Abstract
Prostate-specific membrane antigen (PSMA)-targeted PET imaging is an emerging technique for evaluating patients with prostate cancer (PCa) in a variety of clinical contexts. As with any new imaging modality, there are interpretive pitfalls that are beginning to be recognized. In this image report, we describe the findings in a 63-year-old male with biochemically recurrent PCa after radical prostatectomy who was imaged with 18F-DCFPyL, a small molecule inhibitor of PSMA. Diffuse radiotracer uptake was noted throughout the sacrum, corresponding to imaging findings on contrast-enhanced CT, bone scan, and pelvic MRI consistent with Paget’s disease of bone. The uptake of 18F-DCFPyL in Paget’s disease is most likely due to hyperemia and increased radiotracer delivery. In light of the overlap in patients affected by PCa and Paget’s, it is important for nuclear medicine physicians and radiologists interpreting PSMA PET/CT scans to be aware of the potential for this diagnostic pitfall. Correlation to findings on conventional imaging such as diagnostic CT and bone scan can help confirm the diagnosis.
Keywords: Prostate cancer, PET/CT, PSMA, DCFPyL
Introduction
Limitations in conventional imaging for the evaluation of prostate cancer (PCa) have spurred the development of a number of new positron emission tomography (PET) molecular imaging agents. Among the most extensively studied radiotracers are small molecule inhibitors of prostate-specific membrane antigen (PSMA) (1-3), a transmembrane enzyme that is highly expressed in PCa and the expression of which is positively correlated with aggressive features of the disease (4-6).
These small molecule imaging agents have demonstrated a number of important findings in early clinical studies of PCa patients including: (1) the reliable identification of clinically significant disease in pre-prostatectomy patients (7); (2) greater sensitivity for the identification of sites of disease in patients with metastatic PCa in comparison to conventional imaging with contrast-enhanced computed tomography (CECT) and 99mTc-methylene diphosphonate (MDP) bone scan (BS) (8); and (3) higher sensitivity than conventional imaging for the detection of lesions in patients with biochemical recurrence following prostatectomy (9). The specificity of these agents is also quite high, being reported to be as high as 100% in some series with pathological correlation (10). Despite this high apparent specificity, potential false positives have been noted in the literature including radiotracer uptake in celiac ganglia (11) and an adrenal adenoma (12).
In this image report, we describe uptake of the PSMA-targeted radiotracer 18F-DCFPyL (3) in Paget’s disease of bone, a common condition in the same elderly male population that is at risk for PCa. Particularly in light of the propensity for PCa to metastasize to bone, potential false positive bone lesions could have the ability to significantly confound interpretation of PSMA PET scans.
Methods and Image Report
The patient is a 63-year-old man who underwent a radical retropubic prostatectomy approximately four years prior to the imaging discussed in this report. Final surgical pathology demonstrated Gleason 4+3 = 7, pT3bN1 PCa. Following surgery, his prostate specific antigen (PSA) level fell to undetectable. Within 1 year his PSA began to rise to a most recent value of 0.3 ng/mL. Alkaline phosphatase level at the time of imaging was mildly elevated to 158 IU/L (normal laboratory range 39-117 IU/L).
As part of routine clinical evaluation for biochemically recurrent PCa, the patient underwent imaging with 99mTc-MDP BS. This demonstrated intense radiotracer uptake throughout the sacrum (Figure 1A). Additionally, the patient was imaged with a single venous phase CECT of the abdomen and pelvis revealing sclerosis of the sacrum with thickening of the trabeculae (Figure 1B). Both of these findings are most compatible with Paget’s disease of bone. The CECT and BS were otherwise unremarkable from an oncologic perspective. To further evaluate the patient, magnetic resonance imaging (MRI) of the pelvis was performed. This demonstrated irregular sclerosis and trabecular thickening within the sacrum with preserved high T1 signal compatible with fatty marrow (Figure 1C), further confirming the benign nature of the sacral lesion. Notably, local recurrence within the prostate bed was not seen on the MRI. These findings were clinically deemed consistent with Paget’s disease of the sacrum, with no definitive findings on conventional imaging to localize the site of patient’s recurrent disease.
Figure 1.
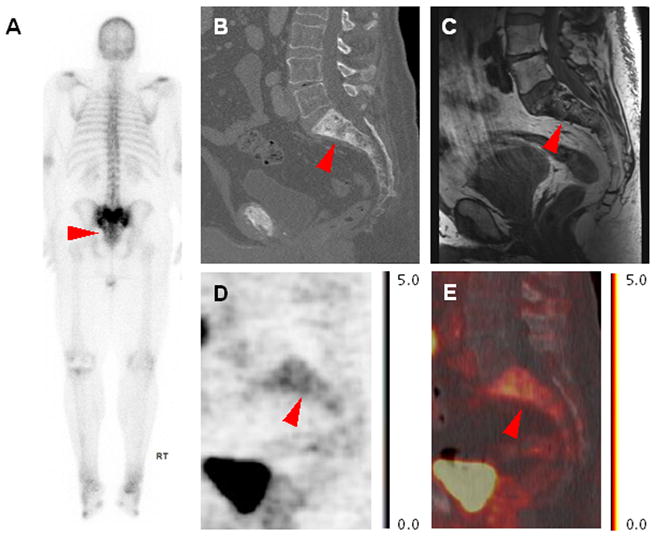
(A) Posterior projection whole-body MDP BS, (B) sagittal CECT image through the sacrum, (C) sagittal T1-weighted non-contrast, non-fat-saturation MRI, (D) sagittal 18F-DCFPyL PET image through the sacrum, and (E) sagittal 18F-DCFPyL PET/CT fused image through the sacrum demonstrating patient’s sacral Paget’s disease. Note the thickened trabecular pattern on CECT corresponding to intense MDP uptake, normal high T1 signal on MRI, and diffuse mild 18F-DCFPyL uptake.
Based upon the patient’s clinical scenario and imaging findings, he qualified for an Institutional Review Board approved prospective trial at our institution evaluating the use of 18F-DCFPyL PET/CT in the context of biochemically recurrent PCa. 18F-DCFPyL was utilized under the auspices of a U.S. Food and Drug Administration Exploratory Investigational New Drug application. Informed consent was obtained from the patient prior to proceeding with the study PET/CT. Briefly, the PET was acquired one hour after the injection of approximately 333 MBq (9 mCi) of 18F-DCFPyL, with the acquisition field-of-view extending from the mid-thighs through the skull vertex, and with a low-dose, non-contrast CT for attenuation correction and anatomic localization also obtained. Details regarding the acquisition protocol have been previously published (3, 13). Standardized uptake values (SUVs) were determined based on lean body mass.
The 18F-DCFPyL PET/CT was notable for two important findings. First, a focus of intense radiotracer uptake was observed within the prostate bed, consistent with a local recurrence of his PCa (maximum SUV 9.3). Second, mild to moderate uptake was seen throughout the sacrum (maximum SUV 4.6, Figure 1D-E), corresponding to the abnormality at this site seen on the other modalities. Figure 2 demonstrates the MRI-occult (A), 18F-DCFPyL-avid presumed local PCa recurrence (B).
Figure 2.

(A) Axial T1-weighted post-contrast MRI and (B) axial 18F-DCFPyL PET/CT images through the prostate bed demonstrating the patient’s presumed local recurrence as intense radiotracer uptake on PET but with no corresponding abnormality on MRI. The relative radiotracer uptake in the prostate bed lesion is visually and quantitatively higher than the uptake in the sacrum in Figure 1D-E.
Discussion
Given the immense volume of data that has been generated on the use of PSMA-targeted radiotracers for PCa PET imaging, it is almost certain that this modality will continue to be used extensively in imaging trials and may become a part of the clinical imaging work-up of patient’s with PCa. As more patients are imaged with these radiotracers, it will become increasingly important for radiologists and nuclear medicine physicians reading these imaging studies to be aware of limitations that might affect their interpretations. To this end, we have described a report of mild to moderate 18F-DCFPyL uptake within the sacrum in a patient with conventional imaging findings compatible with Paget’s disease of the sacrum. This level of uptake will generally be distinguishable from sites of PCa demonstrating intense uptake (compare Figure 1 and Figure 2), however in our group’s experience densely sclerotic bone metastatic PCa lesions that are composed predominantly of osteoblastic reaction and may contain few viable tumor cells can also have relatively low PSMA-targeted radiotracer uptake (8). Ultimately, the determination of true and false positive findings from PSMA scans will best be determined by well-designed prospective trials incorporating large numbers of patients.
Although Paget’s disease is a common benign bone condition in the elderly, affecting up to 10% of the population over the age of 85, disease confined to the sacrum is a somewhat unusual manifestation (14). Elevated serum alkaline phosphate levels can be seen in Paget’s disease, as in this patient, however osteoblastic metastases from PCa can also present with this laboratory abnormality and it should therefore be interpreted with caution in these patients (15). Paget’s disease can be profoundly hyperemic (to the extent of rarely causing high output heart failure (16), and it is this feature that likely leads to mildly increased radiotracer distribution within a Paget’s lesion.
Of interest, a single other case report of Paget’s disease of bone imaged with a 68Ga-labeled PSMA-targeted radiotracer was recently published (17). The authors of this report postulate that the high level of uptake could suggest that the target of the radiotracer may be present within the neovasculature of areas of Padget’s disease. An alternative hypothesis is that radiotracer accumulation is driven by hyperemia and increased radiotracer delivery, If the latter mechanism is accurate, then it is possible that other underlying etiologies that cause hyperemia (e.g. healing bone after trauma or inflammatory processes) may also cause false positive uptake on PSMA PET scans.
In the presented report, the appearance of the sacrum on conventional imaging (Figure 1) was compatible with Paget’s and was not suggestive of PCa bone metastasis. The thickened trabeculae on CECT in combination with diffusely increased MDP uptake on BS and the lack of fatty marrow replacement indicated by preservation of high T1 signal on MRI all suggest the diagnosis. These conventional imaging findings are likely to remain important in distinguishing densely sclerotic metastatic foci with mild PSMA-targeted radiotracer uptake from Paget’s disease.
It should be noted that this is only a single observational image report, with a second similar report recently appearing in the literature (17), and these findings will need to be confirmed in other patients undergoing PSMA-targeted PET studies. Further, given the definitively Pagetoid appearance of the sacrum in this case, it was not felt necessary to confirm the findings by invasive means, leading to a lack of histologic evidence to support the presented conclusions.
In summary, PSMA-based PET/CT is a promising means of evaluating PCa in a variety of clinical contexts; however, as with almost any imaging modality there are potential false-positive findings that can complicate interpretation of these examinations. Uptake of PSMA-targeted agents in Paget’s disease of bone is important to recognize as a potential false positive finding of this imaging technique.
Acknowledgments
We acknowledge financial support from CA134675, CA184228, CA183031 and philanthropic funds to the James Buchanan Brady Urological Institute. We thank Margarita Mana-ay, Akimosa Jeffrey-Kwanisai, and Yavette Morton for clinical trials support.
Footnotes
Disclosures
MGP is a co-inventor on a US Patent covering 18F-DCFPyL and as such is entitled to a portion of any licensing fees and royalties generated by this technology.
This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Cho SY, Gage KL, Mease RC, Senthamizhchelvan S, Holt DP, Jeffrey-Kwanisai A, Endres CJ, Dannals RF, Sgouros G, Lodge M, Eisenberger MA, Rodriguez R, Carducci MA, Rojas C, Slusher BS, Kozikowski AP, Pomper MG. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med. 2012;53:1883–91. doi: 10.2967/jnumed.112.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart G, Hadaschik BA, Holland-Letz T, Giesel FL, Kratochwil C, Haufe S, Haberkorn U, Zechmann CM. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–95. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 3.Szabo Z, Mena E, Rowe SP, Plyku D, Nidal R, Eisenberger MA, Antonarakis ES, Fan H, Dannals RF, Chen Y, Mease RC, Vranesic M, Bhatnagar A, Sgouros G, Cho SY, Pomper MG. Initial evaluation of [18F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17:565–74. doi: 10.1007/s11307-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright GL, Jr, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, Troyer J, Konchuba A, Schellhammer PF, Moriarty R. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–34. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 5.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–40. doi: 10.1016/s0090-4295(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 6.Chang SS, Reuter VE, Heston WD, Gaudin PB. Comparison of anti-prostate-specific membrane antigen antibodies and other immunomarkers in metastatic prostate carcinoma. Urology. 2001;57:1179–83. doi: 10.1016/s0090-4295(01)00983-9. [DOI] [PubMed] [Google Scholar]
- 7.Rowe SP, Gage KL, Faraj SF, Macura KJ, Cornish TC, Gonzalez-Roibon N, Guner G, Munari E, Partin AW, Pavlovich CP, Han M, Carter HB, Bivalacqua TJ, Blackford A, Holt D, Dannals RF, Netto GJ, Lodge MA, Mease RC, Pomper MG, Cho SY. 18F-DCFBC for PSMA-based detection and characterization of primary prostate cancer. J Nucl Med. 2015;56:1003–10. doi: 10.2967/jnumed.115.154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowe SP, Macura KJ, Ciarallo A, Mena E, Blackford A, Nadal R, Antonarakis ES, Eisenberger M, Carducci MA, Ross AE, Kantoff PW, Holt DP, Dannals RF, Mease RC, Pomper MG, Cho SY. Comparison of PSMA-based 18F-DCFBC PET/CT to conventional imaging modalities for detection of hormone-sensitive and castration-resistant metastatic prostate cancer. J Nucl Med. 2015 Oct 22; doi: 10.2967/jnumed.115.163782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, Graner FP, Kübler H, Haberkorn U, Eisenhut M, Wester HJ, Gschwend JE, Schwaiger M. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–74. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 10.Budaüs L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, Graefen M, Steuber T, Rosenbaum C. Initial experience of 68Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2015 Jun 24; doi: 10.1016/j.eururo.2015.06.010. S0302-2838(15)00513-8. [DOI] [PubMed] [Google Scholar]
- 11.Krohn T, Verburg FA, Pufe T, Neuhuber W, Vogg A, Heinzel A, Mottaghy FM, Behrendt FF. [68Ga]PSMA-HBED uptake mimicking lymph node metastasis in coeliac ganglia: an important pitfall in clinical practice. Eur J Nucl Med Mol Imaging. 2015;42:210–4. doi: 10.1007/s00259-014-2915-3. [DOI] [PubMed] [Google Scholar]
- 12.Law WP, Fiumara F, Fong W, Miles KA. Gallium-68 PSMA uptake in adrenal adenoma. J Med Imaging Radiat Oncol. 2015 Sep 23; doi: 10.1111/1754-9485.12357. [DOI] [PubMed] [Google Scholar]
- 13.Rowe SP, Gorin MA, Hammers HJ, Som Javadi M, Hawasli H, Szabo Z, Cho SY, Pomper MG, Allaf ME. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann Nucl Med. 2015 Aug 19; doi: 10.1007/s12149-015-1017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conforti R, Galasso R, Marrone V, Urciuoli L, Cirillo S. Paget’s disease. A case report. Neuroradiol J. 2012;25:475–80. doi: 10.1177/197140091202500410. [DOI] [PubMed] [Google Scholar]
- 15.Berruti A, Cerutti S, Fasolis G, Sperone P, Tarabuzzi R, Bertetto O, Pagani G, Zolfanelli R, Pallotti S, Bumma C, Fontana D, Rosseti SR, Dogliotti L, Angeli A. Osteoblastic flare assessed by serum alkaline phosphatase activity is an index of short duration of response in prostate cancer patients with bone metastases submitted to systemic therapy. Gruppo Onco Urologico Piemontese (G.O.U.P.) Anticancer Res. 1997;17:4697–702. [PubMed] [Google Scholar]
- 16.Anand IS, Florea VG. High output cardiac failure. Curr Treat Options Cardiovasc Med. 2001;3:151–9. doi: 10.1007/s11936-001-0070-1. [DOI] [PubMed] [Google Scholar]
- 17.Artigas C, Alexiou J, Garcia C, Wimana Z, Otte FX, Gil T, Van Velthoven R, Flamen P. Paget bone disease demonstrated on 68Ga-PSMA ligand PET/CT. Eur J Nucl Med Mol Imaging. 2015 doi: 10.1007/s00259-015-3236-x. In press. [DOI] [PubMed] [Google Scholar]


