Abstract
The neurosteroids allopregnanolone and dehydroepiandrosterone (DHEA) are integral components of the stress response and exert positive modulatory effects on emotion in both human and animal studies. Though these antidepressant and anxiolytic effects have been well established, little research to date has examined their neural correlates, and no research has been conducted into the effects of neurosteroids on large-scale networks at rest. To investigate the neurosteroid impact on intrinsic connectivity networks, participants were administered 400 mg of pregnenolone (N = 16), 400 mg of DHEA (N = 14), or placebo (N = 15) and underwent 3T fMRI. Resting-state brain connectivity was measured using amygdala as a seed region. Compared to placebo, pregnenolone administration reduced connectivity between amygdala and dmPFC, between amygdala and precuneus, and between amygdala and hippocampus. DHEA reduced connectivity between amygdala and peri-amygdala and between amygdala and insula. Reductions in amygdala to precuneus connectivity were associated with less self-reported negative affect. These results demonstrate that neurosteroids modulate amygdala functional connectivity during resting-state, and may be a target for pharmacological intervention. Additionally, allopregnanolone and DHEA may shift the balance between salience network and default network, a finding that could provide insight into the neurocircuitry of anxiety psychopathology.
Keywords: Dehydroepiandrosterone, pregnenolone, fMRI, pharmaco-fMRI, neuroactive steroid, anxiety
Introduction
Allopregnanolone and dehydroepiandrosterone (DHEA) are endogenously-produced neurosteroids with neuroprotective, anxiolytic, antidepressant, antioxidant, and antiglucocorticoid effects (Belelli and Lambert, 2005; Maninger, et al., 2009; Patchev, et al., 1994). Deficiencies in allopregnanolone and DHEA are related to anxiety and depressive-like behavior. For instance, endogenous levels of allopregnanolone and DHEA are decreased in animal models of depression, and these animals exhibit behavioral improvements after DHEA administration or allopregnanolone induction (Genud, et al., 2009; Pibiri, et al., 2008). DHEA and allopregnanolone administration reduce immobility in the forced swim test (Frye, et al., 2004; Urani, et al., 2001), and reduce stress and anxiety-like behavior in rodents (Frye and Rhodes, 2006; Melchior and Ritzmann, 1994). In human studies, allopregnanolone and DHEA dysregulation is associated with anxiety and depression. Individuals with major depressive disorder exhibit reduced plasma and cerebrospinal fluid (CSF) levels of allopregnanolone (Romeo, et al., 1998; Uzunova, et al., 1998) and DHEA (Barrett-Connor, et al., 1999; Morsink, et al., 2007; Wong, et al., 2011), and those with posttraumatic stress disorder (PTSD) show reduced CSF allopregnanolone (Rasmusson, et al., 2006). Furthermore, increases in serum allopregnanolone and DHEA over a course of treatment predict symptomatic improvement (Amin, et al., 2006; Olff, et al., 2007; Uzunova, et al., 1998; Yehuda, et al., 2006). Therefore, these neurosteroids show promise as targets for antidepressant and anxiolytic effects in psychiatric disorders.
Converging preclinical and neuroimaging research suggests that the amygdala, a key region in threat detection (Adolphs, et al., 1999), fear conditioning (Armony and LeDoux, 1997), and emotional salience (Whalen, et al., 2001), may be a particular locus of allopregnanolone and DHEA’s effects. Our laboratory has recently demonstrated that single-dose DHEA (Sripada, et al., 2013a) and the allopregnanolone precursor pregnenolone (Sripada, et al., 2013b) decrease amygdala activity and increase activity in medial prefrontal regions during tasks engaging cognition-emotion interactions. It had been reported that progesterone administration (which in turn increases allopregnanolone levels) modulates amygdala responsivity to emotional faces (van Wingen, et al., 2007), and increases functional connectivity between amygdala and dorsal medial prefrontal cortex (dmPFC) (van Wingen, et al., 2008). Allopregnanolone modulates GABA(A) receptor-mediated inhibitory postsynaptic currents in the central nucleus of the amygdala (Wang, et al., 2007), and microinfusions of allopregnanolone directly into the amygdala produce anxiolytic (Engin and Treit, 2007) and antidepressant (Shirayama, et al., 2011) effects. The anxiolytic and antidepressant effects of DHEA may be mediated by the amygdala as well. In animal models, DHEA administration increases BDNF concentration (Naert, et al., 2007) and 5-HT(2A) receptor expression (Cyr, et al., 2000) in the amygdala, and DHEA infusions into the amygdala modulate stress responsivity (Singh, et al., 1994). Thus, the amygdala might be a key region in the anxiolytic and emotion modulatory effects of neurosteroids.
Though mounting evidence suggests that neurosteroids modulate amygdala activity, the reported direction of amygdala modulation is somewhat inconsistent. Activation of amygdala has been reported as increased (van Wingen, et al., 2008), or decreased (Sripada, et al., 2013b; van Wingen, et al., 2007) by allopregnanolone, depending on the task employed. To fully understand the impact of neurosteroids on amygdala function, it may be necessary to examine their effect on amygdala connectivity at rest. Resting-state connectivity offers a powerful way to assess intrinsic connections between brain networks (Fox and Raichle, 2007; Fox, et al., 2005; Raichle, et al., 2001) without external demands or confounds imposed by specific tasks. The amygdala resting-state network encompasses regions associated with emotion generation, including ventral medial prefrontal cortex, insula, thalamus and striatum (Fulwiler, et al., 2012; Kim, et al., 2011; Roy, et al., 2009). Thus, resting-state analyses may provide a fuller understanding of neurosteroid modulation of the amygdala and its associated emotion production network. Previous investigations demonstrate that the amygdala is strongly positively correlated with contralateral amygdala and insula at rest, and strongly anticorrelated with precuneus (Kim, et al., 2011; Roy, et al., 2009). Due to their anxiolytic and antidepressant properties, we predicted that allopregnanolone and DHEA would modulate both positive (amygdala and insula) and negative (precuneus) connectivity within the amygdala functional connectivity network during rest (Roy, et al., 2009), and that this effect would be associated with reduced self-reported negative affect. Furthermore, given the impact of allopregnanolone and DHEA on functional connectivity between amygdala and mPFC (Alhaj, et al., 2006; Sripada, et al., 2013a; Sripada, et al., 2013b; van Wingen, et al., 2008), we hypothesized that these neurosteroids would also modulate resting-state amygdala connectivity with mPFC.
Materials and Methods
1.1 Participants
Study participants were 45 right-handed healthy male volunteers aged 18–34 years (mean ± SD = 22 ± 3.6) recruited from the community via advertisement. Exclusion criteria were a history of head injury, recent steroid use, or current or past psychiatric disorder, as assessed via the Mini-International Neuropsychiatric Interview (M.I.N.I.; Sheehan, et al., 1998). No participant was taking over-the-counter or prescription medication at the time of the experiment. Participants were given full details of the study and provided written informed consent. The study was approved by the Institutional Review Board of the University of Michigan Medical School.
1.2 Procedure
To control for any potential diurnal variation in endogenous DHEA (Stanczyk, 2006), all fMRI scans were conducted in the afternoon, and blood collection times were standardized across subjects. Baseline blood samples were drawn between 11:00 and 11:30 AM, and post-scan blood samples were drawn between 3:00 and 4:00 PM. At one hour post-drug administration, participants completed the Positive and Negative Affect Scale Expanded (PANAS-X; Watson and Clark, 1994), the Drug Effects Questionnaire (Morean, et al., 2013), and a 20-item Visual Analogue Scale. The Drug Effects Questionnaire assessed the extent to which participants (1) felt any substance effects, (2) liked the effects, (3) felt high, and (4) wanted more of the substance. The Visual Analogue Scale included the following items: Clear-headed, Anxious, Stimulated, Tired, Calm, Drowsy, Peaceful, Nervous, Hungry, Energetic, Uneasy, Relaxed, Alert, Contented, Focused, Dreamy, Restless, Nauseous, Worn Out, and Jittery. Each item consisted of a 4-inch bar with ‘Not At All’ and ‘Extremely’ indicated at the extremities.
1.3 Drug Administration
Study drugs (pregnenolone and DHEA) and matching placebo identical in appearance were obtained from Belmar Pharmacy (Lakewood, CO), which provided certificates of analysis. Participants were randomly assigned to receive a single oral dose of 400 mg pregnenolone, 400 mg DHEA, or placebo. Participants and investigators were blind to condition. Pregnenolone was administered as a precursor loading strategy to significantly increase downstream allopregnanolone levels. Although data are limited, allopregnanolone serum levels appear to reach three times baseline levels two hours after oral administration of 400 mg pregnenolone (Marx, 2007), and DHEA serum concentrations peak 60 to 480 minutes after DHEA administration (Arlt, et al., 1998). Thus, drug administration occurred two hours before neuroimaging to ensure elevated levels during the scan.
1.4 Steroid measurements
We used circulating levels of DHEA and allopregnanolone as indicators of central neurosteroid levels. In animal models, serum neurosteroid levels are closely related to levels in the hippocampus (Marx, et al., 2006a), and serum DHEA levels are closely related to levels in CSF (Kancheva, et al., 2011), though this study did not find a correlation between CSF and serum pregnenolone levels. CSF DHEA levels are also correlated with temporal cortex DHEA levels, as are CSF and temporal cortex pregnenolone levels (Naylor, et al., 2008). We collected serum samples for assay once prior to drug administration and once after the scanning session. Serum DHEA levels were determined via enzyme immunoassay (ALPCO Diagnostics, Salem, NH), and serum DHEAS levels were determined by chemiluminescent enzyme immunoassay (IMMULITE) according to the manufacturer’s directions (Siemens Healthcare Diagnostics Inc., Tarrytown, NY). Pregnenolone, pregnanolone, allopregnanolone, and androsterone levels in serum were determined by a highly sensitive and specific gas chromatography-mass spectrometry (GC/MS) method, as previously described (Marx, et al., 2006b; Marx, et al., 2006c), with modifications (the electron impact mode was utilized rather than negative ion chemical ionization). One ml of serum was extracted three times in ethyl acetate before high performance liquid chromatography (HPLC) purification using tetrahydrofuran, ethanol, and hexane in the mobile phase. All samples were injected in duplicate. Mean intra-assay coefficients of variation for pregnenolone and allopregnanolone were 0.9% and 2.9%, respectively. The limit of detection with this method was 1 pg. All neurosteroid values were natural log transformed prior to analyses.
1.5 Resting-State Paradigm
Participants underwent structural (sMRI) and functional (fMRI) scanning that included both an emotion regulation task (Sripada, et al., 2013a; Sripada, et al., 2013b) and resting state procedures. Resting-state scans always occurred prior to tasks. Participants were positioned in the MR scanner and their heads comfortably restrained to reduce head movement. Heart rate and respiration measurements were acquired for group comparisons (via Independent-Samples Kruskal-Wallis tests). A black fixation cross on a white background was displayed in the center of the screen for 8 minutes. Participants were instructed to relax and keep their eyes open and fixed on the cross.
1.6 Image Acquisition
MRI scanning occurred on a Philips 3.0 Tesla Achieva X-series MRI (Philips Medical Systems). After a T1 image (T1-overlay) was obtained, a T2*-weighted, echoplanar acquisition sequence [GRE; repetition time, 2000 ms; echo time, 25 ms; flip angle, 90°; field of view (FOV), 22 cm; 42 slice; thickness/skip, 3.0/0 mm matrix size equivalent to 64 × 64] was collected. After discarding three initial volumes to permit thermal equilibration of the MRI signal, 240 volumes were acquired over 8 minutes. After acquiring the functional volumes, a high-resolution T1 scan was obtained for anatomic normalization [26 FOV; thickness/skip, 1.0/0 mm]. Participants viewed a fixation cross through MR-compatible liquid crystal display goggles (NordicNeuroLabs http://www.nordicneurolab.com).
1.7 Preprocessing
A standard series of processing steps was performed using statistical parametric mapping (SPM8; www.fil.ion.ucl.ac.uk/spm). Scans were reconstructed, motion-corrected, slice-time corrected, realigned to the first scan in the experiment to correct for head motion, and co-registered with the high-resolution sagittal images. Normalization was performed using the voxel-based morphometry toolbox implemented in SPM8 (www.fil.ion.ucl.ac.uk/spm). Scans were normalized to standard space, segmented into tissue types, bias-corrected, and iteratively realigned using DARTEL. Smoothing was performed with an 8 × 8 × 8 mm3 kernel. Motion parameters (mean displacement, mean angle) were compared across drug conditions via Independent-Samples Kruskal-Wallis tests, and runs with any movement greater than 3 mm were excluded.
1.8 Data Analysis
Right and left amygdala seed region ROIs were constructed from cytoarchitectonically determined probabilistic maps of the human basolateral and centromedial amygdala, developed by Amunts and colleagues (2005) within the SPM Anatomy Toolbox (Eickhoff, et al., 2005). These subregions were combined to form a single, whole amygdala seed. We extracted the spatially averaged time series from right and left amygdala ROIs for each participant. Since resting-state functional connectivity measures low-frequency spontaneous BOLD oscillations (.01 – .10 Hz band) (Fox, et al., 2005), the time-course for each voxel was band-passed filtered in this range. Next, motion scrubbing and nuisance regression were performed. During motion scrubbing, individual frames with excessive head motion were censored from the time series, following Power et al. (2012). The target high motion frame, but not those flanking it, was removed in accordance with the findings of Satterthwaite et al. (2013). Nuisance regression was performed to remove the effects of nuisance variables unrelated to neuronal activity. Covariates of no interest included six motion regressors generated from the realignment step noted above. In addition, we included five principal components of the BOLD time series extracted from white matter and cerebrospinal fluid masks, which has been demonstrated to effectively remove signals derived from the cardiac and respiratory cycle (Chang and Glover, 2009). This method is comparable to the COMPCOR method and is complementary to the RETROICOR method (Behzadi, et al., 2007). In generating the white matter and cerebrospinal fluid regressors, subject-specific masks were first created using VBM-based segmentation implemented in SPM8. Masks were eroded using FSL-Erode to eliminate border regions of potentially ambiguous tissue-type. A spatially averaged time series was extracted from these masks, and the first five principal components were included in the regression. The residuals from this regression were then retained for further analysis. We did not perform global-signal regression, as it had been suggested that this may produce spurious anti-correlation with orthogonal networks that increase in proportion to the size of the those networks (Anderson, et al., 2011). Pearson product-moment correlation coefficients were calculated between average time courses in the seed regions of interest (ROIs) and all other voxels of the brain resulting in a 3-dimensional correlation coefficient image (r-image). These r-images were then transformed to z-scores using a Fisher r-to-z transformation.
1.9 Whole Brain and Region of Interest (ROI) Analysis
Z-score images from the individual functional connectivity analyses were entered into second-level random-effects analyses (one-sample and two-sample t-tests) implemented in SPM8. At the second level, for between-group comparisons, we set a whole-brain voxel-wise significance threshold at p < .05, cluster-level corrected for multiple comparisons across the entire brain (cluster volume > 1220 voxels); these cluster-level thresholds were calculated using Monte-Carlo simulations (AFNI AlphaSim, http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim). To exclude a general effect of drug administration on the BOLD response (van Wingen, et al., 2007), the primary visual cortex (V1) was used as the control region (Amunts, et al., 2000). Reported voxel coordinates correspond to standardized Montreal Neurologic Institute (MNI) space. To assess for correlations with mood, PANAS-X scores were correlated with data extracted from regions of group difference (averaged across the entire cluster).
Results
2.1 Participants
Sixteen participants were administered pregnenolone, 14 were administered DHEA, and 15 were administered placebo. Groups did not differ by age (F(2,44) = .71, p = .5). Pregnenolone administration significantly increased serum pregnenolone, pregnanolone, and allopregnanolone levels, and DHEA administration increased serum DHEA, DHEAS, and androsterone levels [p < .001; details can be found in (Sripada, et al., 2013a; Sripada, et al., 2013b)]. There were no significant differences in subjective drug effects (p > .4), and participants’ guesses of which drug they received did not deviate from chance (χ2 (6) = 3.80, p = .7). There were no significant differences between drug administration and placebo groups in self-reported sedation (p > 0.5).
2.2 Motion and Physiological Variables
There were no movements greater than 3 millimeters and no motion differences between DHEA and placebo groups (p > .2). In the pregnenolone administration group, mean displacement was reduced (mean = .066 mm) as compared to the placebo group (mean = .099 mm, p = .006). To correct for this discrepancy, we implemented motion scrubbing procedures. There were no group differences in heart rate or respiration (p > .7).
2.3 fMRI Findings
Under the placebo condition, the left amygdala seed showed positive connectivity with right amygdala, peri-amygdala, bilateral hippocampus and superior temporal gyrus, and anti-correlations with posterior cingulate cortex (see Table 1). The right amygdala seed showed positive connectivity with left amygdala, bilateral peri-amygdala and bilateral hippocampus, and anti-correlation with precuneus (see Table 1).
Table 1.
| Contrast Map and Brain Region | Cluster Size | MNI Coordinates (x y z) | Analysis (z) |
|---|---|---|---|
| Left Amygdala positive correlations under PBO | |||
| Superior Temporal Gyrus/Left Insula | 8153 | −27 −7 −29 | 7.28 |
| Medial Frontal Gyrus | 59 | −6 41 −23 | 4.45 |
| Right Lingual Gyrus | 13 | 15 −40 −2 | 4.29 |
| Right Heschl’s Gyrus | 16 | 39 −25 7 | 3.92 |
| Inferior Frontal Gyrus | 178 | −48 11 28 | 3.83 |
| Right Insula | 14 | 30 −7 10 | 3.71 |
| Left Angular Gyrus | 51 | −57 −70 25 | 3.48 |
| Left Amygdala negative correlations under PBO | |||
| Right Middle Frontal Gyrus | 52 | 36 47 13 | 4.12 |
| Posterior Cingulate Cortex | 13 | 6 −43 22 | 3.61 |
| Right Amygdala positive correlations under PBO | |||
| Right Parahippocampal Gyrus | 1935 | 24 −10 −23 | 7.66 |
| Superior Temporal Gyrus | 1766 | −33 −4 −29 | 6.31 |
| Right Inferior Temporal Gyrus | 13 | 48 −49 −26 | 5.37 |
| Left Inferior Temporal Gyrus | 436 | −54 −67 −5 | 4.71 |
| Right Precentral Gyrus | 1015 | 60 −4 46 | 4.09 |
| Left Precentral Gyrus | 19 | −48 −7 25 | 3.99 |
| Inferior Parietal Lobule | 23 | −48 −34 49 | 3.84 |
| Supplementary Motor Area | 58 | 12 −22 52 | 3.79 |
| Right Paracentral Lobule | 14 | 6 −40 67 | 3.64 |
| Left Paracentral Lobule | 35 | −9 −31 73 | 3.61 |
| Right Amygdala negative correlations under PBO | |||
| Cingulate Gyrus | 71 | −3 −19 25 | 4.27 |
| Precuneus | 111 | 12 −64 31 | 3.98 |
| Left Precuneus | 12 | −9 −70 34 | 3.38 |
Data thresholded at p < 0.001, uncorrected, extent threshold k = 10. MNI, Montreal Neurologic Institute; PBO, placebo.
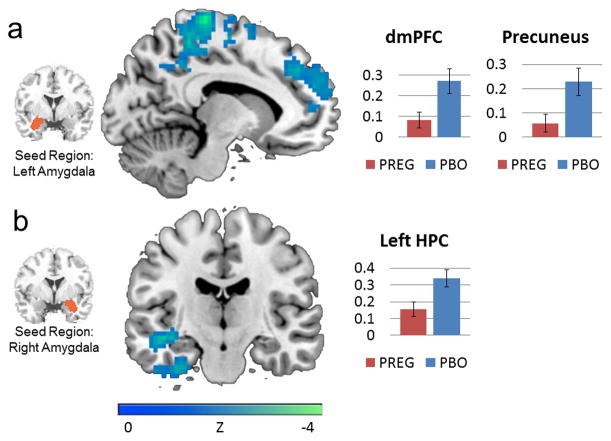
Compared to placebo, pregnenolone administration reduced connectivity between the left amygdala seed and a cluster encompassing dmPFC and precuneus (see Table 2; Figure 1). Pregnenolone also reduced connectivity between the right amygdala seed and left hippocampus (see Figure 1).
Table 2.
| Contrast Map and Brain Region | Cluster Size | MNI Coordinates (x y z) | Analysis (z) |
|---|---|---|---|
| Left Amygdala ALLO > Placebo | |||
| Left Superior Frontal Gyrus | 2687 | −9 −28 76 | 3.84 |
| Superior Medial Frontal Gyrus (dmPFC) | 196 | −12 41 37 | 2.82 |
| Precuneus | 199 | −9 −40 55 | 2.86 |
| Right Amygdala ALLO > Placebo | |||
| Left Middle Temporal Gyrus | 1522 | −45 −19 −11 | 3.91 |
| Left Hippocampus | 18 | −36 −16 −11 | 2.84 |
| Left Amygdala DHEA > Placebo | |||
| Left Supramarginal Gyrus | 2038 | −66 2 7 | 3.62 |
| Left Insula | 165 | −36 −1 −8 | 2.81 |
| Left Amygdala | 8 | −24 −7 −14 | 2.40 |
| Left Amygdala DHEA > ALLO | |||
| Medial Frontal Gyrus | 2049 | −15 50 10 | 3.88 |
| dmPFC | 396 | −12 53 10 | 3.58 |
Cluster-level significance set at p < .05, whole brain corrected for multiple comparisons. Within each significant cluster, Z-score and associated a priori region are noted along with MNI atlas coordinates of peaks, in bold. ALLO, allopregnanolone; dmPFC, dorsal medial prefrontal cortex; MNI, Montreal Neurologic Institute.
Figure 1.
(A) Pregnenolone administration reduced functional connectivity between left amygdala and a cluster encompassing dmPFC and precuneus (x=−11). (B) Pregnenolone administration reduced functional connectivity between right amygdala and left hippocampus (y=−16). Bar graphs depict percent signal change. Error bars depict standard error of the mean. dmPFC = dorsal medial prefrontal cortex. HPC = hippocampus.
Compared to placebo, DHEA reduced connectivity between the left amygdala seed and a cluster encompassing left amygdala, left peri-amygdala, and left insula (see Table 2; Figure 2). There were no significant differences between DHEA and placebo groups in connectivity with a right amygdala seed.
Figure 2.
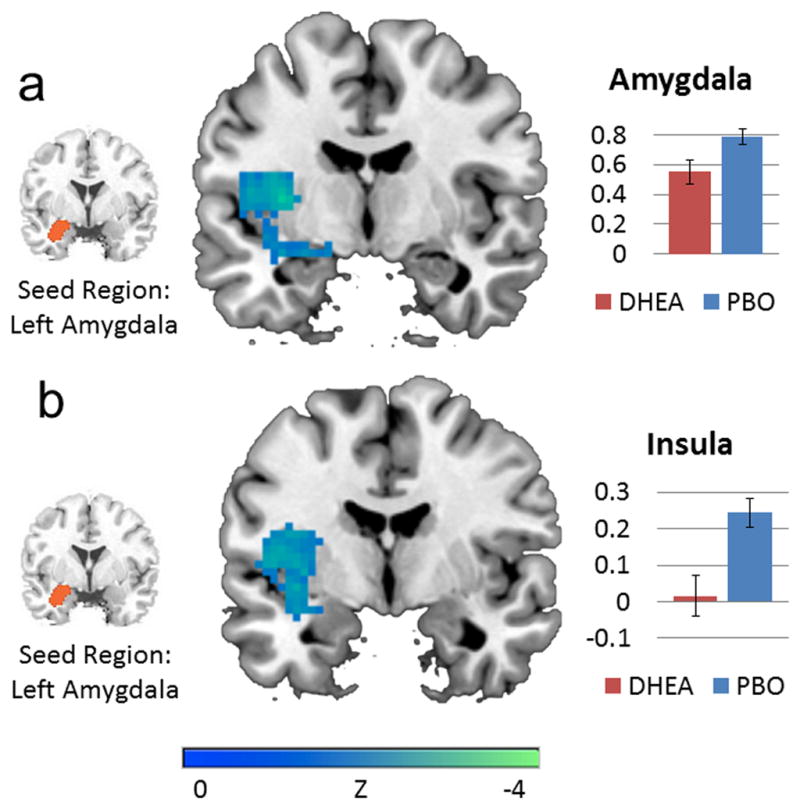
DHEA reduced functional connectivity between left amygdala and (A) peri-amygdala (y=−7) and (B) left insula (y=4). Bar graphs depict percent signal change. Error bars depict standard error of the mean.
We also conducted an exploratory analysis of differences in amygdala functional connectivity between pregnenolone and DHEA administration groups. Compared to DHEA, pregnenolone reduced functional connectivity between left amygdala and dmPFC (see Table 2).
2.4 Correlations with PANAS-X
As compared to placebo, DHEA administration reduced PANAS-X negative affect score at trend level (t(27) = 1.87, p = .07) (as reported in Sripada, et al., 2013a). To probe the neural underpinnings of this effect, correlations were computed between PANAS-X negative affect score and the values extracted from regions of group difference. These included: (1) the dmPFC and precuneus-encompassing cluster that exhibited reduced connectivity with left amygdala in the pregnenolone group, (2) the left hippocampus-encompassing cluster that exhibited reduced connectivity with right amygdala in the pregnenolone group, and (3) the left insula-encompassing cluster that exhibited reduced connectivity with left amygdala in the DHEA group. Across all three groups (pregnenolone, DHEA, and placebo), there was a positive correlation between PANAS-X negative affect score and functional connectivity between right amygdala and the hippocampus-encompassing cluster (r = .294, p = .05), indicating that reduced connectivity with hippocampus was associated with less negative affect. Across groups, there was also a positive correlation between PANAS-X negative affect score and functional connectivity between left amygdala and the insula-encompassing cluster (r = .291, p = .05). Additional correlations were computed between PANAS-X negative affect score and the values extracted from anatomical insula, dmPFC, hippocampus, and precuneus. Across all three groups (pregnenolone, DHEA, and placebo), there was a positive correlation between PANAS-X negative affect score and left amygdala functional connectivity with anatomical precuneus (r = .319, p = .035).
2.5 Correlations with Neurosteroid Levels
To further probe relationships between neurosteroids and connectivity patterns, correlations were computed between delta serum neurosteroid levels (endpoint minus baseline) and the values extracted from regions of group difference. Across all three groups, functional connectivity between the right amygdala seed and left hippocampus-encompassing cluster was negatively associated with delta serum pregnenolone (r = −.513, p < .001), delta serum pregnanolone (r = −.326, p = .031), and delta serum allopregnanolone (r = −.553, p < .001). Across all three groups, functional connectivity between the left amygdala seed and left insula-encompassing cluster was negatively associated with delta serum DHEAS (r = −.436, p = .003), and delta serum androsterone (r = −.390, p = .009). Additionally, functional connectivity between left amygdala and anatomical left insula was negatively correlated with delta serum DHEAS (r = −.302, p = .047), and functional connectivity between right amygdala and anatomical precuneus was negatively correlated with delta serum pregnanolone (r = −.301, p = .047). Neither pregnenolone nor DHEA significantly modulated connectivity between amygdala and primary visual cortex (left amygdala p > .4; right amygdala p > .7), indicating that drug effects on other brain regions were not attributable to general changes in the BOLD response.
Discussion
To interrogate the neural underpinnings of anxiety- and mood-altering properties of neurosteroids, we investigated the impact of single-dose pregnenolone and DHEA on patterns of resting-state functional connectivity of the amygdala. Compared to placebo, pregnenolone administration reduced connectivity between amygdala and dmPFC, between amygdala and precuneus, and between amygdala and hippocampus. Compared to placebo, DHEA reduced amygdala connectivity with insula and with peri-amygdaloid regions. These connectivity patterns were associated with less self-reported negative affect. To our knowledge, this is the first investigation of resting-state functional connectivity after neurosteroid administration, and the first to demonstrate that neurosteroids modulate intrinsic connectivity networks in a way that is relevant to negative affect. Our results demonstrate that allopregnanolone and DHEA reduce connectivity within the resting-state amygdala network, and may also shift the balance between intrinsic connectivity networks, a function that could provide insight into the neurocircuitry of anxiety psychopathology.
We found that DHEA reduced functional connectivity within left amygdala and between left amygdala and left peri-amygdala, suggesting reduced connectivity within this emotion generation network. Converging evidence from multiple lines of investigation suggests that higher anxiety is associated with greater amygdala activity. Greater amygdala activation is associated with greater state (Bishop, et al., 2004) and trait (Etkin, et al., 2004) anxiety, and a metaanalysis of neuroimaging studies in various anxiety disorders concluded that elevated amygdala activity is present across anxiety disorders (Etkin and Wager, 2007). Furthermore, both animal and human connectivity data suggest that amygdala and extended amygdala function as a coordinated unit to process threat (Liberzon, et al., 2003; Oler, et al., 2012). It is still unclear, however, if greater interamygdala connectivity is associated with higher levels of anxiety. For example, Pantzatos and colleagues (2012) have argued that right and left amygdala are more coupled while viewing neutral faces than fearful faces. However, given the strong link between amygdala activation and negative emotional response, it is reasonable to interpret reduced interamygdala connectivity as potentially contributing to reduced emotional reactivity. This reduction of connectivity within amygdala and between amygdala and peri-amygdala may thus be relevant DHEA’s anxiolytic effects (Maninger, et al., 2009; Melchior and Ritzmann, 1994).
DHEA administration also reduced functional connectivity between left amygdala and left insula. The insula is implicated in interoception (Oppenheimer, et al., 1992), disgust (Britton, et al., 2006), emotion processing (Stein, et al., 2007), emotional recall (Phan, et al., 2002), and anticipation of aversive stimuli (Simmons, et al., 2008). Amygdala and insula are structurally interconnected (Aggleton, et al., 1980), and negative emotion induction enhances coupling between insula and amygdala in healthy volunteers (van Marle, et al., 2010). We have previously reported that amygdala exhibits greater resting-state connectivity with insula in PTSD patients as compared to healthy and combat-exposed control participants (Sripada, et al., 2012a; Sripada, et al., 2012b). This finding has been replicated by others (Rabinak, et al., 2011). Thus, reduced connectivity between amygdala and insula could represent a protective/resilience factor in the development of pathological anxiety states. In support of this hypothesis, functional connectivity between amygdala and insula was positively associated with negative affect in our sample. DHEA reduced connectivity between amygdala and insula, and also reduced PANAS-X at trend level. However, given the lack of a statistically significant decrease in PANAS-X scores, it is possible that the association between negative affect and amygdala-insula connectivity may be due to spontaneous variations in mood rather than a drug-induced effect.
During rest, the amygdala shows positive functional connectivity with ventral medial prefrontal regions, insula, thalamus, and striatum, and anti-correlations with superior frontal gyrus, bilateral middle frontal gyrus, posterior cingulate cortex, and precuneus (Fulwiler, et al., 2012; Kim, et al., 2011; Roy, et al., 2009). Notably, the observed amygdala resting-state connectivity map shows substantial overlap with the salience network, an intrinsic connectivity network responsible for detecting and orienting to salient stimuli, implicated in homeostatic regulation, interoceptive, autonomic, and reward processing (Cauda, et al., 2011; Dosenbach, et al., 2007; Seeley, et al., 2007; Sridharan, et al., 2008). Amygdala, along with dorsal anterior cingulate cortex, anterior insula/inferior frontal gyrus, and ventral striatum, are key nodes in this network. Both allopregnanolone and DHEA have demonstrated effects on this circuit in neuroimaging studies. DHEA’s reduction of amygdala to insula connectivity and interamygdala connectivity suggests this neurosteroid may modulate the intrinsic connectivity of the salience network. During task-based studies, there is preliminary evidence to suggest that DHEA modulates anterior cingulate cortex (Alhaj, et al., 2006; Sripada, et al., 2013a) and insula activity (Alhaj, et al., 2006), and that allopregnanolone influences activity in amygdala (Ossewaarde, et al., 2010a; van Wingen, et al., 2007; van Wingen, et al., 2008) and ventral striatum (Ossewaarde, et al., 2010b), all components of the salience network. We have previously suggested that stronger within-salience network connectivity is associated with greater anxiety (Sripada, et al., 2012a; Sripada, et al., 2012b). In addition, both PTSD and major depressive disorder were found to be associated with greater salience network activity in recent meta-analyses (Hamilton, et al., 2012; Patel, et al., 2012). Since elevations in neurosteroids have been associated with symptomatic improvement in anxiety disorders (Rasmusson, et al., 2006), the observed reduction of intrasalience network connectivity could suggest a potential mechanism for this anxiolytic effect.
Finally, pregnenolone reduced amygdala connectivity with dmPFC, precuneus, and hippocampus. Across groups, greater amygdala to precuneus connectivity was associated with greater PANAS-X negative affect [ten items assessing constructs of fear, hostility, guilt, and sadness (Watson and Clark, 1994)], suggesting that a reduction in this connectivity is associated with less negative affect. In monkeys, the amygdala is structurally connected with dorsal precuneus (Leichnetz, 2001), ventral precuneus (Parvizi, et al., 2006), and dmPFC/dorsal anterior cingulate cortex (Ghashghaei, et al., 2007) and typically shows negative functional connectivity with the precuneus and dmPFC at rest (Roy, et al., 2009; Zhang and Li, 2012). This is consistent with the key roles of the dmPFC and precuneus in the default network, a network that is anti-correlated with salience network at rest (Fox, et al., 2005). The default network is associated with stimulus-independent, internally-focused thought, including spontaneous cognition, autobiographical memory, prospection and mind-wandering (Menon, 2011; Qin and Northoff, 2011; Spreng, et al., 2009; Toro, et al., 2008), thus this network is active during non-structured tasks such as resting state. The current data suggest that allopregnanolone might further reduce functional connectivity between amygdala and default network, contributing to enhanced segregation between default network and salience network.
Recently, our group has proposed that decreased segregation, i.e. inappropriate connectivity between salience network and default network, contributes to anxiety psychopathology (Sripada, et al., 2012b). Among participants with PTSD, we found that greater functional connectivity between default network and salience network regions was correlated with elevated PTSD symptoms (Sripada, et al., 2012b). Similarly, enhanced cross-network connectivity between default network and thalamus (a node in salience network) has been observed in PTSD in a different study (Yin, et al., 2011). Furthermore, enhanced connectivity between default network and amygdala predicted the development of PTSD symptoms in acutely traumatized individuals (Lanius, et al., 2010), and enhanced connectivity between default network and insula was associated with higher self-reported anxiety in a separate study (Dennis, et al., 2011). Precuneus exhibits greater connectivity with amygdala in generalized anxiety (Strawn, et al., 2012) and panic disorder (Pannekoek, et al., 2013a), and with anterior cingulate cortex (a key node in salience network) in social anxiety disorder (Pannekoek, et al., 2013b). Moreover, in healthy controls, acute laboratory stress increases amygdala resting-state connectivity with precuneus and posterior cingulate cortex (Veer, et al., 2011), though the authors note that this connectivity pattern might indicate healthy rather than pathologic recovery and adaptation in the post-stressor period. Another study in healthy individuals demonstrated that viewing fearful as compared to neutral face enhanced amygdala to precuneus coupling (Pantazatos, et al., 2012). Together, these data evince a consistent pattern in which anxiety disorders or induction of anxiety is associated with reduced segregation between salience network and default network. Thus, allopregnanolone’s enhancement of segregation between default network and salience network highlights the potential contribution of this neurosteroid to the amelioration of anxiety.
Though both pregnenolone and DHEA reduced amygdala intrinsic connectivity, pregnenolone exerted a greater effect than DHEA on connectivity with medial prefrontal regions. These differences may be due to differing mechanisms of action. DHEA appears to have anxiolytic-like actions (Maninger, et al., 2009; Melchior and Ritzmann, 1994), however it demonstrates modest negative modulation of GABA(A) receptors (Imamura and Prasad, 1998). Thus, its anxiolytic-like actions in rodents may involve other mechanisms. In contrast, allopregnanolone has very pronounced effects at the GABA(A) receptor, and decreases GABA(A) receptor responses with 20-fold higher potency than benzodiazepines and barbiturates (Majewska, et al., 1986). Allopregnanolone’s greater GABAergic activity, in combination with the greater wealth of evidence linking allopregnanolone to amygdala modulation, may help to explain the somewhat stronger modulation of amygdala connectivity under allopregnanolone versus DHEA.
There are several limitations to this study. Limitations of our sample include the fact that the number of participants in each group was relatively small, thus, our results should be considered preliminary. Additionally, our sample consisted of healthy male individuals without mood or anxiety disorder diagnoses. Thus, extrapolations to women or to clinical populations should be made with caution. The between-group design of our study does not allow for a baseline comparison of functional connectivity. Thus, it is difficult to determine whether between-group differences are solely due to the drugs administered, or partially due to natural variation. Future studies should implement a crossover design to address this issue. Additionally, we did not obtain baseline measurements of subjective negative affect, and therefore group differences in PANAS-X score may also be partially attributable to natural variation. Limitations of our intervention include the fact that we measured serum levels of allopregnanolone and DHEA, and not CSF or brain levels. However, in animals, neurosteroid levels are highly correlated (Kancheva, et al., 2011; Marx, et al., 2006a). Additionally, in the allopregnanolone manipulation, we administered pregnenolone, allopregnanolone’s precursor. Though we have framed our results in terms of an allopregnanolone manipulation, it is possible that our results may be attributable to increases in pregnenolone in addition to allopregnanolone. Since allopregnanolone is not commercially available for clinical use, it was necessary to administer pregnenolone as a precursor loading strategy to increase downstream allopregnanolone levels, and our results demonstrate that oral administration of pregnenolone increases allopregnanolone levels sevenfold (see Sripada, et al., 2013b). We also did not measure levels of additional downstream steroids. Pregnenolone administration may increase levels of pregnenolone sulfate and progesterone (Marx, et al., 2009), and DHEA administration may increase estradiol and testosterone (Schmidt, et al., 2005). Thus, it is possible that increases in these downstream steroids, rather than increases in only allopregnanolone and DHEA, contributed to the reported findings. Furthermore, though the bulk of DHEA research supports an anxiolytic effect for DHEA, some studies suggest an anxiogenic role (e.g., Dmitrieva, et al., 2001; van Goozen, et al., 2000). Thus, further studies should assess the behavioral impact of DHEA’s reduction of amygdala resting-state functional connectivity.
Turning to methodological limitations, our results may have been impacted by the reduced average head displacement of .03 mm in the pregnenolone group. For instance, Van Dijk and colleagues have suggested that small head movements can inflate measures of local functional coupling and deflate measure of long-range functional coupling (Van Dijk, et al., 2012). However, we implemented scrubbing procedures as described by Power et al. (2012), which have been demonstrated to significantly reduce confounds introduced by head movement. In addition, it has been reported that DHEA and pregnenolone may potentially impact neurovascular coupling (e.g., see Liu and Dillon, 2004; Naylor, et al., 2010). PET or arterial spin labeling studies could examine this issue directly, however we compared BOLD signal across treatment groups in control regions (i.e. visual cortex) and found no significant differences. Another potential limitation is that different methodologies were used for assaying different neurosteroids: GC/MS for pregnenolone and allopregnanolone, and enzyme immunoassay for DHEA and DHEAS. DHEA values derived from these two assay methods, however, show excellent correlation (Stanczyk, 2006), and immunoassay is much less labor-intensive and less expensive than GC/MS. In contrast, there are currently no commercially available methodologies for allopregnanolone and pregnenolone that approach the sensitivity and specificity of GC/MS, and it remains the ‘gold standard’ quantification approach for these steroids. It also does not appear optimal to use GC/MS for sulfated neurosteroids such as DHEAS, given recent challenges and controversies regarding their quantification (see Ebner, et al., 2006; Higashi, et al., 2003; Liere, et al., 2004; Liu, et al., 2003; Marx, et al., 2009; Schumacher, et al., 2008). Finally, we have conceptualized decreased within-salience network connectivity as relevant to reduced anxiety; however decreased connectivity within this network may also have drawbacks. For instance, stronger salience network connectivity has been associated with better working memory (Li, et al., 2012). Future studies should investigate neurosteroid modulation of salience network in anxiety-disordered populations to further assess the benefits and disadvantages of these patterns.
Conclusion
In summary, allopregnanolone and DHEA reduced amygdala functional connectivity with other regions within amygdala/salience network, and reduced amygdala connectivity with regions of default network in our participants. These findings suggest that neurosteroids modulate amygdala functional connectivity during the resting state, and may shift the balance between salience network and default network at rest. These findings provide insight into the neurocircuitry of anxiety, and suggest that allopregnanolone and DHEA may be potential targets for pharmacological intervention for anxiety psychopathology.
Acknowledgments
Role of the Funding Source:
The research reported in this article was supported by grants from the National Institute of Mental Health (R24 MH075999) to IL, from the Telemedicine and Advanced Technology Research Center (W81XWH-08-2-0208) to IL, from a VA Career Development Transition Award to CM, and by the Veterans Affairs Mid-Atlantic Mental Illness, Research, Education and Clinical Center.
Footnotes
Financial Disclosures:
Dr. Marx discloses that she is an applicant or co-applicant on pending U.S. patent applications on the use of neurosteroids and derivatives for the treatment of central nervous system disorders and for lowering cholesterol (no patents issued, no licensing in place), and she is an unpaid scientific advisor to Sage Therapeutics. The remaining authors have no potential conflicts of interest to disclose.
References
- Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, Anderson A, Lee GP, Damasio AR. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–7. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–68. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Alhaj HA, Massey AE, McAllister-Williams RH. Effects of DHEA administration on episodic memory, cortisol and mood in healthy young men: a double-blind, placebo-controlled study. Psychopharmacology (Berl) 2006;188:541–51. doi: 10.1007/s00213-005-0136-y. [DOI] [PubMed] [Google Scholar]
- Amin Z, Mason GF, Cavus I, Krystal JH, Rothman DL, Epperson CN. The interaction of neuroactive steroids and GABA in the development of neuropsychiatric disorders in women. Pharmacol Biochem Behav. 2006;84:635–43. doi: 10.1016/j.pbb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–52. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann’s areas 17 and 18 brought into stereotaxic space-where and how variable? Neuroimage. 2000;11:66–84. doi: 10.1006/nimg.1999.0516. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong EK, Desai K, Yurgelun-Todd D. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Hum Brain Mapp. 2011;32:919–34. doi: 10.1002/hbm.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt W, Justl HG, Callies F, Reincke M, Hubler D, Oettel M, Ernst M, Schulte HM, Allolio B. Oral dehydroepiandrosterone for adrenal androgen replacement: pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J Clin Endocrinol Metab. 1998;83:1928–34. doi: 10.1210/jcem.83.6.4850. [DOI] [PubMed] [Google Scholar]
- Armony JL, LeDoux JE. How the brain processes emotional information. Ann N Y Acad Sci. 1997;821:259–70. doi: 10.1111/j.1749-6632.1997.tb48285.x. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, von Muhlen D, Laughlin GA, Kripke A. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr Soc. 1999;47:685–91. doi: 10.1111/j.1532-5415.1999.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24:10364–8. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: An fMRI study. Neuroimage. 2006;31:397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47:1448–59. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr M, Landry M, Di Paolo T. Modulation by estrogen-receptor directed drugs of 5-hydroxytryptamine-2A receptors in rat brain. Neuropsychopharmacology. 2000;23:69–78. doi: 10.1016/S0893-133X(00)00085-3. [DOI] [PubMed] [Google Scholar]
- Dennis EL, Gotlib IH, Thompson PM, Thomason ME. Anxiety modulates insula recruitment in resting-state functional magnetic resonance imaging in youth and adults. Brain Connectivity. 2011;1:245–54. doi: 10.1089/brain.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva TN, Oades RD, Hauffa BP, Eggers C. Dehydroepiandrosterone sulphate and corticotropin levels are high in young male patients with conduct disorder: comparisons for growth factors, thyroid and gonadal hormones. Neuropsychobiology. 2001;43:134–40. doi: 10.1159/000054881. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–8. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner MJ, Corol DI, Havlikova H, Honour JW, Fry JP. Identification of neuroactive steroids and their precursors and metabolites in adult male rat brain. Endocrinology. 2006;147:179–90. doi: 10.1210/en.2005-1065. [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Walters NB, Schleicher A, Kril J, Egan GF, Zilles K, Watson JD, Amunts K. High-resolution MRI reflects myeloarchitecture and cytoarchitecture of human cerebral cortex. Hum Brain Mapp. 2005;24:206–15. doi: 10.1002/hbm.20082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: the amygdala, medial prefrontal cortex, or hippocampus. Behav Pharmacol. 2007;18:461–70. doi: 10.1097/FBP.0b013e3282d28f6f. [DOI] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–55. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 5alpha-pregnan-3alpha-ol-20-one (3alpha,5alpha-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3alpha,5alpha-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006;18:960–75. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5 alpha-reductase. Brain Res. 2004;1004:116–24. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, King JA, Zhang N. Amygdala-orbitofrontal resting-state functional connectivity is associated with trait anger. Neuroreport. 2012;23:606–10. doi: 10.1097/WNR.0b013e3283551cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genud R, Merenlender A, Gispan-Herman I, Maayan R, Weizman A, Yadid G. DHEA lessens depressive-like behavior via GABA-ergic modulation of the mesolimbic system. Neuropsychopharmacology. 2009;34:577–84. doi: 10.1038/npp.2008.46. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional Neuroimaging of Major Depressive Disorder: A Meta-Analysis and New Integration of Baseline Activation and Neural Response Data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Higashi T, Daifu Y, Ikeshima T, Yagi T, Shimada K. Studies on neurosteroids XV. Development of enzyme-linked immunosorbent assay for examining whether pregnenolone sulfate is a veritable neurosteroid. J Pharm Biomed Anal. 2003;30:1907–17. doi: 10.1016/s0731-7085(02)00534-4. [DOI] [PubMed] [Google Scholar]
- Imamura M, Prasad C. Modulation of GABA-gated chloride ion influx in the brain by dehydroepiandrosterone and its metabolites. Biochem Biophys Res Commun. 1998;243:771–5. doi: 10.1006/bbrc.1998.8177. [DOI] [PubMed] [Google Scholar]
- Kancheva R, Hill M, Novak Z, Chrastina J, Kancheva L, Starka L. Neuroactive steroids in periphery and cerebrospinal fluid. Neuroscience. 2011;191:22–7. doi: 10.1016/j.neuroscience.2011.05.054. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–73. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, Neufeld RW, Williamson PC, Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR. Connections of the medial posterior parietal cortex (area 7m) in the monkey. Anat Rec. 2001;263:215–36. doi: 10.1002/ar.1082. [DOI] [PubMed] [Google Scholar]
- Li Y, Qin W, Jiang T, Zhang Y, Yu C. Sex-dependent correlations between the personality dimension of harm avoidance and the resting-state functional connectivity of amygdala subregions. PLoS ONE. 2012;7:e35925. doi: 10.1371/journal.pone.0035925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Phan KL, Decker LR, Taylor SF. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28:726–33. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- Liere P, Pianos A, Eychenne B, Cambourg A, Liu S, Griffiths W, Schumacher M, Sjovall J, Baulieu EE. Novel lipoidal derivatives of pregnenolone and dehydroepiandrosterone and absence of their sulfated counterparts in rodent brain. J Lipid Res. 2004;45:2287–302. doi: 10.1194/jlr.M400244-JLR200. [DOI] [PubMed] [Google Scholar]
- Liu D, Dillon JS. Dehydroepiandrosterone stimulates nitric oxide release in vascular endothelial cells: evidence for a cell surface receptor. Steroids. 2004;69:279–89. doi: 10.1016/j.steroids.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Liu S, Sjovall J, Griffiths WJ. Neurosteroids in rat brain: extraction, isolation, and analysis by nanoscale liquid chromatography-electrospray mass spectrometry. Anal Chem. 2003;75:5835–46. doi: 10.1021/ac0346297. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE. 2007 unpublished data. [Google Scholar]
- Marx CE, Keefe RS, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, Strauss JL, Naylor JC, Payne VM, Lieberman JA, Savitz AJ, Leimone LA, Dunn L, Porcu P, Morrow AL, Shampine LJ. Proof-of-Concept Trial with the Neurosteroid Pregnenolone Targeting Cognitive and Negative Symptoms in Schizophrenia. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Shampine LJ, Duncan GE, VanDoren MJ, Grobin AC, Massing MW, Madison RD, Bradford DW, Butterfield MI, Lieberman JA, Morrow AL. Clozapine markedly elevates pregnenolone in rat hippocampus, cerebral cortex, and serum: candidate mechanism for superior efficacy? Pharmacol Biochem Behav. 2006a;84:598–608. doi: 10.1016/j.pbb.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Marx CE, Trost WT, Shampine L, Behm FM, Giordano LA, Massing MW, Rose JE. Neuroactive steroids, negative affect, and nicotine dependence severity in male smokers. Psychopharmacology (Berl) 2006b;186:462–72. doi: 10.1007/s00213-005-0226-x. [DOI] [PubMed] [Google Scholar]
- Marx CE, Trost WT, Shampine LJ, Stevens RD, Hulette CM, Steffens DC, Ervin JF, Butterfield MI, Blazer DG, Massing MW, Lieberman JA. The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer’s disease. Biol Psychiatry. 2006c;60:1287–94. doi: 10.1016/j.biopsych.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Melchior CL, Ritzmann RF. Dehydroepiandrosterone is an anxiolytic in mice on the plus maze. Pharmacol Biochem Behav. 1994;47:437–41. doi: 10.1016/0091-3057(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology (Berl) 2013;227:177–92. doi: 10.1007/s00213-012-2954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsink LF, Vogelzangs N, Nicklas BJ, Beekman AT, Satterfield S, Rubin SM, Yaffe K, Simonsick E, Newman AB, Kritchevsky SB, Penninx BW. Associations between sex steroid hormone levels and depressive symptoms in elderly men and women: results from the Health ABC study. Psychoneuroendocrinology. 2007;32:874–83. doi: 10.1016/j.psyneuen.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Naert G, Maurice T, Tapia-Arancibia L, Givalois L. Neuroactive steroids modulate HPA axis activity and cerebral brain-derived neurotrophic factor (BDNF) protein levels in adult male rats. Psychoneuroendocrinology. 2007;32:1062–78. doi: 10.1016/j.psyneuen.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Naylor J, Li J, Milligan CJ, Zeng F, Sukumar P, Hou B, Sedo A, Yuldasheva N, Majeed Y, Beri D, Jiang S, Seymour VA, McKeown L, Kumar B, Harteneck C, O’Regan D, Wheatcroft SB, Kearney MT, Jones C, Porter KE, Beech DJ. Pregnenolone sulphate- and cholesterol-regulated TRPM3 channels coupled to vascular smooth muscle secretion and contraction. Circ Res. 2010;106:1507–15. doi: 10.1161/CIRCRESAHA.110.219329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor JC, Hulette CM, Steffens DC, Shampine LJ, Ervin JF, Payne VM, Massing MW, Kilts JD, Strauss JL, Calhoun PS, Calnaido RP, Blazer DG, Lieberman JA, Madison RD, Marx CE. Cerebrospinal fluid dehydroepiandrosterone levels are correlated with brain dehydroepiandrosterone levels, elevated in Alzheimer’s disease, and related to neuropathological disease stage. J Clin Endocrinol Metab. 2008;93:3173–8. doi: 10.1210/jc.2007-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Birn RM, Patriat R, Fox AS, Shelton SE, Burghy CA, Stodola DE, Essex MJ, Davidson RJ, Kalin NH. Evidence for coordinated functional activity within the extended amygdala of non-human and human primates. Neuroimage. 2012;61:1059–66. doi: 10.1016/j.neuroimage.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, de Vries GJ, Guzelcan Y, Assies J, Gersons BP. Changes in cortisol and DHEA plasma levels after psychotherapy for PTSD. Psychoneuroendocrinology. 2007;32:619–26. doi: 10.1016/j.psyneuen.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–32. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- Ossewaarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson IM, Backstrom T, Fernandez G. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. 2010a;35:47–55. doi: 10.1016/j.psyneuen.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Ossewaarde L, van Wingen GA, Kooijman SC, Backstrom T, Fernandez G, Hermans EJ. Changes in functioning of mesolimbic incentive processing circuits during the premenstrual phase. Soc Cogn Affect Neurosci. 2010b;6:612–20. doi: 10.1093/scan/nsq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek JN, Veer IM, van Tol MJ, van der Werff SJ, Demenescu LR, Aleman A, Veltman DJ, Zitman FG, Rombouts SA, van der Wee NJ. Aberrant limbic and salience network resting-state functional connectivity in panic disorder without comorbidity. J Affect Disord. 2013a;145:29–35. doi: 10.1016/j.jad.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Pannekoek JN, Veer IM, van Tol MJ, van der Werff SJ, Demenescu LR, Aleman A, Veltman DJ, Zitman FG, Rombouts SA, van der Wee NJ. Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. Eur Neuropsychopharmacol. 2013b;23:186–95. doi: 10.1016/j.euroneuro.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Pantazatos SP, Talati A, Pavlidis P, Hirsch J. Cortical functional connectivity decodes subconscious, task-irrelevant threat-related emotion processing. Neuroimage. 2012;61:1355–63. doi: 10.1016/j.neuroimage.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci U S A. 2006;103:1563–8. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–71. doi: 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36:2130–42. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2008;105:5567–72. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–33. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, Phan KL. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60:704–13. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Romeo E, Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–3. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–26. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–56. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, St Clair LS, Murphy JH, Haq N, Rubinow DR. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. 2005;62:154–62. doi: 10.1001/archpsyc.62.2.154. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Liere P, Akwa Y, Rajkowski K, Griffiths W, Bodin K, Sjovall J, Baulieu EE. Pregnenolone sulfate in the brain: a controversial neurosteroid. Neurochem Int. 2008;52:522–40. doi: 10.1016/j.neuint.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Shirayama Y, Muneoka K, Fukumoto M, Tadokoro S, Fukami G, Hashimoto K, Iyo M. Infusions of allopregnanolone into the hippocampus and amygdala, but not into the nucleus accumbens and medial prefrontal cortex, produce antidepressant effects on the learned helplessness rats. Hippocampus. 2011;21:1105–13. doi: 10.1002/hipo.20824. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biol Psychiatry. 2008;64:681–90. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VB, Kalimi M, Phan TH, Boadle-Biber MC. Intracranial dehydroepiandrosterone blocks the activation of tryptophan hydroxylase in response to acute sound stress. Mol Cell Neurosci. 1994;5:176–81. doi: 10.1006/mcne.1994.1019. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012a;37:241–9. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. 2012b;74:904–11. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rajaram N, Garfinkel SN, Abelson JL, Liberzon I. DHEA Enhances Emotion Regulation Neurocircuits and Modulates Memory for Emotional Stimuli. Neuropsychopharmacology. 2013a doi: 10.1038/npp.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I. Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits. Biol Psychiatry. 2013b;73:1045–53. doi: 10.1016/j.biopsych.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk FZ. Measurement of androgens in women. Semin Reprod Med. 2006;24:78–85. doi: 10.1055/s-2006-939566. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Bitter SM, Weber WA, Chu WJ, Whitsel RM, Adler C, Cerullo MA, Eliassen J, Strakowski SM, Delbello MP. Neurocircuitry of Generalized Anxiety Disorder in Adolescents: A Pilot Functional Neuroimaging and Functional Connectivity Study. Depress Anxiety. 2012;29:939–47. doi: 10.1002/da.21961. [DOI] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18:2553–9. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urani A, Roman FJ, Phan VL, Su TP, Maurice T. The antidepressant-like effect induced by sigma(1)-receptor agonists and neuroactive steroids in mice submitted to the forced swimming test. J Pharmacol Exp Ther. 2001;298:1269–79. [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95:3239–44. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Goozen SH, van den Ban E, Matthys W, Cohen-Kettenis PT, Thijssen JH, van Engeland H. Increased adrenal androgen functioning in children with oppositional defiant disorder: a comparison with psychiatric and normal controls. J Am Acad Child Adolesc Psychiatry. 2000;39:1446–51. doi: 10.1097/00004583-200011000-00020. [DOI] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernandez G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53:348–54. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- van Wingen G, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar J, Fernandez G. How progesterone impairs memory for biologically salient stimuli in healthy young women. J Neurosci. 2007;27:11416–23. doi: 10.1523/JNEUROSCI.1715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar JK, Fernandez G. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. 2008;13:325–33. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NY, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SA. Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. Neuroimage. 2011;57:1534–41. doi: 10.1016/j.neuroimage.2011.05.074. [DOI] [PubMed] [Google Scholar]
- Wang C, Marx CE, Morrow AL, Wilson WA, Moore SD. Neurosteroid modulation of GABAergic neurotransmission in the central amygdala: a role for NMDA receptors. Neurosci Lett. 2007;415:118–23. doi: 10.1016/j.neulet.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA. Manual for the Positive and Negative Affect Schedule - Expanded Form. University of Iowa; 1994. [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Wong SY, Leung JC, Kwok T, Ohlsson C, Vandenput L, Leung PC, Woo J. Low DHEAS levels are associated with depressive symptoms in elderly Chinese men: results from a large study. Asian J Androl. 2011;13:898–902. doi: 10.1038/aja.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2006;114:187–93. doi: 10.1111/j.1600-0447.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- Yin Y, Jin C, Hu X, Duan L, Li Z, Song M, Chen H, Feng B, Jiang T, Jin H, Wong C, Gong Q, Li L. Altered resting-state functional connectivity of thalamus in earthquake-induced posttraumatic stress disorder: a functional magnetic resonance imaging study. Brain Res. 2011;1411:98–107. doi: 10.1016/j.brainres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li CS. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012;59:3548–62. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]