Abstract
The prevailing view in the field of adolescent brain development is that heightened activity in the mesolimbic dopaminergic reward system serves as a liability, orienting adolescents towards risky behaviors, increasing their sensitivity to social evaluation and loss, and resulting in compromised well-being. Several findings inconsistent with this deficit view challenge the perspective that adolescent reward sensitivity largely serves as a liability and highlights the potential adaptive function that heightened striatal reactivity can serve. The goal of this review is to refine our understanding of dopaminergic reward sensitivity in adolescence. I review several studies showing that ventral striatum activation serves an adaptive function for adolescents’ health and well being relating to declines in both risk taking and depression and increases in cognitive persistence and achievement.
Keywords: adolescence, rewards, risk taking, health, brain development
Adolescence is a sensitive and vulnerable developmental period marked by steep increases in risk-taking behavior, emotional lability, and poor behavioral regulation (Steinberg, 2005). Such changes relate to increased rates of depression and anxiety (i.e., internalizing problems) and conduct disorder and rule breaking behaviors (i.e., externalizing problems) that incur significant public health concern, driven by their high prevalence, chronicity, and adverse effects on functioning. For example, the onset of many psychiatric disorders emerges during adolescence, with depression rising 500% from childhood to adolescence and an additional 400% by the young adult years (see Thaper et al., 2012). Morbidity and mortality rates increase 300% from childhood to adolescence (CDC, 2014) with over 70% of adolescent deaths each year due to preventable causes including motor vehicle crashes, unintentional injuries, homicide, and suicide (CDC, 2013). Recent evidence from animal models and developmental neuroscience studies in human youth has shown that disruptions in reward processing may underlie increases in internalizing and externalizing symptoms during adolescence (Spear, 2011).
Adolescent Peaks in Reward Sensitivity
Across many species, including rodents, nonhuman primates, and humans, adolescents show peaks in reward-related behaviors, providing strong evidence for the conservation of reward processing across evolution (Spear, 2011). Adolescent rats are more sensitive than their adult counterparts to the rewarding properties of a variety of positively rewarding stimuli, including novelty-seeking (Douglas et al., 2003), the rewarding effects of social interactions (Douglas et al., 2004), consummatory behavior (Friemel et al., 2010; Spear, 2011), and palatable testants (Vaidya et al., 2004; Wilmouth & Spear, 2009; Friemel et al., 2010). In human primates, inverted U-shaped developmental patterns have been observed in reward seeking behaviors. For example, human adolescents show peaks in self-reported reward-seeking and sensation seeking (Steinberg et al., 2009; Romer et al., 2010), greater sensitivity to positive feedback during a behavioral gambling task (Cauffman et al., 2010), and heightened preferences and reactivity to sweet substances (Galván & McGlennen, 2013; Post & Kemper, 1993). These behavioral shifts in reward seeking behaviors and preferences are driven, in part, by underlying neural changes in frontostriatal circuitry.
Dopaminergic Changes in the Adolescent Brain
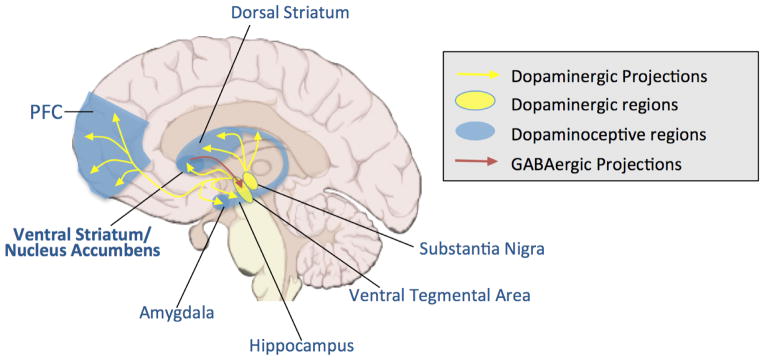
Frontostriatal circuits subserving reward processes are modulated by the neurotransmitter dopamine (DA) The primary reward circuit includes dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens, which release dopamine in response to reward-related stimuli (Russo & Nestler, 2013). The ventral striatum, and the nucleus accumbens in particular, has been recognized as a core node for incentive, reward driven behaviors (see Padmanabhan & Luna, 2014; Galvan, 2015). DA signaling supports reinforcement learning, and DA modulation of striatal and prefrontal function influences affective and motivated behaviors that are altered in adolescence (Padmanabhan & Luna, 2014). Major components of the reward system that undergo particularly dramatic change during adolescence include projections from DA neurons deep in the base of the brain (e.g., VTA; substantia nigra) to subcortical regions including the striatum, as well as the prefrontal cortex (PFC) and other cortical regions including the amygdala and hippocampus. There are also GABAergic projections from the nucleus accumbens to the VTA. These include projections through the direct pathway, which is mediated by D1-type medium spiny neurons, which directing innervate the VTA, and projections through the indirect pathway, which is mediated by D2-type medium spiny neurons, which innervate the VTA through GABAergic neurons in the ventral pallidum (Russo & Nestler, 2013). All of these reward-regions are interconnected in complex ways (see Figure 1).
Figure 1.
Dopaminergic pathways in the brain.
The DA system undergoes significant reorganization over adolescence, which is implicated in the pathophysiology of various disorders that appear during adolescence (see Nelson et al., 2005; Spear, 2000; Wahlstron et al., 2010). Across rodents, non-human primates, and humans, increases in dopamine signaling peak during adolescence (see Wahlstrom et al., 2010b). Adolescent-specific peaks have been observed in the density of dopamine receptors D1 and D2 in the ventral striatum in rodents (Andersen et al., 1997; Tarazi, Tomasini, & Baldessarini, 1999; Teicher, Andersen, & Hostetter Jr., 1995; Badanich, et al., 2006; Philpot, et al., 2009), although a few studies have not found functional differences in the nucleus accumbens of adolescent rodents compared to adults (Matthews et al., 2013; Sturman & Moghaddam, 2012). Moreover, DA concentrations and the density of DA fibers projecting to the PFC increase into adolescence (Benes et al., 2000), as well as the number of PFC projections to the nucleus accumbens (Brenhouse et al., 2008). In non-human primates, region-wide DA innervation peaks in adolescence (see Wahlstrom et al., 2010b).
Studies with humans have reported similar peaks in DA expression during adolescence. For example, in human post-mortem samples, DA levels in the striatum increase until adolescence and then decrease or remain stable (Haycock, et al., 2003), both in terms of length of axons as well as total number of projecting axons (Lambe, et al., 2000; Rosenberg & Lewis, 1994). There is also a peak in glutamatergic connectivity from the PFC to the nucleus accumebens, specifically in D1-expressing neurons (Brenhouse, et al., 2008). Finally, fMRI studies, which allow for assessment of changes in neural systems innervated by DA, have shown that the ventral striatum is significantly more active among adolescents than children or adults when receiving secondary rewards (e.g., money; Ernst et al., 2005; Galvan et al., 2006; Van Leijenhorst et al., 2010), primary rewards (e.g., sweet liquid; Galván & McGlennen 2013), or social rewards (Chein et al., 2011; Guyer et al., 2009) as well as in the presence of appetitive social cues (Somerville et al., 2011). Such peaks in ventral striatum activation are associated with compromised cognitive control (Somerville et al., 2011) and increased self-reported risk taking (Galván et al., 2007). Some studies have also found that adolescents show blunted ventral striatum activation relative to children or adults when anticipating rewards (Bjork et al., 2004; Bjork et al., 2010), and such blunted striatal activation is associated with greater risk taking behaviors (Schneider et al., 2012). Hypoactivation of the ventral striatum is argued to suggest that adolescents may attain less positive feelings from rewarding stimuli, which drives them to seek out greater reward-inducing experiences that increase activity in dopamine-related circuitry (Spear 2000).
It is hypothesized that the DA system is at a functional ceiling during adolescence (Chambers et al., 2003), as evidenced by peaks in DA cell firing, overall higher tonic DA levels, greater DA innervation, and increased DA receptor densities (see Padmanabhan & Luna, 2014). Therefore, the mesolimbic DA system is thought to be in a state of overdrive during adolescence, which has important functional significance for behavioral outcomes. It has also been proposed that tonic DA levels in the PFC rise beyond optimal levels in adolescence, resulting in a DA “overdose” which then biases input from limbic regions such as the nucleus accumbens (e.g., Wahlstrom et al., 2010). This shift in DA functional balance could have marked consequences on the competition between PFC and limbic regions for control of information, such that greater functional levels of DA activity in the nucleus accumbens shifts information flow toward greater limbic and less PFC influence on the nucleus accumbens (Spear, 2011).
Deficit Perspective on Dopaminergic Reactivity in Adolescence
Adolescent-specific peaks in DA activity are widely thought to account for the heightened orientations towards rewards in the environment including preferences for novelty, increased interest in risky situations, and the onset of many psychiatric disorders (Wahlstrom et al., 2010b). The prevailing view in the field of adolescent brain development is that heightened activity in the mesolimbic DA system serves as a liability, orienting adolescents towards risky behaviors, increasing their sensitivity to social evaluation and loss, and resulting in compromised well-being (e.g., Casey et al., 2008; Chambers et al., 2003). Therefore, while adolescent peaks in striatal reactivity may have evolved for adaptive purposes (e.g., facilitating reproductive success, emigration, and decreased inbreeding), striatal reactivity in modern societies may be more of a burden and even life threatening (Spear, 2008). Indeed, across dozens of neuroimaging studies of human adolescents, alterations in ventral striatum activation have been linked to negative outcomes including drug and alcohol use (Jager, et al., 2013), depression (Telzer et al., 2014; Silk et al., 2013), anxiety (Bar-Haim et al., 2009; Guyer et al., 2006), susceptibility to peer influence (Chein et al., 2010), and conduct and rule-breaking behaviors (Galván et al., 2007; Qu et al., 2015). Thus, rewards can profoundly influence important behaviors that constitute modifiable risk factors for chronic disease and mortality.
A Potential Adaptive Role of Dopaminergic Reactivity in Adolescence
The view that peaks in DA activity during adolescence underlie increases in risk taking and adolescent psychopathology suggests that adolescents are predisposed towards mental health problems due to hard-wired developmental constraints via altered neural processing. Such a deficit perspective driven by biologically determined brain immaturities implies a ubiquitous, inevitability to adolescent risk taking and psychopathology that constrains our ability to intervene. Moreover, this view emphasizes adolescent problem behaviors over more positive developments and behaviors. While this view is not wrong (i.e., heightened striatal activation does relate to negative outcomes), this perspective is overly simplified (Pfeifer & Allen, 2012) and does not take into consideration how heightened dopaminergic reactivity may be adaptive during this developmental period. Several findings inconsistent with this deficit view challenge the perspective that adolescent reward sensitivity largely serves as a liability and highlights the potential adaptive function that heightened striatal reactivity can serve.
One view that has been proposed is that heightened dopaminergic sensitivity increases risk-taking behaviors that may be adaptive for promoting survival and skill acquisition (Spear, 2000). The tendency to approach, explore and take risks during adolescence may serve an adaptive purpose that affords a unique opportunity for adolescents to attain new experiences at a time when youth are primed to learn from their environments and leave the safety of their caregivers (Spear, 2000). Thus, ventral striatum responses can facilitate goal attainment and long-term survival, allowing the adolescent to move towards relative autonomy (Wahlstrom et al., 2010). In short, this conceptualization suggests that risk taking itself is a normative and adaptive behavior. Heightened ventral striatum reactivity may therefore be an adaptive response as long as the system is not in overdrive and adolescents only engage in moderate levels of risk taking; high levels of risk taking may be detrimental and even life threatening (Spear, 2008). Moreover, the consequences of risk taking are likely to be context dependent. In our modern society, the environments where adolescents engage in risk taking (e.g., driving cars) may result in maladaptive instead of adaptive outcomes (Spear, 2008).
Moving beyond the theory that risk taking itself is an adaptive behavior, I propose a new conceptualization and adaptive role of reward sensitivity such that striatal reactivity can actually lead adolescents away from risks and psychopathologies. That is, striatal reactivity can direct adolescents away from the very same behavior thought to arise as a result of peaks in DA. Rather than promoting risk taking and psychopathology, recent evidence reveals that heightened striatal reactivity may actually motivate adolescents to engage in more thoughtful, positive behaviors, facilitating improved cognition, and ultimately protecting them from developing depression and engaging in health-compromising risk-taking behavior. Indeed, heightened ventral striatum responses, coupled with effective neural regulation, represent “the translation of positive motivation to adaptive action” (Wahlstrom et al., 2010, pp. 3).
Heightened DA signaling may therefore be a neurobiological marker for approach-related behaviors, regardless of the perceived outcome (i.e., adaptive or maladaptive). On the one hand, DA signaling may be channeled towards motivated behaviors that are highly adaptive, such as an orientation towards motivationally positive behaviors (e.g., striving for academic success, engaging in prosocial behaviors, working towards a goal). On the other hand, DA signaling may be directed towards motivated behaviors that can be highly maladaptive depending on situational and contextual variables (e.g., dangerous driving behaviors, risky sexual behaviors). Ventral striatum sensitivity may therefore represent either a vulnerability or an opportunity depending on the social and motivational context (see Table 1). Thus, developmental trajectories in ventral striatum sensitivity may vary across stimuli and contexts.
Table 1.
Ventral striatum reactivity can be both a source of vulnerability and opportunity
| Vulnerability
|
Opportunity
|
|---|---|
Orientation towards Negative Rewards
|
Orientation towards Positive Rewards
|
Sensitivity to Social Threat
|
Sensitivity to Social Connection
|
Because peaks in dopaminergic sensitivity appear to be universal in adolescence, as seen across species, contexts, and cultures, my goal is to highlight ways that such dopaminergic sensitivity can be redirected towards positive, health promoting behaviors. Several compelling reviews have recently suggested that adolescent neurodevelopment is complex (Crone & Dahl, 2012), and the ventral striatum is sometimes associated with adaptive outcomes (Pfeifer & Allen, 2012). In this review, I move a step further in this conceptualization and suggest that DA hyperactivation can promote adolescent health. Below, I review several recent studies that challenge traditional views that reward sensitivity functions in primarily maladaptive ways during adolescence. Across diverse samples and contexts, ventral striatum activation serves an adaptive function for adolescents’ health and well being relating to declines in both risk taking and depression and increases in cognitive persistence and achievement.
The Ventral Striatum and Reward: Reverse Inference
Before reviewing the current literature, I want to note a few issues with interpreting neuroimaging data. Human fMRI work has identified the ventral striatum as a key region involved in reward processing (see Delgado, 2007). However, an important limitation of the interpretation of the ventral striatum as coding reward is the problem of reverse inference (i.e., inferring cognitive states solely from the activation of a particular brain area; Poldrack, 2011). In the current manuscript, I will make several inferences regarding the ventral striatum and reward. While ventral striatum activation certainly is not synonymous with reward, this link is sometimes necessary to make in describing a cogent story. Nonetheless, it is important to note that other neural regions outside of the ventral striatum (e.g., VTA, vmPFC) and neurochemistry beyond dopamine (e.g., opioids) code for reward, and the ventral striatum is involved in a wide range of sensorimotor, cognitive and motivational functions. For instance, the striatum is involved in motor control (see Groenewegen, 2003), learning including habit formation (Jog et al., 1999), skill learning (Poldrack et al., 1999), and reward-related learning (O’Doherty, 2004), as well as sensitivity to aversion/punishment (Jensen et al., 2003). The striatum, therefore, has been implicated in integrating information regarding cognition, motor control, and motivation (see Delgado, 2007).
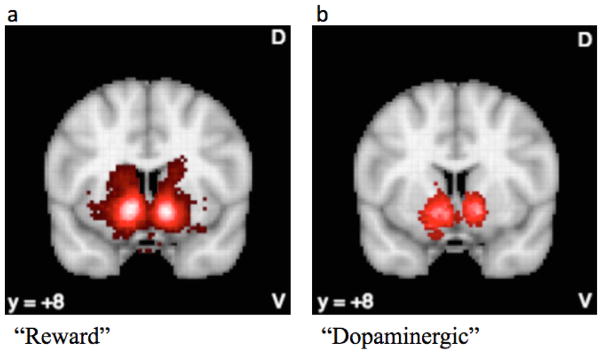
In order to make more confident claims that a given region (e.g., ventral striatum) is involved relatively selectively in a particular process (e.g., reward), I conducted a meta-analysis using Neurosynth, an open source platform for conducting large meta-analyses of fMRI data (http://www.neurosynth.org; Yarkoni et al., 2011). I quantified the forward inference or the probability that there would be activation in specific brain regions given the presence of a particular term P(activation|term), and the reverse inference or the probability that a term would occur in an article given the presence of activation in a particular brain region P(term|activation). I first performed a term-based search on “reward,” which included 671 studies (retrieved August 8, 2015) and generated a reverse inference mask (i.e., probability of the term “reward” given the observed activation). Depicted In Figure 2a is a “reward” reverse inference map (i.e., p[reward|activation]), which indicates the likelihood that studies reporting blood oxygen level-dependent (BOLD) activation in highlighted regions use the term “reward.” As shown in Figure 2a, this search term resulted in a cluster localized in the bilateral ventral striatum (xyz=12 8 −8, Z=26.57). Although activation extended into the VTA and vmPFC, the most robust clusters were in the VS. I conducted a similar search with the term “dopaminergic,” which included 162 studies (retrieved August 8, 2015). This also resulted in a reverse inference map localized in the ventral striatum (xyz=14 8 −8, Z=11.42; Figure 2b), again extending into the VTA. In addition, I examined the probability that the term “reward” would occur in the ventral striatum. To this end, I used the coordinates [12 8 −8; −12 8 −8]. Activation in these voxels are linked to “reward” with a posterior probability of 0.91 and 0.90, respectively, and to “dopaminergic” with a posterior probability of 0.83 and 0.85, respectively.
Figure 2.
Reverse inference maps in NeuroSynth with search terms (a) reward and (b) dopaminergic. Activation is localized in the ventral striatum. The maps are false discovery rate corrected at Z>2.5.
Evidence that Dopaminergic Sensitivity Can Promote Adolescent Health
Ventral striatum sensitivity to prosocial decisions predicts declines in risk-taking behavior
Engagement in prosocial behaviors activates high intensity, rewarding feelings that engage the dopaminergic reward system. For instance, in adults, providing financial support to charities engages the ventral striatum (Moll et al., 2006; Harbaugh et al., 2007). This is thought to indicate a “warm glow” effect, suggesting that it feels good to be prosocial (Moll et al., 2006). Indeed, we have shown that adolescents who report feeling happier on days when they support their family show greater ventral striatum activation when providing monetary support to their family during an fMRI scan (Telzer et al., 2010), supporting the notion that the ventral striatum codes for feelings of happiness linked to being prosocial. Ventral striatum activation to prosocial rewards may therefore represent an adaptive signal that may facilitate well-being. To test this, we followed adolescents over a one-year period to examine how ventral striatum activation during a positive, prosocial context predicted changes in health-risk behaviors. Adolescents completed a task during which they could make costly monetary donations to their family. We found that adolescents who showed heightened activation in the ventral striatum when making prosocial decisions (i.e., costly donations to their family) showed longitudinal declines in risk taking behaviors (e.g., stealing, drinking alcohol, using drugs, and skipping school) over the course of a year (Telzer et al., 2013). Moreover, adolescents who showed greater ventral striatum activation during prosocial family decisions showed less ventral striatum activation during a risk-taking task (Telzer et al., in press), suggesting that prosocial rewards may offset the rewarding nature of engaging in risky behavior. These findings highlight how ventral striatum sensitivity can be an asset for youth depending upon the context in which that activation occurs. The ventral striatum, which has been identified as a risk factor for adolescent risk taking, is also protective against this same behavior when that activation occurs within a meaningful, prosocial context.
Ventral striatum sensitivity to different types of rewards predicts both increases and decreases in depression over time
While optimal well-being may be achieved through meaningful and positive behaviors, adolescents often tend to orient towards more negative, hedonic activities (e.g., risk taking), potentially placing them at risk for ill-being (Chambers et al., 2003; Steinberg, 2008). We examined whether ventral striatum activation to eudaimonic rewards (e.g., prosocial decisions to the family) and hedonic rewards (e.g., risky decisions) differentially predict longitudinal changes in depressive symptoms. Adolescents completed a prosocial task in which they could choose to donate earnings to their family or keep monetary rewards for themselves (Telzer et al., 2010). They also completed a risk taking task (the BART; Lejuez et al., 2002), during which they could inflate a virtual balloon that gave them increasing monetary rewards with each pump but could explode at any point resulting in a loss of all earnings attained for that balloon. Results demonstrated that adolescents who showed heightened ventral striatum activation during prosocial decisions to their family experienced longitudinal declines in depressive symptoms over the course of the following year. In contrast, adolescents who showed heightened ventral striatum activation when keeping earnings for themselves on the prosocial task (i.e., selfish decisions) or who showed heightened ventral striatum activation during the risk taking task experienced longitudinal increases in depressive symptoms (Telzer et al., 2014). Thus, heightened striatal activation to prosocial behavior is an adaptive neural response, potentially serving as a neurobiological signal that codes for an orientation towards positive rewards (e.g., feeling a sense of meaning and purpose; feeling socially connected) and leading to declines in depressive symptomology. Heightened striatal response during selfish decisions (i.e., keeping money for oneself) or when being risky is a maladaptive neural response, potentially serving as a neurobiological signal that codes for an orientation towards more negative rewards (e.g., the thrill of being risky; heightened social threat) and resulting in increases in depressive symptomology. These findings highlight the importance of considering the context in which ventral striatum activation occurs.
Maternal presence redirects adolescent ventral striatum sensitivity away from risky behavior
Parents play an important scaffolding role, helping their children to make adaptive decisions and avoid risks. While parents may decrease adolescent risk-taking merely by serving as gatekeepers and limiting adolescents’ opportunities to make poor decisions, we tested whether maternal presence changes the ways in which adolescents process risks. During an fMRI scan, adolescents completed a simulated driving task during which they could choose to make risky or safe decisions. They played the task alone and in the presence of their mother. We found that adolescents made significantly more risky decisions when alone (55% of decisions) than when their mother was present (45% of decisions). At the neural level, the ventral striatum was significantly less activated during risky decisions and more active during safe decisions when adolescents’ mothers were present (Telzer et al., 2015). Importantly, we found that when adolescents made safe decisions, they showed significant functional coupling (i.e., cross-talk between neural regions) between the ventral striatum and the ventrolateral prefrontal cortex (VLPFC) when their mothers were present but did not when they were alone. In other words, making safe decisions in the presence of their mother may elicit a neural response that codes for reward value (i.e., safe decisions engaged the ventral striatum), and this reward response promotes activation of the prefrontal cortex, a key brain region involved in cognitive control, the braking of motor responses (Gray et al., 2002; Wessel et al., 2013), as well as rule representation and response selection (Souza et al., 2009; Snyder et al., 2011). Therefore striatal responses may facilitate cognitive control and deliberation about response selections, ultimately resulting in safe choices. Theories of adolescent risk-taking propose that heightened ventral striatum sensitivity largely underlies risk-taking during adolescence, and prior work has focused on the contexts in which striatal sensitivity leads to maladaptive, risky behavior. Importantly, the ventral striatum, which has been linked to greater risk-taking, for example when peers are present (Chein et al., 2011), can be redirected away from risky decisions (i.e., less activation during risk taking) and towards more deliberative and safe decisions when mothers are present.
Extrinsic and intrinsic rewards can promote improved cognitive persistence via heightened ventral striatum activation
Although adolescents tend to show detriments in cognitive performance, particularly in highly arousing or emotional states (e.g., Hare et al., 2008; Somerville et al., 2011), adolescents actually show improved performance (i.e., inhibit prepotent responses) when they are rewarded for doing so (Geier & Luna 2009, 2012; Geier et al., 2010; Padmanabhan et al., 2011). In a series of clever experiments, Geier and colleagues had participants complete a simple cognitive task (antisaccade task), and, on some trials, good inhibitory control yielded a monetary reward. Adolescents made fewer errors on rewarded versus neutral trials compared to adults (Padmanabhan et al., 2011), suggesting that adolescents are particularly sensitive to rewards, and that extrinsic motivation (i.e., getting money) may improve their cognitive control. Importantly, at the neural level, adolescents exhibited increased activation compared to children and adults in the ventral striatum on the rewarded trials (Padmanabhan et al., 2011), suggesting that extrinsic rewards and ventral striatum activation may act to improve behavioral regulation in adolescents.
In addition to examining extrinsic motivation (i.e., receiving money), researchers have sought to examine how intrinsic motivation may be associated with striatal activation and enhanced behavioral regulation. Satterthwaite and colleagues (2012) examined how the ventral striatum was activated during a working memory task with different levels of difficulty among participants ranging in age from 8 to 22 years. The authors defined intrinsic motivation as a heightened ventral striatal response when correctly completing the task. Satterthwaite and colleagues (2012) found robust ventral striatum activation that scaled with task difficulty. That is, when participants made correct versus incorrect responses, they showed heightened ventral striatum activation, striatum activation that was higher for more difficult trials. This striatal activation correlated with task performance, suggesting that ventral striatum activation during challenging tasks promotes more effective working memory. In addition, adolescence showed the highest ventral striatum activation to these intrinsic rewards. Importantly, these striatal responses were present despite the lack of explicit feedback or rewards, suggesting that ventral striatal responses in this context may reflect intrinsic reinforcement signals. These are among the first results to demonstrate adolescent peaks in intrinsic rather than explicit reinforcement responses.
Following on the work of Satterthwaite and colleagues (2012), we sought to examine whether there may be cultural differences in the neural correlates of intrinsic motivation. To this end, we recruited two samples of late adolescents who traditionally vary in terms of their self-reported intrinsic school motivation. East Asian adolescents tend to be more motivated in academics than their American counterparts (Pomerantz, Ng, & Wang, 2008) and score significantly higher on cognitive control, executive functioning, and behavioral inhibition compared to their American counterparts (Sabbagh et al., 2006; Lan et al., 2011). To test whether ventral striatum activation may support East Asian students’ motivation, we scanned American and Chinese students as they engaged in a basic cognitive control task (Go-Nogo task) that required persistence over time. Our findings again point towards the adaptive role of the ventral striatum. We found significant differences in behavioral performance across the cognitive control task, such that Chinese and American students did not differ in performance at the beginning of the task, suggesting similar levels of cognitive control. However, across time, Chinese students showed significant improvement in performance whereas American students showed significant declines in performance (Telzer & Qu, 2015). At the neural level, Chinese participants demonstrated increasing ventral striatum activation over the course of the task, whereas American participants’ ventral striatum activation remained low over the task. In addition, Chinese students showed increasing functional connectivity between the ventral striatum and the prefrontal cortex across time, connectivity that was not present in the American students. Importantly, this functional connectivity was associated with behavioral performance on the task, showing that greater VS-PFC connectivity facilitated improvements in cognitive engagement. Thus, the ventral striatum may represent intrinsic reinforcement signals that elicit the PFC to engage in more effective cognitive control. That the ventral striatum was functionally coupled with the PFC suggests that improvements in cognitive engagement among Chinese students may occur via a reinforcement response that boosts their cognitive control system, similar to our findings above on adolescent risk taking in the presence of mothers. Thus, striatal reactivity may be a neurobiological signal representing intrinsic motivation that serves an adaptive function, increasing Chinese students’ motivation to engage in cognitive control. Together with the work of Satterthwaite et al. (2012), these findings suggest that academic achievement may be improved when individuals find their work more rewarding.
Increased striatal reactivity during cognitive control predicts positive peer influence effects
In a clever manipulation, Falk and colleagues (Cascio et al., 2015) implemented a brain-as-predictor framework to examine how neural processes predict later behaviors in adolescents. During an fMRI scan, adolescents completed a basic cognitive control task (Go-Nogo task). One week following the scan, adolescents returned to the lab to undergo a simulated driving session during which they could make decisions to engage in safe and risky behavior. Adolescents completed the simulation in the presence of either a high- or low-risk peer. Behaviorally, adolescents made significantly fewer risky choices (i.e., drove through intersections with red lights) in the presence of low-risk peers compared to high-risk peers. At the neural level, adolescents who displayed relatively greater activation in the ventral striatum when engaging in cognitive control were more influenced by their cautious peers, such that greater ventral striatum activation was associated with engaging in fewer risks in the presence of cautious peers. Ventral striatum activation was not associated with being influenced by risky peers or to driving behavior when alone. These findings suggest that individual differences in adolescents’ recruitment of the ventral striatum during cognitive control are associated with buffering the effects of risk-taking in the presence of cautious peers. Importantly, heightened ventral striatum activation was associated with less risk taking behavior in the presence of prosocial but not risky peers, providing further evidence of the adaptive role of striatal reactivity. These effects are consistent with our findings above which found heightened ventral striatum activation during cognitive control among Chinese students (Telzer & Qu, 2015). In our study, ventral striatum activation during cognitive control served to increase cognitive performance, and Cascio et al (2015) found similar neural responses served to increase the positive influence of peers. While it is not clear whether the ventral striatum here is serving a reward response, the effects nonetheless indicate an adaptive role of the ventral striatum. Together, these findings suggest that individuals who recruit the ventral striatum more when engaging in cognitive control also engage in more adaptive behaviors in their everyday lives resulting in more effective cognitive control and less risk taking behavior. Such findings further emphasize understanding the situational context in which ventral striatum activation occurs.
Increased striatal reactivity can serve a regulatory role
Other work has shown that the ventral striatum may also be involved in emotion regulation. In a recent longitudinal study, Pfeifer and colleagues (2011) examined youth during the transition from childhood to adolescence. Participants were scanned twice to examine how neural response to emotional facial expressions changed over time. Longitudinal increases in ventral striatum reactivity were associated with longitudinal decreases in risky behavior as well as declines in susceptibility to peer influence. Pfeifer et al. (2011) also found that the ventral striatum was negatively functionally coupled with the amygdala when processing emotional faces, suggesting that the ventral striatum may serve to regulate and dampen heightened amygdala response to emotionally arousing stimuli. Others have also reported ventral striatum activation during cognitive reappraisal of negative emotions in adolescent samples (McRae et al., 2012), further highlighting a role of the striatum in emotion regulation. In corroboration of the regulatory role of the ventral striatum, Masten and colleagues (2009) found that heightened ventral striatum activation during social exclusion was associated with dampened self-reported emotional distress and less activation in brain regions involved in “social pain” processing, suggesting that the ventral striatum may be crucial for regulating negative affect during adolescence. Given its role in reward processing, the ventral striatum may serve to aid in the reappraisal of negative experiences into positive interpretations (Masten et al., 2009; Wager et al., 2009). Again, contrary to common interpretations that increased striatal reactivity during adolescence represents a risk factor, these findings highlight an adaptive role of the striatum and specifically implicate it in emotion regulation.
Summary
Together, these studies suggest that heightened ventral striatum activation can be adaptive by increasing cognitive persistence and emotion regulation, and decreasing risk taking behavior and depression. While reward sensitivity can be a marker of negative outcomes as current conceptualizations emphasize, the reviewed research here shows that ventral striatum activation can also serve a positive, adaptive role for teenagers. Although the majority of studies have reported deficits related to heightened striatal activation in adolescence (e.g., drug and alcohol use, depression and anxiety, and conduct and rule-breaking behaviors; Jager, et al., 2013; Telzer et al., 2014; Silk et al., 2104; Galvan et al., 2007; Bar-Haim et al., 2009; Guyer et al., 2006; Qu et al., in press), a burgeoning body of literature reviewed here suggests a more complex role of the ventral striatum. Across several different contexts, the ventral striatum is associated with positive behavioral outcomes. In particular, ventral striatum activation may serve an adaptive role in contexts that promote prosocial decision making (Telzer et al., 2013, 2014), cognitive control (Telzer & Qu, 2015; Cascio et al., 2015; Padmanabhan et al., 2011), working memory (Satterthwaite et al., 2012), emotion regulation (Pfeifer et al., 2011; Master et al., 2009), or which include positive instead of negative social influences (Telzer et al., 2015; Cascio et al., 2015). Based on the specific measurements in the reviewed studies, it is not evident per se whether the ventral striatal activations reported are representing a reward response or another psychological process. Because, the striatum may be involved in behaviors beyond reward processing, such as learning, some of the individual differences in striatal responses to prosocial versus risky contexts may represent individual differences in learning and adaptation. Indeed, an important aspect of adolescence is that it is a time when many new behaviors and contexts are experienced and new behavior patterns are acquired (Dahl. 2008). Thus, future research should disentangle the psychological significance of the ventral striatum across these diverse contexts.
Can we take Advantage of Adolescent Ventral Striatum Sensitivity in Ways that Promote Adaptive Decision Making?
Ventral striatal reactivity in adolescence appears to be universal, as seen across species, contexts, and cultures. Such peaks in dopaminergic activation in adolescence can be channeled into a range of behaviors. On the one hand, if directed towards problematic activities, such as drug experimentation, risky sexual behavior, and engagement with deviant peers, this heightened ventral striatum reactivity is indeed a vulnerability. On the other hand, if directed towards meaningful activities, such as prosocial behaviors or hobbies, positive social relationships, or motivated engagement in school, this heightened ventral striatum reactivity may be a source of protection and reduce susceptibility to compromised health. Therefore, the ways in which adolescents approach and respond to rewards may have significant implications for their well being. Importantly, each of studies reviewed here examined individual differences. That is, not all adolescents showed heightened striatal activation, and not all striatal activation in these contexts was adaptive. Adolescents who showed the greatest ventral striatum activation when being prosocial showed the greatest declines in risk taking and depression (Telzer et al., 2013, 2014); adolescents who showed the greatest ventral striatum activation during cognitive control showed the greatest improvements in cognitive persistence (Telzer & Qu, 2015) and were the most influenced by their prosocial peers to engage in less risk taking (Cascio et al., 2015); adolescents who demonstrated the greatest ventral striatum activation during a difficult working memory task had the highest performance (Satterthwaite et al., 2012); and adolescents who showed the greatest increases in ventral striatum activation when processing emotions showed the greatest declines in risk taking (Pfeifer et al., 2011). These individual differences highlight the need to identify characteristics of adolescents who show these more adaptive ventral striatum responses. Pushing adolescents to engage in prosocial behavior will not be effective unless adolescents value engaging in this behavior. Therefore identifying the particular behaviors and passions that adolescents most value and helping to direct them to those activities may have the most beneficial and lasting outcomes.
This view of ventral striatum sensitivity as being adaptive and directing adolescents away from health-compromising behaviors has significant implications for the development of effective treatment and intervention efforts to prevent upward trajectories of depression, risk-taking behavior, and subsequent morbidity and mortality rates among adolescent populations. Although adolescents may tend to orient towards more maladaptive behaviors (Steinberg, 2005), identifying ways to take advantage of adolescents’ heightened ventral striatum sensitivity in ways that promote their health should be a key aim of ongoing research efforts. Teachers, parents, and clinicians should see it as a central goal to try to tip the balance in teens favor – that is, to direct their heightened ventral striatum sensitivity away from risk and towards opportunity. If we can find more ways to direct adolescents towards the positive aspects of their heightened ventral striatum reactivity, strengthening the pathways by which this system serves as an opportunity, and decreasing the availability or desire for the negative aspects of heightened ventral striatum reactivity, we may reduce mortality and morbidity rates in adolescence.
The Complexity of Ventral Striatum Reactivity in Adolescence
Functional heterogeneity supporting different psychological processing
While the prevailing view suggests that peaks in reward seeking behaviors largely serve as a vulnerability, orienting adolescence towards negative behaviors, the psychological significance of ventral striatum activation varies across contexts. Thus, a more nuanced understanding of the ventral striatum is necessary. While in some contexts, ventral striatum reactivity may be signaling maladaptive reward seeking, in other contexts the striatum may be a neurobiological signal for approach-related behaviors that are highly adaptive and not related to risk taking, such as an orientation towards motivationally positive behaviors (e.g., striving for academic success, working towards a goal). Therefore, the meaning and psychological significance of ventral striatum activation depends on the context and situation in which it occurs and likely varies across individuals.
In addition, ventral striatum activation may have different psychological significance depending on which regions it is coupled with. That is, during different psychological experiences, the ventral striatum may be co-active with different regions. Thus, perhaps which brain regions the VS talks to can disentangle the psychological process occurring. While most prior research focuses on whether the ventral striatum is active or not, or whether the ventral striatum correlates with individual differences in behaviors, it is essential to unpack whether the ventral striatum is differentially coupled with brain regions as a function of context. Indeed, distinct behaviors occur through integration of the ventral striatum with the prefrontal cortex (PFC) via overlapping, but functionally segregated pathways (Alexander et al., 1986; Di Martino et al., 2008; Postuma & Dagher, 2006). For instance, intricate patterns of overlap and segregation exist between afferents from different cortical and subcortical sources. The ventral striatum receives afferents from neural regions traditionally implicated in affective processes, including orbitofrontal cortex (OFC), dorsal anterior cingulate cortex, ventromedial PFC, amygdala, and hippocampus (e.g., Haber & Knutson, 2010; Pennartz et al., 2011; see Delgado, 2007). Therefore, understanding how the ventral striatum communicates with other neural regions may inform our understanding of how the ventral striatum can serve both as a liability but also as an opportunity. Some findings reported in the current review support this idea. For instance, when making risky choices alone (Telzer et al., 2015) or in the presence of peers (Chein et al., 2010), the ventral striatum is significantly active. However, when making safe choices in the presence of mother, the ventral striatum appears to function via communication with the VLPFC, connectivity that is not present when making safe choices alone (Telzer et al., 2015). Therefore, examining the regions that come online with the ventral striatum across different contexts can help us to understand the function and specificity of ventral striatum activation. Perhaps ventral striatum reactivity is adaptive when it occurs in tandem with VLPFC activation but may be maladaptive when it occurs in tandem with limbic activation such as the amygdala. Future research should focus on examining functional connectivity.
Structural heterogeneity supporting different psychological processing
The heterogeneity of cell types within a given reward structure is another likely explanation for the different effects observed in the ventral striatum. Indeed, the ventral striatum may be divided into subregions which each engage in different psychological processes (structural heterogeneity). Based on anatomical, physiological, immunohistochemical and pharmacological studies with rats, primates, and humans (e.g., Cardinal et al. 2002; Koya et al. 2009; Pennartz et al. 1994; Voorne et al., 1989), the VS does not behave as a monolithic structure and may therefore consist of spatially distinct ensembles of neurons with specific functional roles (Kalenscher et al., 2010). Indeed, the striatum encompasses subregions of tissue that have distinct chemical compositions and connections, and the ventral striatum in particular has more differentiation and complexity of neurochemical systems than other regions such as the dorsal striatum (Holt et al., 1997). Within the ventral striatum, the nucleus accumbens has been divided into three subregions including the shell, core, and rostral pole territories (Zaborszky et al., 1985; Meredith et al., 1993). Additional differences within these three subregions exist, including the densities of chemical markers and the distribution of connections (Holt et al., 1997). Moreover, the subregion of the striatum closely related to the limbic system occupies a region that extends beyond the boundaries of what is traditionally considered the nucleus accumbens (Eblen & Graybiel, 1996).
Because the ventral striatum is involved in diverse psychological, cognitive, and motor processes, researchers have proposed that the ventral striatum is composed of functionally distinct ensembles of neurons (Pennartz et al. 1994). An ensemble is defined as a group of neurons characterized by similar afferent/efferent relationships as well as closely related functions in overt behavior, neuroendocrine regulation, and sensorimotor fating (Pennartz et al., 1994). Pennartz and colleagues propose that “the nuccleus accumbens, in its entirety, does not send a monolithic output to its target structures, which would then effectuate a unidirectional change in some behavioral parameter.” Instead “each distinct ensemble is capable of generating output which is then transferred to a specific set of target structures characteristic for this ensemble, and hence may induce behavioral effects that are specifically linked to this ensample” (Pennaetz et al., 1994, pp. 726).
Evidence of structural differentiation of the ventral striatum comes from psychopharmacological research. For example, microinjection of the GABAA agonist muscimol in the rostral medial accumbens shell in rats elicits appetitive behavior, whereas microinjection in the caudal shell instead elicits fearful behavior. Intermediate shell GABAergic activation produces combined positive and negative motivational effects (Reynolds & Berridge, 2002). These results indicate that GABAergic neurotransmission in local microcircuits of the nucleus accumbens mediates motivated and affective behavior that is bivalently organized along rostrocaudal gradients. This bivalent division helps to elucidate how the ventral striatum can participate in both appetitive and aversive motivational functions. In addition, TRKB, a tyrosine kinase receptor, has been found to differentially effect reward-related behaviors. For example, optogenetic stimulation of D1-type medium spiny neurons in rodents enhances reward driven behaviors, whereas stimulations of D2-type medium spiny neurons results in freezing behavior (Lobo et al., 2010).
However, a difference in cell types is not the only explanation for the heterogeneity seen in behavioral outputs. Molecular changes within specific cell types of the brain’s reward regions shape the ways in which adolescents respond to changes in the environment, determining either resilience or susceptibility (Russo & Nestler, 2013). Thus, stressors in ones environment shape molecular mechanisms in the VTA-nucleus accumbens circuit, and can impact whether stimuli are experienced adaptively or maladaptively. Vulnerability for depression, risk taking, or other psychopathologies may be determined by molecular changes in cells that occur following stressors (see Russo & Nestler, 2013). It will therefore be necessary for researchers to utilize genome-wide assays to map the genetic loci in the NAc that are influenced by stressors.
Although significant research in animal models highlights the structural heterogeneity of the nucleus accumbens, translating these models to human research will be more challenging. Unfortunately, fMRI techniques, the most widely used method for examining adolescent brain function and related psychological behaviors, do not allow for the specific differentiation of precise boundaries and heterogeneities in the human striatum. Moreover, the BOLD signal does not provide direct information about neurochemistry, and it remains unclear how BOLD activation relates to striatal dopamine release (Schott et al., 2008). Some researchers have combined fMRI with PET, which allows for the direct measure of dopamine release. Importantly, high reward-related dopamine release is correlated with increased activation in the ventral striatum, providing evidence that dopaminergic neurotransmission plays a key role in ventral striatum activation measured with fMRI (Schott et al., 2008). Finally, most neuroimaging research uses large smoothing kernels that make it nearly impossible to subdivide the ventral striatum into its subparts. Thus it is often unclear if the activation is centralized in the nucleus accumbens or ventral head of the caudate nucleus (Delgado, 2007), or even further subdivisions of the striatum. Despite attempts to improve the spatial resolution of the BOLD signal, particularly in regions like the ventral striatum and VTA (D’Ardenne et al., 2008), we need more direct measures of activity dependent-dopamine release (Schott et al., 2008). Future research and more advanced scientific methods are needed that may reveal more fine-grained subdivisions corresponding to functionally distinct areas of the ventral striatum.
Future Directions
While research has begun to disentangle the complex role of rewards in adolescents’ lives, several contributions are essential to significantly move the field forward and lay the foundation for developing testable hypotheses about the psychological mechanisms that will produce the greatest health impact. Future research should therefore investigate how differing social and motivational contexts influence the neurobiology of adolescent reward sensitivity and subsequent health outcomes. The time is ripe to address these goals in light of evidence indicating rapid brain growth and heightened susceptibility to environmental input during adolescence, coupled with dramatic changes in social behavior that underlie an increased orientation towards rewards as well as steep increases in psychopathology. Thus, future research is needed that continues to disentangle the contexts in which ventral striatum sensitivity is adaptive versus maladaptive. Moreover, given that the body of work highlighting potential adaptive functions of ventral striatum reactivity in adolescence is nascent, research that replicates findings in the studies reviewed here is necessary. Below I outline several other areas of inquiry for future research.
Develop novel and innovative tasks that tap diverse contexts and allows comparison of reward sensitivity across situations
Prior research has largely examined adolescent decision making and reward sensitivity within a social vacuum, although studies are emerging that examine adolescent decision making in a social context (e.g., Peake et al., 2013; Braams et al., 2014; Chein et al., 2010). Given that adolescence is a period marked by increasingly complex social development, and adolescent decision making most often occurs during socioemotional arousal (Dahl, 2008; Gardner & Steinberg, 2005), research needs to incorporate the social and motivational context into experimental tasks in order to tap the complexity of adolescent behavior. If researchers continue to rely on tasks that tap largely negative behaviors (e.g., risk taking) or reward sensitivity in the absence of social processes, ventral striatum sensitivity will appear to be ubiquitously negative. However, if experimental tasks incorporate more complex social processes (e.g., presence of others, prosocial versus antisocial decisions), ventral striatum sensitivity is likely to show unique patterns, sometimes representing a liability but at other times being adaptive. It is essential that this complexity be incorporated into our understanding of adolescent brain development and behavior in order to fully understand the role of ventral striatum reactivity in adolescent decision making and health behaviors.
Focus on individual and cultural differences to identify youth most at risk
Not all striatal activation is good or bad, and it depends on the context and the individual. By identifying individual and cultural group differences in youth for whom rewards serve different functions, we can better tailor programs to direct adolescents towards the behaviors that are most meaningful within appropriate sociocultural contexts. Rewards will take on different meanings for groups who value certain behaviors. For example, we recently examined activation in the mesolimbic reward system among White and Latino youth as they engaged in a task involving personal sacrifices for their family (Telzer et al., 2010). Whereas Latino participants showed more ventral striatum activation when contributing to their family, White participants showed more ventral striatum activation when gaining personal rewards for themselves. These results suggest that decisions to help one’s family may be guided, in part, by the personal rewards one attains from that assistance, and this sense of reward may be modulated by cultural influences. Thus, if intervention efforts rely on findings from a specific cultural group, interventions may not be successful with a different group and may actually have iatrogenic effects. That is, interventions that focus on increasing the participation of behaviors that are valued only by certain adolescents may have negative implications for adolescent health. Future research therefore should carefully unpack how the dopamine reward system functions across diverse groups of adolescents.
Use neural sensitivity to predict changes in real-life health outcomes and behaviors
Research has begun to treat the brain as a predictor variable of future health behaviors. This approach offers unprecedented opportunity to examine how adolescents’ neural sensitivity predicts engagement in real-life health behaviors. Although self-reported intentions predict some variability in future health behavior, evidence suggests that self-reports are not sufficient to capture the multidimensional nature of risk taking (Aklin et al., 2005). Perhaps this is because individuals lack the insight or cognitive ability to provide an accurate report of their own intentions or because they may be untruthful in their self-reports (Aklin et al., 2005). Thus, implicit processes may explain variability in behavior change that is not explained by self-reported measures such as attitudes and intentions. Recent advances in neuroimaging have begun to use neural activation to predict behavior either concurrently or in the future. Importantly, this work has found that neural activation can predict what kind of changes (increases or decreases) in risk taking behaviors and depressive symptoms will be observed one month to over one year (e.g., Cascio et al., 2015; Telzer et al., 2013; Telzer et al., 2014). The ability to prospectively predict future engagement in health behaviors based on adolescents’ neural sensitivity can have profound effects on our ability to develop individualized prevention programs. Thus, a key goal of future research should be to examine how ventral striatum reactivity across different contexts (i.e., reward sensitivity in positive and maladaptive contexts) predicts health-related behavioral changes.
Conclusions
In this review, I have shown that peaks in ventral striatum sensitivity can be adaptive for adolescent functioning and can facilitate improved well-being and health-promoting behaviors. This mechanism of reward sensitivity in adolescence challenges the traditional view that dopaminergic reward sensitivity largely leads to health-compromising behaviors during adolescence. Thus, I have identified a potential neurobiological mechanism by which to decrease the upward trajectories of both depression and risk taking behaviors during a developmental time when these symptoms are usually rising. Modifiable health behaviors are the leading cause of morbidity and mortality among adolescents. Understanding the factors that direct adolescents’ heightened ventral striatal sensitivity away from problematic behaviors and towards more positive, prosocial behaviors will have immense impact across a range of health behaviors and health outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aklin WM, Lejuez CW, Zvolensky MJ, Kahler CW, Gwadz M. Evaluation of behavioral measures of risk taking propensity with innercity adolescents. Behavior Research and Therapy. 2005;43:215–228. doi: 10.1016/j.brat.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, et al. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuro Report. 1997;8:1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, et al. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. European Journal of Pharmacology. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, Ernst M. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychological Science. 2009;20(8):1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, et al. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: Implications for the development of psychopathology. Cerebral Cortex. 2000;10(10):1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Braams BR, Peters S, Peper JS, Güroğlu B, Crone EA. Gambling for self, friends, and antagonists: differential contributions of affective and social brain regions on adolescent reward processing. Neuroimage. 2014;100:281–289. doi: 10.1016/j.neuroimage.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, et al. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: Relationship to enhancedmotivational salience of drug cues in adolescence. Journal of Neuroscience. 2008;28(10):2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. The Journal of Neuroscience. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PloS one. 2010;5(7):e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caouette JD, Guyer AE. Gaining insight into adolescent vulnerability for social anxiety from developmental cognitive neuroscience. Developmental Cognitive Neuroscience. 2014;8:65–76. doi: 10.1016/j.dcn.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience & Biobehavioral Reviews. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124(1):111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CN, Carp J, O’Donnell MB, Tinney FJ, Jr, Bingham CR, Shope JT, Falk EB. Buffering social influence: neural correlates of response inhibition predict driving safety in the presence of a peer. Journal of Cognitive Neuroscience. 2014;27:83–95. doi: 10.1162/jocn_a_00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauffman E, Shulman EP, Steinberg L, Claus E, Banich MT, Graham S, Woolard J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Developmental Psychology. 2010;46(1):193. doi: 10.1037/a0016128. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National vital statistics reports. 2. Vol. 64. Hyattsville, MD: 2014. Available from: http://www.cdc.gov/nchs/data/hus/2014/021.pdf. [Google Scholar]
- Centers for Disease Control and Prevention. Youth Risk Behavior Survey. 2013 Available at: www.cdc.gov/yrbs.
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. American Journal of Psychiatry. 2014;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2010;14(2):F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319(5867):1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Biological, developmental, and neurobehavioral factors relevant to adolescent driving risks. American Journal of Preventive Medicine. 2008;35(3):S278–S284. doi: 10.1016/j.amepre.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Annals of the New York Academy of Sciences. 2007;1104(1):70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, et al. Functional connectivity of human striatum: A resting state fMRI study. Cerebral Cortex. 2008;18(12):2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiology & Behavior. 2003;80(2):317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Developmental Psychobiology. 2004;45(3):153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Eblen F, Graybiel AM. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. Journal of Neuroscience. 1996;15:5999–6013. doi: 10.1523/JNEUROSCI.15-09-05999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Friemel CM, Spanagel R, Schneider M. Reward sensitivity for a palatable food reward peaks during pubertal developmental in rats. Frontiers in Behavioral Neuroscience. 2010;4:1–10. doi: 10.3389/fnbeh.2010.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuligni AJ, Telzer EH. Another way the family can get in the head and under the skin: The neurobiology of family assistance. Perspectives in Child Development. 2013;7:138–142. [Google Scholar]
- Galván A. The Neurobiology of Childhood. Springer; Berlin Heidelberg: 2014. Neural systems underlying reward and approach behaviors in childhood and adolescence; pp. 167–188. [DOI] [PubMed] [Google Scholar]
- Galván A, McGlennen K. Enhanced striatal sensitivity to aversive reinforcement in adolescents versus adults. Journal of Cognitive Neuroscience. 2013;25:284–296. doi: 10.1162/jocn_a_00326. [DOI] [PubMed] [Google Scholar]
- Galván A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Developmental Science. 2007;10(2):F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology. 2005;41(4):625. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Geier C, Luna B. The maturation of incentive processing and cognitive control. Pharmacology Biochemistry and Behavior. 2009;93(3):212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Luna B. Developmental Differences in the Effects of Incentives on Response Inhibition. Child Development. 2012;83(4):1262–1274. doi: 10.1111/j.1467-8624.2012.01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in Reward Processing and its Influence on Inhibitory Control in Adolescence. Cerebral Cortex. 2010;20(7):1613–29. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ. The basal ganglia and motor control. Neural Plasticity. 2003;10:107–120. doi: 10.1155/NP.2003.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80(4):1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Pérez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, Bjork JM, Henderson HA, Pine DS, Fox NA, Ernst M. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26:6399–405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316(5831):1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. Journal of Neurochemistry. 2003;87(3):574–585. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Graybiel AM, Saper CB. Neurochemical architecture of the human striatum. Journal of Comparative Neurology. 1997;384(1):1–25. doi: 10.1002/(sici)1096-9861(19970721)384:1<1::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Tentative evidence for striatal hyperactivity in adolescent cannabis-using boys: a cross-sectional multicenter fMRI study. Journal of Psychoactive Drugs. 2013;45(2):156–167. doi: 10.1080/02791072.2013.785837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40(6):1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Kalenscher T, Lansink CS, Lankelma JV, Pennartz CM. Reward-associated gamma oscillations in ventral striatum are regionally differentiated and modulate local firing activity. Journal of Neurophysiology. 2010;103(3):1658–1672. doi: 10.1152/jn.00432.2009. [DOI] [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Hope BT. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nature Neuroscience. 2009;12(8):1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. The Journal of Neuroscience. 2000;20(23):8780–8787. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan X, Legare CH, Ponitz CC, Li S, Morrison FJ. Investigating the links between the subcomponents of executive function and academic achievement: A cross-cultural analysis of Chinese and American preschoolers. Journal of Experimental Child Psychology. 2011;108(3):677–692. doi: 10.1016/j.jecp.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8(2):75. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Nestler EJ. Cell type–specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4(2):143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M, Bondi C, Torres G, Moghaddam B. Reduced presynaptic dopamine activity in adolescent dorsal striatum. Neuropsychopharmacology. 2013;38(7):1344–1351. doi: 10.1038/npp.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Ochsner KN. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Pennartz CMA, Groenewegen HJ. The cellular fromework for chemical signalling in the nucleus accumbens. Progress in Brain Research. 1993;99:3–24. doi: 10.1016/s0079-6123(08)61335-7. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto–mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences. 2006;103(42):15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure E, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(02):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nicholls JG. Achievement motivation: Conceptions of ability, subjective experience, task choice, and performance. Psychological Review. 1984;91(3):328. [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A, Luna B. Developmental imaging genetics: linking dopamine function to adolescent behavior. Brain and Cognition. 2014;89:27–38. doi: 10.1016/j.bandc.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Geier CF, Ordaz SJ, Teslovich T, Luna B. Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Developmental Cognitive Neuroscience. 2011;1(4):517–529. doi: 10.1016/j.dcn.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake SJ, Dishion TJ, Stormshak EA, Moore WE, Pfeifer JH. Risk-taking and social exclusion in adolescence: Neural mechanisms underlying peer influences on decision-making. Neuro Image. 2013;82:23–34. doi: 10.1016/j.neuroimage.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CMA, Ito R, Verschure PFMJ, Battaglia FP, Robbins TW. The hippocampal–striatal axis in learning, prediction and goal-directed behavior. Trends in Neurosciences. 2011;34(10):548–559. doi: 10.1016/j.tins.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, da Silva FHL. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Progress in Neurobiology. 1994;42(6):719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in Cognitive Sciences. 2012;16(6):322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69(5):1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot RM, Wecker L, Kirstein CL. Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. International Journal of Developmental Neuroscience. 2009;27(8):805–815. doi: 10.1016/j.ijdevneu.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Prabhakaran V, Seger CA, Gabrieli JD. Striatal activation during acquisition of a cognitive skill. Neuropsychology. 1999;13(4):564. doi: 10.1037//0894-4105.13.4.564. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron. 2011;72(5):692–697. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz EM, Ng FF, Wang Q. Culture, parenting, and motivation: The case of East Asia and the United States. Advances in Motivation and Achievement: Social Psychological Perspectives. 2008;15:209–240. [Google Scholar]
- Post G, Kemper H. Nutritional intake and biological maturation during adolescence: the Amsterdam growth and health longitudinal study. European Journal of Clinical Nutrition. 1993;47:400–408. [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cerebral Cortex. 2006;16(10):1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Qu Y, Galvan A, Fuligni AJ, Lieberman MD, Telzer EH. Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. The Journal of Neuroscience. 2015;35(32):11308–11314. doi: 10.1523/JNEUROSCI.1553-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/”disliking” reactions, place preference/avoidance, and fear. The Journal of Neuroscience. 2002;22(16):7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Duckworth AL, Sznitman S, Park S. Can adolescents learn self-control? Delay of gratification in the development of control over risk taking. Prevention Science. 2010;11(3):319–330. doi: 10.1007/s11121-010-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: A tyrosine hydroxylase immunohistochemical study. Biological Psychiatry. 1994;36(4):272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature Reviews Neuroscience. 2013;14(9):609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh MA, Xu F, Carlson SM, Moses LJ, Lee K. The development of executive functioning and theory of mind a comparison of Chinese and US preschoolers. Psychological Science. 2006;17(1):74–81. doi: 10.1111/j.1467-9280.2005.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Ruparel K, Loughead J, Elliott MA, Gerraty RT, Calkins ME, Wolf DH. Being right is its own reward: Load and performance related ventral striatum activation to correct responses during a working memory task in youth. Neuroimage. 2012;61(3):723–729. doi: 10.1016/j.neuroimage.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Miedl SF, Banaschewski T, IMAGEN Consortium. Risk taking and the adolescent reward system: a potential common link to substance abuse. American Journal of Psychiatry. 2012;169:39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. The Journal of Neuroscience. 2008;28(52):14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE. Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive and Affective Neuroscience. 2013;9:1798–1807. doi: 10.1093/scan/nst175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Banich MT, Munakata Y. Choosing our words: Retrieval and selection processes recruit shared neural substrates in left ventrolateral prefrontal cortex. Journal of Cognitive Neuroscience. 2011;23(11):3470–3482. doi: 10.1162/jocn_a_00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza MJ, Donohue SE, Bunge SA. Controlled retrieval and selection of action-relevant knowledge mediated by partially overlapping regions in left ventrolateral prefrontal cortex. Neuroimage. 2009;46(1):299–307. doi: 10.1016/j.neuroimage.2009.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Authoritative Communities. Springer; New York: 2008. The psychobiology of adolescence; pp. 263–280. [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011;1(4):390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Development. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B. Striatum processes reward differently in adolescents versus adults. Proceedings of the National Academy of Sciences. 2012;109(5):1719–1724. doi: 10.1073/pnas.1114137109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Developmental Neuroscience. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Developmental Brain Research. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Gálvan A. Identifying a cultural resource: Neural correlates of familial influence on risk taking among Mexican-origin adolescents. In: Chiao JY, Li S-C, Seligman R, Turner R, editors. The Oxford Handbook of Cultural Neuroscience. Oxford University Press; New York, NY: in press. [Google Scholar]
- Telzer EH, Masten CL, Berkman ET, Lieberman MD, Fuligni AJ. Gaining while giving: An fMRI study of the rewards of family assistance among White and Latino youth. Social Neuroscience. 2010;5:508–518. doi: 10.1080/17470911003687913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Gálvan A. Neural sensitivity to eudaimonic and hedonic rewards differentially predict adolescent depressive symptoms over time. Proceedings of the National Academy of Sciences. 2014;111:6600–6605. doi: 10.1073/pnas.1323014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galvan A. Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Developmental Cognitive Neuroscience. 2013;3:45–52. doi: 10.1016/j.dcn.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Masten CL, Berkman ET, Lieberman MD, Fuligni AJ. Neural regions involved in self-control and mentalizing are recruited during prosocial decisions towards the family. Neuro Image. 2011;58:242–249. doi: 10.1016/j.neuroimage.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Masten CL, Berkman ET, Lieberman MD, Fuligni AJ. Gaining while giving: An fMRI study of the rewards of family assistance among White and Latino youth. Social Neuroscience. 2010;5:508–518. doi: 10.1080/17470911003687913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Ichien NI, Qu Y. Mothers know best: Redirecting adolescent reward sensitivity to promote safe behavior during risk taking. Social Cognitive Affective Neuroscience. 2015 doi: 10.1093/scan/nsv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Qu Y. Neural processes underlying cultural differences in cognitive engagement. doi: 10.1016/j.neuroimage.2017.05.034. under review. [DOI] [PubMed] [Google Scholar]
- Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. The Lancet. 2012;379(9820):1056–1067. doi: 10.1016/S0140-6736(11)60871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya JG, Grippo AJ, Johnson AK, Watson D. A comparative developmental study of impulsivity in rats and humans: the role of reward sensitivity. Annals of the New York Academy of Sciences. 2004;1021:395–398. doi: 10.1196/annals.1308.051. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Moor BG, de Macks ZAO, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51(1):345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Voorn P, Gerfen CR, Groenewegen HJ. Compartmental organization of the ventral striatum of the rat: Immunohistochemical distribution of enkephalin, substance P, dopamine, and calcium-binding protein. Journal of Comparative Neurology. 1989;289(2):189–201. doi: 10.1002/cne.902890202. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2009;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain and Cognition. 2010;72(1):146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience & Biobehavioral Reviews. 2010b;34(5):631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Conner CR, Aron AR, Tandon N. Chronometric electrical stimulation of right inferior frontal cortex increases motor braking. Journal of Neuroscience. 2013;33(50):19611–19619. doi: 10.1523/JNEUROSCI.3468-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Hedonic sensitivity in adolescent and adult rats: taste reactivity and voluntary sucrose consumption. Pharmacology Biochemistry and Behavior. 2009;92(4):566–573. doi: 10.1016/j.pbb.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Alheid GF, Beinfeld MC, Eiden LE, Heimer L, Palkovits M. Cholecystokinin innervation of the ventral striatum: A morphological and radioimmunological study. Neuroscience. 1985;14:427–453. doi: 10.1016/0306-4522(85)90302-1. [DOI] [PubMed] [Google Scholar]