Abstract
Background
Recent studies reported that the CD4/CD8 T-cell ratio is inversely associated with biomarkers traditionally used to measure immune activation and systemic inflammation in highly active antiretroviral therapy (HAART)-treated HIV-infected (HIV+) patients. The relation of HCV co-infection with the CD4/CD8 ratio in HIV+ patients is unknown.
Methods
We examined 50,201 CD4/CD8 ratios measured over 20 years in three groups of HIV+ women enrolled in the Women's Interagency HIV Study (WIHS): HCV antibody negative (n=1,734), cleared HCV (n=231) and chronic HCV (n=751) in multivariate models. IFNL4-ΔG genotype and HCV viral load were also considered.
Results
Compared to HCV antibody negative status, chronic HCV infection was associated with lower CD4/CD8 ratios when HIV viral load was suppressed to the lower limit of quantification (LLQ - β= -0.08; P=0.002). Cleared HCV (β= -0.10; P=0.0009), but not IFNL4-ΔG genotype or HCV viral load, was also associated with lower CD4/CD8 ratios when HIV viral load was suppressed to the LLQ.
Conclusions
The association of HCV co-infection with CD4/CD8 ratio is consistent with previously observed associations of HCV co-infection with biomarkers traditionally used to measure immune activation and systemic inflammation in HIV+ patients. These data provide additional support for the use of CD4/CD8 ratio for routine monitoring of immune activation and inflammation in HIV+ patients, including those with HIV/HCV co-infection, however, the unexpected association between cleared HCV and lower CD4/CD8 ratio requires additional study.
Keywords: CD4/CD8, hepatitis C virus, HCV, HIV, inflammation, immune activation
Introduction
Immune activation and systemic inflammation are hallmark characteristics of untreated HIV infection. Treatment of HIV with highly active antiretroviral therapy (HAART) can suppress HIV replication and reduce immune activation and systemic inflammation. However, these levels do not completely normalize under HAART and it is hypothesized that residual immune activation and inflammation contribute to incidence of non-AIDS clinical events that occur in treated HIV-infected (HIV+) patients.1;2 Understanding factors that contribute to immune activation and systemic inflammation in HIV infection is therefore a priority.
Recent studies suggest that the CD4/CD8 T-cell ratio – which is easily calculable from routine flow cytometry measurements – may represent a way at no additional cost to assess immune activation and inflammation in treated HIV+ patients. Specifically, the CD4/CD8 ratio is inversely associated with biomarkers of CD4+ and CD8+ T-cell activation and systemic inflammation (i.e., ↓ CD4/CD8 ratio = ↑ immune activation/systemic inflammation)3-5 and predicts non-AIDS events and death in treated HIV+ patients.6
New biomarkers including the CD4/CD8 T-cell ratio should be carefully scrutinized prior to adoption for routine use in clinical and research settings. In this manuscript we scrutinize the CD4/CD8 ratio by testing its association with hepatitis C virus (HCV) co-infection. Our group and others have shown that HCV co-infection is associated with higher levels of peripheral blood biomarkers traditionally used to measure immune activation and systemic inflammation in HIV+ patients (e.g., %CD8+ CD38+ HLA-DR+ T-cells).3;7-11 Therefore, if the CD4/CD8 ratio is a good biomarker for immune activation and inflammation in treated HIV+ patients then it should be lower in HIV/HCV co-infected patients as compared to HIV+ patients without HCV.
Specifically, we examine the association of HCV co-infection with CD4/CD8 ratios measured over 20 years in HIV+ women enrolled in the Women's Interagency HIV Study (WIHS), including during study visits at which HIV viral loads were at the lower limit of quantification (LLQ). For completeness, we also consider CD4/CD8 ratios in relation to other HCV-related factors that have been assessed in WIHS women: a) spontaneous HCV clearance, b) the interferon lambda 4 (IFNL4)-ΔG genotype and c) HCV viral load. These analyses may inform our understanding of CD4/CD8 ratio dynamics in HIV+ patients.
Materials and Methods
Study Population
The WIHS is a multicenter, prospective study of HIV+ and HIV- women who were recruited using similar methods at six US sites during two recruitment periods: 1994-1995 and 2001-2002. Detailed methods and characteristics of the study population have been described previously.12;13 At enrollment and then semi-annually interviews were conducted, a physical exam performed, and blood specimens collected. The protocol was approved by the Institutional Review Boards at each study site, and all participants provided written informed consent.
Clinical Laboratory Measurements
HCV serostatus was determined at enrollment (defined as WIHS visits 1-3 for hepatitis testing) using a commercial second- or third-generation enzyme immunoassay, and HCV viremia was determined in HCV-seropositive women using either the COBAS Amplicor Monitor 2.0, which has a linear range of 600–5.0 × 105 IU/ml, as previously described,14 or the COBAS Taqman assay, which has a linear range of 10–2.0 × 108 IU/ml (both from Roche Diagnostics, Branchburg, NJ). Follow-up HCV RNA testing has also been conducted on most women who were HCV RNA positive at enrollment using the Amplicor or Taqman assay, primarily in 1996-2001 and 2006-2007.
Plasma HIV RNA levels were originally measured through visit 6 with a nucleic acid sequence-based amplification method that had 4,000 copies/ml as its lower limit of quantification (LLQ - Organon Teknika Corp., Durham, NC). Similar methods with greater sensitivity were used thereafter as they became clinically available (i.e., the LLQ was 400 copies/ml during visits 7 to 9 and 80 copies/ml thereafter). In the past several years, there has been an effort to re-measure HIV RNA levels with more sensitive assays at WIHS visits where assay LLQ was 400 or 4,000 copies/ml and the HIV viral load was at the LLQ. This effort has been largely successful but re-measurement of HIV RNA levels was not possible at visits with insufficient plasma available in the WIHS repository. Total CD4+ and total CD8+ T-cell counts (cells/μL) were determined in both HIV-infected and HIV-uninfected women by flow cytometry in laboratories participating in the DAIDS Quality Assurance Program at each study visit.15
IFNL4-ΔG Genotyping
Genotyping for IFNL4-ΔG (rs368234815) was performed at the Laboratory of Translational Genomics, National Cancer Institute, Bethesda, USA with custom TaqMan allelic discrimination genotyping assays, as previously described.16
Statistical Methods
We considered the association of HCV infection with the CD4/CD8 ratio among women who enrolled in WIHS with prevalent HIV infection (n=2,791) with HIV seroconverters (n=24) being excluded. We also excluded women with unknown HCV antibody status (n=17), those with positive HCV antibody at enrollment but unknown HCV RNA status (n=42), and those without any CD4/CD8 data (n=16). Women in the “HCV clearance” group (i.e., HCV antibody positive and HCV RNA negative at enrollment (n=204) or HCV RNA positive at enrollment but HCV RNA negative on a follow-up HCV RNA test (n=27) were considered separately from the “Chronic HCV” group (i.e., women persistently positive at every available HCV RNA test). Sensitivity analyses were conducted which excluded the 27 women who were HCV RNA positive at enrollment but HCV RNA negative on follow-up and also those women who reported successful HCV therapy during follow-up. Differences in demographic and HIV characteristics between the three study groups at the WIHS enrollment visit were assessed with Fisher's exact tests (categorical variables) or Mann-Whitney tests (continuous variables).
Associations of chronic HCV and cleared HCV with CD4/CD8 ratios were examined longitudinally using generalized estimating equation (GEE) models. For these models, we chose a correlation structure based on comparison of goodness-of-fit statistics using the Quasi-likelihood under the independence model criterion (QIC)17 and based on plots of within-woman CD4/CD8 ratio correlations over time. Among commonly used GEE correlation structures (i.e., exchangeable, independent, unstructured, autoregressive(1)), the exchangeable and autoregressive(1) structures had the lowest QIC values (lower is better). However, within-woman correlation plots showed that assumptions of autocorrelation were not met for most women. We therefore used exchangeable correlation structures for our GEE models. HCV antibody negative women served as the referent group because our principal scientific question was how women with HIV/HCV co-infection compare to the much larger group of never HCV-infected HIV+ patients. These models included adjustment for demographic and HIV disease characteristics and with the exception of race/ethnicity these characteristics were time-varying along with CD4/CD8 ratios in the GEE analysis.
We then considered associations of CD4/CD8 ratios over time with IFNL4-ΔG genotype (rs368234815) in the subset of women with IFNL4-ΔG data (n=2,175, 80% of the total data set). We considered IFNL4-ΔG under a dominant genetic model (ΔG/ΔG + ΔG/TT vs. TT/TT) because individuals who carry at least one copy of the ΔG allele produce the biologically active IFN-λ4 protein whereas those with the TT/TT homozygous genotype do not. However, IFNL4-ΔG has an additive association with spontaneous HCV clearance and HCV viral load in some populations18 and so for completeness we also considered associations of IFNL4-ΔG under an additive genetic model. Confounding is uncommon for genetic epidemiologic analyses after adjusting for race/ethnicity. However, to increase power and to facilitate interpretation we adjusted analyses of IFNL4-ΔG by race/ethnicity and also demographic and HIV disease characteristics.
HCV viral load levels were analyzed in relation to CD4/CD8 ratios among women with chronic HCV infection (n=747 women with ≥1 contemporaneous HCV viral load and CD4/CD8 ratio measurement). The number of available HCV viral load measurements per woman was: 1 viral load (n=179), 2 viral loads (n=320), 3 viral loads (n=103), ≥4 viral loads (n=145). The distribution of HCV viral load measures over time was: visits 1-3 (1994-1996): 960 measures; visits 4-14 (1996-2001): 583 measures; visits 15-24 (2001-2006): 165 measures; visit 25 (2006-2007): 161 measures. Analyses of HCV viral load were conducted using GEE models with adjustment for demographic and HIV disease characteristics.
Results
Characteristics of the Study Population at Enrollment
We considered associations of HCV infection with CD4/CD8 ratio in three groups of HIV+ women: HCV antibody negative (n=1,734), cleared HCV (n=231) and women with chronic HCV (n=751). These three groups differed by a variety of demographic and HIV disease characteristics at their WIHS enrollment visit (Table 1). For example, women with chronic HCV were older, more likely to be African American and had higher HIV viral loads than HCV antibody negative women and those with cleared HCV. Women with chronic HCV also had lower CD4/CD8 ratios than HCV antibody negative women.
Table 1. Baseline characteristics of HIV-positive women by HCV statusa.
| HCV antibody negative (n=1,734) |
Cleared HCVb (n=231) |
Chronic HCVb (n=751) |
|
|---|---|---|---|
| Demographic characteristics | |||
| Agec,d,e | 34 (8) | 39 (8) | 40 (6) |
| Race/ethnicity | |||
| African Americanc,d,e | 941 (54%) | 104 (45%) | 470 (63%) |
| Hispanicd,e | 489 (28%) | 68 (29%) | 144 (19%) |
| Whitec,e | 235 (14%) | 52 (23%) | 124 (17%) |
| Other | 69 (4%) | 7 (3%) | 13 (2%) |
| Education | |||
| < High schoold | 603 (35%) | 93 (40%) | 331 (44%) |
| High school | 512 (30%) | 65 (28%) | 242 (32%) |
| > High schoold,e | 613 (35%) | 73 (32%) | 178 (24%) |
| Unknown | 6 (0%) | 0 | 0 |
| Household income | |||
| ≤ $12,000c,d | 903 (52%) | 156 (68%) | 533 (71%) |
| $12,001- $30,000c,d | 508 (29%) | 50 (22%) | 155 (21%) |
| > $30,000c,d | 274 (16%) | 20 (9%) | 38 (5%) |
| Unknown | 49 (3%) | 5 (2%) | 25 (3%) |
| Smoking | |||
| Neverc,d,e | 794 (46%) | 32 (14%) | 67 (9%) |
| Former c,d,e | 647 (37%) | 145 (63%) | 584 (78%) |
| Current c,d,e | 293 (17%) | 54 (23%) | 100 (13%) |
| Ever injection drug usec,d | 56 (3%) | 183 (79%) | 632 (84%) |
| Enrollment periodc,d | |||
| 1994-1995 | 1111 (64%) | 202 (87%) | 682 (91%) |
| 2001-2002 | 623 (36%) | 29 (13%) | 69 (9%) |
| HIV disease characteristics | |||
| HAART naïvec,d | 1310 (76%) | 215 (93%) | 710 (95%) |
| Log10 HIV copies/mLd,e | 3.9 (1.2) | 3.9 (1.2) | 4.2 (1.1) |
| CD4+ cells/mLd,e | 420 (293) | 458 (349) | 384 (294) |
| CD4/CD8 ratiod | 0.54 (0.43) | 0.52 (0.38) | 0.47 (0.36) |
Continuous variables expressed as mean (standard deviation). Differences assessed by Fisher's exact test (categorical variables) or Mann-Whitney test (continuous variables). Race/ethnicity was by self-report.
Women with “HCV clearance” were HCV antibody positive and HCV RNA negative at enrollment (n=204) or HCV RNA positive at enrollment but HCV RNA negative on a follow-up HCV RNA test (n=27). Women with “chronic HCV” were persistently positive at every available HCV RNA test. The “cleared HCV” and “chronic HCV” groups were exclusive (no overlap over time).
P<0.05 between HCV antibody negatives vs. women with cleared HCV
P<0.05 between HCV antibody negatives vs. women with chronic HCV
P<0.05 between women with cleared vs. chronic HCV
HCV Infection and CD4/CD8 Ratios Over Time
CD4/CD8 ratios (n=50,201) measured over 39 WIHS visits (20 years) were considered in the current study. As shown in Figure 1, mean CD4/CD8 ratios increased over time from WIHS visit 1 (1994-1995) to WIHS visit 39 (2013-2014) for women in each of the three study groups. Mean CD4/CD8 ratios were, however, lower for women with chronic HCV than for HCV antibody negative women at all study visits. The trajectory of mean CD4/CD8 ratios for women with cleared HCV did not follow a consistent pattern. We note, though, that women with cleared HCV were the smallest study group (Supplementary Table 1).
Figure 1.
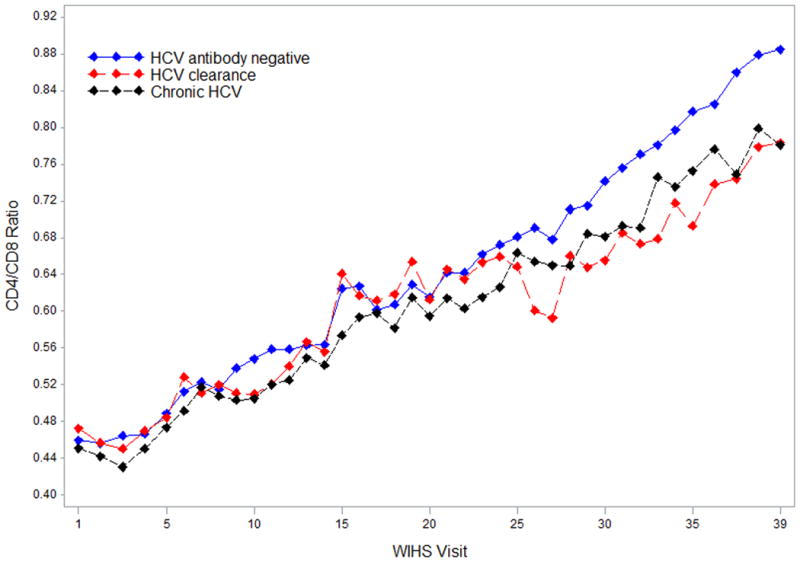
Mean CD4/CD8 ratios over time in HIV-positive WIHS women. Note: Several cross-sectional studies have observed higher CD4/CD8 ratios in older vs. younger healthy adults.28-30 It is unclear whether the higher mean CD4/CD8 ratios observed at later vs. earlier WIHS visits shown in Figure 1 reflect: i) age-related increases in CD4/CD8 ratios, ii) increases in CD4/CD8 ratios due to improvements in HIV treatment and clinical care since 1994, iii) unmeasured factors, or iv) a combination of i-iii.
In multivariate GEE models (Table 2), HIV viral load and CD4 cell count measurements had the strongest associations (highest Z scores) with CD4/CD8 ratio. Characteristics associated with improvements in HIV clinical care over time – age and WIHS enrollment period – were also statistically significant and had high Z scores. Cleared HCV (β = -0.04; 95% CI: -0.08, -0.006; P=0.02) but not chronic HCV infection (β = -0.01; 95% CI: -0.04, 0.01; P=0.36) was significantly associated with lower CD4/CD8 ratios in adjusted GEE analyses.
Table 2. Multivariate associations of chronic HCV, cleared HCV and adjustment characteristics with CD4/CD8 ratios over time in HIV-positive womena,b.
| β | 95% CI | Z score | P value | ||
|---|---|---|---|---|---|
| HCV characteristics | |||||
| HCV antibody (-) | Ref | Ref | Ref | Ref | |
| Cleared HCV | -0.04 | -0.08, -0.006 | -2.3 | 0.02 | |
| Chronic HCV | -0.01 | -0.04, 0.01 | -0.9 | 0.36 | |
| Demographic characteristics | |||||
| Age | 0.005 | 0.004, 0.006 | 6.9 | <0.000001 | |
| Race/ethnicity | |||||
| African American | Ref | Ref | Ref | Ref | |
| Hispanic | 0.01 | -0.01, 0.03 | 0.8 | 0.44 | |
| White | 0.06 | 0.03, 0.09 | 3.6 | 0.0003 | |
| Other | 0.04 | -0.02, 0.10 | 1.2 | 0.23 | |
| Education | |||||
| < High school | Ref | Ref | Ref | Ref | |
| High school | 0.009 | -0.02, 0.03 | 0.7 | 0.50 | |
| > High school | 0.03 | 0.002, 0.05 | 2.1 | 0.04 | |
| Unknown | -0.006 | -0.20, 0.18 | -0.1 | 0.95 | |
| Household income | |||||
| ≤ $12,000 | Ref | Ref | Ref | Ref | |
| $12,001- $30,000 | 0.006 | -0.003, 0.01 | 1.4 | 0.17 | |
| > $30,000 | 0.006 | -0.009, 0.02 | 0.8 | 0.44 | |
| Unknown | -0.0006 | -0.01, 0.01 | -0.1 | 0.93 | |
| Smoking | |||||
| Never | Ref | Ref | Ref | Ref | |
| Former | -0.02 | -0.05, 0.009 | -1.4 | 0.16 | |
| Current | -0.03 | -0.06, 0.004 | -1.7 | 0.08 | |
| Enrollment period | |||||
| 1994-1995 | Ref | Ref | Ref | Ref | |
| 2001-2002 | 0.11 | 0.08, 0.13 | 7.5 | <0.000001 | |
| HIV disease characteristics | |||||
| HAART use | -0.007 | -0.02, 0.006 | -1.0 | 0.30 | |
| Log10 HIV copies/mL | -0.050 | -0.054, -0.045 | -20.9 | <0.000001 | |
| CD4+ cells/mL | 0.0007 | 0.0007, 0.0007 | 38.5 | <0.000001 | |
Analysis includes n=50,201 CD4/CD8 ratios measured over 39 WIHS visits (20 years)
All variables shown in Table 2 were analyzed together in a single multivariate GEE model
Since the association of cleared HCV with CD4/CD8 ratio might have changed over time, we repeated the GEE analysis in both early (WIHS visits 1 – 19) and late time periods (WIHS visits 20 – 39). Cleared HCV was negatively associated with lower CD4/CD8 ratio in the late time period (β = -0.07; 95% CI: -0.13, -0.01; P=0.02) but not in the early time period (P=0.91). There was no statistically significant association of chronic HCV with CD4/CD8 ratio in either the early (P=0.08) or late time periods (P=0.21) in adjusted analyses.
We conducted three sensitivity analyses. First, we excluded n=27 women who were HCV RNA positive at WIHS enrollment but HCV RNA negative at a follow-up visit. Exclusion of these women did not alter the association of cleared HCV with CD4/CD8 ratio in the late time period (β = -0.07 → -0.07; P=0.02 → 0.04). Second, we excluded n=20 women from the chronic HCV group who reported successful HCV therapy during follow-up. Exclusion of these women did not alter the null association of chronic HCV with CD4/CD8 ratio (P=0.36 → 0.30). Third, we removed CD4 cell counts from the multivariate model because to our knowledge there is no agreement in the field as to whether or not CD4 counts should be included as possible predictors of CD4/CD8 ratios. In this third sensitivity analysis, neither chronic nor cleared HCV was significantly associated with the CD4/CD8 ratio (data not shown).
HCV Infection and CD4/CD8 Ratios at Visits with HIV Viral Loads at the Lower Limit of Quantification
We repeated analyses of chronic HCV and cleared HCV with CD4/CD8 ratios over time using data from WIHS visits including women with HIV viral loads at the lower limit of quantification (LLQ). Data from 19,214 visits were used: HCV antibody negative (n=13,657 visits), cleared HCV (n=1,649 visits) and chronic HCV (n=3,908 visits). The distribution of LLQs for these visits was: 20 copies/ml (18%), 48 copies/ml (19%), 80 copies/ml (63%), 400 copies/ml (0.2%) and 4,000 copies/ml (0.1%).
Associations of demographic and HIV disease characteristics with CD4/CD8 ratios at WIHS visits with HIV viral loads at the LLQ were generally similar to those assessed using all WIHS visits (Table 3). However, both chronic HCV (β= -0.08; 95% CI -0.13, -0.03; P=0.002) and cleared HCV (β= -0.10; 95% CI: -0.17, -0.04; P=0.0009) were significantly associated with lower CD4/CD8 ratios at visits with HIV viral loads at the LLQ. Associations of HAART use with CD4/CD8 ratios also differed in analyses of all WIHS visits (no association – P=0.30) vs. visits where HIV viral load was at the LLQ (associated with ↑ CD4/CD8 ratios – P=0.008).
Table 3. Multivariate associations of chronic HCV, cleared HCV and adjustment characteristics with CD4/CD8 ratios over time at WIHS visits where HIV viral loads were at the lower limit of quantification (LLQ)a,b.
| β | 95% CI | Z score | P value | ||
|---|---|---|---|---|---|
| HCV characteristics | |||||
| HCV antibody (-) | Ref | Ref | Ref | Ref | |
| Cleared HCV | -0.10 | -0.17, -0.04 | -3.3 | 0.0009 | |
| Chronic HCV | -0.08 | -0.13, -0.03 | -3.1 | 0.002 | |
| Demographic characteristics | |||||
| Age | 0.01 | 0.01, 0.02 | 9.8 | <0.000001 | |
| Race/ethnicity | |||||
| African American | Ref | Ref | Ref | Ref | |
| Hispanic | 0.02 | -0.03, 0.06 | 0.7 | 0.47 | |
| White | 0.05 | -0.001, 0.10 | 1.9 | 0.06 | |
| Other | 0.07 | -0.04, 0.17 | 1.3 | 0.20 | |
| Education | |||||
| < High school | Ref | Ref | Ref | Ref | |
| High school | 0.01 | -0.03, 0.05 | 0.7 | 0.49 | |
| > High school | 0.06 | 0.02, 0.10 | 2.7 | 0.007 | |
| Unknown | 0.22 | -0.09, 0.53 | 1.4 | 0.17 | |
| Household income | |||||
| ≤ $12,000 | Ref | Ref | Ref | Ref | |
| $12,001- $30,000 | 0.008 | -0.004, 0.02 | 1.3 | 0.20 | |
| > $30,000 | 0.01 | -0.007, 0.03 | 1.3 | 0.18 | |
| Unknown | -0.002 | -0.03, 0.02 | -0.1 | 0.89 | |
| Smoking | |||||
| Never | Ref | Ref | Ref | Ref | |
| Former | -0.01 | -0.08, 0.06 | -0.3 | 0.78 | |
| Current | -0.01 | -0.08, 0.06 | -0.4 | 0.70 | |
| Enrollment period | |||||
| 1994-1995 | Ref | Ref | Ref | Ref | |
| 2001-2002 | 0.16 | 0.12, 0.20 | 7.7 | <0.000001 | |
| HIV disease characteristics | |||||
| HAART use | 0.04 | 0.01, 0.06 | 2.7 | 0.008 | |
| Log10 HIV copies/mL | -0.07 | -0.11, -0.04 | -4.4 | 0.00001 | |
| CD4+ cells/mL | 0.0006 | 0.0005, 0.0006 | 26.3 | <0.000001 | |
Analysis includes n=19,214 CD4/CD8 ratios measured over 39 WIHS visits (20 years)
All variables shown in Table 3 were analyzed together in a single multivariate GEE model
In time period-stratified analyses at visits with HIV viral loads at the LLQ, chronic HCV was significantly associated with lower CD4/CD8 ratios in both the early (visits 1 – 19 – P=0.03) and late (visits 20 – 39 – P=0.001) time periods. In contrast, cleared HCV was associated with lower CD4/CD8 ratios in the late (P=0.0007) but not the early (P=0.07) time period.
In sensitivity analyses neither: i) exclusion of n=20 women from the chronic HCV group who reported successful HCV therapy during follow-up nor ii) nor removal of CD4 cell counts from the multivariate model affected the findings (in both sensitivity analyses: chronic HCV – P<0.01; cleared HCV – P<0.01).
IFNL4-ΔG and CD4/CD8 Ratios Over Time
In adjusted multivariate GEE models (Supplementary Table 2), there was no statistically significant association of IFNL4-ΔG genotype with CD4/CD8 ratios in the HCV antibody negative or chronic HCV groups. A borderline association (P=0.05) was observed under a dominant genetic model in women with cleared HCV.
We also considered the association of IFNL4-ΔG with CD4/CD8 ratios in time period-stratified analyses. Among women with cleared HCV in the late time period (n=120 women with 1,782 total CD4/CD8 ratios), IFNL4-ΔG (dominant model) was negatively associated with CD4/CD8 ratio (β= -0.12; 95% CI: -0.24, -0.008; P=0.04) whereas IFNL4-ΔG (additive model) had a borderline association (P=0.07). No other significant or borderline associations of IFNL4-ΔG were observed in time period-stratified analyses or in analyses of visits with HIV viral loads at the LLQ (data not shown).
HCV Viral Load and CD4/CD8 Ratios Over Time
Among women with chronic HCV infection, 747 had ≥1 contemporaneous HCV viral load and CD4/CD8 ratio measurement. In a time-varying multivariate GEE model adjusted for the demographic and HIV characteristics shown in Table 1, log10 HCV viral load was significantly associated with lower CD4/CD8 ratio (β= -0.04; 95% CI: -0.06, -0.02; P=0.0001). This association remained statistically significant in analyses restricted to both early (P=0.004) and late time periods (P=0.03). However, there was no statistically significant association of log10 HCV viral load with CD4/CD8 ratio when the analysis was restricted to visits with HIV viral loads at the LLQ (data not shown).
HCV Infection and CD4 and CD8 T-Cell Dynamics Over Time
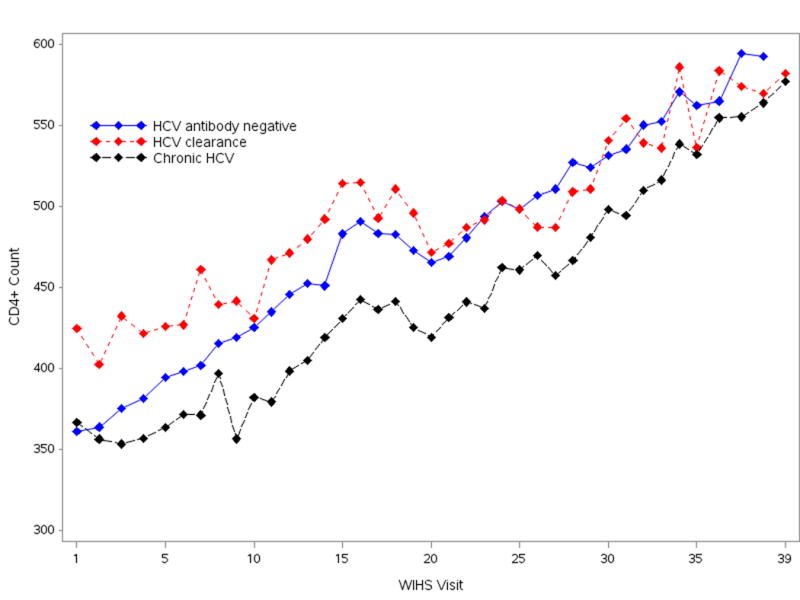
To better understand associations of HCV infection with CD4/CD8 ratios, we considered associations of chronic and cleared HCV with CD4 and CD8 T-cell levels separately. As shown in Figure 2, CD4 levels were lower in women with chronic HCV infection as compared to women without chronic HCV at most WIHS visits. However, in multivariate analyses adjusted for characteristics shown in Table 2 (aside from CD4 level), there were no differences in CD4 T-cells levels between women with chronic HCV (β= -13.0; 95% CI: -33.7, 7.6; P=0.22) or cleared HCV (β= 27.9; 95% CI: -4.9, 60.8; P=0.10) and women without HCV antibody. In contrast, when the subset of WIHS visits where HIV viral load was at the LLQ was considered in adjusted multivariate analyses, CD4 levels were lower for women with chronic HCV infection (β= -76.1; 95% CI: -108.3, -43.9; P<0.0001) as compared to women without HCV antibody. CD4 levels did not differ between women with cleared HCV vs. no HCV antibody in adjusted multivariate analyses in WIHS visits where HIV viral load was at the LLQ (data not shown).
Figure 2. Mean CD4 T-cell levels over time in HIV-positive WIHS women.
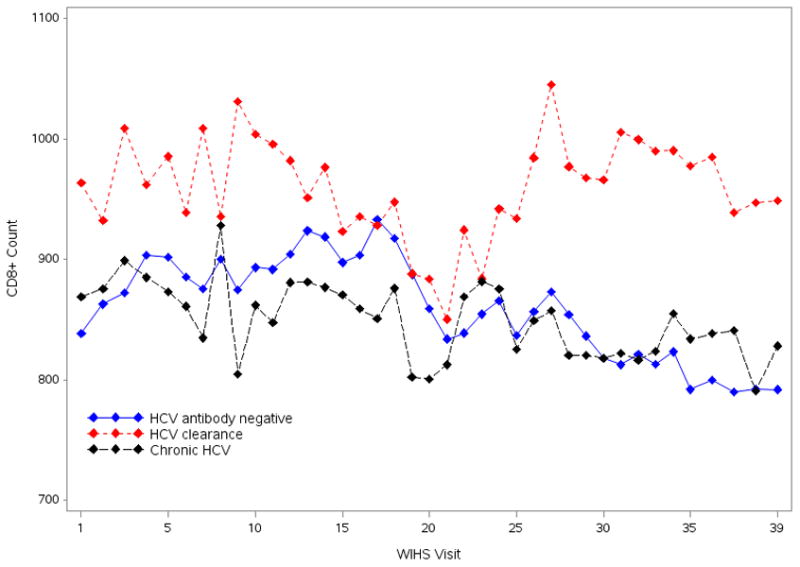
CD8 T-cell levels were higher among women with cleared HCV as compared to women with chronic HCV and women without HCV antibody at most WIHS visits (Figure 3). In multivariate analyses adjusted for characteristics shown in Table 2 (aside from CD4 level), CD8 levels were higher for women with cleared HCV as compared to women without HCV antibody (β= 105.0; 95% CI: 33.8, 176.1; P=0.004) and similar associations were observed in the subset of WIHS visits where HIV viral load was at the LLQ (β= 113.7; 95% CI: 29.3, 198.0; P=0.008). Sensitivity analysis that excluded n=27 women who were HCV RNA positive at WIHS enrollment but HCV RNA negative at a follow-up visit showed similar significant associations of HCV clearance with CD8 levels (data not shown). There were no significant differences in CD8 levels between women with chronic HCV infection and women without HCV antibody overall or in the subset of WIHS visits where HIV viral load was at the LLQ in adjusted multivariate analyses.
Figure 3. Mean CD8 T-cell levels over time in HIV-positive WIHS women.
Discussion
We scrutinized the CD4/CD8 ratio – a putative biomarker of immune activation and systemic inflammation in treated HIV+ patients – by considering the relationship of n=50,201 CD4/CD8 ratios measured over 20 years with HCV phenotypes in HIV+ women. Consistent with our expectations and prior data based on biomarkers traditionally used to measure immune activation and systemic inflammation in HIV+ patients,3;7-11 we observed that chronic HCV infection was associated with low CD4/CD8 ratio in HIV+ women. These data are consistent with those of a recent study19 and provide support for the use of the CD4/CD8 ratio for routine monitoring of immune activation and inflammation in HIV+ and HIV/HCV co-infected patients. However, our observation of low CD4/CD8 ratio in women with cleared HCV infection suggests that T-cell dynamics are incompletely understood in HIV/HCV-infected populations.
The association of chronic HCV infection with lower CD4/CD8 ratio was only observed in women with HIV viral loads at the lower limit of quantification (LLQ). These data suggest that chronic HCV is a lesser influence on CD4/CD8 ratios compared to uncontrolled HIV infection – when HIV replication is suppressed the impact of HCV on CD4/CD8 ratio becomes manifest. However, a significant association of chronic HCV with CD8+ T-cell activation was observed in WIHS women in the pre-HAART era.7
Although the association of chronic HCV infection with CD4/CD8 ratio was independent of CD4 count (Table 3), our analyses of separate CD4 and CD8 T-cell dynamics suggests that this association may in part be linked to lower CD4 levels in women with chronic HCV infection as compared to women without HCV antibody. Whether HCV co-infection accelerates HIV natural history or impairs response to HAART is controversial. However, our observation of lower CD4 levels in suppressed HCV/HIV co-infected patients is consistent with a recent analysis that combined data from four randomized studies in the AIDS Clinical Trial Group (ACTG).20 Lastly, we cannot exclude uncontrolled confounding as an explanation for the observed CD4 differences. WIHS women with and without chronic HCV differ in meaningful ways (Table 1) and it is possible that biological or social characteristics that impact CD4 recovery are not completely captured by WIHS protocols.
Unexpectedly, we also observed that women with cleared HCV had significantly lower CD4/CD8 ratios compared to HCV antibody negative women, specifically in the late follow-up visits when CD4/CD8 ratios were highest across all groups. This association may be linked to higher CD8 levels in women with cleared HCV infection as compared to women without HCV antibody – the first time to our knowledge this observation has been reported. A recent study of a majority male French population observed higher CD8 levels in HCV/HIV co-infected patients compared to HIV monoinfected patients but a limitation of that study was that HCV infection was defined based on HCV serology alone.19
This unexpected finding needs replication but is worth discussing briefly. One hypothesis is that WIHS women with cleared HCV have a genetic predisposition for higher levels of systemic immune activation and inflammation. Studies by our group and others have shown that spontaneous HCV clearance is significantly associated with genetic variants related to the adaptive (HLA) and innate (IFNL4-ΔG) immune responses.18;21 HLA variants are associated with many inflammation disorders and reports associate IFNL4-ΔG with clinical phenotypes other than HCV.22;23 IFNL4-ΔG genotype was inversely associated with CD4/CD8 ratio in this study among women with cleared HCV but why this association was not observed in other study groups is unclear. A second hypothesis is that prior injection drug use (IDU) is linked to higher levels of systemic immune activation and inflammation. However, we were unable to differentiate associations with HCV from IDU because only 3% of HCV antibody negative women reported IDU at baseline (compared to 79% of women with cleared and 84% of women with chronic HCV infection). It is recognized that active IDU is associated with levels of certain immune biomarkers10;24 but whether history of IDU has a long-lasting impact on immune activation/inflammation independent of HCV is less clear. Moreover, if past IDU impacted CD8 dynamics we would expect to see similar patterns in women with chronic and cleared HCV.
HCV viral load had an unequivocal negative association with CD4/CD8 ratio in women with chronic HCV infection. However, most of the WIHS HCV viral load data were from the pre-/early-HAART era (1994-2001) and subset analyses restricted to visits with HIV viral loads at the LLQ were not statistically significant. This said, high HCV viral load predicts HCV treatment failure following HCV therapy25 and study of HCV viral load associations is a priority.
A second unexpected but interesting finding was that HAART use was associated with higher CD4/CD8 ratios among women with HIV viral loads at the LLQ. Among women with HIV viral loads at the LLQ, 10.4% were not receiving HAART. These data support the hypothesis that use of HAART confers an added benefit even to HIV patients with very low or undetectable HIV viral loads and are consistent with data from a recent report that showed higher levels of certain immune activation and inflammation markers in elite controllers vs. ART-treated patients with undetectable HIV viral loads.26 Levels of immune activation and inflammation biomarkers were comparable in elite controllers vs. ART-treated patients in another recent report.27
Limitations must be considered in the interpretation of these data. First, lack of HCV RNA testing data at every WIHS visit limits the precision of our results including our ability to measure successful HCV treatment and spontaneous clearance over time. However, exclusion of those women who reported successful HCV antiviral therapy during follow-up did not affect the findings. Further, because WIHS is an observational cohort that does not link to medical record data we cannot exclude the possibility that some women in the HCV clearance group had antiviral therapy-associated HCV eradication vs. spontaneous HCV clearance. However, the vast majority of women included in the HCV clearance group were HCV antibody positive/HCV RNA negative at their WIHS enrollment visit in 1994-1995. HCV antiviral therapies in use at that time were not efficacious and were rarely prescribed to patients with HIV co-infection.
Second, follow-up HCV antibody testing was not conducted for most women who were HCV antibody negative at enrollment. Most women in the chronic and cleared HCV groups reported IDU at baseline whereas the vast majority of HCV antibody negative women did not. Thus, WIHS is not sufficiently powered to assess the separate contribution of IDU vs. HCV infection acquired via a non-IDU route in relation to CD4/CD8 ratios – IDUs are at increased risk for HCV and also other infectious and non-infectious conditions, some of which might impact CD4/CD8 ratios.
In conclusion, the CD4/CD8 ratio was lower in HIV suppressed women with chronic HCV versus women without HCV antibody, supporting the use of this new biomarker as a surrogate for immune activation and inflammation in treated HIV+ patients. However, cleared HCV infection was also associated with low CD4/CD8 ratio – an unexpected finding likely due to elevated CD8 counts in this population. A timely question is whether we know enough about CD4/CD8 ratios for them to be clinically useful in HIV care. T-cell dynamics are however central to our understanding of HIV, HCV and other immune-mediated diseases and therefore increased attention is warranted to understand these cell populations in afflicted individuals.
Supplementary Material
Acknowledgments
Source of Funding: Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Mary Young), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women's HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA).
Footnotes
Conflicts of Interest: All authors, no conflicts of interest declared.
References
- 1.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633–645. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hearps AC, Martin GE, Rajasuriar R, et al. Inflammatory co-morbidities in HIV+ individuals: learning lessons from healthy ageing. Curr HIV /AIDS Rep. 2014;11:20–34. doi: 10.1007/s11904-013-0190-8. [DOI] [PubMed] [Google Scholar]
- 3.Zheng L, Taiwo B, Gandhi RT, et al. Factors associated with CD8+ T-cell activation in HIV-1-infected patients on long-term antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;67:153–160. doi: 10.1097/QAI.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serrano-Villar S, Gutierrez C, Vallejo A, et al. The CD4/CD8 ratio in HIV-infected subjects is independently associated with T-cell activation despite long-term viral suppression. J Infect. 2013;66:57–66. doi: 10.1016/j.jinf.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014;10:e1004078. doi: 10.1371/journal.ppat.1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mussini C, Lorenzini P, Cozzi-Lepri A, et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV. 2015;2:e98–e106. doi: 10.1016/S2352-3018(15)00006-5. [DOI] [PubMed] [Google Scholar]
- 7.Kovacs A, Al-Harthi L, Christensen S, et al. CD8(+) T cell activation in women coinfected with human immunodeficiency virus type 1 and hepatitis C virus. J Infect Dis. 2008;197:1402–1407. doi: 10.1086/587696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuniholm MH, Xie X, Anastos K, et al. Association of Chronic Hepatitis C Infection With T-Cell Phenotypes in HIV-Negative and HIV-Positive Women. J Acquir Immune Defic Syndr. 2014;67:295–303. doi: 10.1097/QAI.0000000000000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodowanec AC, Brady KE, Gao W, et al. Characterization of CD4(+) T-cell immune activation and interleukin 10 levels among HIV, hepatitis C virus, and HIV/HCV-coinfected patients. J Acquir Immune Defic Syndr. 2013;64:232–240. doi: 10.1097/QAI.0b013e31829c6de0. [DOI] [PubMed] [Google Scholar]
- 10.Salter ML, Lau B, Mehta SH, et al. Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high risk for HCV and HIV infections. J Acquir Immune Defic Syndr. 2013;64:488–495. doi: 10.1097/QAI.0b013e3182a7ee2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armah KA, Quinn EK, Cheng DM, et al. Human immunodeficiency virus, hepatitis C, and inflammatory biomarkers in individuals with alcohol problems: a cross-sectional study. BMC Infect Dis. 2013;13:399. doi: 10.1186/1471-2334-13-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacon MC, von W, V, Alden C, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 14.Al-Harthi L, Voris J, Du W, et al. Evaluating the impact of hepatitis C virus (HCV) on highly active antiretroviral therapy-mediated immune responses in HCV/HIV-coinfected women: role of HCV on expression of primed/memory T cells. J Infect Dis. 2006;193:1202–1210. doi: 10.1086/500843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvelli T, Denny TN, Paxton H, et al. Guideline for flow cytometric immunophenotyping: a report from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry. 1993;14:702–715. doi: 10.1002/cyto.990140703. [DOI] [PubMed] [Google Scholar]
- 16.Prokunina-Olsson L, Muchmore B, Tang W, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45:164–171. doi: 10.1038/ng.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 18.Aka PV, Kuniholm MH, Pfeiffer RM, et al. Association of the IFNL4-DeltaG Allele With Impaired Spontaneous Clearance of Hepatitis C Virus. J Infect Dis. 2014;209:350–354. doi: 10.1093/infdis/jit433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaegel-Faucher O, Bregigeon S, Cano CE, et al. Impact of hepatitis C virus coinfection on T-cell dynamics in long-term HIV-suppressors under combined antiretroviral therapy. AIDS. 2015;29:1505–1510. doi: 10.1097/QAD.0000000000000650. [DOI] [PubMed] [Google Scholar]
- 20.Hua L, Andersen JW, Daar ES, et al. Hepatitis C virus/HIV coinfection and responses to initial antiretroviral treatment. AIDS. 2013;27:2725–2734. doi: 10.1097/01.aids.0000432470.46379.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuniholm MH, Kovacs A, Gao X, et al. Specific human leukocyte antigen class I and II alleles associated with hepatitis C virus viremia. Hepatology. 2010;51:1514–1522. doi: 10.1002/hep.23515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eslam M, Hashem AM, Leung R, et al. Interferon-lambda rs12979860 genotype and liver fibrosis in viral and non-viral chronic liver disease. Nat Commun. 2015;6:6422. doi: 10.1038/ncomms7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manuel O, Wojtowicz A, Bibert S, et al. Influence of IFNL3/4 polymorphisms on the incidence of cytomegalovirus infection after solid-organ transplantation. J Infect Dis. 2015;211:906–914. doi: 10.1093/infdis/jiu557. [DOI] [PubMed] [Google Scholar]
- 24.Strickler HD, Blanchard JF, Vlahov D, et al. Elevated serum levels of neopterin but not beta 2-microglobulin in HIV-1-seronegative injecting drug users. AIDS. 1993;7:361–367. doi: 10.1097/00002030-199303000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Osinusi A, Meissner EG, Lee YJ, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013;310:804–811. doi: 10.1001/jama.2013.109309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereyra F, Lo J, Triant VA, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. 2012;26:2409–2412. doi: 10.1097/QAD.0b013e32835a9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noel N, Boufassa F, Lecuroux C, et al. Elevated IP10 levels are associated with immune activation and low CD4(+) T-cell counts in HIV controller patients. AIDS. 2014;28:467–476. doi: 10.1097/QAD.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 28.Amadori A, Zamarchi R, De SG, et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1:1279–1283. doi: 10.1038/nm1295-1279. [DOI] [PubMed] [Google Scholar]
- 29.Jentsch-Ullrich K, Koenigsmann M, Mohren M, et al. Lymphocyte subsets' reference ranges in an age- and gender-balanced population of 100 healthy adults--a monocentric German study. Clin Immunol. 2005;116:192–197. doi: 10.1016/j.clim.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Yan J, Greer JM, Hull R, et al. The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun Ageing. 2010;7:4. doi: 10.1186/1742-4933-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


