Abstract
Adolescence has been characterized as a period of heightened sensitivity to social contexts. However, adolescents vary in how their social contexts affect them. According to neurobiological susceptibility models, endogenous, biological factors confer some individuals, relative to others, with greater susceptibility to environmental influences, whereby more susceptible individuals fare the best or worst of all individuals, depending on the environment they encounter (e.g., high vs. low parental warmth). Until recently, research guided by these theoretical frameworks has not incorporated direct measures of brain structure or function to index this sensitivity. Drawing on prevailing models of adolescent neurodevelopment and a growing number of neuroimaging studies on the interrelations among social contexts, the brain, and developmental outcomes, we review research that supports the idea of adolescent neurobiological susceptibility to social context for understanding why and how adolescents differ in development and well-being. We propose that adolescent development is shaped in part by brain-based individual differences in sensitivity to social contexts – be they positive or negative – such as those created through relationships with parents/caregivers and peers. As such, we recommend that future research measure brain function and structure to operationalize susceptibility factors that moderate the influence of social contexts on developmental outcomes.
Keywords: Adolescence, Brain development, Social environment, Neuroimaging, Individual differences
1. Introduction
Development proceeds through an intricate weaving of inherent, biologically-guided mechanisms and one’s experiences, good and bad. While much behavioral research shows that adolescence is a developmental period characterized by heightened sensitivity to social experiences in particular (e.g., peer interactions), recent reviews of neuroimaging-based evidence corroborate this characteristic of adolescence (Blakemore & Mills, 2014; Burnett, Sebastian, Cohen Kadosh, & Blakemore, 2011; Crone & Dahl, 2012; Nelson & Guyer, 2011; Nelson, Leibenluft, McClure, & Pine, 2005; Pfeifer & Allen, 2012; Somerville, 2013). Among the behavioral changes unique to adolescence relative to childhood or adulthood are increased self-consciousness, greater orientation away from parents and toward peers, heightened sensitivity to social acceptance, increased risk-taking especially in the presence of peers, and greater emergence of mental health problems that hinder social functioning. These characteristics may partially reflect maturational changes in how the adolescent brain codes and generates responses to social information (Nelson & Guyer, 2011; Steinberg, 2008). As such, individual differences in the structural growth and functional fine-tuning of neural circuitry that underpins social-cognitive and affective processing may relate to adolescents’ increased and differential sensitivity to social influences (Davey, Yucel, & Allen, 2008; Nelson & Guyer, 2011). Indeed, highly salient and impactful social contexts in adolescence, such as being embedded in hostile parent-child interactions or in exciting, accepting peer environments, likely interact with neurobiologically-based individual differences in shaping subsequent outcomes.
Theoretical frameworks concerning neurobiological susceptibility (Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2011), also known as biological sensitivity to context (Boyce & Ellis, 2005), differential susceptibility to environmental influences (Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007; Belsky & Pluess, 2009), and sensory processing sensitivity (Aron & Aron, 1997), provide a valuable model for considering how an adolescent’s level of neurobiological sensitivity might moderate the influence of social contexts on development. Specifically, these models suggest that individuals vary in their sensitivity to their environments, with some more affected than others. An implication of this is that individuals who are particularly sensitive to adverse social environments are also those who are most responsive to supportive social environments. At the same time, several models of adolescent brain development have suggested that changes in brain-based social sensitivity during adolescence promote developmental trajectories that range from a successful transition to adulthood to those culminating in psychopathology or maladaptation. We propose that considering an adolescent neurobiological susceptibility to social context framework (Figures 1 and 2), derived from extant models of neurobiological susceptibility and adolescent neurodevelopment, will yield a fuller characterization of biological sensitivity/susceptibility. By incorporating brain function and structure parameters that might reflect the neural instantiation of this sensitivity, future work can characterize not only those individuals at greatest risk for negative outcomes but also those most likely to benefit from supportive social contexts.
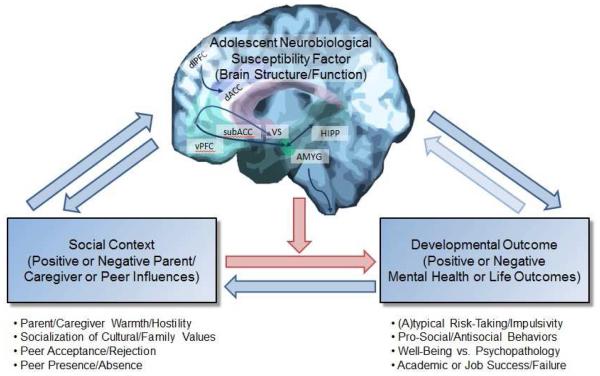
Fig. 1.
Conceptual model depicting our proposed adolescent neurobiological susceptibility to social context framework, whereby the manner and extent to which social contexts shape developmental outcomes is moderated by adolescents’ neurobiological susceptibility to social context as indexed by brain characteristics (e.g., function, structure). The pink arrows represent the moderated link from social context to developmental outcomes. The blue arrows represent additional bidirectional links among components of the model, which, although important, are not the focus of the proposed framework. Amygdala = AMYG; dorsal anterior cingulate cortex = dACC; dorsolateral prefrontal cortex = dlPFC; hippocampus = HIPP; subgenual anterior cingulate cortex = subACC; ventral prefrontal cortex = vPFC; ventral striatum = VS.
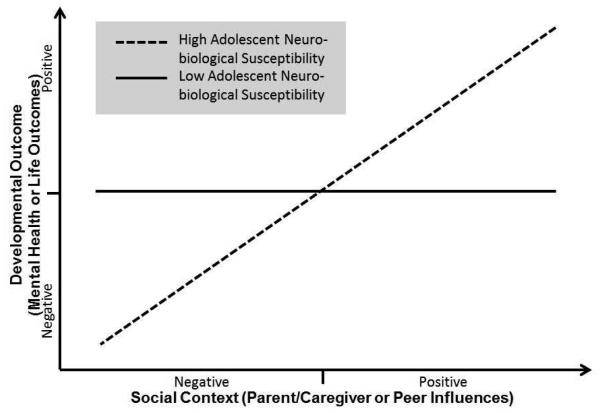
Fig. 2.
Graphical representation of the moderated effect of social contextual influences on developmental outcomes in accordance with adolescent neurobiological susceptibility. The x-axis represents variation in social contextual factors from negative to positive (e.g., harsh parenting vs. supportive parenting; peer victimization vs. peer support); the y-axis represents variation in developmental outcomes from negative to positive (e.g., depressive symptoms); and the two lines represent groups differing on adolescent neurobiological susceptibility, high versus low. Moderation by adolescent neurobiological susceptibility is shown in that the relation between adolescent neurobiological susceptibility and developmental outcomes is significant at both ends of the social-contextual influence.
In this review, we examine evidence from the neuroimaging literature that supports the ideas that adolescence is a period of heightened neurobiological sensitivity to social context and that individual differences in indices of brain structure and function can moderate social-contextual influences on development. By individual differences, we refer to brain-based constructs or characteristics for which there is substantial variability across people. By social contexts, we refer to key social relationships quantified by their positive and negative characteristics and focus on experiences with parents/caregivers and peers. Although several review papers highlight a long-standing empirical literature demonstrating that parent-child and peer relationships help shape adolescent development (Brown & Bakken, 2011; Brown & Larson, 2009; Steinberg & Morris, 2001), only recently has work focused on how these experiences are associated with features of the adolescent brain. This research indicates that adolescents’ social lives both leading up to and during adolescence relate to the sensitivity of the brain when perceiving, processing, and responding to social information (Blakemore & Mills, 2014). Furthermore, individual differences in this neurobiological sensitivity may be captured in adolescence by brain function/structure characteristics that moderate the influence of social contexts, past and present, on subsequent development.
Our review proceeds in the following sections. First, we discuss neurobiological susceptibility models (Ellis et al., 2011). While not traditionally centered on direct assessments of the brain, they have guided work on how endogenous, biological factors, such as genotype, render some individuals relative to others more responsive to and affected by environmental influences. Second, we discuss models of adolescent neurodevelopment that address specific neural circuits that are promising candidates for neurobiological moderators of social influences during this period. Third, we review findings from neuroimaging studies that show associations between adolescents’ brain function/structure and their experiences with parents/caregivers. Fourth, we similarly discuss results demonstrating associations between adolescents’ brain function/structure and experiences with peers. Finally, we offer conceptual and empirical future directions for research in this area. We suggest that the field of developmental cognitive neuroscience pursue research on the adolescent brain within the framework of adolescent neurobiological susceptibility to social context to pinpoint the brain-based circuits (e.g., social-affective; cognitive-regulatory), properties (e.g., volume, activation), and mechanisms (e.g., pruning, connectivity) with which social contexts interact to affect development. These recommendations may advance the field by capturing additional information about the conditions and mechanisms that underlie how individual neurobiological variability relates to outcomes of health and well-being.
Ultimately, the structural and functional properties of the adolescent brain may be critical moderators of social influences on development inasmuch as they (a) generate responses to social and affective signals from the environment, (b) undergo further maturation due to age and puberty, and (c) may be more reflective of, reactive to, and shaped by social influences during this period. Indeed, the brain undergoes fundamental alterations related to puberty (Giedd et al., 2006; Ladouceur et al., 2012; Lenroot & Giedd, 2010), potentially instantiating new neurobiological sensitivities that may inhibit or excite mechanisms of change in neural plasticity and gene expression in response to one’s social environment. Adolescence includes a phase of synaptic pruning, extensive myelination, volumetric changes, and change in balance of excitatory and inhibitory inputs that may render the adolescent brain particularly susceptible to new social contexts (Monahan et al., 2015) through what has been coined the “social re-orientation of adolescence” (Nelson et al., 2005). Because the organization and function of neural systems established early on can shape later stages of neural development, neurobiological sensitivities may partially reflect the influences of earlier social contexts on the brain, especially at the turning point of adolescence (Andersen, 2003). There is even evidence suggesting that the neural plasticity associated with adolescent development makes this a period of renewal and remediation (e.g., Bredy et al., 2004), capable of reprogramming the effects of earlier life in ways consistent with current experience. Thus, this period of marked growth and change in the human brain, second only to that seen in infancy, may have especially important and lasting effects on subsequent development (Andersen, 2003; Crone & Dahl, 2012; Giedd, 2008; Spear, 2000), a view consistent with research on juvenile non-human animals (e.g., Delville, Melloni & Ferris, 1998; Hoeve et al., 2013; Weintraub, Singaravelu & Bhatnagar, 2010).
2. Neurobiological Susceptibility Models
Studies of human development widely acknowledge that individuals vary in whether, how, and how much they are affected by their environment. In clinical and developmental psychology, there is a rich history of research aimed at identifying individual-difference variables that are predictive of a range of responses to environmental influences (Cicchetti & Rogosch, 2002; Masten & Obradovic, 2006; Rutter, Moffitt, & Caspi, 2006). Most of this work tracked development of psychopathology and other problematic outcomes, focusing on vulnerability to the adverse effects of negative experiences or exposures. For example, in Caspi et al.’s (2002) seminal study on the combined contribution of genes and environment to the emergence of antisocial behavior in males, being maltreated as a child was linked with developing violent tendencies. However, this effect was greater in individuals carrying the genetic allele associated with low versus high activity of the neurotransmitter-metabolizing enzyme monoamine oxidase (MAOA) that is associated with aggressive behavior. The dual-risk or diathesis–stress models (Hankin & Abela, 2005, Zubin, Feldman, & Salzinger, 1991) that emerged from this and similar work have suggested that genetic, hormonal, physiological, and other biological vulnerabilities or predispositions (diatheses) interact with environmental triggers (stress) to promote maladaptive trajectories.
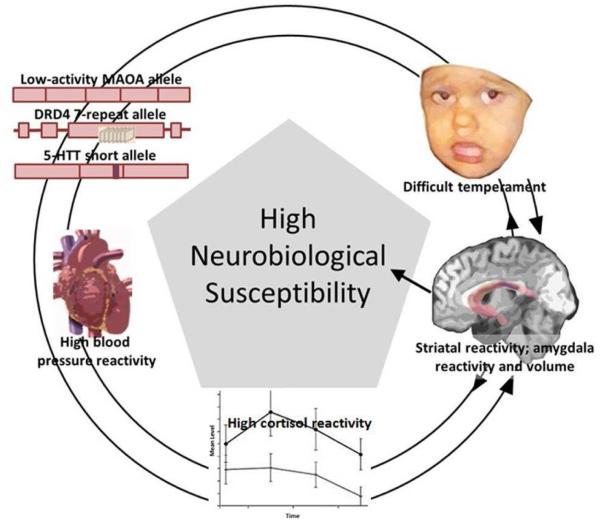
However, an accumulation of evidence later indicated that vulnerable individuals identified in diathesis–stress models might instead be viewed as sensitive, developmentally plastic, and malleable vis-à-vis environmental influences, regardless of their valence. This alternate perspective led to the biological sensitivity to context model (Boyce & Ellis, 2005) and the differential-susceptibility hypothesis (Belsky & Pluess, 2009), both of which share features with the concept of sensory processing sensitivity (Aron & Aron, 1997) from the personality literature. These independently developed but complementary and influential models have been joined under the umbrella term neurobiological susceptibility (Ellis et al., 2011; see also Moore & Depue (in press), Pluess (2015), and Stamps (2015) for highly relevant reviews of this general concept). The central tenet of these models is that individuals vary in their sensitivity to psychosocial contexts as a function of biological factors that are innate and/or conferred by early experience. Individuals low in sensitivity to the environment will fare similarly across all environments, whereas individuals who are highly sensitive to the environment are both more vulnerable to adverse contexts and more responsive to salubrious contexts. For example, for individuals with the low-as opposed to high-activity MAOA genotype, not only have high levels of childhood adversity been associated with extreme antisocial behavior (Caspi et al., 2002) but low levels of adversity have been associated with low or even absent antisocial behavior (Foley et al., 2004).
Based on this and other similar findings, a variety of biologically-rooted sensitivity or susceptibility factors have been identified that include candidate genes (e.g., MAOA; serotonin-transporter-linked polymorphic region, 5-HTTLPR; dopamine D4 receptor gene, DRD4; dopamine D2 receptor gene, DRD2); high stress reactivity in the form of adrenocortical, immune, or physiological response (e.g., higher cortisol reactivity; higher vagal withdrawal or respiratory sinus arrhythmia reactivity; low vagal tone); and biologically-based behavioral phenotypes such as temperament (e.g., behavioral inhibition; difficult temperament) and personality (e.g., neuroticism; sensory-processing sensitivity). These factors are thought to shape the way that individuals perceive, attend and react to, and behave within their environments, and to ultimately moderate associations between the environment and emerging competencies and psychopathologies (Boyce & Ellis, 2005). Moderation is expected because individuals’ underlying biological systems are thought to differentially monitor the environment to match its demands. For example, the biologically-based tendency toward hyperreactivity to novelty in infancy, known as behavioral inhibition, may manifest as social reticence and anxiety in childhood despite a strong motivation to interact with peers (Coplan, Rubin, Fox, Calkins, & Stewart, 1994; Rubin, Coplan, & Bowker, 2009). Conflict between high-avoidance and high-approach motivations may lead individuals to be particularly sensitive to the social milieu as they alternately check cues tapping either motivation, thus reinforcing biases through experience (Caouette & Guyer, 2014). Over time, highly susceptible individuals who encountered supportive environments may learn to take advantage of the positive, supportive features of their surroundings, while those exposed to risk and adversity may be more vigilant for and reactive to environmental threats and hazards. Similar accounts could be generated for other susceptibility factors that tend to be associated with negative emotionality and converge on learning through careful observation – pausing before acting rather than acting first. The ensuing, potent registration of experience upon the nervous system may allow neural processes to track survival-related subtleties (Belsky, 2005; Suomi, 1997; Wolf et al., 2008).
The degree to which individuals “tune” to the environment may be calibrated through genetic expressions, stress reactivity, and, as we propose, structural and functional neural characteristics that are context-sensitive and reactive to environmental cues, particularly within the social domain during adolescence (Meaney, 2001; Nelson & Guyer, 2011; Nelson et al., 2005). This heightened social sensitivity makes adolescence an important and model developmental period for investigating susceptibility at the neurobiological level. Despite the proposal that biological susceptibility comprises a “complex, integrated, and highly conserved repertoire of central neural and peripheral neuroendocrine responses” (Boyce & Ellis, 2005, p. 271; emphasis added), direct measures of brain structure and function have been largely unexamined as sensitivity factors (but see Yap et al., 2008 and Whittle et al., 2011, as exceptions). As interactions between biology and environment sometimes explain more variance in outcomes than do main effects (Beauchaine et al., 2008), accounting for these neural factors can clarify why some adolescents may be more primed for good or bad outcomes given their combination of neural susceptibility and social-contextual exposures.
On the one hand, it is unsurprising that the brain has not been investigated as a source of susceptibility. First, research guided by neurobiological susceptibility models tends to group individuals by susceptibility markers, categorize environments as high vs. low on a valenced dimension (or as high on oppositely valenced dimensions), and examine their interactive effects on a developmental outcome. It is then determined whether the association between the moderator and outcome is significant at both ends of the environmental variable (Roisman et al., 2012). Concrete, reliable indices of an individual’s group membership are readily derived when the susceptibility factor is, for example, genotype or temperament. However, neuroimaging researchers do not typically (but we argue increasingly could) categorize individuals in their samples according to high/low standing on a parameter of brain function, structure, or related properties, and/or examine the interactive effects of brain and social-contextual factors on developmental outcomes (Figure 2). Second, in developmental cognitive neuroscience work, the statistical approaches commonly used in functional neuroimaging analyses identify group-based trends. In fMRI analyses, contrasts between task events within the same group of individuals or between groups of individuals who differ in social context (e.g., maltreated vs. non-maltreated) or developmental outcome (e.g., depressed vs. non-depressed) are typically assessed rather than intragroup variability characterized, which is necessary to examine individual differences. Likewise, researchers rarely use quantified properties of the brain that draw on findings from group-based analyses to guide new work that uses them as markers to index individuals’ susceptibility to social influences. Although these steps can be taken, this renders much extant neuroimaging research lacking with regard to brain structure/function indices as markers of susceptibility. Finally, neuroimaging data are expensive and time-consuming to collect and analyze. These attributes can limit their integration within the longitudinal research designs needed to track developmental outcomes.
On the other hand, it is surprising that the brain has not been investigated as a source of susceptibility. For one, the brain is the primary determinant of behavior. Although changes in behavior are influenced by both congenitally and socially determined factors that create a backdrop for the brain’s influence, both operate through brain circuits to affect behavior. According to the neurosensitivity hypothesis (Pluess, Stevens, & Belsky, 2013), sensitivity of the central nervous system, which is jointly determined by direct and interactive effects of genetic and environmental factors, is the primary mechanism underlying susceptibility. Likewise, in considering that subjective experience of social contexts is central to transmitting their influence, it cannot be ignored that “[a]ll operations of the mind, conscious and unconscious (and that includes the perception and conceptualization of experiences), have to be based on the working of the brain” (Rutter, 2012, p. 17149).
Indeed, while the brain’s influence on behavior is instrumental for considering its role in shaping developmental outcomes, brain indices may be particularly useful for capturing differences in what Pluess (2015) terms sensitivity, the extent to which input coming from external influences is generated, perceived, and internally processed. Notably, sensitivity represents the first, requisite leg of susceptibility and does not necessarily have a one-to-one correspondence with responsivity, or the behavioral output that captures the extent to which one responds to the environment. To this end, focusing on the neural components of behavior is beneficial because assessing the brain allows sensitivity (and possibly the responsivity that follows) to be parsed into elements associated with different functions (e.g., affective reactivity, reward processing, conflict monitoring) that may not be evident through self-reported or observed behavior. Another advantage of using brain indices over other established susceptibility factors (e.g., genes, cortisol reactivity, heart rate variability, temperament) in testing hypotheses about adolescent neurobiological susceptibility to social context is the ability to reveal possible contributions from different classes of emotion, cognition, and motivation.
The brain should also be expected to underlie differential susceptibility inasmuch as it is intrinsically and reciprocally interconnected with genotypic to phenotypic systems already empirically demonstrated to manifest susceptibility. Activation of the anterior cingulate cortex (ACC), for example, is associated with genotypic variations in DRD2 (Pecina et al., 2013) and MAOA (Eisenberger et al., 2007), high skin conductance reactivity (Nagai et al., 2010), low heart rate variability (Gianaros et al., 2004; O’Connor et al., 2007; Lane et al., 2004), and negative emotionality/neuroticism (Haas et al., 2007). All of these are well-established susceptibility markers in contexts of social and affective processing. With the brain as the primary determinant of behavior, it stands to reason that it arbitrates and integrates between these different levels of analysis, which may demonstrate the operation of susceptibility in different domains of functioning and combine in cumulative and/or multiplicative ways. Expanding the range of neurobiological susceptibility factors examined would ultimately be useful for deriving comprehensive, multi-modal profiles regarding which adolescents are likely to experience which outcomes, to the benefit of predictive accuracy and prevention and intervention efforts.
Even within a given level of analysis, established susceptibility factors may act on different underlying neurobiological circuits, resulting in a variety of neurobiological pathways through which susceptibility manifests to impact behavior (Hariri, 2009; Moore & Depue, in press). For example, the DRD2 and DRD4 genes encode types of dopamine receptors that are richly distributed in the striatum and other brain regions and that associate these regions with individual differences in attention and reward-sensitivity (Schultz, 2006; Wise, 2004) and responses to aversive stimuli (Horvitz, 2000). As another example, the 158Met allele of the COMT gene is linked to increased working memory capacity and efficient prefrontal information processing (Tan et al., 2007). Because numerous complex, interactive pathways contribute to neural processing and, through the brain, to behavior, the brain may provide especially effective summary measures of susceptibility. With increasingly advanced methodologies, such as imaging genetics, this can be taken even further by quantifying linkages from genotype to brain to outcome, as indeed “it may be that any type of reactivity pattern may comprise many specific gene-environment-outcome pathways (or be characterized by domain specificity, where different individuals are susceptible for different reasons to different environmental influences for different outcomes” (Moore & Depue, in press, p. 2).
Finally, structural and functional brain indices may be sufficiently stable within and across developmental periods (Caceres et al., 2009; Fair et al., 2012; Forbes et al., 2009; Hariri, 2009; Johnstone et al., 2005; Manuck et al., 2007; Miller et al., 2002, 2009; Wu et al., 2014; Zuo et al., 2010) to warrant treatment as susceptibility factors. The test-retest reliability of fMRI measures is critical to establish in longitudinal developmental work to be able to separate what is stable vs. changing about neural response, such as due to development vs. noise. For example, in adults, high test-retest reliability (e.g., intraclass correlation coefficients (ICCs) > .70) of amygdala response to emotional faces was found across multiple sessions conducted over days (Gee et al., 2015) and months (Johnstone et al., 2005), suggesting that individual differences in certain types of neural response are stable in adults (but see Sauder, Hajcak, Angstadt, & Phan, 2013, for an example of poorer reliability in amygdala reactivity that is affected by stimulus type). Even more imperative for our framework is establishing the reliability of fMRI measures in adolescent samples. Test-retest reliability of the amygdala's response to aversive stimuli over three measurement occasions across six months showed low reliability (ICC < .40) in a sample of adolescents (N= 22; ages 12-19 years) (van den Bulk et al, 2013). Nevertheless, Koolschijn et al. (2011) observed that, in contrast to children (N = 10), adolescents (N = 12) and adults (N = 10) showed fair (ICCs = .41-.59) to good (ICCs = .60-.74) reliabilities for activations in a variety of brain regions (e.g., precuneus, ACC, insula, inferior and superior parietal cortices, angular gyrus) during a rule-switch task separated by ~3.5 years. These values are comparable to the stability of other susceptibility factors (e.g., physiological measures; Cohen & Hamrick, 2003; Cohen et al., 2000), suggesting that brain indices may be sufficiently reliable to join the collection of established susceptibility markers.
3. Neurobiological Models of Adolescent Brain Development
Existing models of adolescent brain development provide a foundation for identifying candidate susceptibility brain circuits that may moderate the influence of different social contexts on functioning. These circuits have tight reciprocal relations with the social sensitivity observed during adolescence, making brain-based susceptibility to social context a plausible marker of risk, resilience, and positive outcomes. Prevailing theories (Casey, Jones, & Hare, 2008; Crone & Dahl, 2012; Nelson & Guyer, 2011; Nelson, Leibenluft, McClure, & Pine, 2005; Pfeifer & Allen, 2012; Steinberg & Morris, 2008) draw on structural and functional differences that distinguish the adolescent brain from the child or adult brain (Casey et al., 2008; Giedd, 2008; Gogtay & Thompson, 2010; Guyer et al., 2008). These models have in common the idea that adolescence is a period of heightened social responsivity due to differential weighting of input from distinct yet interconnected neural circuits, namely, social-affective and cognitive-regulatory systems. These differentials are lessened or come into balance with maturation and experience. Another commonality is that these models were generated primarily to account for the “dark side” of adolescent development, such as normative increases in poor decision-making, risky behavior, and mental health problems (but see Crone & Dahl, 2012, and Pfeifer & Allen, 2012, for neurodevelopmental accounts of adolescence as a time of opportunity). We nevertheless propose that these models leave room for exploring neural moderators of social influences on developmental outcomes in the for-better and for-worse fashion proposed by neurobiological susceptibility models. Below we describe briefly four prominent models of adolescent neurodevelopment.
Dual-systems models (Casey, Jones, & Hare, 2008; Steinberg, 2008) account for the unique changes observed in adolescence by focusing on the temporal disjoint between the development of a social-affective system – comprised of limbic and paralimbic regions such as the amygdala, ventral striatum (VS), orbitofrontal cortex (OFC), medial prefrontal cortex (mPFC), and superior temporal sulcus (STS) – relative to cognitive control systems, which mature at a slower pace and include the lateral and ventral prefrontal and parietal cortices and their interconnections with the anterior cingulate cortex (ACC). A result of this temporal gap is that adolescence, more so than childhood, may be fraught with a heightened sensitivity to affective and motivational cues in salient social contexts that tip behavior in the direction of overreactivity, risk-taking, and impulsivity rather than self-control. Given the ramping up of social sensitivity in adolescence, how social-affective circuitry was shaped by earlier developmental periods might also become manifest in reaction to current contextual influences. Furthermore, the larger the developmental gap or the longer that it exists, the greater the period of vulnerability or plasticity to environmental influences.
Adding nuance to dual-systems models, the Triadic Model (Ernst & Fudge, 2009; Ernst, Pine, & Hardin, 2006) proposed that motivated behavior in adolescence results from the coordination of two social-affective neural circuits via cognitive circuitry. The social-affective circuits include an approach system mediated by the VS and an avoidance system mediated by the amygdala. Reconciliation between these approach and avoidance systems is ascribed to a cognitive regulatory system spearheaded by the PFC. The Triadic Model also speaks to the bivalent effects of adolescent neurobiological susceptibility to social context inasmuch as valence-related biases emerge against the role of both systems in coding positive and negative social experiences. Indeed, the VS reacts to not only positively valenced contexts but also negative ones (e.g., peer acceptance and rejection; Gunther Moor, van Leijenhorst, Rombouts, Crone, & Van der Molen, 2010; Guyer et al., 2015; Guyer, Choate, Pine, & Nelson, 2012), and the amygdala reacts to not only negatively valenced contexts but also positive ones (e.g., fearful and happy faces; Canli, Sivers, Whitfield, Gotlib, & Gabrieli, 2002, or negative/threatening and positive/interesting information; Hamann, Ely, Hoffman, & Kilts, 2002; Vasa et al., 2011). As such, individual differences in ventral striatal and amygdala sensitivity can contribute to both positivity and negativity biases.
The Social Re-Orientation framework (Nelson et al., 2005) focuses on how adolescent social behavior is rooted in the development of brain regions nested across a social information processing network (SIPN) of nodes. The detection node, which is already well-developed in early life, supports the perception and categorization of basic social properties of stimuli by engaging regions such as the superior temporal sulcus (STS), intraparietal sulcus, fusiform face area, and inferior temporal and occipital cortical regions. The affective node processes social information by imbuing it with positive/rewarding or negative/punishing salience by engaging the VS, amygdala, hypothalamus, bed nucleus of the stria terminalis, and the OFC. Finally, the cognitive-regulatory node performs complex cognitive processing of social stimuli (e.g., perceiving others’ mental states, inhibiting prepotent responses, generating goal-directed behavior) via input from the medial and dorsal PFC (mPFC; dPFC) and areas of the ventral PFC (vPFC). The affective node, although somewhat well-established in early life, sees an upsurge in reactivity and sensitivity during adolescence with the influx of gonadal steroids at the onset of puberty (Halpern, Udry, & Suchindran, 1997, 1998; McEwen, 2001; Romeo, Richardson, & Sisk, 2002), whereas the cognitive-regulatory node follows a more protracted developmental course into early adulthood (Casey, Giedd, & Thomas, 2000), supporting increasingly complex and controlled responses to salient social stimuli.
Elaborating on the cognitive-regulatory node, Nelson and Guyer’s (2011) extension of the SIPN model focuses on the gradual attainment of not only cognitive control but flexibility in social behavior. Three aspects of social flexibility are identified. Each is supported by areas within the vPFC. Emotional value computation is supported by the medial part of the OFC, while both rule generation/acquisition and inhibitory control of social behavior are subserved by more lateral areas of the orbital gyrus and inferior frontal gyrus. As flexible social behavior is critical for competently interacting with others and adapting to social contexts, perturbations in the function of the vlPFC, in particular, relate to psychopathology in adolescence, such as social anxiety disorder (Guyer et al., 2008; Monk et al., 2006; Monk et al., 2008). Conversely, achieving social flexibility may protect some adolescents from developing psychopathology and promote their well-being. Such flexibility may even support thriving in the case of highly susceptible adolescents, who are posited to exhibit outcomes at either extreme of the continuum depending on exposure to unsupportive or supportive environments (e.g., see Belsky & Beaver, 2011 regarding differences in adolescent self-regulation as a function of genetically-defined neurobiological susceptibility and quality of parenting).
Across these neurodevelopmental models, maturation of the PFC and its connections with subcortical regions is thought to foster the acquisition of flexible emotional and behavioral regulatory abilities in the face of varied social environments. Adolescents have to navigate and adapt to new social contexts (e.g., managing peer acceptance, finding romantic partners, individuating from parents). These behaviors are guided by input from key brain regions that are reactive to these contexts. Processes related to social status, interpersonal motivation, self-esteem, and social evaluation will be augmented via hot, socially sensitized regions, with hyperresponsivity of implicated neural regions relating to extreme outcomes within these contexts. Our contention is that the intensified salience of social context in adolescence will, particularly for more susceptible adolescents, guide social-affective circuitry toward becoming primarily attuned to what is (or is perceived as) relevant in the social environment – be it negative, threatening, and/or antisocial vs. positive, encouraging, and/or prosocial. This attunement may occur via the brain’s coding of social-contextual cues (e.g., Todd, Cunningham, Anderson, & Thompson, 2012), a process not explicitly articulated in existing neurobiological susceptibility models. In addition, discussed in detail below, a supportive environment that fosters regulatory abilities through development of prefrontal neurocircuitry could help place susceptible adolescents in a prime position to secure the best outcomes of all. Such adolescents would be not only more sensitive to the contingencies of positive social environments through social-affective neurocircuitry but also, through cognitive-regulatory neurocircuitry, better able to control and leverage that sensitivity toward adaptive ends. For example, adolescents who are highly context sensitive and exposed to highly positive environments may gain superior proficiency in using subtle social cues to persist in positive goal pursuit and to model, interact with, and empathize with others. They might also better learn how to downregulate distress and divert away from adverse outcomes (Figure 1).
In sum, we propose that models of adolescent neurodevelopment serve as a basis for exploring neural moderators of social influences on outcomes in the for-better and for-worse fashion proposed by neurobiological susceptibility models. First, through the coordination of different systems (e.g., approach vs. avoidance) that are sensitive and responsive to different contextual cues (e.g., incentives vs. threats), social-affective circuits may collectively mediate an adolescent’s susceptibility to social context. Indeed, social-affective circuitry that is primarily reactive to negative social contexts also shows responsiveness to positive ones, and vice versa, perhaps facilitating the encoding of context overall. Second, each neurodevelopmental model addresses a growing capacity in adolescence for self-regulation and cognitive flexibility – an ability to steer the sensitive ship – in the transition to more agentic and independent behavior. The ability to control one’s thoughts, emotions, and behaviors in response to changes in internal and external conditions is critical to thriving. We highlight that the flexibility of this faculty in adolescence provides another avenue for explaining how adolescents who are highly context sensitive and exposed to supportive environments are best able to secure positive developmental outcomes relative to those in negative environments who show detrimental outcomes.
We now turn to a review of key empirical findings from the neuroimaging literature that illustrate the potential for individual differences in brain structure and function in adolescence to interact with primary social contexts to impact outcomes. First, we review the influence of the family/caregiving context. Then, we proceed to that of the peer environment. The majority of this work was not designed to quantify neural sensitivity as a moderating individual-difference factor nor to assess change in behavior over time. Nevertheless, it offers clues for characteristics of the brain and of social contexts that merit further study, allowing for the consideration of a new model of adolescent neurodevelopment.
4. Social Contexts and the Adolescent Brain
4.1. Family/caregiving contexts
A substantial body of research indicates that the social context created through one’s caregiving experiences, including parenting style, quality of parent-child interactions, family climate, and socialization of family and cultural values, is an important predictor of adolescent development (Collins, Maccoby, Steinberg, Hetherington, & Bornstein, 2000; Darling & Steinberg, 1993; Steinberg, 2001; Steinberg & Morris, 2001). These effects should manifest most robustly in susceptible individuals. Indeed, parenting influences have been demonstrated to be moderated by individual differences in biological sensitivity, such as genetic phenotype (Bakermans-Kranenburg & van Ijzendoorn, 2011; Knafo, Israel, & Ebstein, 2011) and stress reactivity (Hastings, Klimes-Dougan, Brand, Kendziora, & Zahn-Waxler, 2015). Although adolescent neurobiological susceptibility to social context may be a product of these biological factors, early-life experiences, and their interaction (Boyce & Ellis, 2005), it is this susceptibility in adolescence that may be important for later outcomes. In the following sections, we first review research that speaks to individual differences in brain characteristics that may moderate the influence of parenting/caregiver experiences on behavioral and developmental outcomes in adolescence. We then discuss findings that suggest how susceptibility may operate for bivalent outcomes based on how the brain relates to different experiential and experimentally manipulated parenting/caregiving experiences.
The most promising candidate neural susceptibility factors from our review of parent/caregiver influences concern brain structure. Relative to brain function, brain structure has a strong genetic basis and may therefore demonstrate individual differences that are more stable, with more evolutionarily novel areas, like the PFC, showing increasing heritability from childhood to adolescence (Jansen et al., 2015; Lenroot et al., 2009). This is consistent with the idea that neurobiological susceptibility occurs via the influence of genetic variants on neurobiological circuits that respond to caregiving (Bakermans-Kranenburg & van Ijzendoorn, 2007, 2011; Belsky & Beaver, 2011; Belsky & Pluess, 2009; Pluess & Belsky, 2013). In contrast to brain structure, brain function serves to immediately track, respond to, and reflect perceived differences in one’s environment. Brain function has been theorized to be a fitting index of sensitivity, with neural reactivity to contextual factors considered a joint function of the (1) magnitude of one’s characteristic neural reactivity and (2) magnitude and type of eliciting stimuli (Moore & Depue, in press). Because stable individual differences reflect coordinated patterns of thought, emotion, and behavior in the face of eliciting circumstances (Fleeson, 2001), these tendencies are posited to arise from regularities in the functioning of relevant brain systems that have been “tuned” via learning and experience in different social contexts over time. As such, both brain structure and function, which may interrelate and whose development inform each other (Hao et al., 2013; Honey, Thivierge, & Sporns, 2010; Paus, 2013; Power et al., 2010; Zielinski, Gennatas, Zhou, & Seeley, 2010), may both serve as susceptibility mechanisms.
4.1.1. Evidence of individual differences in neurobiological susceptibility: Structural findings
Although direct brain-based indices of differential susceptibility are currently lacking in the literature, a handful of studies highlight a set of promising candidates to examine as neural indices of adolescent susceptibility to social context. This work has documented associations between adolescent brain structure and laboratory measures of parent-adolescent interactions that quantify such aspects as level of parental warmth vs. hostility; adolescent positivity vs. aggression or dysphoria; and parents’ and adolescents’ responses to these behaviors in each other. Because family dynamics remain formative in adolescence, connecting measures of brain structure to observations of parent–adolescent interactions provides an ecologically-valid approach for investigating neurobiological sensitivity to social context in considering their combined effect on later outcomes. These observation measures are treated as a snapshot or window into family processes likely to have been chronically experienced and that may be linked to adolescent neural development. Although assessment of concurrent rather than longitudinal relations in some of these studies limits inferences regarding causality or developmental sequence, and although this research does not control for the potentially confounding genetic influences of the child being nested within the family, findings suggest various operations of neurobiological social sensitivity.
First, individual differences in adolescent brain structure have been linked to affective and behavioral responses to emotionally charged interactions with parents in ways that can inform susceptibility models of positive or negative developmental outcomes. Whittle and colleagues (2008) found that, in the context of a challenging conflict resolution exercise between adolescents (ages 11-13) and their parents, having larger amygdala was associated with adolescents’ maintaining aggressive behaviors toward their mothers for longer duration. Furthermore, in males, decreased leftward ACC volumetric asymmetry was also associated with maintaining aggression toward mothers, and decreased leftward OFC volumetric asymmetry was associated with reciprocating mothers’ dysphoric behavior. This set of findings could suggest a susceptibility effect on the risk-augmenting side of the equation, i.e., diathesis stress, given that (1) volume of the amygdala, a region traditionally associated with reacting to threat cues and generating negative affect, may reflect a history of greater engagement, and (2) structural asymmetries favoring the right PFC have also been associated with both increased negative affect (Canli, 2004; Davidson & Fox, 1989; Fox et al., 2001) and diminished emotion regulation (Jackson et al., 2003).
Other work is more directly demonstrative of individual differences in neurobiological response to family influences in the for-better and for-worse manner described by neurobiological susceptibility models. Whereas Whittle et al. (2008) found that larger amygdala volumes and less leftward ACC asymmetry were associated with more maladaptive responses to maternal aggression in adolescent males, Yap et al. (2008) found that these same exact factors predicted the lowest levels of depression among adolescent males (ages 11 to 13) with low-aggression mothers. Yap et al. also identified a possible neurobiological susceptibility mechanism in females whereby smaller bilateral amygdala volume was associated with less depression in adolescents when mothers were low in aggression but with more depression when mothers were high in aggression. Taken together, these findings illustrate bivalent outcomes in contexts of high and low adversity as moderated by individual differences in brain structure.
In both of the above studies, brain morphology and affective outcomes were measured concurrently. However, Whittle et al. (2011) prospectively examined hippocampal volume as a moderator of the effect of maternal aggression on change in depressive symptoms from early (ages 11-13) to mid (ages 13-15) adolescence. They found that, for girls, larger bilateral hippocampus predicted greater and lesser subsequent depressive symptoms in the context of high and low maternal aggression, respectively, during a parent-child conflict resolution exercise. Thus, at least for females during adolescence, greater hippocampal volume may interact with familial contexts by moderating whether a susceptibility to depression is expressed or inhibited. This finding gives support to the idea that directly measured neural markers of neurobiological sensitivity show differential moderation of parenting contexts. It is interesting to consider whether hippocampal volume also moderates the influence of supportive family characteristics on development. Higher gray matter density in the hippocampus (as well as in the orbitofrontal gyrus) was found in adolescents whose mothers had greater general interpersonal affiliation (Schneider et al., 2012), a finding consistent with work in animal models showing that behaviors denoting a pleasant experience (e.g., appetitive vocalizations while being tickled) were linked with hippocampal cell proliferation and survival (Wohr et al., 2009; Yamamuro et al., 2010). These findings suggest sensitivity of the hippocampus to positive contexts that include supportive parenting.
That the amygdala and hippocampus may be loci of neurobiological susceptibility makes sense. Both the amygdala and hippocampus are known to mediate attentional and learning aspects of emotion (Baxter & Murray, 2002; Calder, Lawrence, & Young, 2001; Kringelbach & Berridge, 2010). It is likely that they have a superordinate function that operates independent of valence, as part of a broad and overlapping affective circuitry (Ernst & Fudge, 2009). More work is needed to explore possible gender effects of amygdala volume as an index of susceptibility to context, as Whittle et al. (2008) and Yap et al. (2008) collectively suggested that larger amygdala volumes in boys and either larger or smaller volumes in girls reflect susceptibility. However, the amygdala’s interactive effects on bivalent outcomes is consistent with its general role in processing the needs, goals, and values of the individual (Cunningham & Brosch, 2012) and in its eliciting positive and negative affect with consequences for avoidance or approach behaviors in different contexts (Bechara, Damasio, Damasio, & Lee, 1999). Furthermore, the social brain hypothesis (Dunbar, 2009) suggests that a greater volume of regions within social-affective circuitry reflects greater processing capacity, consistent with evidence of greater amygdala volume being linked to overall social sensitivity rather than specifically to threats. For example, large amygdala volume is positively associated not only with separation anxiety (Redlich et al., 2015) but also with mental state inference (Rice et al., 2014) and social network size and complexity (Bickart et al, 2010; Kanai et al., 2012), including in adolescents (Von der Heide, Vyas, & Olson, 2014). Likewise, the hippocampus, known for its contextual sensitivity (Hirsh, 1974; Rudy, 2009; Fanselow, 2010), helps encode episodic and emotional information that arises during motivationally relevant events. The hippocampus is thought to carry out this function often independently of valence; that is, it supports binding the elements of scenes, events, and contexts into representations across time, ultimately guiding behavior in line with these representations (Schacter & Addis, 2007). Finally, for both the amygdala and hippocampus, their consideration as regions within a connectome of regions is imperative.
4.1.2. Bivalent caregiving experiences and social-affective circuitry: Affective “tuning” via brain function
Given initial evidence that brain characteristics – such as brain structure – might mark neurobiologically susceptible adolescents, we now consider the paths or mechanisms by which sensitive adolescents who are exposed to bivalent caregiving contexts reach divergent outcomes. Positive versus negative caregiving contexts may sensitize the social-affective circuitry of the brain to their contingencies. Neural processing that assigns value to social-affective information is instantiated in ways consistent with the aspects operative in and goals promoted by different caregiving contexts. As such, an initially neutral social sensitivity may develop into a biased sensitivity that disproportionately registers, processes, and responds to the adverse vs. supportive features of the social environment (Pluess, 2015). This is consistent with the idea that “what one thinks should be attended to in a dangerous world is quite different from what should be attended to in a world of opportunities” (Cunningham & Brosch, p. 56). How this tuning of brain function occurs through learning and experience in different contexts may be revealed, in part, by research examining the moderating effects of brain function on the link between caregiving contexts and behavioral outcomes, including across the lifespan. Indeed, it is important to note that neurobiological susceptibility can operate before adolescence. However, what an adolescent has been tuned to, and what will thus likely contribute to experiences in new social contexts, will become apparent during this period of enhanced social sensitivity.
Consistent with the idea of affective tuning, studies have documented the impact of early-life stress and family adversity on brain function in adolescence and beyond. For example, adolescents (ages 9-18) who experienced caregiver deprivation and emotional neglect in infancy showed amygdala and hippocampus hyperactivation when processing threatening information (Maheu et al., 2010). This finding is consistent with structural evidence demonstrating that more years of orphanage rearing in early childhood were associated with larger amygdala volume decades later that also predicted anxiety symptoms (Tottenham et al., 2010). Associations between unsupportive caregiving contexts and the brain have also been noted in adolescents’ reward circuitry. Among a sample of adolescents (ages 9-17), increased and sustained neural response to maternal criticism in the lentiform nucleus was associated with perceiving criticism more negatively (Lee, Siegle, Dahl, Hooley, & Silk, 2014). Casement and colleagues (2014) found in a sample of girls that low parental warmth in early adolescence (ages 11-12) was associated in mid-adolescence (age 16) with increased sensitization to monetary reward cues in the amygdala, VS, and mPFC; this increased VS and mPFC response mediated the link between low parental warmth and depressive symptoms. The authors speculated that greater activation of these regions, which are generally related to reward-processing and coding social information about oneself and others (Amodio & Frith, 2006; Gallagher & Frith, 2003), may reflect maladaptive valuation of and expectations for performance based on unfavorable past social experiences. Thus, neurobiological susceptibility to social context may become expressed over time through gradual reinforcement of the brain’s coding and valuation of social and evaluative experiences. Taken together, the results of these studies suggest that regions within social-affective circuitry are functionally sensitive to adverse caregiving experiences and could signify a neural marker for highly susceptible individuals.
Experiences of supportive parenting have also been associated with brain characteristics and developmental outcomes, evidence that is important for a framework hinged on the influence of bivalent experiences for susceptible individuals. For example, Morgan, Shaw, & Forbes (2014) found that greater maternal warmth experienced by boys in early childhood (18 and 24 months) was associated with reduced mPFC activation to anticipated and experienced loss of monetary rewards in late adolescence/early adulthood (age 20). These results suggest that parenting characterized by affection and warmth may diminish neural response to negative events in brain regions associated with integrating emotional and social information, including about self and others. This protective effect of maternal warmth was stronger for boys exposed versus not exposed to maternal depression in early childhood, consistent with the notion that susceptibility tends to stem from an early-appearing baseline of negative reactivity and suggesting a neurobiological attunement of the mPFC to bivalent parenting contexts. These results indicate that regions involved in reward learning (e.g., the striatum and mPFC) are sensitive to the nuances of maternal social behavior. That is, the brain function of adolescents whose mothers’ tendency is toward friendly and loving behavior may reflect a learning history initiated since childhood of reward loss vs. receipt as being of low value or importance. This example suggests how the effects of the social environment on the behavior of susceptible adolescents may eventually be conferred – through the shaping of neural responses to certain elicitors over time in regions related to social sensitivity.
Tracking not only adolescents’ familial contexts but also the stimuli that tap their social sensitivity and with what developmental consequences would help illuminate how sensitivity of some brain regions is adaptive or maladaptive depending on context. The VS, which processes reward cues, is one such set of regions. Although some research relates greater VS reactivity to increased risk taking behaviors in adolescence (Bjork, Chen, Smith, & Hommer, 2010; Bjork & Pardini, 2015; Chein et al., 2011; Galvan et al., 2007; Gatzke-Kopp et al., 2009; Somerville et al., 2011), VS response may be sensitive to the socialization of family and cultural values in its linkage to adaptive social behaviors and reduced risk taking. Latino adolescents (ages 14-16) who reported greater family obligation values showed blunted VS response to monetary incentive cues, a response associated with less risk-taking behavior (Telzer, Fuligni, Lieberman, & Galvan, 2013a). Other work found that adolescents (ages 15-17) who previously reported greater identification with, and fulfillment from helping, their family had heightened response in VS when making costly donations to their family as opposed to gaining monetary reward (Telzer, Masten, Berkman, Lieberman, & Fuligni, 2010). Related work found that increased VS response to these prosocial acts predicted decreases in adolescent risk-taking a year later (Telzer, Fuligni, Lieberman, & Galvan, 2013b). Thus, “the very same neural region that has conferred vulnerability for adolescent risk taking may also be protective against risk taking” (Telzer et al., 2013b, p. 45). Furthermore, Telzer, Fuligni, Lieberman, and Galvan (2014) found that VS reactivity to eudaimonic (e.g., meaning/purpose, prosocial) vs. hedonic (e.g., risk-taking, self-gratifying) rewards predicted longitudinal declines and inclines, respectively, in depressive symptoms. This set of findings raises the possibility that neural sensitivity to reward relates to adaptive or maladaptive outcomes depending on the class of reward (e.g., hedonic, monetary, social, eudaimonic) to which sensitivity becomes oriented as a function of family/caregiving socialization experiences and learning.
4.1.3. Bivalent caregiving experiences and cognitive-regulatory circuitry: PFC maturation
As discussed so far, bivalent outcomes may occur for susceptible adolescents because positive contexts promote behavior that is motivated toward socially valued opportunities whereas negative contexts promote behavior defined by threat and health-compromising risks. However, different trajectories might also take shape because the ability to use cognitive regulation to achieve adaptive goals will have been reinforced in positive, not negative, contexts. As such, differential development of cortical versus subcortical circuitry may occur in susceptible adolescents exposed to different family contexts, contributing to divergent outcomes. Behavioral research indicates that individual differences in executive function and self-regulation abilities develop in systematic ways across childhood, stabilizing in early adolescence (Deater-Deckard & Wang, 2012). Findings from cross-sectional and longitudinal studies point to the importance of warm, sensitive, and responsive parenting/caregiving for strengthening these faculties (e.g., Bernier, Carlson, Deschênes, & Matte-Gagné, 2012; Hammond, Müller, Carpendale, Bibok, & Liebermann-Finestone, 2012; Hughes, 2011). Through complex biology-environment interplay, regulatory abilities (or the impairment of these abilities) are transferred through parent/caregiver-youth relationships that provide powerful experiential contexts for scaffolding and practicing them (or not) (Deater-Deckard, 2014).
Neuroimaging studies support this picture. Negative contexts show dysregulating effects. Widespread deficiencies in cortical thickness were observed in children who suffered early-life psychosocial deprivation from institutional rearing, deficiencies that mediated problems with attention and impulsivity (McLaughlin et al., 2014). In adolescence (ages 9-17), exposure to maternal criticism was associated with increased activity in social-affective circuitry (e.g., lentiform nucleus, posterior insula) and decreased activity in cognitive control (e.g., dlPFC, ACC) and social cognitive (e.g., TPJ, posterior cingulate cortex/precuneus) circuitry (Lee, Siegle, Dahl, Hooley, & Silk, 2014). Similarly, being raised with harsh parenting and other family stressors was related to positive connectivity, denoting less differentiated function, of amygdala with right vlPFC in response to emotional stimuli in adulthood (ages 18-36), suggesting that vlPFC was not exerting an inhibitory role on amygdala response (Taylor, Eisenberger, Saxbe, Lehman, & Lieberman, 2006). There is also evidence that early adversity (age 1) is associated with accelerated development of negative amygdala–mPFC coupling in adolescence more typically seen in adults (Gee et al., 2013). Accelerated cortical development may be associated with less optimal behavioral outcomes later on, perhaps because a truncated period of immaturity lessens the opportunity to learn how to regulate oneself in different social environments to reach adult efficiency (Lu et al., 2009; Nelson & Guyer, 2011). Taken as a whole, negative contexts are associated with cognitive and affective dysregulation at the neural level. We propose that, while all adolescents reared in these contexts face disadvantage, greater neurobiological susceptibility to social context renders those adolescents with greater neural sensitivity disadvantaged to a greater extent.
Conversely, positive environments promote the development of cognitive regulatory circuitry that should help adolescents attain positive developmental outcomes. In a direct test of differential susceptibility, genetically-defined susceptible vs. non-susceptible children (age 8) had the highest PFC volume, which was associated with better cognitive functioning, when they were reared in relatively positive environments; at trend levels of significance, they had the lowest PFC volume when reared in negative environments (Brett at al., 2015). In fact, consistent with “vantage sensitivity” (Pluess & Belsky, 2013), which focuses on susceptibility to environmental influences that are supportive, cognitive functioning was best in susceptible children who developed in more positive contexts. Belsky and Beaver (2011) found in adolescent males (but not females) (ages 16-17) that the more plasticity alleles they had, the more and less self-regulated behavior they showed in supportive and unsupportive parenting conditions, respectively (also see Laucht et al., 2007). We propose that enhanced development of PFC circuitry will be enlisted in adolescence to serve salubrious goals. Telzer et al. (2011) found that greater socialization of family values was related to recruitment of cognitive regulatory and mentalizing regions that were functionally connected with VS when adolescents were exposed to the prosocial context of giving to their family. In sum, findings suggest that PFC circuitry that is hypoactive or otherwise compromised in function, structure, or connectivity is manifested in susceptible adolescents exposed to negative environments, whereas susceptible adolescents exposed to enriching environments show PFC characteristics associated with securing positive outcomes (see also Moore & Depue, in press, for discussion of a somewhat related concept, neural constraint, as it relates to susceptibility).
4.2. Peer contexts
Among the most striking changes in adolescence is a shift in social affiliation from being family- to peer-oriented (Rubin, Bukowski, & Parker, 2006; Steinberg & Morris, 2001). Upon entering adolescence, youths spend more time with peers (Csikszentmihalyi & Larson, 1984), increasingly seek out and value peers’ opinions (Brown, 1990), and are generally more preoccupied with peer acceptance (Parkhurst & Hopmeyer, 1998), especially as the risk for peer rejection increases during this period (Coie et al., 1990). Although these social changes are associated with consequences for adolescents’ emotional well-being and mental health, little is known about how individual differences in neurobiological sensitivity to the peer milieu may be linked to adolescent outcomes and subsequent adult trajectories. Nevertheless, research has begun to shed light on the neural underpinnings of adolescent sensitivity to the contexts of peer presence, peer evaluation, and social exclusion, including with regard to how adolescents vary in this sensitivity. Here, we focus on individual differences in adolescent brain function during neural response eliciting situations involving peers, and the associations of the above with emerging psychopathology or competence. To our knowledge, there are not currently research findings relating indices of adolescent brain structure with peer contexts and developmental outcomes (although, as cited above, there is evidence of a relation between amygdala volume and social network complexity in both adolescence and adulthood; Von der Heide et al., 2014).
4.2.1. Peer presence
One important peer context that taps adolescents’ increased neural social sensitivity is simply whether peers are physically present or not. This has been manipulated experimentally. For example, when playing a simulated driving game, Stoplight, with peers watching versus alone, adolescents (ages 14-18) compared to young adults (ages 18-22) showed greater activation in VS and OFC that was associated with greater risk-taking behavior (Chein, DiSorbo, Uckert, Eagan, & Steinberg, 2009). Within the adolescent sample, Chein et al. (2009) found that VS response to peer presence in this risk-taking context was negatively correlated with self-reported resistance to peer influence, suggesting that activation of this region supports the susceptibility of adolescents to peer influences. In related electroencephalography work, the effect of peer presence was exaggerated in adolescent males (ages 15-16) high in trait surgency (a composite of behavioral approach, sensation-seeking, and positive affect) perhaps because the enhancement of peer salience in these individuals may reduce neural activation of regions (e.g., mPFC) that regulate reward-driven and self-monitoring neural and behavioral responses (Segalowitz et al., 2012). Thus, peer presence may increase adolescent risk-taking and reduce attention to negative aspects of risk and performance failure especially among those with heightened neurobiological sensitivity to peers.
4.2.2. Peer evaluation
In adolescence, socially evaluative situations are assigned high salience, arousal, and self-relevance. Adolescents characterized by greater levels of neurobiological susceptibility to social context might be more sensitive to situations in which they believe that they are being evaluated by others. A body of work by Guyer and colleagues has identified neural activation patterns in adolescents when anticipating evaluation from peers that they may interact with as upcoming online “Chatroom” partners. While adolescents (ages 9-17) made predictions about whether peers would be interested in interacting with them, activity in regions associated with social-affective processing, e.g., nucleus accumbens, hypothalamus, hippocampus, and insula, which respectively relate to reward drive, affective engagement, memory and consolidation, and subjective feelings, was heightened in adolescent girls (but not boys), especially older girls (Guyer et al., 2009). This suggests greater salience of peers’ opinions that increases with age for adolescent girls, whose neural sensitivity to this type of social-evaluative context might render them more vulnerable to internalizing forms of psychopathology but also more likely to engage in prosocial and other types of affiliative behavior guided by social awareness.
Other work has concentrated on striatal sensitivity to peer evaluation, consistent with the idea that peers increasingly sway reward-driven processing and behavior in adolescence. For example, adolescents (age 18) categorized across infancy and childhood as behaviorally inhibited, a temperamental trait that increases risk for developing clinical levels of social anxiety and that has been established as a susceptibility factor (Aron, Aron, & Jagiellowicz, 2012), showed heightened levels of striatal activation when anticipating being evaluated by a peer of interest, even in the absence of manifesting psychopathology (Guyer et al., 2014). Striatal sensitivity to social evaluation may thus be prominent in adolescents who started life as sensitive to their environment via behavioral inhibition. Likewise, Powers et al. (2013) showed that, at least by early adulthood (ages 18-24), individual differences in rejection sensitivity, another construct related to caring about social evaluation, were associated with greater activation of VS and dmPFC when anticipating positive versus negative social feedback. That striatal sensitivity may “tune to” either good or bad outcomes is supported by work by Gunther Moor et al. (2010) showing that activation of the striatum, particularly, the putamen, and vmPFC linearly increased across ages 10-21 to both anticipating peer acceptance and receiving peer rejection. This suggests increasing salience of and ability to regulate responses within socially evaluative contexts. On the one hand, exaggerated striatal activation may render social evaluation overly important, locking adolescents into patterns of inflexible responding if they developed in an environment where the tools for competent social behavior were not transferred. On the other hand, in supportive environments, such social sensitivity may culminate in an adaptive and “more responsive strategy [that] is partly characterized by being more prone to ‘pause to check’ in a novel situation, being more sensitive to subtle stimuli, and employing deeper or more complex processing strategies for planning effective action and later revising cognitive maps, all of which is driven by stronger emotional reactions, positive and negative” (Aron, Aron, & Jagiellowicz, 2012, p. 263).
The amygdala is another potential marker of adolescent neurobiological sensitivity to social context that has emerged from work on peer feedback and acceptance. Relative to non-anxious adolescents, socially anxious adolescents, who generally believe that others will be disinterested in interacting with them, demonstrated heightened amygdala activation when anticipating peer evaluation (Guyer et al., 2008; Lau et al., 2012) in combination with sustained amygdala response after being rejected by peers (Lau et al., 2012). However, as mentioned above, the amygdala has been found to be responsive to not only negatively-but also positively-valenced stimuli. For example, it is reactive to not only fearful faces but also happy ones (Canli, Sivers, Whitfield, Gotlib, & Gabrieli, 2002; Guyer et al., 2008; Perez-Edgar et al., 2007). Indeed, the amygdala has been proposed to be a hub of social-affective circuitry that anchors distinct networks that respectively support overall social perception, social affiliation, and social aversion (Bickart, Dickerson, & Barrett, 2014). Thus, a range of developmental outcomes may emerge against the role of this structure in responding to positive and negative experiences. Ultimately, it will be important for future work to examine if variations in amygdala, vlPFC, dmPFC, and striatal reactivity to peer evaluation moderate associations between social contexts and development of psychopathology or social competencies.
4.2.3. Social exclusion
Other neuroimaging research has focused more specifically on adolescent brain response to social exclusion, a pervasive and particularly distressing form of social stress during this developmental stage that has been manipulated in as well as measured outside the laboratory. Using the simulated ball-tossing game Cyberball (Williams & Jarvis, 2006), Masten et al. (2009) found in adolescents (age 12-14) that individual differences in experiencing distress to being excluded from the game, an index of sensitivity to this social context, was positively associated with activation of social-affective regions (e.g., subgenual ACC, or subACC, and insula) and negatively with activation of regions that support regulation (e.g., vlPFC, dmPFC, and VS); these sets of regions showed negative connectivity to each other. Subsequent work found that subACC activation to social exclusion prospectively predicted longitudinal increases in depressive symptoms from early- to mid-adolescence (Masten et al., 2011).
The subACC will be an important brain region to track in work on reacting to positive as well as negative peer contexts. Although the subACC seems to primarily mediate negative affective experience and regulation, its activation to positively valenced emotional processes has also been reported. Laxton et al. (2013) found in adults with depression that, of the neurons in subACC that responded to emotional imagery, two-thirds responded to sad or disturbing content but one third responded to neutral, happy, or exhilarating content. In a cross-sectional study with pre-pubertal children (8-10 years), early adolescents (12-14 years), older adolescents (16-17 years), and young adults (19-25 years), Gunther Moor et al. (2010) found that, in adults, the subACC activated to being accepted when expecting peer acceptance and rejected when expecting peer rejection. Focusing on the subACC’s response to more chronic expectancy biases in adolescence, Spielberg et al. (2014) found that subACC activation to peer evaluation increased across ages 8-17 for healthy and anxious adolescents who anticipated feedback from selected and rejected peers, respectively. Taken together, results suggest valence consistency in what the subACC tracks, in line with our ideas on affective tuning.
Also consistent with a neurobiological susceptibility standpoint, Masten et al. (2009) found that greater activation of the dorsal ACC (dACC) was associated with individual differences in both one arguably maladaptive factor, rejection sensitivity, and one unambiguously adaptive factor, interpersonal competence, with which the subACC was also associated. This set of findings highlights the dACC and subACC as possible neural sensitivity regions that relate to for-better and for-worse propensities. The dACC has been implicated in supervisory cognitive functions such as conflict monitoring, expectancy violation, and decision-making errors (Carter & Van Veen, 2007; Somerville, Heatherton, & Kelley, 2006). What differentiated the patterns of dACC activation associated with the seemingly distinct traits of rejection sensitivity and interpersonal competence was that competence was also related to recruitment of regulatory regions (e.g., vlPFC, dmPFC, VS) whereas rejection sensitivity was not. Thus, the bivalent effects of neurobiological susceptibility to events in the peer milieu may be afforded by high sensitivity in all susceptible individuals. However, in those susceptible individuals who secure positive outcomes, this may also transpire through the ability to channel that sensitivity toward adaptive ends, such as through flexibly regulating behavior in light of important social standards. Activity within brain circuitry that processes psychological pain may lead to positive as well as negative outcomes by helping one carefully monitor, through this social alarm system, one’s alignment with the group, promoting learning and behavior that keeps one in harmony with it (Eisenberger & Lieberman, 2004; MacDonald & Leary, 2005).
Finally, integrative work has examined the neural basis of how social exclusion relates to risk-taking behaviors as a function of susceptibility to peer influences. Peake, Dishion, Stormshak, Moore, and Pfeifer (2013) found that being excluded from Cyberball was related to more risk-taking on Stoplight in adolescents (ages 14-17) who were less able to resist the influence of peers. This effect was mediated by increased activation of rostral TPJ (rTPJ) as adolescents made risky driving decisions while supposedly being watched by the rejecting peers. The “peer influenced” adolescents also showed less activation of dlPFC when experiencing the consequences of said risks. Thus, adolescents' vulnerability to peer influence on risk-taking outcomes may be mediated by attentional and/or mentalizing neural mechanisms that are differentially sensitized to the influence of peers given the role of rTPJ in mentalizing (Gweon et al., 2012; van den Bos et al., 2011) and dlPC in self-regulation and attention control (Aron et al., 2004; Cohen et al., 2012). Similarly, among males aged 16-17, peer context (peer presence vs. absence) and neural response to social exclusion in social-affective networks (e.g., social pain: AI, dACC, subACC, and mentalizing: dmPFC, TPJ, PCC) had an interactive effect on subsequent risk-taking behavior (Falk et al., 2014). This study serves as a “proof of concept” inasmuch as individual differences in neural sensitivity to being socially excluded predicted adolescent risk-taking behaviors in the presence of peers.
4.3. Timing and the convergence of parent/caregiver and peer influences
Putting the two contexts of parenting/caregiving and peers together, and with adolescence as an anchor point, it may be that differential susceptibility to social context unravels with a sensitivity to timing of exposures and in a hierarchical manner such that experiences with parents/caregivers, formative early on and still influential in adolescence, set the stage for neural sensitivities that take root in or get amplified in adolescence. That is, earlier family contexts may help “teach” the susceptible brain what to attend to, respond to, and value. Subsequently, as adolescents increasingly orient to their salient peer environments, susceptibility to experiences with peers may begin to add more weight in what guides outcomes. Ultimately, the confluence of both influences during this sensitive period may last into early adulthood and beyond.
Some neuroimaging research suggests that experiences with parents/caregivers lay the foundation for individual differences in neural sensitivities that influence how adolescents engage with peers. Supportive of this, Tan and colleagues (2014) found that longer lasting maternal negative affect during a challenging mother-adolescent interaction that called for maternal supportiveness was associated with adolescents’ (ages 11-17) dampened neural response to the positive context of peer acceptance in the amygdala, left anterior insula, subACC, and left nucleus accumbens (NAcc), all regions within social-affective circuitry. Associations between parenting and neural response to peers have also been observed within cognitive-regulatory circuits that follow a more protracted path of development. In youth with versus without an early childhood temperament of behavioral inhibition, higher levels of harsh parenting experienced in middle childhood (age 7) were associated with diminished vlPFC response to peer rejection in late adolescence (ages 17-18), suggesting less or less flexible regulation of responses to peer rejection, as a function of adverse parenting, in the behaviorally inhibited group (Guyer et al., 2015). These results were complemented by the finding that youth who experienced high levels of warm parenting in middle childhood showed a decreased caudate response to peer rejection in adolescence (Guyer et al., 2015). Taken together, these results suggest that parenting is associated with adolescent neural response to peers in ways that are (1) valence-specific and that show either (2) moderation of parenting influences by individual differences or (3) parenting as a source of individual differences that operate in adolescence.
In considering how developmental outcomes may stem from adolescent neurobiological susceptibility to both social contexts, it may be that parent experiences are more influential than peer experiences at first and for certain outcomes. Casement et al. (2014) found that peer victimization and low parental warmth in early adolescence (ages 11 and 12) were both associated with aberrant neural response to reward cues in mid-adolescence (age 16), but that only neural response associated with low parental warmth was linked to depression. Still, peer experiences during adolescence may be more influential than parent experiences on subsequent development, especially as social sensitivity increases during adolescence and inasmuch as this social sensitivity is re-oriented to peers. Masten et al. (2012) found that time spent with friends in late adolescence (age 18) predicted dampened neural response to being socially excluded in early adulthood (age 20) in two regions, the anterior insula and dACC, consistently associated with experiencing distress in this context (Eisenberger, Lieberman, & Williams, 2003; Masten et al., 2009). This suggests that past peer contexts in adolescence affect adult outcomes and that neurobiologically-based individual differences from adolescence may moderate the strength of these effects. Thus, experiences in the family may calibrate neurobiological attunement to threat and reward cues from the peer milieu, and, subsequently, susceptibility to peer environments may chiefly guide development, with influences lasting into early adulthood and beyond.
It will be important for future work to focus questions of adolescent neurobiological susceptibility on considerations of timing, such as investigating how and to what extent adolescence represents a sensitive period; the ramifications of different regions maturing at different times and of individual differences in these rates of maturation; the effect of timing of different social-contextual exposures (e.g., parent/caregiver vs. peer contexts in pre-, early, mid-, late, and post-adolescence), and the hierarchical effects of these social-contextual exposures (i.e., that earlier perturbations or advantages may affect subsequent development).
5. Future Directions and Conclusions
Drawing from prevailing models of adolescent neurodevelopment and a growing neuroimaging literature on the interrelations among social contexts, functional and structural properties of the brain, and developmental outcomes, we have proposed from this review of the literature a framework of adolescent neurobiological sensitivity to social context (Figures 1 and 2). Neurobiological susceptibility models (Ellis et al., 2011) focus on how endogenous, biological factors confer some individuals, relative to others, with greater susceptibility to environmental influences. However, the vast majority of empirical work guided by these theoretical frameworks has not incorporated direct measures of the brain as a source of neurobiological moderating factors. Nor has the available neuroimaging literature tended to use neurobiological susceptibility frameworks for interpreting brain function/structure as moderators of social-contextual influences on outcomes (but see Yap et al., 2008, and Whittle et al., 2011, for exceptions).
We found some possible illustrations in adolescence of neural characteristics that moderated family or peer influences in a for-better or for-worse fashion. For brain structure, this included volume of the amygdala with possible gender differences in the directionality of effects (Whittle et al., 2008; Yap et al., 2008), decreased leftward asymmetric ACC volume in males (Whittle et al., 2008; Yap et al., 2008), and larger hippocampi in females (Whittle et al., 2011). For brain function, the subACC and dACC (Masten et al., 2009), VS (Guyer et al., 2006, 2012, 2015; Telzer et al., 2013; 2014), and vlPFC (Guyer et al., 2015) showed sensitivity to peer or parenting cues and contexts and/or were linked to competencies or vulnerabilities aligned with the bivalent outcomes expected by neurobiological susceptibility models. All of these regions fall under the auspices of the social-affective and cognitive-regulatory systems outlined in models of adolescent neurodevelopment reviewed above.
It is imperative to ground the foregoing region of interest findings with the understanding that these regions do not operate in isolation, and to appreciate that characterizing functional and structural connectivity and network patterns will be important for understanding neurobiological susceptibility and for characterizing susceptible individuals. For example, it could be that the extreme bivalent effects of neurobiological social sensitivity predicted by neurobiological susceptibility models are conferred not only by high social sensitivity in all susceptible contributions from cognitive control circuitry. Indeed, it is through the development of cognitive regulation in tandem with high social sensitivity that sensitive adolescents might be poised to experience the best possible outcomes among all adolescents. Based on the literature and ideas described above, in the following section, we make eight recommendations for applying our proposed framework of adolescent neurobiological susceptibility to social context in future work.
5.1. Future Directions
First, given the centrality of individual differences to neurobiological susceptibility models, we suggest that future neuroimaging work explore and leverage these differences. As a first step, youth could be characterized in terms of being high or low on brain indices quantified along such parameters as brain volume or surface area (i.e., folding) or functional reactivity or connectivity in response to certain social cues or at rest. Subsequently, these possible neural phenotypes can be treated as predictors of outcomes to test for the moderating influence of the brain on associations between social contexts and development (Figure 2). Such quantitative characterizations have been shown in past research to be qualitatively meaningful. For example, Gee et al. (2014) found that grouping children (ages 4-10) and adolescents (ages 11-17) simply in terms of positive versus negative amygdala-mPFC connectivity in response to maternal (vs. stranger) stimuli predicted their levels of separation anxiety with a large effect size, η2 = .21. Conversely, using clustering techniques and other person-centered analytic methods, adolescents can be grouped in terms of being susceptible vs. non-susceptible to social context based on their behavioral outcomes (e.g., adolescents showing highest vs. lowest levels of functioning among those who experienced supportive vs. unsupportive social contexts, respectively). Those affected for-better and for-worse may be put in one category, those relatively unaffected in a second, and the brain characteristics that distinguish the two, sought and verified, using such methods as machine learning classification (e.g., Dosenbach et al., 2010). Indeed, one possibility for our framework is its eventual application to the individual prediction of developmental outcomes and tailoring of interventions. While univariate analytic techniques can be used to improve understanding of circuitry abnormalities that distinguish susceptible adolescents as a group, multivariate techniques such as machine learning would allow for characterization of neurobiological susceptibility at the individual-level without the need to place adolescents in the context of an extant sample (the approach described above) given their reliance on algorithms, or classifiers, derived from previous samples. Additionally, machine learning could aid in more precise conceptualization of susceptibility factors themselves, as these methods are sensitive to subtle, spatially distributed effects in the brain that would otherwise be difficult to detect using standard univariate techniques that focus on group-level differences (Orrù,et al., 2012).
Second, candidate indices of adolescent neural susceptibility can be related or compared to established susceptibility factors such as genotypes (e.g., low-activity MAOA genotype), physiological reactivity (e.g., low heart rate variability), and temperament (e.g., behavioral inhibition). This integrative approach may elucidate more precisely what the neural measures characterize about the individual and provide a more unified understanding of environmental and individual differences across development. Future studies are needed to determine whether behavioral, physiological, and genetic markers of sensitivity to contextual factors constitute the same phenomena expressed at different levels of analysis or represent different types or profiles of susceptibility that may have cumulative or multiplicative effects on development (Figure 3). For example, can an adolescent characterized as high in dACC response to social exclusion be expected to also show high levels of physiological reactivity and neuroticism in socially stressful experiences? This type of multi-level, person-centered approach will allow us to determine what distinguishes brain-based sensitivity indices from indices ascertained at other levels of analyses or biological systems. Furthermore, it provides the potential to ultimately create profiles of neurobiologically-oriented sensitivity that integrate across systems.
Fig. 3.
Pictorial representation of the brain along with biological factors that have already been established in the literature as neurobiological susceptibility factors. We propose that the brain, on which these other factors converge and from which they derive, is a primary source of neurobiological susceptibility, including of adolescent neurobiological susceptibility. Ultimately, joint consideration of assessments of neurobiological susceptibility factors across multiple levels of analysis may be useful for creating and honing comprehensive, multi-modal profiles regarding which adolescents are likely to experience what outcomes, to the benefit of predictive accuracy and strengthening efforts at prevention and intervention.
Third, identifying susceptibility factors at the level of the brain can be facilitated by using endophenotypic approaches, such as imaging genetics (Hyde et al., 2011; Meyer-Lindenberg & Weinberger, 2006; Scharinger, Rabl, Sitte, & Pezawas, 2010) and imaging gene x environment frameworks (Bogdan, Hyde, & Hariri, 2012; Hyde et al., 2011) that explore the underlying neurobiological mechanisms by which specific genetic variants and social contexts shape emotional and behavioral outcomes, possibly in ways consistent with neurobiological susceptibility. For example, researchers could examine the associations between established genetic markers of susceptibility and brain structure, function, and connectivity, and link them to adolescent individual differences in cognitive and affective processes (e.g., emotional reactivity, reward processes, inhibitory control), personality traits (e.g., neuroticism), and developmental outcomes (e.g., psychopathology, competencies). Indeed, susceptibility may lie on a continuum, with cumulative indices of plasticity able to be derived based on how many plasticity alleles one has (e.g., Belsky & Beaver, 2001). With individuals varying in their number of plasticity alleles and these alleles working on different neural regions/circuits, methods like imaging genetics could be used to investigate not only whether adolescents are susceptible or not to their social contexts, but, of those who are, whether they are susceptible to different extents, and in different ways (e.g., via reward drive vs. emotional sensitivity or both).
Fourth, as shown in Figures 1 and 2, future work should measure relevant social contexts, defined as the constellation of influences and events outside of the individual (e.g., parenting, maternal care, early adversity, family income, stress levels), across a wide range of valence, from supportive to deleterious attributes (e.g., social acceptance vs. rejection), and across several domains of social functioning (e.g., familial, peer, romantic). This approach will help determine the specific dimensions of social context to which the brain is most responsive and whose influence the brain is most likely to moderate with regard to outcomes in adolescence and beyond. Dimensions of social context can include positive or negative valence, the type of social relationship represented by that context, and the extent of the adolescent’s experience within that context. Indeed, peer influences are not always negative. A social context defined by supportive or positive peers, such as having civic-minded or prosocial friends, could generate outcomes such as academic striving/achievement and mitigate risk for depression for those adolescents characterized by high neurobiological sensitivity. Furthermore, the timing of the social-contextual exposure should be taken into account. Parenting experiences in early childhood may impact adolescent neurobiological sensitivity to social context in a different way than interchanges between parents and their children during adolescence.
Fifth, for functional neuroimaging work, researchers need to delineate the best stimuli and cues to include in tasks used to characterize brain-based neurobiological susceptibility to social context. For example, although an adolescent may be defined by hypersensitivity to reward, the type of reward matters for understanding his or her developmental course. Recall that Telzer and colleagues (2010) showed that greater striatal response to performing the prosocial act of making costly donations to one’s family predicted less risk-taking later on. Moreover, with different classes of stimuli assessed, careful analysis of subject-by-subject patterns of brain response to them may reveal that very few individual patterns look like the average. For example, some adolescents may show a pattern of greater response to negative and positive stimuli than to neutral stimuli, others, heightened responses only to negative stimuli, and still others, the opposite response, with greatest activation to positive stimuli. Such data would help categorize individual neural response to social context and facilitate understanding of how this response guides outcomes.
Sixth, to understand developmental change as it unfolds over time, at least two time points of outcome data must be obtained. This issue emphasizes the importance not only of making hypotheses regarding the timing of influences but also the need to pay attention to the timing of measurements. Data may be collected not only within but beyond the developmental period of interest. For example, it may be genetically- and environmentally-shaped brain development during early childhood that bestows individuals with the neurobiological susceptibility factors that in adolescence, lead them to be differentially sensitive to social contextual exposures (Paus, 2013). Overall, there is a need for longitudinal neuroimaging studies that are sensitive to developmental timing and that address the question of within-person development. To this end, a powerful approach to reveal brain-behavior relationships that change across development is to use person-centered methods that track shifts in structural, functional, or connectivity-based measures with developmentally mediated differences in laboratory-based or everyday behavior. This generally aligns with the idea of using our evolving understanding of the brain as revealed through neuroimaging research to predict behavior (Berkman & Falk, 2013). Only then can we clarify what the moderating and/or mediating processes are, their sequence, and causality.
Seventh, future work would likely benefit from increased cross-talk between researchers who focus on human samples and those who use animal models (Stevens & Vaccarino, 2015). Understandably, in human-based research, it may be difficult to incorporate all aspects needed to test the predicted influences in our proposed framework (i.e., applying longitudinal designs, selecting susceptibility factors a priori, ensuring coverage of social contexts across valence, and probing responses to a variety of stimuli). Animal models can enrich our hypotheses about neurobiological susceptibility in humans through opportunities to directly manipulate valenced social-contextual exposures, take measurements at both varied and multiple points in development, and isolate specific neurobiology susceptibility factors at very mechanistic levels. As several parallels have been established between adolescence in human and non-human animals alike (e.g., increases in exploratory behavior, affective reactivity, social play, reward sensitivity, and risk-taking; Callaghan & Tottenham, 2015; Doremus-Fitzwater et al., 2009; Lee et al., 2015; Muñoz-Cuevas et al., 2013; Schneider et al., 2014; Simon & Moghaddam, 2015; Siviy, Deron, & Kasten, 2011; Spear, 2011; Yu et al., 2014), studying the adolescent period in animal models may provide insights into the operation of neurobiological susceptibility as it relates to adolescence. To this end, work in animal models has been valuable for charting the emergence and influence of sensitive periods, when environmental experiences have the greatest impact on brain circuitry, with effects on later development (Hensch & Bilimoria, 2012).
Finally, because it is critical to establish the reliability of brain indices before treating them as metrics of adolescent neurobiological susceptibility, it is important, as in all research, to understand what optimizes reliability and minimizes the sources of error that impair it. For example, Johnstone et al. (2005) achieved high test-retest reliability for the amygdala across three measurement occasions over two months yet found that reliability was affected by such characteristics as usage of percent signal change vs. z scores, ROIs that were structurally vs. empirically defined, as well as different theoretically sound contrasts (e.g., the contrast of viewing fearful faces vs. a fixation cross produced higher ICCs than that of viewing fearful vs. neutral faces). Indeed, numerous steps can be taken to ensure quality of the signal, of the analyses, and, ultimately, of the results, such as increasing the number of subjects, increasing the number of runs, giving consistent task instructions across all participants, using block as opposed to event-related designs, and keeping in mind which contrasts will be used (see Bennet & Miller, 2010, for several helpful recommendations). As Bennet and Miller (2010) observe, neuroimaging itself has “reached a point of adolescence, where knowledge and methods have made enormous progress but there is still much development left to be done” (p. 150). Nevertheless, neuroimaging is a powerful method, and the prospect of what can be learned about adolescent neurobiological susceptibility with its application an exciting direction.
5.2. Conclusions
In sum, our proposed framework is intended to ignite new theories and empirical tests that build on extant models of neurobiological susceptibility and adolescent brain development. For this kind of work to move forward, interdisciplinary collaboration between cognitive neuroscientists and developmental scientists must increase. Developmental scientists who have existing longitudinal samples could be recruited for scanning, whereas neuroscientists’ extant datasets could be made accessible to developmental scientists. A distal and applied goal of this research is also to foster opportunity for intervention. Using a neurobiological framework and incorporating neurally sensitive designs into interventions to promote resilient functioning or repair conditional adaptations gone awry may contribute to the ability to design individualized interventions that are based on knowledge gleaned from multiple biological and psychological levels of analysis.
The inclusion of neurobiological assessments in the design and evaluation of interventions designed to foster resilience enables scientists to discover whether and which of the various components of multifaceted interventions exert a differential impact on separate brain systems and subsequent outcomes. Most generally, this approach can allow for intervening with specific aspects of the environment and for flagging who may benefit the most or who may face the greatest risk by quantifying individual differences in neural moderators of developmental outcomes in adolescence to help promote adaptive, adult functioning.
Highlights.
We propose a framework, adolescent neurobiological susceptibility to social context. This framework pivots about brain-based individual differences in social sensitivity. Adolescent brain indices may moderate the effect of social context on development. Neuroimaging work on social context, the adolescent brain, and outcomes is reviewed. We suggest that brain measures be used to index neurobiological susceptibility.
Acknowledgment
This work was supported by a William T. Grant Foundation Mentoring Award (A.E.G.; R.A.S.), a William T. Grant Foundation Scholars Award (A.E.G.), and NIH grant R01MH098370 (A.E.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest in relation to the present manuscript
References
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience & Biobehavioral Reviews. 2003;27(1):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aron EN, Aron A. Sensory-processing sensitivity and its relation to introversion and emotionality. Journal of Personality and Social Psychology. 1997;73(2):345. doi: 10.1037//0022-3514.73.2.345. [DOI] [PubMed] [Google Scholar]
- Aron EN, Aron A, Jagiellowicz J. Sensory processing sensitivity: a review in the light of the evolution of biological responsivity. Pers Soc Psychol Rev. 2012;16(3):262–282. doi: 10.1177/1088868311434213. doi: 10.1177/1088868311434213. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to rearing environment depending on dopamine-related genes: new evidence and a meta-analysis. Dev Psychopathol. 2011;23(1):39–52. doi: 10.1017/S0954579410000635. doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat. Rev. Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E, Brenner SL, Gatzke-Kopp L. Ten good reasons to consider biological processes in prevention and intervention research. Dev Psychopathol. 2008;20(3):745–774. doi: 10.1017/S0954579408000369. doi: 10.1017/S0954579408000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. The Journal of Neuroscience. 1999;19(13):5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J. Differential susceptibility to rearing influences: An evolutionary hypothesis and some evidence. In: Ellis B, Bjorklund D, editors. Origins of the social mind: Evolutionary psychology and child development. Guilford; New York: 2005. pp. 139–163. [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16(6):300–304. doi: DOI 10.1111/j.1467-8721.2007.00525.x. [Google Scholar]
- Belsky J, Beaver KM. Cumulative-genetic plasticity, parenting and adolescent self-regulation. Journal of Child Psychology and Psychiatry. 2011;52(5):619–626. doi: 10.1111/j.1469-7610.2010.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135(6):885–908. doi: 10.1037/a0017376. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Miller MB. How reliable are the results from functional magnetic resonance imaging? Annals of the New York Academy of Sciences. 2010;1191(1):133–155. doi: 10.1111/j.1749-6632.2010.05446.x. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Falk EB. Beyond brain mapping using neural measures to predict real-world outcomes. Current directions in psychological science. 2013;22(1):45–50. doi: 10.1177/0963721412469394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, Deschênes M, Matte-Gagné C. Social factors in the development of early executive functioning: a closer look at the caregiving environment. Developmental science. 2012;15(1):12–24. doi: 10.1111/j.1467-7687.2011.01093.x. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Dickerson BC, Barrett LF. The amygdala as a hub in brain networks that support social life. Neuropsychologia. 2014;63:235–248. doi: 10.1016/j.neuropsychologia.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nature Neuroscience. 2011;14(2):163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Mills KL. Is adolescence a sensitive period for sociocultural processing? Annu Rev Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Hyde LW, Hariri AR. A neurogenetics approach to understanding individual differences in brain, behavior, and risk for psychopathology. Molecular psychiatry. 2013;18(3):288–299. doi: 10.1038/mp.2012.35. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brennan RL. Generalizability Theory. Springer; New York: 2001. [Google Scholar]
- Brett ZH, Sheridan M, Humphreys K, Smyke A, Gleason MM, Fox N, Drury S. A neurogenetics approach to defining differential susceptibility to institutional care. International Journal of Behavioral Development. 2014 doi: 10.1177/0165025414538557. 0165025414538557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BB. Peer groups and peer cultures. In: Feldman SS, Elliot GR, editors. At the Threshold: The Developing Adolescent. Harvard University Press; Cambridge, MA: 1990. pp. 171–96. [Google Scholar]
- Brown BB, Bakken JP. Parenting and peer relationships: Reinvigorating research on family–peer linkages in adolescence. Journal of Research on Adolescence. 2011;21(1):153–165. http://dx.doi.org/10.1111/j.1532-7795.2010.00720.x [Google Scholar]
- Brown BB, Larson J. Peer relationships in adolescents. In: Steinberg RML, editor. Handbook of adolescent psychology, Contextual influences on adolescent development. 3rd Vol. 2. John Wiley & Sons; Hoboken, NJ: 2009. pp. 74–103. [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Birn RM. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience. 2012;15(12):1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Cohen Kadosh K, Blakemore SJ. The social brain in adolescence: evidence from functional magnetic resonance imaging and behavioural studies. Neurosci Biobehav Rev. 2011;35(8):1654–1664. doi: 10.1016/j.neubiorev.2010.10.011. doi: 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Smith AR, Hommer DW. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J Child Psychol Psychiatry. 2010;51:827–837. doi: 10.1111/j.1469-7610.2009.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Pardini DA. Who are those “risk-taking adolescents”? Individual differences in developmental neuroimaging research. Developmental cognitive neuroscience. 2015;11:56–64. doi: 10.1016/j.dcn.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A, Hall DL, Zelaya FO, Williams SC, Mehta MA. Measuring fMRI reliability with the intra-class correlation coefficient. Neuroimage. 2009;45:758–768. doi: 10.1016/j.neuroimage.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nature Reviews Neuroscience. 2001;2(5):352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Tottenham N. The neuro-environmental loop of plasticity: A cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JD. Amygdala response to happy faces as a function of extraversion. Science. 2002;296(5576):2191–2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Caouette JD, Guyer AE. Gaining insight into adolescent vulnerability for social anxiety from developmental cognitive neuroscience. Developmental Cognitive Neuroscience. 2014;8:65–76. doi: 10.1016/j.dcn.2013.10.003. doi: 10.1016/j.dcn.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Casement MD, Guyer AE, Hipwell AE, McAloon RL, Hoffmann AM, Keenan KE, Forbes EE. Girls' challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Dev Cogn Neurosci. 2014;8:18–27. doi: 10.1016/j.dcn.2013.12.003. doi: 10.1016/j.dcn.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Geidd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–247. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Chein J, DiSorbo A, Uckert K, Eagan DE, Steinberg L. Paper presented at the Society for Research in Child Development. Denver, CO; 2009. Neural markers of a peer influence on adolescent risk taking. [Google Scholar]
- Cicchetti D, Rogosch FA. A developmental psychopathology perspective on adolescence. J Consult Clin Psychol. 2002;70(1):6–20. doi: 10.1037//0022-006x.70.1.6. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Berkman ET, Lieberman MD. Principles of Frontal Lobe Functions. 2nd Oxford University Press; USA: 2012. Intentional and incidental self-control in ventrolateral PFC. [Google Scholar]
- Cohen S, Hamrick N. Stable individual differences in physiological response to stressors: Implications for stress-elicited changes in immune related health. Brain, behavior, and immunity. 2003;17(6):407–414. doi: 10.1016/s0889-1591(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick NM, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. The stability of and intercorrelations among cardiovascular, immune, endocrine, and psychological reactivity. Annals of Behavioral Medicine. 2000;22(3):171–179. doi: 10.1007/BF02895111. [DOI] [PubMed] [Google Scholar]
- Coie JD, Dodge KA, Kupersmidt JB. Peer group behavior and social status. In: Asher SR, Coie JD, editors. Peer Rejection in Childhood. Cambridge Studies in Social and Emotional Development. Cambridge University Press; New York, NY: 1990. pp. 17–59. [Google Scholar]
- Collins WA, Maccoby EE, Steinberg L, Hetherington EM, Bornstein MH. Contemporary research on parenting: The case for nature and nurture. American Psychologist. 2000;55:218–232. [PubMed] [Google Scholar]
- Coplan RJ, Rubin KH, Fox NA, Calkins SD, Stewart SL. Being alone, playing alone, and acting alone: Distinguishing among reticence and passive and active solitude in young children. Child development. 1994;65(1):129–137. [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R. Being adolescent: Conflict and growth in the teenage years. Basic Books; New York, NY: 1984. [Google Scholar]
- Cunningham WA, Brosch T. Motivational salience amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science. 2012;21(1):54–59. [Google Scholar]
- Darling N, Steinberg L. Parenting style as context: An integrative model. Psychological bulletin. 1993;113(3):487. [Google Scholar]
- Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev. 2008;32(1):1–19. doi: 10.1016/j.neubiorev.2007.04.016. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Frontal brain asymmetry predicts infants' response to maternal separation. Journal of abnormal psychology. 1989;98(2):127–131. doi: 10.1037//0021-843x.98.2.127. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K. Family Matters Intergenerational and Interpersonal Processes of Executive Function and Attentive Behavior. Current directions in psychological science. 2014;23(3):230–236. doi: 10.1177/0963721414531597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deater-Deckard K, Wang Z. Development of temperament and attention: Behavioral genetic approaches. Cognitive neuroscience of attention. 2012:331–342. [Google Scholar]
- Delville Y, Melloni RH, Ferris CF. Behavioral and neurobiological consequences of social subjugation during puberty in golden hamsters. The Journal of Neuroscience. 1998;18(7):2667–2672. doi: 10.1523/JNEUROSCI.18-07-02667.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiology & Behavior. 2009;97(3):484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Schlaggar BL. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RI. The social brain hypothesis and its implications for social evolution. Annals of Human Biology. 2009;36(5):562–572. doi: 10.1080/03014460902960289. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: an evolutionary--neurodevelopmental theory. Dev Psychopathol. 2011;23(1):7–28. doi: 10.1017/S0954579410000611. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: A common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;8(7):294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33(3):367–382. doi: 10.1016/j.neubiorev.2008.10.009. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. doi:10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput. Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, Schlaggar BL, Mennes M, Gutman D, Bangaru S, Buitelaar JK, Dickstein DP, Di Martino A, Kennedy DN, Kelly C, Luna B, Schweitzer JB, Velanova K, Wang YF, Mostofsky S, Castellanos FX, Milham MP. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front. Syst. Neurosci. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. From contextual fear to a dynamic view of memory systems. Trends in Cognitive Sciences. 2010;14(1):7–15. doi: 10.1016/j.tics.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleeson W. Toward a structure-and process-integrated view of personality: Traits as density distributions of states. Journal of personality and social psychology. 2001;80(6):1011–1027. [PubMed] [Google Scholar]
- Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, Riley B. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Arch Gen Psychiatry. 2004;61(7):738–744. doi: 10.1001/archpsyc.61.7.738. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am. J. Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: Who is at risk? Dev Sci. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Beauchaine TP, Shannon KE, Chipman J, Fleming AP, Crowell SE, Liang O, Johnson LC, Aylward E. Neurological correlates of reward responding in adolescents with and without externalizing behavior disorders. J Abnorm Psychol. 2009;118:203–213. doi: 10.1037/a0014378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Tottenham N. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. The Journal of Neuroscience. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, McEwen SC, Forsyth JK, Haut KM, Bearden CE, Addington J, Cannon TD. Reliability of an fMRI paradigm for emotional processing in a multisite longitudinal study. Human brain mapping. 2015 doi: 10.1002/hbm.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P, Van Der Veen F, Jennings J. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. J Adolesc Health. 2008;42(4):335–343. doi: 10.1016/j.jadohealth.2008.01.007. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Chrousos GP. Puberty-related influences on brain development. Molecular and Cellular Endocrinology. 2006;254:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. Brain Cogn. 2010;72(1):6–15. doi: 10.1016/j.bandc.2009.08.009. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther Moor B, van Leijenhorst L, Rombouts SA, Crone EA, Van der Molen MW. Do you like me? Neural correlates of social evaluation and developmental trajectories. Social Neuroscience. 2010;5(5-6):461–482. doi: 10.1080/17470910903526155. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Benson B, Choate VR, Bar-Haim Y, Perez-Edgar K, Jarcho JM, Nelson EE. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Dev Psychopathol. 2014;26(1):229–243. doi: 10.1017/S0954579413000941. doi: 10.1017/S0954579413000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, Ernst M. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am J Psychiatry. 2012;169(2):205–212. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Soc Cogn Affect Neurosci. 2012;7(1):81–92. doi: 10.1093/scan/nsr043. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Jarcho JM, Perez-Edgar KP, Degnan KA, Pine DS, Fox NA, Nelson EE. Temperament and parenting styles in early childhood differentially influence neural response to peer evaluation in adolescence. J Abnorm Child Psych. doi: 10.1007/s10802-015-9973-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Kaufman J, Hodgdon HB, Masten CL, Jazbec S, Pine DS, Ernst M. Behavioral alterations in reward system function: the role of childhood maltreatment and psychopathology. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1059–1067. doi: 10.1097/01.chi.0000227882.50404.11. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Nelson EE. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 2008;65(11):1303–1312. doi: 10.1001/archpsyc.65.11.1303. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 2009;80(4):1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, Ernst M. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci. 2006;26(24):6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gweon H, Dodell-Feder D, Bedny M, Saxe R. Theory of mind performance in children correlates with functional specialization of a brain region for thinking about thoughts. Child Dev. 2012;83:1853–1868. doi: 10.1111/j.1467-8624.2012.01829.x. [DOI] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behavioral Neuroscience. 2007;121(2):249–256. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- Halpern CT, Udry JR, Suchindran C. Testosterone predicts initiation of coitus in adolescent females. Psychosomatic Medicine. 1997;59:161–171. doi: 10.1097/00006842-199703000-00008. [DOI] [PubMed] [Google Scholar]
- Halpern CT, Udry JR, Suchindran C. Monthly measures of salivary testosterone predict sexual activity in adolescent males. Archives of Sexual Behavior. 1998;27:445–465. doi: 10.1023/a:1018700529128. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: Activation of the human amygdala in positive and negative emotion. Psychological Science. 2002;13(2):135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Hammond SI, Müller U, Carpendale J, Bibok MB, Liebermann-Finestone DP. The effects of parental scaffolding on preschoolers’ executive function. Developmental Psychology. 2012;48:271–281. doi: 10.1037/a0025519. [DOI] [PubMed] [Google Scholar]
- Hao X, Xu D, Bansal R, Dong Z, Liu J, Wang Z, Peterson BS. Multimodal magnetic resonance imaging: The coordinated use of multiple, mutually informative probes to understand brain structure and function. Human brain mapping. 2013;34(2):253–271. doi: 10.1002/hbm.21440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annual review of neuroscience. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Klimes-Dougan B, Brand A, Kendziora KT, Zahn-Waxler C. Regulating sadness and fear from outside and within: Mothers' emotion socialization and adolescents' parasympathetic regulation predict the development of internalizing difficulties. Development and Psychopathology. doi: 10.1017/S0954579414001084. in press. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Bilimoria PM. Cerebrum: the Dana forum on brain science. Vol. 2012. Dana Foundation; Jul, 2012. Re-opening windows: manipulating critical periods for brain development. [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Thivierge JP, Sporns O. Can structure predict function in the human brain? NeuroImage. 2010;52(3):766–776. doi: 10.1016/j.neuroimage.2010.01.071. [DOI] [PubMed] [Google Scholar]
- Hirsh R. The hippocampus and contextual retrieval of information from memory: A theory. Behavioral Biology. 1974;12(4):421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Hughes C. Changes and challenges in 20 years of research into the development of executive functions. Infant and Child Development. 2011;20:251–271. [Google Scholar]
- Hyde LW, Bogdan R, Hariri AR. Understanding risk for psychopathology through imaging gene–environment interactions. Trends in cognitive sciences. 2011;15(9):417–427. doi: 10.1016/j.tics.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DC, Muller CJ, Dolski I, Dalton KM, Nitschke JB, Urry HL, et al. Now you feel it, now you don’t: Frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science. 2003;14:612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- Jansen AG, Mous SE, White T, Posthuma D, Polderman TJ. What Twin Studies Tell Us About the Heritability of Brain Development, Morphology, and Function: A Review. Neuropsychology Review. 2015;25(1):27–46. doi: 10.1007/s11065-015-9278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, Kalin NH, Whalen PJ. Stability of amygdala BOLD response to fearful faces over multiple scan sessions. Neuroimage. 2005;25(4):1112–1123. doi: 10.1016/j.neuroimage.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Kanai R, Bahrami B, Roylance R, Rees G. Online social network size is reflected in human brain structure. Proceedings of the Royal Society of London B: Biological Sciences. 2012;279(1732):1327–1334. doi: 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knafo A, Israel S, Ebstein RP. Heritability of children's prosocial behavior and differential susceptibility to parenting by variation in the dopamine receptor D4 gene. Dev Psychopathol. 2011;23(1):53–67. doi: 10.1017/S0954579410000647. doi: 10.1017/S0954579410000647. [DOI] [PubMed] [Google Scholar]
- Koolschijn PCM, Schel MA, de Rooij M, Rombouts SA, Crone EA. A three-year longitudinal functional magnetic resonance imaging study of performance monitoring and test-retest reliability from childhood to early adulthood. The Journal of Neuroscience. 2011;31(11):4204–4212. doi: 10.1523/JNEUROSCI.6415-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Berridge KC. Towards a functional neuroanatomy of pleasure and happiness. Trends in cognitive sciences. 2009;13(11):479–487. doi: 10.1016/j.tics.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44(1):213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Laucht M, Skowronek MH, Becker K, Schmidt MH, Esser G, Schulze TG, Rietschel M. Interacting effects of the dopamine transporter gene and psychosocial adversity on attention-deficit/hyperactivity disorder symp- toms among 15-year-olds from a high-risk community sample. Archives of General Psychiatry. 2007;64:585–590. doi: 10.1001/archpsyc.64.5.585. [DOI] [PubMed] [Google Scholar]
- Laxton AW, Neimat JS, Davis KD, Womelsdorf T, Hutchison WD, Dostrovsky JO, Lozano AM. Neuronal coding of implicit emotion categories in the subcallosal cortex in patients with depression. Biological psychiatry. 2013;74(10):714–719. doi: 10.1016/j.biopsych.2013.03.029. [DOI] [PubMed] [Google Scholar]
- Lee AM, Tai LH, Zador A, Wilbrecht L. Between the primate and ‘reptilian’ brain: Rodent models demonstrate the role of corticostriatal circuits in decision making. Neuroscience. 2015;296:66–74. doi: 10.1016/j.neuroscience.2014.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Siegle GJ, Dahl RE, Hooley JM, Silk JS. Neural responses to maternal criticism in healthy youth. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu133. nsu133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the balloon analogie risk task (BART) as a predictor of adolescent real-world risk-taking behavior. Journal of Adolescence. 2003;26:475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain and Cognition. 2010;72(1):46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, et al. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Human Brain Mapping. 2009;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LH, Dapretto M, O'Hare ED, Kan E, McCourt ST, Thompson PM, Sowell ER. Relationships between brain activation and brain structure in normally developing children. Cerebral Cortex. 2009;19(11):2595–2604. doi: 10.1093/cercor/bhp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychological Bulletin. 2005;131(2):202–23. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Ernst M. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci. 2010;10(1):34–49. doi: 10.3758/CABN.10.1.34. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Brown SM, Forbes EE, Hariri AR. Temporal stability of individual differences in amygdala reactivity. The American Journal of Psychiatry. 2007;164:1613–1614. doi: 10.1176/appi.ajp.2007.07040609. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents' risk for depression. Dev Psychopathol. 2011;23(1):283–292. doi: 10.1017/S0954579410000799. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4(2):143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Fuligni AJ, Lieberman MD, Eisenberger NI. Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Soc Cogn Affect Neurosci. 2012;7(1):106–114. doi: 10.1093/scan/nsq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Obradovic J. Competence and resilience in development. Ann N Y Acad Sci. 2006;1094:13–27. doi: 10.1196/annals.1376.003. doi: 10.1196/annals.1376.003. [DOI] [PubMed] [Google Scholar]
- Muñoz-Cuevas FJ, Athilingam J, Piscopo D, Wilbrecht L. Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nature Neuroscience. 2013;16(10):1367–1369. doi: 10.1038/nn.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Invited Review: Estrogen effects on the brain: multiple sites and molecular mechanisms. Journal of Applied Physiology. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, Nelson CA. Widespread reductions in cortical thickness following severe early-life deprivation: a neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biol Psychiatry. 2014;76(8):629–638. doi: 10.1016/j.biopsych.2013.08.016. doi: 10.1016/j.biopsych.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. doi: 10.1146/annurev.neuro.24.1.116124/1/1161 [pii] [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nature Reviews Neuroscience. 2006;7(10):818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Miller MB, Van Horn JD, Wolford GL, Handy TC, Valsangkar-Smyth M, Inati S, Grafton S, Gazzaniga MS. Extensive individual differences in brain activations associated with episodic retrieval are reliable over time. J. Cogn. Neurosci. 2002;14:1200–1214. doi: 10.1162/089892902760807203. [DOI] [PubMed] [Google Scholar]
- Miller MB, Donovan CL, Van Horn JD, German E, Sokol-Hessner P, Wolford GL. Unique and persistent individual patterns of brain activity across different memory retrieval tasks. NeuroImage. 2009;48:625–635. doi: 10.1016/j.neuroimage.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan K, Guyer AE, Silk J, Fitzwater T, Steinberg LD. Integration of developmental neuroscience and contextual approaches to the study of adolescent psychopathology. In: Cicchetti D, editor. Developmental Psychopathology. 3rd Wiley; 2015. [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Pine DS. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163(6):1091–1097. doi: 10.1176/ajp.2006.163.6.1091. doi: 10.1176/appi.ajp.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SR, Depue RA. Neurobehavioral foundation of environmental reactivity. Psychological Bulletin. doi: 10.1037/bul0000028. in press. [DOI] [PubMed] [Google Scholar]
- Nagai M, Hoshide S, Kario K. The insular cortex and cardiovascular system: a new insight into the brain-heart axis. Journal of the American Society of Hypertension. 2010;4(4):174–182. doi: 10.1016/j.jash.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Guyer AE. The development of the ventral prefrontal cortex and social flexibility. Dev Cogn Neurosci. 2011;1(3):233–245. doi: 10.1016/j.dcn.2011.01.002. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. doi: 10.1017/S0033291704003915. [DOI] [PubMed] [Google Scholar]
- O'Connor M, Guendel H, McRae K, Lane R. Baseline vagal tone predicts BOLD response during elicitation of grief. Neuropsychopharmacology. 2007;32:2184–2189. doi: 10.1038/sj.npp.1301342. [DOI] [PubMed] [Google Scholar]
- Orrù G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using support vector machine to identify imaging biomarkers of neurological and psychiatric disease: A critical review. Neuroscience & Biobehavioral Reviews. 2012;36(4):1140–1152. doi: 10.1016/j.neubiorev.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Paus T. How environment and genes shape the adolescent brain. Hormones and behavior. 2013;64(2):195–202. doi: 10.1016/j.yhbeh.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Peake SJ, Dishion TJ, Stormshak EA, Moore WE, Pfeifer JH. Risk-taking and social exclusion in adolescence: neural mechanisms underlying peer influences on decision-making. Neuroimage. 2013;82:23–34. doi: 10.1016/j.neuroimage.2013.05.061. doi: 10.1016/j.neuroimage.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina M, Mickey BJ, Love T, Wang H, Langenecker SA, Hodgkinson C, Zubieta JK. DRD2 polymorphisms modulate reward and emotion processing, dopamine neurotransmission and openness to experience. Cortex. 2013;49(3):877–890. doi: 10.1016/j.cortex.2012.01.010. doi: 10.1016/j.cortex.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn Sci. 2012;16(6):322–329. doi: 10.1016/j.tics.2012.04.011. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M, Belsky J. Vantage sensitivity: Individual differences in response to positive experiences. Psychological Bulletin. 2013;139(4):901–916. doi: 10.1037/a0030196. [DOI] [PubMed] [Google Scholar]
- Power JD, air DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich R, Grotegerd D, Opel N, Kaufmann C, Zwitserlood P, Kugel H, Dannlowski U. Are you gonna leave me? Separation anxiety is associated with increased amygdala responsiveness and volume. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu055. nsu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice K, Viscomi B, Riggins T, Redcay E. Amygdala volume linked to individual differences in mental state inference in early childhood and adulthood. Developmental Cognitive Neuroscience. 2014;8:153–163. doi: 10.1016/j.dcn.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, Haydon KC. Distinguishing differential susceptibility from diathesis–stress: Recommendations for evaluating interaction effects. Development and psychopathology. 2012;24(02):389–409. doi: 10.1017/S0954579412000065. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Richardson HN, Sisk CL. Puberty and the maturation of the male brain and sexual behavior: Recasting a behavioral potential. Neuroscience and Biobehavioral Reviews. 2002;26:381–391. doi: 10.1016/s0149-7634(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Bukowski W, Parker JG. Peer interactions, relationships, and groups. In: Damon W, editor. Handbook of Child Psychology. fifth Wiley; New York: 1998. pp. 619–700. [Google Scholar]
- Rubin KH, Coplan RJ, Bowker JC. Social withdrawal in childhood. Annual review of psychology. 2009;60:141. doi: 10.1146/annurev.psych.60.110707.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal–hippocampal system. Learning & Memory. 2009;16(10):573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Achievements and challenges in the biology of environmental effects. Proceedings of the National Academy of Sciences. 2012;109(Supplement 2):17149–17153. doi: 10.1073/pnas.1121258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry. 2006;47(3-4):226–261. doi: 10.1111/j.1469-7610.2005.01557.x. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Sauder CL, Hajcak G, Angstadt M, Phan KL. Test-retest reliability of amygdala response to emotional faces. Psychophysiology. 2013;50(11):1147–1156. doi: 10.1111/psyp.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362(1481):773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharinger C, Rabl U, Sitte HH, Pezawas L. Imaging genetics of mood disorders. Neuroimage. 2010;53(3):810–821. doi: 10.1016/j.neuroimage.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Hannusch C, Schmahl C, Bohus M, Spanagel R, Schneider M. Adolescent peer-rejection persistently alters pain perception and CB1 receptor expression in female rats. European Neuropsychopharmacology. 2014;24(2):290–301. doi: 10.1016/j.euroneuro.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Schneider S, Brassen S, Bromberg U, Banaschewski T, Conrod P, Flor H, Buchel C. Maternal interpersonal affiliation is associated with adolescents' brain structure and reward processing. Transl Psychiatry. 2012;2:e182. doi: 10.1038/tp.2012.113. doi: 10.1038/tp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Willoughby T, Reker DL, Campbell K, Chalmers H, Rose-Krasnor L. Adolescent peer interaction and trait surgency weaken medial prefrontal cortex responses to failure. Soc Cogn Affect Neurosci. 2012;7(1):115–124. doi: 10.1093/scan/nsq090. doi: 10.1093/scan/nsq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA. Variation in neural development as a result of exposure to institutionalization early in childhood. Proc Natl Acad Sci U S A. (3rd) 2012;109(32):12927–12932. doi: 10.1073/pnas.1200041109. doi: 10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, How J, Araujo M, Schamberg MA, Nelson CA. What are the links between maternal social status, hippocampal function, and HPA axis function in children? Dev Sci. 2013;16(5):665–675. doi: 10.1111/desc.12087. doi: 10.1111/desc.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Moghaddam B. Neural processing of reward in adolescent rodents. Developmental Cognitive Neuroscience. 2015;11:145–154. doi: 10.1016/j.dcn.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siviy SM, Deron LM, Kasten CR. Serotonin, motivation, and playfulness in the juvenile rat. Developmental Cognitive Neuroscience. 2011;1(4):606–616. doi: 10.1016/j.dcn.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cognit Neurosci. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH. The teenage brain sensitivity to social evaluation. Current directions in psychological science. 2013;22(2):121–127. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat. Neurosci. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011;1(4):390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, Jarcho JM, Dahl RE, Pine DS, Ernst M, Nelson EE. Anticipation of peer evaluation in anxious adolescents: Divergence in neural activation and maturation. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu165. nsu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A Social Neuroscience Perspective on Adolescent Risk-Taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annu Rev Psychol. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Stevens HE, Vaccarino FM. How animal models inform child and adolescent psychiatry. Journal of the American Academy of Child & Adolescent Psychiatry. 2015;54(5):352–359. doi: 10.1016/j.jaac.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi S. Early determinants of behaviour: Evidence from primate studies. British Medical Bulletin. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Sust S, Buckholtz JW, Meyers JD, Egan MF, et al. Epistasis between catechol-O-methyltransferase and type II metabotropic glutamate receptor 3 genes on working memory brain function. Proc Natl Acad Sci, USA. 2007;104:12536–12541. doi: 10.1073/pnas.0610125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PZ, Lee KH, Dahl RE, Nelson EE, Stroud LJ, Siegle GJ, Silk JS. Associations between maternal negative affect and adolescent's neural response to peer evaluation. Dev Cogn Neurosci. 2014;8:28–39. doi: 10.1016/j.dcn.2014.01.006. doi: 10.1016/j.dcn.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry. 2006;60(3):296–301. doi: 10.1016/j.biopsych.2005.09.027. doi: DOI 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galván A. Meaningful family relationships: Neurocognitive buffers of adolescent risk taking. Journal of cognitive neuroscience. 2013a;25(3):374–387. doi: 10.1162/jocn_a_00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galván A. Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Dev Cogn Neurosci. 3:45–52. doi: 10.1016/j.dcn.2012.08.004. doi: 10.1016/j.dcn.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galván A. Neural sensitivity to eudaimonic and hedonic rewards differentially predict adolescent depressive symptoms over time. Proceedings of the National Academy of Sciences. 2014;111(18):6600–6605. doi: 10.1073/pnas.1323014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Miernicki ME, Galván A. The quality of adolescents’ peer relationships modulates neural sensitivity to risk taking. Social cognitive and affective neuroscience. 2014 doi: 10.1093/scan/nsu064. nsu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Ichien NT, Qu Y. Mothers know best: Redirecting adolescent reward sensitivity toward safe behavior during risk taking. Social cognitive and affective neuroscience. 2015 doi: 10.1093/scan/nsv026. nsv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Masten CL, Berkman ET, Lieberman MD, Fuligni AJ. Gaining while giving: an fMRI study of the rewards of family assistance among white and Latino youth. Soc Neurosci. 2010;5(5-6):508–518. doi: 10.1080/17470911003687913. doi: 10.1080/17470911003687913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Masten CL, Berkman ET, Lieberman MD, Fuligni AJ. Neural regions associated with self control and mentalizing are recruited during prosocial behaviors towards the family. Neuroimage. 2011;58(1):242–249. doi: 10.1016/j.neuroimage.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RM, Cunningham WA, Anderson AK, Thompson E. Affect-biased attention as emotion regulation. Trends in cognitive sciences. 2012;16(7):365–372. doi: 10.1016/j.tics.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare T, Quinn B, McCarry T, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically larger amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, van Dijk E, Westenberg M, Rombouts SARB, Crone EA. Changing brains, changing perspectives: The neurocognitive development of reciprocity. Psychol Sci. 2011;22:60–70. doi: 10.1177/0956797610391102. [DOI] [PubMed] [Google Scholar]
- van den Bulk BG, Koolschijn PCMP, Meens PH, van Lang ND, van der Wee NJ, Rombouts SA, Crone EA. How stable is activation in the amygdala and prefrontal cortex in adolescence? A study of emotional face processing across three measurements. Developmental Cognitive Neuroscience. 2013;4:65–76. doi: 10.1016/j.dcn.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa RA, Pine DS, Thorn JM, Nelson TE, Spinelli S, Nelson E, Mostofsky SH. Enhanced right amygdala activity in adolescents during encoding of positively valenced pictures. Developmental Cognitive Neuroscience. 2011;1(1):88–99. doi: 10.1016/j.dcn.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ver Hoeve ES, Kelly G, Luz S, Ghanshani S, Bhatnagar S. Short-term and long-term effects of repeated social defeat during adolescence or adulthood in female rats. Neuroscience. 2013;249:63–73. doi: 10.1016/j.neuroscience.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Research. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- Whittle S, Bartholomeusz C, Yucel M, Dennison M, Vijayakumar N, Allen NB. Orbitofrontal sulcogyral patterns are related to temperamental risk for psychopathology. Soc Cogn Affect Neurosci. 2014;9(2):232–239. doi: 10.1093/scan/nss126. doi: 10.1093/scan/nss126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Yap MB, Sheeber L, Dudgeon P, Yucel M, Pantelis C, Allen NB. Hippocampal volume and sensitivity to maternal aggressive behavior: a prospective study of adolescent depressive symptoms. Dev Psychopathol. 2011;23(1):115–129. doi: 10.1017/S0954579410000684. doi: 10.1017/S0954579410000684. [DOI] [PubMed] [Google Scholar]
- Whittle S, Yap MB, Yucel M, Fornito A, Simmons JG, Barrett A, Allen NB. Prefrontal and amygdala volumes are related to adolescents' affective behaviors during parent-adolescent interactions. Proc Natl Acad Sci U S A. 2008;105(9):3652–3657. doi: 10.1073/pnas.0709815105. doi: 10.1073/pnas.0709815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD, Jarvis B. Cyberball: A program for use in research on interpersonal ostracism and acceptance. Behav Res Methods. 2006;38(1):174–180. doi: 10.3758/bf03192765. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nature Reviews Neuroscience. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Kehl M, Borta A, Schänzer A, Schwarting RKW, Höglinger GU. New insights into the relationship of neurogenesis and affect: tickling induces hippocampal cell proliferation in rats emitting appetitive 50-kHz ultrasonic vocalizations. Neuroscience. 2009;163(4):1024–1030. doi: 10.1016/j.neuroscience.2009.07.043. [DOI] [PubMed] [Google Scholar]
- Wolf M, Van Doorn GS, Weissing FJ. Evolutionary emergence of responsive and unresponsive personalities. Proceedings of the National Academy of Sciences. 2008;105:15825–15830. doi: 10.1073/pnas.0805473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Samanez-Larkin GR, Katovich K, Knutson B. Affective traits link to reliable neural markers of incentive anticipation. NeuroImage. 2014;84:279–289. doi: 10.1016/j.neuroimage.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamuro T, Senzaki K, Iwamoto S, Nakagawa Y, Hayashi T, Hori M, Urayama O. Neurogenesis in the dentate gyrus of the rat hippocampus enhanced by tickling stimulation with positive emotion. Neuroscience research. 2010;68(4):285–289. doi: 10.1016/j.neures.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Yap MB, Whittle S, Yucel M, Sheeber L, Pantelis C, Simmons JG, Allen NB. Interaction of parenting experiences and brain structure in the prediction of depressive symptoms in adolescents. Arch Gen Psychiatry. 2008;65(12):1377–1385. doi: 10.1001/archpsyc.65.12.1377. doi: 10.1001/archpsyc.65.12.1377. [DOI] [PubMed] [Google Scholar]
- Yu Q, Teixeira CM, Mahadevia D, Huang Y, Balsam D, Mann JJ, Ansorge MS. Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Molecular Psychiatry. 2014;19(6):688–698. doi: 10.1038/mp.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou J, Seeley WW. Network-level structural covariance in the developing brain. Proceedings of the National Academy of Sciences. 2010;107(42):18191–18196. doi: 10.1073/pnas.1003109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubin J, Feldman RS, Salzinger S. A developmental model for the etiology of schizophrenia. In: Grove WM, Cicchetti D, editors. Thinking clearly about psychology: Vol. 2. Personality and psychopathology: Essays in honor of Paul E. Meehl. Univ. of Minnesota Press; Minneapolis, MN: 1991. pp. 410–429. [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test–retest evaluation using ICA and dual regression approach. NeuroImage. 2010;49:2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]