Abstract
Purpose
To implement the maximum level of statistical iterative reconstruction that can be used to establish dose-reduced head CT protocols in a primarily pediatric population.
Methods
Select head examinations (brain, orbits, sinus, maxilla and temporal bones) were investigated. Dose-reduced head protocols using an adaptive statistical iterative reconstruction (ASiR) were compared for image quality with the original filtered back projection (FBP) reconstructed protocols in phantom using the following metrics: image noise frequency (change in perceived appearance of noise texture), image noise magnitude, contrast-to-noise ratio (CNR), and spatial resolution. Dose reduction estimates were based on computed tomography dose index (CTDIvol) values. Patient CTDIvol and image noise magnitude were assessed in 737 pre and post dose reduced examinations.
Results
Image noise texture was acceptable up to 60% ASiR for Soft reconstruction kernel (at both 100 and 120 kVp), and up to 40% ASiR for Standard reconstruction kernel. Implementation of 40% and 60% ASiR led to an average reduction in CTDIvol of 43% for brain, 41% for orbits, 30% maxilla, 43% for sinus, and 42% for temporal bone protocols for patients between 1 month and 26 years, while maintaining an average noise magnitude difference of 0.1% (range: −3% to 5%), improving CNR of low contrast soft tissue targets, and improving spatial resolution of high contrast bony anatomy, as compared to FBP.
Conclusion
The methodology in this study demonstrates a methodology for maximizing patient dose reduction and maintaining image quality using statistical iterative reconstruction for a primarily pediatric population undergoing head CT examination.
I. Introduction
Use of statistical iterative reconstruction (IR) has been demonstrated as an effective method for lowering radiation exposure in thoracic and abdominopelvic CT1-8. Recently, several studies have investigated reduced dose in head CT using statistical IR9-14; however, only two studies examined a pediatric population10, 11. These studies investigated the effect of statistical IR on image quality using metrics such as noise magnitude, by measuring the inter-pixel variation or standard deviation within a region of interest (ROI). Measuring noise magnitude is simple, but does not fully describe the effect statistical IR algorithms have on the texture, or appearance of the pixelated noise, as has been reported previously2, 15, 16.
Current institutional examinations for chest and abdomen-pelvis are performed on a Lightspeed VCT-XTe (GE Healthcare, Waukesha WI) and incorporate adaptive statistical iterative reconstruction (ASiR; GE Healthcare)1, 2, but protocols involving the head (brain, orbits, sinus, maxilla and temporal bone) are reconstructed using filtered back projection (FBP). The purpose of this study was to implement the maximum level of statistical IR for dose-reduced head protocols using ASiR in a primarily pediatric population while maintaining similar image noise magnitude. Fourier based image quality metrics, such as noise power spectrum (NPS) and modulation transfer function (MTF), were used to fully characterize effects of ASiR on noise and spatial resolution. Dose reduction estimates are based on a comparison of pre and post dose-reduced examination volume computed tomography dose index (CTDIvol) values.
II. Materials and Methods
II. A. Head CT image quality analyzed in phantom
To determine the maximum possible level of statistical IR and tube current (i.e., mA) reduction, image quality from ASiR reconstruction was analyzed and compared to image quality from the original head protocols using FBP. Image quality was assessed in phantom based on the measured change of: image noise frequency (i.e., change in perceived appearance of noise texture as quantified by calculating the NPS), image noise magnitude (i.e., calculated using standard deviation of an ROI), contrast-to-noise ratio (CNR), and spatial resolution (calculated using MTF).
The NPS was calculated using a 20 cm diameter uniform water phantom (Quality Assurance Phantom; GE Healthcare). The water phantom was scanned to produce 12, 2.5 mm images using tube potential (i.e., kVp) and other acquisition factors from the original head protocols [Table 1]. The images were averaged together, and the center of the averaged image was used to calculate a single NPS curve2. Initially, the uniform water phantom was imaged at the CTDIvol and mA, or, in the case of tube current modulated (TCM) examinations, the Noise Index value recorded for the original clinical FBP protocol. To produce a series of noisier images, the mA setting was decremented in steps of 10 mA until the original CTDIvol decreased by ~70% (e.g., for > 19 year old brain protocol, the initial CTDIvol and mA was 36.6 mGy and 200 mA, both were decremented until 10.04 mGy and 60 mA); for head scan techniques imaged using TCM, the Noise Index value was incremented2 (thus allowing a lower mA) in steps of three. All other acquisition parameters were held constant, [Table 1]. Each mA-reduced image was reconstructed using the Soft, Standard, and Bone reconstruction kernels at every level of ASiR (0-100%; where 0% ASiR represents 100% FBP). Image noise magnitude, variance, and NPS were calculated using a script written in MATLAB (R2014b, Mathworks, Natick, MA).
Table 1.
Head CT examination parameters. All protocols were imaged with a Scan field of view (SFOV) using “Head” unless otherwise indicated. All helical acquisitions were scanned with a pitch of 0.984 unless otherwise indicated.
| Patient Age (yrs) | (A)xial (H)elical | Rotation (sec) | Collimation (mm) | Slice (mm) | Reconstruction Kernel | kVp | Pre-ASiR mA | Post-ASiR mA |
|---|---|---|---|---|---|---|---|---|
| Brain | ||||||||
| 0-2a | A | 0.5 | 20 | 5 | Soft & Bone | 100 | 280 | 150 |
| 2-5 | 1 | 200 | 120 | |||||
| 6-10 | 220 | 130 | ||||||
| 11-18 | 240 | 140 | ||||||
| > 19 | 120 | 200 | 105 | |||||
| Sinus | ||||||||
| ≥ 19 | H | 0.5 | 40 | 2.5 | Soft & Bone | 120 | NId = 7.5 | 155 |
| 0-18 | 100 | 220 | 130 | |||||
| Orbits | ||||||||
| 0-18b | Hc | 0.5 | 20 | 1.25 | Standard & Bone | 100 | 240 | 155 |
| Temporal Bone | ||||||||
| ≥ 19 | H | 1 | 20 | 1.25 | Standard & Bone | 120 | 250 | 150 |
| 2-18 | 0.5 | 120 | 400 | 230 | ||||
| Maxillary Bone | ||||||||
| ≥ 19 | Hc | 0.5 | 20 | 2.5 | Standard & Bone | 120 | NId = 7.5 | NId = 9.25 |
| 0-18 | H | 100 | 300 | 180 | ||||
SFOV used “Ped Head”
SFOV used “Small Head”
Pitch = 0.516
Noise Index (NI)
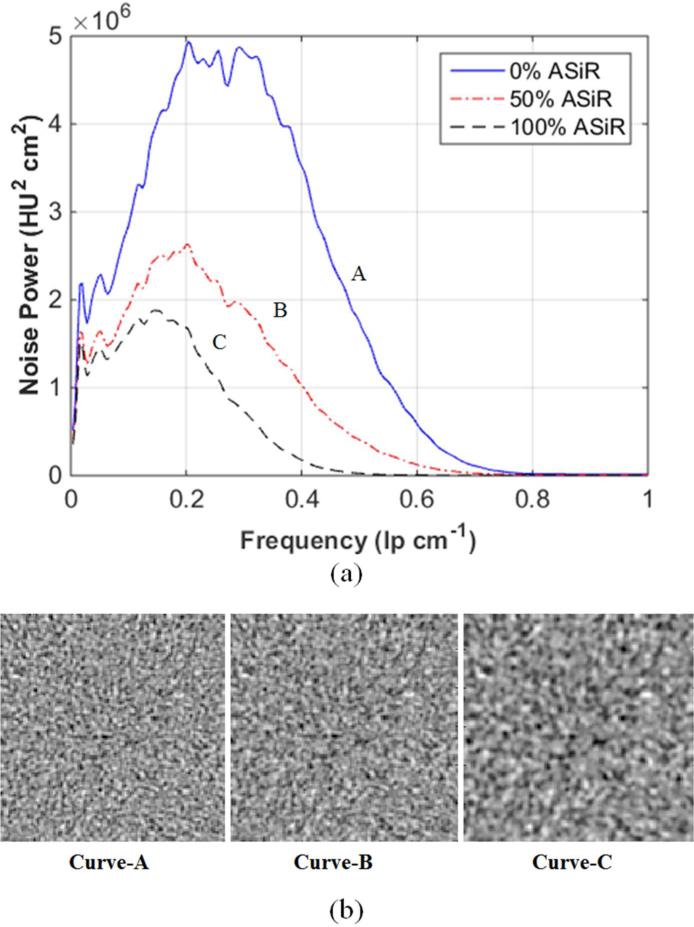
The NPS of dose-reduced statistical IR data were grouped according to similar amplitudes (i.e., the measure of noise variance), by reconstruction kernel type (i.e., Soft, Standard, or Bone), and kVp level (i.e., 100 and 120). From these matched NPS curves, the shift in mean NPS frequency was calculated at each level of ASiR reconstruction. The texture of the image noise, as it appeared in reconstructed images, changed as the mean of the NPS curve shifted along the abscissa; thus, shifts in mean NPS frequency were associated with changes in image noise texture, [Fig 1], as has been shown in previous studies2, 16.
Fig 1.
Texture of image noise, as it appears in reconstructed images, changes as the mean of the NPS curve shifts along the abscissa; shifts in mean NPS frequency are associated with changes in appearance of image noise texture. (a) NPS curves of the Standard reconstruction kernel are reconstructed at three levels of ASiR. (b) A corresponding ROI of 128 × 128 pixels from the center of a water phantom shows the appearance of the noise texture as it correlates with a 32% shift in NPS mean frequency along the abscissa from curve A to B and 52% shift in curve A to C.
A literature search was conducted to determine the level of acceptable shift in mean NPS frequency in lieu of a receiver operating characteristic (ROC) test performed by institutional radiologists. Acceptable changes in perceived noise texture determined by an institutional ROC would not be generalizable, whereas a literature search represented a multi-institutional consensus. The resulting literature search indicated for soft tissue reconstruction kernels, typical of body imaging (i.e., the Standard kernel), an average implementation of 40% ASiR reconstruction1-3, 6-8, 17-19 correlated with an acceptable change in perceived image noise texture, or mean NPS frequency shift of 25% (range 16%-40%)2, 4, 5, 20, 21. No level of acceptable shift in mean NPS frequency was reported for the Soft reconstruction kernel typical for brain CT. The tolerance of 25% reported for the Standard reconstruction kernel was adopted for the Soft reconstruction kernel.
Images of low contrast targets were acquired to qualitatively compare noise texture. The low contrast targets were imaged at multiple mA-reduced, ASiR reconstructed levels, and compared to the original full dose protocol using FBP. Images of low contrast targets were acquired with the Soft and Standard reconstruction kernels using a Catphan 700 phantom (The Phantom Laboratory, Salem NY), and CNR of the 3 mm diameter target were calculated. Additionally, a qualitative assessment of low contrast target was performed on a diagnostic quality display (Dome S3c, NDSsi, San Jose, CA) under reading room ambient light control (i.e., illuminance average ~ 20 lx).
Fine detail spatial resolution was evaluated for the Bone reconstruction kernel by calculating MTF from images of high contrast targets using the Catphan 700 phantom. FBP and mA-reduced statistical IR images were used to image the phantom. Twelve scans of the first test module were acquired and averaged. The Fourier transform of the derivative of an ensemble of one-dimensional edge spread functions sampled radially across the bone circular boundary insert was used to calculate the MTF22.
The percent difference between mA values from the FBP image and the matched NPS curve reconstructed with statistical IR was used to determine new dose-reduced mA settings for all head protocols. All changes to protocols were reviewed by the chief neuroradiologist prior to implementation.
II.B. Image quality and dosimetry analyzed from patient examination
Our institutional review board deemed this quality assurance analysis exempt from the need to obtain informed consent. All data were managed in compliance with the Health Insurance Portability and Accountability Act. Head protocols were selected on the basis of each patient's age, which was obtained immediately prior the examination. Pre dose-reduced examination CTDIvol values were analyzed from June 2013-2014. Post dose-reduced values were analyzed from June 2014-2015.
Reconstructed image noise magnitude from pre and post dose-reduced patient examination images was assessed based on an ROI analysis. Multiple ROIs were placed in regions of uniformity within the brain and averaged; the locations varied depending on the examination type. Image noise analysis was only for images reconstructed with soft tissue reconstruction kernels (i.e., Soft or Standard).
III. Results
III.A. Head CT image quality analyzed in phantom
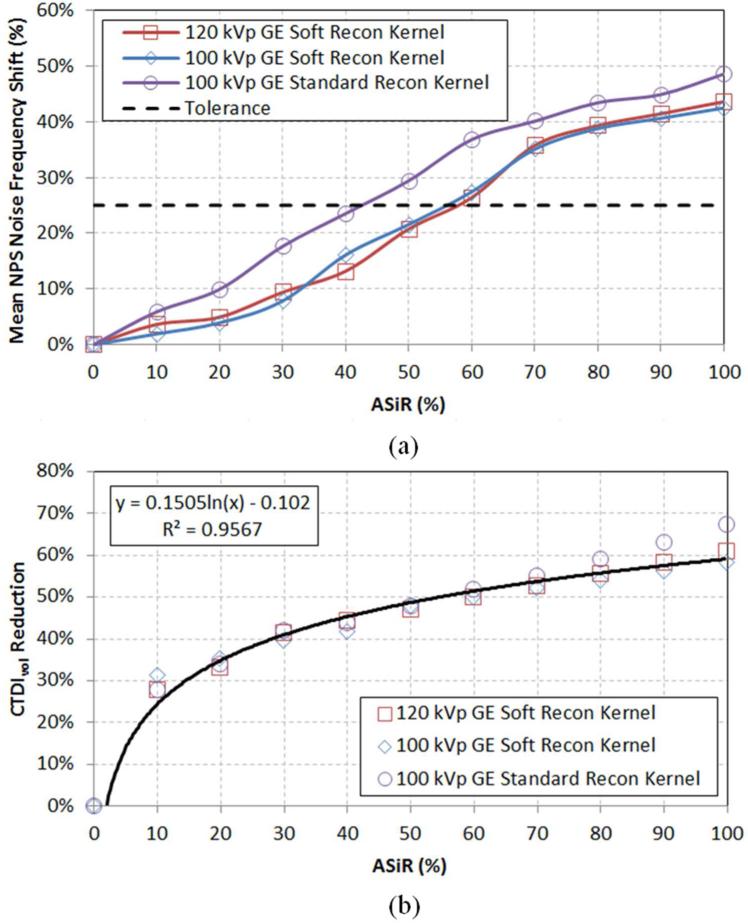
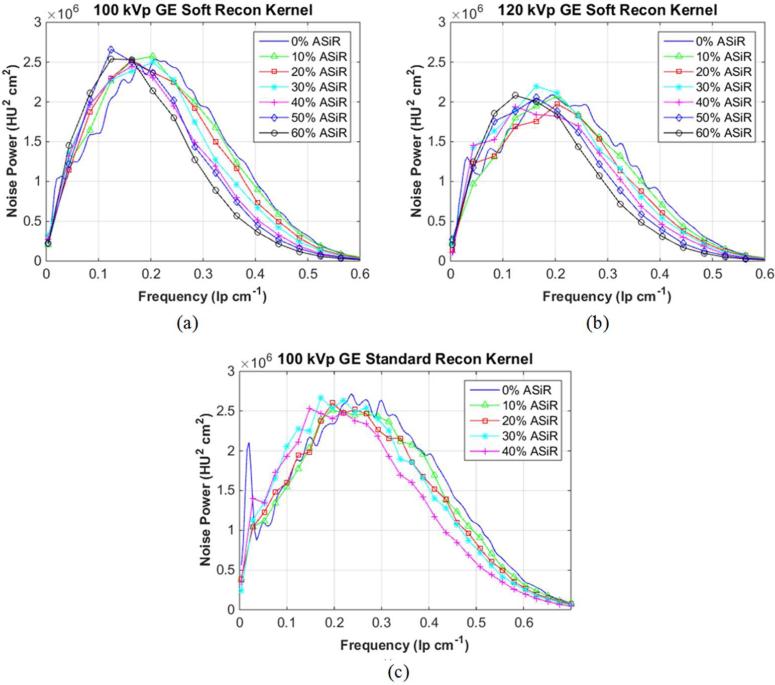
Eleven NPS (1 FBP, 10 ASiR spectra) were calculated for the Soft reconstruction kernel (at both 100 and 120 kVp) and the Standard reconstruction kernel (100 kVp). The percent shift in mean NPS frequency for each spectrum was plotted as a function of level of ASiR, [Fig 2(a)] with its accompanying reduction in CTDIvol, [Fig 2(b)]. Shift of mean NPS frequency (i.e., noise texture) was impacted mostly by selection of reconstruction kernel, and not level of kVp. Based on the reported4, 5, 20, 21 25% threshold for acceptable change in perceived noise texture (dashed line in [Fig 2(a)]), an implementation of 60% ASiR was chosen for the Soft reconstruction kernel and 40% ASiR was chosen for the Standard reconstruction kernel, which data for the Standard reconstruction kernel agrees with previous publications3, 6-8, 17-19. Dose-reduced NPS curves, for Soft reconstruction kernel was calculated up to 60% ASiR, and for the Standard reconstruction kernel, up to 40% ASiR [Fig 3]. The overall noise magnitude and variance for the dose-reduced ASiR spectra were matched to the original FBP noise amplitude to a mean (± 1 standard deviation) of 4.8 HU (±0.4), 4.1 HU (±0.3), and 5.7 HU (±0.5) for protocols acquired with the Soft reconstruction kernel at 100 kVp [Fig 3(a)] and 120 kVp [Fig 3(b)], and the Standard reconstruction kernel at 100 kVp [Fig 3(c)], respectively.
Fig. 2.
Dose-reduced ASiR protocols compare (a) the mean NPS frequency shift as a function of level of ASiR reconstruction. An acceptable tolerance for the appearance of noise texture in the reconstructed image is reported in the literature [ref 4, 5, 20, 21] based on a 25% shift of NPS noise frequency (dashed line). (b) Corresponding reductions of CTDIvol for the protocols using ASiR are plotted and fit using a log-regression function.
Fig. 3.
Noise power spectra acquired with the (a) Soft reconstruction kernel at 100 kVp from 240-120 mA, (b) with the Soft reconstruction kernel at 120 kVp from 200-110 mA, and the (c) Standard reconstruction kernel at 100 kVp from 250-140 mA. The calculated spectra are reconstructed at 0-60% ASiR (Soft reconstruction kernel) and at 0-40% ASiR (Standard reconstruction kernel).
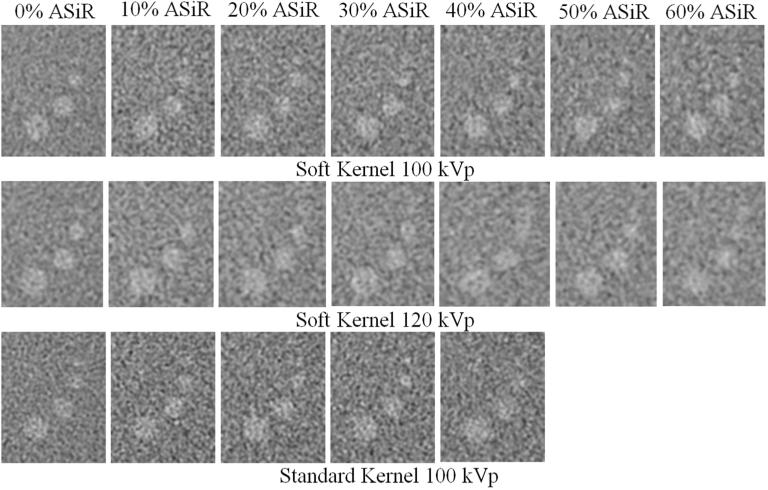
The visual assessment of low contrast targets demonstrates a slight degradation in lesion boundary sharpness with ASiR reconstruction, [Fig. 4]. However, for images reconstructed with the Soft reconstruction kernel, CNR improved with increasing level of ASiR reconstruction. The smallest low contrast target (3 mm) acquired at 100 kVp had a CNR calculated to be 1.2 at 0% ASiR and 2.4 at 60% ASiR. For targets acquired at 120 kVp, CNR was calculated to be 1.7 at 0% ASiR and 2.3 at 60% ASiR. For targets acquired with the Standard reconstruction kernel, CNR improved up to the level of 30% ASiR, CNR was 1.4 at 0% ASiR and 1.9 at 30% ASiR; however, CNR was only 1.8 at 40% ASiR, a slight decrease from 30% ASiR.
Fig. 4.
Images of the 3, 5, 7, and 9 mm low contrast targets in the Catphan 700 phantom are acquired with FBP and dose-reduced ASiR reconstruction up to 60% ASiR for the Soft reconstruction kernels at both 100 and 120 kVp and up to 40% for the Standard reconstruction kernel at 100 kVp.
For image quality measurements of the Bone reconstruction kernel, the dose-reduced NPS demonstrated an overall average reduction in noise variance by 26% (range: 7%-36%) compared to non-dose-reduced FBP protocols. Additionally, spatial resolution calculated for the dose-reduced 60% ASiR protocol improved by an average 26% (range: 24%-30%) when compared at the 50% MTF level, and 113% (range: 101%-123%) at the 10% MTF level.
III.B. Image quality and dosimetry analyzed from patient examination
Total number of pre dose-reduced examinations analyzed was 376 (242 male); the mean age was 9.6 ± 6.2 years (1 month to 24 years). The number of examinations analyzed per protocol was: 220 brain, 11 orbits, 98 sinus, 37 maxilla, and 10 temporal bones. Total number of post dose-reduced examinations analyzed was 361 (212 male); the mean age was 10.7 ± 6.6 (1 month to 26 years). The number of examinations analyzed per protocol was: 193 brain, 3 orbits, 127 sinus, 35 maxilla, and 3 temporal bones. Lowering the protocol mA [Table 1] and implementing 40% or 60% ASiR, for image noise control, resulted in lowered CTDIvol values as shown in Figure 3(b). The percent reduction in CTDIvol for all examinations is shown in Table 2. Image noise magnitude from the dose-reduced patient examinations was shown to change by an average difference of 0.1% (range: −3% to 5%) compared to the original FBP patient examinations, [Table 2]
Table 2.
Original and dose-reduced CTDIvol values for all head protocols
| CTDIvol (mGy) | Noise (HU) | ||||||
|---|---|---|---|---|---|---|---|
| Patient Age Category | Protocol | Original | Dose-reduced | Difference | Original | Dose-reduced | Difference |
| 0-23 mon | Brain | 15.0±0.7 | 8.0±0.4 | −47% | 4.4±1.0 | 4.2±0.7 | −3% |
| 2-5 yrs | Brain | 24.1±0.9 | 14.6±0.6 | −39% | 4.2±0.7 | 4.1±0.7 | −3% |
| 6-10 yrs | Brain | 26.3±1.3 | 15.9±0.4 | −40% | 4.1±0.5 | 4.2±0.6 | 4% |
| 11-18 yrs | Brain | 29.1±0.9 | 17.0±0.5 | −42% | 4.4±0.6 | 4.5±0.6 | 3% |
| ≥ 19 yrs | Brain | 36.6±0.8 | 18.9±0.5 | ≥48% | 4.3±0.6 | 4.4±0.4 | 3% |
| 0-18 yrs | Maxilla | 19.4±0.0 | 11.5±0.1 | −41% | 11.2±2.8 | 11.6±1.6 | 3% |
| ≥ 19 yrs | Maxilla | 22.8±0.0 | 18.7±0.2 | −18% | 9.6±1.4 | 9.2±1.7 | −3% |
| 0-18 yrs | Orbits | 26.9±8.0 | 15.8±0.5 | −41% | 7.5±1.2 | 7.2±0.1 | −4% |
| 0-18 yrs | Sinus | 13.1±0.0 | 7.2±0.3 | −45% | 8.5±1.2 | 8.9±1.1 | 5% |
| ≥ 19 yrs | Sinus | 22.8±0.0 | 13.7±0.1 | −40% | 8.3±0.9 | 8.2±0.6 | −1% |
| 2-18 yrs | Temporal | 40.7±0.0 | 22.8±0.0 | −44% | 9.3±1.4 | 9.2±1.2 | −2% |
| ≥ 19 yrs* | Temporal | 49.9±0.0 | 29.7±0.0 | −40% | 9.3±1.0 | ||
No dose reduced patient examinations were available for comparison. Dose-reduced CTDIvol value is calculated based on scan parameters. Dose difference is a theoretical calculation.
IV Discussion
The purpose of this study was to implement the maximum level of statistical IR that could be used to establish dose-reduced pediatric head protocols (i.e., brain, orbits, sinus, maxilla, and temporal bone), while maintaining acceptable image quality. The use of NPS to evaluate image quality is a departure from the more commonly used metrics of CNR, SNR, and standard deviation as previously reported11-14. Using NPS allowed the definition of acceptable image quality to be based on the results from multiple published observer studies instead of a single institute analysis; thus, the results of this analysis will be more generalizable across pediatric imaging institutions. The results of this study provide a more in-depth description of image appearance and noise texture, and demonstrate a methodical approach for application of the highest possible dose reduction using statistical IR while maintaining similar noise magnitude in the reconstructed image.
Images acquired with higher levels of statistical IR can appear overly smooth, leading to concerns about visibility of anatomic structures. This change in image appearance is likely a visual manifestation of a shift in the spatial frequency distribution of the image noise. By measuring the mean frequency of NPS curves, the image noise texture produced by ASiR for the dose-reduced protocols could be compared to the image texture produced by the original FBP protocols, allowing the selection of acceptable change in noise texture. While the dose-reduced protocols did result in changes in the spatial frequency, these shifts were similar to the reported tolerance for soft tissue imaging in the body4, 5, 20, 21, and were not detrimental for image diagnosis as determined by the radiologists at our institution.
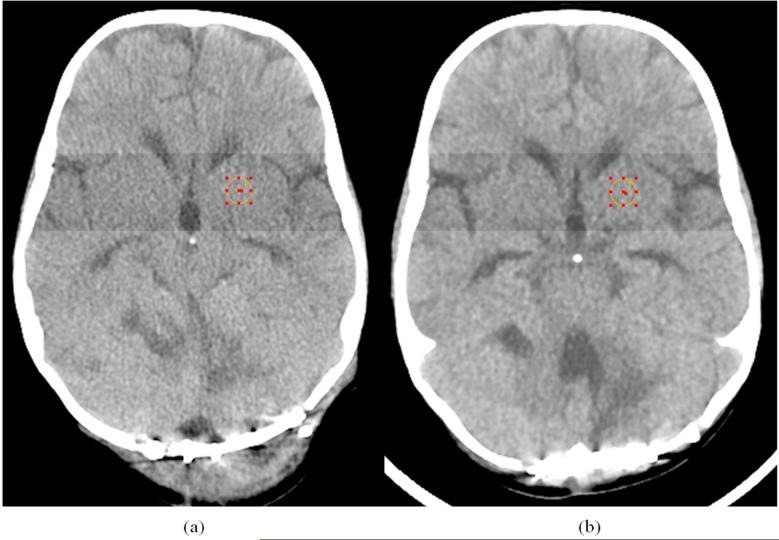
In one clinical example, image noise magnitude was measured in two axial brain examinations of a 16 kg (3 year old) patient performed approximately 6 months apart. The first scan [Fig. 5(a)] was acquired with the original institutional protocol, and the second [Fig. 5(b)], with the dose-reduced protocol at 60% ASiR. Noise texture appearance was slightly coarser, but the noise magnitude, as measured by the standard deviation of a 1 cm2 ROI, was 3.8 HU in the in the pre-ASiR image and 4.0 HU in the post-ASiR image. The pre-ASiR image was acquired at 200 mA, and the post-ASiR image at 120 mA (both at 100 kVp); all other scan parameters were constant with a minor difference in gantry tilt angle to align with the orbitomeatal line. The change in mA represented a decrease in CTDIvol from 25.1 mGy to 15.0 mGy, a dose reduction of 40%.
Fig. 5.
3 year old male scanned 6 months apart. (a) The original brain protocol is acquired at 200 mA and 100 kVp with a CTDIvol of 25.1 mGy using FBP. (b) The patient is reexamined post-surgical operation with the dose-reduced brain protocol using 60% ASiR, 120 mA, 100 kVp, with a CTDIvol of 15.0 mGy, Both examinations are acquired using the GE Soft reconstruction kernel.
A comparison of radiation dose reduction between FBP and dose-reduced ASiR brain protocols with previously published studies follows. By implementing 30% ASiR reconstruction, Kilic et. al11 reported a reduction of an adult brain protocol of 35% (CTDIvol: 59.4 to 38.6 mGy); whereas this study achieved a 48% dose reduction from 36.6 to 18.9 mGy in a population of patients ≥ 19 years utilizing 60% ASiR. For pediatric brain scans, Vorona et. al9 reported a reduction of 22% (CTDIvol: 28.8 to 22.4 mGy) for patients 3-18 years old using 20% ASiR, as compared to the average reduction of 40% (CTDIvol: 26.5 to 15.8 mGy) in this study for the same age range using 60% ASiR. Also, for pediatric brain scans, McKnight et. al10, using 30% ASiR, reported a reduction of CTDIvol of 28% (30.0 to 21.5 mGy) for patients 3-12 years old and 48% (49.9 to 25.7 mGy) for patients > 12 years old, as compared to the 40% (25.2 to 15.3 mGy) and 45% (32.9 to 18.0 mGy) dose reduction reported in this study utilizing 60% ASiR, respectively. Percent reductions are relative to the initial CTDIvol calculated using FBP reconstruction. Similarities in dose reduction between this study and other previous studies, despite differences in level of statistical IR implementation, are due to differences in the initial FBP CTDIvol values.
In this study, the statistical IR technique ASiR was used to mitigate increased image noise from reductions of tube current allowing reduce patient examination radiation dose. The use of ASiR is only available on GE scanners. Other statistical IR algorithms are available for use with other CT manufacturers and may be used for potential head CT dose reduction purposes. The implementation of these statistical IR algorithms will be subtly different; thus, the description of image noise texture and amount of dose reduction reported in this study may not be identical of other scanners using statistical IR algorithms for dose-reduced head CT. However, the principles outlined in the methodology of this study are universal, namely: the need to analyze both image noise magnitude (i.e., using traditional ROI analysis) and the visual perception of the noise texture (i.e., using Fourier analysis techniques such as NPS) for a more complete understanding of the impact on reconstructed patient image quality from statistical IR. The use of Fourier image quality metrics, such as NPS and MTF, will allow a more detailed analysis and customization of a statistical IR algorithm, no matter the application.
V. Conclusion
Substantial dose reduction can be achieved at higher levels of ASiR reconstruction than previously reported for head CT protocols. An analysis of the effects on the perceived appearance of noise texture from implementation of statistical IR was performed. In this study, it was shown that an implementation of 60% ASiR (Soft reconstruction kernel) and 40% ASiR (Standard reconstruction kernel) will produce acceptable changes in image noise texture in the reconstructed mage as defined in the scientific literature, and may be used for greater dose reduction. Head CT images acquired with the Soft and Standard reconstruction kernel demonstrated an overall improvement of CNR of the image. For all head protocols, the average reduction in CTDIvol was 43% for brain, 41% for orbits, 30% for maxilla, 43% for sinus, and 42% for temporal bone.
Acknowledgement
The authors would like to acknowledge Zoltan Patay, MD PhD for his advice and expertise. This work was supported by American Lebanese Syrian Associated Charities (ALSAC) and NCI R25E Grant 5R25CA23944.
DISCLOSURES: Amy Mirro, Samuel Brady—RELATED: Grant: NCI.* Robert Kaufman—RELATED: Grant: POE students are supported in part by grant R25CA23944 from the National Cancer Institute,* Comments: This funds partial support of the summer student POE program at St. Jude which helped to support co-author Amy Mirro, an undergraduate student at Washington University in St. Louis.
*money paid to institution
Abbreviations
- ASiR
Adaptive statistical iterative reconstruction
- CNR
Contrast-to-noise ratio
- FBP
Filtered back projection
- IR
Iterative Reconstruction
- NI
Noise index
- NPS
Noise power spectrum
- MTF
Modulation transfer function
- ROC
Receiver operating characteristic
- ROI
Region of Interest
- TCM
Tube current modulated
- CTDIvol
Volume computed tomography dose index
References
- 1.Brady S, Moore B, Yee B, et al. Implementation of ASiR™ reconstruction for substantial dose reduction in pediatric CT without affecting image noise. Radiology. 2014;270:223–231. doi: 10.1148/radiol.13122578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady SL, Yee BS, Kaufman RA. Characterization of adaptive statistical iterative reconstruction algorithm for dose reduction in CT: A pediatric oncology perspective. Medical physics. 2012;39:5520–5531. doi: 10.1118/1.4745563. [DOI] [PubMed] [Google Scholar]
- 3.Hara AK, Paden RG, Silva AC, et al. Iterative reconstruction technique for reducing body radiation dose at CT: feasibility study. AJR American journal of roentgenology. 2009;193:764–771. doi: 10.2214/AJR.09.2397. [DOI] [PubMed] [Google Scholar]
- 4.Hong SS, Lee JW, Seo JB, et al. Evaluation of image quality and radiation dose by adaptive statistical iterative reconstruction technique level for chest CT examination. Radiation protection dosimetry. 2013;157:163–171. doi: 10.1093/rpd/nct127. [DOI] [PubMed] [Google Scholar]
- 5.Sagara Y, Hara AK, Pavlicek W, et al. Abdominal CT: comparison of low-dose CT with adaptive statistical iterative reconstruction and routine-dose CT with filtered back projection in 53 patients. AJR American journal of roentgenology. 2010;195:713–719. doi: 10.2214/AJR.09.2989. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Kalra MK, Gilman MD, et al. Adaptive statistical iterative reconstruction technique for radiation dose reduction in chest CT: a pilot study. Radiology. 2011;259:565–573. doi: 10.1148/radiol.11101450. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Kalra MK, Hsieh J, et al. Abdominal CT: comparison of adaptive statistical iterative and filtered back projection reconstruction techniques. Radiology. 2010;257:373–383. doi: 10.1148/radiol.10092212. [DOI] [PubMed] [Google Scholar]
- 8.Vorona GA, Ceschin RC, Clayton BL, et al. Reducing abdominal CT radiation dose with the adaptive statistical iterative reconstruction technique in children: a feasibility study. Pediatr Radiol. 2011;41:1174–1182. doi: 10.1007/s00247-011-2063-x. [DOI] [PubMed] [Google Scholar]
- 9.Vorona GA, Zuccoli G, Sutcavage T, et al. The use of adaptive statistical iterative reconstruction in pediatric head CT: a feasibility study. AJNR American journal of neuroradiology. 2013;34:205–211. doi: 10.3174/ajnr.A3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKnight CD, Watcharotone K, Ibrahim M, et al. Adaptive statistical iterative reconstruction: reducing dose while preserving image quality in the pediatric head CT examination. Pediatr Radiol. 2014;44:997–1003. doi: 10.1007/s00247-014-2943-y. [DOI] [PubMed] [Google Scholar]
- 11.Kilic K, Erbas G, Guryildirim M, et al. Lowering the dose in head CT using adaptive statistical iterative reconstruction. AJNR American journal of neuroradiology. 2011;32:1578–1582. doi: 10.3174/ajnr.A2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korn A, Fenchel M, Bender B, et al. Iterative reconstruction in head CT: image quality of routine and low-dose protocols in comparison with Standard filtered back-projection. AJNR American journal of neuroradiology. 2012;33:218–224. doi: 10.3174/ajnr.A2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu YT, Mehta D, Zhang ZR, et al. Radiation dose reduction in temporal bone CT with iterative reconstruction technique. AJNR American journal of neuroradiology. 2012;33:1020–1026. doi: 10.3174/ajnr.A2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rapalino O, Kamalian S, Kamalian S, et al. Cranial CT with adaptive statistical iterative reconstruction: improved image quality with concomitant radiation dose reduction. AJNR American journal of neuroradiology. 2012;33:609–615. doi: 10.3174/ajnr.A2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samei E, Richard S. Assessment of the dose reduction potential of a model-based iterative reconstruction algorithm using a task-based performance metrology. Medical physics. 2015;42:314–323. doi: 10.1118/1.4903899. [DOI] [PubMed] [Google Scholar]
- 16.Solomon J, Samei E. Quantum noise properties of CT images with anatomical textured backgrounds across reconstruction algorithms: FBP and SAFIRE. Medical physics. 2014;41:091908. doi: 10.1118/1.4893497. [DOI] [PubMed] [Google Scholar]
- 17.Cornfeld D, Israel G, Detroy E, et al. Impact of Adaptive Statistical Iterative Reconstruction (ASIR) on radiation dose and image quality in aortic dissection studies: a qualitative and quantitative analysis. AJR American journal of roentgenology. 2011;196:W336–340. doi: 10.2214/AJR.10.4573. [DOI] [PubMed] [Google Scholar]
- 18.Flicek KT, Hara AK, Silva AC, et al. Reducing the radiation dose for CT colonography using adaptive statistical iterative reconstruction: A pilot study. AJR American journal of roentgenology. 2010;195:126–131. doi: 10.2214/AJR.09.3855. [DOI] [PubMed] [Google Scholar]
- 19.Leipsic J, Nguyen G, Brown J, et al. A prospective evaluation of dose reduction and image quality in chest CT using adaptive statistical iterative reconstruction. AJR American journal of roentgenology. 2010;195:1095–1099. doi: 10.2214/AJR.09.4050. [DOI] [PubMed] [Google Scholar]
- 20.Marin D, Nelson RC, Schindera ST, et al. Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm--initial clinical experience. Radiology. 2010;254:145–153. doi: 10.1148/radiol.09090094. [DOI] [PubMed] [Google Scholar]
- 21.Mieville FA, Gudinchet F, Brunelle F, et al. Iterative reconstruction methods in two different MDCT scanners: physical metrics and 4-alternative forced-choice detectability experiments--a phantom approach. Physica medica : PM : an international journal devoted to the applications of physics to medicine and biology : official journal of the Italian Association of Biomedical Physics. 2013;29:99–110. doi: 10.1016/j.ejmp.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Richard S, Husarik DB, Yadava G, et al. Towards task-based assessment of CT performance: System and object MTF across different reconstruction algorithms. Medical physics. 2012;39:4115–4122. doi: 10.1118/1.4725171. [DOI] [PubMed] [Google Scholar]