Abstract
This study investigated whether TNF-α, Toll-like receptors (TLRs) 7/8 agonist resiquimod (R848), the TLR4 agonist lipopolysaccharide (LPS) and their combinations can enhance autologous AML-reactive T cell generation in an in vitro culture. AML peripheral blood or bone marrow mononuclear cells were cultured in medium supplemented with GM-CSF/IL-4 to induce dendritic cell (DC) differentiation of AML blasts (AML-DC). The impact of TNF-α, LPS, R848 and their combinations on AML-DC cultures was analyzed. Significantly enhanced CD80, CD40, CD83, CD54, HLADR and CD86 expression of AML cells was observed by addition of TNF-α, LPS, R848 alone or combinations. Induced CD80 expression of AML cells was significantly higher through the combination of TNF-α, LPS and R848 (T + L + R) than that by T alone. CTL induced from T + L + R, T + R, T + L, L + R and R, but not T, L alone stimulated cultures showed significantly higher IFN-γ release than the medium control in response to autologous AML cells. IFN-γ release by T + L + R was significantly higher than T or L alone, and T + R was significantly higher than T alone. CTL generated from T + L + R, T + L, T + R, L + R and L alone exerted significantly higher AML cell killing than medium control. AML cell killing by T + L + R and T + R was significantly higher than T or R alone. These results indicate that the combination of T + L + R induces a significantly enhanced antigen presentation effect of AML-DC. We speculate that the complementary effects of reagent combinations may better address the heterogeneity of responses to any single agent in AML cells from different patients.
Keywords: Acute myeloid leukemia, Dendritic cells, Toll-like receptor, Cytotoxic T lymphocytes, Resiquimod, Lipopolysaccharide
Introduction
Toll-like receptors (TLRs) recognize pathogen-associated molecular patterns (PAMPs), the conserved motifs shared by many bacteria, viruses, protozoa and fungi, activate and initiate innate and adaptive immune responses [1, 2]. Ten human TLRs have been identified. TLR-1, TLR-2, TLR-4, TLR-5 and TLR-6 mainly recognize microbial membrane components including lipids, lipoproteins and flagella. TLR-3, TLR-7, TLR-8 and TLR-9 recognize both microbial and viral nucleic acids [2–13]. Considering that there would be a mixture of various pathogen products in a pathogen infection site, the PAMPs produced by pathogens are a mixture of various TLR agonists. These TLR agonists trigger a spectrum of TLRs of the immune cells opsonized around infection sites. The immune response and memory established during and after the infection are actually products of the total effects of various activated TLRs [14,15]. TLRs are primarily expressed in hematopoietic cells, especially the myeloid lineage. The expression of TLRs is different among different sub-lineages of hematopoietic cells. Myeloid dendritic cells express TLR-1–6 and TLR-8, whereas plasmacytoid DC expresses TLR-7 and TLR-9. Neutrophils express TLR-1, TLR-2 and TLR-4–10, natural killer (NK) cells express TLR-1, monocytes express all TLRs except TLR-3 and B lymphocytes express TLR-7, TLR-9 and TLR-10; regulatory T cells (Tregs) can express TLR-8 and TLR-10 [1, 2, 16, 17].
Ligand recognition and signal transduction from TLRs ultimately induce the expression of numerous genes required for the inflammatory response, including inflammatory cytokines, chemokines, antimicrobial molecules, as well as major histocompatibility and costimulatory molecules important for triggering DC activation, promoting DC differentiation and maturation, enhanced antigen uptake and cell surface antigen presentation. These processes stimulate expansion and differentiation of naive T cells toward a T helper 1 (Th1) phenotype, establishing long-standing adoptive immunity to the host [18–20].
AML is a heterogeneous disease that can be classified by morphology, lineage, and genetics and reflects the diversity of malignant transformation at differentiation stages of myeloid precursors [21, 22]. AML cells express TLR-2, TLR-4, TLR-7, TLR-8 and TLR-9. The expression and the level of TLRs in AML vary among patients [23, 24]. Differentiation of AML cells into DC-like cells can be induced with cytokines. A DC phenotype with increased CD80, CD83, CD86, CD40 expression can be induced after in vitro exposure of native AML blasts to several cytokine combinations. Such phenotypically altered AML cells (AML-DC) appear to be more efficient than native blasts as antigen-presenting cells for the presentation of leukemia-restricted peptides to T cells [25–27]. AML-DC can be used to activate leukemia-specific T cells from allogeneic PBMNC obtained from healthy BM donors or autologous PBMNC obtained from patients with AML in complete remission (CR) [28, 29]. Intact autologous AML cells are likely the best source of leukemia-associated antigens (LAAs) since all relevant candidate LAA should be contained on AML blasts. DC-differentiated AML cells are effective whole cell vaccines without the need to define the unique LAA expressed and are ideal antigen-presenting cells to activate anti-AML adoptive immunity [30, 31]. Based on our previous studies of an AML-PBMNC culture system that induced AML cell DC differentiation for generating autologous AML-reactive T cells [32], the aim of the current study was to investigate whether the addition of the TLR agonists LPS and R848 or the cytokine TNF-α or combinations of these reagents will more effectively induce AML-DC maturation and further augment the AML reactivity of CTLs that are generated by AML-PBMC culture system of this study.
Materials and methods
Patient samples and cells
PB or BM samples of primary or relapsed AML patients were obtained with informed consent under an institutional review board (IRB) approved protocol. After Ficoll–Hypaque gradient centrifugation, the MNC were collected and cryopreserved.
Evaluation of effects TLR agonists on AML-DC culture
AML-MNC cultures were generated as previously described [32]. Briefly, MNC of AML patients were suspended in 1 × 106/ml in culture medium containing 45 % AIM-V medium, 45 % RPMI-1640, 12 mM l-glutamine, 55 μM β-mercaptoethanol, 100 units/ml penicillin, 100 μg/ml streptomycin, 10 % heat-inactivated human AB serum, 20 IU/ml IL-2, 1,000 U/ml IL-4 and 50 ng/ml GM-CSF. 0.2 ml of AML-MNC suspension was added to 96-well U-bottom culture plates. At day 7 of culture in a humidified 37 °C incubator with 5 % CO2, various reagents and combinations of reagents were used to stimulate the maturation of AML-DC. There were eight stimulation conditions, including culture medium control, TNF-α (20 ng/ml) (Invitrogen), LPS (500 pg/ml) (Sigma), R848 (5 μg/ml) (Invitrogen) or their combinations, TNF-α LPS (T + L), TNF-α + R848 (T + R), LPS + R848 (L + R), TNF-α + LPS + R848 (T + L + R). After 24 h of incubation, the culture medium was removed from each well and the plates were washed twice (2×). Fresh culture medium supplemented with 6000 IU ml IL-2 was added to each well. After 2–3 weeks of culture, when most wells contained confluent proliferating lymphocytes, 0.1 ml cell suspensions were taken from each well and transferred to another culture plate. Both culture plates were then filled with 0.1 ml culture medium with 6000 IU/ml IL-2. After continuous culture for 2–3 days, 2 × 104 autologous AML-MNC were added to each well of the daughter plates. After overnight incubation, 100 μl of culture supernatant was obtained from each well for ELISA assays of IFN-γ concentration. Cells obtained from the original plates were used for CTL assays.
Flow cytometry analysis
For determination of DC differentiation of AML blasts and AML-specific T cell proliferation, cells were stained with antibodies to CD3, CD4, CD8, CD33, CD34, CD54, CD80, CD86, CD83 and HLA-DR at day 0 and day 8 of culture. For evaluating T cell priming and proliferation, CD3, CD4, CD8 expression was examined weekly after day 14 to the end of culture. Monoclonal antibodies were purchased from BD Biosciences (San Jose, CA).
CTL assay of AML-reactive CTL by FACS
CTLs generated from 12 replicates of one treatment culture condition in a 96-well plate were mixed for flow cytometry CTL assays. CTL assays by FACS were performed according to a 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE)-based cytotoxicity assay reported by Jedema et al. [33] with modification. Briefly, after washing with phosphate-buffered saline (PBS), AML-MNC suspensions as targets were re-suspended at 10 × 106 cells/ml in PBS. CFSE (eBioscience, San Diego, CA) was then added to the cell suspension to a final concentration of 10 μM, mixed immediately and incubated at room temperature in the dark for 10 min. The labeling was stopped by adding 5 volumes of cold complete media and incubated on ice for 5 min. After three washes with complete medium, the CFSE-labeled target cells were re-suspended in complete medium and directly used for CTL assay. CFSE-labeled AML-MNC, 0.1 ml in a cell concentration of 1 × 106/ml and autologous CTLs, 0.1 ml in a cell concentration of 1–2 × 106/ml were separately seeded in wells of 96-well U-bottom plates in an effector-to-target cell ratio of 1:1–2:1. Separately seeded CFSE-labeled AML-PBMNC and autologous CTL were mixed immediately after culture plate setup as time 0 to determine total AML cells left (percentage) after 24 or 48 h of culture. This tube permitted determination of CTL-dependent cytotoxic effects on AML cells by assessment of cells killed by CTL, as well as non-cytotoxic effects, e.g., growth of AML cells and/or CTLs, or cell apoptosis during 24 or 48 h of culture. This tube was defined as “Mixed at T0.” Separately seeded CFSE-labeled AML cells and autologous CTL were mixed at 24 or 48 h after culture as controls to determine the effect of autonomous growth or apoptosis of AML cells or CTLs during 24 or 48 h of culture without the cytotoxic effect of AML-reactive CTLs. The tubes were defined as “Mixed at T24” or “Mixed at T48.” The CFSE-labeled target cells were AML-PBMNC including AML cells, normal monocytes, T and B cells. The percentage of AML cells in AML-PBMC varied widely among different patients. To determine the exact percentage of AML cells after co-culture with CTL cells in “Mixed at T0” and “Mixed at T24 or T48” tubes, the cells therein were stained with CD33-PE and CD34-PE at 24 and 48 h after initiation of the CTL assay. Only CD33/CD34-PE and CSFE double-positive cells by flow cytometry were counted as AML cells in the co-culture of “Mixed at T0” and “Mixed at T24 or T48” tubes. The percentage of AML cell elimination by autologous AML-reactive CTL was calculated using the following equation:
Statistical analysis
Throughout the analysis, p < 0.05 was considered to be statistically significant. Linear mixed effect models with subject as a random effect were fitted. Post hoc contrasts were tested following the modeling. The original data were transformed (square root or log transformation) when needed. If a parametric model was not appropriate, the nonparametric Wilcoxon signed rank test was used to compare the effects of test agents on the same patient samples. Holm’s step-down method was used to adjust the p values after multiple comparisons. The analysis was done using R version 3.1.2 [34].
Results
Enhancement of AML-DC maturation
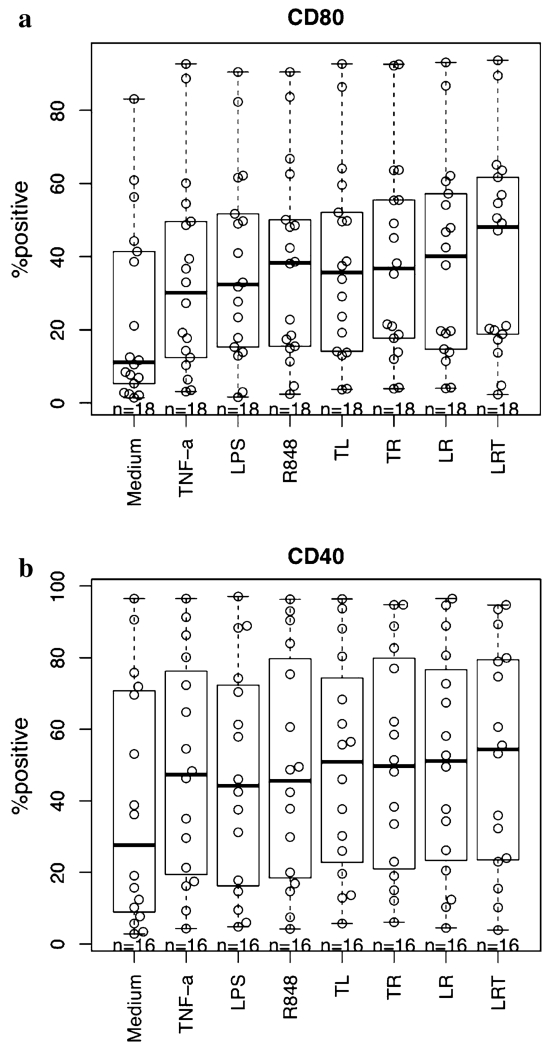
We studied 18 primary AML patient samples (Three patient samples were studied repeatedly, and the mean value of the repeated experiments was used). MNC from AML patients at first presentation or relapse were obtained and immunophenotyped. The cells consisted of 90 ± 8 % CD33+ and/or CD34+ AML blasts (range 69–98 %) and 3 ± 3 % CD3+ T cells (range 1–13 %). AML-MNC were cultured in 96-well plates as described in “Materials and methods” section. TNF-α, LPS, R848 and combinations were added at day 7 and analyzed at day 8 by flow cytometry. The percent positive for CD80 expression at day 8 of culture with different treatment conditions is shown in Fig. 1a. All single reagents and reagent combinations added at day 7 induced significantly higher CD80, CD86, CD83, CD40, CD54 and HLA-DR expression of AML cells compared to the medium control (p < 0.01 for all; for CD80, 86, 83, 40 and HLA-DR, based on contrasts after linear mixed effect models, CD83 was square root transformed; for CD54, Wilcoxon signed rank test was used; the Holm method was employed for multiple comparison adjustments). Comparing single reagents with their combinations, the enhancing effect of T + L + R on CD80 expression was significantly higher than T alone (p < 0.001). T + R had a better CD54 enhancing effect than R alone (p = 0.04). Other than the above, reagent combinations always had nonsignificant small sub-additive effects for CD80 and CD40 expression, but not for CD54, CD86, CD83 and HLA-DR.
Fig. 1.
Enhancement of co-stimulatory molecule expression on AML-DC stimulated by TNF-α, LPS, R848 and their combinations. AML-MNC were stimulated to differentiate into mature DC as described in “Materials and methods” section (8 days in culure). a CD80 expression (%). All reagents and combinations vs medium, p < 1e–09; among reagents: T + L + R vs T, p < 0.001). b CD40 expression. All reagents and combinations versus medium, p < 1e–05; no significant difference was noted among reagents when analyzed by FACS on day 8 (TL: TNF-α + LPS, TR: TNF-α + R848, LR: LPS + R848, LRT: LPS + R848 + TNF-α)
Enhancement of IFN-γ release response of AML-reactive CTL
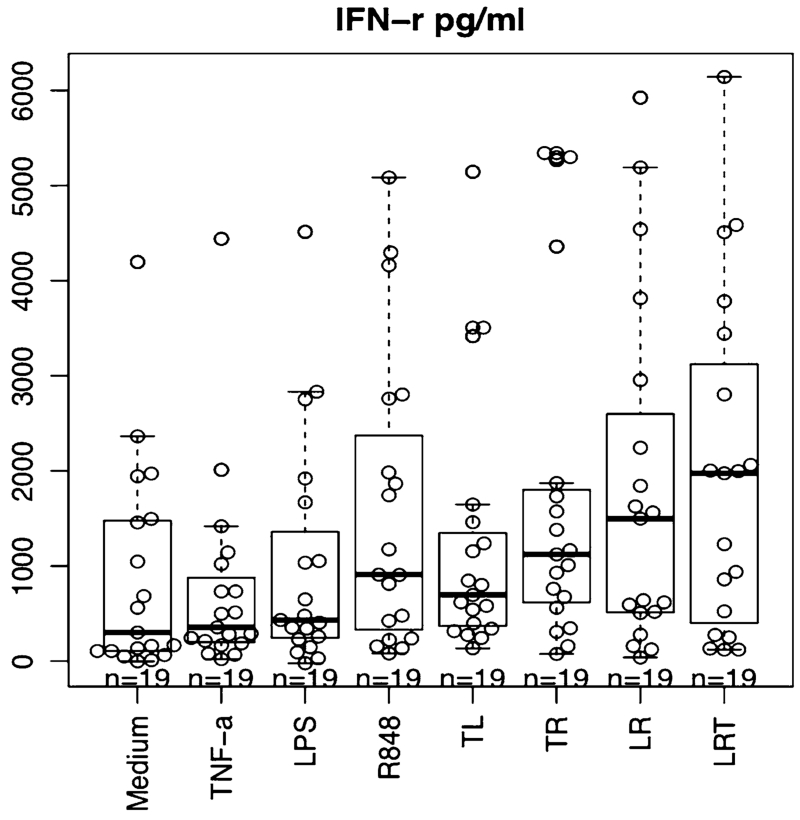
High-dose IL-2 was added on day 8 to expand T cells in the culture. After 2–3 weeks, T cells obtained from each culture well were tested for reactivity by ELISA assay of IFN-γ release in response to autologous AML cells. For every experiment, the mean of 10 replicates of each treatment condition was calculated. The results from 19 independent experiments are summarized in Fig. 2. CTLs primed and activated by AML cells that were stimulated by all the combinations or R alone released significantly higher IFN-γ in response to autologous AML cells than that of medium control (p < 0.01 for all, contrasts after linear mixed effect model with Holm’s adjustment, IFN-γ is log transformed). But T or L alone did not show a significant difference from the culture medium control. Among reagents and combinations, T + L + R was statistically significantly higher than T (p = 0.02) or L (p = 0.03) alone; T + L + R showed a small, but nonsignificant increase compared with R alone. These results suggested the trend that the combination of these reagents, especially T + L + R, stimulated AML cells toward better AML-DC differentiation with higher CTL stimulating efficiency than any single reagent.
Fig. 2.
Enhancement of IFN-γ release by AML-reactive CTL. CTLs generated from AML-MNC cultures under different conditions were compared for INF-γ release in response to autologous AML by ELISA assay (T + L + R, T + R, T + L, L + R, or R vs medium, p < 0.01; no significant difference for T, L vs medium. Among reagents: T + L + R vs T or L, T + R vs T, < 0.05)
Increased autologous AML elimination by AML-reactive CTL
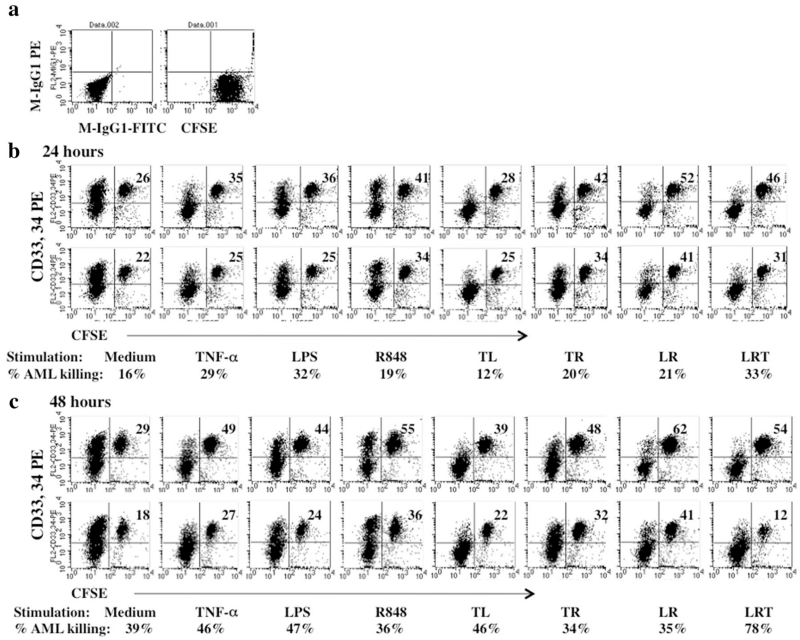
CTLs generated from nine patient samples were analyzed for cytotoxic effect against autologous AML cells. One example of 24- and 48-h flow cytometry CTL assays from patient #2010-8 is shown in Fig. 3. CTL stimulated by a combination of T + L + R achieved greater AML cell elimination than single reagents and other reagent combinations.
Fig. 3.
CTL activity against AML cells analyzed by FACS (patient #2010-8). CTLs were obtained from AML-MNC culture under different culture conditions. CTL assays were performed as described in “Materials and methods” section. a Isotype antibody and CFSE control. b Percentage of AML elimination by CTL at 24 h of incubation. The top panel shows the percentages of AML cells after 24 h in cultures separate from the AML-CTLs but after mixing with the CTLs just prior to the FACS analysis. The bottom panel shows the percentages of AML cells remaining after 24 h of co-culture with AML-CTLs. c Percent of AML elimination by CTL at 48 h of incubation. The top panel shows the percentages of AML cells after 48 h in cultures separate from the AML-CTLs but after mixing with the CTLs just prior to the FACS analysis. The bottom panel shows the percentages of AML cells remaining after 48 h of co-culture with AML-CTLs. The percentages of AML cell elimination calculated using the equation in “Materials and methods” section are indicated underneath the corresponding FACS plots for each treatment
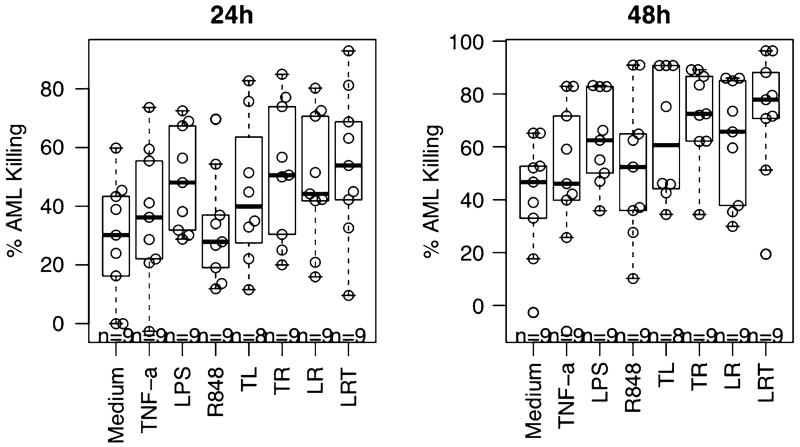
Results of nine CTL assays showed that the AML cell elimination by CTLs achieved by a single reagent varied from patient to patient. However, reagent combinations almost always demonstrated better stimulation than single reagents (eight of nine), suggesting that the reagent combinations have complementary or additive effects in inducing AML-reactive CTLs. For most patient samples, the reagent combination T + L + R was the most effective inducer of AML-reactive CTLs. However, for patient #2011-2, single-reagent LPS was most effective and the combination of T + L + R was least effective. The overall effect of the reagents and reagent combinations to stimulate AML-specific CTL from nine patient samples is summarized in Fig. 4. Comparing reagent or reagent combinations to the medium control, all the reagent combinations along with single-reagent L showed significantly more AML cell killing than the medium control (p < 0.001 for all, contrasts after linear mixed effect modeling with Holm adjustment). The T and R combination significantly improved cell killing compared with either T or R alone (p < 0.01 for both). Also, the T + L + R three agent combination improved cell killing compared with each reagent alone, significantly so for T or R (p < 0.01 for both).
Fig. 4.
Summary of CTL assays for AML elimination by autologous AML-reactive T cells generated under various conditions (T + L + R, T + L, T + R, L + R, or L vs medium, p < 0.01; T + R vs T or R, p < 0.01 for both; T + L + R vs T or R, p < 0.01 for both). The data shown represent the combined data from studies of nine AML patients
Discussion
AML cells can be differentiated to DC-like cells possessing the ability to stimulate antileukemic immunity by antigen-specific T cell responses [29–32]. As a group of diseases, the heterogeneous nature of AML predicts a diversity of responses to agents stimulating DC differentiation and DC maturation of AML cells. For a meaningful therapeutic effect, it would be clinically useful to develop an effective AML-DC maturation induction protocol that reliably promotes effective anti-AML immunity for most AML patients.
TNF-α is well recognized for playing a significant role in inducing DC maturation and has been used for DC maturation in many studies [35–37]. In our previous studies of an AML-PBMC culture system, TNF-α was tested for inducing AML-DC maturation. Although TNF-α enhanced CD80, CD86, CD83 expression, it did not significantly enhance IFN-γ release or cytolytic activity by AML-DC-induced CTL in response to autologous AML (unpublished data). For this reason, we hypothesized that combinations of stimulatory molecules and cytokines would improve maturation of the AML-DC and thus improve the immune response in a wider range of patients with AML. Upon recognition and activation by microbial products, TLRs activate the immune system and initiate innate and adaptive immune responses. The powerful immunostimulatory properties of TLR agonists were exploited and compared with TNF-α for stimulating complete maturation of AML-DC and augmentation of active immunity against AML in this study. TLR-3, TLR-4, TLR-7/8 and TLR-9 agonists are in the National Cancer Institute’s list of immunotherapeutic agents ranked with the highest potential to treat cancer [38]. Since TLR-4, TLR-7/8 and TLR-9 were reported to be expressed in AML cells with very low expression of TLR-9 [23, 24], we investigated TNF-α, LPS, R848 and their combinations for their ability to stimulate activation of AML cells toward mature DC differentiation. LPS used in this AML-MNC culture was 500 pg/ml. After 1-day stimulation, the cells were washed twice to remove the TLR agonists. The culture was continued for 3–4 weeks with repeated changing of culture medium. After three final washes, the residual LPS in harvested CTLs is likely negligible. The efficiency of single or combinations of reagents in stimulating AML blast DC differentiation and maturation was evaluated by three criteria: (1) stimulating phenotypic change of AML cells toward mature DC; (2) IFN-γ release by culture-expanded CTL in response to autologous AML cells; and (3) killing of autologous AML blasts by CTLs generated in our AML-MNC culture.
AML blasts are of low immunogenicity, probably because of the lack of the co-stimulatory molecules. We showed that treating AML-DC with single-reagent TNF-α, LPS, R848 and their various combinations could significantly increase CD80, CD86, CD40, CD54, CD83 and HLA-DR compared to medium control. CD80 expression on AML cells was higher using the combination of T + L + R compared with single reagents, and it was significant for T. CTLs that were derived from AML-PBMNC culture stimulated by all the combinations and single-reagent R released significantly higher IFN-γ in response to autologous AML cells compared with medium control, but T or L alone did not. Among reagents and combinations, T + L + R was significantly higher than T or L alone and T + R was significantly higher than T alone. Comparing CTL activity stimulated with single reagents or combinations versus the medium control, again the overall effect of AML-specific CTL of nine patients showed significantly enhanced killing of AML cells by all reagent combinations and R alone. AML cell killing by CTL using T and L was higher, but not significantly so, compared with medium. The results indicated that the effects of cooperatively triggering multiple TLRs promoted the most consistently effective immune responses to the heterogeneous AML cells from different patients, presumably due to the genetic heterogeneity of AML.
The significantly enhanced mature DC phenotype, IFN-γ release response and autologous AML cell killing by the T + L + R combination may due to the complementary effects of reagent combination on the cells from different AML patients. In contrast to normal myeloid DC differentiation, heterogeneity of AML patient cells, with variability in the phenotype, various genetic mutations and clone diversity, may predict different responses to individual reagents and/or variability in T cell immune responses to culture-derived AML-DC. Because of the heterogeneous nature of AML, the advantage of stimulating AML cells with T + L + R agonist combinations is that the mixture of T + L + R agonists may be effective in triggering TLR-induced activation over a wider range of TLR expression of AML cells from different patients.
These results may have important implications with regard to the design of clinical trials in humans. We speculate that the combination of T + L + R may be the best method for AML-DC and CTL stimulation for most AML patients, but that pretesting of each patient may be necessary to select an optimal TLR agonist or combination to generate optimal AML-reactive CTLs for immunotherapy. In addition, our results suggest that the criteria for selection of the optimal culture conditions may require testing of all three assays employed in this study, i.e., AML-DC phenotype and both IFN-γ release and cytotoxicity by CTL in response to autologous AML cells. However, given the uncertain predictability of in vitro assays of CTL effectiveness to in vivo results [39], additional investigation will be required to assess which of the above assays or combination of assays correlates best with in vivo elimination of AML.
In conclusion, these studies demonstrated that the combination of R848, LPS and TNF-α stimulated AML-DC with optimal maturation and higher CTL priming and cytotoxic activity in our AML-MNC culture. However, there was significant heterogeneity among patients with respect to generating the most effective CTL. Moreover, additional investigation will be required in order to assess the extent to which these findings translate to in vivo elimination of AML.
Acknowledgments
This study was funded in part by the Federico Foundation and the Acute Myeloid Leukemia Research Fund at the University of California, San Diego (UCSD).
Abbreviations
- AML
Acute myeloid leukemia
- BM
Bone marrow
- CD
Cluster differentiation
- CFSE
5-(and 6)-Carboxyfluorescein diacetate succinimidyl ester
- CR
Complete remission
- CTL
Cytotoxic T lymphocyte
- DC
Dendritic cell
- AML-DC
Dendritic cells differentiated AML cells
- FACS
Fluorescence-activated cell sorting
- GM-CSF
Granulocyte/monocyte colony-stimulating factor
- IFN
Interferon
- IRB
Institutional review board
- LPS (L)
Lipopolysaccharide
- MNC
Mononuclear cells
- NK
Natural killer
- PAMP
Pathogen-associated molecular patterns
- PB
Peripheral blood
- R848 (R)
Resiquimod
- TLR
Toll-like receptors
- TNF-α (T)
Tumor necrosis factor
Footnotes
Conflict of interest The authors declare that they have no conflict of interest to report.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi ST, Lariviere L, Leveque G. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (TLR4) J Exp Med. 1999;189(4):615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoshino K, Takeuchi O, Kawai T. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J Immunol. 1999;162(7):3749–3752. [PubMed] [Google Scholar]
- 6.Grote K, Schütt H, Schieffer B. Toll-like receptors in angiogenesis. Sci World J. 2011;11:981–991. doi: 10.1100/tsw.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 8.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 9.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636–1642. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 10.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 11.Mansson A, Cardell LO. Role of atopic status in Toll-like receptor (TLR)7- and TLR9-mediated activation of human eosinophils. J Leukoc Biol. 2009;85:719–727. doi: 10.1189/jlb.0808494. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen G, Andresen L, Matthiessen MW, Rask-Madsen J, Brynskov J. Expression of Toll-like receptor 9 and response to bacterial CpG oligodeoxynucleotides in human intestinal epithelium. Clin Exp Immunol. 2005;141:298–306. doi: 10.1111/j.1365-2249.2005.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gribar SC, Anand RJ, Sodhi CP, Hackam DJ. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J Leukoc Biol. 2008;83:493–498. doi: 10.1189/jlb.0607358. [DOI] [PubMed] [Google Scholar]
- 14.Olson JK, Miller S. Microglia initiate central nervous system innate and adaptive immune response through multiple TLRs. J Immunol. 2004;173(6):3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 15.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Antonio Lanzavecchia. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24(6):801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boiko Julie R, Borghesi Lisa. Hematopoiesis sculpted by pathogens: Toll-like receptors and inflammatory mediators directly activate stem cells. Cytokine. 2012;57:1–8. doi: 10.1016/j.cyto.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19(1):24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Latz E, Schoenemeyer A, Visintin A. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5(2):190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Amakawa R, Kaisho T. Interferon-α and interleukin-12 are induced differentially by Toll like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195(11):1507–1512. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubnitz JE, Gibson B, Smith FO. Acute myeloid leukemia. Hematol Oncol Clin N Am. 2010;24(1):35–63. doi: 10.1016/j.hoc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Baldus CD, Bullinger L. Gene expression with prognostic implications in cytogenetically normal acute myeloid leukemia. Semin Oncol. 2008;35(4):356–364. doi: 10.1053/j.seminoncol.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Smits EL, Cools N, Lion E, Van Camp K, Ponsaerts P, Berneman ZN, Van Tendeloo VF. The Toll-like receptor 7/8 agonist resiquimod greatly increases the immunostimulatory capacity of human acute myeloid leukemia cells. Cancer Immunol Immunother. 2010;59(1):35–46. doi: 10.1007/s00262-009-0721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt A, Li L, Giannopoulos K, Greiner J, Reinhardt P, Wiesneth M, Schmitt M. Quantitative expression of Toll-like receptor-2, -4, and -9 in dendritic cells generated from blasts of patients with acute myeloid leukemia. Transfusion. 2008;48(5):861–870. doi: 10.1111/j.1537-2995.2007.01616.x. [DOI] [PubMed] [Google Scholar]
- 25.Robinson SP, English N, Jaju R, Kearney L, Knight SC, Reid CDL. The in vitro generation of dendritic cells from blast cells in acute leukemia. Brit J Hematol. 1998;103:763–771. [PubMed] [Google Scholar]
- 26.Charbonnier A, Gaugler B, Sainty D, Lafage-Pochitaloff M, Olive D. Human acute myeloblastic leukemia cells differentiate in vitro into mature dendritic cells and induce the differentiation of cytotoxic T cells against autologous leukemias. Eur J Immunol. 1999;29:2567–2578. doi: 10.1002/(SICI)1521-4141(199908)29:08<2567::AID-IMMU2567>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 27.Cignetti A, Bryant E, Allione B, Vitale A, Foa R, Cheever MA. CD34+ acute myeloid and lymphoid leukemic blasts can be induced to differentiate into dendritic cells. Blood. 1999;94:2048–2055. [PubMed] [Google Scholar]
- 28.Choudhury BA, Liang JC, Thomas EK. Dendritic cells derived in vitro from acute myelogenous leukemia cells stimulate autologous, antileukemic T-cell responses. Blood. 1999;93:780–786. [PubMed] [Google Scholar]
- 29.Woiciechowsky A, Regn S, Kolb HJ, Roskrow M. Leukemic dendritic cells generated in the presence of FLT3 ligand have the capacity to stimulate an autologous leukemia-specific cytotoxic T cell response from patients with acute myeloid leukemia. Leukemia. 2001;15:246–255. doi: 10.1038/sj.leu.2402013. [DOI] [PubMed] [Google Scholar]
- 30.Gruijl TD, Eertwegh AJM, Pinedo HM, Scheper RJ. Whole-cell cancer vaccination: from autologous to allogeneic tumor- and dendritic cell-based vaccines. Cancer Immunol Immunother. 2008;57:1569–1577. doi: 10.1007/s00262-008-0536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheuk ATC, Ba Guinn. Immunotherapy of acute myeloid leukaemia: development of a whole cell vaccine. Front Biosci. 2008;13:2022–2029. doi: 10.2741/2820. [DOI] [PubMed] [Google Scholar]
- 32.Zhong RK, Lane TA, Ball ED. Generation of T cell lines to autologous acute myeloid leukemia (AML) cells by competitive limiting dilution culture of AML mononuclear cells (MNC) Exp Hematol. 2008;36(4):486–494. doi: 10.1016/j.exphem.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Jedema I, van der Werff NM, Barge RM, Willemze R, Falkenburg JH. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood. 2004;103(7):2677–2682. doi: 10.1182/blood-2003-06-2070. [DOI] [PubMed] [Google Scholar]
- 34.R Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. http://www.R-project.org/ [Google Scholar]
- 35.McIlroy D, Gregoire M. Optimizing dendritic cell-based anticancer immunotherapy: maturation state does have clinical impact. Cancer Immunol Immunother. 2003;52(10):583–591. doi: 10.1007/s00262-003-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thurner B, Roder C, Dieckmann D, Heuer M, Kruse M, Glaser A. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods. 1999;223(1):1–15. doi: 10.1016/s0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- 37.Jonuleit H, Giesecke-Tuettenberg A, Tuting T, Thurner-Schuler B, Stuge TB, Paragnik L, Kandemir A, Lee PP, Schuler G, Knop J, Enk AH. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer. 2001;93:243–251. doi: 10.1002/ijc.1323. [DOI] [PubMed] [Google Scholar]
- 38.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 39.Schürch C, Riether C, Amrein M, Ochsenbein A. Cytotoxic T cells induce proliferation of chronic myeloid leukemia stem cells by secreting interferon-γ. J Exp Med. 2013;210(3):605–621. doi: 10.1084/jem.20121229. [DOI] [PMC free article] [PubMed] [Google Scholar]