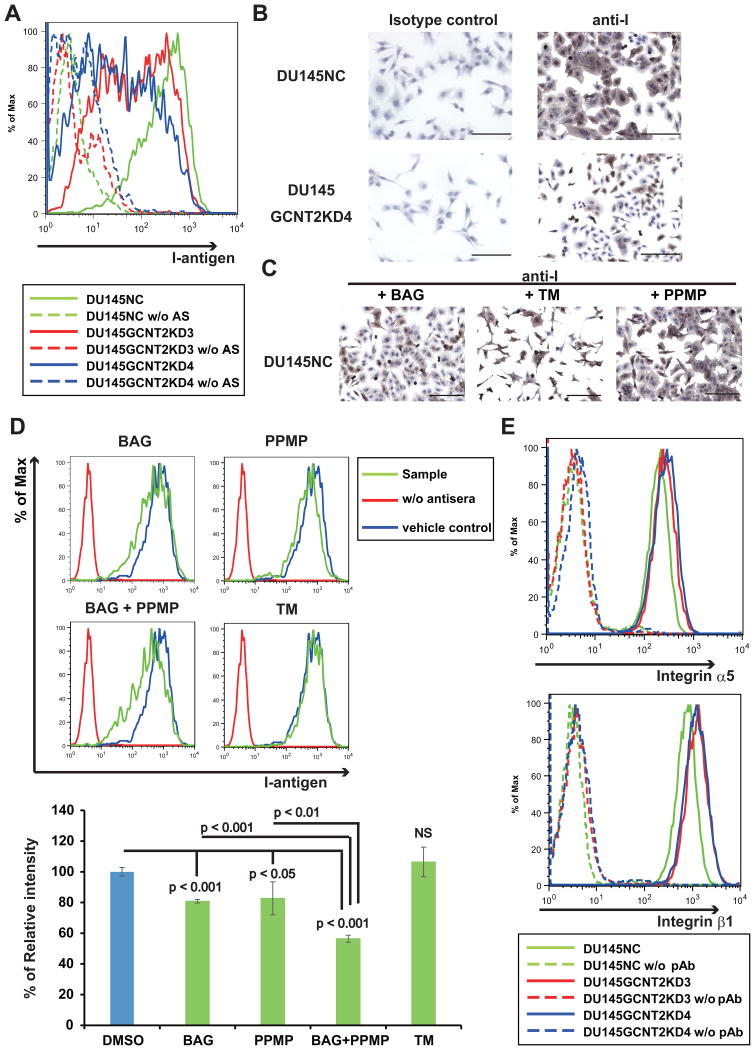
Interactions between cells and the extracellular matrix (ECM) have been shown to regulate cell motility.(23) Moreover, cell surface glycan modifications have reported biological functions during adhesion to the ECM and selectins and inhibits NK cell cytotoxicity.(24, 25) Thus, we investigated the effects of GCNT2 on cell surface glycans, and confirmed that GCNT2 converts i-antigen to I-antigen on cell surface carbohydrate structures (Fig. 1A). Specifically, GCNT2-expressing PCa cell lines showed pronounced cell surface presentation of I-antigen (Figure S3A and 3B). In contrast, GCNT2 knockdown cells had limited I-antigen presentation on cell surfaces (Figs. 3A and 3B). GCNT2 reportedly acts on O-glycans, N-glycans, and glycolipids to form GlcNAc-Gal branches (Fig. 1A).(12) Accordingly, after treatment of DU145NC cells with the inhibitors BAG or PPMP, significantly decreased I-antigen expression was observed. Moreover, co-treatment with BAG and PPMP led to greater inhibition of I-antigen presentation than individual treatments (Fig. 3D). In contrast, treatment with TM did not decrease I-antigen presentation on cell surfaces (Figs. 3C and 3D). Treatment with these inhibitors decreased each glycan on the cell surface (Figure S4), suggesting that I-antigens were formed on O-glycan and glycolipid molecules on PCa cell surfaces.
Figure 3.
I-branching N-acetylglucosaminyltransferase (GCNT2) expression regulates I-antigen presentation on prostate cancer cells. Cell surface I-antigen expression was determined using flow cytometry (FC) and immunocytochemistry (IHC) with anti-I antigen human antisera (Ma). (A) DU145GCNT2KD3 and DU145GCNT2KD4 showed decreased cell surface I-antigen expression in FC analyses. (B) DU145NC and DU145GCNT2KD4 cells were cultured on glass slides and were stained with anti-I antigen human antisera (Ma) or the human IgM isotype control. The brown color indicates I-antigen expression and the blue color indicates nuclear staining. DU145GCNT2KD4 cells had strongly reduced I-antigen expression. DU145 cells were cultured with benzyl-α-GalNAc (BAG), tunycamycin (TM), or DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol hydrochloride (PPMP) for 48 h. I-antigen expression was determined using IHC (C) and FC (D). I-antigen expression was significantly reduced in BAG-treated cells and PPMP-treated cells. TM-treated cells had no effect on I-antigen presentation. Co-treatment with BAG and PPMP strongly reduced I-antigen expression compared with either treatment alone. Population comparison was performed using Flowjo softwear. Assays were conducted in triplicate. (E) Integrin expression was determined using FC, and expression of α5 and β1 integrins was similar in DU145NC and GCNT2 knockdown cell lines. Scale bars: 200 μm.
I-antigen enhances α5β1 integrin signaling
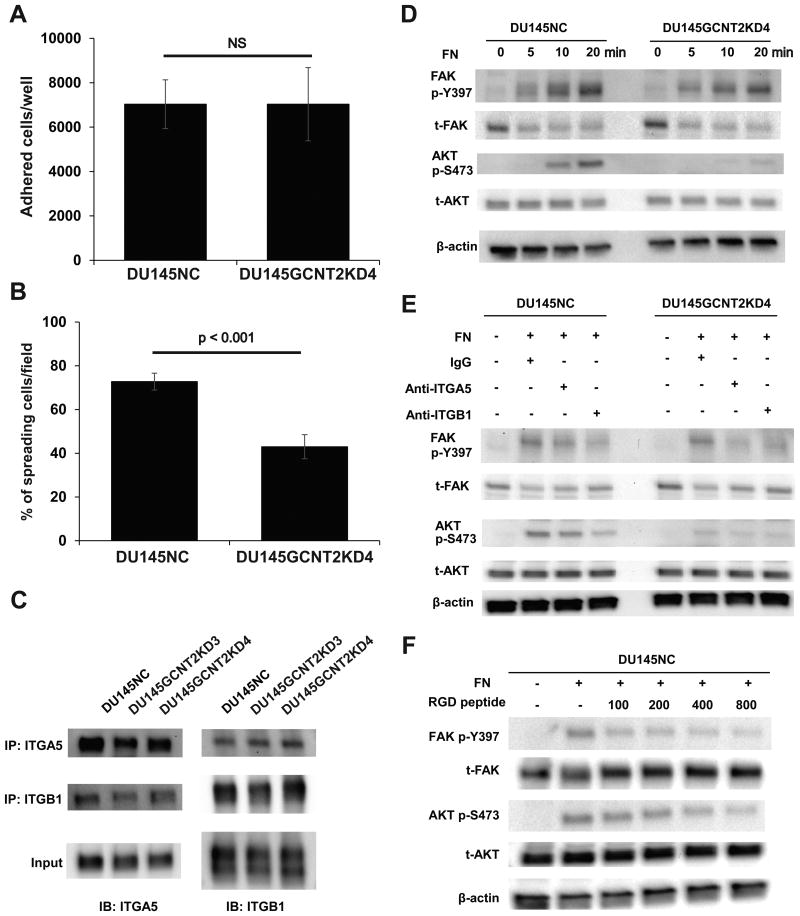
Integrin is a well-known heterodimeric receptor of the ECM and has reported roles in cell adhesion.(26) The major stromal ECM motility factor fibronectin has been shown to interact with the integrin α5β1 heterodimer in a glycan-structure dependent manner.(27, 28) In the present experiments, α5 integrin and β1 integrin were expressed at similar levels in DU145NC and GCNT2 knockdown cells (Fig. 3E). Thus, to confirm the role of I-antigen in cell–ECM interactions, adhesion and spreading assays were performed using DU145 cell lines on fibronectin-coated plates. Although adhesion on fibronectin did not differ between DU145NC and DU145GCNT2KD4 cells, significantly fewer DU145GCNT2KD4 cells demonstrated spreading activity (Figs. 4A and 4B). Integrin–ECM interactions that mediate outside–inside signals play important roles in cell spreading and migration.(29, 30) Moreover, abnormal heterodimeric forms of these molecules have been shown to inhibit integrin-mediated signaling.(31, 32) However, heterodimeric forms of α5β1 integrin did not differ between GCNT2 knockdown and DU145NC cells (Fig. 4C). In previous studies, FAK and phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) are reported downstream targets of integrin-mediated signaling.(29, 32, 33) Although FAK phosphorylation at tyrosine 397 (p-Y397) was not affected by GCNT2 expression on fibronectin-coated plates, AKT phosphorylation at serine 473 (p-S473) was significantly less in DU145GCNT2KD4 cells than in DU145NC cells (Figs. 4D). Moreover, AKT p-S473 was inhibited by antibody blocking and RGD peptide of the fibronectin–integrin interaction (Fig. 4E and 4F), suggesting that I-antigens enhance integrin mediated PI3K/AKT signaling.
Figure 4.
I-antigen regulates cell spread and α5β1 integrin–fibronectin interactions that are mediated by AKT phosphorylation in DU145NC and DU145GCNT2KD4 cells. Cell adhesion and spreading on fibronectin was examined by (A) harvesting cells after a 30 min culture on fibronectin-coated 96-well plates. DU145NC and DU145GCNT2KD4 cells showed no significant differences in adhesion potential. (B) Cells were harvested from fibronectin-coated glass slides after 30 min of incubation, and spreading cells were visualized using crystal violet solution. Percentages of spreading cells were significantly less in DU145GCNT2KD4 cells than in DU154NC cells. (C) Cell lysates from DU145 cells were immunoprecipitated using anti-integrin α5 or β1 antibodies, and heterodimers were detected. GCNT2 expression had no effect of heterodimerization of α5β1 integrin. (D) Focal adhesion kinase (FAK) and protein kinase B (AKT) are downstream targets of integrin signaling. FAK phosphorylation at tyrosine 397 (p-Y397) was similar in DU145NC and DU145GCNT2KD4 after integrin–fibronectin interactions. In contrast, AKT phosphorylation at serine 473 (p-S473) was strongly inhibited by GCNT2 knockdown in DU145 cells. (E) A functional blocking antibody against α5 and β1 integrins inhibited α5β1 integrin–fibronectin interactions. Pre-treatment of DU145NC and DU145GCNT2KD4 cells with anti-integrin α5 or β1 led to lower FAK p-Y397 and AKT p-S473 levels compared those after pre-treatment with IgG isotype control. (F) Pre-treatment of DU145NC cells with RGD peptide (100, 200, 400 or 800 μM) inhibited FAK p-Y397 and AKT p-S473 in a concentration-dependent manner.
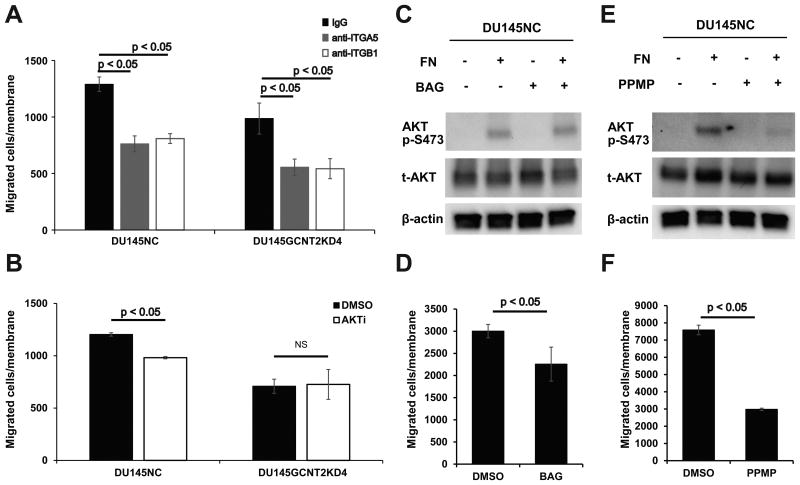
I-antigens support α5β1integrin–fibronectin induced cell migration
To demonstrate the roles of I-antigens, cell migration assays were performed after treatments of DU145 cells with various inhibitors. In these experiments, function-blocking antibodies against α5 integrin and β1 integrin strongly inhibited DU145NC and DU145GCNT2KD4 cell migration (Fig. 5A). Moreover, the AKT phosphorylation inhibitor had high efficacy in DU145 cells without causing cytotoxicity (Figures S5A and S5B), although numbers of migrant cells were significantly fewer among DU145NC cells in which AKT is strongly activated than in DU145GCNT2KD4 cells that express AKT p-S473 at low levels (Fig. 5B). Because I-antigens are carried on O-glycan and glycolipids, we determined which glycan is more important for AKT p-S473 and cell migration. Although BAG treated DU145NC cells had significantly reduced migration potential (Fig. 5D), AKT p-S473 did not differ between vehicle control and BAG-treated cells (Fig. 5C). However, after PPMP treatments, DU145NC cells showed strongly inhibited AKT p-S473 and cell migration (Figs. 5E and 5F). In addition, cell viability was >90% after BAG and PPMP treatments (Figures S5C and S5D), indicating that O-glycans carrying I-antigens support cell migration, and that glycolipids carrying I-antigens play important roles in integrin–fibronectin-mediated PI3K/AKT activation and cell migration.
Figure 5.
Glycolipid carrying I-antigens play important roles in integrin-mediated AKT phosphorylation and migration of prostate cancer cells. (A) Functional blocking antibodies against α5 and β1 integrins significantly inhibited cell migration in DU145NC and DU145GCNT2KD4 cells. (B) AKT phosphorylation at serine 473 (p-S473) was inhibited by the AKT inhibitor VIII (10 mM), and cell migration was inhibited in DU145NC cells but not DU145GCNT2KD4 cells. (C) DU145NC cells were cultured with benzyl-α-GalNAc (BAG), DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol hydrochloride (PPMP) or with dimethyl sulfoxide (DMSO) for 48 h. (C) DU145NC cells were cultured with DMSO or BAG on fibronectin-coated dishes for 20 min. Depletion of O-glycan had no effect on AKT p-S473. (D) BAG treated DU145NC cells had significantly inhibited cell migration. (E) Depletion of glycolipids in DU145NC cells significantly inhibited AKT p-S473 and (F) migration. Migration assays were conducted in triplicate.
Discussion
This study demonstrates that highly metastatic PCa cell lines express GCNT2 at high levels. On the basis of immunohistochemical analyses of radical prostatectomy specimens, GCNT2 expression on PCa cell surfaces closely correlated with extra-capsular extensions of PCa. It is also noteworthy that patients with GCNT2-negative PCa exhibited better PSA-free survival compared with patients with GCNT2-positive tumors (Fig. 1). Moreover, multivariate analysis revealed that GCNT2 is an independent predictor for PSA recurrence of PCa (Table 1). Subsequent experiments also indicated strong correlations between GCNT2 expression and malignant potential of PCa.
Integrin–ECM-mediated signaling is reportedly central to solid tumor locomotion,(29, 30) and integrin function is reportedly regulated by glycan structure.(32) Integrin α5β1 is a well-known fibronectin receptor and its binding function is regulated by N-glycan. Moreover, depletion of N-glycan from α5β1 integrin by N-glycosidase F inhibited α5β1 integrin–fibronectin interactions,(28) and cell motility was positively associated with the formation of MGAT5-mediated GlcNAcβ1-6 branching N-glycans.(22) Inhibition of α-mannosidase I by 1-deoxymannojirimycin in fibroblasts led to the formation of high mannose type N-glycan, and although immature α5β1 integrin was overexpressed on cell surfaces, the immature form strongly inhibited fibronectin binding affinity.(27) GCNT2 also transformed GlcNAc β1-6 residues to galactose and formed the I-antigen on N-glycans (Fig. 1).(12) In the present study, we demonstrated that I-antigens predominantly carried O-glycans and glycolipids (Fig. 3), and were not affected by α5β1 integrin heterodimerization and fibronectin binding affinity. Knockdown of GCNT2 also led to similar α5β1 integrin expression levels, suggesting that GCNT2-mediated branched-form I-antigen indirectly regulates α5β1 integrin function in PCa cells.
Previous studies showed associations of glycolipid with malignant potential of melanomas,(34) breast cancers,(35, 36) and prostate cancers.(37) In accordance, glycolipids were expressed on various cell surfaces, and their glycan structures were modified by several glycosyltransferases. Moreover, these glycolipids regulated not only cell–cell interactions by glycan–glycan interactions (38) but also regulated cell adhesion and migration by glycolipid/tetraspanin or glycolipid/caveola interactions.(39) Glycosphingolipid GM3 (NeuAcα2-3Galβ1-4Glcβ1-Cer) was also predominantly expressed at cell adhesion cites and inhibited cell migration. In a previous study of bladder carcinomas, high expression of GM3 blocked integrin and tetraspanin interactions and inhibited Src kinase signaling.(40) In the present study, GCNT2 had the potential to modify glycolipids, and its inhibition strongly reduced cell migration (Fig. 2). In addition, GCNT2 knockdown PCa cells showed strongly reduced α5β1 integrin–fibronectin-mediated AKT phosphorylation (Fig. 4). Previously, AKT phosphorylation was regulated by integrin linked kinase, which is a known downstream target of integrin activated Src kinase.(33, 41) Moreover, effects of AKT activation on proliferation, survival, and migration have been reported (42) and suggest that glycolipid-bearing I-antigens stabilize integrin-mediated signaling and/or integrin/tetraspanin-mediated Src kinase activation. Because inhibition of AKT phosphorylation inhibited PCa cell migration, glycolipid-bearing I-antigens enhance AKT phosphorylation and cell migration.
To establish a new diagnostic marker and therapeutic target, Carroll et al. immunized mice against the androgen-independent cell line PC3 and established a monoclonal antibody (mAb) against F77.(43) Subsequently, high expression of the F77 antigen was shown in androgen-independent prostate cancer cell lines (PC3 and DU145), which had reduced cell proliferation in vitro and in vivo in the presence of the F77 mAb. In a subsequent study, the F77 antigen was shown to be carried by glycolipids.(44) Moreover, a recent study showed that the F77 mAb recognized blood group H antigen-like glycan structures and GlcNAc β1-6Gal/GalNAc branching structures.(21, 45) In the present study, GCNT2, which catalyzes GlcNAc β1-6Gal branching, was more strongly expressed in androgen-independent cell lines than in an androgen-sensitive cell line (LNCaP). Moreover, I-antigen-expressing PCa cells showed strongly activated AKT. Taken together, these data suggests that glycolipid-carrying I-antigens play important roles in PCa proliferation and migration.
Although the mechanisms of GCNT2-mediated cancer progression remain poorly understood, the present experiments demonstrate that GCNT2-formed I-antigens are predictive of the malignant potential of PCa cells. However, further research is necessary to determine the utility of GCNT2 as a therapeutic target and biomarker for PCa.
Supplementary Material
Supporting Information
Doc. S1. Supplementary Materials and Methods
Fig. S1. Nodal metastatic prostate cancer expresses I-branching N-acetylglucosaminyltransferase (GCNT2).
Fig. S2. I-branching N-acetylglucosaminyltransferase (GCNT2) expression regulates prostate cancer cell migration and invasion without affecting the epithelial mesenchymal transition.
Fig. S3. I-antigen expression on the cell surface of GCNT2-expressing prostate cancer cell lines.
Fig. S4. Treatment with glycosylation inhibitor decreases presentation of O-glycan, N-glycan and glycolipid on prostate cancer cell surface.
Fig. S5. The AKT inhibitor, the O-glycosylation inhibitor, and the glucosylceramide synthetase inhibitor had no effects on cell viability during assays.
Table S1. Primer list
Table S2. I-branching N-acetylglucosaminyltransferase status and patient data
Table S3. I-branching N-acetylglucosaminyltransferase status and pathological parameters
Acknowledgments
The authors thank Dr. Shigeru Tsuboi and Ms. Sayaka Yamada for useful discussions and technical supports. This work was supported by JSPS KAKENHI Grant Numbers 24791631 and 15K10569 to YT and 15H02563 to CO, the Hirosaki University Grant for Exploratory Research by Young Scientists (to YT), and a National Institutes of Health Grant (U01 CA168925; to MF).
Abbreviations
- PCa
prostate cancer
- TM
tunicamycin
- PPMP
DL-threo-1-phenyl-2-palmitoylamino-3-morpholino-1-propanol hydrochloride
- BAG
benzyl-α-GalNAc
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
References
- 1.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer research. 1996;56:5309–18. [PubMed] [Google Scholar]
- 2.Fukuda M. Possible roles of tumor-associated carbohydrate antigens. Cancer research. 1996;56:2237–44. [PubMed] [Google Scholar]
- 3.Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochimica et biophysica acta. 1999;1473:21–34. doi: 10.1016/s0304-4165(99)00167-1. [DOI] [PubMed] [Google Scholar]
- 4.Stenzl A, Studer UE. Outcome of patients with untreated cancer of the prostate. European urology. 1993;24:1–6. doi: 10.1159/000474253. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European journal of cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Baade PD, Youlden DR, Cramb SM, Dunn J, Gardiner RA. Epidemiology of prostate cancer in the Asia-Pacific region. Prostate international. 2013;1:47–58. doi: 10.12954/PI.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalona WJ. Management of cancer of the prostate. The New England journal of medicine. 1994;331:996–1004. doi: 10.1056/NEJM199410133311507. [DOI] [PubMed] [Google Scholar]
- 9.Drabik A, Ciolczyk-Wierzbicka D, Dulinska-Litewka J, et al. A comparative study of glycoproteomes in androgen-sensitive and -independent prostate cancer cell lines. Molecular and cellular biochemistry. 2014;386:189–98. doi: 10.1007/s11010-013-1857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen GY, Kurosawa N, Muramatsu T. A novel variant form of murine beta-1, 6-N-acetylglucosaminyltransferase forming branches in poly-N-acetyllactosamines. Glycobiology. 2000;10:1001–11. doi: 10.1093/glycob/10.10.1001. [DOI] [PubMed] [Google Scholar]
- 11.Inaba N, Hiruma T, Togayachi A, et al. A novel I-branching beta-1,6-N-acetylglucosaminyltransferase involved in human blood group I antigen expression. Blood. 2003;101:2870–6. doi: 10.1182/blood-2002-09-2838. [DOI] [PubMed] [Google Scholar]
- 12.Bierhuizen MF, Mattei MG, Fukuda M. Expression of the developmental I antigen by a cloned human cDNA encoding a member of a beta-1,6-N-acetylglucosaminyltransferase gene family. Genes & development. 1993;7:468–78. doi: 10.1101/gad.7.3.468. [DOI] [PubMed] [Google Scholar]
- 13.Wiener AS, Unger LJ, Cohen L, Feldman J. Type-specific cold auto-antibodies as a cause of acquired hemolytic anemia and hemolytic transfusion reactions: biologic test with bovine red cells. Annals of internal medicine. 1956;44:221–40. doi: 10.7326/0003-4819-44-2-221. [DOI] [PubMed] [Google Scholar]
- 14.Muramatsu H, Kusano T, Sato M, Oda Y, Kobori K, Muramatsu T. Embryonic stem cells deficient in I beta1,6-N-acetylglucosaminyltransferase exhibit reduced expression of embryoglycan and the loss of a Lewis X antigen, 4C9. Glycobiology. 2008;18:242–9. doi: 10.1093/glycob/cwm138. [DOI] [PubMed] [Google Scholar]
- 15.Ohyama C, Tsuboi S, Fukuda M. Dual roles of sialyl Lewis X oligosaccharides in tumor metastasis and rejection by natural killer cells. The EMBO journal. 1999;18:1516–25. doi: 10.1093/emboj/18.6.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsui KH, Chang PL, Feng TH, Chung LC, Sung HC, Juang HH. Evaluating the function of matriptase and N-acetylglucosaminyltransferase V in prostate cancer metastasis. Anticancer research. 2008;28:1993–9. [PubMed] [Google Scholar]
- 17.Hagisawa S, Ohyama C, Takahashi T, et al. Expression of core 2 beta1,6-N-acetylglucosaminyltransferase facilitates prostate cancer progression. Glycobiology. 2005;15:1016–24. doi: 10.1093/glycob/cwi086. [DOI] [PubMed] [Google Scholar]
- 18.Hatakeyama S, Kyan A, Yamamoto H, et al. Core 2 N-acetylglucosaminyltransferase-1 expression induces aggressive potential of testicular germ cell tumor. International journal of cancer Journal international du cancer. 2010;127:1052–9. doi: 10.1002/ijc.25117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Meng F, Wu S, et al. Engagement of I-branching {beta}-1, 6-N-acetylglucosaminyltransferase 2 in breast cancer metastasis and TGF-{beta} signaling. Cancer research. 2011;71:4846–56. doi: 10.1158/0008-5472.CAN-11-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojima Y, Yoneyama T, Hatakeyama S, et al. Detection of Core2 beta-1,6-N-Acetylglucosaminyltransferase in Post-Digital Rectal Examination Urine Is a Reliable Indicator for Extracapsular Extension of Prostate Cancer. PloS one. 2015;10:e0138520. doi: 10.1371/journal.pone.0138520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nonaka M, Fukuda MN, Gao C, et al. Determination of carbohydrate structure recognized by prostate-specific F77 monoclonal antibody through expression analysis of glycosyltransferase genes. The Journal of biological chemistry. 2014;289:16478–86. doi: 10.1074/jbc.M114.559047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo HB, Lee I, Kamar M, Akiyama SK, Pierce M. Aberrant N-glycosylation of beta1 integrin causes reduced alpha5beta1 integrin clustering and stimulates cell migration. Cancer research. 2002;62:6837–45. [PubMed] [Google Scholar]
- 23.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bao X, Kobayashi M, Hatakeyama S, et al. Tumor suppressor function of laminin-binding alpha-dystroglycan requires a distinct beta3-N-acetylglucosaminyltransferase. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12109–14. doi: 10.1073/pnas.0904515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuboi S, Sutoh M, Hatakeyama S, et al. A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. The EMBO journal. 2011;30:3173–85. doi: 10.1038/emboj.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 27.Isaji T, Gu J, Nishiuchi R, et al. Introduction of bisecting GlcNAc into integrin alpha5beta1 reduces ligand binding and down-regulates cell adhesion and cell migration. The Journal of biological chemistry. 2004;279:19747–54. doi: 10.1074/jbc.M311627200. [DOI] [PubMed] [Google Scholar]
- 28.Zheng M, Fang H, Hakomori S. Functional role of N-glycosylation in alpha 5 beta 1 integrin receptor. De-N-glycosylation induces dissociation or altered association of alpha 5 and beta 1 subunits and concomitant loss of fibronectin binding activity. The Journal of biological chemistry. 1994;269:12325–31. [PubMed] [Google Scholar]
- 29.Yamada KM, Geiger B. Molecular interactions in cell adhesion complexes. Current opinion in cell biology. 1997;9:76–85. doi: 10.1016/s0955-0674(97)80155-x. [DOI] [PubMed] [Google Scholar]
- 30.Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549–54. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 31.Isaji T, Sato Y, Zhao Y, et al. N-glycosylation of the beta-propeller domain of the integrin alpha5 subunit is essential for alpha5beta1 heterodimerization, expression on the cell surface, and its biological function. The Journal of biological chemistry. 2006;281:33258–67. doi: 10.1074/jbc.M607771200. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Hatakeyama S, Yu SY, et al. Core3 O-glycan synthase suppresses tumor formation and metastasis of prostate carcinoma PC3 and LNCaP cells through down-regulation of alpha2beta1 integrin complex. The Journal of biological chemistry. 2009;284:17157–69. doi: 10.1074/jbc.M109.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu C, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. The Journal of cell biology. 2001;155:505–10. doi: 10.1083/jcb.200108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamamura K, Furukawa K, Hayashi T, et al. Ganglioside GD3 promotes cell growth and invasion through p130Cas and paxillin in malignant melanoma cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11041–6. doi: 10.1073/pnas.0503658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu Y, Zhang J, Mi W, et al. Silencing of GM3 synthase suppresses lung metastasis of murine breast cancer cells. Breast cancer research : BCR. 2008;10:R1. doi: 10.1186/bcr1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang YJ, Ding Y, Levery SB, Lobaton M, Handa K, Hakomori SI. Differential expression profiles of glycosphingolipids in human breast cancer stem cells vs. cancer non-stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4968–73. doi: 10.1073/pnas.1302825110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimada S, Ito A, Kawasaki Y, et al. Ganglioside disialosyl globopentaosylceramide is an independent predictor of PSA recurrence-free survival following radical prostatectomy. Prostate cancer and prostatic diseases. 2014;17:199–205. doi: 10.1038/pcan.2014.9. [DOI] [PubMed] [Google Scholar]
- 38.Hakomori S. Carbohydrate-to-carbohydrate interaction, through glycosynapse, as a basis of cell recognition and membrane organization. Glycoconjugate journal. 2004;21:125–37. doi: 10.1023/B:GLYC.0000044844.95878.cf. [DOI] [PubMed] [Google Scholar]
- 39.Hakomori SI. Structure and function of glycosphingolipids and sphingolipids: recollections and future trends. Biochimica et biophysica acta. 2008;1780:325–46. doi: 10.1016/j.bbagen.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hakomori SI. Glycosynaptic microdomains controlling tumor cell phenotype through alteration of cell growth, adhesion, and motility. FEBS letters. 2010;584:1901–6. doi: 10.1016/j.febslet.2009.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochimica et biophysica acta. 2013;1833:3481–98. doi: 10.1016/j.bbamcr.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 42.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Advances in cancer research. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 43.Carroll AM, Zalutsky M, Schatten S, et al. Monoclonal antibodies to tissue-specific cell surface antigens. I. Characterization of an antibody to a prostate tissue antigen. Clinical immunology and immunopathology. 1984;33:268–81. doi: 10.1016/0090-1229(84)90081-3. [DOI] [PubMed] [Google Scholar]
- 44.Zhang G, Zhang H, Wang Q, et al. Suppression of human prostate tumor growth by a unique prostate-specific monoclonal antibody F77 targeting a glycolipid marker. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:732–7. doi: 10.1073/pnas.0911397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao C, Liu Y, Zhang H, et al. Carbohydrate sequence of the prostate cancer-associated antigen F77 assigned by a mucin O-glycome designer array. The Journal of biological chemistry. 2014;289:16462–77. doi: 10.1074/jbc.M114.558932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Doc. S1. Supplementary Materials and Methods
Fig. S1. Nodal metastatic prostate cancer expresses I-branching N-acetylglucosaminyltransferase (GCNT2).
Fig. S2. I-branching N-acetylglucosaminyltransferase (GCNT2) expression regulates prostate cancer cell migration and invasion without affecting the epithelial mesenchymal transition.
Fig. S3. I-antigen expression on the cell surface of GCNT2-expressing prostate cancer cell lines.
Fig. S4. Treatment with glycosylation inhibitor decreases presentation of O-glycan, N-glycan and glycolipid on prostate cancer cell surface.
Fig. S5. The AKT inhibitor, the O-glycosylation inhibitor, and the glucosylceramide synthetase inhibitor had no effects on cell viability during assays.
Table S1. Primer list
Table S2. I-branching N-acetylglucosaminyltransferase status and patient data
Table S3. I-branching N-acetylglucosaminyltransferase status and pathological parameters