Abstract
The standard of care for newly diagnosed glioblastoma (GBM) is surgery, then radiotherapy (RT) with concurrent temozolomide (TMZ), followed by adjuvant TMZ. We hypothesized patients with low diffusivity measured using apparent diffusion coefficient (ADC) histogram analysis evaluated after RT+TMZ, prior to adjuvant TMZ, would have a significantly shorter progression-free (PFS) and overall survival (OS). To test this hypothesis we evaluated 120 patients with newly diagnosed GBM receiving RT+TMZ followed by adjuvant TMZ. MRI was performed after completion of RT+TMZ, prior to initiation of adjuvant TMZ. A double Gaussian mixed model was used to describe the ADC histograms within the enhancing tumor, where ADCL and ADCH were defined as the mean ADC value of the lower and higher Gaussian distribution, respectively. An ADCL value of 1.0 um2/ms and ADCH value of 1.6 um2/ms were used to stratify patients into high and low risk categories. Results suggest patients with low ADCL had significantly shorter PFS (Cox Hazard Ratio = 0.12, P = 0.0006). OS was significantly shorter with low ADCL tumors, showing a median OS of 407 vs. 644 days (Cox Hazard Ratio = 0.31, P = 0.047). ADCH was not predictive of PFS or OS when accounting for age and ADCL. In summary, newly diagnosed glioblastoma patients with low ADCL after completion of RT+TMZ are likely to progress and die earlier than patients with higher ADCL. Results suggest ADC histogram analysis may be useful for patient risk stratification following completion of RT+TMZ.
Keywords: Glioblastoma, ADC Histogram Analysis, Diffusion MRI
Introduction
GBM is the most common and deadly form of primary brain tumor in adults. The current standard, aggressive therapy consisting of maximal surgical resection followed by concurrent RT and TMZ chemotherapy and adjuvant TMZ has shown a median survival of only 14.6 months (1-3). Although GBMs generally have a very poor prognosis, there are clearly cohorts of patients that benefit from specific therapies. Thus, there is great interest in identifying risk factors and biomarkers for predicting response to therapy prior to therapeutic initiation. Patient age at diagnosis, neurological performance status, extent of surgical resection, radiographic composition of the tumor, tumor volume and location, IDH1 mutation status, gene expression subtype, and MGMT promoter methylation are commonly assessed prognostic characteristics for GBM (4-12).
The use of imaging features to phenotype tumors and predict response from therapy is an attractive option compared to more invasive approaches based on tissue-derived biomarkers. By non-invasively characterizing the composition of the tumor microenvironment, features associated with particular response patterns can be identified leading to the potential for patient cohort enrichment for use in clinical trials. Recently, we have shown the ADC characteristics measured using diffusion MRI can be used to predict both PFS and OS in GBM patients treated with bevacizumab at recurrence (13-15). Specifically, results in both single and multicenter trials have shown high ADC measurements within the contrast-enhancing tumor regions are prognostic of a favorable response to bevacizumab treatment at recurrence as indicated by a longer PFS and OS, whereas patients with low ADC measurements have a significantly shorter PFS and OS. Importantly, results have also suggested the prognostic capabilities of ADC measurements may be specific to bevacizumab therapy at recurrence, since no difference in PFS or OS were noted in bevacizumab-naïve patients treated with chemotherapy at recurrence (13). However, it is conceivable that ADC measurements may also be prognostic when used to evaluate the phase of adjuvant TMZ prior to the first recurrence, since various studies have suggested a general increase in ADC following successful RT+TMZ (16-21).
In the current study we examined a cohort of 120 patients with a newly diagnosed GBM that underwent tumor resection followed by RT+TMZ. We then evaluated the diffusion MR characteristics within the tumor 4 weeks following completion of RT+TMZ, just prior to starting the adjuvant phase of TMZ therapy. We hypothesized high ADC measurements within contrast-enhancing voxels following completion of RT+TMZ would be indicative of a longer PFS and OS.
Methodology
Patient Characteristics
All patients participating in this study signed institutional review board-approved informed consent. Data acquisition was performed in compliance with all applicable HIPAA regulations. Patients were retrospectively selected from our institution's neuro-oncology database. Initially, a total of n = 169 patients who met the following criteria were selected: 1) pathology confirmed glioblastoma, 2) treatment with standard external beam radiotherapy and concurrent TMZ, followed by adjuvant TMZ, 3) MRI scans obtained after surgical resection and within 4 weeks following completion of RT+TMZ, just prior to the adjuvant phase of TMZ. Average age for this population was 58.4 years old (± 11 years standard deviation), average KPS was 86 (± 10 standard error of the mean, S.E.M.), and 57% of the patients were male (97/169). Seventy (n = 70) patients had a gross total resection at the time of initial surgery, n = 73 patients had a subtotal resection, and n = 26 patients had only a biopsy prior to radiochemotherapy.
Of all patients enrolled, n = 120 patients had good quality diffusion-weighted images and were included in the final analyses for this study. Exclusions were based on gross geometric distortions or low signal-to-noise ratio in the raw DWI datasets or patients with contrast enhancing tumor less than 0.1 cc on the first MRI scan following RT+TMZ. These follow-up scans were obtained approximately 10 weeks from the time of treatment initiation (mean = 75 days ± 2.6 days SEM), or approximately 4 weeks from the end of initial radiochemotherapy. At the time of last assessment, 104 of the 120 patients had died.
Treatment Paradigm
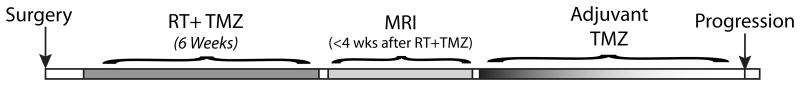
Patients were treated with 60Gy external beam radiation therapy (2Gy fractions given one daily for five days over a six week period) with concomitant TMZ (75 mg/m2 orally or intravenously for 42 consecutive days), followed by a 28 day break then the start of adjuvant TMZ (150 mg/m2 orally or intravenously for 5 consecutive days in the first 28 day cycle, followed by 200 mg/m2 orally or intravenously for 5 consecutive days in the first 28 day cycle for a maximum of 6 cycles). Diffusion and standard anatomical MRI were performed within 10 weeks after the start of RT+TMZ, or within 4 weeks from the end of RT+TMZ, just prior to adjuvant TMZ (Figure 1). Start of adjuvant TMZ and the MRI evaluation were performed on the same day. This is typically the first imaging evaluation after completion of RT+TMZ and therefore is an important clinical decision-making time point.
Figure 1. Treatment and MR Evaluation Timeline.
Patients were treated with 60Gy external beam radiation therapy (2Gy fractions given one daily for five days over a six week period) with concomitant TMZ (75 mg/m2 orally or intravenously for 42 consecutive days), followed by a 28 day break then the start of adjuvant TMZ (150 mg/m2 orally or intravenously for 5 consecutive days in the first 28 day cycle, followed by 200 mg/m2 orally or intravenously for 5 consecutiave days in the first 28 day cycle for a maximum of 6 cycles). Diffusion and standard anatomical MRI were performed within 10 weeks after the start of RT+TMZ, or within 4 weeks from the end of RT+TMZ, just prior to adjuvant TMZ.
Magnetic Resonance Imaging
Diffusion and structural MRI were obtained on either a 1.5T (GE Signa Excite HDx or Lx; GE Medical Systems, Waukesha, WI; Siemens Avanto or Sonata; Siemens Healthcare, Erlangen, Germany) or 3T MR system (Siemens Trio, Allegra, or Verio; Siemens Healthcare, Erlangen, Germany). Standard anatomical MRI consisted of pre- and post-contrast (Gd-DTPA at a dose of 0.1 mmol/kg body weight; Magnevist, Bayer Schering Pharma, Leverkusen, Germany) axial T1-weighted images along with pre-contrast axial T2-weighted, and FLAIR sequences with standard sequence parameters. Patients also received DWIs with echo time TE/TR = 80-120ms/>5000ms, matrix size = 128×128, slice thickness = 3mm with no interslice gap, and b-values of 0 and 1000 s/mm2 in three orthogonal directions. ADC maps were calculated for each image voxel as ADC(x,y,z) = -1/1000 • ln[S(x,y,z)/S0(x,y,z)], where S(x,y,z) is the signal intensity of the voxel at coordinate (x,y,z) with b=1000 s/mm2, S0(x,y,z) is the signal intensity at voxel (x,y,z) with b=0 s/mm2.
ADC Histogram Analysis
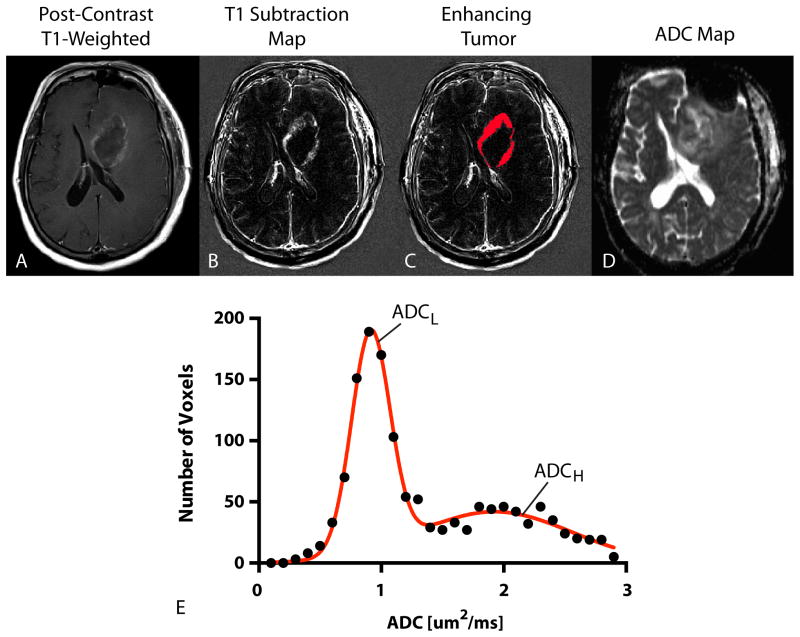
ADC histogram analysis was performed using techniques previously described. (13-15) Briefly, contrast enhancing tumor regions were segmented on T1 subtraction images calculated by subtracting pre-contrast from post-contrast T1-weighted images.(22) ADC characteristics from within enhancing regions were then extracted. A double Gaussian mixed model was used to describe the ADC histogram using nonlinear regression, where ADCH reflects the mean ADC in the larger of the two Gaussian distributions and ADCL is the mean ADC value of the lower Gaussian distribution (Figure 2). Both ADCL and ADCH were used as the primary imaging biomarker for the current study using GraphPad Prism v6 (GraphPad Software, Inc., La Jolla, CA).
Figure 2. ADC Histogram Analysis.
A) Post-contrast T1-weighted image showing ring-enhancing tumor in the left frontal lobe. B) T1 subtraction map generated from subtracting pre-contrast from post-contrast T1-weighted images. C) Enhancing tumor mask extracted from T1 subtraction maps. D) ADC map used for diffusion MR phenotyping. E) Resulting ADC histogram (raw data = black closed circles; double Gaussian mixed model fit = solid red line). ADCL = mean of the lower Gaussian distribution estimated the double Gaussian mixed model fit. ADCH = mean of the higher Gaussian distribution estimated from the double Gaussian mixed model fit.
Definition of Tumor Progression
Progression was defined prospectively by the treating neuro-oncologists if subsequent scans showed an increase in imaging-evaluable tumor (≥ 25% increase in the sum of enhancing lesions, new enhancing lesions > 1 cm2, an unequivocal qualitative increase in nonenhancing tumor, or an unequivocal new area of noncontrast enhancing tumor). Patients were required to have stable or decreasing contrast agent dose before partial or complete response could be determined. Additionally, patients requiring increased dosage of steroids in order to maintain neurologic function, even in the absence of worsening on anatomical images, were considered to be stable, but required early reevaluation. Patients who experienced significant neurologic decline were also declared to have progressed at the time of irreversible decline. Landmark PFS was defined as the time between the MRI scan following completion of RT+TMZ and progression. Landmark OS was defined as the time between the MRI scan and death.
Statistical Analyses
ROC analysis was used to determine whether a low ADCL could identify patients who progressed within 6 months from starting adjuvant TMZ (i.e. PFS6) and patients who died within 12 months from starting adjuvant TMZ (e.g. OS12) using area under the ROC curve (AUC) as a measure of performance. An ADCL value of 1.0 μm2/ms and an ADCH value of 1.6 μm2/ms were chosen as the primary biomarkers of interest, since these values were near the median of the patient distribution and found to have the highest likelihood ratio [sensitivity / (1-specificity)] for both PFS6 and OS12. This cutoff was then used to stratify PFS and OS using both Log-rank analysis on Kaplan-Meier data and multivariate Cox regression analysis using age as an additional covariate. A p-value less than 0.05 was considered statistically significant and a p-value less than 0.10 was considered trending toward significance.
Results
ROC Analysis
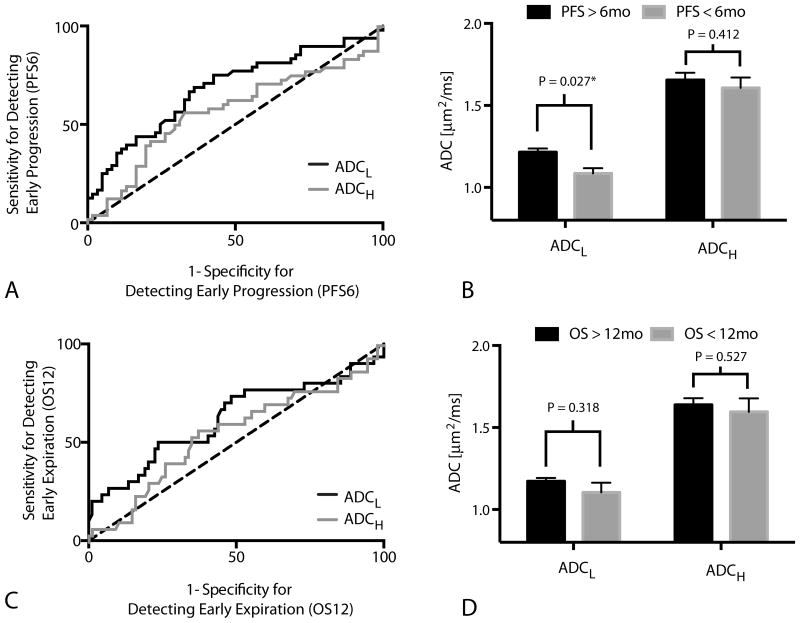
Results suggest ADCL is a significant predictor of patients that will progress within 6 months of starting adjuvant temozolomide (Figure 3A; ROC AUC = 0.68 ± 0.053 S.E.M., P = 0.0011); however, ADCH was not a significant predictor of progression by 6 months (Figure 2A; ROC AUC = 0.5768 ± 0.057 S.E.M., P = 0.2187). A threshold of ADCL < 1.0 um2/ms had a low sensitivity (34%) and high specificity (90%) for identifying patients that would progress within 6 months, meaning a high proportion of patients with low ADCL after RT+TMZ will progress early after starting adjuvant TMZ (Figure 3B; t-test, P = 0.027). (For reference, ADCL < 1.2 um2/ms used in previous studies showed a sensitivity of 71% and specificity of 57% for PFS6).
Figure 3. Receiver-Operator Characteristic (ROC) Curves.
A) ROC curve showing the sensitivity and specificity of ADCL and ADCH in detecting patients who progressed within 6 months of starting adjuvant TMZ (PFS6; ADCL; ROC AUC = 0.6820 ± 0.05252 S.E.M., P = 0.0011; ADCH; ROC AUC = 0.5768 ± 0.057 S.E.M., P = 0.2187). B) ADCL and ADCH measurements for individual tumors categorized based on progression before or after 6 months from the start of adjuvant TMZ (PFS6). Significant differences in ADCL (t-test, P = 0.027), but not ADCH (t-test, P = 0.412), were observed between patients progressing before and after 6 months from the start of adjuvant TMZ. ( C) ROC curve showing the sensitivity and specificity of ADCL and ADCH in detecting patients who died within 12 months of starting adjuvant TMZ (OS12; ADCL; ROC AUC = 0.6176 ± 0.06551 S.E.M., P = 0.0547; ADCH; ROC AUC = 0.55 ± 0.064 S.E.M., P = 0.4104). D) ADCL and ADCH measurements for individual tumors categorized based on death before or after 12 months from the start of adjuvant TMZ (OS12). No significant differences were observed in measurements of ADCL (t-test, P = 0.318) or ADCH (t-test, P = 0.527) when patients were stratified by OS12.
ADCL also trended toward being a significant predictor of OS12 (Figure 3C; ROC AUC = 0.62 ± 0.066 S.E.M., P = 0.0547), while ADCH did not predict OS12 (Figure 3C; ROC AUC = 0.55 ± 0.064 S.E.M., P = 0.4104). ADCL showed a relatively low sensitivity (33%) but high specificity (82%) of predicting OS12 when using ADCL < 1.0 um2/ms for patient stratification. (For ADCL < 1.2 um2/ms, sensitivity/specificity = 73%/48%).
Progression-Free Survival
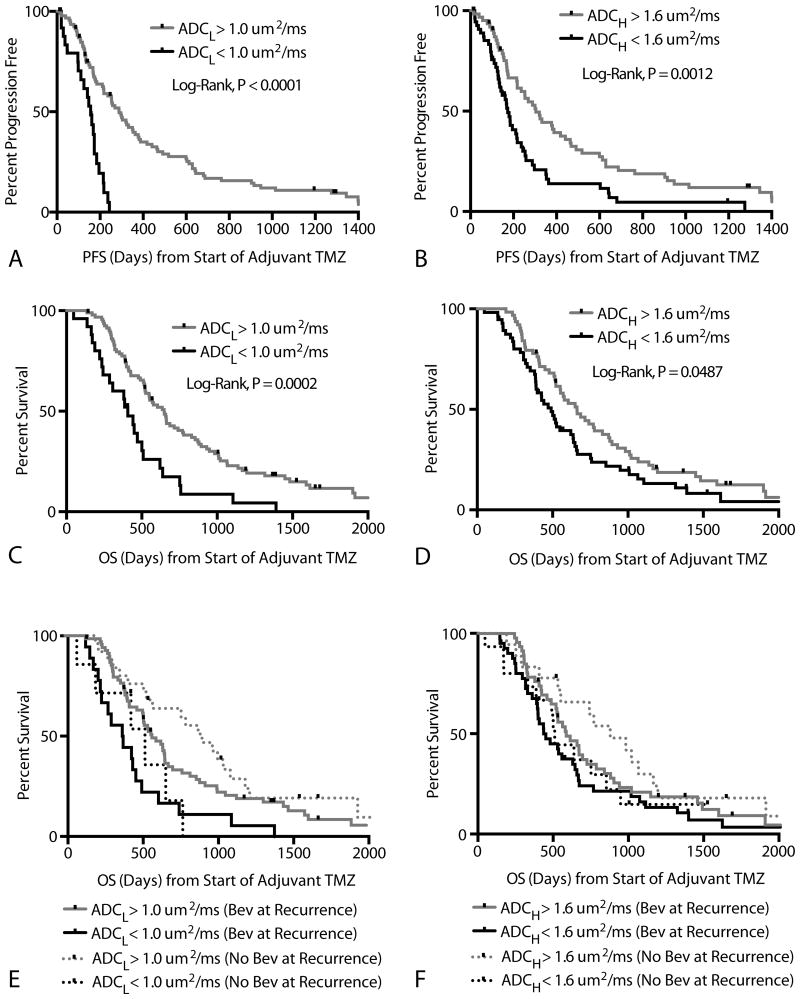
Patients with an ADCL < 1.0 um2/ms had a significantly shorter PFS compared with the rest of the patients (Figure 4A; Log-rank, P < 0.0001). Median PFS for patients exhibiting a low ADCL (< 1.0 um2/ms) was 156 days compared to a median PFS of 288 days for patients with a high ADCL (> 1.0 um2/ms). Similarly, patients with an ADCH < 1.6 um2/ms also demonstrated a significantly shorter PFS compared with the rest of the patients (Figure 4B; Log-rank, P = 0.0012), with a median PFS of 173 days compared with 304 days for patients with ADCH > 1.6 um2/ms. Cox multivariate regression examining the effects of age, ADCL, and ADCH on PFS confirmed that ADCL was a significant predictor of PFS (Cox Regression, HR = 0.11 (0.03 – 0.39), P = 0.0006) and age trended toward significance (Cox Regression, HR = 1.02 (1.00 – 1.04), P = 0.0521). No association between ADCH and PFS was observed when accounting for age and ADCL (Cox Regression, P = 0.9498). No differences in ADCL were observed between MGMT methylated and unmethylated tumors (t-test, P=0.38), but methylated tumors had signficiantly higher values of ADCH (t-test, P = 0.0443; mean ADCH for methylated = 1.50 um2/ms; mean ADCH for unmethylated tumors = 1.72 um2/ms).
Figure 4. Progression-Free and Overall Survival.
A) Kaplan-Meier curves showing significantly lower PFS in patients with ADCL < 1.0 um2/ms (Log-rank, P < 0.0001; Cox Multivariate, P=0.0002). B) Kaplan-Meier curves showing significantly lower PFS in patients with ADCH < 1.6 um2/ms in univariate analysis (Log-rank, P = 0.0012); however, ADCH was not significant during multivariate analysis (Cox Multivariate, P=0.9498). C) Kaplan-Meier curves showing significantly lower OS in patients with ADCL < 1.0 um2/ms (Log-rank, P=0.0002; Cox Multivariate, P = 0.0487). D) Kaplan-Meier curves showing significantly lower OS in patients with ADCH < 1.6 um2/ms in univariate analysis (Log-rank, P = 0.0487), but not when accounting for age and ADCL (Cox Multivariate, P=0.5478). E) Kaplan-Meier curves showing differences in OS based on ADCL higher or lower than 1.0 um2/ms for both bevacizumab-naïve (Log-rank, P=0.0130) and bevacizumab treated (Log-rank, P=0.0029) patients at recurrence. No differences in OS were observed between patients treated with bevacizumab and those without being treated with bevacizumab within high ADCL (Log-rank, P=0.1977) or low ADCL (Log-rank, P=0.8959) groups. F) Kaplan-Meier curves showing no differences in OS based on ADCH higher or lower than 1.6 um2/ms for both bevacizumab-naïve (Log-rank, P=0.1330) and bevacizumab treated (Log-rank, P=0.1510) patients at recurrence.
Overall Survival
Patients with ADCL < 1.0 um2/ms had a significantly shorter OS compared with patients having higher ADCL (Figure 4C; Log-rank, P=0.0002), with median OS for patients with low ADCL (< 1.0 um2/ms) around 407 days compared to a 648 days for patients with a high ADCL (> 1.0 um2/ms). Similarly, patients with ADCH < 1.6 um2/ms also had a significantly shorter OS compared with patients exhibiting a higher ADCH (Figure 4D; Log-rank, P = 0.0487), with median OS for patients with a low ADCH around 491 days compared with 662 days for patients with a high ADCH. Cox multivariate regression confirmed that both age (Cox, HR= 1.03 (1.01 -1.05 95% C.I.), P = 0.001) and ADCL (Cox, HR = 0.31 (0.09 – 0.98 95% C.I.), P = 0.047) were correlated with OS. ADCH did not have a significant association with OS when accounting for age and ADCL (Cox, P = 0.5478).
Significant OS differences were observed between high and low ADCL in both patients receiving bevacizumab at first recurrence (Figure 4E; N=87, Log-rank, P=0.003) and patients who did not receive bevacizumab at first recurrence (Figure 4E; N = 33, Log-rank, P=0.01). No differences were observed between high and low ADCH in patients receiving bevacizumab at first recurrence (Figure 4F; Log-rank, P = 0.1510) and patients who did not receive bevacizumab at first recurrence (Figure 4F; Log-rank, P = 0.1330). No differences in OS were observed between patients treated with bevacizumab and those without being treated with bevacizumab within high ADCL (Log-rank, P=0.20) or low ADCL (Log-rank, P=0.90) groups.
Discussion
Diffusion MR measures of ADC have been shown to be correlated with both tumor cellularity(19, 23-25) and mitotic activity.(26) Therefore, successful radiochemotherapy would be expected to result in a relatively higher amount of tumor cell destruction, leading to an increase in the diffusivity of water within the tumor due to lack of restrictions to diffusion from structures such as cell membranes. Tumors with a low ADC following combined RT+TMZ, on the other hand, may consist of a more cellular, more aggressive, or possibly more treatment resistant tumor phenotype. Results from the current study support this hypothesis, suggesting patients with an ADCL < 1.0 um2/ms in contrast enhancing tumor have a significantly shorter PFS and OS after starting adjuvant TMZ compared with other patients.
Previous studies using ADC histogram analysis have shown that tumor ADCL values higher than 1.2 um2/ms have a significantly longer PFS and OS in recurrent GBM treated with bevacizumab.(13-15, 27). Using the threshold of 1.0 um2/ms in the current study we did not observe any difference in OS between patients treated with bevacizumab and those that were not treated with bevacizumab. However, we did observe this trend at recurrence when using a threshold of 1.2 um2/ms (data not shown), but this threshold was not significant when used for the evaluation of adjuvant TMZ so it was not used in the current study. Together, these results may suggest patients with a low ADCL (< 1.0 um2/ms) after RT+TMZ are likely to be non-responsive to any subsequent therapies including bevacizumab or additional chemotherapies, patients with a high ADCL (>1.2 um2/ms) are likely to respond favorably to bevacizumab at first recurrence, and patients with an intermediate ADCL (1.0 um2/ms < ADCL < 1.2 um2/ms) may benefit from a subsequent chemotherapy prior to treatment with bevacizumab.
There are a few limitations to the current study that should be noted. It is important to point out that there was a potential selection bias in the current study, as only patients who successfully completed surgical resection and RT+TMZ with measurable contrast-enhancing tumor (>0.1 cc) were eligible for ADC histogram analysis. Additionally, it is conceivable that some patients determined to have early progression following completion of RT+TMZ actually had pseudoprogression, or treatment-related changes in vascular permeability mimicking radiographic changes similar to treatment failure or tumor growth. The addition of multimodal imaging techniques, including perfusion MRI (28), may have allowed for more accurate delineation of pseudoprogression from true progression. Despite this potential confound, we found significant differences in both PFS and OS in all patients based on diffusion characteristics as well as patients with PFS greater than 3 months from the end of RT+TMZ, where the incidence of pseudoprogression is likely to be the highest. Additionally, the current study involved acquisition of diffusion MR images using variety of MR systems and field strengths for the purpose of mimicking a clinical trial environment. Recent studies have shown that errors in ADC measurements vary nonlinearly from the scanner isocenter and different MR systems have different degrees of nonlinearity (29). Thus, the current study would have benefited from the use of a temperature-controlled water phantom in order to account for system-specific errors in ADC measurements.
In summary, the current study demonstrates that diffusion characteristics obtained using ADC histogram analysis can be used to predict PFS and OS following completion of RT+TMZ, prior to adjuvant TMZ therapy. Results suggest patients with ADCL < 1.0 um2/ms are at increased risk for early progression and early death, indicating that ADC histogram analysis may be useful for patient risk stratification following completion of RT+TMZ. Future studies aimed at integrating ADC histogram analysis into clinical decision-making as well as identifying biological correlates of diffusion characteristics are warranted.
Acknowledgments
We would like to thank our funding sources including the National Brain Tumor Society Research Grant (BME, TFC); NIH/NCI 1 R21 CA167354-01 (BME); UCLA Institute for Molecular Medicine Seed Grant (BME); UCLA Radiology Exploratory Research Grant (BME); University of California Cancer Research Coordinating Committee Grant (BME); ACRIN Young Investigator Initiative Grant (BME); Art of the Brain (TFC); Ziering Family Foundation in memory of Sigi Ziering (TFC); Singleton Family Foundation (TFC); and Clarance Klein Fund for Neuro-Oncology (TFC). National Institute of Health National Instititute of General Medical Sciences training grant, GM08042 (KL) and the University of California Los Angeles Medical Scientist Training Program (KL). We would also like to thank the volunteers, technologists, and research coordinators in the UCLA Brain Tumor Imaging Laboratory (BTIL) and the UCLA Center for Computer Vision and Imaging Biomarkers (CVIB) for their support.
Abbreviations
- ADCL
ADC within the lower distribution of the bimodal histogram model
- AUC
area under the curve
- GBM
Glioblastoma Multiforme
- GE
General Electric
- HIPAA
Health Insurance Portability and Accountability Act
- HR
hazard ratio
- IDH1
isocitrate dehydrogenase 1
- MGMT
O6-methylguanine methyltransferase
- OS
overall survival
- PFS
progression-free survival
- ROC
receiver-operator characteristics
- RT
Radiation Therapy
- SEM
standard error of the mean
- TE
echo time
- TMZ
temozolomide
- TR
repetition time
Footnotes
Conflicts of Interest: Drs. Timothy F. Cloughesy, Albert Lai, Whitney Pope, and Benjamin Ellingson are paid consultants for Genentech, Inc., and Hoffman-La Roche, Ltd. Drs. Ellingson and Pope are also a paid consultant for MedQIA, LLC.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Park CK, Lee SH, Kim TM, Choi SH, Park SH, Heo DS, et al. The value of temozolomide in combination with radiotherapy during standard treatment for newly diagnosed glioblastoma. Journal of neuro-oncology. 2013;112(2):277–83. doi: 10.1007/s11060-013-1060-3. [DOI] [PubMed] [Google Scholar]
- 3.Sher DJ, Henson JW, Avutu B, Hochberg FH, Batchelor TT, Martuza RL, et al. The added value of concurrently administered temozolomide versus adjuvant temozolomide alone in newly diagnosed glioblastoma. Journal of neuro-oncology. 2008;88(1):43–50. doi: 10.1007/s11060-008-9530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhry NS, Shah AH, Ferraro N, Snelling BM, Bregy A, Madhavan K, et al. Predictors of long-term survival in patients with glioblastoma multiforme: advancements from the last quarter century. Cancer investigation. 2013;31(5):287–308. doi: 10.3109/07357907.2013.789899. [DOI] [PubMed] [Google Scholar]
- 5.Kumar N, Kumar P, Angurana SL, Khosla D, Mukherjee KK, Aggarwal R, et al. Evaluation of outcome and prognostic factors in patients of glioblastoma multiforme: A single institution experience. Journal of neurosciences in rural practice. 2013;4(Suppl 1):S46–55. doi: 10.4103/0976-3147.116455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parks C, Heald J, Hall G, Kamaly-Asl I. Can the prognosis of individual patients with glioblastoma be predicted using an online calculator? Neuro-oncology. 2013;15(8):1074–8. doi: 10.1093/neuonc/not033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazaris P, Hong X, Altshuler D, Schultz L, Poisson LM, Jain R, et al. Key determinants of short-term and long-term glioblastoma survival: a 14-year retrospective study of patients from the Hermelin Brain Tumor Center at Henry Ford Hospital. Clinical neurology and neurosurgery. 2014;120:103–12. doi: 10.1016/j.clineuro.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Ellingson BM, Lai A, Harris RJ, Selfridge JM, Yong WH, Das K, et al. Probabilistic radiographic atlas of glioblastoma phenotypes. AJNR American journal of neuroradiology. 2013;34(3):533–40. doi: 10.3174/ajnr.A3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(8):774–82. doi: 10.1200/JCO.2013.51.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(25):4189–99. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 11.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–12. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. The New England journal of medicine. 2009;360(8):765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellingson BM, Sahebjam S, Kim HJ, Pope WB, Harris RJ, Woodworth DC, et al. Pretreatment ADC histogram analysis is a predictive imaging biomarker for bevacizumab treatment but not chemotherapy in recurrent glioblastoma. AJNR American journal of neuroradiology. 2014;35(4):673–9. doi: 10.3174/ajnr.A3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pope WB, Kim HJ, Huo J, Alger J, Brown MS, Gjertson D, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252(1):182–9. doi: 10.1148/radiol.2521081534. [DOI] [PubMed] [Google Scholar]
- 15.Pope WB, Qiao XJ, Kim HJ, Lai A, Nghiemphu P, Xue X, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. Journal of neuro-oncology. 2012;108(3):491–8. doi: 10.1007/s11060-012-0847-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellingson BM, Cloughesy TF, Zaw T, Lai A, Nghiemphu PL, Harris R, et al. Functional diffusion maps (fDMs) evaluated before and after radiochemotherapy predict progression-free and overall survival in newly diagnosed glioblastoma. Neuro-oncology. 2012;14(3):333–43. doi: 10.1093/neuonc/nor220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamstra DA, Chenevert TL, Moffat BA, Johnson TD, Meyer CR, Mukherji SK, et al. Evaluation of the functional diffusion map as an early biomarker of time-to-progression and overall survival in high-grade glioma. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(46):16759–64. doi: 10.1073/pnas.0508347102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moffat BA, Chenevert TL, Lawrence TS, Meyer CR, Johnson TD, Dong Q, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(15):5524–9. doi: 10.1073/pnas.0501532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chenevert TL, Stegman LD, Taylor JM, Robertson PL, Greenberg HS, Rehemtulla A, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. Journal of the National Cancer Institute. 2000;92(24):2029–36. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 20.Hein PA, Eskey CJ, Dunn JF, Hug EB. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR American journal of neuroradiology. 2004;25(2):201–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Castillo M, Smith JK, Kwock L, Wilber K. Apparent diffusion coefficients in the evaluation of high-grade cerebral gliomas. AJNR American journal of neuroradiology. 2001;22(1):60–4. [PMC free article] [PubMed] [Google Scholar]
- 22.Ellingson BM, Kim HJ, Woodworth DC, Pope WB, Cloughesy JN, Harris RJ, et al. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–10. doi: 10.1148/radiol.13131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellingson BM, Malkin MG, Rand SD, Connelly JM, Quinsey C, LaViolette PS, et al. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging. 2010;31(3):538–48. doi: 10.1002/jmri.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9(1):53–60. doi: 10.1002/(sici)1522-2586(199901)9:1<53::aid-jmri7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Guo AC, Cummings TJ, Dash RC, Provenzale JM. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002;224(1):177–83. doi: 10.1148/radiol.2241010637. [DOI] [PubMed] [Google Scholar]
- 26.Karavaeva E, Harris RJ, Leu K, Shabihkhani M, Yong WH, Pope WB, et al. Relationship Between [F]FDOPA PET Uptake, Apparent Diffusion Coefficient (ADC), and Proliferation Rate in Recurrent Malignant Gliomas. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2014 doi: 10.1007/s11307-014-0807-3. [DOI] [PubMed] [Google Scholar]
- 27.Pope WB, Mirsadraei L, Lai A, Eskin A, Qiao J, Kim HJ, et al. Differential gene expression in glioblastoma defined by ADC histogram analysis: relationship to extracellular matrix molecules and survival. AJNR American journal of neuroradiology. 2012;33(6):1059–64. doi: 10.3174/ajnr.A2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsien C, Galban CJ, Chenevert TL, Johnson TD, Hamstra DA, Sundgren PC, et al. Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(13):2293–9. doi: 10.1200/JCO.2009.25.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malyarenko DI, Newitt D, L JW, Tudorica A, Helmer KG, Arlinghaus LR, et al. Demonstration of nonlinearity bias in the measurement of the apparent diffusion coefficient in multicenter trials. Magn Reson Med. 2015 doi: 10.1002/mrm.25754. [DOI] [PMC free article] [PubMed] [Google Scholar]