Abstract
Acute allograft rejection is mediated by host CD8+ cytotoxic T lymphocytes (CTL) targeting graft class I major histocompatibility complex (MHC) molecules. In experimental rodent models, rejection requires differentiation of naive CD8+ T cells into alloreactive CTL within secondary lymphoid organs, whereas in humans, CTL may alternatively develop within the graft from circulating CD8+ effector memory T cells (TEM) that recognize class I MHC molecules on graft endothelial cells (EC). This latter pathway is poorly understood. Here, we show that host CD4+ TEM, activated by EC class II MHC molecules, provide critical help for this process. First, blocking HLA-DR on EC lining human artery grafts in immunodeficient mice reduces CD8+ CTL development within and acute rejection of the artery by adoptively transferred allogeneic human lymphocytes. Second, siRNA knockdown or CRISPR/Cas9 ablation of class II MHC molecules on EC prevents CD4+ TEM from helping CD8+ TEM to develop into CTL in vitro. Finally, implanted synthetic microvessels, formed from CRISPR/Cas9-modified EC lacking class II MHC molecules, are significantly protected from CD8+ T cell–mediated destruction in vivo. We conclude that human CD8+ TEM–mediated rejection targeting graft EC class I MHC molecules requires help from CD4+ TEM cells activated by recognition of class II MHC molecules.
Introduction
Solid organ transplantation is the most effective therapy for end-stage organ failure of the heart, liver, kidneys, and lungs (1, 2), but despite advances in clinical management, acute allograft rejection remains a major cause of early graft loss (3). This process is principally mediated by host T cells that directly recognize nonself allelic forms of class I and class II major histocompatibility complex (MHC) molecules expressed by graft cells (4, 5). A high frequency (>1%) of host T cells, selected to recognize microbial peptides bound to self-allelic forms of MHC molecules, cross-react against nonself (graft) MHC molecules associated with many different peptides, although different T cell clones respond to different allogeneic donors (6). As a result of prior infections, a large percentage (>50%) of T cells circulating in adult humans are memory T cells, and because the allogeneic response is a cross-reaction of T cells that recognize microbial peptides, a comparably high percentage of the circulating memory T cell population is alloreactive (6). Furthermore, the pretransplant frequency of alloantigen-reactive memory, but not naive T lymphocytes, correlates with both the severity and frequency of acute rejection episodes (7), suggesting that acute graft rejection in adult humans may in fact be a memory response.
The actual process of acute graft rejection in humans correlates with and is probably mediated by infiltrating host CD8+ cytotoxic T lymphocytes (CTL) that recognize nonself class I MHC molecules and express transcripts encoding perforin, granzyme B, and IFN-γ (5). In typical rodent transplant models, alloreactive CTL arise solely from naive CD8+ T cells that differentiate within the secondary lymphoid organs where they encounter donor-derived professional antigen presenting cells (APCs), more specifically DC that have migrated from the graft (passenger leukocytes) (6). Host CD4+ T cells, also activated by the same graft DC within the secondary lymphoid organs, may provide help for the activation of naive CD8+ T cells. A need for CD4+ T cell help is established in certain rodent models (8), but there are exceptions that lead to different conclusions as to the nature of help in different CD8+ T cell–mediated immunopathologies. For example, CTL-mediated graft rejection of pancreatic islet allografts from BALB/c recipients require CD4+ T cells for rejection, whereas C57BL/6 recipients may still reject after CD4+ T cells have been depleted (9). Furthermore, sterile allografts such as heart, kidneys, or liver may also differ from organs that are colonized with commensal microorganisms, such as the skin, bowel or lungs, as microbes present in the latter group of organs may license graft DC to better activate host CD8+ T cells, reducing the need for CD4+ T cell help (8).
In contrast to most laboratory rodents, adult humans have a subset of alloreactive circulating CD8+ memory T cells, called CD8+ effector memory T cells (TEM), that can home directly into allografts, bypassing secondary lymphoid organs, and that can mature into CTL within the graft. The conditions required for human CD8+ TEM conversion to CTL is not completely understood, especially in vivo, and may significantly differ from the processes that have been studied for differentiation of naive T cells. It is known that memory T cells in general have activation requirements that differ from naive T cells such that they do not require professional APCs, and they can often be refractory to conventional immune suppressants that potently affect naive T cells (10–12). These important differences are thought to contribute to the failure of rodent models to predict the efficacy of therapeutic interventions in human transplantation.
If development into CTL occurs within the graft, the question arises as to which types of graft cells stimulate this process. In human grafts, endothelial cells (EC) that line the vasculature of the organ are primary cellular targets of acute rejection responses (10, 13, 14). Furthermore, both in vitro and in vivo human EC effectively activate allogeneic TEM to proliferate and produce cytokines (15). This capacity of human EC is thought to explain why depletion of professional APC from a human graft prior to transplantation and treatment with fingolimod, a drug that traps differentiating effector T cells in secondary lymphoid organs, fail to fully prevent human transplant rejection (16, 17). The response of circulating human TEM to human graft EC is an alternative initiator of acute rejection in the clinical setting that does not occur in commonly studied rodent models. However, while recognition of alloantigen and costimulator molecules on allogeneic human EC can activate isolated CD8+ TEM to secrete effector cytokines and undergo a limited degree of replication in vitro, such cultures are insufficient to induce CTL development without provision of exogenous IL-2 (10, 18–20). Activated CD4+ T cells are a possible endogenous source of IL-2 or other signals that can complement those provided by allogeneic EC.
The roles of TEM and of graft EC are difficult to study in humans in vivo but may be successfully modeled in humanized mice. Rejection of human skin grafts by adoptively transferred human T cells in such mice can be mediated by TEM lymphocytes but not central memory or naive T cell subsets (15). Adoptive transfer of purified CD8+ T cells into mouse hosts has been unsuccessful, so it is unclear what roles are performed by CD4+ TEM in this humanized mouse model (21). Human but not rodent EC have a capacity to activate effector CD4+ T cell responses (22), and these cells, in addition to promoting engraftment, could provide help for the conversion of CD8+ TEM into CTL. Human artery segments interposed into the infra-renal aortae of immunodeficient mice also can be rejected by adoptively transferred human T cells from a donor allogeneic to the source of the artery segment (23–27), providing a simple model system for studying human T cell–EC interactions in vivo. Unlike skin grafts, these transplanted arteries do not contain any detectable leukocytes by 3–7 days after transplantation, and luminal EC are the only cells that display significant levels of both class I and II MHC molecules. Thus, initiation of rejection in this model can be attributed to recognition of allogeneic EC, supporting the conclusion that graft EC can initiate cell-mediated vascular rejection (intimal arteritis) in the absence of graft DC (28).
Based on these observations, we make two important predictions of clinical relevance. First, if CD4+ TEM are required to provide help that drives expansion of alloreactive CD8+ TEM to effector CTL, then blocking activation of CD4+ TEM should limit this process and reduce the severity of rejection. Second, if graft EC are a primary activator of host CD4+ TEM, then reducing recognition of class II MHC molecules expressed by EC should result in reduced rejection by CD8+ CTL, even though CTL identify their targets by recognition of class I MHC. In this study, we use humanized mouse models as well as cell culture systems to test both of these propositions.
Results
MHC class II blockade reduces allogeneic T cell–mediated injury and CD8+ CTL–associated transcripts in human arterial grafts in vivo
We first investigated the contribution of graft EC expression of class II MHC molecules in our model of human intimal arteritis/endothelialitis. A small segment of human epicardial coronary artery is grafted end-to-end into the infrarenal aorta of an immunodeficient C.B-17 SCID/bg mouse (23). Subsequent adoptive transfer of human peripheral blood mononuclear cells (PBMC) allogeneic to the artery donor leads to engraftment of circulating CD4+ and CD8+ T lymphocytes, but not neutrophils, monocytes, DC, or natural killer cells (23, 29). Over the next 3–4 weeks, circulating human T cells infiltrate the intima of the human artery graft and destroy the luminal EC but generally spare the vessel media. We implanted adjacent human coronary artery grafts from the same donor into pairs of mice, following which recipient mice were adoptively transferred with human PBMC allogeneic to the artery donor and given either F(ab)′2 fragments of mouse anti–HLA-DRα or of mouse IgG control for 21 days. We selectively targeted HLA-DR because, even though human EC also express HLA-DP and HLA-DQ molecules, HLA-DR is the dominant stimulator of allogeneic T cell responses (30). Furthermore, we used F(ab)′2 fragments because intact anti–HLA-DRα antibody reduced the numbers of human T cells present in the circulation following adoptive transfer, whereas F(ab)′2 fragments blocked CD4+ T cell activation by EC in vitro but did not reduce circulating human T cell numbers in vivo (Supplemental Figure 1, A and B; supplemental material available online with this article; doi:10.1172/jci.insight.85293DS1).
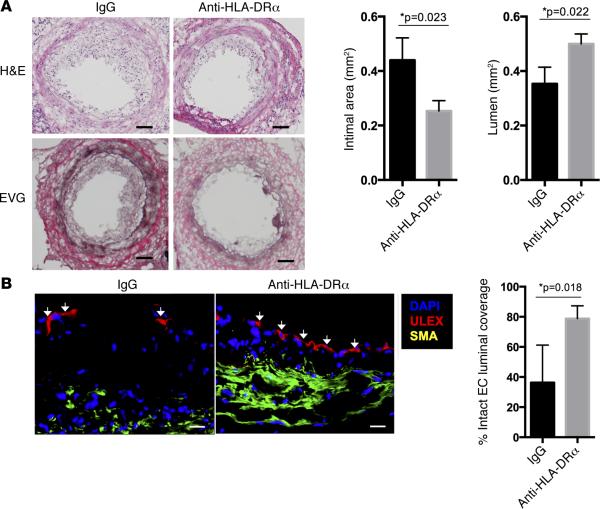
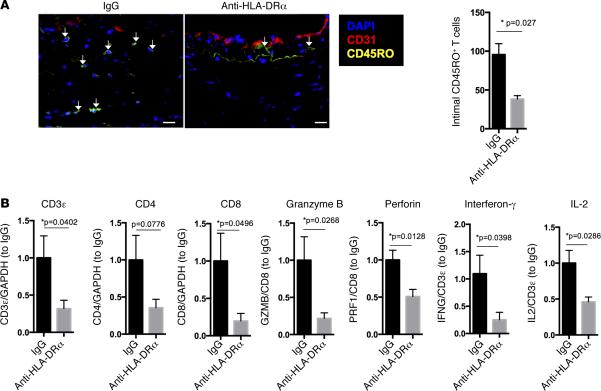
Blocking HLA-DR recognition significantly reduced the intimal area and luminal narrowing and preserved the human EC lining of the graft compared with control F(ab)′2 treatment (Figure 1, A and B). There was also a significant reduction in the total T cell infiltration and cytokine transcripts within the intima of anti–HLA-DRα F(ab)′2–treated animals (Figure 2, A and B). Despite the fact that this anti–HLA-DR antibody does not prevent CD8+ T cells from interacting with class I MHC molecules (31), there was a decrease in perforin and granzyme B transcripts when normalized to a CD8-specific marker (Figure 2B), consistent with inhibition of CTL development. Collectively, these data support the conclusion that recognition of class II MHC molecules on the EC in this model promotes acute CTL-mediated rejection, consistent with our hypothesis that activation of CD4+ TEM in response to class II MHC molecules expressed by EC is needed to provide help for development of CD8+ TEM into effector CTL. However, protection was incomplete, perhaps because we only blocked HLA-DR and not HLA-DP and -DQ, which are also expressed by human EC, albeit at lower levels. Furthermore, despite preserving circulating T cell numbers, we also cannot rule out a more subtle effect of the antibody on T cell function. These limitations of antibody-mediated effects led us to explore genetic approaches to human immunology both in vitro and in vivo.
Figure 1. HLA-DR blockade reduces acute T cell–mediated injury to implanted allogeneic vessel segments in vivo.
(A and B) In an MHC-mismatched model of arterial rejection by allogeneic T cells (n = 6 per group), blockade of class II MHC by anti–HLA-DRα F(ab)′2 fragment reduces intimal area expansion and increases lumen area as measured by H&E and EVG staining (scale bar: 50 μm) (A) and reduces disruption of EC lining the vessel lumen, a hallmark of intimal arteritis/endothelialitis, as measured by percent of circumferential coverage (scale bar: 20 μm) (B), both at 21 days. Arrows indicate areas of intact endothelial lining. *P < 0.05, 2-tailed Student's t test. EVG, Elastia-van Gieson; MHC, major histocompatibility complex; EC, endothelial cells.
Figure 2. HLA-DR blockade reduces CD8+ T cell infiltration into and CTL development within implanted allogeneic vessel segments in vivo.
(A and B) Anti–HLA-DRα F(ab)′2 fragment significantly (n = 6 per group) reduces total intimal T cell infiltration as detected by immunofluorescence (scale bar: 20 μm) (A) and CD8+ T cell–associated transcripts of cytokines and CTL effector molecules in rejected artery grafts at 21 days (B) as detected by qPCR normalized to either total mRNA or T cell–specific mRNA as indicated on the y axis. Arrows indicate infiltrating intimal CD45RO+ T cells. *P < 0.05, 2-tailed Student's t test. CTL, cytotoxic T cell.
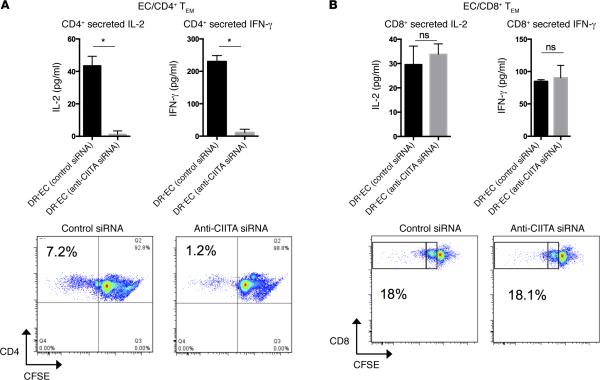
Silencing class II MHC expression in EC directly inhibits CD4+ but not CD8+ TEM responses in vitro
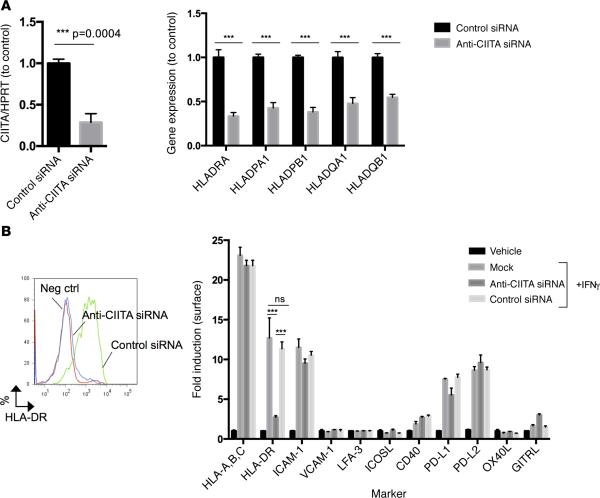
We modeled human CD4+ and CD8+ TEM interactions with EC using mixed EC-lymphocyte reactions in vitro and initially reduced class II MHC molecule expression with an siRNA specific for the class II MHC trans-activator (CIITA). Knockdown of CIITA inhibits the IFN-γ–dependent induction of CIITA and blocks transcription at all three class II MHC loci (HLA-DR, -DP, and -DQ) as detected by quantitative PCR (qPCR) (Figure 3A and refs. 32–35). Consequently, HLA-DR surface protein is effectively reduced (Figure 3B). Treatment with anti-CIITA siRNA did not affect expression of class I MHC or other immunologically relevant adhesion or costimulatory molecules (Figure 3B). CD4+ TEM (CD45RO+CCR7−CD62L−CD4+) isolated from human PBMC (Supplemental Figure 2) and cocultured with allogeneic EC, sequentially pre-treated with anti-CIITA siRNA and IFN-γ, secrete significantly less IL-2 and IFN-γ and proliferate less when compared with CD4+ TEM cocultured with sequential control siRNA and IFN-γ–treated EC (Figure 4A). The same comparison showed no differences in IL-2 or IFN-γ secretion or in proliferation (Figure 4B) by CD8+ TEM (CD45RO+CCR7−CD62L−CD8+). These data suggest that changes we had observed within the vessel wall in vivo by anti–HLA-DR antibody are likely due to inhibition of CD4+ T cell recognition of class II MHC on graft EC.
Figure 3. anti-CIITA siRNA selectively inhibits induction of CIITA and class II MHC molecules in human EC.
Cultured human EC were transfected with anti-CIITA siRNA or control siRNA on day 0, treated with IFN-γ at 24 hours, and analyzed on day 3. (A and B) Specific transcripts were measured by qPCR (A), and protein was assessed by FACS (B). Similar results were seen in four independent experiments. ***P < 0.0005, 2-tailed Student's t test. CIITA, class II MHC transactivator; MHC, major histocompatibility complex; EC, endothelial cells.
Figure 4. siRNA-mediated knockdown of CIITA in EC directly inhibits CD4+ but not CD8+ TEM alloresponses.
(A and B) Anti-CIITA siRNA inhibits activation of CD4+ TEM (10:1 T cell/EC ratio) (A) but not activation of CD8+ TEM (30:1 T cell/EC ratio) (B), as measured by ELISA at 24 hours for IL-2 and IFN-γ and proliferation by CFSE dilution and flow cytometric analysis at 7 days. Note that the majority of CD8+ TEM have divided only once, whereas CD4+ TEM undergo multiple rounds of proliferation. Similar results were seen in three independent experiments. *P < 0.05, 2-tailed Student's t test. CIITA, class II MHC trans-activator; TEM, effector memory T cell; EC, endothelial cells.
EC-activated CD4+ TEM enhance CD8+ TEM responses to allogeneic EC
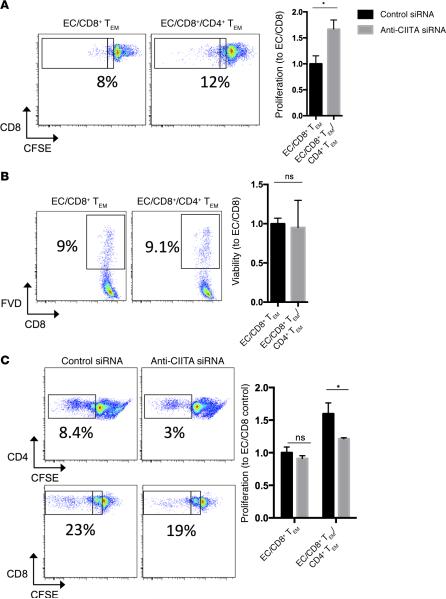
To determine if CD4+ TEM activated by EC can provide help for CD8+ TEM, we examined the effect of adding CD4+ TEM to EC–CD8+ TEM mixed cocultures. The addition of CD4+ TEM enhanced CD8+ TEM proliferation (Figure 5A). In the absence of CD4+ TEM, proliferation typically stopped after a single division, whereas the presence of CD4+ TEM promoted additional rounds of division indicated by further dilution of CFSE dye. These differences were not due to changes in TCR engagement of MHC class I alloantigens, as addition of CD4+ TEM did not significantly change the number of CD8+ TEM cells that initially upregulated CD25, the inducible component of the high-affinity IL-2 receptor and an early activation marker that reflects TCR engagement (Supplemental Figure 3). The enhanced proliferation was also not due to increased survival of CD8+ TEM cells, as there was no significant difference in viability at 7 days with or without CD4+ TEM present (Figure 5B). To determine if CD4+ TEM enhancement of EC-reactive CD8+ TEM required concomitant activation of CD4+ TEM by the allogeneic EC, we cocultured CD4+ and CD8+ TEM with allogeneic EC that were pretreated with anti-CIITA siRNA to uncouple MHC class II–dependent activation of CD4+ TEM from MHC class I–dependent activation of CD8+ TEM. We observed that the increase in proliferation by CD8+ TEM due to the addition of CD4+ TEM to the cultures was attenuated by pretreatment of the EC with anti-CIITA siRNA (Figure 5C). This result implies that CD4+ TEM must first be activated by allogeneic EC before they can help CD8+ TEM.
Figure 5. Activation of CD4+ TEM by allogeneic EC is necessary to enhance CD8+ TEM responses to the same EC.
(A and B) Addition of CD4+ TEM to EC/CD8+ TEM cocultures enhances CD8+ expansion as measured by CFSE dilution and flow cytometry at 7 days (A), but CD4+ TEM do not enhance survival of CD8+ TEM (B) as measured by viability. (C) Knockdown of CIITA expression by siRNA, to prevent CD4+ TEM activation, inhibits CD4+ TEM enhancement of CD8+ TEM expansion at 7 days as measured by CFSE dilution and flow cytometric analysis at 7 days. Note that the inclusion of activated CD4+ TEM increases the rounds of replication of CD8+ TEM (inset boxes). Similar results were seen in three independent experiments. *P < 0.05, 2-tailed Student's t test. TEM, effector memory T cell; CIITA, class II MHC transactivator; FVD, fixable viability dye; EC,endothelial cells.
We then further analyzed the phenotype of EC-reactive CD8+ TEM using their dilution of CFSE to separate the reactive (CFSElo) and nonreactive (CFSEhi) populations. Perforin expression appeared restricted to the CFSEloCD8+ TEM population, and the number of perforin+CFSEloCD8+ T cells increased with the addition of CD4+ TEM (Supplemental Figure 4). Inhibiting MHC class II expression on allogeneic EC attenuated the enhancement of perforin expression in the reactive population. In contrast, few cells in the nonreactive CD8+ TEM population expressed perforin, and this was unchanged by the addition of CD4+ TEM with or without pretreatment of the EC with anti-CIITA siRNA (Supplemental Figure 4). These results suggest that EC-reactive CD4+ TEM promote perforin expression, a marker of CTL maturation, within the CD8+ TEM population.
CD4+ TEM provide help to CD8+ TEM via paracrine provision of IL-2
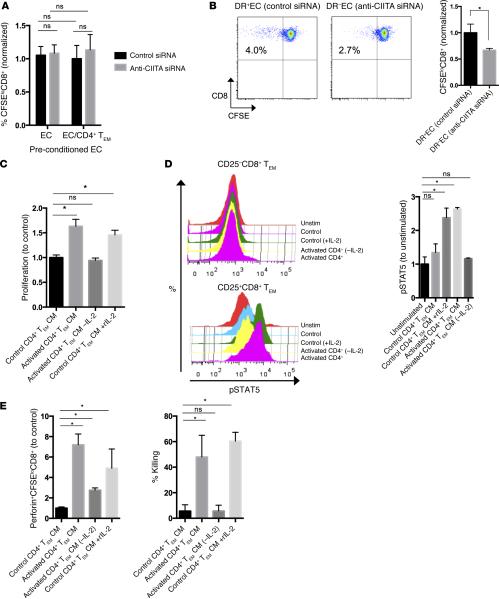
We next investigated possible mechanisms by which allogeneic EC-activated CD4+ TEM help CD8+ TEM activation and CTL development, including: (i) modifying (licensing) EC to become more immunogenic to CD8+ TEM (e.g., upregulation of EC MHC class I, costimulatory molecules, and/or transpresentation of cytokines such as IL-15); (ii) enhancing CD8+ TEM responses in a contact-dependent manner (e.g., upregulation of CD4+ T cell costimulatory molecules like CD40L facilitating T-T interactions); or (iii) enhancing CD8+ TEM in a contact-independent manner (e.g., CD4+ T cell paracrine release of IL-2 or other signals). To determine if CD4+ TEM license EC (36), EC cultures were pretreated with either anti-CIITA or control siRNA, activated with IFN-γ, cocultured CD4+ TEM, and FACS isolated for subsequent culture with CD8+ TEM. We observed no significant difference in CD8+ TEM alloresponses between control EC or EC preconditioned with CD4+ TEM (Figure 6A), ruling out any functional changes in EC immunogenicity toward CD8+ TEM. To examine if CD4+ TEM–driven enhancement of EC-reactive CD8+ TEM responses is contact dependent, we separated CD4+ and CD8+ TEM cocultures with EC by a semipermeable transwell membrane. CD4+ TEM cocultured with control siRNA/IFN-γ–treated EC were able to enhance the CD8+ TEM response compared with CD4+ TEM cocultured with EC pretreated anti-CIITA siRNA/IFN-γ, even across a semipermeable membrane, providing evidence for the existence of a soluble “helper” factor (Figure 6B).
Figure 6. CD4+ TEM enhancement of CD8+ TEM responses are mediated by secreted IL-2.
(A) EC precultured with CD4+ TEM and subsequently isolated by FACS have not increased their capacity to activate CD8+ TEM. (B) When separated by a semipermeable transwell membrane, CD4+ TEM enhance CD8+ TEM alloresponses. (C) CM generated by EC-activated CD4+ TEM is sufficient to enhance proliferation of CD8+ TEM. Immunoabsorption of IL-2 removes that capacity, whereas addition of IL-2 to control CM mimics activated CM. (D) Similarly, CM induces phosphorylates STAT5 in CD25+ but not CD25−CD8+ TEM in an IL-2–dependent manner. (E) In addition, CM enhances expression of perforin and promotes CTL killing in an IL-2–dependent manner. Similar results were seen in three independent experiments. *P < 0.05, by 2-tailed Student's t test (B) or by 1-way ANOVA with Bonferroni post-hoc test (A and C–E). TEM, effector memory T cell; CIITA, class II MHC transactivator; CM, conditioned medium; EC,endothelial cells; CTL, cytotoxic T cell.
To identify the potential factor(s) responsible for help in this assay, we generated conditioned medium by coculture of CD4+ TEM with EC transduced to express an Fc receptor (FcR; CD32) and overlayed with anti-CD3 mAb, a procedure in which T cells are polyclonally activated in the context of costimulators expressed by human ECs, enhancing the generation of soluble mediators. After 24 hours of primary coculture, conditioned medium from EC-activated CD4+ TEM or control medium was collected and used in EC-CD8+ TEM cocultures. Consistent with the results generated by transwell experiments, activated CD4+ TEM–conditioned medium enhanced proliferation of EC-reactive CD8+ TEM (Figure 6C). Since IL-2 is produced by activated CD4+ TEM and has previously been shown to enhance CD8+ responses to allogeneic EC (18), we tested whether IL-2 contributed to the effects observed with conditioned medium. Removal of IL-2 by immunoabsorption neutralized the effect of the conditioned medium, and addition of IL-2 to the control medium replicated the effect of activated conditioned medium. STAT5 is a transcription factor that is known to control cell cycle progression in effector T cells and is phosphorylated with IL-2–, IL-7–, or IL-15–initiated common γ chain cytokine signaling (37). We observed phosphorylation of STAT5 in CD25+ but not CD25−CD8+ TEM with activated conditioned medium, and this response was abrogated with IL-2 immunoabsorption (Figure 6D). We then asked if EC-activated CD4+ TEM conditioned medium was sufficient to promote CTL development from circulating CD8+ TEM and observed an increase in perforin expression when CD8+ TEM were cocultured with EC in the presence of CD4+ TEM conditioned medium, again in only the EC-reactive (CFSElo) population (Figure 6E). This response was also largely abrogated with IL-2 immunoabsorption. Similarly, when adapted to a CTL killing assay of allogeneic EC, CD8+ TEM expanded with the same allogeneic EC either in the presence of activated CD4+ TEM conditioned medium or the exogenous IL-2, which both demonstrated significant cytotoxicity (Figure 6E).
Genetic ablation of CIITA in synthetic blood vessels limits T cell–mediated destruction in vivo
We extended our genetic approach to confirm the effects of EC-mediated activation of CD4+ T cells on cell-mediated rejection in vivo. siRNA effects are generally too short-lived and shRNA effects are too incomplete for effective use over the timeframe required to study graft rejection in vivo. Recently, we developed a method to genetically modify untransformed human EC derived from endothelial colony-forming cells (HECFC, also known as human cord blood EC) using CRISPR/Cas9 to specifically ablate CIITA and demonstrated that inactivating mutations in CIITA prevented IFN-γ–induced class II MHC molecule expression, but not class I MHC molecule expression, and reduced allogeneic CD4+ memory T cell responses (38). Importantly, CIITAnull EC otherwise retained phenotypic functions of unmodified cells, including the ability to form synthetic microvascular beds when implanted into C.B-17 SCID/bg mice. We confirmed that CD4+ TEM enhance CD8+ TEM responsiveness to HECFC-derived EC in vitro and that this effect is attenuated by CIITA ablation due to effects on class II MHC molecule expression (Supplemental Figure 5, A and B), recapitulating the results we had observed with siRNA treatment of human umbilical vein EC (HUVEC).
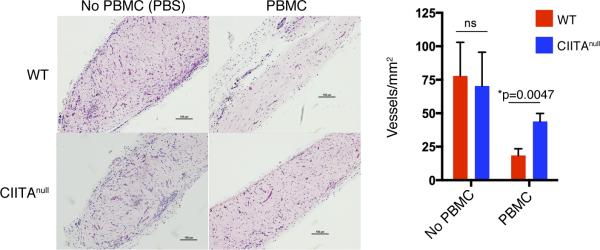
When synthetic vessels are formed from human EC following implantation into C.B-17 SCID/bg mouse hosts and then challenged with adoptive transfer of human PBMC allogeneic to the EC, the human EC-lined microvessels are largely destroyed over a period of 10 days (39, 40). Experiments using alloreactive T cell lines generated against cultured human EC suggested that this effect is likely mediated by direct recognition of alloantigen on the EC (39). To determine if this model could be used to quantify CTL-mediated killing using PBMC, we conducted experiments using selective adoptive transfers. In prior studies, we had found that adoptive transfer of CD8+ T cells is inefficient in the absence of CD4+ T cells but that CD4+ T cells readily engraft in the absence of CD8+ T cells (21). Inoculation of mice with unfractionated PBMC led to the appearance of both human CD4+ and CD8+ T cells in the circulation, whereas PBMC depleted of CD8+ cells prior to adoptive transfer produced a circulating human T cell population consisting wholly of CD4+ T cells (Supplemental Figure 6A). The absence of CD8+ T cells sharply reduced the destruction of synthetic vessels at 10 days following adoptive transfer, despite similar numbers of circulating human T cells, implying that most of the graft destruction is CD8+ T cell–mediated (Supplemental Figure 6B). We then engineered synthetic human EC-lined vessels using either unmodified (WT) or CIITAnull EC and then adoptively transferred human PBMC allogeneic to the EC to determine if CD4+ T cell activation played any role in this CD8+ T cell response. The destruction of synthetic microvessels formed from CIITAnull EC was markedly attenuated compared with WT EC (Figure 7). There was still some destruction of microvessels formed from CIITAnull EC in the absence of CD8+ T cells (82% ± 20% of control, n = 3 mice), suggesting that some rejection may not involve direct recognition of EC MHC molecules; however, the difference between vessel loss following adoptive transfer of whole or CD8+-depleted PBMC was ablated (WT: 75% ± 12%, CIITAnull: 82% ± 20%, n = 3 mice each), suggesting that in this in vivo model, recognition of allogeneic class II MHC molecules on EC by human CD4+ T cells is once again required for provision of CD4+ T cell help for human CD8+ T cell activation and efficient expansion into CTL. As these animals do not have functional secondary lymphoid organs, this process, as in the human artery graft model, likely takes place within the graft itself.
Figure 7. Genetic ablation of CIITA in EC by CRISPR/Cas9 reduces T cell–mediated destruction of synthetic vessels in vivo.
Synthetic human microvessels formed from WT or CIITAnull EC were implanted s.c. in the abdominal wall of C.B-17 SCID/bg mice. After 2 weeks, mice were adoptively transferred with peripheral blood mononuclear cells (PBMC). In the absence of circulating T cells, WT and CIITAnull EC form equivalent numbers of vessels (n = 3 each). In the presence of circulating T cells, however, there is a significantly higher loss of perfused vessels formed from WT compared with CIITAnull EC (n = 3 each). Scale bar: 100 μm. *P < 0.05, 2-tailed Student's t test. CIITA, class II MHC transactivator; bg, beige; EC,endothelial cells.
Discussion
The importance of CD4+ T cell help for generation of CTL in some but not other rodent models of transplantation is well established and primarily involves conversion of alloreactive naive CD8+ T cells into CTL as a result of recognition of graft DC that have migrated from the graft into the host's secondary lymphoid organs (6). Rejection can usually be prevented by depletion of DC from the graft prior to transplantation (41) or by use of recipients that lack secondary lymphoid organs (42). In human transplantation, depletion of DC from grafts or treatment of graft recipients with fingolimod can reduce but not prevent rejection (16, 17). This difference between humans and mice likely arises because adult humans have circulating alloreactive CD8+ TEM that can develop into effector CTL within the allograft itself, likely in response to direct recognition of class I MHC molecules expressed by graft EC and because human EC can effectively recruit and activate TEM. This difference does not mean that activation and differentiation of naive CD8+ TEM cells into CTL is unimportant in human transplantation but rather that prevention of this process will be insufficient on its own to prevent graft loss. A successful strategy in humans will additionally need to prevent the development of circulating CD8+ TEM into effector CTL that can occur in the graft. The questions addressed by our study are whether human CD4+ TEM are necessary to provide help to CD8+ TEM to promote CTL-mediated graft rejection in vivo and, if so, whether graft EC can sufficiently activate CD4+ TEM in vivo to provide such help.
Previous studies have shown human EC are competent stimulators of memory CD8+ T cells but only promote expansion of alloreactive CTLs when cocultures of purified memory CD8+ T cells with allogeneic EC are supplemented with exogenous IL-2, suggesting that EC alone do not have the capacity to efficiently promote pre-CTL differentiation (18). In our study, we show that CD4+ TEM can replace the need for exogenous IL-2 but only when they are activated by recognition of class II MHC molecules on the EC. The necessary signal can be found in conditioned medium and appears to be IL-2. One of the measures that we used to follow CTL development was expression of perforin. Interestingly, the majority of freshly isolated CD8+ TEM express perforin but lose expression in culture unless appropriately activated. Furthermore, very few of these perforin-expressing cells show killing of allogeneic cells from a particular donor prior to culture, and only proliferating CD8+ T cells demonstrate allospecific killing, consistent with the idea that alloreactive T cell clones require expansion in order to see killing of specific targets. In this study, we used several systems to show that concomitant activation of CD4+ TEM by recognition of class II MHC molecules on EC could promote EC-reactive CD8+ TEM expansion and CTL development. IL-2 present in medium conditioned by activated CD4+ TEM was critical for this enhancement in vitro, but our data do not preclude the possibility of other soluble or contact-dependent signals contributing to the effects in vivo. Our in vitro studies also raise the question of where might interactions between CD4+ and CD8+ TEM take place in vivo because grafts typically lack the highly specialized organization of the secondary lymphoid organs. In the case of the artery model, infiltrating CD4+ and CD8+ T cells have been observed to form clusters within the intima (25). It is less clear where this occurs in our synthetic microvessel system as lymphocytic infiltrates are not observed.
The artery model we describe here is best understood as a model of acute cell-mediated vascular rejection. The destruction of luminal EC in this model correlates with the infiltration of CD8+ T cells that express activation and cytolytic markers (e.g., perforin) and recapitulates the features of clinical intimal arteritis (23, 43). The Banff criteria for scoring rejection in transplanted kidneys classifies the involvement of vessels in acute T cell–mediated rejection as type II (intimal arteritis) or type III (transmural arteritis) (44). The presence and degree of such vascular lesions is reported to correlate with the degree of steroid-resistant rejection and worse outcomes (45–47). These data suggest normal endothelial and vascular function is essential for allograft survival. In these vessel segments, the only cells that express significant and functional levels of MHC molecules and costimulators are the EC lining the lumen. As the development of intimal arteritis may be uncoupled from parenchymal cell rejection, our studies are particularly relevant for understanding the pathogenesis of this form of rejection.
The synthetic microvessels we used in our final series of experiments were formed from HECFC. This raises a question as to whether other sources of EC might give alternative results. It has been our experience that cultured human EC from different sources behave very similarly with regard to their ability to stimulate allogeneic T cells and that HECFC are not unique (40). Moreover, with time, the entire vascular tree of an allograft appears to become a target for the host immune system, consistent with a common pattern of EC interactions.
Our study addressed a second major question with translational implications: if in fact CD4+ TEM are the key cellular players in intimal arteritis, will preventing their activation by blocking recognition of class II MHC molecules on allogeneic EC limit CTL-mediated rejection in vivo? This is the likeliest interpretation of our finding that blockade of HLA-DR recognition by antibody limited molecular and pathological signs of intimal arteritis. We acknowledge that the antibody used does not prevent recognition of HLA-DP or -DQ and could have other effects. To more definitively address the role of EC class II MHC molecules, we also genetically ablated CIITA from HECFC-derived EC by CRISPR/Cas9 genomic editing and used these modified EC to create implantable synthetic microvessels that do not express class II MHC molecules. This allowed us to examine the specific role of class II MHC presentation by EC in driving human acute cell–mediated rejection in vivo in immunodeficient mouse hosts. We showed, through selective adoptive transfers, that ablation of CIITA limits vessel destruction that had a large but not complete dependency on the presence of circulating CD8+ T lymphocytes, suggesting CD4+ T cells not only provide critical help for the CD8+ T cell response but may also directly contribute to EC destruction. The finding that preventing CD8+ TEM from accessing IL-2 elaborated by activated CD4+ TEM results in impaired CTL development reinforces the value of using anti-CD25 induction therapies, which, despite their potential to interfere with Tregs, have been shown to reduce the incidence of acute rejection in kidneys (48–50).
Collectively, our results have additional implications for transplantation of allogeneic human grafts. Despite record transplant waitlists, many donated organs are underutilized due to their perceived unsuitability for transplantation. This is because unavoidable nonimmune injuries, such as ischemia reperfusion injury, are thought to cause delayed graft function and accelerate acute rejection due to transient increases in proinflammatory cytokines that shape a pathologic T cell response (51, 52). Several approaches have shown promise in reducing the intensity of allograft rejection in various models, including modifying the organ as well as dampening the host immune response through adoptive transfer of Tregs or agents that favor Treg activation and expansion (26, 53, 54). It is not known whether ablation of class II MHC molecules on EC can impair the alloantigen-specific functions of Tregs or the overall response to such therapies, and this requires further study. Recently, a number of transplant centers have experimented with pretrans-plant ex vivo normothermic perfusion to reduce postoperative inflammation in the grafted organ, with notable improvements in outcomes with several systems including the kidney, lung, and liver (51, 55, 56). It may be possible during ex vivo conditioning of the graft to further reduce its immunogenicity with the introduction of a therapeutic agent that can dampen the T cell response during this critical period. While there are many cellular and molecular targets for possible intervention, several recent studies have reported robust nanoparticle systems that can encapsulate siRNA and target human EC to deliver their payloads over an extended period of time (57, 58). Such systems could be repurposed to transfect graft EC, as well as DCs, with anti-CIITA siRNA — as described in this report — to turn off allogeneic class II MHC molecule expression in both passenger leukocytes and graft vasculature ex vivo in preparation for transplantation. The studies reported here provide strong preclinical justification for such strategies.
Methods
Isolation of human EC
HUVEC were isolated from umbilical cords following collagenase digestion and serially cultured on 0.1% gelatin-coated tissue culture plates in M199 (Invitrogen) supplemented with 20% FBS (Invitrogen), l-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), 0.1% EC growth supplement (Corning Inc.), and porcine heparin (100 μg/ml; Sigma-Aldrich). HECFC were differentiated from cord blood mononuclear cells in vitro, as previously described (59). Briefly, umbilical cord blood was anticoagulated with heparin and enriched for mononuclear cells by density centrifugation using Lymphocyte Separation Medium (MP Biomedicals). These cells were then plated onto gelatin (0.1%, Avantor Performance Materials) and human-plasma fibronectin–coated (20 μg/ml, Millipore) tissue culture plates in EGM-2M media supplemented with 10 ng/ml VEGF (Lonza). Nonadherent cells were removed by washing after 4 days. Colonies of proliferating, differentiated cells were identified at 7–10 days, at which time the media was changed to EGM-2/15% FCS for expansion. HECFC-derived EC cultures were serially propagated in gelatin-coated tissue culture flasks with EGM-2/5% FBS (Lonza). After transduction with tetracycline-inducible Cas9 lentiviral vector, cultures were maintained in EGM-2 media using tetracycline-free FBS (Clontech).
siRNA knockdown
To silence CIITA, we used the follow siRNA sequence: 5′-GAAGUGAUCGGUGAGAGUAUU-3′ (ON-TARGETplus; Dharmacon, GE Healthcare). A nontargeting control sequence (Dhar-macon, GE Healthcare) was used as a control. siRNA (25 nM) were introduced in EC using Lipofectamine RNAiMax (Invitrogen) according to manufacturer's instructions and then, at 24 hours, stimulated with human IFN-γ for approximately 48 hours and used for mixed-EC lymphocyte reactions, qPCR, or FACS analysis.
TEM isolation
Human PBMC were collected with informed consent by leukapheresis from anonymized healthy volunteer donors under a protocol approved by the Yale Human Investigation Committee. Mono-nuclear cells were further enriched by density gradient centrifugation of leukapheresis products using Lymphocyte Separation Medium according to the manufacturer's protocol. Purified PBMCs were cryopre-served in 10% DMSO-90% FBS in liquid nitrogen before use. CD4+ and CD8+ T cells were isolated from PBMC using Dynabeads CD4+ and CD8+ Positive Isolation Kits (Invitrogen) per manufacturer's protocol. Naive, activated T cells, monocytes, and central memory T cells were removed by negative selection using anti-CD45RA (clone H100, eBioscience), anti–HLA-DR (clone LB3.1, a gift from J. Strominger, Harvard University, Cambridge, Massachusetts, USA), anti-CCR7 (clone G043H7, BioLegend), and anti-CD62L mAb (clone DREG-56, eBioscience) at a concentration of 10 μg/ml for 20 minutes, washed twice, and magnetically depleted using pan-mouse IgG beads (Invitrogen). The population obtained by this procedure was routinely >95% HLA-DR−CD45RA−CCR7−CD62L−CD45RO+CD4+ and HLA-DR−CD45RA−CCR7− CD62L−CD45RO+CD8+ TEM lymphocytes as analyzed by flow cytometry.
Flow cytometric analysis
To analyze EC with anti-CIITA or control siRNA treatment or CRISPR/Cas9 ablation, HUVEC- or HECFC-derived EC were harvested with trypsin (TrypLE Express, Invitrogen), washed in 1× PBS, and subsequently stained with directly conjugated PB anti–PECAM-1 (clone WM-59), FITC anti–HLA-A, -B, -C (clone W6/32), FITC or APC anti–HLA-DR (clone LN3) (all from eBioscience), PE anti-GITRL (clone 109101, R&D Systems), FITC anti–ICAM-1 (clone LB-2), PE anti–PD-L1 (clone MIH5), PE anti-CD40 (clone 5C3), FITC anti–LFA-3 (clone 1C3), PE anti–PD-L2 (clone MIH18), PE anti-OX40L (clone ik-1), or PE anti-ICOSL (clone 2D3) (all from BD Biosciences).
To analyze T lymphocytes, cells were collected after isolation or mixed EC-lymphocyte cocultures, washed in 1× PBS, and stained in FACS-staining buffer (PBS/1% BSA) with eFluor 450 fixable viability dye (eBioscience), anti-CD25 (clone BC96), PerCp-Cy5.5 anti-CD4 (clone RPA-T4), APC or PB anti-CD8 (clone SK1), PE anti-Granzyme B (clone GB11), PE or PB anti-Perforin (clone dG9,), FITC anti-CCR7 (clone G043H7), FITC anti-pSTAT5 (clone Y694), APC anti-CD62L (clone DREG56) (all from eBioscience), or PE anti-CD45RO (clone UCHL1, BioLegend). For experiments requiring intracellular staining (perforin, granzyme B, pSTAT5), cells were fixed in 2% paraformaldehyde and subsequently permeabilized (0.1% saponin/FACS buffer or BD Phosflow Perm Buffer III, BD Biosciences) before staining. Viability assay was performed according to manufacturer's instructions (eBioscience). All samples were analyzed on an LSR II flow cytometer (BD Biosciences) with postacquisition analysis using FlowJo software (FlowJo LLC).
Cytokine measurement by ELISA
Cell culture supernatants were collected from mixed EC-lymphocyte reactions at 24 hours and were assayed by sandwich ELISA for secreted human IL-2 or IFN-γ (Platinum ELISA kits, eBioscience), according to the manufacturer's instructions. CD8+ TEM were cultured at 30:1 ratio with EC to detect elaborated IL-2 and IFN-γ.
Mixed EC-lymphocyte reactions
CD4+ TEM activation by allogeneic EC requires recognition of nonself class II MHC molecules, principally HLA-DR, on the EC, which is lost on in vitro cultured cells. To rein-duce EC expression of class II MHC, EC or HECFC-derived EC were typically treated with 50 ng/ml recombinant human IFN-γ (Invitrogen) for 48 hours.
For in vitro stimulation, EC were washed with HBSS, plated to confluence in gelatin-coated 24-well plates, and then 1 × 106 allogeneic CD8+ TEM and/or 1 × 106 allogeneic CD4+ TEM were added to each well in RPMI 1640 (Invitrogen), supplemented with 10% FBS (Invitrogen), l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). T cells were labeled with 250 nM CFSE (Molecular Probes, Invitrogen) prior to coculture and assessed at 7 days for CFSE dilution. In some experiments, EC were pretreated with anti-CIITA or control siRNA prior to IFN-γ treatment. Where indicated, cocultures were supplemented 25 U/ml recombinant human IL-2 (BioLegend), 1:1 dilution of CD4+ TEM conditioned medium, or 10 μg/ml of blocking anti–HLA-DRα or irrelevant isotype control were added into allogeneic cocultures. For transwell experiments, CFSE-labeled CD8+ TEM (10:1 ratio) were cultured in a transwell insert (0.4-μM pore size, Corning Inc.) above a in C-24 well monolayer of CD4+ TEM and EC (10:1 ratio) that were either pretreated with anti-CIITA or control siRNA.
For generation of conditioned medium, EC were retrovirally transduced with the extracellular domain of Fcγ receptor IIA (CD32), as previously described (60). CD32+ EC were then overlayed with 2.5 μg/ml of isotype control (Mouse IgG2a,k, BioLegend) or anti-CD3 (clone OKT3, BioLegend) for 4 hours, washed, and then purified CD4+ TEM were added. After 24 hours, medium was collected, and excess antibody was removed by protein G–coated beads (Invitrogen) for 1 hour at 4°C on continuous shaking and then frozen for subsequent use or analysis by ELISA. In some experiments, IL-2 was immunoabsorbed from the activated CD4+ TEM conditioned with 20 μg/ml anti–IL-2 (clone AB12-3G4, eBioscience) and subsequently immunoabsorbed with protein G beads, resulting in specific depletion of IL-2 below limits detectable by ELISA.
For cytotoxicity assays, EC were plated onto C-96 U-well microtiter plates (Corning) and after 24 hours, approximately 200 × 103 allogeneic CD8+ TEM were added to each well. At 72 hours, RPMI medium, activated CD4+ TEM conditioned medium, activated CD4+ TEM conditioned medium depleted of IL-2, control conditioned medium, or control conditioned medium with 25 U/ml IL-2 (eBioscience) were added at 1:1 dilution to the wells. At 7 days, CD8+ TEM were collected and restimulated with fresh EC containing 1:1 medium conditions. Approximately 72 hours after replating, additional medium conditions were added. At 14 days, cells were collected, purified by CD8+ isolation, and assayed by FACS or for cytotoxicity as described previously (20, 61). Briefly, target EC were preloaded with 500 nM calcein AM (Molecular Probes, Invitrogen) and CD8+ TEM were added in AIMV medium supplemented 10 mM HEPES (Invitrogen) at a 30:1 ratio and assayed for calcein release on a fluorescent plate reader (BioTek) in bottom reading mode at 37°C for 4 hours. The percentage of cytotoxicity was defined as (live – experimental)/(live – lysed) × 100, where live is the values of calcein-loaded EC without CTL, experimental is with CTL, and lysed is lysed with 1% Triton-X 100 (Sigma-Aldrich).
Arterial transplantation
Arterial transplantation was performed as previously described (23). In brief, 1- to 3-mm segments of diameter-matched human epicardial coronary arteries harvested from explanted human hearts of organ donors or recipients were interposed into the infrarenal aortae of female C.B-17 SCID/bg mice (Taconic Biosciences) by end-to-end microsurgical anastomotic technique. Adjacent human artery segments were transplanted in groups of 2–5 mice for each experiment, and data from individual experiments were pooled for analysis. Approximately 2–7 days after transplantation, 2 × 108 human PBMC allogeneic to the artery graft were adoptively transferred into mice i.p. As previously described, only T and B lymphocytes are successfully engrafted by this procedure, and only T cells appear in the circulation (23). Successful engraftment, achieved in all mice in this study, was defined as detection by flow cytometry of a distinct population of human CD3+ cells, ranging from 0.5%–10% of the total circulating mononuclear cells.
In vivo HLA-DR blockade
For HLA-DR blockade experiments, we observed that injection of intact blocking HLA-DR antibodies depleted mice of circulating human T cells. To overcome this problem, we prepared F(ab)′2 fragments from this antibody using a F(ab)′2 preparation kit (Thermo Scientific) per manufacturer's instructions. Mice bearing human artery grafts were injected s.c. with 200 μg loading and 100 μg every 48 hours maintenance doses of anti–HLA-DRα (clone LB3.1) or with irrelevant IgG control (Jackson ImmunoResearch Laboratories Inc.) starting on the day before allogeneic PBMC transfer. After 21 days of treatment, animals were anesthetized and human arterial grafts were perfused with normal saline and excised before death. Arterial grafts were snap frozen in optimum cutting temperature compound (OCT compound), and serial 5-μm transverse sections were cut for morphometric, immunofluorescence, and qPCR analyses.
CRISPR/Cas9-mediated ablation of CIITA in EC
CIITAnull HECFC-derived ECs were generated as previously described (38). Briefly, early-passage HECFC were transduced with lentiviral constructs encoding doxycycline-inducible Cas9 and CIITA specific guide sequence (GATATTGGCATAAGCCTCCC). After 48 hours, cells were drug selected and Cas9 expression was induced with doxycycline and blasticidine treatment for 7 days and then stimulated with 50 ng/ml IFN-γ. CIITA loss-of-function EC were identified by FACS gated on HLA-DR−cells, isolated using a 100-μm low-pressure nozzle on BD FACSAria II, then expanded and used for qPCR, in vitro mixed EC-lymphocyte reactions, and formation of synthetic vessels.
Synthetic microvessel formation and transplantation
Human microvessels were generated and implanted s.c. in the abdominal wall of female 6- to 8-week-old C.B-17 SCID/bg mice as previously described (59). Briefly, HECFC-derived EC were suspended in a rat tail type I collagen gel, and 400 μl of cell suspension was gently poured into a single well of a 48-well tissue culture plate. The protein gel was polymerized at 37°C/5% CO2 and then implanted. Each mouse received a single implant containing either unmodified EC or CRISPR/Cas9-modified CIITAnull EC. Approximately 14 days after implantation, WT or CIITAnull gel implanted mice were distributed into three groups categorized by inoculation: no PBMC (PBS), PBMC, or with PBMC depleted of CD8+ cells. In the PBMC group, approximately 2 × 108 human PBMC allogeneic to the EC graft were adoptively transferred into mice i.p. In the CD8+-depletion group, 2 × 108 human PBMC were depleted of CD8+ with Dynabeads CD8+ beads and then adoptively transferred. Approximately 10 days after inoculation, animals were euthanized and grafts were harvested for analysis of human microvasculature. Gels and surrounding soft tissues were fixed in 10% neutral buffered formalin and embedded in paraffin, and 5-μm thick sections were cut for H&E staining or immunostaining for human CD31 (BioGenex). Vessel number was quantified by number of perfused vessels (containing murine erythrocytes) normalized to gel area, and only vascularized implants were included for analysis. Previous experience with the collagen implant model indicated that n = 3–5 per group are needed to obtain statistical significance (40).
Histology and immunofluorescence
Cross sections (5-μm) of artery grafts were stained with Elastica van Gieson (EVG) as well as H&E and used to quantify the luminal and intimal areas using ImageJ (NIH) for morphometry. The intimal area was defined as bound by internal elastic lamina and the lumen. The EC lining of grafts were identified by Rhodamine ulex europaeus agglutinin 1 (Vector Laboratories) and/or CD31 expression, which yielded equivalent results, and quantified by tracing the circumference of lumen using ImageJ. Graft-infiltrating human lymphocytes were quantified by staining for human CD45RO+ (BD Biosciences) or CD3+ (Dako), which yielded equivalent results, by indirect immunofluorescence (donkey anti-rabbit or donkey anti-mouse AlexaFluor-594, Invitrogen). The numbers of intimal CD45RO+ were counted in two cross sections per graft. All sections were mounted on slides using mounting medium (Pro-Long Gold, Invitrogen) and examined by microscopy with an Axiovert 200M microscope (Zeiss).
qPCR analysis
RNA from cultured EC was isolated using RNeasy Mini Kit (QIAGEN) and used to make cDNA with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) per manufacturer's protocols. To isolate RNA from artery grafts, serial sections of flash-frozen artery were immersed briefly in water, centrifuged, and resuspended in RLT lysis buffer and then isolated by RNeasy kit.
qPCR reactions were assembled with TaqMan 2× Gene Expression Master Mix (Applied Biosystems) and predeveloped Taqman gene expression probes and analyzed on a CFX96 Real-Time system using CFX Manager Software (Bio-Rad). Probes used in this study: GAPDH (Hs02758991_g1), HPRT1 (Hs02800695_m1), CIITA (Hs00172094_m1), HLA-DRA (Hs00219575_m1), HLA-DPA1 (Hs01072899_m1), HLA-DPB1 (Hs03045105_m1), HLA-DQA1 (Hs03007426_mH), HLA-DQB1 (Hs03054971_m1), CD3e (Hs01062241_m1), CD4 (Hs01058407_m1), CD8 (Hs00233520_m1), PRF1 (Hs00169473_m1), GZMB (Hs00188051_m1), CXCL10 (Hs01124251_g1), IFN-γ (Hs00989291_m1), and IL-2 (Hs00174114_m1), all from Applied Biosystems. Gene expression levels were normalized to GAPDH, HPRT, CD3e, or CD8 where indicated.
Statistics
All data are expressed as mean ± SD. Statistical comparisons were made using Student's t test (2-tailed) or 1-way ANOVA with Bonferroni post-hoc test. P values less than 0.05 was considered statistically significant. All results were analyzed with Prism v6.0 (GraphPad).
Study approval
All human cells (cord blood, PBMC), tissues (umbilical cords), and vessels were obtained with informed consent under protocols approved by the Yale Human Investigation Committee and the New England Organ Bank. All animal protocols were approved by the Yale Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
We thank Louise Benson, Nancy Kirkiles-Smith, and Gwendolyn Davis-Arrington for assistance with cell culture and animal care. This work is supported by NIH grants R01 HL036003, R01 HL051014, R01 HL085416, and R01 HL109455. P.A. was supported by an NIH Medical Scientist Training Program grant (T32-GM007205) and the Paul and Daisy Soros Fellowship for New Americans and is currently supported by an NIH National Research Service Award predoctoral fellowship (F30AI112218).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Author contributions
PA and JSP conceived the study and wrote the manuscript. PA, LQ, and WGC conducted the experiments. PA, LQ, WGC, ALMB, GT, WMS, and JSP designed experiments.
References
- 1.Suthanthiran M, Strom TB. Renal transplantation. N Engl J Med. 1994;331(6):365–376. doi: 10.1056/NEJM199408113310606. [DOI] [PubMed] [Google Scholar]
- 2.Stehlik J, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report — 2011. J Heart Lung Transplant. 2011;30(10):1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Taylor DO, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Heart Transplant Report — 2009. J Heart Lung Transplant. 2009;28(10):1007–1022. doi: 10.1016/j.healun.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Colvin RB, et al. Evaluation of pathologic criteria for acute renal allograft rejection: reproducibility, sensitivity, and clinical correlation. J Am Soc Nephrol. 1997;8(12):1930–1941. doi: 10.1681/ASN.V8121930. [DOI] [PubMed] [Google Scholar]
- 5.Strehlau J, et al. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc Natl Acad Sci U S A. 1997;94(2):695–700. doi: 10.1073/pnas.94.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakkis FG, Lechler RI. Origin and biology of the allogeneic response. Cold Spring Harb Perspect Med. 2013;3(8):a014993. doi: 10.1101/cshperspect.a014993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heeger PS, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immuno-logic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163(4):2267–2275. [PubMed] [Google Scholar]
- 8.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 9.Makhlouf L, et al. Allorecognition and effector pathways of islet allograft rejection in normal versus nonobese diabetic mice. J Am Soc Nephrol. 2003;14(8):2168–2175. doi: 10.1097/01.asn.0000079041.15707.a9. [DOI] [PubMed] [Google Scholar]
- 10.Shiao SL, McNiff JM, Pober JS. Memory T cells and their costimulators in human allograft injury. J Immunol. 2005;175(8):4886–4896. doi: 10.4049/jimmunol.175.8.4886. [DOI] [PubMed] [Google Scholar]
- 11.Weaver TA, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med. 2009;15(7):746–749. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearl JP, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5(3):465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 13.Jutte NH, et al. Human heart endothelial-cell-restricted allorecognition. Transplantation. 1996;62(3):403–406. doi: 10.1097/00007890-199608150-00017. [DOI] [PubMed] [Google Scholar]
- 14.Adams PW, et al. Alloantigenicity of human endothelial cells. 1. Frequency and phenotype of human T helper lymphocytes that can react to allogeneic endothelial cells. J Immunol. 1992;148(12):3753–3760. [PubMed] [Google Scholar]
- 15.Shiao SL, Kirkiles-Smith NC, Shepherd BR, McNiff JM, Carr EJ, Pober JS. Human effector memory CD4+ T cells directly recognize allogeneic endothelial cells in vitro and in vivo. The Journal of Immunology. 2007;179(7):4397–4404. doi: 10.4049/jimmunol.179.7.4397. [DOI] [PubMed] [Google Scholar]
- 16.Brewer Y, et al. Effect of graft perfusion with two CD45 monoclonal antibodies on incidence of kidney allograft rejection. Lancet. 1989;2(8669):935–937. doi: 10.1016/s0140-6736(89)90951-3. [DOI] [PubMed] [Google Scholar]
- 17.Salvadori M, et al. FTY720 versus MMF with cyclosporine in de novo renal transplantation: a 1-year, randomized controlled trial in Europe and Australasia. Am J Transplant. 2006;6(12):2912–2921. doi: 10.1111/j.1600-6143.2006.01552.x. [DOI] [PubMed] [Google Scholar]
- 18.Biedermann BC, Pober JS. Human vascular endothelial cells favor clonal expansion of unusual alloreactive CTL. J Immunol. 1999;162(12):7022–7030. [PubMed] [Google Scholar]
- 19.Biedermann BC, Pober JS. Human endothelial cells induce and regulate cytolytic T cell differentiation. J Immunol. 1998;161(9):4679–4687. [PubMed] [Google Scholar]
- 20.Dengler TJ, Pober JS. Human vascular endothelial cells stimulate memory but not naive CD8+ T cells to differentiate into CTL retaining an early activation phenotype. J Immunol. 2000;164(10):5146–5155. doi: 10.4049/jimmunol.164.10.5146. [DOI] [PubMed] [Google Scholar]
- 21.Murray AG, et al. Dermal microvascular injury in the human peripheral blood lymphocyte reconstituted-severe combined immunodeficient (HuPBL-SCID) mouse/skin allograft model is T cell mediated and inhibited by a combination of cyclosporine and rapamycin. Am J Pathol. 1998;153(2):627–638. doi: 10.1016/S0002-9440(10)65604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreisel D, et al. Vascular endothelium does not activate CD4+ direct allorecognition in graft rejection. J Immunol. 2004;173(5):3027–3034. doi: 10.4049/jimmunol.173.5.3027. [DOI] [PubMed] [Google Scholar]
- 23.Lorber MI, et al. Human allogeneic vascular rejection after arterial transplantation and peripheral lymphoid reconstitution in severe combined immunodeficient mice1. Transplantation. 1999;67(6):897–903. doi: 10.1097/00007890-199903270-00018. [DOI] [PubMed] [Google Scholar]
- 24.Rao DA, et al. Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection. J Exp Med. 2008;205(13):3145–3158. doi: 10.1084/jem.20081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi T, et al. Reperfusion injury intensifies the adaptive human T cell alloresponse in a human-mouse chimeric artery model. Arterioscler Thromb Vasc Biol. 2012;32(2):353–360. doi: 10.1161/ATVBAHA.111.239285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadig SN, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16(7):809–813. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yacoub-Youssef H, et al. Chronic vascular rejection: histologic comparison between two murine experimental models. Transplant Proc. 2005;37(6):2886–2887. doi: 10.1016/j.transproceed.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 28.Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012;33(1):49–57. doi: 10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7(2):118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 30.Hirschberg H, Braathen LR, Thorsby E. Antigen presentation by vascular endothelial cells and epidermal Langerhans cells: the role of HLA-DR. Immunol Rev. 1982;66(1):57–77. doi: 10.1111/j.1600-065x.1982.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 31.Epperson DE, Pober JS. Antigen-presenting function of human endothelial cells. Direct activation of resting CD8 T cells. J Immunol. 1994;153(12):5402–5412. [PubMed] [Google Scholar]
- 32.Chang CH, Fontes JD, Peterlin M, Flavell RA. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. J Exp Med. 1994;180(4):1367–1374. doi: 10.1084/jem.180.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steimle V, Siegrist C-A, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-γ mediated by the transactivator gene CIITA. Science. 1994;265(5168):106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 34.Masternak K, Reith W. Promoter-specific functions of CIITA and the MHC class II enhanceosome in transcriptional activation. EMBO J. 2002;21(6):1379–1388. doi: 10.1093/emboj/21.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boss JM, Jensen PE. Transcriptional regulation of the MHC class II antigen presentation pathway. Curr Opin Immunol. 2003;15(1):105–111. doi: 10.1016/s0952-7915(02)00015-8. [DOI] [PubMed] [Google Scholar]
- 36.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper a T-killer cell. Nature. 1998;393(6684):474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 37.Moriggl R, et al. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10(2):249–259. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- 38.Abrahimi P, et al. Efficient gene disruption in cultured primary human endothelial cells by CRISPR/Cas9. Circ Res. 2014;117(2):121–128. doi: 10.1161/CIRCRESAHA.117.306290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng L, Gibson TF, Schechner JS, Pober JS, Bothwell AL. Bcl-2 transduction protects human endothelial cell synthetic microvessel grafts from allogeneic T cells in vivo. J Immunol. 2004;173(5):3020–3026. doi: 10.4049/jimmunol.173.5.3020. [DOI] [PubMed] [Google Scholar]
- 40.Suárez Y, Shepherd BR, Rao DA, Pober JS. Alloimmunity to human endothelial cells derived from cord blood progenitors. J Immunol. 2007;179(11):7488–7496. doi: 10.4049/jimmunol.179.11.7488. [DOI] [PubMed] [Google Scholar]
- 41.Lafferty KJ, Bootes A, Dart G, Talmage DW. Effect of organ culture on the survival of thyroid allografts in mice. Transplantation. 1976;22(2):138–149. doi: 10.1097/00007890-197608000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6(6):686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 43.Meehan SM, et al. Cytotoxicity and apoptosis in human renal allografts: identification, distribution, and quantitation of cells with a cytotoxic granule protein GMP-17 (TIA-1) and cells with fragmented nuclear DNA. Lab Invest. 1997;76(5):639–649. [PubMed] [Google Scholar]
- 44.Racusen LC, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 45.Hirsch HH, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79(10):1277–1286. doi: 10.1097/01.tp.0000156165.83160.09. [DOI] [PubMed] [Google Scholar]
- 46.Haas M, Kraus ES, Samaniego-Picota M, Racusen LC, Ni W, Eustace JA. Acute renal allograft rejection with intimal arteritis: histologic predictors of response to therapy and graft survival. Kidney Int. 2002;61(4):1516–1526. doi: 10.1046/j.1523-1755.2002.00254.x. [DOI] [PubMed] [Google Scholar]
- 47.Gaber LW, et al. Correlation between Banff classification, acute renal rejection scores and reversal of rejection. Kidney Int. 1996;49(2):481–487. doi: 10.1038/ki.1996.68. [DOI] [PubMed] [Google Scholar]
- 48.Ponticelli C, et al. A randomized, double-blind trial of basiliximab immunoprophylaxis plus triple therapy in kidney transplant recipients1, 2. Transplantation. 2001;72(7):1261–1267. doi: 10.1097/00007890-200110150-00014. [DOI] [PubMed] [Google Scholar]
- 49.Mourad G, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. Sequential protocols using basiliximab versus anti-thymocyte globulins in renal-transplant patients receiving mycophenolate mofetil and steroids. Transplantation. 2004;78(4):584–590. doi: 10.1097/01.tp.0000129812.68794.cc. [DOI] [PubMed] [Google Scholar]
- 50.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355(19):1967–1977. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 51.Hosgood SA, Heurn E, Nicholson ML. Normothermic machine perfusion of the kidney: better conditioning and repair? Transpl Int. 2015;28(6):657–664. doi: 10.1111/tri.12319. [DOI] [PubMed] [Google Scholar]
- 52.Abrahimi P, Liu R, Pober J. Blood vessels in allotransplantation. Am J Transplant. 2015;15(7):1748–1754. doi: 10.1111/ajt.13242. [DOI] [PubMed] [Google Scholar]
- 53.Wang C, et al. Rapamycin-treated human endothelial cells preferentially activate allogeneic regulatory T cells. J Clin Invest. 2013;123(4):1677–1693. doi: 10.1172/JCI66204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng G, et al. Functional regulatory T cells produced by inhibiting cyclic nucleotide phosphodiesterase type 3 prevent allograft rejection. Sci Transl Med. 2011;3(83):83ra40. doi: 10.1126/scitranslmed.3002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cypel M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364(15):1431–1440. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 56.St Peter SD, Imber CJ, Lopez I, Hughes D, Friend PJ. Extended preservation of non-heart-beating donor livers with normothermic machine perfusion. Br J Surg. 2002;89(5):609–616. doi: 10.1046/j.1365-2168.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- 57.Devalliere J, et al. Sustained delivery of proangiogenic microRNA-132 by nanoparticle transfection improves endothelial cell transplantation. FASEB J. 2014;28(2):908–922. doi: 10.1096/fj.13-238527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dahlman JE, et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. 2014;9(8):648–655. doi: 10.1038/nnano.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shepherd BR, Enis DR, Wang F, Suarez Y, Pober JS, Schechner JS. Vascularization and engraftment of a human skin substitute using circulating progenitor cell-derived endothelial cells. FASEB J. 2006;20(10):1739–1741. doi: 10.1096/fj.05-5682fje. [DOI] [PubMed] [Google Scholar]
- 60.Manes TD, Shiao SL, Dengler TJ, Pober JS. TCR signaling antagonizes rapid IP-10-mediated transendothelial migration of effector memory CD4+ T cells. J Immunol. 2007;178(5):3237–3243. doi: 10.4049/jimmunol.178.5.3237. [DOI] [PubMed] [Google Scholar]
- 61.Kummerow C, Schwarz EC, Bufe B, Zufall F, Hoth M, Qu B. A simple, economic, time-resolved killing assay. Eur J Immunol. 2014;44(6):1870–1872. doi: 10.1002/eji.201444518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.