Abstract
Background & Aims
Intestinal epithelial stem cells that express Lgr5 and/or Bmi1 continuously replicate and generate differentiated cells throughout life1. Previously, Paneth cells were suggested to constitute an epithelium-intrinsic niche that regulates the behavior of these stem cells2. However, ablating Paneth cells has no effect on maintenance of functional stem cells3-5. Here, we demonstrate definitively that a small subset of mesenchymal, subepithelial cells expressing the winged-helix transcription factor Foxl1 are a critical component of the intestinal stem cell niche.
Methods
We genetically ablated Foxl1+ mesenchymal cells in adult mice using two separate models by expressing either the human or simian diphtheria toxin receptor (DTR) under Foxl1 promoter control.
Conclusions
Killing Foxl1+ cells by diphtheria toxin administration led to an abrupt cessation of proliferation of both epithelial stem- and transit-amplifying progenitor-cell populations that was associated with a loss of active Wnt signaling to the intestinal epithelium. Therefore, Foxl1-expressing mesenchymal cells constitute the fundamental niche for intestinal stem cells.
Keywords: Intestinal stem cell niche, Wnt, mesenchyme
INTRODUCTION
Adult multipotent stem cells replenish the gut epithelium both during homeostasis and following injury throughout life. The mammalian intestinal epithelium undergoes complete regeneration every three to five days; this renewal is supported by multipotent intestinal stem cells (ISCs) 1, 6-10. Epithelial stem cell populations reside in two zones: at the crypt-base and at the “+4” position, 4-6 cell widths above the crypt-base. Cells in both zones express markers associated with stem-cell behavior, such as CD24 and Lrig18, 9. There are, in addition, zone-specific markers. For the small intestine, the crypt-base cells are marked by Lgr51 and Olfm411, and the “+4” position cells express Bmi-1, HopX, and mTert6, 7, 12. These epithelial stem cells divide to produce progenitor (or transit-amplifying) cells that proliferate rapidly and then differentiate into Paneth cells, which are located at the crypt base, or into goblet cells, enteroendocrine cells, M-cells, and absorptive enterocytes that are located closer to the gut lumen.
Stem cell niches are defined as local microenvironments that provide physical support and/or molecular signals necessary for proper stem- and progenitor-cell replication and differentiation. Wnt signaling has been established as the major driver of intestinal stem- and progenitor-cell proliferation, as evidenced for instance by a rapid loss of proliferation when the secreted Wnt inhibitor Dickkopf-1 is over-expressed in the epithelium13, 14. However, the cellular identity of the intestinal stem-cell niche has remained controversial. Clevers and colleagues concluded that epithelial Paneth cells constitute the niche for Lgr5+ stem cells in intestinal crypts, based on the fact that Paneth cells elaborate important signaling molecules such as Wnt3 and EGF and on the observation that in vitro organoid formation by Lgr5+ stem cells was enhanced by co-culture with a Paneth cell-enriched population2. However, complete and permanent absence of Paneth cells in mice deficient for the transcription factor Math1 (Atoh1) has no impact on intestinal stem cell maintenance and proliferation3, 5. The latter studies support earlier work by Gordon and colleagues who employed two independent methods to ablate mature Paneth cells and concluded that “stemness in the crypt is not defined by instructive interactions involving the Paneth cells”4. Moreover, epithelial-specific deletion of Wnt3 had no effect on intestinal stem cells in mice, suggesting the presence of other Wnt source(s)15. These findings point to the existence of an extraepithelial source of Wnt and other signaling molecules necessary to maintain epithelial homeostasis.
METHODS
Derivation of Foxl1-hDTR mice
All protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. The human diphtheria toxin receptor (hDTR) coding sequence (NM_001945.1) was introduced into the coding region of the mouse Foxl1 gene in the bacterial artificial chromosome (BAC) RP23-446J14 by means of BAC recombineering as described previously 16. The targeting primers were as follows:
Forward: GGGGCAAAGTCCTTAGGACTCCCCGGTGGAGCGGAGAGGCTGCTGTCGCCGAATTCGGCACGAGGGCTACGCGGG
Reverse: AGGCCCCTCAGTGCACGACTTTGGCCGGCACGGGTACGCTGCTCCAAACCAGCTCCACCGCGGTGGCGGCCGCTC.
The resulting Foxl1-hDTR BAC was linearized and microinjected into the pronucleus of C57Bl/6 mice. The positive transgenic founders were identified by genomic PCR and crossed to C57Bl/6 mice for at least 5 generations. Animals were euthanized at 2-6 months of age for subsequent experiments.
Generation of Foxl1-Cre; Rosa-iDTR/YFP, Foxl1-Cre;Rosa-YFP, and Foxl1-Cre;Rosa-mT/mG mice
Foxl1-Cre mice were generated and characterized previously16. Foxl1-Cre mice were crossed to Rosa-iDTR/YFP mice (Jackson Laboratories) in order to obtain Foxl1-Cre; Rosa-iDTR/YFP mice. Foxl1-Cre;Rosa-mT/mG mice, Foxl1-Cre mice resulted from crossing to Rosa-mT/mG mice (Jackson Laboratories). Animals were sacrificed at 2-6 months of age for subsequent experiments.
Diphtheria toxin treatment
For Foxl1-hDTR mice, diphtheria toxin (Sigma-Aldrich) dissolved in 0.9% sodium chloride was administered intraperitoneally at 20 ng/g body weight. Mice were injected on day 0 and day 2 and sacrificed on day 3. Foxl1-Cre; Rosa-iDTR/YFP mice and their control littermate (Rosa-iDTR/YFP) mice were administered with diphtheria toxin at 22 ng/g body weight, twice daily from day 0 to day 3 and sacrificed on day 4.
Anti-Foxl1 antibody production
A Foxl1-pUC57 codon-optimized plasmid containing amino acids 150-255 of mouse Foxl1 (avoiding regions of similarity with other Fox proteins, especially focusing on non-winged-helix domains with good antigenicity prediction and likely subdomain folding) with flanking BamHI/HindIII sites was generated (GenScript USA Inc.). The BamHI/HindIII fragment was inserted into pGexKG17, confirmed by sequencing, and used to produce soluble GST-Foxl1 fusion protein from bacteria. GST-Foxl1 was dialyzed against PBS and used as an antigen in goat and guinea pig (SDIX, LLC) or chicken (Aves Labs). Raw sera/antibodies were used to purify Foxl1-specific antibodies via GST/E. coli-extract depletion and GST-Foxl1 matrix-based affinity purification. GST-Foxl1 protein production and antibody purification were performed as described previously 18.
Fluorescent activated cell sorting, RNA isolation, and sequencing library preparation
Isolation of Foxl1+ cells using fluorescent-activated cell sorting (FACS) was performed using Foxl1-Cre;Rosa-YFP mice. Small intestines were dissected and washed thoroughly with PBS. Intestinal villi were scraped using a coverslip and the remaining tissue was incubated in 30mM EDTA plus 1.5mM DTT HBSS on ice for 20 min and subsequently incubated in 30mM EDTA at 37 degrees for 8 minutes to completely remove the epithelium. After vigorous washes, the remaining mesenchymal fraction was collected and cut into small pieces. The mesenchymal tissue was collected by centrifugation and resuspended in 7 mg/ml Dispase II/0.05% Trypsin solution at 37 degrees until the solution became cloudy and the mesenchyme was dissociated. A single-cell suspension was obtained by collecting the supernatant and washing with HBSS prior to cell sorting using a BD influx instrument (BD Biosciences). For RNA isolation, YFP+ cells were lysed and total RNA was isolated by column purification (Agilent Technologies). mRNA was isolated using Poly(A) mRNA isolation magnetic beads and an mRNA sequencing library prepared using the NEBNext RNA library prep kit (New England BioLabs Inc.). RNAseq was performed on an Illumina HiSeq instrument.
RNA isolation and Library formation from crypt cells
In order to isolate RNA from intestinal crypts after diphtheria toxin injection, Foxl1-hDTR and control mice were injected with diphtheria toxin at 20 ng/g body weight at day 0 and euthanized at day 3.. Dissected intestines were washed in PBS to remove the luminal content, incubated in 5 mM EDTA in PBS for 10 minutes, and scraped with a coverslip gently to remove villus cells. The remaining tissue was cut into 5 mm pieces and incubated in 5 mM EDTA for 20 minutes with vigorous pipetting every 2-3 minutes. The tissue was washed and pipetted vigorously, and floating crypts were collected. Crypt RNA was isolated using the TRIZOL reagent (Life Technologies) and subjected to Poly-A selection using magnetic isolation (New England BioLabs). Libraries were prepared with the RNA Library Prep Kit (New England BioLabs) and sequenced using an Illumina HiSeq instrument.
Histology
For hematoxylin and eosin staining, paraffin tissue sections were rehydrated, incubated in hematoxylin for 2.5 min, rinsed in water, dipped quickly in 0.5% acid alcohol, and washed in water. Tissues were then immersed in 0.2% NaHCO3, rinsed in water, dipped in eosin for 15 sec, and briefly rinsed in water before dehydration and mounting.
Immunofluorescence and Immunohistochemistry
At times of sacrifice, mouse intestines were rinsed in phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde overnight, rinsed in PBS, and either dehydrated for paraffin embedding or immersed in 30% sucrose for 4 hours at 4°C, embedded in OCT and frozen for cryosectioning. Antigen retrieval was achieved using citrate buffer, pH 6.0, with a pressure cooker (PickCell Laboratories). Foxl1 staining required the use of cryosections ,antigen retrieval, and amplification of signal using tyramide (TSA systems PerkinElmer). Antibodies used were: Ki67 (BD Pharmingen 1:500), Lysozyme (Dako 1:1000), Sox9 (Millipore 1:300), Mucin 2, HBEGF (Santa Cruz Biotechnology 1:50) Guinea Pig Foxl1 (1:1500), Goat GFP (Abcam 1:200), Mouse E-cadherin (BD transduction 1:250), Mouse β catenin (BD transduction 1:200), Rabbit EpCAM (Abcam 1:100), Rabbit αSMA (Abcam 1:100), Rabbit Myh11 (Abcam 1:100). Cy2-, Cy3- and Cy5-conjugated donkey secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. For immunohistochemistry, horseradish peroxidase (HRP)-conjugated secondary antibodies were incubated at room temperature for 2 hours. VECTASTAIN ABC kit (Vector Laboratories) was then used to detect the signal, and the slides were washed with PBS. For signal development, the DAB substrate was used.
In situ hybridization
Olfm4 RNA in situ hybridization was performed as previously described using OCT-embedded frozen tissues 11, 19. For other in situ hybridization experiments, double DIG-labeled locked nucleic acid probes were designed (Exiqon). Paraffin sections were re-hydrated, fixed, and pretreated as described by Silahtaroglu20 with the following modifications: in addition to acetylation, slides were pretreated with 10 ng/mg Proteinase K for 10 minutes at 37 degrees, and 80 nM of probe mixture was used for hybridization. DAPI was used to mark the nuclei (Vector Laboratories).
Western Blotting
Small and large intestines were dissected from C57Bl/6 control and Foxl1-null 7 mice. Isolated tissues were washed with PBS and scraped with a coverslip to remove the villus epithelium. Intestinal epithelial cells were separated from mesenchymal cells as described in Fluorescent activated cell sorting and RNA isolation. Tissues were then subjected to multiple rounds of sonication to lyse the cells in buffer containing 50mM Tris (pH8.0), 150mM NaCl, 1mM EDTA, 1% Triton-X 100, 0.5% deoxycholic acid, and 1% SDS, supplemented with protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktails 1 and 2 (Sigma). Cell lysates were separated by SDS-PAGE and transferred to PVDF membranes with the iBlot system (Life Technologies). Membranes were blocked in blocking buffer consisting of 5% milk and 0.1% Tween-20 in PBS for 2 hours and incubated with antibodies at 4 degrees overnight in blocking buffer. Antibodies used were: Chicken Foxl1 (1:500), Goat Foxl1 (1:500), Mouse α-SMA (Sigma 1:10,000), Mouse E-cadherin (BD transduction 1:5000), Rabbit β-actin (Cell Signaling 1:10,000). Membranes were then washed and incubated with HRP-conjugated goat-anti-rabbit IgG.
Fractional separation of mesenchyme and RT-PCR
Intestines from adult Foxm1-hDTR mice were cut open and washed in PBS. Intestinal epithelial cells were separated from mesenchymal cells as described in Fluorescent activated cell sorting and RNA isolation. The crypt epithelium was scraped off and collected. To ensure isolation of pure epithelium, epithelial cells were incubated in 0.05% Trypsin to obtain single cells, followed by incubation with an Alexa 488 mouse EpCAM (Biolegend 1:250) antibody, and sorted in a BD Influx instrument (BD Biosciences). The remaining mesenchymal tissue was washed to remove any epithelial contamination. From the epithelial and mesenchymal fractions, RNA was isolated and cDNA synthesized. For mesenchymal and epithelial-specific markers as well as hDTR, RT-PCR was performed using GoTaq (Promega). Primers used in this experiment are listed in Table 1.
Table 1.
| Cdh1 Fw | GGCTTCAGTTCCGAGGTCTA |
| Chd2 Rv | CTCTGGGTTGGATTCAGAGG |
| Vim Fw | GATCAGCTCACCAACGACAA |
| Vim Rv | TCCGGTACTCGTTTGACTCC |
| HBEGF Fw | ACTTTATCCTCCAAGCCACAAG |
| HBEGF RV | CCCAGATACCATCGGACATACT |
RESULTS
Foxl1 is expressed in a subset of mesenchymal cells
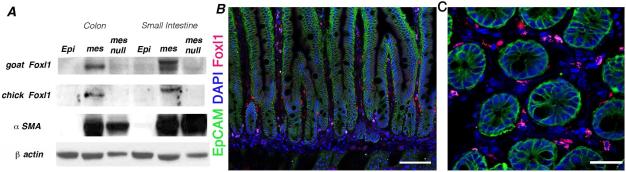
We hypothesized that mesenchymal cells in close apposition to the crypt epithelium constitute the intestinal stem cell niche in vivo. We and others have shown that the winged-helix transcription factor Foxl1 (previously known as Fkh6) is expressed specifically in the gastrointestinal mesenchyme during fetal development and in adulthood21-23. We developed new Foxl1-specific antibodies to more precisely map the location of this cell type in the mammalian gut. We established the specificity of these affinity-purified antibodies through immunoblot analysis of Foxl1-null gut tissue (Figure 1A). Foxl1 protein was confined to the nuclei of a subset of sub-epithelial cells that are tightly apposed to the epithelium of small intestinal crypts, with some cells extending into the villus tip (Fig. 1 B,C).
Figure 1. Antibodies to the mouse Foxl1 protein are specific, and Foxl1 is expressed in a small subset of subepithelial fibroblasts in the intestine.
(A) Immunoblot analysis for the Foxl1 protein in epithelial and mesenchymal fractions of mouse small intestine (jejunum) and colon, as indicated. Two different affinity-purified anti-Foxl1 antibodies were employed. Note the absence of the Foxl1 protein band in mesenchymal fractions from Foxl1 null mice. Immunoblot analysis of α-smooth muscle actin (αSMA) confirms separation of epithelium and mesenchyme. (B) Immunostaining demonstrating expression of Foxl1 (red) in the adult small intestine in a subset of mesenchymal cells located in close apposition to the epithelium (outlined with immunostaining for EpCAM, green). Nuclei labeled with DAPI (blue). Scale bar = 50μm (C) Cross section of crypt region showing the localization of Foxl1+ cells (red) in pericryptal mesenchymal cells. Nuclei labeled with DAPI (blue). Scale bar = 25μm.
Foxl1+ cells are distinct from myofibroblasts and express potential niche-supporting factors
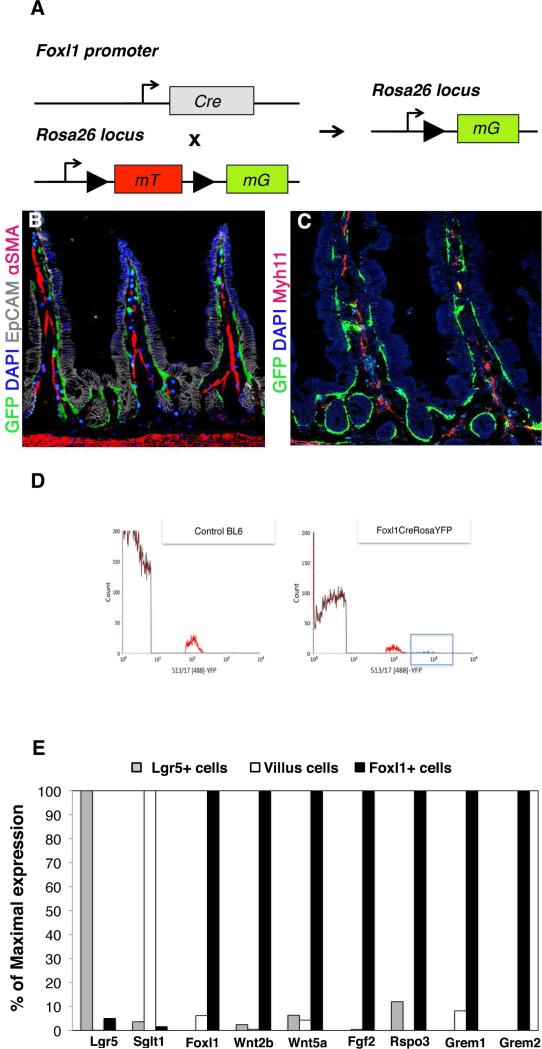
To better define the relationship of Foxl1-expressing cells to established mesenchymal cell types, we employed Foxl1-Cre; Rosa-mT/mG mice, in which Foxl1 promoter-dependent Cre activity results in the expression of a plasma membrane-bound green fluorescence protein (scheme in Fig. 2A)(see methods section for details), which allowed us to visualize the Foxl1-expressing cells in their full extension. As shown in Figure 2B, Foxl1+ cells elaborate long processes that span the width of multiple epithelial cells, suggesting that Foxl1+ cells are placed in an optimal position for short-range signaling to the epithelium. Dual-label immunofluorescence staining clearly demonstrated that the Foxl1+ cell is distinct from mesenchymal cells expressing the smooth muscle markers α-smooth muscle actin (Fig. 2B) and myosin heavy chain 11 (Myh11) (Fig. 2C).
Figure 2. Foxl1+ cells have long processes and express potential niche supporting factors.
(A) Schema of Foxl1-Cre; Rosa-mTmG mouse used to drive expression of a membrane-bound green fluorescence protein (GFP) in Foxl1-Cre positive cells. (B) Foxl1-Cre-driven expression of membrane-bound GFP is restricted to pericryptal mesenchymal cells, which elaborate long extensions into the villus tips and surrounding intestinal crypts. Immunofluorescence staining for GFP (green) and αSMA (red) shows very limited overlap between the two markers, indicating that Foxl1+ cells represent a unique cell population. αSMA expression is present in the core of the lamina propria, whereas GFP expression is located at the pericryptal sheath. (C) Immunofluorescence staining for Foxl1-Cre driven GFP (green) and Myh11 (red) indicates very limited overlap between the two markers. (D) A histogram of fluorescence-activated cell sorting of intestinal mesenchymal cells isolated from C57BLl6 control and Foxl1Cre; Rosa-YFP mice using YFP fluorescence. The x-axis shows YFP fluorescence intensity, and the gate indicated in the Foxl1Cre; Rosa-YFP plot shows the sorted cells from which RNA was isolated for RNA sequence analysis. Note the absence of high-intensity YFP+ cells in the control histogram. (E) Relative mRNA levels of various markers and key signaling molecules in sorted Lgr5+-epithelial stem cells, differentiated villus epithelial cells, and sorted Foxl1+ cells.
Next, we employed fluorescence-activated cell sorting (FACS) to enrich for mesenchymal cells expressing Foxl1-Cre (Fig. 2D). When we compared the mRNA levels of key genes between Foxl1+ mesenchymal cells and either Lgr5+ epithelial stem cells or differentiated intestinal villus cells (data from Sheaffer et al., 2014), we found, as expected, that the Foxl1+ fraction does not express Lgr5 or the sodium/glucose cotransporter Sglt1, markers of epithelial stem and differentiated villus cells, respectively (Fig. 2E). However, the mesenchymally expressed Wnt genes Wnt2b and Wnt5a were highly enriched in Foxl1+ pericryptal fibroblasts, suggesting that it is these cells that provide critical Wnt signals to the intestinal stem cell compartment. In addition, Foxl1+ cells express high levels of R-spondin 3 (Rspo3), an activator of Went signaling24, fibroblast growth factor 2 (Fgf2), and Gremlin 1 and 2, antagonists of the BMP pathway that are expressed in intestinal reticular stem cells 25.
Ablating Foxl1+ cells leads to disrupted epithelium
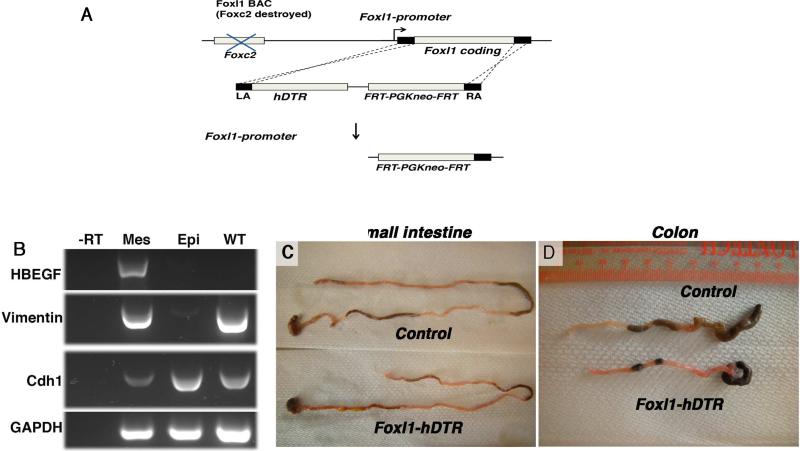
Because of this unique expression pattern, the elaboration of key signaling molecules, and because ablation of the Foxl1 gene itself causes altered Wnt signaling in stomach and intestine26, we surmised that Foxl1-expressing mesenchymal cells might constitute the intestinal stem cell niche. To test this hypothesis, we derived mice transgenic for a bacterial artificial chromosome comprising 170 kb of the Foxl1 locus in which the Foxl1 coding region was replaced by the human diphtheria toxin receptor (hDTR) cDNA (Foxl1-hDTR mice, Fig. 3A). While mice are normally resistant to diphtheria toxin, expression of the human diphtheria toxin receptor in Foxl1-expressing (Foxl1+) mesenchymal cells renders these cells diphtheria-toxin-sensitive, leading to cell-specific apoptosis following toxin administration. As expected, expression of the human diphtheria toxin receptor was confined to the subepithelial mesenchyme (Fig. 3B, 4A).
Figure 3. Ablation of Foxl1+ mesenchymal cells in diphtheria-toxin treated Foxl1-hDTR mice results in shortening of the gut tube.
(A) Schema for the generation of Foxl1–hDTR mice using BAC recombineering. The coding sequence of exon 1 of Foxl1 was targeted by the sequence of the human diphtheria toxin receptor (hDTR) following destruction of the upstream FoxC2 coding sequence. (B) Expression of the human diphtheria toxin receptor (the HBEGF gene) is confined to the mesenchyme in Foxl1-hDTR mice. Mesenchyme and epithelium of the colon from Foxl1-hDTR mice were separated and cDNA prepared. RT-PCR analysis was performed to detect mesenchymal Vimentin, epithelial E-cadherin, and ubiquitous GAPDH transcripts. Note that the HBEGF/hDTR mRNA is present exclusively in the mesenchyme. Abbreviations: -RT, without reverse transcriptase; Mes, mesenchyme; Epi, epithelium; WT, whole colon from control, non-transgenic mice. (C,D) Representative images of small intestine and colon from DT-treated Foxl1-hDTR and control mice. Note the shortening of the gut tube in the DT-treated Foxl1-hDTR mice.
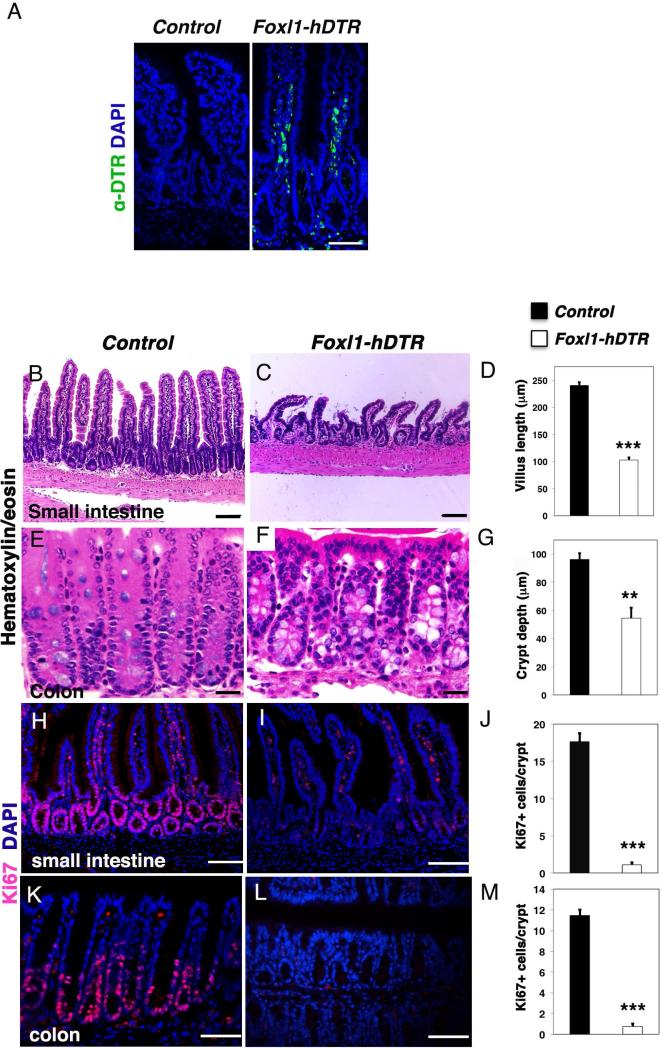
Figure 4. Intestinal epithelial architecture and proliferation are dependent on Foxl1+ mesenchymal cells.
(A) Representative immunofluorescence images of human diphtheria toxin receptor (green) staining in the jejunum of control and Foxl1–hDTR mice, counterstained with DAPI to label nuclei (blue). (B,C) Intestinal morphology of the jejunum of DT-treated control mice (B) or Foxl1–hDTR mice (C) as assessed by hematoxylin/eosin (H/E) staining. Note the reduced number and length of intestinal villi in Foxl1–hDTR mice. (D) Quantification of small intestinal villus length. (n = 4, in each animal > 20 villi were measured; ***, P < 0.001) (E,F) Intestinal morphology of the proximal colon from DT-treated control (E) and Foxl1–hDTR mice (F). Note the decreased depths of the colonic crypts and the irregular localization of epithelial nuclei in Foxl1–hDTR mice. (G) Quantification of colonic crypt depth. (n = 4, in each animal > 20 crypts were measured; **, P < 0.005). (H,I) Representative immunofluorescence images of the proliferation marker Ki67 (red) counterstained with DAPI to label nuclei (blue) in the jejunum of control (H) and Foxl1–hDTR (I) mice after toxin injection. (J) Quantification of the number of proliferating cells per crypt unit in jejunum of DT-treated control and Foxl1–hDTR mice. (n = 20 crypts each) ***, P < 0.001. (L,M) Ki67 labeling (red) overlaid with DAPI for nuclei (blue) in the colon of DT-treated control (K) and Foxl1–hDTR (L) mice. The number of Ki67-positive cells is dramatically reduced in Foxl1–hDTR mice. Scale bars: 50 μm. (M) Quantification of the number of proliferating cells per crypt unit in the proximal colon of DT-treated control and Foxl1–hDTR mice. (n = 20 crypts each) ***, P < 0.001; **, P < 0.005.
Strikingly, after three days of exposure to diphtheria toxin, the length of the small and large intestine was reduced in Foxl1-hDTR mice (Fig. 3C,D).
Foxl1-hDTR mice displayed dramatic changes to the architecture of the small and large intestine (Fig. 4B,C,E,F). The length of the villi was dramatically reduced in the jejunum (Fig. 4D), and epithelial architecture was abnormal in colonic crypts, which exhibited reduced depth (Fig. 4G). To test our hypothesis that Foxl1+ mesenchymal cells constitute an essential part of the intestinal stem and progenitor cell niche, we next analyzed gut tissue for epithelial proliferation. Immunofluorescence labeling for the proliferation marker Ki67 showed that the deletion of Foxl1+ mesenchymal cells resulted in a dramatic and highly significant reduction of epithelial cell proliferation in the jejunum (Fig. 4H-J) and colon (Fig. 4K-M).
Ablating Foxl1+ cells by a separate model leads to similar epithelial disruption
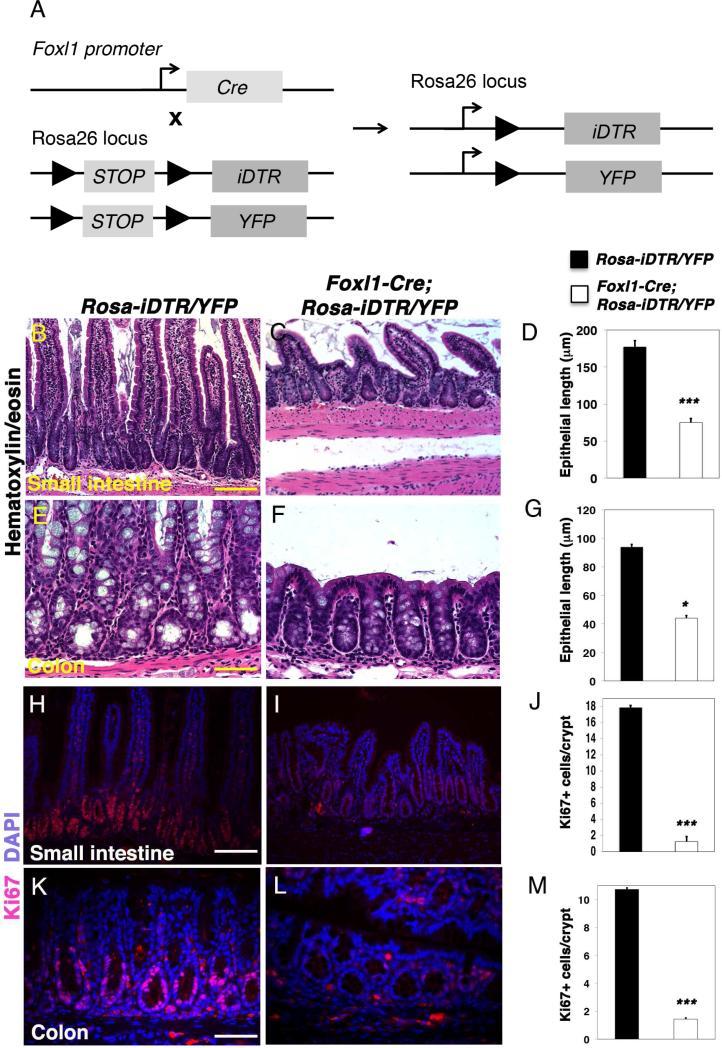
Next, we employed an independent paradigm (Fig. 5A; Foxl1-Cre;Rosa-iDTR mice; see Methods section for experimental details) in which expression of the simian DTR is activated in Foxl1-positive cells through Cre-mediated deletion of a STOP cassette, again making cells susceptible to diphtheria toxin27, 28. Remarkably similar results were obtained with this second cell-ablation model, where again villus length and crypt depth was significantly reduced in in the jejunum and colon, respectively (Fig. 5B-G). Likewise, epithelial proliferation was reduced dramatically in diphtheria toxin-treated Foxl1-Cre, Rosa-iDTR mice (Fig. 5H-M). Taken together, these results demonstrate that Foxl1-expressing mesenchymal cells are required for maintaining proliferation of the intestinal epithelium, both in stem cells and in the derived transit-amplifying cell population.
Figure 5. Targeted ablation of Foxl1+ mesenchymal cells using the iDTR model causes loss of epithelial proliferation.
(A) Schema for the generation of Foxl1-iDTR mice. Foxl1-Cre mice were crossed to mice carrying a loxP-stop-loxP Simian diphtheria toxin receptor construct in the Rosa26 locus (RosaiDTR mice). (B,C) Small intestinal (Jejunum) morphology was assessed by hematoxylin/eosin (H/E) staining of DT-treated control (B) and Foxl1 iDTR mice (C). Note the reduced number and length of intestinal villi. (D) Quantification of small intestinal villus length. (n = 4, in each animal; > 20 villi were evaluated per mouse; ***, P < 0.001). (E,F) Colonic morphology of DT-treated control (E) and Foxl1-iDTR mice (F). Decreased depths of the colonic crypts is apparent in Foxl1-iDTR mice. (G) Quantification of colonic crypt depth. (n = 4, in each animal; >20 crypts were evaluated per mouse; *, P < 0.05). (H,I) Representative immunofluorescence images of small intestinal (jejunum) sections stained for the proliferation marker Ki67 (red) and counterstained with DAPI to label nuclei (blue) of control (H) and Foxl1-iDTR (I) mice. The number of Ki67-positive epithelial cells is dramatically reduced in Foxl1 iDTR mice. (J) Quantification of the number of proliferating cells per crypt unit in the jejunum of DT-treated control and Foxl1 iDTR mice. (n = 20 crypts each) ***, P < 0.001. (K,L) Ki67 labeling (red) overlaid with DAPI to mark nuclei (blue) in the colon of control DT-treated control (K) and Foxl1 iDTR (L) mice. The number of Ki67-positive cells is dramatically reduced in Foxl1 iDTR mice. (M) Quantification of the number of proliferating cells per crypt unit in the colon of DT-treated control and Foxl1 iDTR mice. (n = 20 crypts each) ***, P < 0.001. Scale bars: 100 μm in the small intestine, 50μm in the colon.
Stem cells but not Paneth cells are affected by ablating Foxl1+ cells
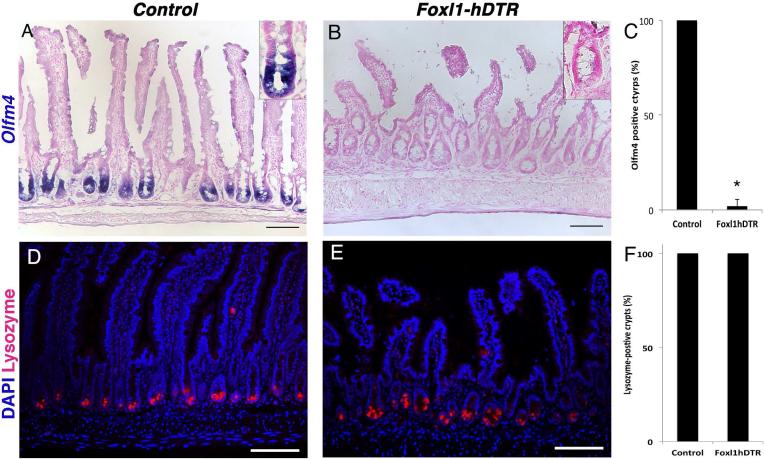
In addition to loss of proliferation, expression of the stem cell marker Olfm4 was dramatically reduced in mice with ablation of Foxl1+ subepithelial mesenchymal cells (Fig. 6A-C). However, the long-lived lysozyme-expressing Paneth cells were retained in the small intestinal epithelium of diphtheria toxin-treated Foxl1-hDTR mice, indicating that loss of epithelial proliferation was not secondary to loss of Paneth cells (Fig. 6D-F).
Figure 6. Foxl1+ mesenchymal cells are required for intestinal stem cell marker expression independent of Paneth cells.
(A,B) Expression of the intestinal stem-cell marker Olfm4 is reduced in DT-treated Foxl1-hDTR mice (B) compared to non-transgenic controls (A) as determined by in situ hybridization. Insets show crypts at high magnification. (C) Quantification of the percentage of small intestinal crypts that are positive for Olfm4 mRNA (> 200 crypts were counted for both DT-treated control and Foxl1-hDTR mice). ***, p < 0.01. (D,E) Expression of lysozyme (red), a Paneth-cell marker, is not affected by ablating Foxl1+ cells. Scale bar: 50μm. (F) Quantification of lysozyme-positive cells per crypt unit. (n = 3 per genotype; > 20 crypts were evaluated per animal.
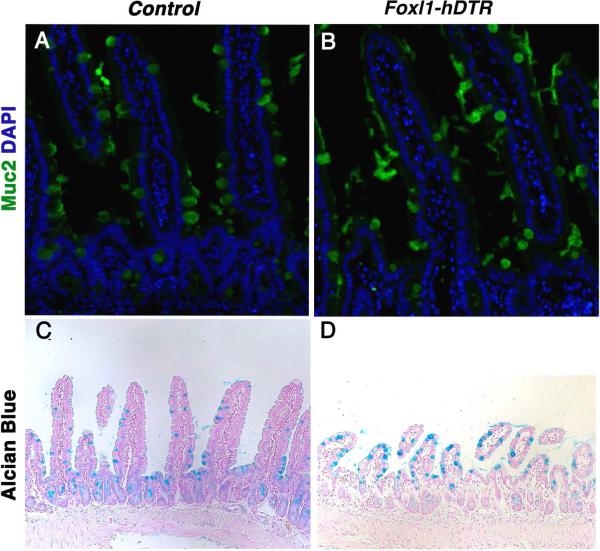
Moreover, cell-lineage allocation among the differentiated lineages was not affected globally in the three days from toxin administration to analysis, as evidenced by the presence of Mucin 2 and Alcian Blue-positive goblet cells (Fig. 7A-D).
Figure 7. The frequency of mucin-secreting goblet cells is unaffected by ablation of Foxl1+ cells.
(A,B) Detection of goblet cells by immunostaining for Muc2 (mucin 2; green) and (C,D) Alcian Blue histochemistry (blue), indicates that the frequency of goblet cells in (B,D) DT-treated DT-treated Foxl1-hDTR mice is not different from that present in control mice (A,C).
Ablating Foxl1+ cells leads to reduced Wnt signaling
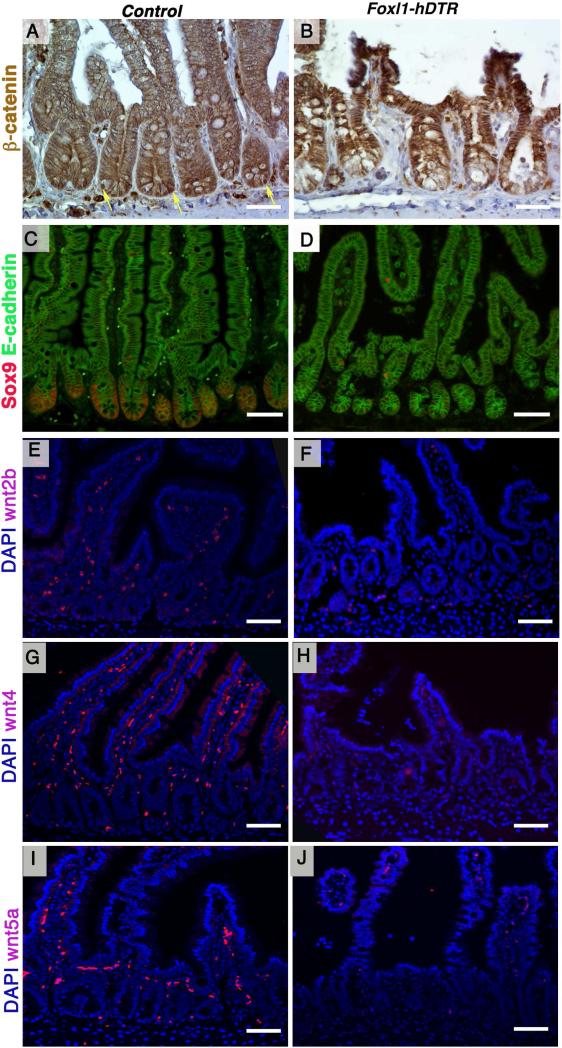
Proliferation of the intestinal epithelium is driven by Wnt signaling13, 14, 29, and we hypothesized that Foxl1-expressing subepithelial cells serve as an essential source of these signals. To determine if Wnt signaling is affected by loss of Foxl1+ cells, we evaluated nuclear ß-catenin staining. As shown in Fig. 8A and 8B, nuclear ß-catenin expression was greatly reduced in the crypts of Foxl1-hDTR mice after diphtheria-toxin administration, indicative of loss of canonical Wnt signaling activity. Next, we investigated the expression of the Wnt target gene Sox9 in Foxl1-hDTR mice after diphtheria toxin administration. Sox9 protein is normally present in the entire crypt, including intestinal stem cells and Paneth cells30. After diphtheria toxin treatment, we noted a striking decrease in the number of Sox9-expressing cells in Foxl1-hDTR crypts as compared to controls (Fig. 8C,D), again supporting reduced Wnt signaling activity.
Figure 8. Foxl1+ mesenchymal cells provide essential Wnt ligands to the intestinal stem cell compartment.
(A,B) Activation of the Wnt pathway in epithelial cells of the jejunum was analyzed by immunohistochemistry for ß-catenin. Reduced nuclear ß-catenin localization is apparent in toxin-treated Foxl-hDTR mice (B) compared to toxin-treated controls (A). Yellow arrows point to nuclei positive for ß-catenin. Scale bars: 25 μm. (C,D) Immunofluorescence staining of the Wnt target Sox9 (red) shows decreased expression in the jejunum of DT-treated Foxl1-hDTR mice (D) compared to non-transgenic controls (C). The epithelium is delineated with E-cadherin immunostaining (green). Scale bars: 50 μm. (E,F) Mesenchymal Wnt2b mRNA was detected by in situ hybridization (red) and nuclei were counterstained with DAPI (blue) Wnt2b mRNA is dramatically reduced in the mesenchyme of DT-treated Foxl1-hDTR mice (F) compared to controls (E). Scale bars: 50 μm. (G,H) Mesenchymal Wnt4 and (I,J) Wnt5a mRNAs were detected by in situ hybridization (red) and nuclei were counterstained with DAPI (blue). Wnt4 and Wnt5a mRNA levels were dramatically reduced in the mesenchyme of DT-treated Foxl1-hDTR mice (H and J) compared to controls (G and I).
Both canonical and non-canonical Wnt factors are produced by the intestinal mesenchyme15, 19. Farin and colleagues established Wnt2b as a mesenchymal Wnt protein that is sufficient to support intestinal organoid growth in culture15. Strikingly, Wnt2b, Wnt4 and Wnt5a signals that are normally detected in the subepithelial mesenchyme were dramatically decreased in Foxl1-hDTR mice after diphtheria toxin administration (Fig. 8E-J). These data suggest that production of Wnt2b, Wnt4 and Wnt5a by Foxl1-expressing mesenchymal cells, which are located in close proximity to the basement membrane of the epithelial crypt, is part of the signal required to maintain the intestinal stem cell niche.
DISCUSSION
Wnt signaling is required for the maintenance of the adult intestine; however, the cellular source of this ligand has not been satisfactorily defined. While the Clevers group deduced that Paneth cells are this source since they express and secrete Wnt ligands, and since co-culture of Paneth cells and Lgr5+ cells allows the formation of intestinal organoids, several lines of evidence challenged the view of the Paneth cell as an absolutely required in vivo source of signals required to maintain the intestinal stem cell. For example, loss of Paneth cells3,4 or loss of the essential Wnt-processing protein Porcupine (Porcn) in Paneth cells31, 32 has no effect on intestinal stem cell maintenance.
An alternative source for the signals required for stem cell maintenance is the intestinal mesenchyme. Recently, however, San Roman and colleagues demonstrated that Porcn, and therefore the Wnt signal, does not originate solely from Myh11-expressing intestinal smooth muscle cells and myofibroblasts or, independently, from Villin-expressing epithelial cells28. Kabiri and colleagues ablated Porcn expression from the entire intestinal epithelium using Villin-Cre and demonstrated normal intestinal morphogenesis27. Together, these studies suggested that either redundant Wnt signaling from both epithelial and mesenchymal compartments or a rare Wnt-producing cell type in one of these compartments that had not been targeted with the existing Cre-lines is responsible for crypt maintenance.
We demonstrate in this manuscript that Foxl1+ mesenchymal cells exist independently of the Myh11-expresssing subepithelial myofibroblasts targeted by San Roman and colleagues (Fig. 1). Thus, the rare Foxl1+ cells that reside in the intestinal mesenchyme, in close apposition to the intestinal crypt, are outstanding candidates to be the minor Wnt-producing cell type in the intestine postulated by San Roman and colleagues32.
These Foxl1+ cells are located directly adjacent to the epithelium and extend long processes that span the length of multiple epithelial cells. They express Wnt2b, Wnt4 and Wnt5a, and the loss of these Foxl1+ cells results in decreased β-cateinin nuclear localization and proliferation within the intestinal epithelium, reduced villi length, diminished crypts depths, and an overall shortening of the intestine. Therefore, Foxl1+ cell are required for intestinal stem cell maintenance and are not functionally redundant with Wnt-expressing Paneth cells.
We considered whether the diphtheria-toxin-mediated killing of Foxl1+ cell might have indirect effects on the intestinal stem cell niche not mediated by controlling physiological signaling components such as the Wnt proteins described above. For instance, one might envisage a “bystander” effect in which debris from dying Foxl1+ cells is taken up by intestinal stem cells, leading to their death. We consider this scenario exceedingly unlikely, considering that diphtheria toxin mediated ablation of Paneth cells, which after all are directly abutting Lgr5+ stem cells, is without effect on crypt health 4.
Of note, mice deficient for the winged helix transcription factor Foxl1, while displaying abnormal gastrointestinal development 23, are viable and maintain functioning crypts. In fact, the role of Foxl1 and its direct targets is in limiting Wnt signaling to the epithelial compartment 23. Of course, it is not surprising that the genetic ablation of one gene within a cell does not have the same consequences as ablating that cell type entirely, as each transcription factor controls only part of the transcriptional program within the cell.
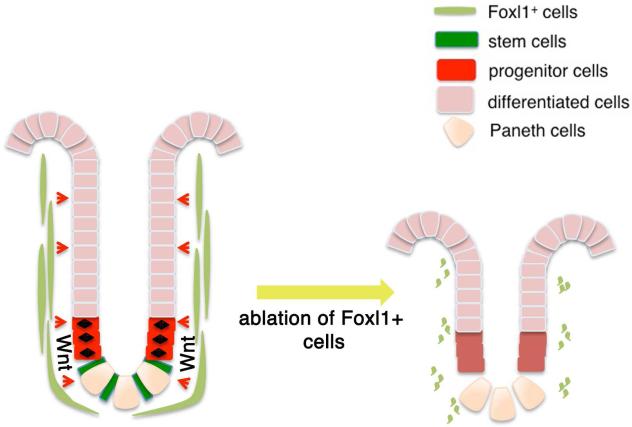
Although the cellular identity of the intestinal stem cell niche has been controversial until this point, using two novel, independent cell-ablation models, we demonstrate that Foxl1+ subepithelial mesenchymal cells are the source of essential Wnt signals and thus constitute the intestinal stem and progenitor cell niche (see model in Fig. 9). Positive identification of the cells that provide the intestinal stem-cell niche might aid in future development of regenerative strategies for intestinal disorders.
Figure 9.
Proposed model of Foxl1-positive subepithelial mesenchymal cells as the intestinal stem cell niche, providing essential Wnt signals, and possibly other signaling molecules, to the epithelium.
Synopsis.
Foxl1+ cells are a small subset of mesenchymal subepithelial fibroblasts. These cells are a critical component of the intestinal stem cell niche as demonstrated by using two separate models to ablate these cells.
ACKNOWLEDGMENTS
We thank Tia Bernard-Banks for technical assistance with the mouse colony. We acknowledge support of the Transgenic Mouse and Molecular Pathology & Imaging Cores of the Center for Molecular Studies in Digestive and Liver Diseases (NIH P30 DK050306). This work was supported by NIH grants NIDDK R37-DK053839 to KH Kaestner, and 5U01-DK089570 and 5U01-DK072473 to CVE Wright.
Abbreviations used in this paper
- FACS
fluorescently activated cell sorting
- Foxl1
Forkhead Box l1
- H&E
hematoxylin and eosin stain
- HCP
hereditary chronic pancreatitis
- IHC
immunohistochemistry
- P
postnatal
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- qRT-PCR
quantitative reverse transcriptase PCR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
Author contributions:
RA: Study concept, design, and implementation; acquisition of data; analysis and interpretation of data; statistical analysis; drafting of manuscript
MS: Study concept, design, and implementation; acquisition of data; analysis and interpretation of data; statistical analysis; drafting of manuscript
NG: Acquisition of data; analysis and interpretation of data
SS: Acquisition of data; analysis and interpretation of data
CLM: Acquisition of data; analysis and interpretation of data
AMZ: Acquisition of data; analysis and interpretation of data
MR: Acquisition of data; analysis and interpretation of data
CLW: Acquisition of data; analysis and interpretation of data
CVEW: Study supervision; critical revision of the manuscript
KHK: Study concept and design; obtained funding; study supervision; critical revision of manuscript
REFERENCES
- 1.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 2.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, Perret C, Shroyer NF, Romagnolo B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garabedian EM, Roberts LJ, McNevin MS, Gordon JI. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. The Journal of biological chemistry. 1997;272:23729–23740. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- 5.Kim TH, Escudero S, Shivdasani RA. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3932–3937. doi: 10.1073/pnas.1113890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nature genetics. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Furstenberg RJ, Gulati AS, Baxi A, Doherty JM, Stappenbeck TS, Gracz AD, Magness ST, Henning SJ. Sorting mouse jejunal epithelial cells with CD24 yields a population with characteristics of intestinal stem cells. American journal of physiology. Gastrointestinal and liver physiology. 2011;300:G409–417. doi: 10.1152/ajpgi.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL, Coffey RJ. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright NA, Irwin M. The kinetics of villus cell populations in the mouse small intestine. I. Normal villi: the steady state requirement. Cell and tissue kinetics. 1982;15:595–609. doi: 10.1111/j.1365-2184.1982.tb01066.x. [DOI] [PubMed] [Google Scholar]
- 11.van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes & development. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farin HF, Van Es JH, Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529. e1517. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Sackett SD, Fulmer JT, Friedman JR, Kaestner KH. Foxl1-Cre BAC transgenic mice: a new tool for gene ablation in the gastrointestinal mesenchyme. Genesis. 2007;45:518–522. doi: 10.1002/dvg.20315. [DOI] [PubMed] [Google Scholar]
- 17.Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Analytical biochemistry. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 18.Candia AF, Wright CV. Differential localization of Mox-1 and Mox-2 proteins indicates distinct roles during development. The International journal of developmental biology. 1996;40:1179–1184. [PubMed] [Google Scholar]
- 19.Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Silahtaroglu AN. LNA-FISH for detection of microRNAs in frozen sections. Methods in molecular biology. 2010;659:165–171. doi: 10.1007/978-1-60761-789-1_11. [DOI] [PubMed] [Google Scholar]
- 21.Fukamachi H, Fukuda K, Suzuki M, Furumoto T, Ichinose M, Shimizu S, Tsuchiya S, Horie S, Suzuki Y, Saito Y, Watanabe K, Taniguchi M, Koseki H. Mesenchymal transcription factor Fkh6 is essential for the development and differentiation of parietal cells. Biochemical and biophysical research communications. 2001;280:1069–1076. doi: 10.1006/bbrc.2001.4247. [DOI] [PubMed] [Google Scholar]
- 22.Kaestner KH, Bleckmann SC, Monaghan AP, Schlondorff J, Mincheva A, Lichter P, Schutz G. Clustered arrangement of winged helix genes fkh-6 and MFH-1: possible implications for mesoderm development. Development. 1996;122:1751–1758. doi: 10.1242/dev.122.6.1751. [DOI] [PubMed] [Google Scholar]
- 23.Kaestner KH, Silberg DG, Traber PG, Schutz G. The mesenchymal winged helix transcription factor Fkh6 is required for the control of gastrointestinal proliferation and differentiation. Genes & development. 1997;11:1583–1595. doi: 10.1101/gad.11.12.1583. [DOI] [PubMed] [Google Scholar]
- 24.de Lau W, Peng WC, Gros P, Clevers H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes & development. 2014;28:305–316. doi: 10.1101/gad.235473.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, Levin D, Schwartz MG, Uygur A, Hayakawa Y, Gross S, Renz BW, Setlik W, Martinez AN, Chen X, Nizami S, Lee HG, Kang HP, Caldwell JM, Asfaha S, Westphalen CB, Graham T, Jin G, Nagar K, Wang H, Kheirbek MA, Kolhe A, Carpenter J, Glaire M, Nair A, Renders S, Manieri N, Muthupalani S, Fox JG, Reichert M, Giraud AS, Schwabe RF, Pradere JP, Walton K, Prakash A, Gumucio D, Rustgi AK, Stappenbeck TS, Friedman RA, Gershon MD, Sims P, Grikscheit T, Lee FY, Karsenty G, Mukherjee S, Wang TC. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perreault N, Sackett SD, Katz JP, Furth EE, Kaestner KH. Foxl1 is a mesenchymal Modifier of Min in carcinogenesis of stomach and colon. Genes & development. 2005;19:311–315. doi: 10.1101/gad.1260605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nature methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- 28.Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nature biotechnology. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman J, Kuhnert F, Davis CR, Kuo CJ. Wnts as essential growth factors for the adult small intestine and colon. Cell Cycle. 2004;3:554–557. [PubMed] [Google Scholar]
- 30.Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, Clevers H, Jay P. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. The Journal of cell biology. 2004;166:37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison, Aliyev J, Wu Y, Bunte R, Williams BO, Rossant J, Virshup DM. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141:2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 32.San Roman AK, Jayewickreme CD, Murtaugh LC, Shivdasani RA. Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem cell reports. 2014;2:127–134. doi: 10.1016/j.stemcr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]